Abstract
Purpose
To assess the safety and tolerability of pre-operative cryoablation-mediated tumor antigen presentation and/or ipilimumab-mediated immune modulation in women with operable breast cancer.
Experimental design
In this pilot study, 19 women with breast cancer for whom mastectomy was planned were treated with pre-operative tumor cryoablation (n=7), single-dose ipilimumab at 10mg/kg (n=6), or both (n=6). The primary outcome for this pilot study was safety/tolerability as defined as freedom from delays in pre-planned, curative-intent mastectomy. Exploratory studies of immune activation were performed on peripheral blood and tumor.
Results
Pre-operative cryoablation and/or ipilimumab were safe and tolerable, with no delays in pre-planned surgery. Grade III toxicity was seen in 1/19 (unrelated rash after ipilimumab). Combination therapy was associated with sustained peripheral elevations in: Th1-type cytokines, activated (ICOS+) and proliferating (Ki67+) CD4+ and CD8+ T cells, and post-treatment proliferative T-effector cells relative to T-regulatory cells within tumor.
Conclusions
Pre-operative cryoablation and single-dose ipilimumab are safe alone or in combination with no surgical delays incurred. Potentially favorable intra-tumoral and systemic immunologic effects were observed with the combination, suggesting the possibility for induced and synergistic anti-tumor immunity with this strategy.
Keywords: Cryoablation, ipilimumab, breast cancer, immunotherapy
Introduction
Therapeutic antibodies against immune checkpoint molecules such as cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death-1/ligand-1 (PD-1/PD-L1) provide significant clinical benefits in a growing number of malignancies. For example, CTLA-4-directed antibodies release T cell inhibition thereby promoting effector T cell activation and proliferation, and have demonstrated significant disease specific benefits in melanoma.(1, 2) In other diseases, for example Hodgkin’s Lymphoma, objective response rates have ranged as high as 87% with PD-1/PD-L1 directed strategies.(3) However, the experience in breast cancer is relatively limited thus far. In advanced hormone receptor positive breast cancer CTLA-4 blockade in combination with an aromatase inhibitor yielded a 42% disease stability rate with no objective responses observedm,(4) and responses to PD-1 pathway blockade in metastatic triple negative breast cancer have been relatively modest (19% in two cohorts of heavily-pretreated triple negative disease).(5, 6)
One explanation for the modest response rates in breast and some other solid tumors is that these modern checkpoint blockade strategies require pre-existing immune “recognition” of the tumor and that some tumor types are less inherently recognizable by the immune system due to either low antigenicity related to mutational load and/or a hostile tumor microenvironment.(7) Reflecting this, surrogate measures of immune recognition including the extent of tumoral infiltration by lymphocyte and tumoral PD-L1 expression are relatively low in the majority of breast cancer specimens.(8–10)
Since checkpoint blockade alone may be an insufficient therapeutic approach in most breast cancers, one promising alternative is to combine checkpoint blockade with methods that engender de novo immune responses against tumor-associated antigens. Cryoablation—the process of freezing a tumor with a thermal probe—induces cell lysis and may optimize the presentation of tumor specific information to the immune system.(11) This tumor-specific immune response may in turn be augmented by checkpoint blockade. Tumor cryoablation administered in conjunction with a checkpoint blocking antibody against CTLA-4 improved survival in a TRAMP C2 mouse model of prostate cancer, also generating intratumoral and systemic expansion of CD8+ T-cells against the SPAS-1 tumor-specific antigen.(12) Thus, cryoablation combined with immune modulation has the potential to generate a potent, persistent, synergistic and tumor-specific immune response, which could thereby confer long- term breast cancer specific immunity.
As an initial step, we sought to confirm the safety and tolerability of immune checkpoint blockade with tumor cryoablation in women with newly diagnosed, operable breast cancer. In designing this trial, one important consideration was the selection of an immune modulating antibody. Ipilimumab, an FDA-approved antibody against CTLA-4, has a well-established safety profile, induces long-term remissions lasting ≥10 years in 10–20% of treated patients in advanced metastatic melanoma,(13) and prolongs progression free survival in the adjuvant setting in high- risk melanoma.(14) Furthermore, because T-cells acutely upregulate expression of CTLA-4 after being exposed to antigens(15) and because CTLA-4 signaling may blunt T-cell responses against these antigens, ipilimumab may be uniquely suited to facilitate priming of antigen-exposed T- cells following cryoablation.(16) For this reason, we aimed to recapitulate the pre-clinical model in humans, and evaluate the safety of ipilimumab in combination with tumor cryoablation.
A second critical design consideration was the selection of an appropriate patient population. Most early phase drug development trials are conducted in patients with metastatic disease; however, this population may be the least amenable to response to immunotherapy as a result of larger tumor burdens and/or iatrogenic immune suppression. Furthermore, although there is significant focus on immunotherapy strategies in the treatment of triple negative breast cancer because of the innate association with tumor infiltrating lymphocytes (TILs), there is no evidence to date that this population will exclusively benefit from strategies incorporating checkpoint blockade with or without tumor ablation. For these reasons, we evaluated cryo- immunotherapy in women with early stage breast cancer (ESBC) of any histology, aiming to facilitate anti-tumor immunity against tumor micrometastases and increase the likelihood of long term disease control. Correlative studies were conducted on serially collected blood and tumor specimens in order to explore the local and systemic impact of the intervention(s), with a goal of directly informing future studies. Here, we report the results from the first study combining cryoablation with checkpoint blockade women with breast cancer treated with curative intent.
Materials and Methods
Study design and participants
Between April 2012 and October 2013, women with biopsy-proven invasive breast cancer planning mastectomy with curative intent at Memorial Sloan Kettering Cancer Center (MSKCC), were considered for enrollment. Inclusion criteria included: tumor ≥1.5cm (by either radiography or clinical exam), mastectomy planned in ≥14 days, feasibility of cryoablation (as determined by a study interventional radiologist), and age 18 years or older. Exclusion criteria included: inflammatory breast cancer, history of autoimmune disease, history of chronic immunosuppression, prior immunotherapy, recent vaccination (<4 weeks), prior radiation therapy, or prior investigational agent. Any hormone receptor (HR), human epidermal receptor growth factor 2 (HER2), and nodal status were permitted. HR-positivity was defined as ≥1% expression of either estrogen receptor or progesterone receptor by immunohistochemistry (IHC). HER2-positivity was defined as either 3+ expression by IHC and/or ≥2.0 HER2 to chromosome 17 centromere signals by fluorescence in situ hybridization (FISH). Multifocal, multicentric and synchronous bilateral invasive disease was permitted.
After providing informed consent, women were sequentially assigned to receive preoperative tumor cryoablation (cryo) alone (group A, n=7), single-dose intravenous ipilimumab (ipi) 10mg/kg alone (group B, n=6), or both cryo and single-dose ipi (group C, n=6). Although enrollment of six patients per group was planned, one subject treated with cryo alone was replaced because of a cryo probe malfunction that resulted in incomplete tumor freezing as reported by the treating interventional radiologist and later confirmed by pathology review. Consequently, all seven patients contribute to the primary safety endpoint but only the six patients in group A for whom cryo was successful are included in the analyses exploring changes in immune correlatives in response to the specific interventions.
The treatment schedule was designed to optimize antigen exposure time without necessitating a delay in the average 2-week lead time between breast cancer diagnosis and standard-of-care surgical resection at our center (figure 1). In groups A and C, cryo was performed a median of 7 days prior to mastectomy (range, 4–10 days), and in groups B and C, ipi was administered a median of 10 days prior to mastectomy (range 8–13 days). In group C, ipi was administered a median of 3 days before cryo (range, 1–5 days).
Figure 1.
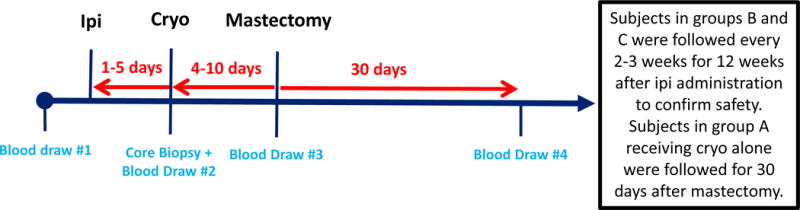
Study design. The timing of all study interventions (tumor biopsy, cryoablation, ipilimumab administration) was defined by the pre-determined, standard-of-care, non-study mastectomy date.
The study was performed in accordance with ethical principles of the Declaration of Helsinki and the International Conference on Harmonization of Good Practice, and approved by the MSKCC Institutional Review Board. All patients provided written informed consent.
Safety and Feasibility Assessments
The primary objective of this pilot study was to assess the feasibility of administering cryo and/or immunotherapy in early stage breast cancer prior to surgical mastectomy. Success was predefined as having at least 5/6 subjects receiving their assigned therapy without grade III/IV adverse events necessitating a delay in the pre-established surgical mastectomy date (a clinically driven end-point defined by the multi-disciplinary MSKCC Breast Cancer Disease Management Team). Toxicities were assessed using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events v4.0 (CTCAE). Subjects receiving ipi were followed clinically and with serum and blood counts (complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone) to confirm safety every 2–3 weeks for 12 weeks after ipilimumab administration (groups B and C) in order to document any potential delayed immune related AEs. Subjects receiving cryo alone were followed for 30 days after mastectomy (group A).
Cryoablation Procedure
Percutaneous cryoablation was performed under MRI guidance 4 to 10 days prior to mastectomy. Research biopsy with intravenous gadolinium was performed in the same session before cryoablation (Appendix). All cases were performed under general anesthesia in the prone position with a breast coil in an interventional MR scanner (Optima MR450w, GE Medical Systems, Milwaukee, WI, USA). Compression grids used for biopsy were either left deployed or released for cryoablation at the discretion of interventional radiologist. A prophylactic dose of intravenous cefazolin was used. Due to the presence of the breast coil and grids only a lateral approach was possible. One to two MR-compatible applicators (IceRod or IceSeed, Galil Medical, Inc., Arden Hills, MN, USA) were used for each case. Cryoablation procedures consisted of two 8–10 minute freeze cycles each followed by a 6–8 minute passive or active thaw cycle. Warm compress and saline injection were used to protect the overlying skin. The length of freeze-thaw cycle were individualized to the patient and their tumor based on intra procedural MR imaging. Complete tumor ablation was not required. After recovery patients were discharged home.
Peripheral Blood and Intratumoral Lymphocyte Isolation
Peripheral blood was obtained at the time of consent, time of biopsy/cryoablation, time of mastectomy, and at the 30 days post-mastectomy safety follow-up visit. Adjuvant systemic therapy or radiation therapy was not initiated until after the safety follow-up visit.
Research biopsies were obtained at the time of cryo (for groups A and C) or during a research biopsy (group B) within 2 days of ipi administration (median 0 days, range 1–3). Fresh mastectomy tumor tissue was submitted immediately following surgery to the MSKCC Ludwig Center Immune Monitoring Core Facility, where TILs were extracted and cryopreserved using previously described methods.(17, 18)
Flow Cytometry
Flow cytometry was performed on both PBMCs and harvested TILs as previously described.(17, 18) Briefly, one million PBMCs/TILs were washed with 2 mL FACS buffer (PBS containing bovine 1% serum albumin and 0.05 mM EDTA), resuspended in 50 μL FACS buffer and stained with a panel of antibodies (CD3-BV570, Biolegend, San Diego, CA; CD4-Qdot655, CD8-Qdot605 and Live/Dead Fixable Dead Cell Stain, Invitrogen/ThermoFisher Scientific, Waltham, MA; FoxP3-eFluor450 and ICOS-PE-Cy7, eBioscience, San Diego, CA; Ki-67-Alexa Fluor700, BD Bioscience, San Diego, CA.). A second flow panel was used to evaluate the effect of therapy on peripheral myeloid derived suppressor cells, phenotypically characterized using the following antibodies: Lineage (CD3/CD16/CD19/CD20/CD56) cocktail FITC (BD Pharmingen), CD14-PerCP Cy5.5, and HLA-DR-ECD (Beckman Coulter, Pasadena, CA). Isotype controls included the appropriate fluorochrome-conjugated mouse IgG1, IgG1 κ, IgG2a, or IgG2b κ antibodies (BD Pharmingen; Beckman Coulter). Stained cells were detected using a LSRFortessa flow cytometer with FACSDiva software (BD Biosciences). Analyses were performed using FlowJo software (version 8.1l TreeStar, Inc., Ashland, OR). The percentages of various T-cell subsets were calculated as a proportion of live CD3+ T cells (i.e., the proportion of CD4+ T cells of live CD3+ T cells, etc.) for each time point. To account for baseline variability across subjects, the effect of therapy was described as percentage fold-change of each marker relative to baseline. Ratios of effector T cells to regulatory T cells (Teff/Treg) were calculated by dividing the frequency of CD8+ T cells by the frequency of FoxP3+CD4+ T cells.
Cytokine Analysis
A Meso Scale Discovery multiplex cytokine immunoassay panel was used to quantitate serum concentrations of various Th1/Th2/proinflammatory cytokines at baseline and following therapy, including IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, and TNF-α. To account for baseline variability across subjects, the effect of therapy was described as percentage fold-change of each analyte relative to baseline. When calculating fold-changes, all concentrations that registered below the lower limit of detection (LLD) for the analyte were replaced with the LLD, and therefore the reported fold-changes represent minimum fold changes.
Statistical Analysis
The primary goal of this study was to assess safety in 6 patients per treatment group, and secondarily to conduct exploratory analyses on immune profile markers. Because of the modest sample size together with the heterogeneity across patients and tumors, correlative study comparisons across groups are descriptive. Safety data, as well as medians/ranges for continuous parameters and frequencies/percentages for categorical parameters, are summarized descriptively for each treatment group. For exploratory analyses where median values are reported, 95% confidence intervals were calculated to offer the degree of precision afforded in our analyses.
Results
Study population
All 19 study participants contributed to the primary endpoint of safety and tolerability. One subject treated with cryo alone was replaced because of a cryo probe malfunction that resulted in incomplete tumor freezing. Baseline characteristics for the 18 women who successfully completed the planned interventions and contributed to the correlative studies are outlined in Table 1. The median age at diagnosis was 49 years (range 34–73 years). Of the 18 study patients: 13 (72%) had HR-positive, HER2-negative disease; 2 (11%) had HR-positive, HER2- positive disease; and 3 (17%) had HR-negative, HER2-negative (triple negative) disease. Receptor status was not balanced across the groups: both subjects with ER positive, HER2 positive disease received ipi alone; 2 subjects with triple negative disease received ipi alone, and 1 subject with triple negative disease received cryo+ipi.
Table 1.
Baseline characteristics for subjects successfully treated with cryo +/− ipi and contributing to the immune correlative studies.
| Group | All patients n=18 |
Cryo (A) n=6 |
Ipi (B) n=6 |
Cryo+Ipi (C) n=6 |
|---|---|---|---|---|
|
Median age years (range) |
49 (34–73) |
42 (39–69) |
52 (34–73) |
49 (34–56) |
|
| ||||
| Clinical stage | ||||
| I | 3 (17%) | 2 (33%) | 1 (17%) | 0 (0%) |
| II | 15 (83%) | 4 (67%) | 5 (83%) | 6 (100%) |
|
| ||||
| Grade | ||||
| 1–2 | 7 (39%) | 3 (50%) | 1 (17%) | 3 (50%) |
| 3 | 11 (61%) | 3 (50%) | 5 (83%) | 3 (50%) |
|
| ||||
| Histology | ||||
| IDC | 11 (61%) | 4 (67%) | 4 (67%) | 3 (50%) |
| ILC | 2 (11%) | 1 (17%) | 0 (0%) | 1 (17%) |
| Mixed/other | 5 (28%) | 1 (17%) | 2 (33%) | 2 (33%) |
|
| ||||
| Receptor Type | ||||
| HR+/HER2− | 13 (72%) | 6 (100%) | 2 (33%) | 5 (83%) |
| HR−/HER2+ | 2 (11%) | 0 (0%) | 2 (33%) | 0 (0%) |
| HR−/HER2− | 3 (17%) | 0 (0%) | 2 (33%) | 1 (17%) |
|
| ||||
| Adjuvant hormone therapy | ||||
| Yes | 14 (78%) | 6 (100%) | 3 (50%) | 5 (83%) |
| No | 4 (22%) | 0 (0%) | 3 (50%) | 1 (17%) |
|
| ||||
| Adjuvant radiation | ||||
| Yes | 6 (33%) | 1 (17%) | 3 (50%) | 2 (33%) |
| No | 12 (67%) | 5 (83%) | 3 (50%) | 4 (67%) |
|
| ||||
| Adjuvant chemo | ||||
| Yes | 13 (72%) | 4 (67%) | 5 (83%) | 4 (67%) |
| No | 5 (28%) | 2 (33%) | 1 (17%) | 2 (33%) |
|
| ||||
|
Median follow-up months as of 1/1/16 (range) |
31 (9–43) |
40 (35–43) |
32 (18–37) |
20 (9–30) |
Abbreviations: cryo, cryoablation; ipi, ipilimumab; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
Although only 3 women had clinically node-positive disease, 8 women had pathologic node involvement. One woman with clinical stage IIB (cT2N1) ER-positive, HER2-positive disease treated with ipi alone in Group B had pathologic metastases in 22 of 23 evaluated lymph nodes. PET/CT was obtained for staging soon thereafter and revealed a right lung nodule with ipsilateral thoracic adenopathy. Transbronchial lymph node fine needle aspiration and wedge resection confirmed breast cancer metastases, and thus, the cancer was metastatic at the time of intervention. She had a complete response to first-line palliative paclitaxel and HER2-directed therapy and remains without evidence of disease after 34 months of follow-up on maintenance HER2-directed therapy alone. Otherwise, after a median follow-up period of 31 months after surgery (range, 9–43), none of the other study participants have experienced a locoregional or distant recurrence, including the three patents with triple negative disease (2 treated with ipi alone and 1 treated with ipi/cryo) after a median follow-up of 33 months (range, 30–35).
Safety and tolerability
Cryo and ipi were safe/well tolerated alone and in combination. The primary endpoint of this trial was reached, with all 19 enrolled patients receiving standard-of-care mastectomy without delay. No treatment-associated grade III/IV adverse events, and one unrelated grade III/IV adverse event, were recorded on study (Table 2). One subject from group C (cryo+ipi) developed a grade 3 maculopapular rash originating at the site of the mastectomy drain, which erupted hours after mastectomy and spread to involve the face, neck and chest. The rash resolved within several days with a topical corticosteroid (clobetasol) and oral antihistamine (hydroxyzine). Based upon the timing, appearance, and distribution of the rash, the dermatologist (M.E.L.) determined the rash was of unlikely relatedness to ipilimumab and possibly related to peri-operative anti-septic chlorhexidine wash and/or cephalexin administration.
Table 2.
Toxicity. All potential treatment-related adverse events are depicted (N=19). Toxicities were grades by the Common Terminology Criteria for Adverse Events, Version 4.0;
| Event | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| All | 16 | 4 | 1* |
| Rash | 3 | 1* | |
| Diarrhea | 3 | ||
| Constipation | 2 | ||
| Nausea | 2 | ||
| Weight loss | 1 | ||
| Dry eyes | 2 | ||
| Fatigue | 3 | 1 | |
| Headache | 2 | ||
| Fever | 1 |
One patient from group C developed grade 3 maculopapular rash that was attributable to peri-operative antibiotic or antiseptic.
A 69 year old woman with a history of coronary artery disease, prior myocardial infarction, hypertension, dyslipidemia, and a more than 50 pack-year smoking history developed flap dehiscence after cryo alone which the surgeon (V.S.) deemed unrelated to the study intervention.
A 72 year old woman with triple negative breast cancer and a self-reported history of irritable bowels but no history of inflammatory bowel disease, developed grade I diarrhea approximately 6 weeks after receiving ipi alone in group B. CT scan was consistent with mild inflammatory colitis. The reaction was deemed likely related to ipi as it is consistent with the known toxicity profile of this agent. She received a course of oral corticosteroids (prednisone 30mg twice daily) with significant clinical improvement after 2 days, and standard taper over 3 weeks. Colonoscopy completed 16 days after completion of the corticosteroid taper and approximately 11 weeks after ipi administration, revealed mildly congested, erythematous and friable mucosae in the entire colon and mildly congested, erythematous mucosa in the terminal ileum. Terminal ileum biopsy was unremarkable, but a colon biopsy revealed diffuse infiltration by CD4+ and to a lesser degree CD8+ T cells by immunohistochemistry. Notably, breast MRI at baseline and biopsy compared with breast MRI obtained the day prior to mastectomy and 8 days after ipi administration revealed a 24% decrease in the size of the tumor (i.e. the tumor decreased in size from 1.7cm in maximal diameter at baseline to 1.3cm in maximal diameter 8 days after ipi administration).
Phenotypic characterization of immune cells in peripheral blood by flow cytometry
No consistent changes were observed in peripheral blood CD3+, CD4+, CD8+, or regulatory (CD4+FoxP3+ Treg) T-cells over time. Specifically, median absolute and relative counts (with relative counts measured as proportion of live cells or CD3+ T cells) remained within 1-fold of baseline across the three treatment groups at all 3 subsequent timepoints (time of biopsy/cryoablation, time of mastectomy, and 30 days post-mastectomy). However, when ICOS expression was evaluated as a surrogate of T cell activation, an increase in the proportion of activated T-cells was observed in both CD4+ and CD8+ subsets in the ipi-treated groups (groups B and C) at the time of surgical mastectomy (median fold-change in ICOShi cells among CD4+: −.1 cryo, +2.0 ipi, +1.8 cryo+ipi; among CD8+: −.1 cryo, +5.0 ipi, +5.3 cryo+ipi). ICOS expression subsequently declined in all treatment groups by 30 days post-mastectomy; however, elevations from baseline were sustained in the cryo+ipi group at this time point (median fold-change in ICOShi cells among CD4+: +.1 cryo, +1.1 ipi, +3.0 cryo+ipi; among CD8+: +.1 cryo, +.7 ipi, +2.8 cryo+ipi). When Ki67 expression was evaluated as a marker of T cell proliferation, a similar trend was observed, with an increase in proliferation of CD4+ and CD8+ T cell subsets in the ipi-treated groups, followed by more sustained proliferation of CD4+ and CD8+ cells in the cryo+ipi group at 30 days post-mastectomy when compared with baseline (Figure 2). Because frequencies of myeloid derived suppressor cells (MDSC) have been correlated with response to ipi, we evaluated trends in MDSCs over time. We did not observe any consistent changes in MDSC frequencies across each of the three treatment groups (data not shown).
Figure 2.
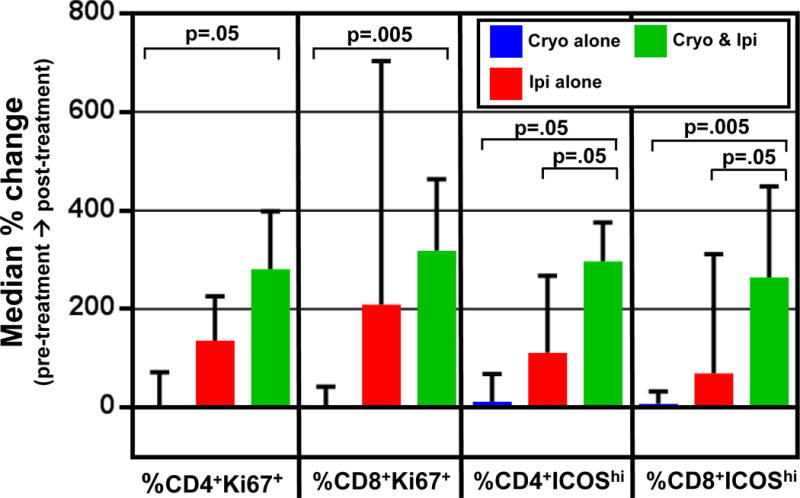
Changes in peripheral blood mononuclear cells (PBMCs) by flow cytometry. An increased frequency of activated T cells (as indicated by high ICOS expression) and proliferating T cells (as indicated by Ki67-positivity) is observed in peripheral blood mononuclear cells at 30 days post-mastectomy follow-up versus baseline in cryo plus ipi treated groups. Medians with interquartile ranges are depicted. Abbreviations: cryo, cryoablation; ipi, ipilimumab, ICOS, inducible co-stimulator.
Peripheral Th1 and Th2 cytokine response following therapy
We characterized the effect of therapy over time on peripheral levels of Th1 and Th2- type cytokines. Cytokine levels were stable over time in the absence of treatment, as evidenced by the absence of >1-fold change in any of the evaluated cytokines across the two baseline blood draws obtained in the cryo-alone treatment group (data not shown). Early changes in cytokines, measured at the time of surgical mastectomy (median 7 days following cryo and 10 days following ipi), were modest with the exception of increased IL-5 (a Th2-type cytokine attributed to maturation and activation of eosinophils),(19,20) observed in most ipi-treated patients (5/6 showing >2-fold increases following ipi, 4/6 following cryo+ipi, versus 1/6 following cryo).
Cytokine changes appeared to increase over time, with the most profound increases detected at 30 days post-mastectomy. Figure 3 illustrates a heat map demonstrating fold-changes in peripheral cytokines at this time point. Increases in interferon gamma (IFN-γ), the prototypical Th1-type cytokine, were observed most commonly following cryo+ipi: 4 of 6 cryo+ipi treated patients exhibited >2-fold increases in IFN-γ, versus none for cryo and 2 of 6 for ipi. Modest but detectable increases in other Th1-type cytokines were also observed following cryo+ipi but not monotherapy, including IL-2 and IL-12 (Figure 3). Sustained elevations in the Th2-type cytokine, IL-10, were observed following ipi alone (>2-fold in 2/6) and cryo+ipi (>2-fold in 1/6).
Figure 3.
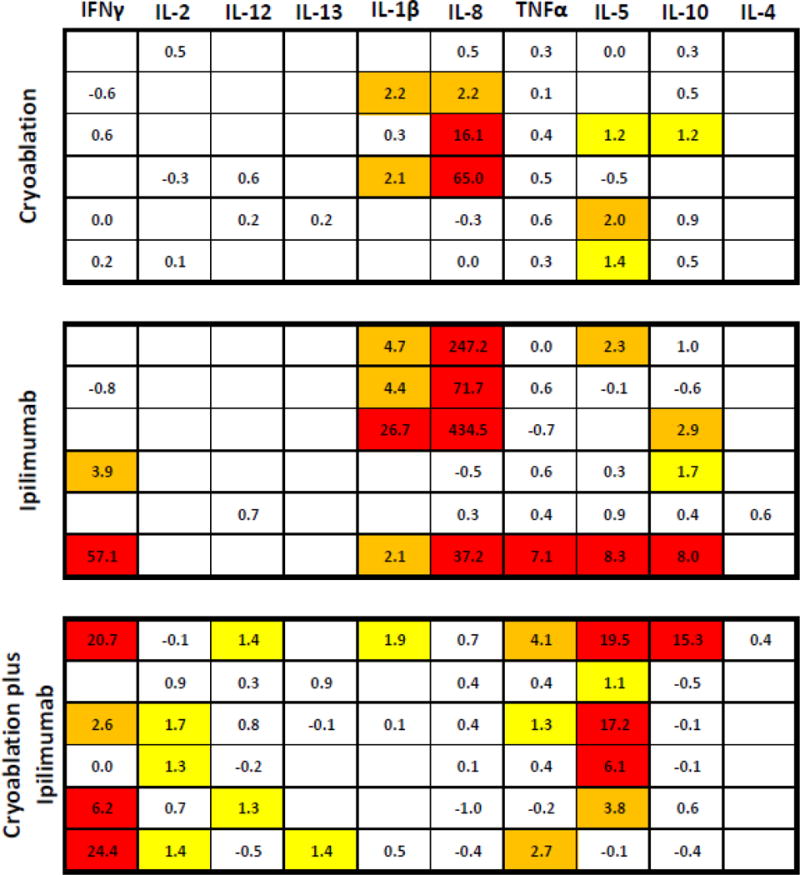
Changes in serum cytokines by Meso Scale Discovery serum based multiplex assay. Fold change in cytokines at one month post-mastectomy versus baseline are depicted, with each row representing an individual patient. Blank cells indicate that cytokine concentrations were below the lower limit of detection for both baseline and followup. Yellow indicates a 1 to 2-fold increase from baseline, orange indicates a 2 to 5-fold increase from baseline, and red indicates a greater than 5-fold increase from baseline. Th1-associated cytokines (IFN-γ, IL-2, IL-12) are oriented leftward, and Th2-associated cytokines (IL-5, IL-10, IL-4) are oriented rightward. Abbreviations: IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
Notably, cytokine and flow cytometric analysis from the previously described patient who developed grade II diarrhea/colitis 6 weeks after ipi administration (4 weeks after mastectomy) revealed a notable increase in peripheral IFN-γ, with concentrations increasing to 57-fold of baseline at the time of her 30-day post-mastectomy safety follow-up assessment, which roughly coincided with onset of symptoms. This was accompanied by smaller elevations in Th2 cytokines such as IL-10 (8-fold of baseline). By flow cytometry, peripheral ICOS-expressing CD4 and CD8 cells increased to levels 641% of baseline, and Ki67-positive CD3 cells increased to 1000% of baseline.
Phenotypic characterization of tumor infiltrating lymphocyte (TILs) s by flow cytometry
The majority of cryoablated tumor specimens exhibited extensive necrosis and loss of microarchitecture, thus making it challenging to employ conventional histologic methods such as haematoxylin and eosin (H&E) or IHC to assess effects on tumor microenvironment. As an alternative, we characterized TILs isolated from tumor resection specimens using flow cytometry. Singlets, followed by viable cells, and then lymphocytes were gated prior to determining the % CD3+ cells. By this method, no significant differences were observed in the proportion of CD4+ T-cells (as defined by proportion of CD4+ cells in live CD3+ cells), CD8+ T-cells (proportion of CD8+ of live CD3+ cells), or regulatory T-cells (proportion of CD4+FoxP3+ of live CD3+ cells) across treatment groups. Similarly, there were no differences in the ratio of effector T-cells (CD8+CD3+) to regulatory T-cells (FOXP3+CD4+CD3+) across groups (Figure 4).
Figure 4.
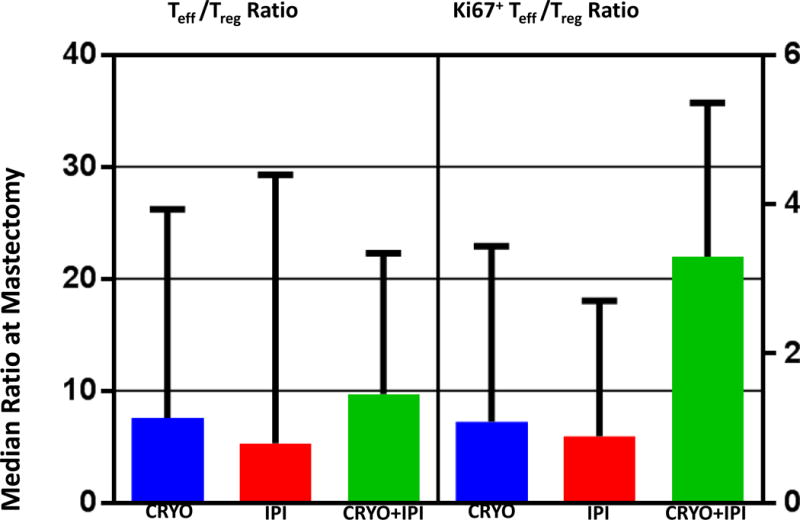
Assessment of tumor infiltrating lymphocytes (TILs) from mastectomy specimens by flow cytometry: The TIL ratio of CD8+ Teff to Tregs appeared greater in mastectomy specimens treated with both cryoablation and ipilimumab when compared with either treatment alone, but only when evaluated by ki67, a marker of proliferation. Medians with interquartile ranges are depicted. Abbreviations: Teff, effector T-cell; Treg, regulatory T-cell; cryo, cryoablation; ipi, ipilimumab.
Ki67 gating was employed to evaluate for potential differences in proliferation status across these subsets. Using this approach, the proportion of Ki67-positive CD4+ cells and CD8+ T cells appeared to be greater among subjects treated with combination therapy compared to either therapy alone. Furthermore, the ratio of Ki67-positive effector T-cells to regulatory T-cells was elevated in the combination-treatment arm (p=0.05, Figure 4). No significant differences in expression of immune checkpoint markers CTLA-4, PD-1, LAG-3 or Tim-3 were observed on CD4 and CD8 TILs at the time of tumor resection across treatment groups (data not shown).
Discussion
In this pilot study, we showed that cryo-immunotherapy can be safely incorporated pre- operatively in women with operable breast cancer without interfering with the timing of standard surgery. This critical observation enables us to consider further studies in the pre-operative setting utilizing combination cryoablative immune-stimulating strategies. Pre-surgical treatment also allows for serial assessment of TILs, which may be useful for demonstrating favorable on- target treatment effects such as expansion of effector T-cells within the tumor microenvironment. While some successes have been reported in adjuvant immunotherapy design (namely, vaccination incorporating known target antigens such as HER2),(21) the inherent limitations of post-operative immunotherapy include the lack of surrogate tissue markers to buttress clinical findings, as well as the possibility of enhanced “off-target” (i.e. directed towards self antigens) immune activation and toxicity. The latter has been suggested to be the cause of excess toxicities observed in melanoma patients treated with adjuvant ipilimumab.(14)
The principal limitation of our pilot study is the lack of statistical power to assess clinical benefit, but this was not the primary aim. The primary aim was to confirm that the interventions could be administered without compromising standard-of-care, curative-intent surgery as a proof-of-principle prior to embarking on a larger study powered to evaluate clinical benefit. However, another critical aim was to explore the impact of the interventions on a comprehensive panel of potential immunologic correlative biomarkers in women with breast cancers of any histology in order to inform the correlative studies in the planned randomized study comparing the intervention with standard peri-operative care. In the context of anti-CTLA-4 therapy, the most extensively reported immune-based biomarker is T-cell expression of inducible costimulator (ICOS), a CD28/CTLA4 family surface molecule that is upregulated only after T- cell activation.(22) Signaling via the ICOS pathway has been shown in mice to promote anti-tumor activity associated with anti-CTLA-4.(23) ICOS expression is a reproducible pharmacodynamic marker following ipilimumab(24) and sustained elevations in T-cell ICOS expression in the peripheral blood were associated with clinical benefit in melanoma patients treated with ipilimumab.(18,25) In this pilot study, we observed sustained elevations in ICOS expression among specific CD4+ and CD8+ peripheral T-cell subsets, persisting for at least one month following therapy, and occurring most obviously in the ipi+cryo arm. Furthermore, there was also evidence of sustained CD4+ and CD8+ proliferation after ipi+cryo, as measured by the proportion of cells expressing the Ki67 marker. These findings, while not definitive, support the hypothesis that cryo+ipi results in activation and proliferation of T-cell subsets that may be implicated in tumor immunity.
In addition to ICOS up-regulation and signaling, another reported mechanism of anti-CTLA-4 therapy is to deplete suppressive T-regulatory cells from the tumor microenvironment, which is thought to occur in part by mediating Fc-dependent phagocytic clearance of Tregs by direct binding of antibody to CTLA-4 on Tregs.(26) In the clinic, the degree of tumoral necrosis in response to ipilimumab has been associated with the ratio of T-effector cells to T-regulatory cells.(27,28) Because of these findings, we evaluated T-effector enrichment following cryo- immunotherapy. Unfortunately, histologic quantification of FoxP3 and other T-regulatory markers proved less informative in our study, as the cryoablation procedure rendered post- treatment samples difficult to assess by IHC. By flow cytometry on TILs, we observed that the Teff/Treg ratio of lymphocytes did not appear to differ across groups. However, when gated for Ki67, we observed a statistically significant increase in proliferating effector cells relative to T- regulatory cells in the ipi+cryo group, compared to cryo or ipi alone. One potential explanation for this discrepancy is that the post-therapy surgical resection occurred too early in time for the changes in TILs to fully manifest, whereas the Ki67 expression identified an active process of preferential expansion of effectors to regulatory cells. While speculative, this observation supports further study of Ki67 as a marker for detecting early intratumoral T cell responses following immunotherapy during window-of-opportunity pre-operative studies.
Peripheral cytokines have been less extensively evaluated as biomarkers of response to immune checkpoint blockade. In our trial, we observed sustained increases in IL-2, IL-12, and IFN-γ in the cryo+ipi arm relative to cryo or ipi alone arms, suggesting that cryo+ipi may alter the peripheral cytokine milieu.
Any conclusions about clinical outcomes are limited by the sample size, the heterogeneity of the patient population, and differences in standard-of-care locoregional and systemic therapies. However, the heterogeneity of the population is also a strength in that it permitted exploratory correlative studies across tumor subtypes and a number of intriguing clinical observations. First, one patient with weakly ER-positive (5%), weakly PR-positive (5%), HER2-positive disease treated with ipi alone was diagnosed with biopsy-proven lung metastases 2 weeks after mastectomy, indicating that the cancer was metastatic at the time of intervention. She had a complete response to first line palliative paclitaxel with HER2-directed therapy and remains without evidence of disease with maintenance trastuzumab and pertuzumab after 34 months of follow-up. Whether the administration of ipi contributed to the ongoing response to HER2-directed therapy in this specific case cannot be determined. Second, the remaining 17 subjects remain relapse-free after a median follow-up of 31 months (range, 9–43 months), including the three patients with triple negative disease (2 treated with ipi alone and 1 treated with ipi/cryo) after a median follow-up of 33 months (range, 30–35 months). Third, one patient with triple negative disease experienced steroid- responsive, ipi-related diarrhea and an apparent radiographic tumor shrinkage of 24% 8 days after ipi administration, suggesting early clinical response. This patient also exhibited robust IFN-γ production and T-cell ICOS expression, supporting the possibility that ipilimumab alone was clinically active.
The constellation of favorable correlative findings, including evidence of peripheral blood T-cell activation and proliferation, Th1-type cytokine responses, and intratumoral skewing towards proliferating effector cells, suggest that cryo-immunotherapy may produce a favorable immune environment. Unfortunately, one of the biggest limitations of our study is that we have no direct, definitive evidence of tumor-specific immune activity due to limited numbers of specimens. A future direction for study would be to conduct high-throughput screening to identify putative biomarkers of tumor-specific T-cell activation. Possible methods include T-cell receptor DNA sequencing (for which our preliminary data are described separately), whole- exome sequencing coupled with tetramer staining to identify T-cell responses specific to tumor neoantigens(29,30) or a seromics approach to identify humoral responses that may be used to guide exploration of T-cell responses.
In light of the limitations of this small pilot study, our findings must be interpreted with caution and should be validated in another prospective study. Extensive immune monitoring, while providing rich datasets that may inform our understanding of mechanisms of response, may be prone to data fitting and misinterpretation. To minimize the potential for this bias, we restricted our analyses primarily to immune parameters that have sound biologic rationale and that have been previously reported in the literature. Other potential limitations of the dataset include the sample size and heterogeneity of tumor characteristics across treatment groups.
Our ultimate goal is to improve the outcomes for women with early stage breast cancer by adopting an immunotherapeutic approach. This trial establishes the clinical feasibility and safety of a pre-operative approach, but raises new questions. The most fundamental question— clinical efficacy—must be asked in the context of a larger clinical trial with a homogenous treatment population. Triple negative breast cancers may be the ideal study population to assess efficacy, owing to their inherent immunogenicity (as exemplified by the prognostic association of TIL quantity),(31–36) high risk of relapse, and early time course of relapse (most commonly in the first three years). However, before such a trial is launched, the optimal immunotherapeutic regimen must be explored. While anti-CTLA-4 and anti-PD-1 both have proven efficacy in metastatic melanoma and preliminary evidence of clinical activity in breast cancer, the combination may be another potential regimen for tumors that are not inherently immunogenic at baseline. In melanoma, ipilimumab plus nivolumab was equally effective in PD-L1-negative and PD-L1 positive tumors,(37) suggesting that the combination may be capable of engendering de novo immune responses against immunologically inert tumors. Our pilot study indicates that cryoablation may serve as an effective method of releasing tumor associated antigens and releasing danger signals at the site of the tumor which may in turn be therapeutically exploited when combined with systemic immune stimulation. These findings support further study of cryoablation with checkpoint blockade as a curative intent strategy in operable breast cancer.
Statement of Translational Relevance.
Because most breast cancers may not exhibit inherent immunogenicity, and clinical responses to immune checkpoint blockade may be limited to more immunologically-active or inflamed tumors, a strategy that combines physical tumor disruption as a means of enhancing antigen presentation with checkpoint blockade may overcome relative resistance to immunotherapy. In this pilot study, we show that preoperative treatment with ipilimumab alone or in combination with cryoablation is feasible and safe in early stage breast cancer. We also demonstrate, using previously described correlative markers, favorable effects for this combination strategy with increased Th1-cytokine production, peripheral T-cell proliferation/activation (as measured by Ki67 and ICOS expression, respectively), and intratumoral proliferation of effector T-cells relative to regulatory T-cells. These findings support further study of this approach in the treatment of early stage breast cancer as a strategy to confer long-term tumor-specific immunity and improved outcomes.
Acknowledgments
None.
Footnotes
Conflicts of Interest:
Heather L. McArthur has research supported by Bristol-Myers Squibb (BMS); Jianda Yuan has a commercial grant from BMS; Padmanee Sharma has consulted for BMS, has research supported by BMS, and has a spouse with patents licensed to BMS; James P. Allison has intellectual property and royalties from BMS; and Jedd D. Wolchok has grant support from BMS and has consulted for BMS.
Disclosures:
This is an original manuscript which is neither in press nor published elsewhere. Data presented in part at the 2014 American Society of Clinical Oncology (ASCO) Annual Meeting (Abstract #1098), the 2013 ASCO Annual Meeting (Abstract #TPS3120), the 2013 San Antonio Breast Cancer Symposium (SABCS, Abstract #P4–13–01), the 2012 SABCS (Abstract #OT3-1-01), the 2014 Breast Cancer Symposium (BCS, Abstract #64), and the 2013 BCS (Abstract #67).
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vonderheide RH, LoRusso PM, Khalil M, Gartner EM, Khaira D, Soulieres D, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16:3485–94. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 5.Emens LA. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC). AACR Annual Meeting; Philadelphia, PA. April 20, 2015. [Google Scholar]
- 6.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.64.8931. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 8.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. Harmonization of the evaluation of tumor infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs-working group 2014. Ann Oncol. 2015;26:259–71. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD- L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–70. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabel MS, Nehs MA, Su G, Lowler KP, Ferrara JL, Chang AE. Immunologic response to cryoablation of breast cancer. Breast Cancer Res Treat. 2005;90:97–104. doi: 10.1007/s10549-004-3289-1. [DOI] [PubMed] [Google Scholar]
- 12.Waitz R, Solomon SB, Petre EN, Trumble AE, Fasso M, Norton L, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012;72:430–9. doi: 10.1158/0008-5472.CAN-11-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolchok JD, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, et al. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol. 2013;24:2174–80. doi: 10.1093/annonc/mdt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–30. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 15.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6:254ra128. doi: 10.1126/scitranslmed.3008918. [DOI] [PubMed] [Google Scholar]
- 17.Yuan J, Page DB, Ku GY, Li Y, Mu Z, Ariyan C, et al. Correlation of clinical and immunological data in a metastatic melanoma patient with heterogeneous tumor responses to ipilimumab therapy. Cancer Immun. 2010;10:1. [PMC free article] [PubMed] [Google Scholar]
- 18.Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16:2861–71. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, et al. Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127:701–21. doi: 10.1016/j.jaci.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 20.Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21:1303–9. doi: 10.1093/intimm/dxp102. [DOI] [PubMed] [Google Scholar]
- 21.Mittendorf EA, Clifton GT, Holmes JP, Schneble E, van Echo D, Ponniah S, et al. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol. 2014;25:1735–42. doi: 10.1093/annonc/mdu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, et al. ICOS co- stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 23.Fan X, Quezada SA, Sepulveda MA, Sharma P, Allison JP. Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J Exp Med. 2014;211:715–25. doi: 10.1084/jem.20130590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng Tang D, Shen Y, Sun J, Wen S, Wolchok JD, Yuan J, et al. Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res. 2013;1:229–34. doi: 10.1158/2326-6066.CIR-13-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Giacomo AM, Calabro L, Danielli R, Fonsatti E, Bertocci E, Pesce I, et al. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother. 2013;62:1021–8. doi: 10.1007/s00262-013-1418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, et al. Fc- dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti- CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci USA. 2008;105:14987–92. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31:e439–42. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–66. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–50. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 33.Tian T, Ruan M, Yang W, Shui R. Evaluation of the prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers. Oncotarget. 2016 doi: 10.18632/oncotarget.10054. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25:611–8. doi: 10.1093/annonc/mdt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 36.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 37.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]


