Abstract
Purpose
There is no consensus on adequate negative margins in breast-conserving surgery (BCS) for ductal carcinoma in-situ (DCIS). We systematically review the evidence on margins in BCS for DCIS.
Methods
Study-level meta-analysis of local recurrence (LR), microscopic margin status and threshold distance for negative margins. LR proportion was modelled using random-effects logistic meta-regression (frequentist), and network meta-analysis (Bayesian) that allows for multiple margin distances per study, adjusting for follow-up time.
Results
Based on 20 studies (LR: 865 of 7,883), odds of LR were associated with margin status (logistic: odds ratio (OR) 0.53 for negative vs positive/close [P<0.001]; network: OR=0.45 for negative vs positive). In logistic meta-regression, relative to >0 or 1mm, ORs for 2mm (0.51), 3 or 5mm (0.42) and 10mm (0.60) showed comparable significant reductions in the odds of LR. In the network analysis, ORs relative to positive margins for 2mm (0.32), 3mm (0.30) and 10mm (0.32) showed similar reductions in the odds of LR that were greater than for >0 or 1mm (0.45). There was weak evidence of lower odds at 2mm compared to >0 or 1mm (Relative OR=0.72, 95% credible interval [CrI] 0.47-1.08), and no evidence of a difference between 2mm and 10mm (Relative OR=0.99, 95% CrI 0.61-1.64). Adjustment for covariates, and analyses based only on studies using whole-breast radiotherapy, did not change findings.
Conclusion
Negative margins in BCS for DCIS reduce the odds of LR; however, minimum margins distances above 2mm are not significantly associated with further reduced odds of LR in women receiving radiation.
Introduction
Breast cancer-specific mortality for women with ductal carcinoma in-situ (DCIS) is low, regardless of whether breast conserving surgery (BCS) or mastectomy is performed1. However, BCS is associated with higher rates of local recurrence (LR), and therefore has the potential to lead to additional treatment. Approximately half of all LRs are invasive, with an associated risk of breast cancer mortality2; therefore, it is critical that BCS is optimized to reduce the risk of LR, while maintaining its benefits to cosmesis and quality of life relative to more extensive surgery3.
Negative margins in BCS for DCIS have been shown to reduce the risk of LR4;5; however, the optimal margin distance (i.e. threshold to declare a negative margin) remains a topic of debate6. Guidelines for BCS in invasive cancer, which recommend a minimum margin of no ink on tumour (>0mm)7, are not directly applicable to DCIS given differences in the use of adjuvant whole breast radiation (WBRT) and systemic therapies. Furthermore, studies of the growth pattern of DCIS have found that multifocal lesions with intervening normal ductal segments are relatively common6. Therefore, while some guidelines have specified a minimum margin >0mm8, wider thresholds of 1mm9 and 2mm10-12 have been adopted by others, and 10mm has been recommended by a previous meta-analysis4.
Given the lack of consensus on what constitutes an adequate negative margin, we undertook a systematic review of the association between margins and LR in DCIS to determine the optimal minimum negative margin width and support the development of consensus guidelines13. Using study-level meta-analysis, the evidence on surgical margins in women with DCIS treated with BCS was systematically examined to: a) estimate the effect of microscopic margin status on LR; b) investigate the effect of various thresholds to define negative margins; and c) define a minimum negative margin distance to maximize local control.
Methods
Criteria for Study Eligibility
Inclusion and exclusion criteria are presented in Appendix A. Eligible studies enrolled women with DCIS undergoing BCS, and allowed calculation of the crude proportion of LR in relation to microscopic margin status and the threshold distance used to declare a negative margin. Only numerically defined margin thresholds (or negative margins defined as “no ink on tumour”, interpreted as >0mm) were included; studies that did not quantify negative margin distance, or used unclear margin definitions were excluded. Studies were excluded if all patients had the same margin status.
Eligible studies were required to report mean or median age for the study population (based on the relationship between age and risk of LR14;15); to present mean or median follow-up of at least four years to allow sufficient time for clinical endpoints to have occurred16;17; and to enrol a minimum of 50 women with DCIS undergoing BCS.
Studies that reported LR rates derived from Kaplan-Meier analysis, from which crude LR data could not be derived, were ineligible. Where studies fulfilled all other inclusion criteria but crude LR by margin status was not presented, study authors were contacted to obtain those data.
Literature search
A systematic search of the biomedical literature was undertaken in October 2014. MEDLINE and EMBASE were searched via EMBASE.com; PREMEDLINE and ALL EBM REVIEWS were searched via Ovid. Search terms were selected to link margins and DCIS. Keywords and medical subject headings included ‘ductal carcinoma in situ’, ‘intraductal carcinoma’, ‘DCIS’, and ‘margin’. The full search strategy is available in Appendix B. Reference lists were searched and content experts consulted to identify additional studies.
Non-duplicate abstracts (N=1,577) were screened for eligibility by one author (MLM), and a sample (N=135) was assessed independently by another author (NH) to ensure consistent application of eligibility criteria. Full text of potentially eligible studies (N=108) was assessed by one author (MLM). Where two or more papers reported the same cohort, the most recent study providing margin-specific crude LR data was used to avoid duplication. Appendix C summarizes the screening and inclusion process. Comparison of eligible studies with those in a previous meta-analysis4 is presented in Appendix D.
Data extraction
Data were extracted independently by two authors (MLM, and either MM or LS) using predefined data extraction forms. Discrepancies were resolved through consensus with a third author (NH). Variables derived from each study were: margin status (positive, close, negative); numeric margin distance(s) (mm); margin-specific LR; patient recruitment period (start/end years); number of patients included/excluded; age (mean/median); duration of follow-up (mean/median); proportion with invasive vs DCIS recurrence; proportion with WBRT; proportion with RT boost; WBRT, boost, and total doses (mean or median, Gy); proportion received endocrine therapy; proportion with screen-detected DCIS; proportion with comedonecrosis; nuclear grade (low, intermediate, high); proportion estrogen receptor positive; proportion hormone receptor positive; tumour size (mean/median, mm); and proportion with multifocal DCIS. Data on the proportion of patients receiving Accelerated Partial Breast Irradiation (APBI) was also collected; however, since only one study of APBI was eligible18, those data have not been analysed separately.
Definition of key variables
Margins
Study-specific information on the definition of final microscopic margins, from excision or re-excision, was extracted based on margin status (negative, close, positive) and margin distance (the threshold for declaring negative margins relative to positive or close). A standard definition of positive margins was considered to be the presence of DCIS at the transected or inked margin; however, alternative definitions of positive margins were also extracted. Such alternative definitions combined positive and close margins, where a close margin indicated the presence of tumour within a specified distance of the resection margin. When margins were not positive (or not positive/close), margins were considered to be negative (i.e. no tumour at resection margin, or no tumour within specified close distance). Where close margins were reported separately from positive margins, those data were extracted as multiple, distance-specific negative margins categories. Data from multiple, discrete close categories were extracted when available.
Where reported, data from unknown margins were extracted. Because the unknown category cannot contribute data on the effect of margins, it has not been included in models16;17; however, these data were included in descriptive analyses.
Local recurrence
Data were extracted using individual study definitions of LR (either a ‘first’ event, or ‘any’ LR), but commonly the definition of LR was not specified. LR included both DCIS and invasive recurrences.
Statistical Analysis
Descriptive analyses examined the distribution of study characteristics. Categorical study-level variables were summarized as percentages; for continuous measures, the median, range, and interquartile range (IQR) were calculated.
Data categorisation
Positive margins
Due to heterogeneity in margin definitions across studies, positive margins were re-categorized as either 0mm (ink on tumour, nine studies19-27) or <1mm (seven studies28-34). In addition, margins of <2mm (three studies18;35;36) or <3mm (one study37) were considered positive when 0mm or <1mm were not reported.
Negative margins
Negative margin of >0 or 1mm were combined into one category due to variability in those definitions; negative distances 3 or 5mm were also combined due to lack of data. Thus, negative margins were categorized as: >0 or 1mm; 2mm; 3 (or 5)mm; and 10mm. Using that classification, nine studies18;23-25;29;31;32;35;36 reported one cut-point for margin distance and 11 studies19-22;26-28;30;33;34;37 reported multiple cut-points within each study.
Two complementary meta-analytic approaches were used to investigate data from all 20 studies. Random effects logistic modelling dichotomized studies at one cut-point, creating a combined positive/close category and an “open-ended” negative category (see Houssami et al.16;17). Bayesian network meta-analysis incorporated single and multiple cut-points per study following the approach used by Wang et al.4. Multiple cut-points resulted in “closed” negative categories with an upper bound. Therefore, for all but 10mm (open-ended), these distance classifications included a combination of open-ended and closed categories (Appendix E).
Random effects logistic meta-regression (frequentist models)
The proportion of women who had LR was modelled using random effects logistic meta-regression (Proc NLmixed in SAS). Random study effects, assumed to follow a normal distribution, were included in all models to allow for anticipated heterogeneity between studies beyond what would arise from within study sampling error alone, thereby taking account of both within and between study variability. Explanatory variables included in the models (margin status, distance and covariates) were fitted as a fixed effect. Statistical significance was set at P<0.05 (two-sided); P<0.10 was considered weak evidence of association.
The association between LR and margin status and distance was estimated by including both as categorical variables in the model. One margin distance could be included for each study. When multiple distances were available, the largest was chosen (with the exception of the only study to apply a 5mm distance19, and a large study defining “close” margins as <2mm27). The effect of alternative distance categorisations for potentially influential studies was investigated. Effect modification between margin status and distance was tested for statistical interaction between these variables.
In addition to margin status analysed as negative versus positive/close, models of positive, close and negative margins as separate categories were attempted. However, those models failed to converge due to few studies reporting separate categories19-23;26;27, and are not reported.
Network meta-analysis
Network meta-analysis allows data for more than one margin distance per study to be utilized, and takes account of the correlations between multiple observations within studies. The approach used (Mixed Treatment Comparison [MTC]38) considers margin thresholds as different “treatments” tested in different studies, and compares them through a network structure informed by both direct (within study) and indirect comparisons (between studies using a common comparator, i.e. positive margins) (Appendix F).
To compare the probability of LR between margin status (negative versus positive), a simplified version of the MTC for two “treatments” was used. An extended version of the MTC for multiple “treatments” was used to compare the probability of LR between all pairs of margin distances (positive; >0 or 1mm; 2mm; 3mm; 10mm). The MTC is a Bayesian random effects hierarchical model where the probability of LR within each margin distance was modelled using a Binomial likelihood at the first level. A logit-normal random effects model was used to link the probability of LR with covariates of interest in a combined MTC and meta-regression framework. All models are adjusted for median follow-up time (centred to their mean), fitted as a fixed effect. Appendix G provides further technical details of this model, including explanation of estimates and 95% credible intervals (CrIs; analogous to 95% confidence intervals [CIs] in providing a range of likely values for a statistical estimate).
The rjags package implemented in the R package was used for all Bayesian analyses.
Assessment of covariates
All models were adjusted for study-specific follow-up time, based on prior evidence that LR increases with longer follow-up, and evidence of association in the random effects logistic meta-regression analysis (see ‘Results’). Other potential study-level confounders of the relationship between margins and LR (median age [years]; median year of recruitment; radiotherapy [%]; radiotherapy boost [%]; total radiotherapy dose [Gy]; DCIS recurrence [%]; endocrine therapy [%]; screen-detection [%]; comedonecrosis [%]; and high grade [%]) were also fitted in univariate logistic meta-regression models (not including margins). Covariates that showed at least a weak association (p<0.10) with LR were adjusted for in both the logistic and Bayesian network models to assess the effect on estimates for margin distance (these were: age; median year of recruitment; endocrine therapy; high grade; see Appendix H). The effect of radiotherapy was also investigated on prior grounds. Variables that were extracted but reported in less than half of studies were not considered reliable for modelling.
Two covariates had missing data (endocrine therapy; high grade). In the network model, a number of statistical techniques for dealing with missing data were investigated, but the results were equivalent to models restricted to studies with non-missing covariate data.
Results
Study characteristics
Twenty studies were eligible for inclusion18-37, reporting data on 8,651 patients with DCIS; 7,883 had known margin status (865 LRs) and were included in our models. Two studies were prospectively designed18;31; the remaining 18 were retrospective. Study characteristics are summarized in Table 1. Studies enrolled patients between 1968 and 2010 (median mid-point of recruitment 1991). The median proportion of patients receiving WBRT across studies was 100% (IQR 50.3–100.0%); 71% of all patients in eligible studies received WBRT. Median study-level proportion of patients receiving endocrine therapy was 20.8% (IQR 0.0-31.4%). Median follow-up time was 78.3 months (IQR 59.0-94.7), and prevalence of LR was 8.3% (IQR 5.0-11.9%) in 7,883 patients with margins data. In 768 patients with unknown margins (not included in our models), the prevalence of LR was 12.4% (95 LRs).
Table 1.
Patient and study characteristics
| N Included | |||||
|---|---|---|---|---|---|
| Variable | Studies | Patients | Median | Interquartile Range (IQR) | (Range) |
| N patients (total)a | 20 | 8651 | 226 | 108-439 | (50-2996) |
| N with known margin status | 7883 | 210 | 98-422 | (50-2788) | |
| Recruitment timeframe (year) | 20 | 8651 | - | - | - |
| Start | 8651 | 1984 | 1977-1988 | (1968-2003) | |
| End | 8651 | 2001 | 1995-2007 | (1990-2010) | |
| Mid-interval | 8651 | 1991 | 1987-1996 | (1979-2006) | |
| Age (years; median or mean) | 20 | 8651 | 53.7 | 53.0-56.7 | (43.0-62.1) |
| Follow-up (months; median or mean) | 20 | 8651 | 78.3 | 59.0-94.7 | (51.5-126.0) |
| Prevalence of LR (patients with known margin status) | 20 | 7883 | 8.3% | 5.0-11.9% | (2.2-24.0%) |
| Total number of LR | 865 | - | - | - | |
| Type of LR | 17 | 952 | - | - | - |
| DCIS | 479 | 50.0% | 42.9-57.1% | (0.0-75.0%) | |
| Invasive | 458 | 50.0% | 41.7- 56.5% | (25.0-100.0%) | |
| Unknown | 15 | 0.0% | 0.0-0.0% | (0.0-7.1%) | |
| WBRT | 20 | 8920 | - | - | - |
| Yesb | 6353 | 100.0% | 50.3-100.0% | (0.0-100.0%) | |
| No | 2533 | 0.0% | 0.0-53.4% | (0.0-100.0%) | |
| Unknown | 34 | 0.0% | 0.0-0.0% | (0.0-1.1%) | |
| WBRT dose (Gy, median) | 11 | 3990 | 50.0 | 50.0-50.0 | (42.5-50.0) |
| Radiation boost | 19 | 5925 | - | - | - |
| Yes | 3207 | 70.9% | 28.4-95.5% | (0.0-100.0%) | |
| No | 2715 | 29.1% | 4.5-71.6% | (0.0-100.0%) | |
| Unknown | 3 | 0.0% | 0.0-0.0% | (0.0-0.6%) | |
| Boost dose (Gy, median) | 8 | 2734 | 10.0 | 10.0-10.0 | (10.0-10.8) |
| Total dose (Gy, median) | 12 | 3890 | 60.0 | 60.0-60.4 | (50.0-64.0) |
| Endocrine therapy | 19 | 8392 | - | - | - |
| Yesd | 1563 | 20.8% | 0.0-31.4% | (0.0-83.2%) | |
| No | 6722 | 79.2% | 68.6-100.0% | (16.8-100.0%) | |
| Unknown | 107 | 0.0% | 0.0-0.0% | (0.0-13.6%) | |
| Screen detected | 14 | 7661 | - | - | - |
| Yes | 6520 | 85.8% | 71.6-89.9% | (45.6-100.0%) | |
| No | 1106 | 14.2% | 10.1-27.2% | (0.0 - 54.4%) | |
| Unknown | 35 | 0.0% | 0.0-0.1% | (0.0-2.8%) | |
| Comedonecrosis | 14 | 6465 | - | - | - |
| Present | 3085 | 37.5% | 27.1-46.0% | (10.4-60.1%) | |
| Absent | 2713 | 55.5% | 34.3-61.5% | (2.0-81.6%) | |
| Unknown | 667 | 5.3% | 0.0-15.9% | (0.0-61.1%) | |
| Grade | 16 | 7225 | - | - | - |
| I-II | 4033 | 57.3% | 37.0-65.5% | (7.3-92.5%) | |
| I c | 901 | 17.5% | 9.1-25.2% | (1.8-64.5%) | |
| II c | 1163 | 28.0% | 23.6-34.9% | (5.5-45.0%) | |
| III | 2243 | 28.4% | 17.9-35.4% | (3.5-45.6%) | |
| Unknown | 949 | 9.2% | 0.0-37.3% | (0.0-87.3%) | |
| Hormone receptor | 5 | 1479 | - | - | - |
| Positive | 740 | 50.4% | 43.7-70.9% | (23.0-80.4%) | |
| Negative | 142 | 8.7% | 7.3-9.7% | (2.8-14.3%) | |
| Unknown | 597 | 40.9% | 16.8-46.6% | (14.8-69.8%) | |
| ER receptor | 3 | 1023 | - | - | - |
| Positive | 522 | 46.8% | 14.9-70.7% | (14.9-70.7%) | |
| Negative | 117 | 12.3% | 3.1-14.3% | (3.1-14.3%) | |
| Unknown | 384 | 40.9% | 15.0-82.0% | (15.0-82.0%) | |
| Tumour size (mm) | 8 | 1880 | 10.9 | 8.0-14.9 | (8.0-20.5) |
| Multifocality | 2 | 286 | - | - | - |
| Present | 46 | 12.6% | 0.0-25.1% | (0.0-25.1%) | |
| Absent | 134 | 58.5% | 16.9-100.0% | (16.9-100.0%) | |
| Unknown | 106 | 29.0% | 0.0-58.0% | (0.0-58.0%) | |
Total number of patients included in margins analyses by eligible studies, including those with unknown margin status. Excludes 269 patients with unconfirmed DCIS from one study (Bijker et al., 2006); those patients did not contribute to the analysis of margins in that study, but were included in descriptive covariate information. Hence, patient numbers for covariates in this table may include those patients, and may sum to more than 8651.
Includes 194 patients with APBI from one study.
From subset of 13 studies reporting grade I and II separately.
Of 11 studies using endocrine therapy, 7 used tamoxifen, 1 used either tamoxifen or other, and 3 did not report the type of endocrine therapy.
Abbreviations: APBI = accelerated partial breast irradiation; DCIS = ductal carcinoma in situ; ER = estrogen receptor; Gy = gray; IQR = interquartile range; LR = local recurrence; mm = millimetres; WBRT = whole-breast radiotherapy.
Random-effects logistic meta-regression modelling
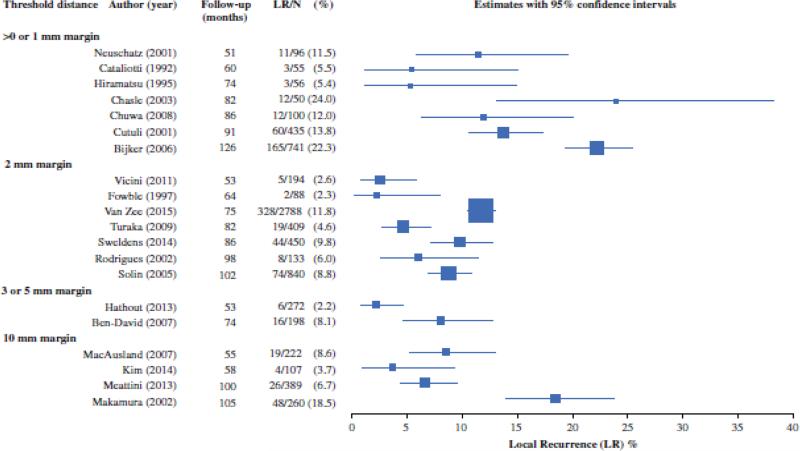
Figure 1 presents study-specific prevalence of LR, stratified by margin threshold used in the models and ordered by follow-up time. Heterogeneity was evident within margins categories; however, prevalence was generally higher at >0 or 1mm relative to wider thresholds.
Figure 1.
Forest plot of study-specific prevalence of LR
Models were adjusted for median follow-up time, given strong evidence that LR increased with follow-up (OR for every additional year of follow-up=1.29 [95% CI 1.11-1.51]; P=0.002). In multivariable models, there was no evidence that the effect of margin status was modified by distance (P for interaction=0.26). Table 2 presents ORs from a model of the main effects of margin status and distance. The OR for negative versus positive/close margin status was 0.53 (95% CI 0.45-0.62; P<0.001). The odds of LR were also associated with margin distance (P for association=0.046; df=3). Relative to >0 or 1mm, ORs for 2mm (0.51 [95% CI 0.31-0.85], P=0.01), 3 or 5mm (0.42 [95% CI 0.18-0.97], P=0.04) and 10mm (0.60 [0.33-1.08], P=0.09) showed comparable, statistically significant reductions in the odds of LR. Pairwise comparisons found no evidence of differences in ORs between the 2mm, 3 or 5mm, and 10mm thresholds (all P>0.40). There was no evidence for a trend in ORs across distance thresholds (P=0.11; df=1). Predicted probabilities of LR at 10-years derived from this model are presented in Table 3.
Table 2.
Main effects of margin status and distance on LR (random-effects logistic meta-regression)
| Negative margin relative to positive/close: OR (95% CI)** | Threshold distance for negative margins relative to >0 or 1 mm: N patients, and OR (95% CI) adjusted for follow-up | |||||
|---|---|---|---|---|---|---|
| >0 or 1 mm | 2 mm | 3 or 5 mm | 10 mm | P value [P for trend] | ||
| Main model (N patients) | 7883 | 1533 | 4902 | 470 | 978 | |
| 0.53 (0.45-0.62) | referent | 0.51 (0.31-0.85)* | 0.42 (0.18-0.97)* | 0.60 (0.33-1.08) | 0.046 [0.11] | |
| Sensitivity analysis (N patients) | 6042 | 1503 | 3420 | 470 | 649 | |
| WBRT cohorts only | 0.52 (0.43-0.63) | referent | 0.50 (0.32-0.79)* | 0.43 (0.20-0.92)* | 0.54 (0.30-0.97)* | 0.02 [0.03] |
| Sensitivity analysis (N patients) | 5115 | 1553 | 2114 | 470 | 978 | |
| Van Zee excluded | 0.45 (0.37-0.56) | referent | 0.44 (0.30-0.65)* | 0.43 (0.22-0.83)* | 0.59 (0.39-0.89)* | 0.004 [0.11] |
| Sensitivity analysis (N patients) | 9220 | 1853 | 4902 | 1042 | 1423 | |
| Adding studies with no summary age dataa | 0.53 (0.46-0.62) | referent | 0.49 (0.26-0.93)* | 0.81 (0.36-1.82) | 0.76 (0.38-1.52) | 0.16 [0.65] |
| Adjustment for covariates (based on main model) | ||||||
| Age | 0.53 (0.45-0.63) | referent | 0.51 (0.31-0.85)* | 0.42 (0.18-0.97)* | 0.60 (0.33-1.08) | 0.046 [0.11] |
| Median recruitment year | 0.53 (0.45-0.62) | referent | 0.56 (0.31-0.99)* | 0.45 (0.19-1.06) | 0.63 (0.34-1.15) | 0.15 [0.19] |
| Proportion with radiotherapy | 0.53 (0.45-0.62) | referent | 0.54 (0.32-0.89)* | 0.49 (0.20-1.19) | 0.58 (0.33-1.03) | 0.07 [0.08] |
| Proportion with endocrine therapyb | 0.55 (0.46-0.65) | referent | 0.52 (0.31-0.86)* | 0.46 (0.19-1.13) | 0.65 (0.34-1.23) | 0.07 [0.24] |
| Proportion with high grade DCISb | 0.55 (0.45-0.66) | referent | 0.55 (0.32-0.96)* | 0.47 (0.19-1.17) | 0.61 (0.27-1.38) | 0.16 [0.25] |
Statistically significantly different from >0 or 1 mm at P < 0.05.
All statistically significant at P < 0.001.
These studies (from the meta-analysis by Wang et al.) were ineligible for inclusion in our meta-analysis because of lack of summary age data (see eligibility criteria); hence, sensitivity analysis reports estimates if these were included in models.
Due to missing covariate information, these analyses were undertaken in a reduced number of studies (19 for endocrine therapy; 16 for high grade DCIS), therefore numbers analysed in these models will be less than those shown in main models.
Abbreviations: CI = confidence interval; DCIS = ductal carcinoma in situ; LR = local recurrence; mm = millimetre; OR = odds ratio; WBRT = whole-breast radiotherapy.
Table 3.
Predicted probabilities of local recurrence (LR) at 10 years from random-effects logistic meta-regression model (adjusted for follow-up)
| Threshold distance for negative margins | Overall probability (%) of 10-year LR as end-point (95% CI) | |||
|---|---|---|---|---|
| All studies | Cohorts with WBRT | |||
| Margin status | Margin status | |||
| Positive/close | Negative | Positive/close | Negative | |
| >0 or 1 mm | 29.4 (20.0-41.0) | 18.1 (11.7-26.7) | 30.1 (21.3-40.6) | 18.3 (12.5-26.0) |
| 2 mm | 17.6 (11.1-26.7) | 10.1 (6.3-16.0) | 17.8 (11.6-26.4) | 10.1 (6.5-15.5) |
| 3 or 5 mm | 14.9 (6.5-30.6) | 8.5 (3.6-18.9) | 15.6 (7.1-31.1) | 8.8 (3.8-18.9) |
| 10 mm | 20.0 (12.1-31.2) | 11.7 (6.7-19.4) | 18.9 (12.1-28.2) | 10.8 (6.7-17.1) |
Abbreviations: CI = confidence interval; LR = local recurrence; mm = millimetre; WBRT = whole-breast radiotherapy.
Table 2 presents results for models adjusted for covariates. The proportion of patients with high grade DCIS was the only statistically significant covariate in multivariable models (P<0.03). For all analyses, adjustment for covariates did not substantially change model estimates.
Network meta-analysis
For direct comparisons between positive and negative margins (adjusted for median follow-up), patients with negative margins were significantly less likely to experience LR than patients with positive margins (OR=0.45, 95% CrI 0.30-0.62).
Table 4 shows estimated relative margin threshold effect parameters on the OR scale compared to the reference category (positive margin group) provided by Bayesian analysis of the network. ORs for 2mm (0.32, 95% CrI 0.21-0.48), 3mm (0.30, 95% CrI 0.12-0.76) and 10mm (0.32, 95% CrI 0.19-0.49) all showed similar reductions in the odds of LR that were greater than for >0 or 1mm (0.45, 95% CrI 0.32-0.61). Probabilities for each threshold being the “best” option were inconclusive because the model was not able to reliably rank them during the iterative process for the reasons outlined by Jansen et al.39
Table 4.
Estimated treatment (margin threshold) effects on LR from the Bayesian network meta-analysis
| Threshold distance for negative margins relative to positive: mean OR (95% CrI) adjusted for follow-up | ||||
|---|---|---|---|---|
| >0 or 1 mm | 2 mm | 3 mm | 10 mm | |
| Main model (N patients) | 2230 | 2412 | 289 | 1963 |
| 0.45 (0.32-0.61) | 0.32 (0.21-0.48) | 0.30 (0.12-0.76) | 0.32 (0.19-0.49) | |
| Sensitivity analysis (N patients) | 1957 | 1851 | 272 | 1079 |
| WBRT cohorts only | 0.45 (0.34-0.61) | 0.33 (0.23-0.47) | 0.22 (0.08-0.53) | 0.37 (0.24-0.57) |
| Sensitivity analysis (N patients) | 1781 | 1524 | 289 | 616 |
| Van Zee et al. excluded | 0.43 (0.31-0.57) | 0.29 (0.19-0.45) | 0.32 (0.14-0.75) | 0.27 (0.16-0.47) |
| Sensitivity analysis (N patients) | 2692 | 2555 | 322c | 2160 |
| Adding studies with no summary age dataa | 0.44 (0.30-0.63) | 0.31 (0.19-0.51) | 0.32 (0.14-0.73) | 0.20 (0.11-0.35)* |
| Adjustment for covariates (based on main model) | ||||
| Age | 0.46 (0.33-0.63) | 0.34 (0.22-0.51) | 0.33 (0.13-0.83) | 0.33 (0.20-0.51) |
| Median recruitment year | 0.45 (0.31-0.62) | 0.31 (0.19-0.46) | 0.29 (0.12-0.68) | 0.32 (0.20-0.49) |
| Proportion with radiotherapy | 0.46 (0.33-0.63) | 0.33 (0.22-0.49) | 0.29 (0.12-0.74) | 0.32 (0.20-0.50) |
| Proportion with endocrine therapyb | 0.45(0.29-0.70) | 0.33 (0.18-0.57) | 0.29(0.10-0.79) | 0.31(0.17-0.57) |
| Proportion with high grade DCISb | 0.45 (0.32-0.62) | 0.33 (0.21-0.48) | 0.31(0.12-0.74) | 0.39 (0.25-0.59) |
95% CrI for ROR of 10 mm versus >0 or 1 mm did not cross 1.
These studies (from the meta-analysis by Wang et al.) were ineligible for inclusion in our meta-analysis because of lack of summary age data (see eligibility criteria); hence, sensitivity analysis reports estimates if these were included in models
Due to missing covariate information, these analyses were undertaken in a reduced number of studies (19 for endocrine therapy; 16 for high grade DCIS) therefore numbers analysed in these models will be less than those shown in main models. Alternative methods to deal with missing data produced similar results.
Two studies using a 5 mm threshold were included with the 3 mm threshold group.
Abbreviations: CrI = credible interval; DCIS = ductal carcinoma in situ; LR = local recurrence; mm = millimetre; OR = odds ratio; ROR = relative odds ratio; WBRT = whole-breast radiotherapy.
Comparisons between 10mm and 2mm showed no meaningful difference in the odds of LR (relative OR [ROR]=0.99, 95% CrI 0.61-1.64). Comparing >0 or1 mm and 2mm showed weak evidence of lower odds of LR for 2mm (ROR=0.72, 95% CrI 0.47-1.08). A similar ROR was observed for 10mm compared with >0 or 1mm (ROR=0.71, 95% CrI 0.44-1.11). Comparisons involving 3mm were not informative as just three studies contributed to that threshold.
Adjustment for covariates (age; mid-point of recruitment period; endocrine therapy; high grade) using different techniques to deal with missing data found that in all cases, the model with no covariates gave the best fit. Estimates from adjusted and unadjusted models were similar (Table 4).
Sensitivity analyses
Sensitivity analyses were conducted that included only study cohorts with adjuvant WBRT. The pattern of results was not altered in either logistic meta-regression (Tables 2 and 3) or network models (Table 4). Models in patients without WBRT failed to converge due to the small number of studies. Sensitivity analyses excluding the potentially influential study by Van Zee et al.27 resulted in similar ORs to those in the main analyses. Logistic meta-regression investigating the effect of reclassifying Van Zee et al.27 at 10mm resulted in a complex model (Appendix I), highlighting the limitations of modelling a single threshold per study.
Additional sensitivity analysis explored the effect of introducing four studies from the meta-analysis by Wang et al. not included in our analysis because they did not report summary age data40-43. Similar results to the main analysis were found for the network model for all but the 10mm threshold group, for which a lower OR was observed (Table 4). There was evidence of a lower OR for 10mm relative to >0 or 1mm (ROR=0.46, 95% CrI 0.26-0.77), attributable to inclusion of one non-WBRT study43 at 10mm. There was no evidence of a difference in the OR for 10mm relative to 2mm (ROR=0.66, 95% CrI 0.35-1.23), or in the OR for 2mm relative to >0 or 1mm (ROR=0.70, 95% CrI 0.42-1.16). In logistic models (Table 2), ORs for the 3 or 5mm (0.81 [95% CI 0.36-1.82]) and 10mm (0.76 [95% CI 0.38-1.52]) thresholds were larger than for the main analysis and not significantly different from >0 or 1mm (P>0.42), reflecting the inclusion of non-WBRT studies at 3 or 5mm28 and 10mm43 with relatively high prevalence of LR (31.0% and 17.8%, respectively). Pairwise comparisons found no evidence that 2mm, 3 or 5mm, and 10mm were different from one another (P>0.20 for all).
Network models were not sensitive to assumptions on the prior distributions and the parameters of these distributions, or to the assumed correlation structure for multiple thresholds within studies.
Discussion
We sourced data on 8,651 patients with DCIS from 20 studies, and meta-analysed these for 7,883 with known margins with a median follow-up of 78 months. Most study cohorts received WBRT, but not endocrine therapy. Two analytic approaches explored how best these heterogeneous data could be modelled: the Bayesian network approach supported more robust and efficient meta-analysis that could utilize data at all margin thresholds compared with conventional random-effects logistic meta-regression. The network analysis showed that the odds of LR are reduced in negative margins relative to positive margins (OR=0.45, 95% CrI 0.30-0.62). It also showed that, relative to positive margins, the 2mm (OR=0.32, 95% CrI 0.21-0.48), 3mm (OR=0.30, 95% CrI 0.12-0.76) and 10mm thresholds (OR=0.32, 95% CrI 0.19-0.49) all had a similar reduction in the odds of LR that was lower than for >0 or 1mm (OR=0.45, 95% CrI 0.32-0.61). These findings were largely consistent with the logistic meta-regression analyses when we classified a large study27 at 2mm.
Our results differ to those of Wang et al.4, who found decreasing ORs as the threshold distance increased up to 10mm and hence recommended a 10mm margin for DCIS. In contrast, the odds of LR in our analysis did not decrease beyond a distance of 2mm. Compared with our network analysis, Wang et al.4 included fewer studies, patients and events at 10mm. Our sensitivity analyses incorporating studies included by Wang et al.4 that did not meet our eligibility criteria suggested that a single no-WBRT study43 (the only study in Wang et al. directly comparing 2 and 10mm, and the only study to contribute additional data at 10mm in our sensitivity analysis), was influential in lowering the odds of LR at 10mm.
When we restricted analyses to only those cohorts receiving WBRT, the pattern of results was unchanged, highlighting the applicability of our findings to patients who receive adjuvant WBRT. Models in patients without WBRT failed to converge due to the small number of studies; therefore, our analysis is unable to investigate whether the effect of margins is modified by receipt or non-receipt of WBRT. However, a recent, large study comparing WBRT and no-WBRT cohorts provides evidence that the effect of margins on LR is modified by adjuvant WBRT; larger margin distances were significantly associated with lower rates of LR in those without WBRT but not those with WBRT27.
There are limitations to analysis of study-level covariates in this meta-analysis, in particular where aggregate data are similar across studies (e.g. age), or where a specific therapy is (or is not) received in the majority (e.g. WBRT and APBI)16;17. An individual patient data meta-analysis of four randomized controlled trials found BCS and adjuvant WBRT to be significantly associated with a reduction in any LR compared with BCS alone (15.2% absolute reduction in 10-year risk)44. However, the study-level proportion of patients receiving WBRT was found not to be univariately associated with LR in our analysis, and therefore did not meet the criterion for inclusion in multivariable models. This is likely to be due to WBRT being used in a majority of patients (71%). Nevertheless, we investigated the effect of WBRT on prior grounds, and both modelling approaches found no substantial differences between models with or without adjustment for WBRT. In addition, given the fact that only one study using APBI was included in the analysis, we are unable to draw conclusions about the effect of margins in patients treated with APBI.
A strength of the Bayesian network model is its capacity to include multiple distance thresholds per study, maximising comparisons to inform conclusions about appropriate negative margin thresholds in DCIS. A possible limitation of that approach is that multiple thresholds result in “closed” distance categories for smaller thresholds, potentially attenuating their effect on LR. In our analysis, this applied particularly to the >0 or 1mm threshold, for which 10 of 16 data points are “closed” categories (four with an upper bound of 2mm). In contrast, of seven data points at 2mm, there was just one closed category (10mm upper bound). Therefore, the network analysis may have exaggerated differences between the 2mm and >0 or 1mm thresholds. However, a similar pattern of results was observed in our logistic meta-regression models, where the limitation of closed margins categories did not apply. These complementary analyses therefore suggest that our results showing lower odds of LR at 2mm compared to >0 or 1mm are likely to be robust.
Heterogeneity in margin definitions among included studies lead to thresholds of >0mm and 1mm being combined in our analysis. This ameliorated the effect of “closed” categories associated with a distance of >0mm, thereby minimising heterogeneity between thresholds. This approach also maximized direct comparisons in the network models; a network structure including separate >0mm and 1mm categories would result in models driven by indirect comparisons, which are potentially unreliable. However, as a result, this analysis has the limitation of being unable to compare margins of >0mm and 1mm.
Notwithstanding the limitations inherent in study-level analyses, the two alternative but complementary meta-analytic approaches reported in our work were consistent in finding reductions in LR at a threshold distance of 2mm relative to smaller thresholds in BCS for DCIS. There was no evidence that minimum margins wider than 2mm were associated with additional reductions in LR in women receiving adjuvant WBRT. Therefore, our meta-analysis indicates that a negative margin threshold of 2mm is an appropriate recommendation for surgical management of DCIS in women receiving BCS with WBRT.
Supplementary Material
Synopsis.
This study-level meta-analysis modelled local recurrence relative to margin distance in breast conserving surgery for DCIS. Odds of local recurrence were reduced at 2mm relative to smaller margins. Larger distances were not associated with lower odds in women receiving radiation.
Acknowledgments
ML Marinovich is supported by a Cancer Institute NSW (CINSW) Early Career Fellowship. N Houssami receives research support via a National Breast Cancer Foundation (NBCF Australia) Breast Cancer Research Leadership Fellowship. This work was partly supported by a NHMRC program grant to the STEP.
References
- 1.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in Situ of the breast: A systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 2.Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103:478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irwig L, Bennetts A. Quality of life after breast conservation or mastectomy: A systematic review. Aust New Zealand J Surg. 1997;67:750–754. doi: 10.1111/j.1445-2197.1997.tb04573.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang S-Y, Chu H, Shamliyan T, et al. Network meta-analysis of margin threshold for women with ductal carcinoma in situ. J Natl Cancer Inst. 2012;104:507–516. doi: 10.1093/jnci/djs142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunne C, Burke JP, Morrow M, Kell MR. Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol. 2009;27:1615–1620. doi: 10.1200/JCO.2008.17.5182. [DOI] [PubMed] [Google Scholar]
- 6.Pilewskie M, Morrow M. Extent and role of margin control for DCIS managed by breast-conserving surgery. In: Newman LA, Bensenhaver JM, editors. Ductal carcinoma in situ and microinvasive/borderline breast cancer. Springer; New York: 2015. pp. 67–83. [Google Scholar]
- 7.Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32:1507–1515. doi: 10.1200/JCO.2013.53.3935. [DOI] [PubMed] [Google Scholar]
- 8.American Society of Breast Surgeons . The American Society of Breast Surgeons Position Statement on Breast Cancer Lumpectomy Margins. American Society of Breast Surgeons; 2013. https://www.breastsurgeons.org/statements/PDF_Statements/Lumpectomy_Margins.pdf. 12-14-2015. [Google Scholar]
- 9.National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology, Breast Cancer Version 1.2016. National Comprehensive Cancer Network; 2015. http://www.nccn.org . 12-14-2015. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence (NICE) Early and locally advanced breast cancer: diagnosis and treatment: NICE guidelines [CG80] National Institute for Health and Care Excellence; 2009. https://www.nice.org.uk/guidance/cg80/chapter/guidance#surgery-to-the-breast. 12-14-2015. [Google Scholar]
- 11.New Zealand Guidelines Group (NZGG) Ductal carcinoma in situ. Management of early breast cancer: Evidence-based best practice guideline. New Zealand Guidelines Group; Wellington: 2015. pp. 133–141. [Google Scholar]
- 12.Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v8–v30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 13.Morrow M, et al. Society of Surgical Oncology - American Society for Radiation Oncology - American Society of Clinical Oncology Consensus Guideline on Margins for Breast Conserving Surgery with Whole Breast Irradiation in Ductal Carcinoma In Situ. Under review by SSO/ASTRO/ASCO. 2016 doi: 10.1016/j.prro.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vicini FA, Recht A. Age at diagnosis and outcome for women with ductal carcinoma-in-situ of the breast: A critical review of the literature. J Clin Oncol. 2002;20:2736–2744. doi: 10.1200/JCO.2002.07.137. [DOI] [PubMed] [Google Scholar]
- 15.Kong I, Narod SA, Taylor C, et al. Age at diagnosis predicts local recurrence in women treated with breast-conserving surgery and postoperative radiation therapy for ductal carcinoma in situ: A population-based outcomes analysis. Curr Oncol. 2014;21:e96–e104. doi: 10.3747/co.21.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houssami N, Macaskill P, Marinovich ML, Morrow M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: A meta-analysis. Ann Surg Oncol. 2014;21:717–730. doi: 10.1245/s10434-014-3480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010;46:3219–3232. doi: 10.1016/j.ejca.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 18.Vicini F, Beitsch P, Quiet C, et al. Five-Year analysis of treatment efficacy and cosmesis by the American society of breast surgeons mammosite breast brachytherapy registry trial in patients treated with accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;79:808–817. doi: 10.1016/j.ijrobp.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 19.Hathout L, Hijal T, Theberge V, et al. Hypofractionated radiation therapy for breast ductal carcinoma in situ. Int J Radiat Oncol Biol Phys. 2013;87:1058–1063. doi: 10.1016/j.ijrobp.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Ben-David MA, Sturtz DE, Griffith KA, et al. Long-term results of conservative surgery and radiotherapy for ductal carcinoma in situ using lung density correction: The University of Michigan experience. Breast J. 2007;13:392–400. doi: 10.1111/j.1524-4741.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- 21.Solin LJ, Fourquet A, Vicini FA, et al. Long-term outcome after breast-conservation treatment with radiation for mammographically detected ductal carcinoma in situ of the breast. Cancer. 2005;103:1137–1146. doi: 10.1002/cncr.20886. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues N, Carter D, Dillon D, Parisot N, Choi DH, Haffty BG. Correlation of clinical and pathologic features with outcome in patients with ductal carcinoma in situ of the breast treated with breast-conserving surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2002;54:1331–1335. doi: 10.1016/s0360-3016(02)03747-1. [DOI] [PubMed] [Google Scholar]
- 23.Cutuli B, Cohen-Solal-Le NC, De LB, et al. Ductal carcinoma in situ of the breast results of conservative and radical treatments in 716 patients. Eur J Cancer. 2001;37:2365–2372. doi: 10.1016/s0959-8049(01)00303-3. [DOI] [PubMed] [Google Scholar]
- 24.Hiramatsu H, Bornstein BA, Recht A, et al. Local recurrence after conservative surgery and radiation therapy for ductal carcinoma in situ: Possible importance of family history. Cancer J Sci Am. 1995;1:55–61. [PubMed] [Google Scholar]
- 25.Cataliotti L, Distante V, Ciatto S, et al. Intraductal breast cancer: Review of 183 consecutive cases. Eur J Cancer. 1992;28:917–920. doi: 10.1016/0959-8049(92)90150-z. [DOI] [PubMed] [Google Scholar]
- 26.Turaka A, Freedman GM, Li T, et al. Young age is not associated with increased local recurrence for DCIS treated by breast-conserving surgery and radiation. J Surg Oncol. 2009;100:25–31. doi: 10.1002/jso.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Zee KJ, Subhedar P, Olcese C, Patil S, Morrow M. Relationship between margin width and recurrence of ductal carcinoma in situ: Analysis of 2996 women treated with breast-conserving surgery for 30 years. Ann Surg. 2015;262:623–631. doi: 10.1097/SLA.0000000000001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meattini I, Livi L, Franceschini D, et al. Role of radiotherapy boost in women with ductal carcinoma in situ: A single-center experience in a series of 389 patients. Eur J Surg Oncol. 2013;39:613–618. doi: 10.1016/j.ejso.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Chuwa EWL, Tan VHS, Tan P-H, Yong W-S, Ho G-H, Wong C-Y. Treatment for ductal carcinoma in situ in an Asian population: Outcome and prognostic factors. ANZ J Surg. 2008;78:42–48. doi: 10.1111/j.1445-2197.2007.04354.x. [DOI] [PubMed] [Google Scholar]
- 30.MacAusland SG, Hepel JT, Chong FK, et al. An attempt to independently verify the utility of the Van Nuys Prognostic Index for ductal carcinoma in situ. Cancer. 2007;110:2648–2653. doi: 10.1002/cncr.23089. [DOI] [PubMed] [Google Scholar]
- 31.Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: Ten-year results of european organisation for research and treatment of cancer randomized phase III trial 10853 - A study by the EORTC breast cancer cooperative group and EORTC radiotherapy group. J Clin Oncol. 2006;24:3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 32.Chasle J, Delozier T, Denoux Y, Marnay J, Michels J-J. Immunohistochemical study of cell cycle regulatory proteins in intraductal breast carcinomas - A preliminary study. Eur J Cancer. 2003;39:1363–1369. doi: 10.1016/s0959-8049(02)00774-8. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura S, Woo C, Silberman H, Streeter J, Lewinsky BS, Silverstein MJ. Breast-conserving therapy for ductal carcinoma in situ: A 20-year experience with excision plus radiation therapy. Am J Surg. 2002;184:403–409. doi: 10.1016/s0002-9610(02)01011-5. [DOI] [PubMed] [Google Scholar]
- 34.Neuschatz AC, DiPetrillo T, Safaii H, Lowther D, Landa M, Wazer DE. Margin width as a determinant of local control with and without radiation therapy for ductal carcinoma in situ (DCIS) of the breast. Int J Cancer. 2001;96:97–104. doi: 10.1002/ijc.10357. [DOI] [PubMed] [Google Scholar]
- 35.Fowble B, Hanlon AL, Fein DA, et al. Results of conservative surgery and radiation for mammographically detected ductal carcinoma in situ (DCIS). Int J Radiat Oncol Biol Phys. 1997;38:949–957. doi: 10.1016/s0360-3016(97)00153-3. [DOI] [PubMed] [Google Scholar]
- 36.Sweldens C, Peeters S, Van LE, et al. Local relapse after breast-conserving therapy for ductal carcinoma in situ: A European single-center experience and external validation of the memorial Sloan-Kettering cancer center DCIS nomogram. Cancer J. 2014;20:1–7. doi: 10.1097/PPO.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 37.Kim H, Noh JM, Choi DH, et al. Excision alone for small size ductal carcinoma in situ of the breast. Breast. 2014;23:586–590. doi: 10.1016/j.breast.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 39.Jansen JP, Trikalinos T, Cappelleri JC, et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: An ISPOR-AMCP-NPC good practice task force report. Value Health. 2014;17:157–173. doi: 10.1016/j.jval.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Fisher ER, Dignam J, Chiu E, et al. Pathologic findings from the national surgical adjuvant breast project (NSABP) eight-year update of protocol b-17: Intraductal carcinoma. Cancer. 1999;86:429–438. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 41.Fish EB, Chapman J-A, Miller NA, et al. Assessment of treatment for patients with primary ductal carcinoma in situ in the breast. Ann Surg Oncol. 1998;5:724–732. doi: 10.1007/BF02303484. [DOI] [PubMed] [Google Scholar]
- 42.Sahoo S, Recant WM, Jaskowiak N, Tong L, Heimann R. Defining negative margins in DCIS patients treated with breast conservation therapy: The University of Chicago experience. Breast J. 2005;11:242–247. doi: 10.1111/j.1075-122X.2005.21617.x. [DOI] [PubMed] [Google Scholar]
- 43.Macdonald HR, Silverstein MJ, Mabry H, et al. Local control in ductal carcinoma in situ treated by excision alone: Incremental benefit of larger margins. Am J Surg. 2005;190:521–525. doi: 10.1016/j.amjsurg.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Correa C, McGale P, Taylor C, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010:162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cutuli B, Cohen-Solal-Le NC, De LB, et al. Breast-conserving therapy for ductal carcinoma in situ of the breast: The French Cancer Centers' experience. Int J Radiat Oncol Biol Phys. 2002;53:868–879. doi: 10.1016/s0360-3016(02)02834-1. [DOI] [PubMed] [Google Scholar]
- 46.Tunon-De-Lara C, De-Mascarel I, Mac-Grogan G, et al. Analysis of 676 cases of ductal carcinoma in situ of the breast from 1971 to 1995: Diagnosis and treatment - The experience of one institute. Am J Clin Oncol Cancer Clin Trials. 2001;24:531–536. doi: 10.1097/00000421-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: An update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28:400–418. doi: 10.1016/s0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 48.Chan KC, Fiona KW, Sinha G, et al. Extent of excision margin width required in breast conserving surgery for ductal carcinoma in situ. Cancer. 2001;91:9–16. doi: 10.1002/1097-0142(20010101)91:1<9::aid-cncr2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 49.Warneke J, Grossklaus D, Davis J, et al. Influence of local treatment on the recurrence rate of ductal carcinoma in situ. J Am Coll Surg. 1995;180:683–688. [PubMed] [Google Scholar]
- 50.Kestin LL, Goldstein NS, Martinez AA, et al. Mammographically detected ductal carcinoma in situ treated with conservative surgery with or without radiation therapy. Patterns of failure and 10-year results. Ann Surg. 2000;231:235–245. doi: 10.1097/00000658-200002000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ringberg A, Idvall I, Ferno M, et al. Ipsilateral local recurrence in relation to therapy and morphological characteristics in patients with ductal carcinoma in situ of the breast. Eur J Surg Oncol. 2000;26:444–451. doi: 10.1053/ejso.1999.0919. [DOI] [PubMed] [Google Scholar]
- 52.Rudloff U, Brogi E, Reiner AS, et al. The influence of margin width and volume of disease near margin on benefit of radiation therapy for women with DCIS treated with breast-conserving therapy. Ann Surg. 2010;251:583–591. doi: 10.1097/SLA.0b013e3181b5931e. [DOI] [PubMed] [Google Scholar]
- 53.Gelman A, Rubin DB. Inference from Iterative Simulation Using Multiple Sequences (with discussion). Statistical Science. 1992;7:457–511. [Google Scholar]
- 54.Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2002;64:583–639. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.