Abstract
Avian leucosis virus subgroup J (ALV-J) can cause lifelong infection and can escape from the host immune defenses in chickens. Since macrophages act as the important defense line against invading pathogens in host innate immunity, we investigated the function and innate immune responses of chicken primary monocyte-derived macrophages (MDM) after ALV-J infection in this study. Our results indicated that ALV-J was stably maintained in MDM cells but that the viral growth rate was significantly lower than that in DF-1 cells. We also found that ALV-J infection significantly increased nitric oxide (NO) production, but had no effect on MDM phagocytic capacity. Interestingly, infection with ALV-J rapidly promoted the expression levels of Myxovirus resistance 1 (Mx) (3 h, 6 h), ISG12 (6 h), and interleukin-1β (IL-1β) (3 h, 12 h) at an early infection stage, whereas it sharply decreased the expression of Mx (24 h, 36 h), ISG12 (36 h), and made little change on IL-1β (24 h, 36 h) production at a late infection stage in MDM cells. Moreover, the protein levels of interferon-β (IFN-β) and interleukin-6 (IL-6) had sharply increased in infected MDM cells from 3 to 36 h post infection (hpi) of ALV-J. And, the protein level of interleukin-10 (IL-10) was dramatically decreased at 36 hpi in MDM cells infected with ALV-J. These results demonstrate that ALV-J can induce host innate immune responses and we hypothesize that macrophages play an important role in host innate immune attack and ALV-J immune escape.
Keywords: ALV-J, macrophage, innate immune response
INTRODUCTION
Avian leucosis virus subgroup J (ALV-J) is an alpharetrovirus that causes immunosuppression and can induce tumors in infected chickens (Payne and Nair, 2012). There is no available vaccine to prevent ALV-J infection and new virus strains are often isolated in China and other parts of the world (Dong et al., 2015; Malhotra et al., 2015). ALV-J has an uncertain length of latency and replicates at variable rates during the course of a persistent, lifelong infection. Previous studies have primarily focused on the impact that ALV-J has on the host while ignoring the host response to virus, especially host innate immune responses.
Macrophages play a crucial role in both the innate and adaptive immune responses. These cells belong to the mononuclear phagocytic system lineage (Qureshi, 2003) but recent studies suggest that tissue-resident macrophages are derived from embryonic precursors and rarely from circulating monocytes (Hashimoto et al., 2013; Yona et al., 2013). Even so, a prevailing model is that most tissue macrophages are derived from circulating monocytes (Davies et al., 2013).
As a major part of the first line of immune defense against pathogens, macrophages are phagocytes that secrete cytokines and chemokines, are the primary antigen-presenting cells and can kill pathogens via nitric oxide (NO) production (Qureshi et al., 2000; Braukmann et al., 2015). However, macrophages can also serve as a retroviral reservoir and directly transmit virus to T cells (Kilareski et al., 2009). In mammals, macrophages are susceptible to lentiviral infection which often results in persistent infections (Narayan and Clements, 1989). In contrast, chicken macrophages are resistant to infection by common avian oncogenic viruses such as infectious bronchitis virus, Marek's disease virus, and reticuloendotheliosis virus (von Bulow and Klasen, 1983a; von Bulow and Klasen, 1983b). Additionally, chicken primary macrophages also have effect on bacterial-disease with innate immune responses (Zhou et al., 2013; Braukmann et al., 2015).
Chicken cytokine responses such as type I interferon (IFN) production are induced by the low pathogenicity avian influenza virus (LPAIV) in macrophage-like cells. In contrast, the highly pathogenic avian influenza virus does not induce IFN in chicken macrophage cell lines (Xing et al., 2008; Liniger et al., 2012). Early studies have demonstrated that macrophages from several chicken genetic lines are susceptible to avian leucosis virus subgroups B and C, but resistant to avian leucosis virus subgroups A, D, E, F, and G infection (Gazzolo et al., 1975). However, the interaction of ALV-J with chicken macrophages, particularly the innate immune responses of macrophages induced by ALV-J, has not been explored. In the present study, we examined the relationship between chicken primary monocyte-derived macrophages (MDM) and ALV-J infection and focused on the cytokine responses induced by ALV-J.
MATERIALS AND METHODS
Ethics Statement
All animal research protocols were approved by South China Agriculture University's Institutional Animal Care and Use Committee. All animal procedures were performed according to the regulations and guidelines established by this committee and the international standards for animal welfare.
Virus
ALV-J strain SCAU-HN06 was provided by the College of Veterinary Medicine, South China Agricultural University. Chickens infected with SCAU-HN06 strain can induce hemangiomas (Zhang et al., 2011).
Experimental Animals
Six-week-old specific-pathogen-free White Leghorn chickens, half males and half females, were purchased from Guangdong DaHuaNong Animal Health Products Co., Ltd and housed in isolator cages. Whole blood samples were collected in EDTA anticoagulation tubes with negative pressure via jugular venipuncture. Collected blood volumes never exceeded 3% of the total blood volume (Dawes et al., 2014).
Isolation and Culture Conditions of Chicken MDM
Chicken primary MDM were isolated from peripheral blood mononuclear cells (PBMC). PBMC from individual chickens were separated according to previously described methods and the manufacturer's instructions of chicken lymphocyte separation medium (Solarbio, Beijing, China) (Dawes et al., 2014). Cells were seeded in 6-well plates (Corning, NY, USA) at a density of 2.5 × 106 PBMC per well. The PBMC suspension was incubated at 37°C in the presence of 5% CO2. After 6 h of incubation, supernatants were removed and adherent cells were washed twice with warm PBS to remove thrombocytes, non-adherent lymphocytes, and other semi-adherent cells. Fresh RPMI-1640 medium (15% chicken serum, 100 U/mL penicillin, and 100 mg/mL streptomycin) was then added to the remaining adherent cells which were primarily monocytes. These cells were then cultured for 6 d to maturity for macrophage differentiation. The culture medium was changed every 2 d in order to ensure stable and consistent conditions.
Cell Identification
MDM identity was confirmed by visual microscopy using inverted microscope (Nikon, Tokyo, Japan) scanning electron microscopy (Fei-XL30 ESEM, Fei, Hillsboro, USA) and flow cytometry. The mouse anti-chicken monocyte/macrophage antibody KUL01-FITC (Southern BioTech, Birmingham, AL) was used to evaluate the cell surface expression of markers typically expressed on chicken macrophage by flow cytometry (Dawes et al., 2014). A mouse anti-MHC Class II monoclonal antibody (Abcam, Cambridge, UK) that cross-reacts with chicken was also used to examine MDM cells by flow cytometry. Flow Jo software was used to analyze the data collected using the Flow Cytometer BD Accuri C6 instrument (BD Biosciences, New Jersey, USA).
Infection of MDM with ALV-J
Six-day-old primary MDM were washed with warm PBS and infected with a 1.3 × 105 TCID50 (50% tissue culture infective dose) /mL dosage of ALV-J strain SCAU-HN06. After 2 h incubation, the virus in the cell medium was discarded and replaced with fresh RPMI-1640 medium containing 5% chicken serum, 100 U/mL penicillin, and 100 mg/mL streptomycin. The infected MDM were maintained at 37°C with 5% CO2 for 7 d with daily monitoring and sample collection. After 3 h, 6 h, and 1 to 7 d infection, 100 μL supernatant per well of the cell culture was collected from 6-well cell culture plates and replaced with an equal volume of fresh cell culture medium. Samples were tested for ALV group-specific antigen (p27) by antigen-capture enzyme-linked immunosorbent assay (ELISA) (IDEXX, ME, USA) according to the manufacturer's instructions. The results were expressed as s/p ratios where s/p = (Sample Mean –Kit Negative Control Mean) / (Kit Positive Control Mean –Kit Negative Control Mean). DF1 cells were used as a control and infected as described above for MDM. DF1 cells were obtained from ATCC (Manassas, VA).
DNA was extracted from MDM after 3 to 36 h infection using a commercial kit (Omega, Norcross, GA, USA). Specific PCR primers were employed to identify ALV-J (Smith et al., 1998). The supernatant of the MDM culture was tested by inoculating them into DF1 cells to identify whether ALV-J had proliferated in chicken MDM cells. All experiments were performed in triplicate.
Detection of NO Levels
The level of NO production in the MDM culture supernatant was measured after 12, 24, and 36 h ALV-J infection using a commercial NO assay kit (Beyotime Biotechnology, Shanghai, China). The concentration of nitrite was measured using absorbance readings at 540 nm. Sodium nitrite was used as a standard curve. Uninfected MDM cells were used as control and all reactions were performed in triplicate.
Capacity of Phagocytosis
Effects of ALV-J infection on the phagocytic ability of infected MDM cells was measured by adding 0.5 mg/mL FITC-dextran (40,000D) (Sigma-Aldrich, Santa Clara, CA, USA) to MDM infected with ALV-J for 24 h (Wang et al., 2013). Infected and uninfected MDM were then incubated at 37°C for 1 h. One uninfected MDM culture plate was incubated at 4°C for 1 h as a control. All experiments were performed in triplicate. After 1 h, the cells were washed extensively to remove excess FITC-dextran and the phagocytosis capacity was measured using flow cytometry and processed as described above using Flow Jo software.
Quantitative Real-time Polymerase Chain Reaction
Total RNA was extracted from infected and uninfected MDM cells at 3, 6, 12, 24, and 36 h post-infection using the RNAfast200 kit (Fastagen, Shanghai, China). cDNA was synthesized using the RevertAid First strand cDNA synthesis kit (Thermo, Waltham, MA, USA) according to the manufacturer's instructions. The primers used for quantitative real time polymerase chain reaction (qRT-PCR) have been previously reported (Kint et al., 2015). GAPDH was used as an internal control (Dai et al., 2015). qRT-PCR was performed on a Bio-Rad CFX96 Real-Time Detection System using iTaqTM Universal SYBR Green Supermix Kit reagents (BIO-RAD, California, USA) according to the manufacturer's specifications. Data analyses were performed using the 2−ΔΔCt method (Livak and Schmittgen, 2001). All experiments were performed in triplicate.
Cytokine Measurements by ELISA
Samples were taken at 3, 6, 12, 24, and 36 h after SCAU-HN06 infection and lysed by freezing and thawing three times. The MDM cell supernatants and cell lysates were used at each time point to measure cytokine expression. IFN-β, IL-1β, IL-6, and IL-10 were examined by ELISA using a commercial kit (USCN, Wuhan, China) according to the manufacturer's specifications.
Statistical Analysis
The significance of the variability among the trials was analyzed by GraphPad Prism (version 5.0) software. The results were presented as means ± SEM and the statistical significance was represented by P values of < 0.05, 0.01, or 0.001.
RESULTS
Characterization of Cultured Chicken MDM
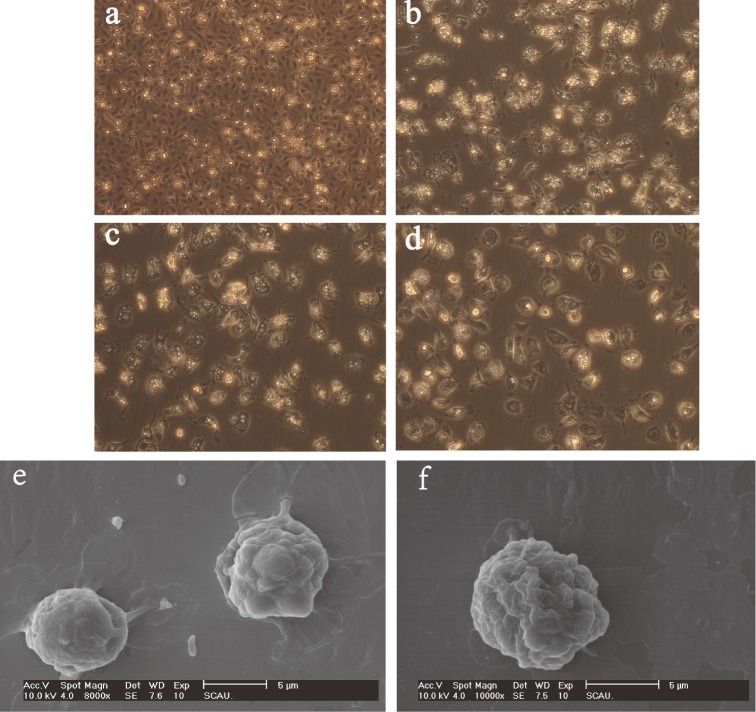
Monocytes after 6 days in culture showed clear and distinct macrophage-like morphologies (Figure 1a-e). As described previously (Qureshi, 2003), as the cytoplasmic volume of these cells increased, the MDM cells spread on the plate surface and exhibited cytoplasmic vacuolation coupled with a reduction in the nucleo-cytoplasmic ratio. Compared to the uninfected MDM cells, ALV-J infected cells appeared as though there were pleats on the cell surface (Figure 1f).
Figure 1.
Morphological changes of chicken MDM. Chicken MDM cells at 6 h, 2 d, 4 d, and 6 d post-adherence as viewed using an inverted microscope (a, b, c, d) (300×). Morphological comparisons between normal MDM (e, 8,000×) and ALV-J infected MDM (f, 10,000×) using scanning electron microscopy.
We used flow cytometry to characterize surface antigen expression of the MDM at 6 d of culture to ensure that these cells were MDM. The cells were positively stained with the monocyte/macrophage antibody KUL01-FITC (97.30 ± 0.26%) and MHC-II (30.87 ± 2.12%) (Figure 2). This indicated that our cell separation procedure resulted in true MDM cells.
Figure 2.
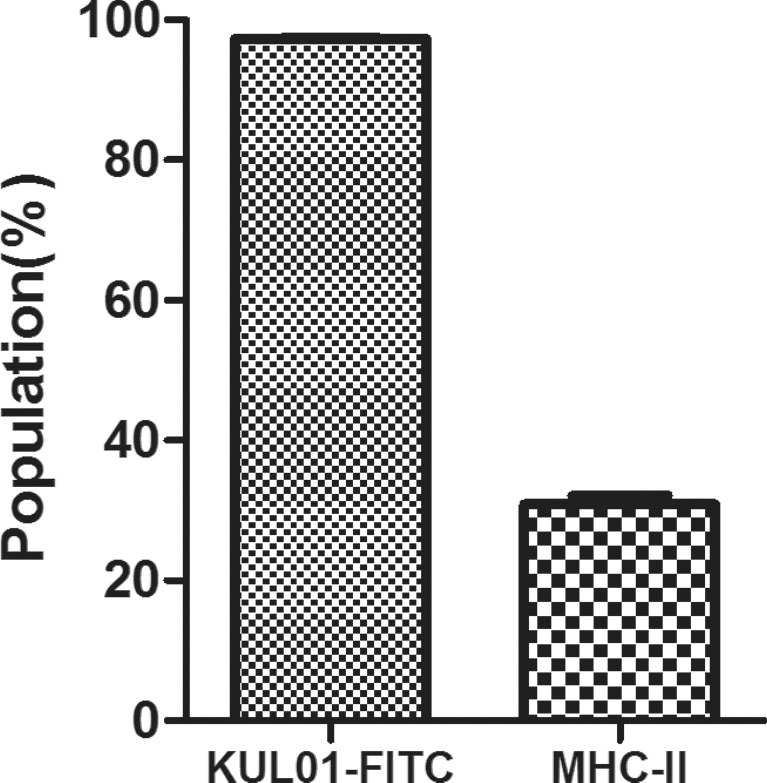
Expression of surface molecules on chicken MDM cells. Cultured MDM were stained with monoclonal antibody KUL01-FITC and MHC-II and analyzed using flow cytometry.
Growth Kinetics of SCAU-HN06 Infected MDM and DF1 Cells
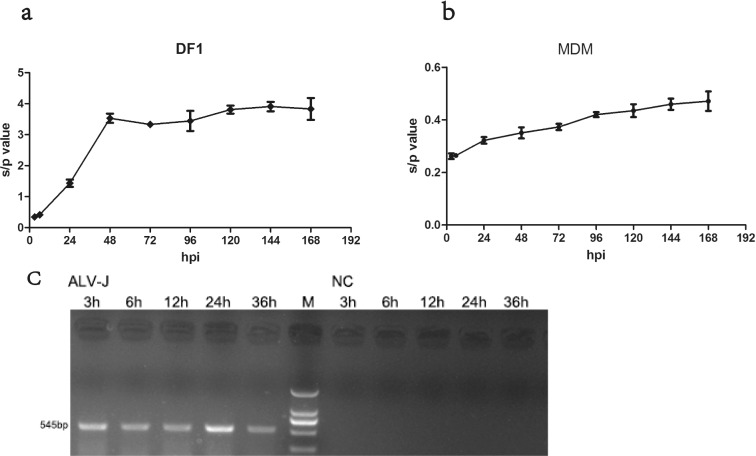
The chicken fibroblast cell line DF1 was developed from Line 0 (EV-0) chick embryo and is known to be susceptible only to exogenous ALV (Himly et al., 1998). MDM and DF1 cells were infected with a 1.3 × 105 TCID50/mL dosage of ALV-J strain SCAU-HN06. We measured growth kinetics after SCAU-HN06 infection by daily testing up to 7 d (Figure 3a, b). The s/p value of virus propagated in DF1 cells were much larger than the s/p value tested in MDM cells. The s/p value of virus propagated in MDM cells just exceeded the threshold value (0.2) and increased slightly over time.
Figure 3.
Growth kinetics of SCAU-HN06 propagated in DF1 and MDM cells. Growth curves for SCAU-HN06 in (a) DF1 and (b) MDM cells by measuring expression of the viral group-specific antigen p27 by ELISA from cell culture supernatants collected at 3 to 168 hpi. (c) PCR results using primers specific for SCAU-HN06 in infected MDM and uninfected MDM at 3 to 36 h.
The stable replication of SCAU-HN06 propagated in MDM cells was further confirmed by reinfecting DF1 cells. ELISA testing resulted in an s/p value of 2.830 for DF1 cells with SCAU-HN06 propagated in MDM cells. PCR tests of MDM after 3 to 36 h infection using ALV-J-specific primers were all positive (Figure 3c, left). ALV-J could not be detected in the control cells (Figure 3c, right).
NO Production after SCAU-HN06 Infection
Supernatants from the MDM cells infected with SCAU-HN06 at 12, 24, and 36 h post infection (hpi) were tested for the presence of NO. Compared to the uninfected controls, NO produced by the MDM cells infected with SCAU-HN06 increased significantly at each time point (Figure 4a).
Figure 4.
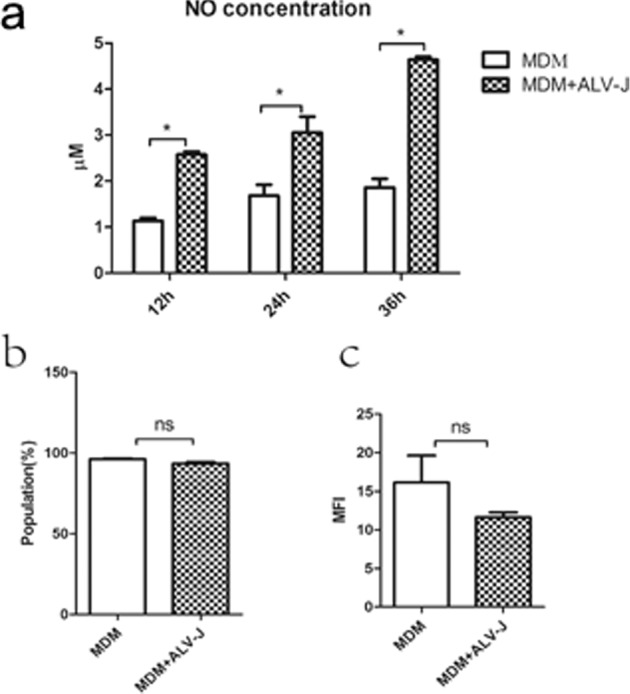
Detection of NO and phagocytic capacity in MDM after SCAU-HN06 infection. (a) NO measurements using a commercial kit (b) Flow cytometry of FITC-dextran loaded normal MDM and infected MDM at 24 hpi. (c) Median fluorescence intensity (MFI) values between control and infected groups (*P < 0.05, ns P > 0.05).
Phagocytic Capacity
Phagocytic capacity of the MDM cells infected with SCAU-HN06 and uninfected MDM cells were examined by flow cytometry at 24 hpi. The amount of FITC-dextran taken up by infected MDM cells (93.43 ± 1.50% of positive cells) showed slightly lower phagocytic ability compared to uninfected MDM cells (96.13 ± 0.71%) (Figure 4b). The median fluorescence intensity (MFI) of infected MDM and uninfected MDM cells was 11.63 ± 1.07 and 16.17 ± 6.01, respectively (Figure 4c). This data indicated that the virus infection had a negligible effect on MDM phagocytic ability.
Transcription of Interferon-stimulated Genes
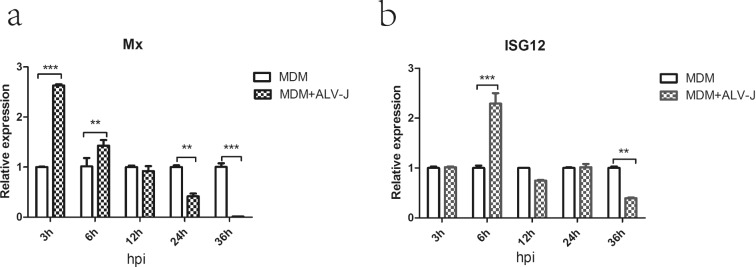
In order to verify whether ALV-J can lead to the transcription of interferon-stimulated genes (ISGs), the relative expression of ISG12 and Mx was measured by qRT-PCR. The expression of ISG12 at 6 hpi and Mx at 3 and 6 hpi in infected MDM cells were significantly up-regulated compared to controls (Figure 5a, b). However, the transcription of these genes was significantly down-regulated at 36 hpi (ISG12) and 24 and 36 hpi (Mx) respectively (Figure 5a, b).
Figure 5.
Relative quantification of mRNA expression of Mx and ISG12 in MDM infected with SCAU-HN06. (a) Mx; (b) ISG12 (**P < 0.01; ***P < 0.001).
Cytokine Expression of MDM Cells with SCAU-HN06 Infection
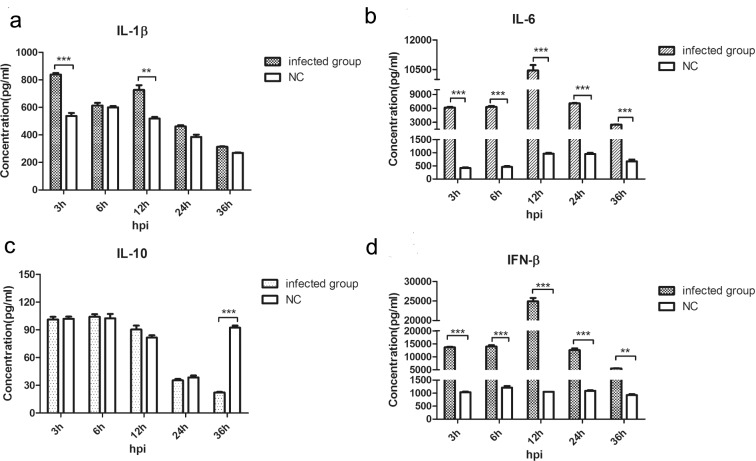
We next measured production of IFN-β, IL-1β, IL-6, and IL-10 to determine if virus infection modulates cytokine production. Compared to uninfected controls, IFN-β and IL-6 protein levels in infected MDM cells increased throughout the study period. The overall pattern was biphasic with peaks and minima at 12 and 36 hpi, respectively (Figure 6b, d). On the other hand, IL-1β levels increased at 3 and 12 hpi (Figure 6a). In contrast, the immunosuppressive cytokine IL-10 was significantly reduced at 36 hpi without significant differences between infected and uninfected MDM cells (Figure 6c).
Figure 6.
Cytokine production in MDM cells at 3 to 36 hpi. Cytokines were measured by ELISA. (a) IL-1β; (b) IL-6; (c) IL-10; (d) IFN-β (**P < 0.01; ***P < 0.001).
DISCUSSION
Infection of chickens by ALV-J can cause tumors as well as immunosuppression. However, the mechanisms of ALV-J induced immunosuppression are not fully understood. Prior immune studies related to ALV have mainly focused on adaptive, and only rarely, innate immunity (Gao et al., 2015). Macrophages are the most plastic cells in the hematopoietic system and show great functional diversity in various tissues (Wynn et al., 2013). As professional antigen-presenting cells, these cells play an important role in both innate immunity and adaptive immunity (Wynn et al., 2013).
In chicken macrophage studies, experimental procedures have used macrophage cell lines such as HD11, HTC, and MQ-NCSU (Xing et al., 2008; Liniger et al., 2012; Haddadi et al., 2013). However, the immune functions of macrophage cell lines may be impaired and genetic differences develop with continuous subculture (Wang et al., 2013). Furthermore, the chicken macrophage cell lines mentioned above are transformed by ALV or Marek's disease virus (Beug et al., 1979; Rath et al., 2003). Therefore, some viral genes may be present in the genomes of these cell lines and may have negative effects on innate immune responses following ALV-J infection. Therefore, we used chicken primary MDM to negate these potential adverse effects.
In the present study, chicken MDM cells had high level of KUL01 antigen and high MHC-II expression. Monoclonal antibody KUL01 has been successfully applied to identify immune cell populations including monocyte/macrophage and NK cell subsets (Stewart et al., 2013). MHC-II is highly expressed on mature and fully functional macrophages (Wang et al., 2009). Compared to DF1 cells, the MDM cells used in our experiments had lower s/p values after ALV-J infection. Nonetheless, our PCR results indicated that chicken macrophages can be infected by ALV-J. Macrophages play key roles in HIV transmission, viral spread in early infection, and can act as a viral reservoir throughout infection (Campbell et al., 2014). ALV-J is a retrovirus and the effects of chicken MDM in ALV-J transmission as well as latent infection deserves further investigation.
NO is secreted by activated macrophages and plays a critical role in innate and adaptive immune immunity including control and escape of infectious pathogens, T and B cell differentiation, and tumor defense (Bogdan, 2015). Our results showed that NO production significantly increased in the SCAU-HN06 infected MDM cells at 12, 24, and 36 hpi demonstrating that chicken MDM cells are activated by ALV-J. However, the amount of NO produced in this study was low. We speculate that this phenomenon was related to the pattern recognition receptor for ALV-J. The TLR4 agonist lipopolysaccharide is the most potent stimulus for NO production in chicken monocytes. Other agonists such as the TLR3 agonist poly I:C as well as the TLR7 agonist loxoribine stimulated little or no NO production (He et al., 2006). In one of our previous studies, we found that ALV-J was primarily recognized by chicken TLR7 and MDA5 (unpublished data). Therefore we speculate that this is a reason for the lack of NO production in this study. In addition, the phagocytic capacity of MDM cells after ALV-J infection was also unchanged at 24 hpi.
Macrophages are the first cells to encounter pathogens and respond by secreting various cytokines such as IL-1, IL-6 and IL-10 (Xing et al., 2008). The pro-inflammatory cytokine IL-1β is a part of the IL-1 family and its presence correlated with some disease states such as influenza and dengue (Xing et al., 2008; Callaway et al., 2015). IL-6 is also a pro-inflammatory cytokine and is multifunctional in the innate immune response (Schaper and Rose-John, 2015). In chickens infected with ALV-J, IL-6 was shown to play a key role in inhibiting ALV-J infection (Gao et al., 2015). In the present study, IL-6 was sustained at high levels from 3 to 36 hpi and IL-1β production increased at early stages of infection (3 and 12 hpi). However, virus production was not reduced as indicated by the presence of the p27 vial antigen. In contrast, IL-10 production did not change after ALV-J infection except at 36 hpi where IL-10 protein levels were significantly reduced. IL-10 production was inhibited in the ALV-J infected MDM cells. IL-10 is the most important anti-inflammatory cytokine and has immunosuppressive effects (Sabat et al., 2010). It is produced primarily by activated macrophages and has negative effects by reducing inflammatory cytokines such as IL-6 and IL-1β and inhibiting antigen presentation as well as phagocytosis (Sabat et al., 2010). Previous reports had demonstrated that IL-1β either alone or in combination with IL-6 can enhance HIV-1 replication in promonocytic cell lines (Poli et al., 1994). IL-10 can inhibit HIV-1 replication in human macrophages (Tanaka et al., 2005) and HIV-1 infected macrophages showed an early reduction of IL-10 secretion (Tomlinson et al., 2014). Our findings indicates that SCAU-HN06 can induce IL-1β and IL-6 production and block IL-10 production in chicken MDM. These results might indicate that this is a strategy for ALV-J to evade host innate immunity and this deserves further investigation. In addition, IL-1 and IL-6 have been linked with tumorigenesis, which suggests that inflammation is associated with tumor development (Aggarwal et al., 2006). SCAU-HN06 can induce IL-1β and IL-6 expression in chicken MDM. Therefore we hypothesize that macrophages play an important role in tumorigenesis induced by ALV-J.
Type I IFN are universally acknowledged for their antiviral function (Davidson et al., 2015). IFN-β belongs to the type I IFN and has antiviral activity in chickens (Sick et al., 1996). The type I IFN system is triggered by virus and induces hundreds of ISGs such as Mx and ISG12 activated via JAK-STAT pathway. The products of these ISGs exert antiviral effector functions (Schoggins and Rice, 2011). The Mx proteins have antiviral activity for AIV in mammals (Haller et al., 2007). However, there is no consistent conclusion about antiviral activity of the chicken Mx protein (Ewald et al., 2011) and some studies suggest that chicken Mx protein lacks antiviral function (Schusser et al., 2011). ISG12 has been shown to inhibit Newcastle disease virus replication (Liu et al., 2014). Avian infectious bronchitis virus and infectious bursal disease virus escape from the host antiviral immune response by avoiding the activation of the IFN-β response (Ye et al., 2014; Kint et al., 2015). In this study, IFN-β protein levels were sustained at high level in the infected MDM at 3 to 36 hpi. Mx and ISG12 transcription increased at 3 hpi and 6 hpi, and expression was significantly inhibited at 36 hpi. These result showed that SCAU-HN06 can induce the expression of type I IFN and ISGs. However, SCAU-HN06 can also inhibit ISG expression at late infection stages by unknown mechanisms. IFN-β and ISGs have no effect on the replication of the ALV-J. Our previous study found that microRNA-23b can promote SCAU-HN06 replication by targeting IRF1 in vitro (Li et al., 2015). IRF-1 is a transcription factor that restricts virus replication by regulating ISG expression (Stirnweiss et al., 2010). We speculate that ALV-J escapes from or inhibits the host antiviral immune responses via blocking ISG expression and we are investigating this further.
CONCLUSION
In summary, chicken MDM cells were susceptible to ALV-J strain SCAU-HN06. The virus significantly increased the NO production of MDM cells, but had no effect on phagocytic capacity. The innate immune responses of chicken MDM cells were induced by ALV-J strain SCAU-HN06. MDM infected with SCAU-HN06 produced high levels of IFN-β and IL-6 as well as inhibiting IL-10 production in infected MDM cells.
Acknowledgments
This work was supported by Natural Scientific Foundation of China (31571269), and the China Agriculture Research System (CARS-42-G05).
REFERENCES
- Aggarwal B. B., Shishodia S., Sandur S. K., Pandey M. K., Sethi G. Inflammation and cancer: How hot is the link? Biochemical pharmacology. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Doderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979;18:375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends in immunology. 2015;36:161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Braukmann M., Methner U., Berndt A. Immune reaction and survivability of salmonella typhimurium and salmonella infantis after infection of primary avian macrophages. PloS one. 2015;10:e0122540. doi: 10.1371/journal.pone.0122540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway J. B., Smith S. A., McKinnon K. P., de Silva A. M., Crowe J. E., Jr., Ting J. P. Spleen Tyrosine Kinase (Syk) Mediates IL-1beta Induction by Primary Human Monocytes during Antibody-enhanced Dengue Virus Infection. J. Biol. Chem. 2015;290:17306–17320. doi: 10.1074/jbc.M115.664136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. H., Hearps A. C., Martin G. E., Williams K. C., Crowe S. M. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. Aids. 2014;28:2175–2187. doi: 10.1097/QAD.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M., Feng M., Liu D., Cao W., Liao M. Development and application of SYBR Green I real-time PCR assay for the separate detection of subgroup J Avian leukosis virus and multiplex detection of avian leukosis virus subgroups A and B. Virology journal. 2015;12:52. doi: 10.1186/s12985-015-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S., Maini M. K., Wack A. Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2015;35:252–264. doi: 10.1089/jir.2014.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies L. C., Jenkins S. J., Allen J. E., Taylor P. R. Tissue-resident macrophages. Nature immunology. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes M. E., Griggs L. M., Collisson E. W., Briles W. E., Drechsler Y. Dramatic differences in the response of macrophages from B2 and B19 MHC-defined haplotypes to interferon gamma and polyinosinic: polycytidylic acid stimulation. Poul. Sci. 2014;93:830–838. doi: 10.3382/ps.2013-03511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Zhao P., Li W., Chang S., Li J., Li Y., Ju S., Sun P., Meng F., Liu J., Cui Z. Diagnosis and sequence analysis of avian leukosis virus subgroup J isolated from Chinese Partridge Shank chickens. Poul. Sci. 2015;94:668–672. doi: 10.3382/ps/pev040. [DOI] [PubMed] [Google Scholar]
- Ewald S. J., Kapczynski D. R., Livant E. J., Suarez D. L., Ralph J., McLeod S., Miller C. Association of Mx1 Asn631 variant alleles with reductions in morbidity, early mortality, viral shedding, and cytokine responses in chickens infected with a highly pathogenic avian influenza virus. Immunogenetics. 2011;63:363–375. doi: 10.1007/s00251-010-0509-1. [DOI] [PubMed] [Google Scholar]
- Gao Y., Liu Y., Guan X., Li X., Yun B., Qi X., Wang Y., Gao H., Cui H., Liu C., Zhang Y., Wang X., Gao Y. Differential expression of immune-related cytokine genes in response to J group avian leukosis virus infection in vivo. Molecular immunology. 2015;64:106–111. doi: 10.1016/j.molimm.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Gazzolo L., Moscovici M. G., Moscovici C., Vogt P. K. Susceptibility and resistance of chicken macrophages to avian RNA tumor viruses. Virology. 1975;67:553–565. doi: 10.1016/0042-6822(75)90455-9. [DOI] [PubMed] [Google Scholar]
- Haddadi S., Kim D. S., Jasmine H., van der Meer F., Czub M., Abdul-Careem M. F. Induction of Toll-like receptor 4 signaling in avian macrophages inhibits infectious laryngotracheitis virus replication in a nitric oxide dependent way. Veterinary immunology and immunopathology. 2013;155:270–275. doi: 10.1016/j.vetimm.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Haller O., Staeheli P., Kochs G. Interferon-induced Mx proteins in antiviral host defense. Biochimie. 2007;89:812–818. doi: 10.1016/j.biochi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Hashimoto D., Chow A., Noizat C., Teo P., Beasley M. B., Leboeuf M., Becker C. D., See P., Price J., Lucas D., Greter M., Mortha A., Boyer S. W., Forsberg E. C., Tanaka M., van Rooijen N., Garcia-Sastre A., Stanley E. R., Ginhoux F., Frenette P. S., Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Genovese K. J., Nisbet D. J., Kogut M. H. Profile of Toll-like receptor expressions and induction of nitric oxide synthesis by Toll-like receptor agonists in chicken monocytes. Molecular immunology. 2006;43:783–789. doi: 10.1016/j.molimm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Himly M., Foster D. N., Bottoli I., Iacovoni J. S., Vogt P. K. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology. 1998;248:295–304. doi: 10.1006/viro.1998.9290. [DOI] [PubMed] [Google Scholar]
- Kilareski E. M., Shah S., Nonnemacher M. R., Wigdahl B. Regulation of HIV-1 transcription in cells of the monocyte-macrophage lineage. Retrovirology. 2009;6:118. doi: 10.1186/1742-4690-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kint J., Fernandez-Gutierrez M., Maier H. J., Britton P., Langereis M. A., Koumans J., Wiegertjes G. F., Forlenza M. Activation of the chicken type I interferon response by infectious bronchitis coronavirus. J. Virol. 2015;89:1156–1167. doi: 10.1128/JVI.02671-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Chen B., Feng M., Ouyang H., Zheng M., Ye Q., Nie Q., Zhang X. MicroRNA-23b Promotes Avian Leukosis Virus Subgroup J (ALV-J) Replication by Targeting IRF1. Scientific reports. 2015;5:10294. doi: 10.1038/srep10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liniger M., Moulin H. R., Sakoda Y., Ruggli N., Summerfield A. Highly pathogenic avian influenza virus H5N1 controls type I IFN induction in chicken macrophage HD-11 cells: a polygenic trait that involves NS1 and the polymerase complex. Virology journal. 2012;9:7. doi: 10.1186/1743-422X-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Long Y., Liu B., Yang D., Li C., Chen T., Wang X., Liu C., Zhu H. ISG12a mediates cell response to Newcastle disease viral infection. Virology. 2014;462–463:283–294. doi: 10.1016/j.virol.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Malhotra S., t. Justice J., Lee N., Li Y., Zavala G., Ruano M., Morgan R., Beemon K. Complete genome sequence of an american avian leukosis virus subgroup j isolate that causes hemangiomas and myeloid leukosis. Genome announcements. 2015;3 doi: 10.1128/genomeA.01586-14. doi:10.1128/genomeA.01586-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Clements J. E. Biology and pathogenesis of lentiviruses. J. Gen. Virol. 1989;70:1617–1639. doi: 10.1099/0022-1317-70-7-1617. [DOI] [PubMed] [Google Scholar]
- Payne L. N., Nair V. The long view: 40 years of avian leukosis research. Avian pathology: journal of the W.V.P.A. 2012;41:11–19. doi: 10.1080/03079457.2011.646237. [DOI] [PubMed] [Google Scholar]
- Poli G., Kinter A. L., Fauci A. S. Interleukin 1 induces expression of the human immunodeficiency virus alone and in synergy with interleukin 6 in chronically infected U1 cells: inhibition of inductive effects by the interleukin 1 receptor antagonist. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:108–112. doi: 10.1073/pnas.91.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi M. A. Avian macrophage and immune response: an overview. Poul. Sci. 2003;82:691–698. doi: 10.1093/ps/82.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi M. A., Heggen C. L., Hussain I. Avian macrophage: effector functions in health and disease. Developmental and comparative immunology. 2000;24:103–119. doi: 10.1016/s0145-305x(99)00067-1. [DOI] [PubMed] [Google Scholar]
- Rath N. C., Parcells M. S., Xie H., Santin E. Characterization of a spontaneously transformed chicken mononuclear cell line. Vet. Immunol. Immunopathol. 2003;96:93–104. doi: 10.1016/s0165-2427(03)00143-0. [DOI] [PubMed] [Google Scholar]
- Sabat R., Grutz G., Warszawska K., Kirsch S., Witte E., Wolk K., Geginat J. Biology of interleukin-10. Cytokine & growth factor reviews. 2010;21:331–344. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Schaper F., Rose-John S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine & growth factor reviews. 2015 doi: 10.1016/j.cytogfr.2015.07.004. doi:10.1016/j.cytogfr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Schoggins J. W., Rice C. M. Interferon-stimulated genes and their antiviral effector functions. Current opinion in virology. 2011;1:519–525. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schusser B., Reuter A., von der Malsburg A., Penski N., Weigend S., Kaspers B., Staeheli P., Hartle S. Mx is dispensable for interferon-mediated resistance of chicken cells against influenza A virus. J. Virol. 2011;85:8307–8315. doi: 10.1128/JVI.00535-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sick C., Schultz U., Staeheli P. A family of genes coding for two serologically distinct chicken interferons. J. Biol. Chem. 1996;271:7635–7639. doi: 10.1074/jbc.271.13.7635. [DOI] [PubMed] [Google Scholar]
- Smith L. M., Brown S. R., Howes K., McLeod S., Arshad S. S., Barron G. S., Venugopal K., McKay J. C., Payne L. N. Development and application of polymerase chain reaction (PCR) tests for the detection of subgroup J avian leukosis virus. Virus research. 1998;54:87–98. doi: 10.1016/s0168-1702(98)00022-7. [DOI] [PubMed] [Google Scholar]
- Stewart C. R., Keyburn A. L., Deffrasnes C., Tompkins S. M. Potential directions for chicken immunology research. Developmental and comparative immunology. 2013;41:463–468. doi: 10.1016/j.dci.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Stirnweiss A., Ksienzyk A., Klages K., Rand U., Grashoff M., Hauser H., Kroger A. IFN regulatory factor-1 bypasses IFN-mediated antiviral effects through viperin gene induction. J. Immunol. 2010;184:5179–5185. doi: 10.4049/jimmunol.0902264. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Hoshino Y., Gold J., Hoshino S., Martiniuk F., Kurata T., Pine R., Levy D., Rom W. N., Weiden M. Interleukin-10 induces inhibitory C/EBPbeta through STAT-3 and represses HIV-1 transcription in macrophages. American journal of respiratory cell and molecular biology. 2005;33:406–411. doi: 10.1165/rcmb.2005-0140OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson G. S., Bell L. C., Walker N. F., Tsang J., Brown J. S., Breen R., Lipman M., Katz D. R., Miller R. F., Chain B. M., Elkington P. T., Noursadeghi M. HIV-1 infection of macrophages dysregulates innate immune responses to Mycobacterium tuberculosis by inhibition of interleukin-10. J. Infect. Dis. 2014;209:1055–1065. doi: 10.1093/infdis/jit621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bulow V., Klasen A. Effects of avian viruses on cultured chicken bone-marrow-derived macrophages. Avian pathology : journal of the W.V.P.A. 1983a;12:179–198. doi: 10.1080/03079458308436162. [DOI] [PubMed] [Google Scholar]
- von Bulow V., Klasen A. Growth inhibition of Marek's disease T-lymphoblastoid cell lines by chicken bone-marrow-derived macrophages activated in vitro. Avian pathology : journal of the W.V.P.A. 1983b;12:161–178. doi: 10.1080/03079458308436161. [DOI] [PubMed] [Google Scholar]
- Wang C., Yu X., Cao Q., Wang Y., Zheng G., Tan T. K., Zhao H., Zhao Y., Wang Y., Harris D. Characterization of murine macrophages from bone marrow, spleen and peritoneum. BMC immunology. 2013;14:6. doi: 10.1186/1471-2172-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Cui X., Tai G., Ge J., Li N., Chen F., Yu F., Liu Z. A critical role of activin A in maturation of mouse peritoneal macrophages in vitro and in vivo. Cellular & molecular immunology. 2009;6:387–392. doi: 10.1038/cmi.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T. A., Chawla A., Pollard J. W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z., Cardona C. J., Li J., Dao N., Tran T., Andrada J. Modulation of the immune responses in chickens by low-pathogenicity avian influenza virus H9N2. J. Gen. Virol. 2008;89:1288–1299. doi: 10.1099/vir.0.83362-0. [DOI] [PubMed] [Google Scholar]
- Ye C., Jia L., Sun Y., Hu B., Wang L., Lu X., Zhou J. Inhibition of antiviral innate immunity by birnavirus VP3 protein via blockage of viral double-stranded RNA binding to the host cytoplasmic RNA detector MDA5. J. Virol. 2014;88:11154–11165. doi: 10.1128/JVI.01115-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S., Kim K. W., Wolf Y., Mildner A., Varol D., Breker M., Strauss-Ayali D., Viukov S., Guilliams M., Misharin A., Hume D. A., Perlman H., Malissen B., Zelzer E., Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. N., Lai H. Z., Qi Y., Zhang X. T., Ning Z. Y., Luo K. J., Xin C. A., Cao W. S., Liao M. An ALV-J isolate is responsible for spontaneous haemangiomas in layer chickens in China. Avian pathology : journal of the W.V.P.A. 2011;40:261–267. doi: 10.1080/03079457.2011.560142. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Wang Z., Cao L., Hu S., Zhang Z., Qin B., Guo Z., Nie K. Upregulation of chicken TLR4, TLR15 and MyD88 in heterophils and monocyte-derived macrophages stimulated with Eimeria tenella in vitro. Experimental parasitology. 2013;133:427–433. doi: 10.1016/j.exppara.2013.01.002. [DOI] [PubMed] [Google Scholar]