Abstract
DNA ligases, found in both prokaryotes and eukaryotes, covalently link the 3′-hydroxyl and 5′-phosphate ends of duplex DNA segments. This reaction represents a completion step for DNA replication, repair and recombination. It is well established that ligases are sensitive to mispairs present on the 3′ side of the ligase junction, but tolerant of mispairs on the 5′ side. While such discrimination would increase the overall accuracy of DNA replication and repair, the mechanisms by which this fidelity is accomplished are as yet unknown. In this paper, we present the results of experiments with Tth ligase from Thermus thermophilus HB8 and a series of nucleoside analogs in which the mechanism of discrimination has been probed. Using a series of purine analogs substituted in the 2 and 6 positions, we establish that the apparent base pair geometry is much more important than relative base pair stability and that major groove contacts are of little importance. This result is further confirmed using 5-fluorouracil (FU) mispaired with guanine. At neutral pH, the FU:G mispair on the 3′ side of a ligase junction is predominantly in a neutral wobble configuration and is poorly ligated. Increasing the solution pH increases the proportion of an ionized base pair approximating Watson–Crick geometry, substantially increasing the relative ligation efficiency. These results suggest that the ligase could distinguish Watson–Crick from mispaired geometry by probing the hydrogen bond acceptors present in the minor groove as has been proposed for DNA polymerases. The significance of minor groove hydrogen bonding interactions is confirmed with both Tth and T4 DNA ligases upon examination of base pairs containing the pyrimidine shape analog, difluorotoluene (DFT). Although DFT paired with adenine approximates Watson–Crick geometry, a minor groove hydrogen bond acceptor is lost. Consistent with this hypothesis, we observe that DFT-containing base pairs inhibit ligation when on the 3′ side of the ligase junction. The NAD+-dependent ligase, Tth, is more sensitive to the DFT analog on the unligated strand whereas the ATP-dependent T4 ligase is more sensitive to substitutions in the template strand. Electrophoretic gel mobility-shift assays demonstrate that the Tth ligase binds poorly to oligonucleotide substrates containing analogs with altered minor groove contacts.
INTRODUCTION
DNA ligases play an essential role in DNA replication, repair and recombination (1–3). Ligases carry out the covalent linkage of the 3′-hydroxyl and 5′-phosphate ends of duplex DNA segments generated in several DNA processes. This reaction requires the activation of the 5′-phosphate by ligase-mediated conversion to the 5′-AMP intermediate. The ligation of the 3′ and 5′ ends is metal-ion-dependent and appears to be mechanistically similar to the incorporation of deoxynucleotide triphosphates by DNA polymerases (1).
It is well established that ligases are sensitive to the identity of the base pairs at the ligase junction (4–14). Ligases generally do not ligate if a base mispair is on the 3′ side of the junction; however, ligases appear much more tolerant of the same mispairs when located on the 5′ side. The biological importance of the apparent ligase fidelity is that newly incorporated, and misincorporated nucleotides, would be found on the 3′ side of the junction following polymerase-mediated DNA replication or repair. The inefficient ligation of a mispair on the 3′ side would provide an additional opportunity for correction of the original misinsertion event (4). Ligase discrimination against 3′ mispairs forms the basis for several in vitro DNA diagnostic assays (15). Despite the biological and practical importance of ligase fidelity, the mechanistic basis for this discrimination has not yet been explained.
DNA ligases are found in essentially all organisms, and fall into two general categories depending upon the cofactor required (1,2,16). The ATP-dependent ligases are found in both eukaryotes and prokaryotes, whereas the NAD+-dependent DNA ligases are found primarily in eubacteria. Despite the wide diversity of amino acid sequences, sizes and properties, all DNA ligases share a conserved three-step catalytic mechanism, which involves self-adenylation of the ligase, transfer of the adenylate moiety to the 5′-phosphate, and the ligation step (1–3,16). Recently, the crystal structures of several DNA ligases have been solved and a common core structure has been revealed (16–21). It is likely that the mechanisms for junction recognition and sealing may also be conserved for all DNA ligases (22).
Here, we report our study on Tth DNA ligase with a battery of base pair analogs aimed at understanding the mechanisms of 3′-mismatch discrimination. Tth DNA ligase is a member of the NAD+-dependent ligase family that was originally isolated from Thermus thermophilus HB8 (7,8,23,24). Our data suggest that base pair geometry of 3′ base pairs, as recognized by hydrogen bond interactions in the minor groove between DNA ligase and the DNA duplex, ensures Tth ligase fidelity. Our results are confirmed in studies with the ATP-dependent T4 DNA ligase (25,26), suggesting that the recognition of minor groove contacts might be a common mechanism by which DNA ligases discriminate against ligation of mispaired substrates.
MATERIALS AND METHODS
Materials
Tth DNA ligase also known as Taq ligase (7,8,23,24), T4 DNA ligase and T4 polynucleotide kinase were obtained from New England BioLabs (Beverley, MA). Adenosine 5′-[γ-32P] triphosphate ([γ-32P]ATP) was obtained from MP Biomedicals (Costa Mesa, CA). Micro Bio-Spin P-6 chromatography columns were purchased from Bio-Rad (Hercules, CA). Phosphoramidites, the universal support, and Poly-Pak II cartridges were obtained from Glen Research (Sterling, VA).
Synthesis of oligonucleotides
Oligonucleotides were prepared by standard solid-phase synthesis (27) using a Gene Assembler Plus (Pharmacia). Oligonucleotides containing 5-fluoro-2′-deoxyuridine (FU) and 2,4-difluorotoluene (DFT) deoxynucleotide on the 3′-hydroxyl end were synthesized using a universal support. Following synthesis and standard deprotection, oligonucleotides were purified with Poly-Pak II cartridges according to the manufacturer's instructions, and further purified with 20% denaturing polyacrylamide gel (7 M urea). Bands containing oligonucleotides of interest were cut and soaked in the elution buffer (0.5 M ammonia acetate, 1 mM EDTA, 0.5% SDS) overnight followed by 1-butanol extraction.
Oligonucleotide labeling and annealing procedures
Oligonucleotide 5′-end radiolabeling was performed using [γ-32P]ATP and T4 polynucleotide kinase under conditions recommended by the enzyme supplier. T4 polynucleotide kinase was subsequently inactivated by heating at 95°C for 10 min. Duplex oligonucleotides containing a ligase junction were generated by mixing the labeled single strand (downstream of the ligase junction) with 2-fold molar excess of the full-length template and the strand upstream of the ligase junction. Annealing mixtures were heated at 95°C for 5 min and then cooled slowly to room temperature (∼3 h). Excess unincorporated radioactive nucleotide was removed by centrifuging through a Bio-Gel P6 column. The annealed duplexes are written as 3′ (5′)-X:Y, where X is on the3′-hydroxyl (5′-phosphate) end of the ligase junction, and Y is on the full-length template.
Tth DNA ligase assay
Substrates (500 fmol) were incubated with 10 U of Tth DNA ligase (∼75 fmol) at 26.5°C for 30 min in 10 μl reaction mixtures containing 1× ligase buffer (20 mM Tris–HCl, pH 7.6, 25 mM potassium acetate, 10 mM magnesium acetate, 10 mM dithiothreitiol, 1 mM NAD+ and 0.1% Triton X-100). One unit of the Tth DNA ligase (New England BioLabs) is defined as the amount of the enzyme required to give 50% ligation of the 12 bp cohesive ends of 1 μg of BstE II-digested λ DNA in a total reaction volume of 50 μl. Reactions were terminated by adding an equal volume of Maxam–Gilbert loading buffer (98% formamide, 0.01 M EDTA, 1 mg/ml xylene cyanol and 1 mg/ml bromphenol blue). Samples were denatured by heating at 95°C for 5 min, and then quickly placed on ice for 1 min before loaded onto a 20% denaturing gel (19:1 acrylamide:bis-acrylamide) containing 7 M urea. Electrophoresis was conducted in 1× TBE at room temperature. The bands corresponding to substrate and ligated product were visualized using a Molecular Dynamics PhosphorImager and the ImageQuant 5.0 software. In order to maintain the ligase junction in duplex form, our assays were conducted at 26.5°C, instead of the optimal operating temperature of the enzyme (65°C). Control experiments performed at different temperature (26.5°C, 37°C and 45°C) show no significant difference in the ligation patterns.
Time course experiments were performed essentially as described above by incubating at 26.5°C for different time periods. Non-linear regression of the data was performed using SigmaPlot 8.0, and the ligation rate was determined as V = F(t) t=0, where F(t) is the exponential fitting function Y = a (1 −e−b×). When a biphasic kinetic curve was observed, the ligation rate was determined for the slow phase, rather than the initial burst phase. The fidelity of Tth DNA ligase is defined as the ratio of the initial rate of perfect match ligation over the initial rate of mismatch ligation (7). pH-dependent ligase assays were conducted with Tris–HCl buffer (pH 7.1–9.0, room temperature).
T4 DNA ligase assay
Standard T4 DNA ligase assays (20 μl) were performed in 50 mM Tris–HCl, pH 7.5, 10 mM MgCl2, 150 mM NaCl, 10 mM DTT, 1 mM ATP, 25 μg/ml BSA and 100 fmol of duplex DNA. Reactions were initiated by adding 100 fmol of T4 DNA ligase, and incubated at 16°C for 10 min. Termination of reactions and electrophoresis were conducted as described above for Tth DNA ligase.
Electrophoretic mobility-shift assay (EMSA)
EMSA was conducted with equimolar Tth DNA ligase and duplex DNA (100 fmol each) in 20 mM Tris–HCl, pH 7.6, 10 mM dithiothreitiol, 1 mM NAD+ and 0.1% Triton X-100. Reactions were incubated at 26.5°C for 5 min, adjusted to 10% glycerol, and then electrophoresed at 150 V for 2 h through a native 10% polyacrylamide gel (37.5:1 acrylamide:bis-acrylamide) in 0.25× TBE at 4°C. In reactions supplemented with Mg2+, 10 mM magnesium acetate was added.
RESULTS
Tth DNA ligase activity on the U:purine base pair
Previous studies have consistently shown that mispairs located on the 3′-hydroxyl end of the ligase junction strongly inhibit Tth ligase activity (6–9,11). This feature may be attributed to either the decreased thermal stability or the wobble geometry of mispairs. To discriminate between these two possibilities, we have prepared a series of purine analogs modified in the 2 and 6 positions to alter the base pair geometry and base pair stability when paired with uracil (Figure 1). U:A, U:2AP, U:DAP and U:R approximate Watson–Crick geometry, and U:G, U:Hx base pairs are in wobble geometry. The relative thermal stability of the U:purine base pairs are in the order of U:DAP > U:A ∼ U:2AP > U:R > U:G ∼ U:Hx (29–31).
Figure 1.
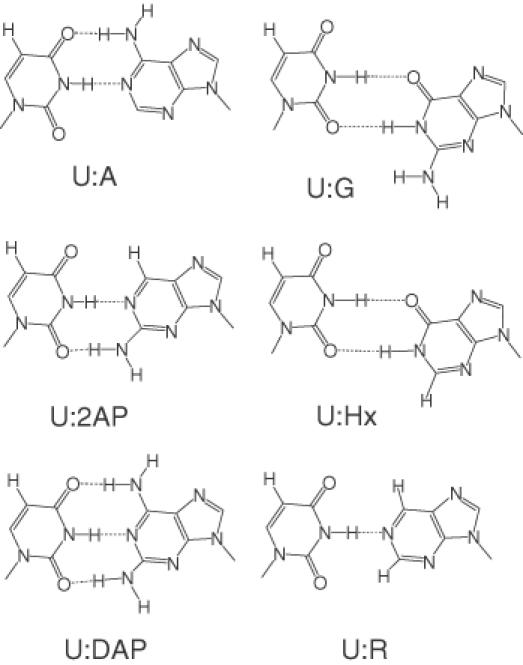
Proposed conformations of the analog base pairs employed in this study. The abbreviations of the analogs are as follows: U, uracil; G, guanine; Hx, hypoxanthine (deoxyinosine); R, nebularine (purine); A, adenine; 2AP, 2-aminopurine; DAP, 2,6-diaminopurine. U:A, U:2AP and U:DAP are of normal Watson–Crick geometry. U:R is stabilized by one hydrogen-bonding interaction in a pseudo Watson–Crick geometry. U:G and U:Hx exhibit wobble geometry [adapted from (28)].
Consistent with previous observations, we show that Tth DNA ligase exhibits significantly higher fidelity against mispairs located on the 3′-hydroxyl end than those on the 5′-phosphate end (Figure 2). The ligation rates of 3′-U:A, U:DAP, U:2AP and U:R by Tth DNA ligase vary by a factor of less than 2. However, the ligation rates of 3′-U:G and U:Hx are significantly slower (Figure 2A). A fidelity ratio of 8.6 × 102 was obtained for the ligation rate of 3′-U:A over that of 3′-U:G for Tth DNA ligase under our assay conditions, corresponding well with the reported 4.5 × 102 for 3′-C:G over 3′-T:G (7). In contrast, different U:purine base pairs on the 5′-phosphate end were ligated at similar rates by Tth DNA ligase (Figure 2B). Trace amounts of the 5′-AMP intermediate were detected in all of the substrates under our assay conditions except 3′-U:Hx, where the accumulation of the 5′-AMP intermediate is slightly more significant (Figure 2A).
Figure 2.
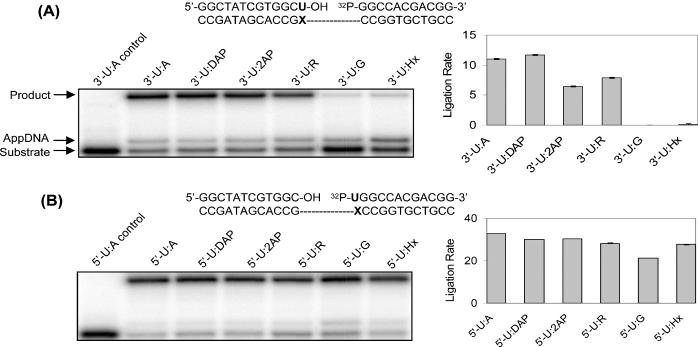
Tth ligase activity on terminal U:purine base pairs on the 3′-hydroxyl end (A) and the 5′-phosphate end (B) of a ligase junction. The gel pictures were performed at 26.5°C for 30 min with 0.5 pmol of duplexes and 10 U of Tth DNA ligase in a total volume of 10 μl. AppDNA indicates the 5′-AMP intermediate of the ligation reaction. The sequences used are listed above the gel pictures, where X = A, DAP, 2AP, R, G and Hx. In order to keep the surrounding sequence constant, the ligase junction was shifted by one base when U was moved from the 3′-hydroxyl end to the 5′-phosphate end. The gray bars represent the ligation rates (femtomoles ligated per minute per unit ligase), which were averaged from two independent measurements. 3′ (5′)-X:Y indicates that X is on the 3′-hydroxyl (5′-phosphate) end of the ligase junction, and Y is on the template.
Effect of solution pH on the ligation rate of FU:G
5-Fluorouracil:guanine (FU:G) base pair exhibits a pH-dependent equilibrium between a neutral wobble geometry and an ionized base pair that approximates Watson–Crick geometry [Figure 3; (32)]. Using this property of the FU:G pair, we further investigated the influence of base pair geometry on Tth DNA ligase activity. The ligation rates of Tth ligase toward C:G, FU:G and U:G located on either end of the ligase junction were measured as a function of pH (sample kinetic data of 3′ base pairs shown in Figure 4). The time course of Tth ligase activity on 3′-U:G exhibits biphasic kinetics at all pHs examined. For the 3′-FU:G base pair, similar biphasic kinetics was observed at pH 7.1–8.2, but monophasic kinetics was observed above pH 8.2. Two kinetic phases were previously reported for T4 DNA ligase toward mispair-containing substrates (33). In contrast, 3′-C:G and 5′ base pairs exhibit only monophasic kinetics. Under our assay conditions, the optimal pH of Tth DNA ligase is around pH 8.0 using C:G containing duplexes (data not shown). This number falls between two previously reported values [pH 7.5 (24) and pH 8.5 (11)]. From pH 7.1 to 9.0, the ligation rate of FU:G and U:G on the 3′-hydroxyl end increases by ∼65- and 8-fold, respectively, after normalized to the ligation rate of 3′-C:G to eliminate pH effects on Tth ligase (Figure 5A). However, < 3-fold changes in ligation rates were observed when FU:G and U:G were placed on the 5′-phosphate end (Figure 5B). Similarly, the ligation rates of FU:A and U:A do not change significantly with solution pH when located on either end of the ligase junction (data not shown).
Figure 3.
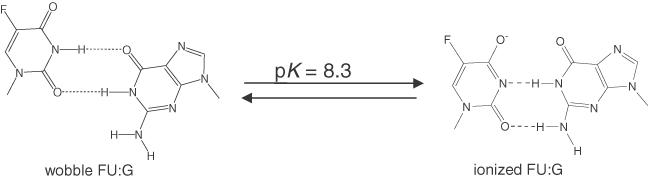
pH-dependent equilibrium between neutral wobble and ionized Watson-Crick structures of FU:G base pair. The pKa value of the N3 proton of FU is 8.3 (32).
Figure 4.
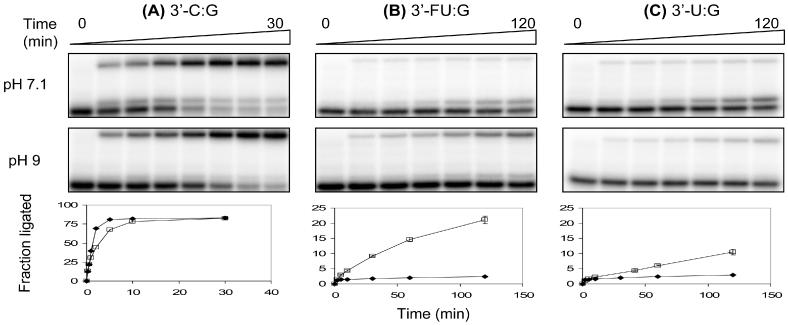
Sample experiments of the ligation time course for (A) 3′-C:G, (B) 3′-FU:G and (C) 3′-U:G as a function of pH. Time courses at pH 7.1 (closed diamond) and pH 9 (open square) are shown. Fraction ligated was averaged from three independent measurements.
Figure 5.
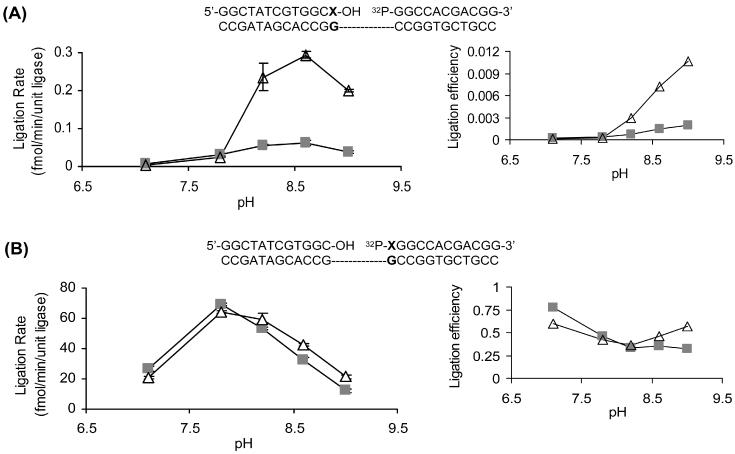
The ligation rates of Tth ligase on FU:G (open triangle) and U:G (shaded square) as a function of solution pH. Results on the 3′ end of the ligase junction are presented in (A) while on the 5′-end in (B). Ligation efficiency is the ligation rate normalized to that of C:G, which is shown in the insets. Sequences used are listed where X = U or FU.
Effect of minor groove interactions on ligase activity
DFT is a non-polar isosteric analog of thymine, which contains fluorine atoms in the place of hydrogen-bonding oxygens on the pyrimidine ring [Figure 6A; (34)]. When base paired with A, the DFT:A pair mimics the Watson–Crick geometry; however, a minor groove hydrogen bond acceptor is lost on DFT. To probe the importance of minor groove interactions in ligase fidelity, we prepared a series of DNA duplexes where DFT was incorporated into the 5′-phosphate end, 3′-hydroxyl end or the template strand. Consistent with tolerance of Tth DNA ligase toward 5′-mispairs, Tth ligase activity is only partially impaired by the presence of DFT on the 5′-phosphate end (Figure 6B), perhaps related to the relative instability of DFT-containing base pairs (35). Inhibition by the DFT analog is more complex when on the 3′-hydroxyl side of the ligase junction. When the DFT is on the unligated strand, essentially no ligation is observed, although some 5′-AMP intermediate is formed. In contrast, when the DFT is on the template strand opposite the 3′-adenine at the ligase junction, partial ligation is observed with substantial formation of the 5′-AMP intermediate (Figure 6C).
Figure 6.
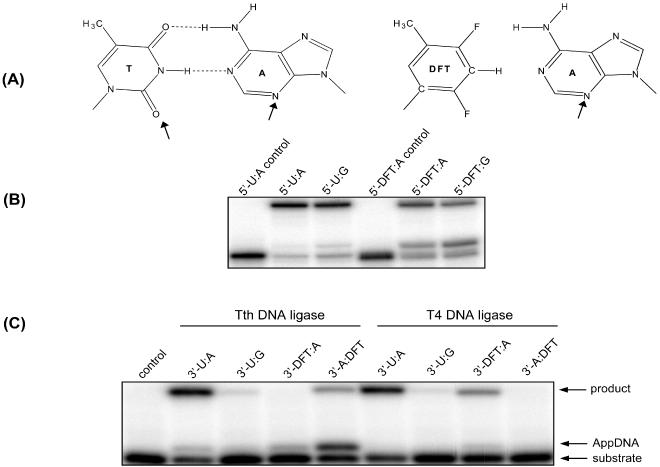
DNA ligase assays with substrates containing DFT. (A) The structure of DFT is shown above the gel, and hydrogen bonding acceptors in the minor groove of an A:T pair are indicated by arrows. (B) Tth DNA ligase assays with DFT on the 5′-phosphate end of a ligase junction. The experiments were conducted at 26.5°C for 30 min with 0.5 pmol of duplexes and 10 U of Tth DNA ligase in a total volume of 10 μl. Control lanes contain no protein. (C) Tth and T4 DNA ligase assays with DFT on the 3′-hydroxyl end of a ligase junction. Both ligase experiments were conducted with equimolar DNA duplex and enzyme (100 fmol each) in a total volume of 20 μl. Tth ligase reactions were incubated at 26.5°C for 5 min, and T4 ligase at 16°C for 10 min in the corresponding reaction buffer as described in Materials and Methods. 3′ (5′)-X:Y indicates that X is on the 3′-hydroxyl (5′-phosphate) end of the ligase junction, and Y is on the template.
In order to determine whether the minor groove contacts on the 3′-hydroxyl side of the ligase junction, as revealed in the study with Tth ligase, were also important with other ligases, the studies described above were repeated with the ATP-dependent T4 DNA ligase. As shown in Figure 6C, we observed that the presence of the DFT analog also inhibited T4 ligase. However, T4 ligase appears to be much more sensitive to the alteration of minor groove contacts when on the template strand.
Effect of minor groove contacts on Tth DNA ligase binding
In order to determine whether minor groove contacts were also important in ligase binding, we conducted EMSA studies with Tth ligase and the series of oligonucleotides containing base analogs. Binding reactions were performed initially in the absence of Mg2+ to prevent conversion of substrate to product during the incubation. DNA ligase assays were performed in parallel to the EMSA assays as shown in Figure 7A. When Mg2+ was excluded from the reaction, Tth DNA ligase was observed to form a tight-binding complex with the substrate containing the 3′-U:A base pair, but only weak complexes were observed with substrates containing a U:G mispair or the DFT–adenine base pair (Figure 7A). Although Tth ligase binds to the substrate containing the correct base pair in the absence of Mg2+, transfer of the AMP moiety from the enzyme to the 5′-phosphate at the ligase junction is not observed. The importance of Mg2+ in the final step of the ligase reaction has been discussed previously (13,16,20,36,37), and our results here provide direct evidence that Mg2+ is also important for the transfer of the AMP moiety from the ligase to the 5′-phosphate at the ligase junction.
Figure 7.
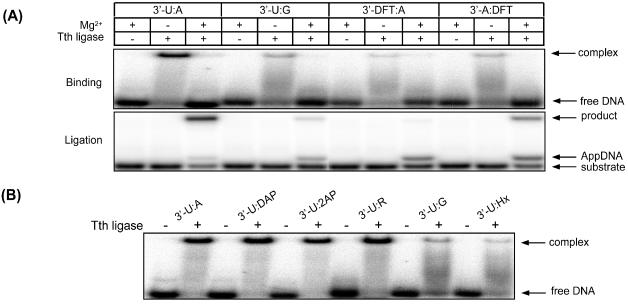
EMSA of Tth DNA ligase bound to duplex DNA. (A) Effects of minor groove interactions as well as Mg2+ on Tth ligase binding and ligation. DNA samples (100 fmol) were incubated with 100 fmol of Tth DNA ligase at 26.5°C in the presence or absence of Mg2+ in 20 μl total volume. After 5 min incubation, half of the samples were adjusted to 10% glycerol, and electrophoresed through a 10% native gel to detect ligase binding. The other half were heat-denatured, and resolved on a 20% denaturing gel to detect ligation. (B) Tth DNA ligase binds to duplexes containing the uracil:purine base pair on the 3′-hydroxyl end. The experiments were performed with equimolar DNA substrates and the ligase (100 fmol each) at 26.5°C for 5 min in the absence of Mg2+. 3′ (5′)-X:Y indicates that X is on the 3′-hydroxyl (5′-phosphate) end of the ligase junction, and Y is on the template.
When Mg2+ was added to the reaction containing the correct base pair, ligation was observed to proceed with dissociation of the ligase from the ligated duplex. In contrast, the addition of Mg2+ to substrates containing the U:G mispair or the DFT–adenine base pair did not result in ligation but did increase formation of the 5′-AMP intermediate. The formation of a ligase–DNA complex is reduced in all cases upon addition of Mg2+ (Figure 7A).
In order to probe more fully the role of minor groove contacts on ligase binding, we repeated the ligase-binding assay, in the absence of Mg2+, with the battery of oligonucleotide substrates containing the purine analogs with functional group substitution shown in Figure 1. Consistent with studies with the DFT analog described above, the formation of a ligase–DNA complex was observed with all base pairs approximating Watson–Crick geometry, irrespective of relative thermal stability. In contrast, substantially less complex formation was observed with duplexes containing the U:G and U:Hx mispairs that assume a wobble geometry and thus have altered minor groove contacts (Figure 7B).
DISCUSSION
The DNA of all living organisms is continuously damaged by a multitude of reactive molecules (38,39). Damaged DNA bases are recognized and removed by an array of glycosylases and nucleases, followed by resynthesis of the excised segment (38,40). The diverse repair pathways ultimately converge on the final step of the repair cycle, the ligation of the 3′-hydroxyl end of the newly synthesized DNA segment with an activated 5′-phosphate of the preexisting DNA duplex (1,2). DNA ligases from both prokaryotes and eukaryotes enhance the overall fidelity of the DNA repair process by selectively ligating DNA segments in which the 3′-terminal base pair at the ligase junction is correctly paired (4–14). Although the structures of several DNA ligases have been solved (16–21), it has not yet been possible to solve the structure of a DNA ligase positioned on a ligase junction. In the absence of such structural information, the mechanism by which ligase discrimination is achieved has not been explained.
In this paper, we addressed the question of ligase fidelity using a series of nucleoside analogs selected to probe specific structural questions regarding the interactions of DNA ligase with correct and aberrant base pair structures. The Tth ligase from T.thermophilus HB8 was chosen because this NAD+-dependent enzyme has very high fidelity and has been well characterized (7–9,11). To extend our observations to other DNA ligases, T4 DNA ligase was included as a representative member of the ATP-dependent ligase subfamily (25,26).
In the first series of experiments, uracil was paired opposite a series of purine analogs. Uracil was selected as the pyrimidine at the ligase junction so that comparisons could be made with other pyrimidines in later experiments. The structures of the uracil:purine base pairs are shown in Figure 1. The functional groups in the 2 and 6 positions on the purine ring were systematically changed to alter the thermal stability of the base pair, the base pair geometry, and the presence or absence of functional groups in the major and minor grooves. Several previous studies have indicated the importance of base pair thermal stability and base pair geometry for the efficiency of both DNA polymerases and nucleases (34,35,41,42). Functional group interactions in the minor groove have also been implicated in DNA polymerase fidelity (43–47).
The results of our experiments with the battery of purine analogs and Tth ligase are shown in Figure 2. In the four cases where the base pair on the 3′-side approximates Watson–Crick geometry, the 5′-AMP is transiently formed with the generation of the full-length ligated product (Figure 2A). When the base pair geometry is predominantly wobble, as is the case for the guanine and hypoxanthine base pairs, formation of the 5′-AMP intermediate is very slow, and very little ligation is observed (Figure 2A). These results suggest that base pair geometry of the 3′ terminal base pair predominates over thermal stability or the placement of functional groups in the 2 and 6 positions in achieving ligase discrimination against 3′ mispairs. In accord with the results of previous studies (6–9,11), similar rates of ligation are observed for all base pairs examined when placed on the 5′ side of the ligase junction (Figure 2B).
We then focused on the 5-fluorouracil:guanine base pair to further examine the role of base pair geometry in ligase fidelity. In contrast to normal base pairs, the FU:G mispair undergoes a pH-dependent transition near neutral pH from a neutral wobble configuration to an ionized base pair that approximates Watson–Crick geometry. The pK for the transition in duplex DNA is measured by solution NMR to be approximately 8.3 [Figure 3; (32)]. The rate of the ligase reaction was monitored as a function of solution pH as shown in Figures 4 and 5. We observe that the rate of ligation for a substrate containing a correct C:G base pair is highest at physiological pH, decreasing at both higher and lower solution pH. In contrast, the rate of ligation with both the FU:G and U:G mispairs on the 3′ end of the ligase junction increase with solution pH by ∼65- and 8-fold, respectively. The magnitude of the pH-dependent increase for the 3′-FU:G mispair is greater than for the U:G mispair, reflecting the lower pK of 5-fluorouracil. These observations are in accord with the previous study by Goodman and coworkers demonstrating that the DNA polymerase-dependent mispairing frequencies of the 5-halouracils increased with solution pH (48). Our results indicate that ligation of the neutral wobble configuration of the FU:G base pair, when placed on the 3′ end of the ligase junction is inhibited. However, by shifting the predominant base pair configuration to the ionized Watson–Crick geometry by increasing solution pH, ligation can proceed. In contrast, such pH-dependent increase of ligation activity was not detected when FU:G and U:G were placed on the 5′-phosphate end.
The observation of a pH-dependent effect with the FU:G mispair further confirms the importance of the correct geometry of 3′ base pairs in ligase fidelity. If the ligase requires the 3′ base pair to conform to Watson–Crick geometry, how is it measured? In previous studies with DNA polymerase, the importance of hydrogen-bond acceptors in the minor groove has been proposed to explain how the polymerase could interrogate the geometry of the base pair at the replication fork (42–47). Both G:C and A:T base pairs have hydrogen-bond acceptors in the minor groove of a B-form DNA duplex, the N3 nitrogen of the purine and the C2 carbonyl of the pyrimidine (49). The positions of these hydrogen bond acceptors are nearly symmetric with respect to the helical axis, so that from the minor groove, all of the normal base pairs could form similar hydrogen bonding interactions. Recently, DFT has been introduced as a pyrimidine shape analog (34). DFT can form a base pair with adenine that approximates Watson–Crick geometry as shown in Figure 6. Although base pair hydrogen bonding interactions are essentially eliminated with DFT, and base stacking interactions are dramatically reduced, DFT analogs can be utilized as substrates by some DNA polymerases (34). We therefore placed DFT into a ligase junction to directly test the importance of hydrogen-bonding interactions of the ligase with the pyrimidine C2 carbonyl.
When a pyrimidine is replaced by DFT on the 3′ side of the ligase junction, essentially no ligation is observed (Figure 6) with Tth ligase despite the fact that the DFT:A base pair has a similar shape to a normal T:A base pair. This result provides direct evidence for the role of specific hydrogen bonding interactions between the minor groove of the DNA duplex and the ligase. When the DFT:A base pair is inverted, placing the DFT analog on the template side, ligation does occur, but at a substantially reduced rate. This result confirms the importance of hydrogen bonding interactions with both bases of the base pair on the 3′ side of the ligase junction, but further suggests that the interactions are more critical for the unligated 3′ base. Similar inhibitory effects of the DFT analog on the 3′ side of the ligase junction were also observed with T4 DNA ligase, indicating the importance of minor groove contacts on ligase fidelity. However, T4 ligase appears to be much more sensitive to the alteration of minor groove contacts when on the template strand.
The results of the ligase assays reported here demonstrate that minor groove contacts are critical for the covalent condensation of the ligase junction with both Tth and T4 ligases. These observations alone cannot reveal if the observed inhibition resulting from the base analog substitution is dependent upon structural perturbations that interfere with the initial ligase binding or the catalysis step. However, our binding studies with Tth ligase demonstrate that the formation of the initial ligase–substrate complex is dependent upon minor groove interactions, suggesting that ligase can distinguish correct from incorrect substrates in the initial binding event. The low affinity of ligase for the mispaired or structurally altered substrates would create the opportunity for other DNA repair proteins to access the altered ligase junction and affect further repairs (4). Interestingly, several antitumor compounds that bind to the minor groove of DNA also inhibit DNA ligases (12,50–52). Our results, demonstrating the critical role of minor groove contacts on ligase binding, are likely related to the observed activities of these minor groove binding compounds.
Our results reported here allow us to explain, for the first time, the mechanism by which the ligase discriminates against mispairs on the 3′ side of a ligase junction. Using a series of nucleoside analogs, we show that relative thermal stability and specific functional groups in the 2 and 6 position of the purine residue and in the 4 position of the pyrimidine residue contribute little to discrimination. In contrast, we show that the presence of hydrogen bond acceptors properly positioned in the minor groove are necessary for efficient ligation and that such interactions allow the ligase to determine the base pair geometry. As the position of the hydrogen bond acceptors in the minor groove is a common feature of correct Watson–Crick base pairs (49), and is sufficient to distinguish correct from mispaired bases, interactions between these nucleoside functional groups and specific amino acid residues of the ligase are likely to be the key to understanding ligase fidelity.
Our results with both Tth DNA ligase and T4 DNA ligase indicate that minor groove contact is likely to be a general mechanism for the fidelity of both NAD+ and ATP-dependent DNA ligases. In mispaired structures, both bases are displaced from the positions occupied when in Watson–Crick geometry. In the more commonly formed wobble mispairs, one of the bases is displaced toward the major groove whereas the other is pushed into the minor groove, altering the minor groove contacts on both the template and primer sides of the duplex (53). Interestingly, the Tth ligase interrogates the contacts on the unligated strand of a ligase junction whereas the T4 ligase interrogates the template side. Both classes of ligase appear to have solved the fidelity question by a similar mechanism, while approaching the helix from different positions. Future structural studies are needed to confirm the key interactions suggested by the studies reported here.
Acknowledgments
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Katherine Noyes Rogstad and Victoria Valinluck for critical reading and helpful discussions. This study was supported by the National Institute of Health (GM40351 and CA112293).
REFERENCES
- 1.Lehman I.R. (1974) DNA ligase: structure, mechanism, and function. Science, 186, 790–797. [DOI] [PubMed] [Google Scholar]
- 2.Timson D.J., Singleton,M.R. and Wigley,D.B. (2000) DNA ligases in the repair and replication of DNA. Mutat. Res., 460, 301–318. [DOI] [PubMed] [Google Scholar]
- 3.Tomkinson A.E. and Mackey,Z.B. (1998) Structure and function of mammalian DNA ligases. Mutat. Res., 407, 1–9. [DOI] [PubMed] [Google Scholar]
- 4.Bhagwat A.S., Sanderson,R.J. and Lindahl,T. (1999) Delayed DNA joining at 3′ mismatches by human DNA ligases. Nucleic Acids Res., 27, 4028–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husain H., Tomkinson,A.E., Burkhart,W.A., Moyer,M.B., Ramos,W., Mackey,Z.B., Besterman,J.M. and Chen,J. (1995) Purification and characterization of DNA ligase III from bovine testes. Homology with DNA ligase II and vaccinia DNA ligase. J. Biol. Chem., 270, 9683–9690. [DOI] [PubMed] [Google Scholar]
- 6.Pritchard C.E. and Southern,E.M. (1997) Effects of base mismatches on joining of short oligodeoxynucleotides by DNA ligases. Nucleic Acids Res., 25, 3403–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo J., Bergstrom,D.E. and Barany,F. (1996) Improving the fidelity of Thermus thermophilus DNA ligase. Nucleic Acids Res., 24, 3071–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo J. and Barany,F. (1996) Identification of essential residues in Thermus thermophilus DNA ligase. Nucleic Acids Res., 24, 3079–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong J., Barany,F. and Cao,W. (2000) Ligation reaction specificities of an NAD+-dependent DNA ligase from the hyperthermophile Aquifex aeolicus. Nucleic Acids Res., 28, 1447–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakatani M., Ezaki,S., Atomi,H. and Imaaka,T. (2002) Substrate recognition and fidelity of strand joining by an archaeal DNA ligase. Eur. J. Biochem., 269, 650–656. [DOI] [PubMed] [Google Scholar]
- 11.Tong J., Cao,W. and Barany,F. (1999) Biochemical properties of a high fidelity DNA ligase from Thermus species AK16D. Nucleic Acids Res., 27, 788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuman S. (1995) Vaccinia virus DNA ligase: specificity, fidelity, and inhibition. Biochemistry, 34, 16138–16147. [DOI] [PubMed] [Google Scholar]
- 13.Sriskanda V. and Shuman,S. (1998) Chlorella virus DNA ligase: nick recognition and mutational analysis. Nucleic Acids Res., 26, 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sriskanda V. and Shuman,S. (1998) Specificity and fidelity of strand joining by Chlorella virus DNA ligase. Nucleic Acids Res., 26, 3536–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barany F. (1991) Genetic disease detection and DNA amplification using cloned thermostable ligase. Proc. Natl Acad. Sci. USA, 88, 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doherty A.J. and Suh,S.W. (2000) Structural and mechanistic conservation in DNA ligases. Nucleic Acids Res., 28, 4051–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramanya H.S., Doherty,A.J., Ashford,S.R. and Wigley,D.B. (1996) Crystal structure of an ATP-dependent DNA ligase from bacteriophage T7. Cell, 85, 607–615. [DOI] [PubMed] [Google Scholar]
- 18.Singleton M.R., Hakansson,K., Timson,D.J. and Wigley,D.B. (1999) Structure of the adenylation domain of an NAD-dependent DNA ligase. Structure, 7, 35–42. [DOI] [PubMed] [Google Scholar]
- 19.Odell M., Sriskanda,V., Shuman,S. and Nikolov,D.B. (2000) Crystal structure of eukaryotic DNA ligase-adenylate illuminates the mechanism of nick sensing and strand joining. Mol. Cell., 6, 1183–1193. [DOI] [PubMed] [Google Scholar]
- 20.Lee J.Y., Chang,C., Song,H.K., Moon,J., Yang,J.K., Kim,H.-K., Kwon,S.-T. and Suh,S.W. (2000) Crystal structure of NAD+-dependent DNA ligase: modular architecture and functional implications. EMBO J., 19, 1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odell M., Malinina,L., Sriskanda,V., Teplova,M. and Shuman,S. (2003) Analysis of the DNA joining repertoire of Chlorella virus DNA ligase and a new crystal structure of the ligase-adenylate intermediate. Nucleic Acids Res., 31, 5090–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherepanov A.V. and de Vries,S. (2002) Dynamic mechanism of nick recognition by DNA ligase. Eur. J. Biochem., 269, 5993–5999. [DOI] [PubMed] [Google Scholar]
- 23.Barany F. and Gelfand,D.H. (1991) Cloning, overexpression and nucleotide sequence of a thermostable DNA ligase-encoding gene. Gene, 109, 1–11. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi M., Yamaguchi,E. and Uchida,T. (1984) Thermophilic DNA ligase. Purification and properties of the enzyme from Thermus Thermophilus HB8. J. Biol. Chem., 259, 10041–10047. [PubMed] [Google Scholar]
- 25.Landegren U., Kaiser,R., Sanders,J. and Hood,L. (1988) A ligase-mediated gene detection technique. Science, 241, 1077–1080. [DOI] [PubMed] [Google Scholar]
- 26.Wu D.Y. and Wallace,R.B. (1989) Specificity of the nick-closing activity of bacteriophage T4 DNA ligase. Gene, 76, 245–254. [DOI] [PubMed] [Google Scholar]
- 27.Gait M.J. (1984) Oligonucleotide Synthesis, A Practical Approach. IRL Press, Oxford, UK, pp. 35–81.
- 28.Liu P., Burdzy,A. and Sowers,L.C. (2002) Substrate recognition by a family of uracil-DNA glycosylases: UNG, MUG, and TDG. Chem. Res. Toxicol., 15, 1001–1009. [DOI] [PubMed] [Google Scholar]
- 29.Hoheisel J.D. and Lehrach,H. (1990) Quantitative measurements on the duplex stability of 2,6-diaminopurine and 5-chloro-uracil nucleotides 1using enzymatically synthesized oligomers. FEBS Lett., 274, 103–106. [DOI] [PubMed] [Google Scholar]
- 30.Eritja R., Kaplan,B.E., Mhaskar,D., Sowers,L.C., Petruska,J. and Goodmam,M.F. (1986) Synthesis and properties of defined DNA oligomers containing base mispairs involving 2-amionpurine. Nucleic Acids Res., 14, 5869–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dritja R., Horowitz,D.M., Walker,P.A., Ziehler-Martin,J.P., Boosalis,M.S., Goodman,M.F., Itakura,K. and Kaplan,B.E. (1986) Synthesis and properties of oligonucleotides containing 2′-deoxynebularine and 2′-deoxyxanthosine. Nucleic Acids Res., 14, 8135–8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sowers L.C., Eritja,R., Kaplan,B., Goodman,M.F. and Fazakerly,G.V. (1988) Equilibrium between a wobble and ionized base pair formed between fluorouracil and guanine in DNA as studied by proton and fluorine NMR. J. Biol. Chem., 263, 14794–14801. [PubMed] [Google Scholar]
- 33.Cherepanov A.V. and de Vries,S. (2003) Kinetics and thermodynamics of nick sealing by T4 DNA ligase. Eur. J. Biochem., 270, 4315–4325. [DOI] [PubMed] [Google Scholar]
- 34.Moran S., Ren,R.X.-F. and Kool,E.T. (1997) A thymindine triphosphate shape analog lacking Watson–Crick pairing ability is replicated with high sequence selectivity. Proc. Natl Acad. Sci. USA, 94, 10506–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales J.C. and Kool,E.T. (2000) Importance of terminal base pair hydrogen-bonding in 3′-end proofreading by the Klenow fragment of DNA polymerase I. Biochemistry, 39, 2626–2632. [DOI] [PubMed] [Google Scholar]
- 36.Yang S.-W. and Chan,J.Y.H. (1992) Analysis of the formation of AMP-DNA intermediated and the successive reaction by human DNA ligases I and II. J. Biol. Chem., 267, 8117–8122. [PubMed] [Google Scholar]
- 37.Magnet S. and Blanchard,J.S. (2004) Mechanistic and kinetic study of the ATP-dependent DNA ligase of Neisseria meningitides. Biochemistry, 43, 710–717. [DOI] [PubMed] [Google Scholar]
- 38.Friedberg E.C., Walker,G.C. and Siede,W. (1995) DNA Repair and Mutagenesis. ASM Press, Washington, DC. [Google Scholar]
- 39.Lindahl T. (1993) Instability and decay of the primary structure of DNA. Nature, 362, 709–715. [DOI] [PubMed] [Google Scholar]
- 40.Hoeijmakers J.H.J. (2001) Genome maintenance mechanisms for preventing cancer. Nature, 411, 366–374. [DOI] [PubMed] [Google Scholar]
- 41.Petruska J., Goodman,M.F., Boosalis,M.S., Sowers,L.C., Cheong,C. and Tinoco,I.Jr. (1988) Comparison between DNA melting thermodynamics and DNA polymerase fidelity. Proc. Natl Acad. Sci. USA, 85, 6252–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodman M.F. (1997) Hydrogen bonding revisited: geometric selection as a principal determinant of DNA replication fidelity. Proc. Natl Acad. Sci. USA, 94, 10493–10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morales J.C. and Kool,E.T. (1999) Minor groove interactions between polymerase and DNA: more essential to replication than Watson–Crick hydrogen bonds? J. Am. Chem. Soc., 121, 2323–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morales J.C. and Kool,E.T. (2000) Functional hydrogen-bonding map of the minor groove binding tracks of six DNA polymerases. Biochemistry, 39, 12979–12988. [DOI] [PubMed] [Google Scholar]
- 45.Kiefer J.R., Mao,C., Braman,J.C. and Beese,L.S. (1998) Visualizing DNA replication in a catalytically active Bacillus DNA polymerase crystal. Nature, 391, 304–307. [DOI] [PubMed] [Google Scholar]
- 46.Doublié S., Tabor,S., Long,A.M., Richardson,C.C. and Ellenberger,T. (1998) Crystal structure of a bacteriophage T7 DNA replication complex at 2.2 Å resolution. Nature, 391, 251–258. [DOI] [PubMed] [Google Scholar]
- 47.Kunkel T.A. and Bebenek,K. (2000) DNA replication fidelity. Annu. Rev. Biochem., 69, 497–529. [DOI] [PubMed] [Google Scholar]
- 48.Yu H., Eritja,R., Bloom,L.B. and Goodman,M.F. (1993) Ionization of bromouracil and fluorouracil stimulates base mispairing frequencies with guanine. J. Biol. Chem., 268, 15935–15943. [PubMed] [Google Scholar]
- 49.Seeman N.C, Rosenberg,J.M and Rich,A. (1976) Sequence-specific recognition of double helical nucleic acids by proteins. Proc. Natl Acad. Sci. USA, 73, 804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montecucco A., Pedrali-Noy,G., Spadari,S., Zanolin,E. and Ciarrocchi,G. (1988) DNA unwinding and inhibition of T4 DNA ligase by anthracyclines. Nucleic Acids Res., 16, 3907–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montecucco A., Fontana,M., Focher,F., Lestingi,M., Spadari,S. and Ciarrocchi,G. (1991) Specific inhibition of human DNA ligase adenylation by a distamycin derivative possessing antitumor activity. Nucleic Acids Res., 19, 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciarrocchi G., Fontana,M., Spadari,S. and Montecucco,A. (1991) Inhibition of human DNA ligase by anthracyclines and distamycins. Anticancer Res., 11, 1317–1322. [PubMed] [Google Scholar]
- 53.Carbonnaux C., Fazakerley,G.V. and Sowers,L.C. (1990) An NMR structural study of deaminated base pairs in DNA. Nucleic Acids Res., 18, 4075–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]


