Abstract
The development of pharmacotherapeutic treatments of psychostimulant abuse has remained a challenge, despite significant efforts made towards relevant mechanistic targets, such as the dopamine transporter (DAT). The atypical DAT inhibitors have received attention due to their promising pharmacological profiles in animal models of cocaine and methamphetamine abuse. Herein we report a series of modafinil analogues that have an atypical DAT inhibitor profile. We extended SAR by chemically manipulating the oxidation states of the sulfoxide and the amide functional groups, halogenating the phenyl rings, and/or functionalizing the terminal nitrogen with substituted piperazines, resulting in several novel leads such as 11b, which demonstrated high DAT affinity (Ki=2.5 nM) and selectivity without producing concomitant locomotor stimulation in mice, as compared to cocaine. These results are consistent with an atypical DAT inhibitor profile and suggest that 11b may be a potential lead for development as a psychostimulant abuse medication.
Keywords: Modafinil, dopamine transporter, addiction, psychostimulant, cocaine, atypical DAT inhibitor
INTRODUCTION
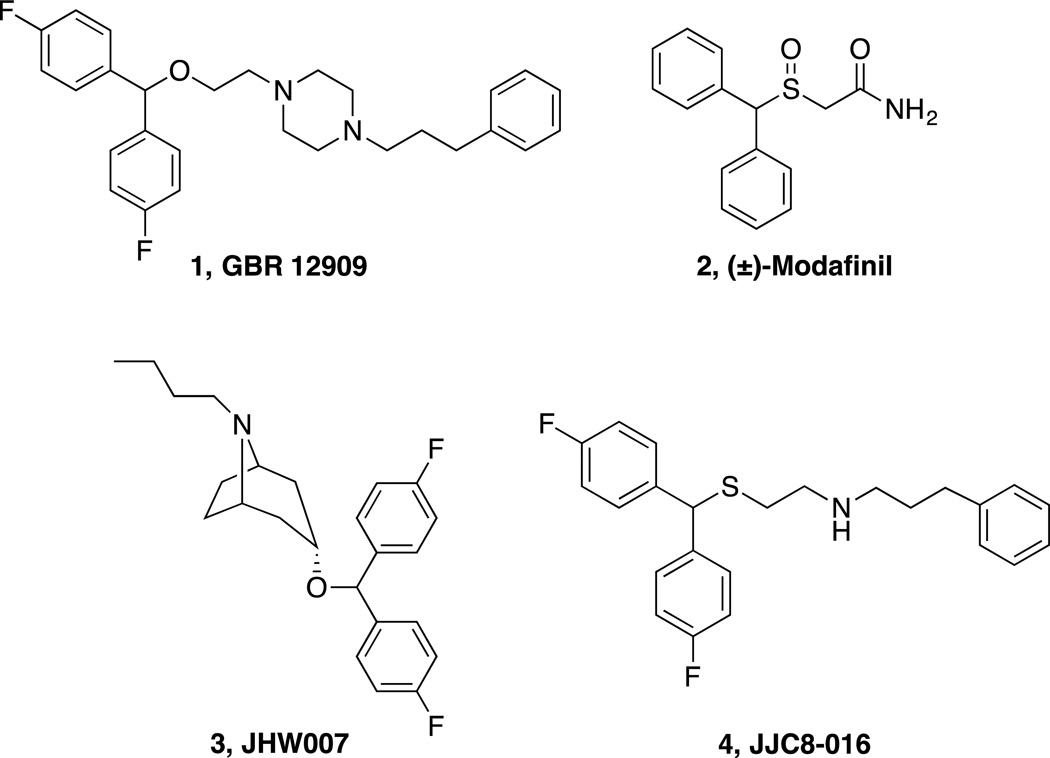
With millions of people worldwide suffering from substance use disorders, the development of pharmacotherapeutics for the treatment of addiction is imperative. While medications exist for treating alcohol and opiate addiction, there remains no FDA-approved medication to treat psychostimulant use disorder.1,2 Psychostimulant drugs of abuse, such as cocaine and methamphetamine, bind to the dopamine transporter (DAT), inhibiting the reuptake of dopamine (DA) into the presynaptic neuron, increasing extracellular dopamine levels, and resulting in the behavioral activation and euphoria that can lead to addiction.3–7 Although tremendous efforts have been made to develop dopamine uptake inhibitors to block the effects of cocaine and methamphetamine, most of these compounds share the psychostimulant effects and addictive liability of cocaine and have not been developed beyond preclinical studies.8 One DAT inhibitor, GBR 12909 [1, 1-(2-(bis(4-fluorophenyl)methoxy)ethyl)-4-(3-phenylpropyl)piperazine, Fig. 1] was taken on into a Phase 1 clinical trial but failed before being tested for efficacy in the cocaine-abusing population.9 These results dampened interest in the DAT as a potential therapeutic target for psychostimulant abuse and indeed, negatively impacted the development of any drugs toward this target, despite the great success of methylphenidate, for example, a DAT inhibitor that has been used for decades to treat Attention Deficit Hyperactivity Disorders (ADHD).10,11
Figure 1. Chemical structures of known DAT inhibitors.
Another DAT inhibitor, (±)-modafinil (2, 2-[(diphenylmethyl)sulfinyl]acetamide, Fig. 1) and its (R)-enantiomer (armodafinil) are FDA-approved to treat narcolepsy and other sleep disorders; however, they are also used off-label as cognitive enhancers, with little evidence of abuse liability in humans.12 Though modafinil primarily targets the DAT, it does not share the same psychostimulant and abuse liability profile of cocaine.13 Parallels to its atypical behavior in both humans14–16 and psychostimulant abuse animal models,17 along with its unique DAT binding profile have piqued interest for further drug development. In addition, as sleep and cognitive disorders have been linked to chronic drug abuse and the inability to remain abstinent,18,19 modafinil has additional therapeutic attributes that may be useful in preventing relapse. Nevertheless, in clinical trials, modafinil has demonstrated limited effectiveness in treating cocaine abuse,14–16,20,21 although a recent clinical study showed promising results in non-alcohol-dependent cocaine abusers.22
(±)-Modafinil and its (R)-enantiomer display unique pharmacological profiles, suggesting they may bind to a conformation of the DAT dissimilar to the cocaine-bound conformation and more similar to a class of “atypical” DAT inhibitors, based on benztropine (e.g. JHW 007, 3, (3-(bis(4-fluorophenyl)methoxy)-8-butyl-8-azabicyclo[3.2.1]octane,23 Fig. 1).13 An independent study on modafinil24 supported these findings and led us to further investigate (R)-modafinil in rodent models of nicotine abuse.25 These studies and previous investigations of several benztropine analogues suggested that atypical DAT inhibitors might attenuate the psychostimulant effects of cocaine and methamphetamine but may not have abuse liability, thereby leading us to further explore modafinil for the treatment of psychostimulant abuse.
Modafinil is essentially insoluble in water and has relatively low affinity for DAT (Ki = 2 µM), which in some ways makes it an excellent therapeutic, as it cannot be illicitly diverted into an injectable and/or abused drug; however, clinical studies in cocaine addicts who also abuse other substances suggest that modafinil is not an ideal medication for this patient population, especially for those who also abuse alcohol.20–22 One possibility for its lack of effectiveness in reducing cocaine-taking in this patient population might be due to modafinil’s low affinity for the DAT relative to cocaine, precluding it from efficiently blocking cocaine’s rapid inhibition of dopamine uptake. Moreover, its low DAT affinity and poor water solubility make modafinil a challenging compound to study preclinically and thus our previous efforts26,27 were aimed at improving its pharmacological profile via a structure-activity relationship (SAR) analyses at the DAT, norepinephrine transporter (NET), and serotonin transporter (SERT). Several compounds from those libraries showed improved water solubility and higher affinity for DAT (Ki<1µM) relative to modafinil; one lead compound arose and is currently being evaluated in behavioral models of cocaine and methamphetamine abuse (4, JJC8-016, N-(2-((bis(4-fluorophenyl)methyl)thio)ethyl)-3-phenylpropan-1-amine, Fig. 1).27
In order to further improve DAT binding affinity and selectivity, we designed a series of compounds that extended SAR by chemically manipulating the oxidation states of the sulfoxide and the amide functional groups, halogenating the phenyl rings, and/or functionalizing the terminal nitrogen, borrowing the piperazine ring from 1, as part of the scaffold.28–34 All final compounds were evaluated for DAT, NET, and SERT binding in rat brain, and a subset were tested for binding to the σ1 receptor, as it has been previously posited that a dual DAT/σ1 profile may have therapeutic advantages over a highly DAT selective compound.35–41 This subset was also tested for binding affinities at the dopamine D2, D3 and D4 receptor subtypes and for efficacy in a D2 Gi1 Bioluminescence Resonance Energy Transfer (BRET) assay. In addition, we tested this subset of analogues for inhibition of [3H]DA uptake and in a binding assay previously shown to determine if a compound possesses a classical or an atypical DAT binding profile, as reported for (±)-modafinil and its (R)-enantiomer.13 These data suggest that the analogues did indeed appear to bind the DAT in this desired atypical fashion like modafinil and the benztropines but unlike cocaine. This subset of compounds was also evaluated for metabolic stability in mouse liver microsomes and the compound with highest DAT affinity and selectivity in this series, 11b, was tested for its effects on locomotor activity in mice.
CHEMISTRY
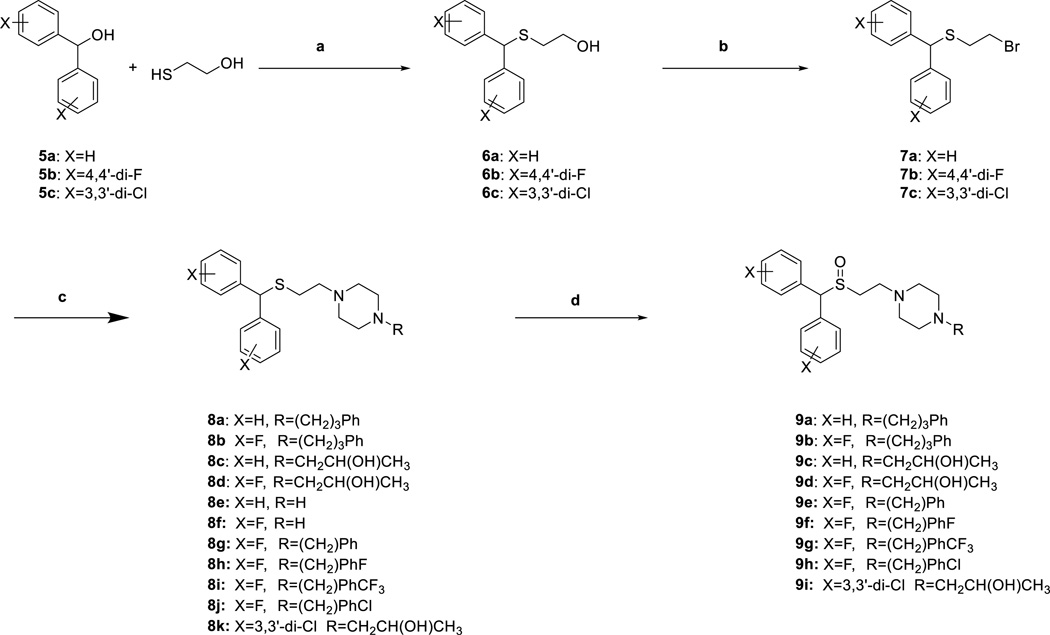
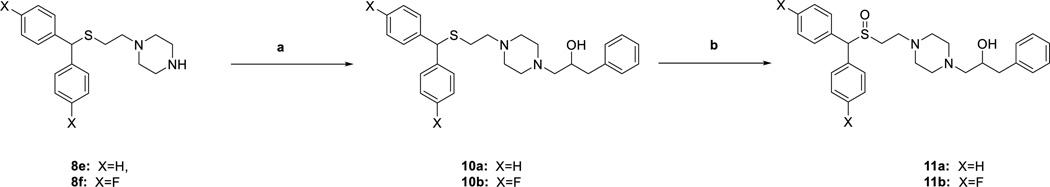
Syntheses of novel sulfenylethanamine (8a–8k, 10a,b) and sulfinylethanamine analogues (9a–9i, 11a,b) were achieved as depicted in Schemes 1 and 2. Dehydration of commercially available benzhydrols 5a–c with 2-mercaptoethan-1-ol in the presence of trifluoroacetic acid followed by treatment with K2CO3 in H2O/acetone provided sulfide alcohols 6a–6c in 61–95% yield. Appel reaction on 6a–6c with triphenylphosphine and tetrabromomethane gave bromides 7a–7c in 61–82% yield. Next, alkylation with the appropriate piperazine provided the sulfenylethanamine analogues 8a–8k in 50–87% yield. Epoxide ring opening of 2-benzyloxirane using unsubstituted piperazine analogues 8e and 8f gave alcohols 10a and 10b, respectively (84% and 86% yield, respectively). Lastly, oxidation of the appropriate sulfenylethanamine was achieved using hydrogen peroxide in an acetic acid/methanol solution to give sulfinylethanamine (9a–9i, 11a,b) in 43–78% yield.
Scheme 1. Synthesis of compound 8a–8k and 9a–9ia.
a Reagents and conditions: (a) (i) TFA, CH2Cl2, r.t. overnight; (ii) K2CO3, H2O/acetone, overnight; (b) PPh3, CBr4, CH3CN, r.t. overnight; (c) Appropriate piperazine, K2CO3, acetone, reflux, overnight; (d) H2O2, AcOH/MeOH, 40 °C, overnight.
Scheme 2. Synthesis of Compounds 10a,b and 11a,ba.
a Reagents and conditions: (a) 2-benzyloxirane, isopropanol, reflux, overnight; (b) H2O2, AcOH/MeOH, 40 °C, overnight.
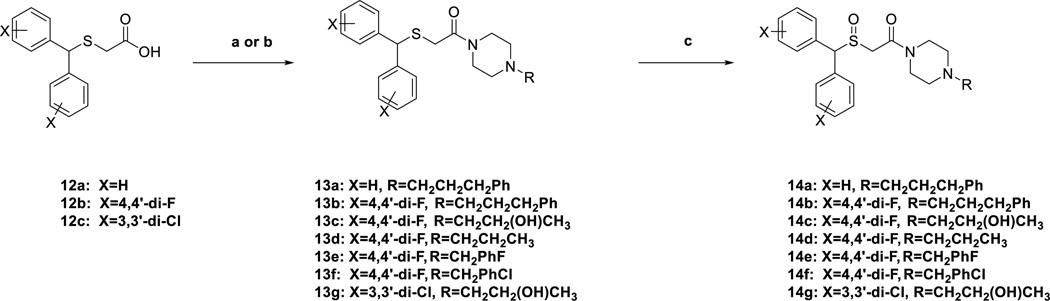
Piperazine-substituted sulfenylacetamides 13a–13g were generated via either synthetic routes ‘a’ or ‘b,’ outlined in Scheme 3. Compounds 13a–13c, and 13g were synthesized through route ‘a’ by amidation of carboxylic acids 12a–12c using 1,1’-carbonyldiimidazole (CDI) and the appropriate amines in 71–90% yield. Synthetic route ‘b’ utilized thionyl chloride to form the acid chloride intermediate of carboxylic acids 12a,b, followed by coupling with the respective amine to afford 13d–13f in 54–67% yield. Oxidation of the sulfenyl moiety gave the desired sulfinylacetamide 14a–14g in 11–72% yield. All final compounds were purified and characterized in their free base form and then converted to the oxalate salts for testing. Note that all final compounds with sulfoxide groups and/or hydroxylated linking chains are the racemic or diastereomeric mixtures.
Scheme 3. Synthesis of Compounds 13a–g and 14 a–ga.
a Reagents and conditions: (a) (i) THF, CDI, r.t. 2h; (ii) substituted piperazine analogue, THF, r.t. overnight; (b) (i) SOCl2, reflux, 2h; (ii) appropriate piperazine; (c) H2O2, AcOH/MeOH, 40 °C, overnight.
SAR at DAT, SERT and NET
All final compounds (8, 9, 11, 13, 14) were evaluated for binding at the monoamine transporters (DAT, SERT and NET) in rat brain membranes and compared to known DAT inhibitors 1–4 (Fig. 1) Changes in DAT binding affinity and selectivity as a result of modifications to the modafinil structure were evaluated; SAR analyses explored the effects of 1) the sulfur oxidation state, 2) reducing the terminal amide to a secondary amine, 3) halogenating the benzhydrol, and 4) varying the amide/amine substituent. The binding affinities (Ki values) are presented in Table 1, where, in general, these novel analogues displayed low nanomolar DAT affinities and showed >100-fold selectivity for DAT over NET and/or SERT.
Table 1.
Binding Data for Sulfenyl- and Sulfinylacetamide, Sulfenyl- and Sulfinylethanamine Analoguesa
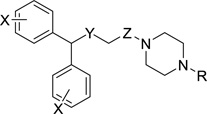 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ki (nM) ± S.E.M. | ||||||||
| Compound | X | Y | Z | R | clogP | DAT | NET | SERT |
| GBR12909,1 | 1.77±0.181b | 497±17.0b | 104±11.4b | |||||
| Modafinil, 2 | 2520±204c | IAd | IAd | |||||
| 3 | 10.5 ± 0.748 | 1670±232 | IAd | |||||
| 4 | - | - | - | - | 6.43 | 116 ± 16.3 | 3,848 ± 21.7 | 360 ± 48.3 |
| 8a | H | S | C | 3-phenylpropyl | 5.87 | 3.58 ± 0.157 | 988 ± 22.8 | 1,050 ±152 |
| 8b | 4,4’-diF | S | C | 3-phenylpropyl | 6.12 | 4.50 ± 0.344 | 1,890 ± 116 | 285 ± 35.4 |
| 8c | H | S | C | 2-OH-propyl | 3.13 | 49.6 ± 4.31 | 44,500± 2400 | 26,700 ± 2,630 |
| 8d | 4,4’-diF | S | C | 2-OH-propyl | 3.42 | 16.7 ± 1.22 | 17,800 ± 885 | 1,770 ± 234 |
| 8g | 4,4’-diF | S | C | benzyl | 6.86 | 7.40±1.04 | 8,240 ± 1,240 | 949 ± 122 |
| 8h | 4,4’-diF | S | C | 4-F-benzyl | 6.92 | 4.98 ± 0.716 | 6,850 ± 633 | 463 ± 69.1 |
| 8i | 4,4’-diF | S | C | 4-CF3-benzyl | 7.74 | 26.6 ± 2.09 | 15,400 ± 1,950 | 2,020 ± 266 |
| 8j | 4,4’-diF | S | C | 4-Cl-benzyl | 7.57 | 19.3 ± 1.91 | 7,410 + 102 | 905 ± 121 |
| 8k | 3,3’-diCl | S | C | 2-OH-propyl | 4.56 | 143 ± 19.2 | 31,300 ± 2,980 | 10,800 ± 1,030 |
| 9a | H | S=O | C | 3-phenylpropyl | 3.91 | 3.17 ± 0.112 | 5,540 ± 808 | 7,360 ± 881 |
| 9b | 4,4’-diF | S=O | C | 3-phenylpropyl | 4.20 | 2.92 ± 0.383 | 4281±343 | 678±66.1 |
| 9c | H | S=O | C | 2-OH-propyl | 1.21 | 636 ± 14.7 | NDe | NDe |
| 9d | 4,4’-diF | S=O | C | 2-OH-propyl | 1.49 | 289 ± 43.0 | NDe | 50,300 ± 5,760 |
| 9e | 4,4’-diF | S=O | C | benzyl | 4.93 | 27.2 ± 1.29 | 45,700 ± 8,480 | 11,200 ± 195 |
| 9f | 4,4’-diF | S=O | C | 4-F-benzyl | 5.00 | 9.67 ± 1.37 | >50 uM | 8,520 ± 349 |
| 9g | 4,4’-diF | S=O | C | 4-CF3-benzyl | 5.81 | 39.4 ± 1.45 | >50 uM | 6,800 ± 468 |
| 9h | 4,4’-diF | S=O | C | 4-Cl-benzyl | 5.64 | 7.62 ± 0.900 | >50 uM | 4,940 ± 665 |
| 9i | 3,3’-diCl | S=O | C | 2-OH-propyl | 2.63 | 403 ± 23.8 | >50 uM | 58,900 ± 7,310 |
| 10a | H | S | C | 2-OH, 3- phenylpropyl |
4.70 | 2.54 ± 0.233 | 1,430 ± 118 | 1,630 ± 169 |
| 10b | 4,4’-diF | S | C | 2-OH, 3- phenylpropyl |
5.00 | 6.72 ± 0.977 | 1,950 ± 227 | 213 ± 13.2 |
| 11a | H | S=O | C | 2-OH, 3- phenylpropyl |
2.78 | 3.43 ± 0.499 | 25,300 ± 2,040 | 21,700 ± 2,020 |
| 11b | 4,4’-diF | S=O | C | 2-OH, 3- phenylpropyl |
3.06 | 2.53 ± 0.250 | 15,000 ± 575 | 4,610 ± 562 |
| 13a | H | S | C=O | 3-phenylpropyl | 6.37 | 47.2 ± 5.56 | 22,600 ± 3,010 | 9,320 ± 932 |
| 13b | 4,4’-diF | S | C=O | 3-phenylpropyl | 6.66 | 28.6 ± 1.52 | 20,200 ± 1,410 | 3,170 ± 275 |
| 13c | 4,4’-diF | S | C=O | 2-OH-propyl | 4.03 | 214 ± 20.7 | NDe | 11,400 ± 902 |
| 13d | 4,4’-diF | S | C=O | propyl | 5.36 | 277 ± 12.5 | NDe | 5,970 ± 497 |
| 13e | 4,4’-diF | S | C=O | 4-F-benzyl | 6.16 | 53.7 ± 1.89 | NDe | 6,210 ± 579 |
| 13f | 4,4’-diF | S | C=O | 4-Cl-benzyl | 6.74 | 58.7 ± 5.05 | NDe | 2,200 ± 177 |
| 13g | 3,3’-diCl | S | C=O | 2-OH-propyl | 5.17 | 915± 84.5 | >50 uM | NDe |
| 14a | H | S=O | C=O | 3-phenylpropyl | 4.67 | 33.0±2.83 | 54,300 ± 3,210 | 15,200 ± 1,100 |
| 14b | 4,4’-diF | S=O | C=O | 3-phenylpropyl | 5.00 | 37.6±1.86 | 12,000 ± 1,430 | 1,320 ± 152 |
| 14c | 4,4’-diF | S=O | C=O | 2-OH-propyl | 2.33 | 752± 87.4 | NDe | 32,800 ± 4,430 |
| 14d | 4,4’-diF | S=O | C=O | propyl | 3.66 | 279 ± 23.4 | NDe | 14,500 ± 1,430 |
| 14e | 4,4’-diF | S=O | C=O | 4-F-benzyl | 4.47 | 74.3 ± 9.64 | NDe | 23,600 ± 1,030 |
| 14f | 4,4’-diF | S=O | C=O | 4-Cl-benzyl | 5.04 | 56.3 ± 6.60 | 63,500 ± 5,290 | 9,460 ± 386 |
| 14g | 3,3’-diCl | S=O | C=O | 2-OH-propyl | 3.47 | 1,380 ± 191 | >50 uM | NDe |
Each Ki value represents data from at least three independent experiments, each performed in triplicate. Ki values were analyzed by PRISM. Binding assays are described in detail in Experimental Methods,
Previously reported in Cao et al.,35
Previously reported in Cao et al.,26
IA; less than 50% inhibition at 100 µM,
ND; not determined
In general, the sulfoxides had similar affinities at DAT relative to their sulfide counterparts; however, affinities at SERT and NET generally decreased, resulting in more DAT-selective sulfoxides. The largest sulfide to sulfoxide improvement in DAT selectivities was seen for the N-bearing 2-hydroxypropyl and 2-hydroxyphenylpropyl derivatives (compared to their deshydroxy counterparts). In particular, oxidation of hydroxyl-containing sulfide 10b to its sulfoxide 11b gave nominal improvement in DAT affinity (Ki=6.72 to 2.53 nM, respectively), but a 7- and 20-fold decrease in SERT and NET affinities, respectively, were observed for sulfoxide 11b, improving DAT selectivity. In contrast, the deshydroxy counterparts sulfide 8b and sulfoxide 9b showed no significant difference in DAT Ki values, while SERT/NET selectivities decreased only ~2-fold for the oxidized compound.
To prevent potential metabolism on the benzhydrol moiety, 4,4’-difluorination was probed, resulting in varying effects on the monoamine transporter binding affinities. When the unsubstituted 8a, 9a, 10a, and 11a were 4,4’-difluoro-substituted to give 8b, 9b, 10b, 11b, respectively, no appreciable change was observed in DAT and SERT Ki values, although NET affinity improved between 5- and 10-fold. DAT affinity (Ki = 143–1380 nM) was lost when the benzhydrol was 3,3’-dichloro-substituted in 8k, 9i, 13g and 14g.
The phenylpropyls (8a, 8b, 9a, 9b, 13a, 13b, 14a, and 14b) and 2-hydroxyphenylpropyls (10a, 10b, 11a, 11b) generally demonstrated high binding affinities for DAT, with DAT Ki = 2.5–47 nM. The removal of the phenyl group resulted in a loss of affinity at DAT, implying that an N-arylalkyl substituent may be optimal. For example, when the phenyl group is removed from 10a and 11a (Ki = 2.5 and 3.4, respectively) to give 8c and 9c, respectively, DAT affinity decreases by 25- and 187-fold. In general, the N-benzyl analogues (e.g. 8g–8j and 9e–9h) also demonstrated high DAT affinities, but metabolic instability was problematic in this subset (see below).
The 2-hydroxy analogues were prepared based on previous SAR42 to give compounds with lower cLogP values (Table 1). While 2-hydroxylation of the propyl and phenylpropyl substitutions did not appreciably change Ki values at DAT, the 2-hydroxy sulfoxides 9d, 11a, 11b, and 14c resulted in a significant decrease in NET and SERT affinities. In contrast, 2-hydroxylation of the linker in sulfides 10a, 10b gave only nominal loss in NET or SERT Ki values. Of note, cLogP values were typically in the >5-range for 4,4’-difluoro substituted-analogues without the sulfoxide function or hydroxylated terminal N-substituents.
Off-target binding profiles – dopamine D2, D3, D4 and σ1 receptors
Based on the DAT, SERT and NET binding results and cLogP values, a subset of analogues (8d, 9d and 11b) was then evaluated for binding affinities at dopamine D2, D3 and D4 receptors in HEK 293 cells, as well as σ1 receptors in rat brain and compared to known compounds (R)-modafinil [(R)-2], 3 and 4. As can be seen in Table 2, (R)-modafinil is highly selective for DAT compared to these off-target receptors but has relatively low affinity for the DAT. In contrast, the atypical DAT inhibitor in the benztropine class, 3, has relatively high affinities for dopamine D2 and D3 receptors and has higher affinity for σ1 than for DAT (σ1/DAT =0.2). Likewise, our previous lead compound 427 had low affinity for D2 receptors, but moderate affinities for D3 and D4 and had comparable affinities at σ1 and DAT (σ1/DAT=1.4). Compound 8d was selective for DAT over the D2-like receptors, but had the highest affinity for σ1 in the series (Ki= 4 nM) resulting in a σ1/DAT ratio similar to 3. Compound 9d had the lowest affinity for DAT in the group (although ~10-fold higher than (R)-2) and its selectivity across the off-target sites was relatively low. Nevertheless, compared to the classic D2 antagonist, eticlopride, the D2-like receptor affinities are very low. Indeed, in Table 3, it can be seen that although all of these compounds were inhibitors of quinpirole-stimulated D2 receptor activation of Gi1, all of them had very low potency. Hence we conclude that they are very weak antagonists at D2 receptors and this off-target activity would likely not affect their behavioral profiles. Finally, in this series, compound 11b emerged as the highest affinity and most selective DAT inhibitor (Tables 2 and 3).
Table 2.
Off target binding affinities for a selected subset of analoguesa
| Compd | Ki ± SEM (nM) | |||||
|---|---|---|---|---|---|---|
| DAT | D2Ra | D3Ra | D4Ra | σ1 | σ1/DAT | |
| (R)-2 | 3050±258 | >100,000 | 39,000±1050 | >100,000 | >100,000 | >300 |
| 3 | 10.5 ± 0.748 | 68.1±7.81 | 34.4±6.47 | 583±128 | 2.4 | 5 |
| 4 | 116±16.3 | 228±27.6 | 65.9±12.1 | 28.1±5.22 | 159±22.7 | 1.4 |
| 8d | 16.7±1.22 | 693±159 | 424±117 | 6300±1460 | 4.03±0.22 | 0.24 |
| 9d | 289±43.0 | 298±61.1 | 480±184 | 3820±876 | 1010±134 | 3.5 |
| 11b | 2.53±0.25 | 78.6±16.6 | 652±105 | 1750±593 | 336±42.2 | 133 |
| eticlopride | NDb | 0.26±0.06 | 0.11±0.03 | 70.4±2.33 | NDb | - |
Each Ki value represents data from at least three independent experiments, each performed in duplicate. Ki values were analyzed by PRISM. Binding assays are described in detail in Experimental Methods.
ND; not determined.
Table 3.
Inhibition of Dopamine D2 receptor Gi1 activation using 1 µM quinpirolea
| Compound | IC50 (nM) |
|---|---|
| (R)-2 | >10,000 |
| 3 | 2000 ± 730 |
| 4 | >10,000 |
| 8d | >10,000 |
| 9d | >10,000 |
| 11b | >10,000 |
| eticlopride | 2.1 ± 0.8 nM |
Results were obtained from G protein activation-BRET experiments in HEK-293T cells transiently transfected with Gαi1-RLuc8 and γ2-GFP10 in cells expressing D2R. Data were fit by nonlinear regression to a sigmoidal dose–response relationship against the ligand concentration (prestimulated with 1 µM quinpirole). The IC50 values are the mean ± S.E.M. of 5 experiments performed in triplicate.
Molecular Pharmacology and Mutagenesis Studies
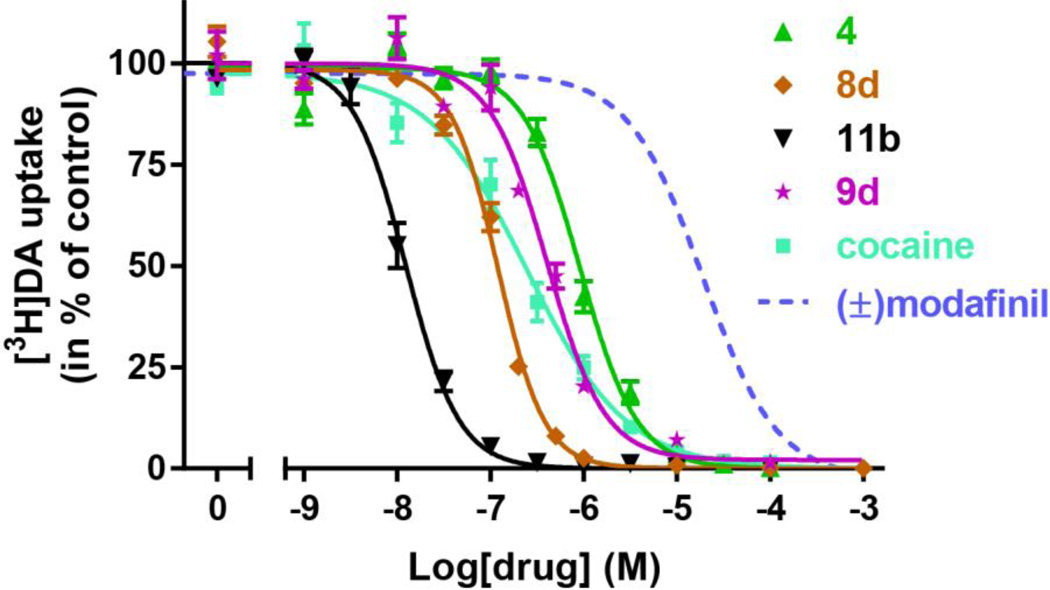
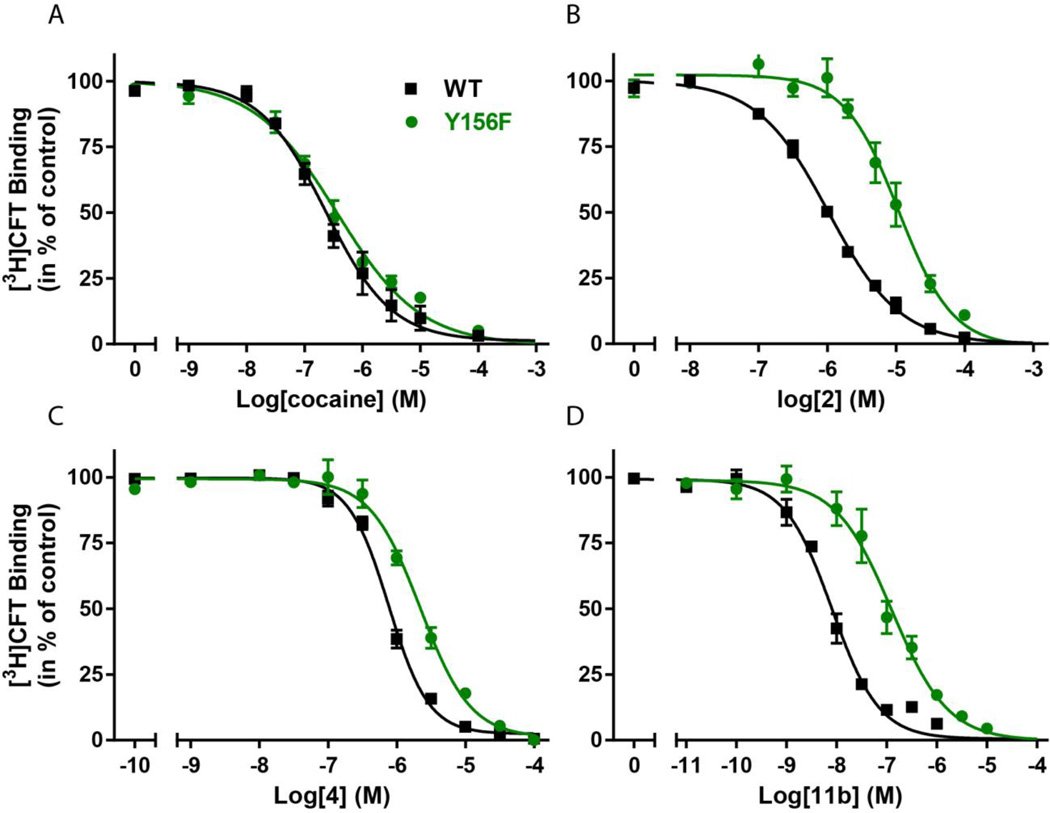
To assess the effect on DAT function, this subset of analogues (4, 8d, 9d and 11b) was tested for inhibition of [3H]DA uptake. Accordingly, COS7 cells transiently expressing DAT were employed. The analogues were added in increasing concentrations followed by a fixed concentration of [3H]DA to allow transport. The reaction was stopped after 5 min and the amount of [3H]DA taken up by the COS7 cells was determined by scintillation counting and plotted as a function of the concentration of added analogue (Fig. 2). All analogues from this subset possessed higher inhibition potency than (±)-modafinil. In line with the binding data from rat brain membranes, compound 11b emerged as the DA uptake inhibitor with the highest potency (Ki= 12 [10;14] nM, mean [s.e.m. interval], n = 3). Compound 8d had an inhibitory potency similar to cocaine (Ki = 200 [140;290] nM and 200 [110;350] nM, respectively, mean [s.e.m. interval], n = 3–4) whereas both compounds 4 and 9d had significantly lower Ki values than cocaine (890 [810;980] nM and 1730 [1370;2170] nM, respectively, mean [s.e.m. interval], n = 6–8). Although all DAT inhibitors, by definition block DA uptake, cocaine’s binding preference to an outward facing conformation of DAT has been described experimentally, computationally43,44 and more recently demonstrated in the X-ray crystal structure of the dDAT.45 It has been proposed that the stabilization of the outward facing DAT conformation by cocaine is, at least in part, mediated by its interference with a H-bond that forms between the OH-group of Tyr156 in TM3 and Asp79 in TM1. This has been substantiated experimentally by showing that the binding mode of cocaine is independent of the OH-group in Tyr156;44 however, when the DAT moves to a more outward occluded and ultimately closed conformation, a critical H-bond forms between Y156 and D79, closing the outer “gate.” This conformation seems to be preferred by substrates, such as dopamine, amphetamine and MDMA and also the atypical DAT inhibitors, such as the benztropines44,46 [e.g. 3]. Accordingly, in contrast to cocaine, the binding of modafinil and other atypical DAT inhibitors does depend on the presence of the OH-group since the Y156F mutation affects their binding affinity13,43 To assess whether or not compounds 4, 8d, 9d and/or 11b demonstrated an atypical DAT inhibitor binding mode, their binding affinities were evaluated in wild type (WT) DAT and the Y156F mutant (Fig. 3 and Table 4). The results were compared to the effects of (±)-modafinil (2), 3 and cocaine (Fig. 3 and Table 4). Inhibition of [3H]WIN35,428 binding on COS7 cells transiently expressing WT DAT or Y156F was determined. In contrast to cocaine, (±)-modafinil (2) and all the tested analogues showed a significant decrease in binding affinity for Y156F relative to WT DAT. Remarkably, 11b was particularly affected with a Y156F/WT ratio of ~15, similar to that found for (R)-modafinil [(R)-2] (Fig. 3 and Table 4.). Also 9d show a marked change (7.6-fold, Table 4), whereas 4 and 8d were affected to a lesser degree (2.8 and 2.6-fold, respectively, Fig. 4 and Table 4). Interestingly, the sulfoxide function was present in the three compounds [(R)-2, 9d and 11b)] that were most impacted by the Y156F mutation. For comparison the classical atypical DAT inhibitor, 3, had a Y156F/WT ratio of 5.5.
Figure 2. Inhibition potency of [3H]DA uptake by modafinil analogues and cocaine.
COS7 cells transiently expressing DAT wild type was incubated with [3H]DA and the indicated concentrations of 4 (green), 8d (brown), 9d (magenta), 11b (black) and cocaine (cyan). The inhibition potency of 2 as assessed previously13 is inserted for comparison (dotted line). Data are means ± s.e.m. of 3–10 experiments performed in triplicates.
Figure 3. Assessment of DAT binding profiles by modafinil analogues.
As demonstrated previously,13,43 a characteristic feature for atypical DAT inhibitors is that, in contrast to cocaine, their binding depends on the presence of the OH-group on Tyr156. Inhibition of [3H]WIN35,428 binding to DAT WT (black) or the Y156F (green) mutant by (A) cocaine, (B) 2, (C) 4 and (D) 11b. The fold change in IC50 values between WT and Y156F is 1.4, 10, 2.8 and 14.9 for cocaine, 2, 4 and 11b, respectively. Binding assays are performed as triplicates on COS7 cells transiently expressing DAT WT or Y156F (n = 3–6). Data are shown as means ± s.e.m.
Table 4.
Assessment of atypical binding properties for selected analogues.a
| WT | Y156F | Y156F/WT | |
|---|---|---|---|
| Compd | Ki (nM) | Ki (nM) | Affinity ratio |
| 2 | 927 [834; 1030] | 9250 [7350; 11700] | 10.0 |
| (R)-2 | 647 [593;704] | 8920 [6930;11500] | 13.8 |
| (S)-2 | 1960 [1700;2270] | 5910 [4640; 7520] | 3.0 |
| 3 | 47 [33;66] | 260 [229;296] | 5.5 |
| 4 | 720 [661;784] | 2050 [1840;2280] | 2.8 |
| 8d | 101 [82;125] | 267 [227; 314] | 2.6 |
| 9d | 1200 [824;1760] | 9170 [7680; 10900] | 7.6 |
| 11b | 6.5 [5.1;8.3] | 97 [67;140] | 14.9 |
| cocaine | 223 [160;309] | 321 [235; 438] | 1.4 |
Inhibition of [3H] WIN35,428 binding to DAT WT or the Y156F mutant. COS7 cells transiently expressing DAT WT or Y156F were assessed for affinity (Ki) for the indicated compounds. Data were analyzed by nonlinear regression analysis using Prism 6.0 (GraphPad). The IC50 values were calculated from means of pIC50 values and s.e.m. interval from pIC50 ± s.e.m. The Ki values were calculated from the IC50-values using the equation Ki=IC50/(1+(L+Kd), where ‘Kd’ is the affinity for WIN35,428 and ‘L’ is the concentration of added [3H]WIN35,428). All data are performed in triplicate, n = 3–6.
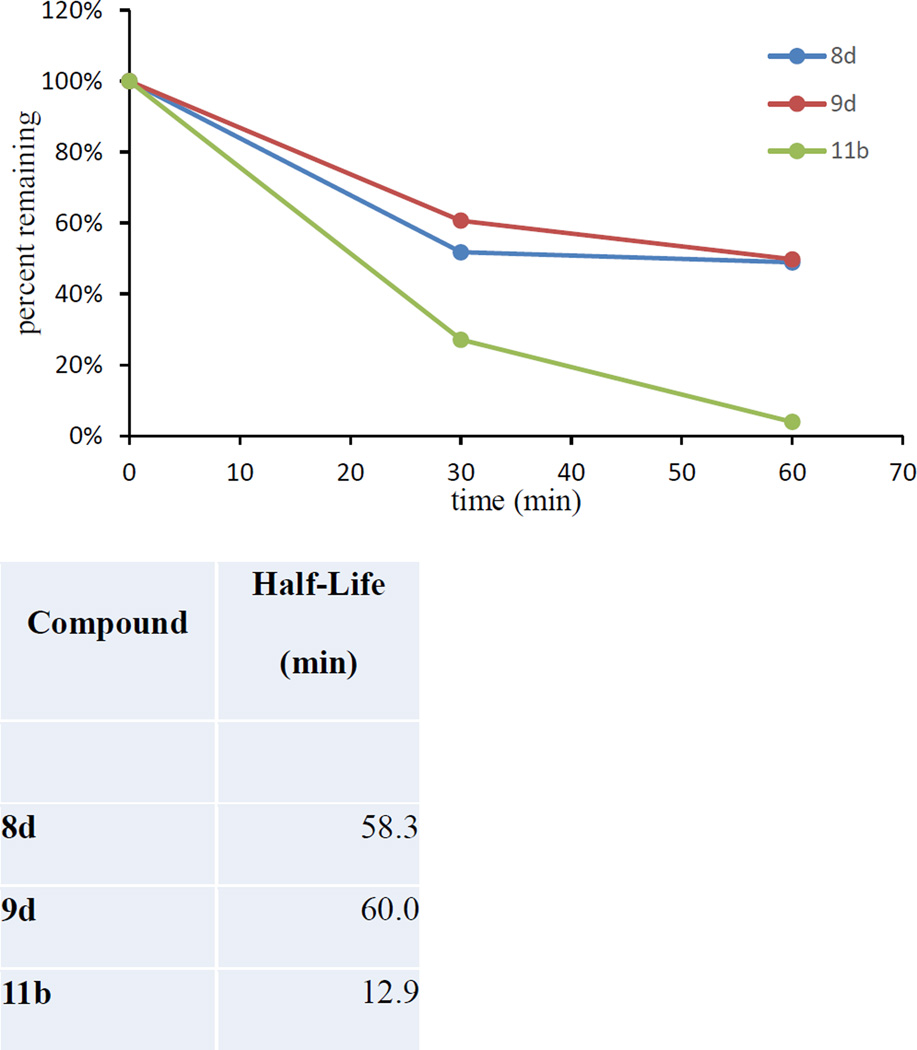
Figure 4. Phase I Metabolic stability of selected analogues in mouse liver microsomes.
Compounds 8d, 9d, and 11b were tested in mouse liver microsomes fortified with NADPH. Compounds 8d and 9d both showed slow metabolism with a half life of approximately 60 min suggesting the compounds to be stable. Compound 11b, on the contrary, had a much shorter half life of 13 min suggesting rapid metabolism. Note: control experiments without cofactors were conducted in parallel and in all cases >95% compound remained at 60 min suggesting the compounds undergoing Cyp dependent metabolism. Testosterone was used a positive control.
Metabolic stability in mouse microsomes
Based on their in vitro profiles, compounds 8d, 9d, and 11b were tested for phase I metabolism following procedures previously described47 to predict the susceptibility to metabolism following in vivo administration. As depicted in Fig. 4, compounds 8d and 9d exhibited good stability with approximately 50% of both compounds remaining in mouse microsomes fortified with NADPH. Corresponding in vitro half-lives for 8d and 9d were calculated to be ~60 min confirming compounds stable to CYP dependent phase I metabolism. In contrast, compound 11b undergoes substantial phase I metabolism with only ~4% remaining in microsomes fortified with NADPH at 1 h, suggesting CYP dependent metabolism of the compound and a relatively short in vitro half-life of 13 min. Of note, compounds 9f, 9g, 9h, 11a, 13c, 14b, 14e were all metabolized in <30 min in the mouse liver microsomes.
Locomotor activity in mice compared to cocaine
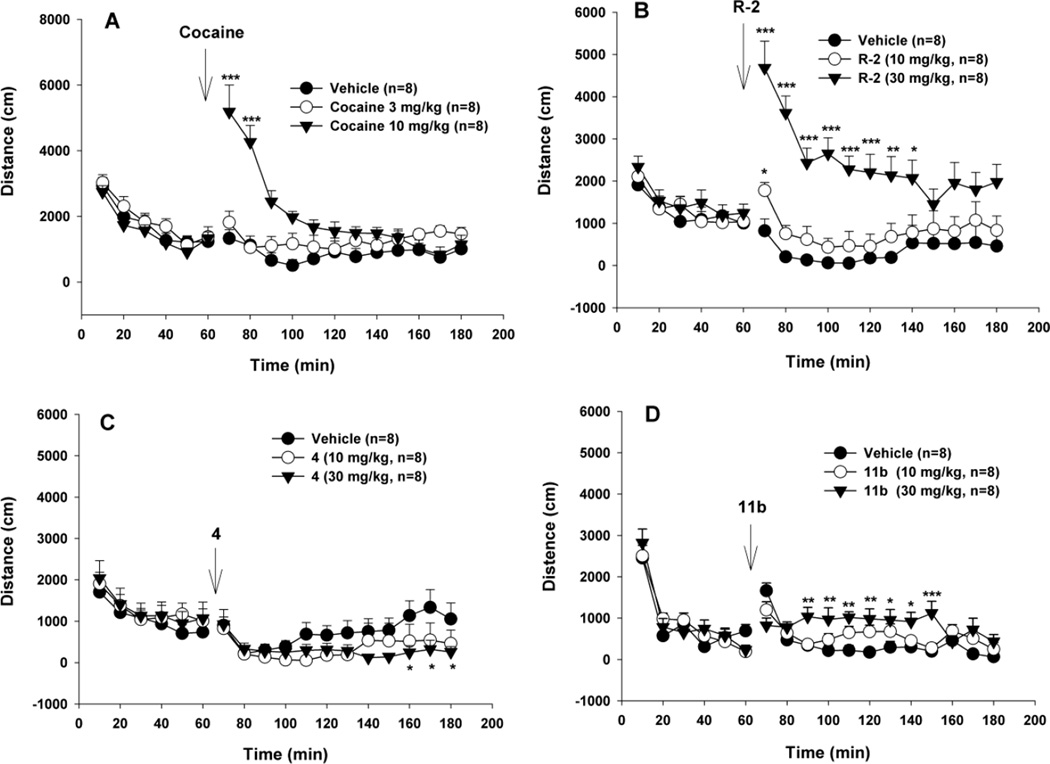
Figure 5 shows the effects of (R)-modafinil and its analogues 4 and 11b on locomotor activity compared to cocaine. Systemic administration (i.p.) of cocaine (3, 10 mg/kg) produced a robust dose-dependent increase in locomotion (Fig. 5A, cocaine treatment main effect, F2,21 =10,82, p<0.001, two-way ANOVA), while (R)-modafinil, and compounds 4 and 11b, at the same dose (10 mg/kg), produced no significant or a very mild locomotor response. When the dose was increased to 30 mg/kg, (R)-modafinil and 11b produced a significant dose-dependent increase, while 4 did not. Two-way ANOVA for repeated measures over time revealed a statistically significant treatment main effect for (R)-modafinil (Fig. 5B, F2,21 =10.01, p<0.001), for compound 11b (Fig. 5D, F2,21 =4.35, p<0.005), but not for compound 4 (Fig. 5C, F2,21 = 0.65, p=ns). Although the assay did not show a significant treatment main effect for compound 4, it revealed a significant time main effect (F11,231 = 5.11, p<0.001) and treatment × time interactions (F21,231 = 1.73, p<0.05). Therefore, post-hoc individual group comparisons revealed statistically significant differences between the different dose groups at certain time points (Fig. 5C).
Figure 5.
Locomotor effects of (R)-modafinil [(R)-2] and its analogs 4 and 11b compared to cocaine in mice. Systemic administration of cocaine (3, 10 mg/kg, i.p.) produced a robust dose-dependent increase in locomotion (A). However, (R)-2 and its analogs 4 and 11b, at the same dose of 10 mg/kg, did not (C, D) or produced a very mild increase (B) in locomotion. When the dose was increased to 30 mg/kg, (R)-2 and 11b produced a significant dose-dependent increase in locomotion, while its analog 4 did not. *p<0.05, **p<0.01, ***p<0.001, compared to the vehicle control group at each time point marked.
Most typical DAT inhibitors increase locomotor activity in mice; indeed this is one behavioral hallmark of this class of agents. In contrast, we and others have reported “atypical” DAT inhibitors that, despite binding with high affinity and selectivity to the DAT and inhibiting DA uptake, these agents are not efficacious locomotor stimulants and do not exhibit other cocaine-like behaviors. (±)-Modafinil and its (R)-enantiomer display unique pharmacological profiles12,13,24,48 that suggest they may be atypical. Herein, compound 11b displayed the highest DAT affinity in the series with good selectivity over other off-targets (Tables 2 and 3). As seen in Fig. 5, compound 11d, does increase locomotor activity in mice, but it is far less efficacious than cocaine suggesting that this modafinil analogue is an atypical DAT inhibitor. In addition, 11b demonstrated the highest Y156F/WT ratio in the mutagenesis study (Table 4, Fig. 3) suggesting that it binds the DAT in a more occluded conformation, unlike cocaine.
CONCLUSION
In summary, we have designed and synthesized a series of modafinil analogues that have higher DAT affinity than the parent molecule and have extended SAR at the DAT by manipulating the oxidation states of the sulfoxide and the amide, halogenating the phenyl rings, and/or functionalizing the terminal nitrogen with N-substituted piperazines. Compounds 8d, 9d and 11b were selected as lead compounds from this series and were tested for binding to σ1, as well as dopamine D2, D3 and D4 receptor subtypes, and for efficacy in a D2 Gi1 BRET assay. In addition, we tested this subset of analogues in cell-based [3H]DA uptake and binding assays for affinities at both the WT and the Y156F DAT mutant, in order to determine if they demonstrated an atypical binding profile, as previously reported for (±)-modafinil and its (R)-enantiomer.13 These data suggest that all three lead compounds did indeed bind the DAT in a conformation that was more like the atypical benztropines and unlike cocaine, with 11b having the highest DAT affinity and selectivity in this series. We discovered, that similar to previously reported atypical DAT inhibitors,49–53 compound 11b produced only moderate locomotor stimulation in mice and was substantially less efficacious than cocaine. These results are consistent with an atypical DAT inhibitor profile and suggest that 11b may be a potential lead for development as a psychostimulant abuse therapeutic. Further investigation of this compound in rodent models of cocaine and methamphetamine abuse, along with compounds 4, 8d and 9d are underway to extend testing of the atypical DAT inhibitor hypothesis, as well as to further investigate the role of σ1 receptors in the behavioral profile of these agents. Finally, although 11b showed significant metabolism in mouse microsomes, preliminary data show a rapid and high brain to plasma ratio, suggesting that 11b can penetrate the blood brain barrier sufficiently to block DA uptake via the DAT. Nevertheless, efforts to modify this structural template in order to improve metabolic stability are also ongoing and will be reported in due course.
EXPERIMENTAL METHODS
Synthesis
1H and 13C NMR spectra were acquired using a Varian Mercury Plus 400 spectrometer at 400 and 100 MHz, respectively. Chemical shifts are reported in parts-per-million (ppm) and referenced according to deuterated solvent for 1H NMR spectra (CDCl3, 7.26 or DMSO-d6, 2.50) and 13C NMR spectra (CDCl3, 77.2 or DMSO-d6, 39.5). Gas chromatography-mass spectrometry (GC/MS) data were acquired (where obtainable) using an Agilent Technologies (Santa Clara, CA) 6890N GC equipped with an HP-5MS column (cross-linked 5% PH ME siloxane, 30 m × 0.25 mm i.d. × 0.25 µm film thickness) and a 5973 mass-selective ion detector in electron-impact mode. Ultrapure grade helium was used as the carrier gas at a flow rate of 1.2 mL/min. The injection port and transfer line temperatures were 250 and 280 °C, respectively. Combustion analysis was performed by Atlantic Microlab, Inc. (Norcross, GA) and the results agree within ±0.5% of calculated values. Melting point determination was conducted using a Thomas-Hoover melting point apparatus and are uncorrected. On the basis of NMR and combustion data, all final compounds are >95% pure.
2-(Benzhydrylthio)ethan-1-ol (6a)
2-Mercaptoethan-1-ol (7.8 g, 100 mmol) was added to commercially available diphenylmethanol (3.7 g, 20 mmol) in TFA (40 mL) and CH2Cl2 (40 mL) at 0 °C. The solution was warmed to room temperature and stirred overnight. The solvent was removed, K2CO3 (11 g, 80 mmol), H2O (7 mL) and acetone (25 mL) were added to the reaction residue, and the mixture stirred at room temperature overnight. The solvent was removed, H2O (100 mL) was added to the residue obtained, and the aqueous mixture was extracted with ethyl acetate (3 × 100 mL). The organic layer was dried over MgSO4 and the solvent was removed in vacuo. The crude product was purified by flash column chromatography (hexane/ethyl acetate = 6:4) to give 6a (3.0 g, 61% yield) as a clear oil. GC/MS (EI) m/z 244 (M+). 1H NMR (400 MHz, CDCl3) δ 7.43–7.45 (m, 4H), 7.30–7.34 (m, 4H), 7.21–7.26 (m, 2H), 5.21 (s, 1H), 3.64–3.69 (m, 2H), 2.62–2.65 (m, 2H).
2-((Bis(4-fluorophenyl)methyl)thio)ethan-1-ol (6b)
Compound 6b was prepared as described for 6a using bis(4-fluorophenyl)methanol (6.6 g, 30 mmol) to give the product (6.9 g, 82% yield) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.35–7.39 (m, 4H), 6.99–7.03 (m, 4H), 5.20 (s, 1H), 3.68–3.70 (m, 2H), 2.59–2.62 (m, 2H).
2-((Bis(3-chlorophenyl)methyl)thio)ethan-1-ol (6c)
Compound 6c was synthesized as described for compound 6a using bis(3-chlorophenyl)methanol (2.84 g, 11.2 mmol) to give the product (3.34 g, 95% yield) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.40 (s, 2H), 7.31-7.22 (m, 6H), 5.14 (s, 1H), 3.71 (t, J = 5.8 Hz, 2H), 2.63 (t, J = 6.0 Hz, 2H), 1.93 (br s, 1H); 13C NMR (100 MHz, CDCl3) δ 142.5, 134.6, 130.0, 128.3, 127.9, 126.4, 60.6, 52.8, 35.3.
Benzhydryl(2-bromoethyl)sulfane (7a)
Triphenylphosphine (1.4 g, 5.3 mmol) was added to a solution of 6a (890 mg, 3.64 mmol) in CH3CN (12 ml), followed by the addition of carbon tetrabromide (1.77 g, 5.34 mmol). The reaction was stirred at room temperature overnight. The solvent was removed and the crude product was purified by flash column chromatography (hexane/ethyl acetate = 5:1) to give 6a (850 mg, 76% yield) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.23–7.45 (m, 10H), 5.23 (s, 1H), 3.33–3.39 (m, 2H), 2.80–2.90 (m, 2H); GC/MS (EI) m/z 307 (M+).
(Bis(4-fluorophenyl)methyl)(2-bromoethyl)sulfane (7b)
Compound 7b was prepared as described for 7a using 6b (6.9 g, 25mmol) to give the product (7.0 g, 83% yield) as a light yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.34–7.37 (m, 4H), 6.00–7.04 (m, 4H), 5.21 (s, 1H), 3.36–3.40 (m, 2H), 2.81–2.85 (m, 2H); GC/MS (EI) m/z 343 (M+).
(Bis(3-chlorophenyl)methyl)(2-bromoethyl)sulfane (7c)
7c was prepared as described for 7a using 6c (3.23 g, 10.3 mmol) to give product as colorless oil (3.03 g, 78% yield). 1H NMR (400 MHz, CDCl3) δ 7.38 (s, 2H), 7.28-7.22 (m, 6H), 5.14 (s, 1H), 3.39 (t, J = 8.0 Hz, 2H), 2.86 (t, J = 8.0 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 142.3, 134.9, 130.2, 128.5, 128.2, 126.5, 53.6, 34.5, 30.2.
1-(2-(Benzhydrylthio)ethyl)-4-(3-phenylpropyl)piperazine (8a)
A mixture of 7a (850 mg, 2.76 mmol), commercially available 1-(3-phenylpropyl)piperazine (564 mg, 2.76 mmol), K2CO3 (1.52 g, 11.0 mmol) and KI (catalytic) in acetone (30 mL) was refluxed overnight. The solvent was removed, H2O (50 mL) was added to the residue, and the aqueous mixture was extracted with ethyl acetate (3 × 50 ml). The organic layer was dried over MgSO4, the solvent was removed in vacuo, and the crude product was purified by flash column chromatography [ethyl acetate/triethylamine = 95:5] to give 8a (810 mg, 61% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. Mp 210 °C (dec.); 1H NMR (400 MHz, CDCl3) δ 7.16–7.43 (m, 15H), 5.22 (s, 1H), 2.33–2.64 (m, 16H), 1.78–1.82 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 142.1, 141.4, 128.5, 128.4, 128.3, 127.2, 125.7, 58.0, 54.5, 54.4, 53.1, 53.0, 33.7, 29.3, 28.6; Anal. (C28H34N2S · 2C2H2O4 · 0.25H2O) C, H, N.
1-(2-((Bis(4-fluorophenyl)methyl)thio)ethyl)-4-(3-phenylpropyl)piperazine (8b)
Compound 8b was prepared as described for 8a using 7b (950 mg, 2.76 mmol) to give the product (940 mg, 73% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. Mp 216–217 °C; 1H NMR (400 MHz, CDCl3) δ 7.34–7.37(m, 4H), 7.24–7.29(m, 2H), 7.15–7.19(m, 3H), 6.97–7.01(m, 4H), 5.20 (s, 1H), 2.33–2.64 (m, 16H), 1.67–1.82 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 163.1, 160.7, 142.1, 137.0, 129.8, 129.7, 128.4, 128.3, 125.7, 115.6, 115.3, 58.0, 53.1, 52.9, 52.8, 33.7, 29.4, 28.6; Anal. (C28H32F2N2S · 2C2H2O4) C, H, N.
1-(4-(2-(Benzhydrylthio)ethyl)piperazin-1-yl)propan-2-ol (8c)
Compound 8c was prepared as described for (8a) using 7a (848 mg, 2.76 mmol) and 1-(piperazin-1-yl)propan-2-ol (398 mg, 2.76 mmol) to give the product (850 mg, 83% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. Mp 209–210 °C; 1H NMR (400 MHz, CDCl3) δ 7.41–7.43 (m, 4H), 7.19–7.32 (m, 6H), 5.21 (s, 1H), 3.77–3.82 (m, 1H), 3.41 (br, 1H), 2.17–2.67 (m, 14H), 1.11–1.14 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 141.4, 128.6, 128.5, 128.3, 127.2, 65.6, 62.2, 57.9, 54.5, 54.4, 53.1, 29.3, 20.0; Anal. (C22H30N2OS · 2C2H2O4 · 0.25H2O) C, H, N.
1-(4-(2-((Bis(4-fluorophenyl)methyl)thio)ethyl)piperazin-1-yl)propan-2-ol (8d; JJC 8-089)
Compound 8d was prepared as described for 8a using 7b (950 mg, 2.76 mmol) and 1-(piperazin-1-yl)propan-2-ol (398 mg, 2.76 mmol) to give the product (880 mg, 79% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. Mp 205–206 °C; 1H NMR (400 MHz, CDCl3) δ 7.33–7.37 (m, 4H), 6.96–7.02 (m, 4H), 5.19 (s, 1H), 3.77–3.82 (m, 1H), 3.41 (br,1H), 2.18–2.69 (m, 14H), 1.11–1.13 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 163.1, 160.7, 137.0, 129.8, 129.7, 115.7, 115.6, 115.5, 115.4, 65.5, 62.2, 57.8, 53.1, 52.9, 29.4, 20.0; Anal. (C22H28F2N2OS · 2C2H2O4) C, H, N.
1-(2-(Benzhydrylthio)ethyl)piperazine (8e)
A mixture of 7a (1.4 g, 4.6 mmol), piperazine (2.35 g, 27.3 mmol), and K2CO3 (1.05 g, 9.12 mmol) in acetonitrile (25 mL) was refluxed overnight. The solvent was removed, H2O (100 mL) was added to the residue, and the aqueous mixture was extracted with ethyl acetate (3 × 100 mL). The organic layer was dried over MgSO4 and solvent was removed in vacuo. The crude product was purified by flash column chromatography (CHCl3/MeOH/NH4OH = 90/10/0.5) to give 8e (710 mg, 50% yield) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.41–7.43 (m, 4H), 7.19–7.32 (m, 6H), 5.22 (s, 1H), 2.83–2.85 (m, 4H), 2.52–2.54 (m, 4H), 2.34–2.37 (m, 4H).
1-(2-((Bis(4-fluorophenyl)methyl)thio)ethyl)piperazine (8f)
Compound 8f was prepared as described for 8e using 7b (1.03 g, 3.00 mmol) to give the product (910 mg, 87% yield) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.34–7.37 (m, 4H), 6.97–7.26 (m, 4H), 5.20 (s, 1H), 2.84–2.86 (m, 4H), 2.50–2.52 (m, 4H), 2.35–2.37 (m, 4H).
1-Benzyl-4-(2-((bis(4-fluorophenyl)methyl)thio)ethyl)piperazine (8g)
Compound 8g was prepared as described as 8a using 7b (687 mg, 2.00mmol) and 1-benzylpiperazine (353 mg, 2.00 mmol) to give the product as yellow oil (530 mg, 60%). The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. Mp 224–226 °C; 1H NMR (400 MHz, CDCl3) δ 7.22–7.38 (m, 9H), 6.96–7.02 (m, 4H), 5.20 (s, 1H), 3.45–3.51 (m, 2H), 2.43–2.68 (m, 12H); 13C NMR (100 MHz, CDCl3) δ 163.1, 160.4, 138.0, 137.0, 129.8, 129.7, 129.2, 128.2, 127.0, 115.7, 115.6, 115.5, 115.4, 63.0, 57.9, 53.1, 53.0, 52.9, 29.4; Anal. Calc.: C, 58.24, H, 5.21, N, 4.53. Found: C, 57.98, H, 5.16, N, 4.63. Anal. (C26H28F2N2S · 2C2H2O4) C, H, N.
1-(2-((Bis(4-fluorophenyl)methyl)thio)ethyl)-4-(4-fluorobenzyl)piperazine (8h)
Compound 8h was prepared as described as 8a using 7b (515 mg, 1.50 mmol) and 1-(4-fluorobenzyl)piperazine (194 mg, 1.50 mmol) to give the product as yellow oil (450 mg, 65.7%). The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid Mp 221–223 °C; 1H NMR (400 MHz, CDCl3) δ 7.33–7.37 (m, 4H), 7.23–7.27 (m, 2H), 6.95–7.00 (m, 6H), 5.18 (s, 1H), 3.44–3.46 (m, 2H), 2.41–2.56 (m, 12H); 13C NMR (100 MHz, CDCl3) δ 163.1, 160.4, 137.0, 133.8,130.6, 130.5, 129.8, 115.6, 115.4, 115.1, 114.9, 62.2, 57.9, 53.0, 52.9, 29.4; GC/MS (EI) m/z 456 (M+); Anal. Calc.: C, 56.6, H, 4.91, N, 4.40. Found: C, 56.35, H, 4.91, N, 4.38. Anal. (C26H27F3N2S · 2C2H2O4) C, H, N.
1-(2-((Bis(4-fluorophenyl)methyl)thio)ethyl)-4-(4-(trifluoromethyl)benzyl)piperazine (8i)
Compound 8i was prepared as described as 8a using 7b (515 mg, 1.50 mmol) and commercially available 1-(4-(trifluoromethyl)benzyl)piperazine (367 mg, 1.50 mmol) to give the product as yellow oil (500 mg, 66%). The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid Mp 223–224 °C; 1H NMR (400 MHz, CDCl3) δ 7.55–7.57 (m, 2H), 7.41–7.43 (m, 2H), 7.33–7.37 (m, 4H), 6.97–7.01 (m, 4H), 5.19 (s, 1H), 3.53–3.54 (m, 2H), 2.43–2.55 (m, 12H); 13C NMR (100 MHz, CDCl3) δ 163.1, 160.7, 142.4, 137.0, 129.8, 129.7, 129.2, 125.2, 125.1, 115.6, 115.4, 62.4, 57.8, 53.0, 52.9, 29.4; GC/MS (EI) m/z 506 (M+); Anal. Calc.: C, 53.87, H, 4.59, N, 4.05. Found: C, 53.78, H, 4.69, N, 4.05. Anal. (C27H27F5N2S · 2C2H2O4 · 0.25H2O) C, H, N.
1-(2-((Bis(4-fluorophenyl)methyl)thio)ethyl)-4-(4-chlorobenzyl)piperazine (8j)
Compound 8j was prepared as described as 8a using 7b (515 mg, 1.50 mmol) and commercially available1-(4-chlorobenzyl)piperazine (316 mg, 1.50 mmol) to give the product as a yellow oil (500 mg, 67.7%). The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid Mp 224–225 °C; GC/MS (EI) m/z 473 (M+); 1H NMR (400 MHz, CDCl3) δ 7.33–7.37 (m, 4H), 7.22–7.28 (m, 4H), 6.97–7.01 (m, 4H), 5.19 (s, 1H), 3.44–3.45 (m, 2H), 2.41–2.54 (m, 12H); 13C NMR (100 MHz, CDCl3) δ 163.1, 160.6, 137.0, 136.6, 132.8, 130.4, 129.8, 129.7, 128.3, 115.7, 115.6, 115.5, 115.4, 62.2, 57.8, 52.9, 29.4; Anal. Calc.: C, 54.80, H, 4.83, N, 4.26. Found: C, 54.61, H, 4.80, N, 4.28. Anal. (C26H27ClF2N2S · 2C2H2O4 · 0.25H2O) C, H, N.
1-(4-(2-((Bis(3-chlorophenyl)methyl)thio)ethyl)piperazin-1-yl)propan-2-ol (8k)
Compound 8k was prepared as described for 8a using 7c (866 mg, 2.30 mmol) and commercially available 1-(piperazin-1-yl)propan-2-ol (335 mg, 2.32 mmol) to give the product as a yellow oil (650 mg, 64% yield). The free base was converted to the oxalate salt in a 2-propanol/acetone solvent mixture and recovered as a white solid. Mp 119–121 °C; 1H NMR (400 MHz, CDCl3) δ 7.39 (s, 2H), 7.29-7.18 (m, 6H), 5.15 (s, 1H), 3.85-3.76 (m, 1H), 2.72-2.62 (br m, 2H), 2.59-2.18 (m, 13H), 1.13 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 142.8, 134.5, 129.96, 128.4, 127.7, 126.4, 65.5, 62.2, 60.4, 57.8, 53.5, 53.1, 29.5, 19.9; Anal. (C22H28Cl2N2OS · 2C2H2O4) C, H, N.
1-(2-(Benzhydrylsulfinyl)ethyl)-4-(3-phenylpropyl)piperazine (9a)
Compound 9a was prepared as previously described8 using 8a (431mg, 1.00 mmol) to give the product (250 mg, 56% yield) as yellow oil. The free base was converted to the hydrochloride salt and recrystallized from methanol to give a white solid. Mp 210 °C (dec.); 1H NMR (400 MHz, CDCl3) δ 7.15–7.50 (m, 15H), 4.96 (s, 1H), 2.33–2.82 (m, 16H), 1.76–1.84 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 142.1, 136.0, 135.2, 129.3, 129.2, 128.7, 128.4, 128.3, 128.2, 125.8, 72.1, 57.9, 53.4, 53.0, 51.0, 48.2, 33.7, 28.6; Anal. (C28H34N2OS · 2HCl· 0.5H2O) C, H, N.
1-(2-((Bis(4-fluorophenyl)methyl)sulfinyl)ethyl)-4-(3-phenylpropyl)piperazine (9b)
Compound 9b was prepared as described for 9a using 8b (466 mg, 1.00 mmol) to give the product (290 mg, 60% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. Mp 204 °C (dec.); 1H NMR (400 MHz, CDCl3) δ 7.37–7.40(m, 4H), 7.24–7.28(m, 2H), 7.16–7.19(m, 3H), 7.05–7.11(m, 4H), 4.95 (s, 1H), 2.34–2.79 (m, 16H), 1.76–2.04 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 164.0, 163.8, 161.6, 142.1, 131.0, 130.6, 130.3, 128.4, 128.3, 125.8, 116.4, 116.2, 115.9, 115.6, 68.8, 68.2, 65.8, 57.9, 53.0, 50.9, 48.1, 33.7, 28.5; Anal. (C28H32F2N2OS · 2C2H2O4 · H2O) C, H, N.
1-(4-(2-(Benzhydrylsulfinyl)ethyl)piperazin-1-yl)propan-2-ol (9c)
Compound 9c was prepared as described for 9a using 8c (556 mg, 1.50 mmol) to give the product (450 mg, 78% yield) as a white solid. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. Mp 195–197 °C (dec.); 1H NMR (400 MHz, CDCl3) δ 7.30–7.49 (m, 10H), 4.95 (s, 1H), 3.78–3.82 (m, 1H), 3.39 (br s, 1H), 2.18–2.83 (m, 14H), 1.11–1.12 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 136.0, 135.1, 129.3, 129.2, 128.7, 128.6, 128.3, 72.2, 65.5, 62.2, 53.1, 50.9, 48.3, 20.0; Anal. (C22H30N2O2S · 2C2H2O4 · 0.25H2O) C, H, N.
1-(4-(2-((Bis(4-fluorophenyl)methyl)sulfinyl)ethyl)piperazin-1-yl)propan-2-ol (9d; JJC8-091)
Compound 9d was prepared as described for 9a using 8d (610 mg, 1.50 mmol) to give the product (340 mg, 54% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. Mp 190–191 °C (dec.); 1H NMR (400 MHz, CDCl3) δ 7.36–7.43 (m, 4H), 7.04–7.12 (m, 4H), 4.92 (s, 1H), 3.77–3.83 (m, 1H), 2.22–2.84 (m, 14H), 1.11–1.12 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 164.0, 163.8, 161.6, 161.3, 131.7, 131.0, 130.6, 130.5, 130.4, 130.3, 116.4, 116.2, 115.9, 115.6, 69.9, 69.8, 65.5, 62.3, 53.1, 50.8, 48.3, 20.0; Anal. (C22H28F2N2O2S · 2C2H2O4) C, H, N.
1-Benzyl-4-(2-((bis(4-fluorophenyl)methyl)sulfinyl)ethyl)piperazine (9e)
Compound 5e was prepared as described for 9a using 8g (307 mg, 0.700 mmol) to give the product (150 mg, 47.1% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. Mp 219–220 °C (dec.); 1H NMR (400 MHz, CDCl3) δ 7.37–7.44 (m, 4H), 7.24–7.33 (m, 5H), 7.05–7.11 (m, 4H), 5.30 (s, 1H), 3.50 (s, 2H), 2.45–2.80 (m, 12H); 13C NMR (100 MHz, CDCl3) δ 165.9, 163.7, 161.5, 159.3, 138.0, 131.8, 129.2, 127.1, 115.6, 69.7, 63.0, 52.9, 50.9, 48.1; Anal. (C26H28F2N2OS · 2C2H2O4) C, H, N.
1-(2-((Bis(4-fluorophenyl)methyl)sulfinyl)ethyl)-4-(4-fluorobenzyl)piperazine (9f)
Compound 9f was prepared as described for 9a using 8h (251 mg, 0.549 mmol) to give the product (100 mg, 38.5% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. Mp 216–218 °C (dec.); 1H NMR (400 MHz, CDCl3) δ 7.37–7.43 (m, 4H), 7.24–7.27 (m, 2H), 6.96–7.11 (m, 6H), 4.94 (s, 1H), 3,46 (s, 2H), 2.44–2.78 (m, 12H); 13C NMR (100 MHz, CDCl3) δ 164.0, 163.7, 163.2, 161.5, 161.3, 160.8, 133.5, 131.8, 131.7, 131.0, 130.7, 130.6, 130.5, 130.4, 130.3, 116.4, 116.2, 115.9, 115.6, 115.1, 114.9, 69.7, 62.1, 53.0, 52.7, 50.8, 48.1; Anal. (C26H27F3N2OS · 2C2H2O4 · 0.5H2O) C, H, N.
1-(2-((Bis(4-fluorophenyl)methyl)sulfinyl)ethyl)-4-(4-(trifluoromethyl)benzyl)piperazine (9g)
Compound 9g was prepared as described for 9a using 8i (304 mg, 0.601 mmol) to give the product (100 mg, 47.8% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. Mp 216–218 °C (dec.); 1H NMR (400 MHz, CDCl3) δ 7.55–7.57 (m, 2H), 7.37–7.43 (m, 6H), 7.04–7.11 (m, 4H), 4.94 (s, 1H), 3.54 (s, 2H), 2.45–2.83 (m, 12H); 13C NMR (100 MHz, CDCl3) δ 164.0, 163.8, 161.6, 161.3, 142.3, 131.8, 131.0, 130.5, 130.4, 130.3, 129.5, 129.2, 128.6, 125.6, 125.2, 125.1, 122.9, 116.4, 116.2, 115.9, 115.669.8, 62.3, 53.0, 52.9, 50.8, 48.2; Anal. (C27H27F5N2OS · 2C2H2O4) C, H, N.
1-(2-((Bis(4-fluorophenyl)methyl)sulfinyl)ethyl)-4-(4-chlorobenzyl)piperazine (9h)
Compound 9h was prepared as described for 9a using 8j (360 mg, 0.761 mmol) to give the product (160 mg, 43.0% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from methanol to give a white solid. Mp 217–219 °C (dec.); 1H NMR (400 MHz, CDCl3) δ 7.37–7.43 (m, 4H), 7.22–7.26 (m, 4H), 7.04–7.10 (m, 4H), 4.94 (s, 1H), 3.45 (s, 2H), 2.44–2.80 (m, 12H); 13C NMR (100 MHz, CDCl3 δ 164.4, 164.0, 163.8, 161.5, 161.3, 136.4, 132.8, 131.7, 131.7, 131.0, 130.5, 130.4, 130.3, 128.4, 116.4, 116.2, 115.9, 115.6, 69.8, 62.1, 52.9, 52.8, 50.8, 48.1; Anal. (C26H27ClF2N2OS · 2C2H2O4 ·0.25H2O) C, H, N.
1-(4-(2-((Bis(3-chlorophenyl)methyl)sulfinyl)ethyl)piperazin-1-yl)propan-2-ol (9i)
Compound 9i was prepared as described for 9a using 8k (400 mg, 1.00 mmol) The free base (190 mg, 46% yield) was obtained as a yellow oil, which was converted to the oxalate salt in a 2-propanol/acetone solvent mixture and recovered as a cream-colored solid. Mp 192–194 °C; 1H NMR (400 MHz, CDCl3) δ 7.42-7.31 (m, 8H), 4.91 (s, 1H), 3.85-3.80 (m, 1H), 2.84-2.21 (m, 15H), 1.12 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 137.5, 136.4, 135.3, 134.7, 130.6, 130.1, 129.3, 128.80, 128.77, 127.5, 126.7, 70.4, 65.5, 62.3, 53.2, 50.7, 48.3, 19.9; Anal. (C22H28Cl2N2O2S · 2C2H2O4) C, H, N.
1-(4-(2-(Benzhydrylthio)ethyl)piperazin-1-yl)-3-phenylpropan-2-ol (10a)
A solution of compound 8e (710 mg, 2.27 mmol) and 2-benzyloxirane (304.6 mg, 2.27 mmol) in isopropanol (24 mL) was refluxed overnight. Solvent was removed and the reaction residue was purified by flash column chromatography (hexane/ethyl acetate/ triethylamine = 49:49:2) to give 10a (850 mg, 84% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot isopropanol to give a white solid. Mp 210–211 °C; 1H NMR (400 MHz, CDCl3) δ 7.40–7.43 (m, 4H), 7.20–7.32 (m, 11H), 5.22 (s, 1H), 3.88–3.93 (m, 1H), 3.45 (br s, 1H), 2.27–2.83 (m, 16H); 13C NMR (100 MHz, CDCl3) δ 141.4, 138.3, 129.3, 128.6, 128.5, 128.3, 127.3, 127.2, 126.3, 67.2, 63.4, 57.9, 54.4, 53.1, 41.3, 29.3; Anal. (C28H34N2OS · 2C2H2O4 · 0.5H2O) C, H, N.
1-(4-(2-((Bis(4-fluorophenyl)methyl)thio)ethyl)piperazin-1-yl)-3-phenylpropan-2-ol (10b)
Compound 10b was prepared as described for 10a using 8f (455 mg, 1.31 mmol) to give the product (540 mg, 86% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot acetone to give a white solid. Mp 206–207 °C; 1H NMR (400 MHz, CDCl3) δ 7.19–7.37 (m, 9H), 6.96 – 7.02 (m, 4H), 5.18 (s, 1H), 3.88–3.93 (m, 1H), 3.45 (br s, 1H), 2.31–2.81 (m, 16H); 13C NMR (100 MHz, CDCl3) δ 163.1, 160.7, 138.2, 137.0, 129.8, 129.7, 129.3, 128.3, 126.3, 115.6, 115.4, 67.2, 63.4, 57.8, 53.1, 52.9, 41.3, 29.4; Anal. (C28H32F2N2OS · 2C2H2O4 · 0.25H2O) C, H, N.
1-(4-(2-(Benzhydrylsulfinyl)ethyl)piperazin-1-yl)-3-phenylpropan-2-ol (11a)
Compound 11a was prepared as described for 9a using 10a (534mg, 1.20 mmol) to give the product (340 mg, 61% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot methanol to give a white solid. Mp 198–200 °C (dec.); 1H NMR (400 MHz, CDCl3) δ 7.20–7.49 (m, 15H), 4.95 (s, 1H), 3.88–3.92 (m, 1H), 2.31–2.83 (m, 16H); 13C NMR (100 MHz, CDCl3) δ 138.2, 136.0, 135.2, 129.3, 129.2, 128.9, 128.7, 128.6, 128.4, 128.3, 126.3, 72.1, 67.3, 63.4, 53.0, 50.8, 48.2, 41.3; Anal. (C28H34N2O2S · 2C2H2O4 · 0.25H2O) C, H, N.
1-(4-(2-((Bis(4-fluorophenyl)methyl)sulfinyl)ethyl)piperazin-1-yl)-3-phenylpropan-2-ol (11b; JJC 8-088)
Compound 11b was prepared as described for 11a using 10b (400 mg, 0.83 mmol) to give the product (280 mg, 68% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot methanol to give a white solid. Mp 198–200 °C (dec.); 1H NMR (400 MHz, CDCl3) δ 7.20–7.43 (m, 9H), 7.05–7.11 (m, 4H), 4.93 (s, 1H), 3.88–3.92 (m, 1H), 2.29–2.84 (m, 16H); 13C NMR (100 MHz, CDCl3) δ 161.6, 161.3, 138.2, 131.8, 131.0, 130.9, 130.5, 130.3, 129.3, 128.4, 126.3, 116.4, 116.2, 115.9, 115.6, 69.4, 67.3, 63.4, 53.1, 50.8, 48.2, 41.3, Anal. (C28H32F2N2O2S · 2C2H2O4) C, H, N.
2-(Benzhydrylthio)-1-(4-(3-phenylpropyl)piperazin-1-yl)ethan-1-one (13a)
A mixture of CDI (583mg, 3.60 mmol) and 12a27 (775 mg, 3.00 mmol) in THF (24 mL) was stirred at room temperature under argon. After 2 h of reaction time, 1-(3-phenylpropyl)piperazine (613mg, 3.00 mmol) in THF (15 mL) was added and the reaction mixture was stirred overnight. Solvent was removed and the reaction residue was purified by flash column chromatography (ethyl acetate/ triethylamine 95:5) to give 13a (1.2 g, 90% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot isopropanol to give a white solid. Mp 92–95 °C; 1H NMR (400 MHz, CDCl3) δ 7.42–7.45 (m, 4H), 7.17–7.33(m, 11H), 5.34 (s, 1H), 3.57–3.60 (t, 2H, J = 5.0 Hz), 3.37–3.40 (t, 2H, J = 5.2 Hz), 3.18 (s, 2H), 2.62–2.66 (m, 2H), 2.34–2.39 (m, 6H), 1.78–1.84 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 167.2, 141.9, 140.7, 128.6, 128.5, 128.4, 128.3, 127.3, 125.8, 57.7, 54.1, 53.2, 52.7, 46.3, 41.9, 33.5, 28.4; Anal. (C28H32N2OS · C2H2O4 · 0.5H2O) C, H, N.
2-((Bis(4-fluorophenyl)methyl)thio)-1-(4-(3-phenylpropyl)piperazin-1-yl)ethan-1-one (13b)
Compound 13b was prepared as described for 13a using 12b27 (588 mg, 2.00 mmol) and 1-(3-phenylpropyl)piperazine (408 mg, 2.00 mmol) to give the product (650 mg, 71% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot methanol to give a white solid. Mp 123–125 °C; 1H NMR (400 MHz, CDCl3) δ 7.36–7.39 (m, 4H), 7.26–7.30 (m, 2H), 7.17–7.20 (m, 3H), 6.98–7.02 (m, 4H), 5.34 (s, 1H), 3.58–3.60 (t, 2H, J=5.0 Hz), 3.39–3.42 (t, 2H, J = 4.8 Hz), 3.15 (s, 2H), 2.62–2.66 (m, 2H), 2.34–2.41 (m, 6H), 1.79–1.83 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 167.1, 163.2, 160.7, 141.9, 136.3, 130.0, 128.4, 125.9, 115.6, 115.4, 57.7, 53.1, 52.7, 52.4, 46.4, 41.9, 33.5, 33.3, 28.4; Anal. (C28H30F2N2OS · C2H2O4 · 0.25H2O) C, H, N.
2-((Bis(4-fluorophenyl)methyl)thio)-1-(4-(2-hydroxypropyl)piperazin-1-yl)ethan-1-one (13c)
Compound 13c was prepared as described for 13a using 12b (588 mg, 2.00 mmol) and 1-(piperazin-1-yl)propan-2-ol (288 mg, 2.00 mmol) to give the product (630 mg, 75% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot methanol to give a white solid. Mp 163–165 °C; 1H NMR (400 MHz, CDCl3) δ 7.36–7.37 (m, 4H), 6.99–7.03 (m, 4H), 5.33 (s, 1H), 3.82–3.87 (m, 1H), 3.57–3.61 (m, 2H), 3.40–3.43 (m, 2H), 3.22 (s, 1H), 3.16 (s, 2H), 2.59–2.60 (m, 2H), 2.23–2.38 (m, 4H), 1.13–1.27 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 167.2, 163.2, 160.7, 136.2, 130.0, 115.6, 115.4, 65.5, 62.4, 53.2, 52.7, 52.4, 46.4, 41.9, 33.2, 21.1; Anal. (C22H26F2N2O2S · C2H2O4) C, H, N.
2-((Bis(4-fluorophenyl)methyl)thio)-1-(4-propylpiperazin-1-yl)ethan-1-one (13d)
Compound 12b (294 mg, 1.00 mmol) was refluxed in SOCl2 (3 mL) for 2h. The solvent was removed. The reaction mixture was added to 1-propylpiperazine (128 mg, 1 mmol), NaHCO3 (500 mg, 6.00 mmol) in amylene-stabilized CHCl3 (10 mL) and H2O (5 mL) at 0 °C and stirred at room temperature for 2 hours. H2O (5 mL) was added to the reaction mixture, and the aqueous mixture was extracted with chloroform (3 × 10 mL). The organic layer was dried over MgSO4 and the solvent was removed in vacuo. The crude product was purified by flash column chromatography (ethyl acetate/triethylamine = 95:5) to give 13d (220 mg, 54% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from methanol and acetone to give a white solid. Mp 167–168 °C; 1H NMR (400 MHz, CDCl3) δ 7.36–7.40 (m, 4H), 6.98–7.03 (m, 4H), 5.34 (s, 1H), 3.58–3.60 (m, 2H), 3.40–3.42 (m, 2H), 3.16 (s, 2H), 2.37–2.41 (m, 4H), 2.28–2.31 (m, 2H), 1.47–1.52 (m, 2H), 1.13–1.27 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 167.1, 163.2, 160.7, 136.3, 130.0, 115.6, 115.4, 60.4, 53.2, 52.7, 52.4, 46.3, 41.9, 33.3, 19.9, 11.9; Anal. (C22H26F2N2OS · C2H2O4) C, H, N.
2-((Bis(4-fluorophenyl)methyl)thio)-1-(4-(4-fluorobenzyl)piperazin-1-yl)ethan-1-one (13e)
Compound 13e was prepared as described for 13d using 12b (589 mg, 2.00 mmol) and 1-(4-fluorobenzyl)piperazine (389 mg, 2.00 mmol) to give the product (600 mg, 64% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from acetone to give a white solid. Mp 168–170 °C; 1H NMR (400 MHz, CDCl3) δ 7.35–7.39 (m, 4H), 7.25–7.28 (m, 2H), 6.98–7.03 (m, 6H), 5.34 (s, 1H), 3.57–3.60 (m, 2H), 3.46 (s, 2H), 3.38–3.41 (m, 2H), 3.15 (s, 2H), 2.37–2.40 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 167.1, 163.3, 163.2, 160.9, 160.7, 136.3, 133.3, 133.2, 130.6, 130.5, 130.0, 115.6, 115.4, 115.3, 115.1, 62.0, 52.8, 52.5, 52.4, 46.3, 41.9, 33.3; Anal. (C26H25F3N2OS·C2H2O4·1.25H2O) C, H, N.
2-((Bis(4-fluorophenyl)methyl)thio)-1-(4-(4-chlorobenzyl)piperazin-1-yl)ethan-1-one (13f)
Compound 13f was prepared as described for 13d using 12b (589 mg, 2.00 mmol) and 1-(4-chlorobenzyl)piperazine (411 mg, 2.00 mmol) to give the product (650 mg, 67% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from acetone/ethyl ether to give a white solid. Mp 165–167 °C; 1H NMR (400 MHz, CDCl3) δ 7.36–7.40 (m, 4H), 7.25–7.28 (m, 4H), 6.98–7.02 (m, 4H), 5.33 (s, 1H), 3.58–3.59 (m, 2H), 3.38–3.46 (m, 4H), 3.15 (s, 2H), 2.37–2.40 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 167.1, 163.2, 160.7, 136.3, 136.1, 133.0, 130.3, 130.0, 128.5, 115.6, 115.4, 62.0, 52.9, 52.6, 52.4, 46.3, 41.8, 33.3; Anal. (C26H25ClF2N2OS·C2H2O4) C, H, N.
2-((Bis(3-chlorophenyl)methyl)thio)-1-(4-(2-hydroxypropyl)piperazin-1-yl)ethan-1-one (13g)
Compound 13g was prepared as described for 13a using 12c27 (654 mg, 2.00 mmol) and 1-(piperazin-1-yl)propan-2-ol (288 mg, 2.00 mmol) to give the product (770 mg, 75% yield) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot methanol to give a white solid. Mp 160–161 °C; 1H NMR (400 MHz, CDCl3) δ 7.28–7.41 (m, 2H), 7.22–7.24 (m, 6H), 5.30 (s, 1H), 3.82–3.87 (m, 1H), 3.56–3.62 (m, 2H), 3.40–3.43 (m, 2H), 3.20–3.22 (m, 3H), 2.59–2.65 (m, 2H), 2.23–2.40 (m, 4H), 1.13–1.15 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 166.9, 142.1, 134.6, 130.0, 128.4, 127.9, 126.7, 65.5, 62.3, 53.1, 52.9, 52.7, 46.4, 41.9, 33.2, 19.9; Anal. (C22H26Cl2N2O2S·C2H2O4) C, H, N.
2-(Benzhydrylsulfinyl)-1-(4-(3-phenylpropyl)piperazin-1-yl)ethan-1-one (14a)
Compound 14a was prepared as described for 9a using 13a (667mg, 1.50 mmol) to give the product (500 mg, 72%) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot acetone to give a white solid. Mp 180–182 °C; 1H NMR (400 MHz, CDCl3) δ 7.49–7.55 (m, 4H), 7.16–7.43 (m, 11H), 5.30 (s, 1H), 3.27–3.70 (m, 6H), 2.61–2.65 (m, 2H), 2.33–2.47 (m, 6H), 1.75–1.83 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 163.1, 141.9, 136.0, 133.6, 130.0, 129.1, 129.0, 128.7, 128.5, 128.4, 128.3, 128.2, 125.8, 70.1, 70.0, 57.5, 53.1, 52.6, 46.4, 42.0, 33.5, 28.4; Anal. (C28H32N2O2S·C2H2O4·0.25H2O) C, H, N.
2-((Bis(4-fluorophenyl)methyl)sulfinyl)-1-(4-(3-phenylpropyl)piperazin-1-yl)ethan-1-one (14b)
Compound 14b was prepared as described for 9a using 13b (480 mg, 1.00 mmol) to give the product (200 mg, 40%) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot acetone to give a white solid. Mp 135–136 °C; 1H NMR (400 MHz, CDCl3) δ 7.45–7.52 (m, 4H), 7.06–7.30 (m, 9H), 5.36 (s, 1H), 3.54–3.69 (m, 3H), 3.27–3.45 (m, 3H), 2.61–2.65 (m 2H), 2.35–2.45 (m, 6H), 1.76–1.84 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 164.2, 163.8, 162.8, 162.7, 161.7, 161.3, 160.2, 141.8, 131.8, 131.7, 130.7, 130.6, 129.0, 128.4, 125.9, 116.2, 116.0, 115.9, 115.7, 67.7, 57.5, 53.1, 52.7, 52.6, 46.4, 42.0, 33.5, 28.3; Anal. (C28H30F2N2O2S · 2C2H2O4) C, H, N.
2-((Bis(4-fluorophenyl)methyl)sulfinyl)-1-(4-(2-hydroxypropyl)piperazin-1-yl)ethan-1-one (14c)
Compound 14c was prepared as described for 9a using 13c (600 mg, 1.43 mmol) to give the product (70 mg, 16%) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot acetone to give a white solid. Mp 162–164 °C; 1H NMR (400 MHz, CDCl3) δ 7.44–7.51 (m, 4H), 7.06–7.13 (m, 4H), 5.36 (s, 1H), 3.83–3.88 (m, 1H), 3.24–3.67 (m, 7H), 2.60–2.67 (m, 2H), 2.26–2.42 (m, 4H), 1.13–1.14 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 164.2, 163.8, 162.9, 161.7, 161.3, 131.7, 131.6, 131.5, 130.7, 129.1, 116.3, 116.1, 116.0, 65.4, 62.4, 53.3, 53.2, 52.7, 52.6, 52.4, 52.3, 46.4, 42.0, 20.0; Anal. (C22H26F2N2O3S·C2H2O4) C, H, N.
2-((Bis(4-fluorophenyl)methyl)sulfinyl)-1-(4-propylpiperazin-1-yl)ethan-1-one (14d)
Compound 14d was prepared as described for 9a using 13d (120 mg, 0.297 mmol) to give the product (40 mg, 32%) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot acetone and methanol to give a white solid. Mp 170–171 °C; 1H NMR (400 MHz, CDCl3) δ 7.45–7.52 (m, 4H), 7.06–7.13 (m, 4H), 5.36 (s, 1H), 3.36–3.67 (m, 6H), 2.30–2.45 (m, 6H), 1.47–1.53 (m, 2H), 0.90–0.92 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 164.2, 163.8, 162.8, 161.7, 161.3, 131.8, 131.7, 130.7, 130.6, 129.0, 116.2, 116.0, 115.9, 115.7, 67.7, 60.2, 53.1, 52.6, 46.3, 41.9, 19.8, 11.8; Anal. (C22H26F2N2O2S · C2H2O4) C, H, N.
2-((Bis(4-fluorophenyl)methyl)sulfinyl)-1-(4-(4-fluorobenzyl)piperazin-1-yl)ethan-1-one (14e)
Compound 14e was prepared as described for 9a using 10e (510 mg, 1.09 mmol) to give the product (120 mg, 23%) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot acetone and methanol to give a white solid. Mp 158–159 °C; 1H NMR (400 MHz, CDCl3) δ 7.44–7.48 (m, 4H), 7.24–7.26 (m, 2H), 6.98–7.13 (m, 6H), 5.36 (s, 1H), 3.27–3.68 (m, 8H), 2.34–2.45 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 166.2, 164.2, 163.8, 162.8, 161.7, 161.3, 160.9, 133.1, 131.7, 130.7, 130.6, 130.5, 129.0, 116.2, 116.0, 115.9, 115.7, 115.3, 115.1, 67.7, 61.8, 52.9, 52.7, 52.4, 46.4, 42.0; Anal. (C26H25F3N2O2S·C2H2O4·1.5H2O) C, H, N.
2-((Bis(4-fluorophenyl)methyl)sulfinyl)-1-(4-(4-chlorobenzyl)piperazin-1-yl)ethan-1-one (14f)
Compound 14f was prepared as described for 9a using 13f (487 mg, 1.00 mmol) to give the product (200 mg, 40%) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot acetone and methanol to give a white solid. Mp 157–158 °C; 1H NMR (400 MHz, CDCl3) δ 7.44–7.52 (m, 4H), 7.22–7.30 (m, 4H), 7.06–7.13 (m, 4H), 5.36 (s, 1H), 3.30–3.65 (m, 8H), 2.37–2.43 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 164.2, 163.8, 162.8, 161.7, 161.3, 136.0, 133.1, 131.7, 130.7, 130.3, 129.1, 129.0, 128.5, 116.2, 116.0, 115.9, 115.7, 67.7, 61.9, 52.9, 52.7, 52.4, 46.4, 42.0; Anal. (C26H25ClF2N2O2S·C2H2O4·1.5H2O) C, H, N.
2-((Bis(3-chlorophenyl)methyl)sulfinyl)-1-(4-(2-hydroxypropyl)piperazin-1-yl)ethan-1-one (14g)
Compound 14g was prepared as described for 9a using 13g (590 mg, 1.15 mmol) to give the product (70 mg, 11%) as a yellow oil. The free base was converted to the oxalate salt and recrystallized from hot acetone and methanol to give a white solid. Mp 169–170 °C; 1H NMR (400 MHz, CDCl3) δ 7.31–7.43 (m, 8H), 5.33 (s, 1H), 3.82–3.84 (m, 1H), 3.59–3.67 (m, 3H), 3.33–3.47 (m, 3H), 3.16–3.19 (m, 1H), 2.61–2.66 (m, 2H), 2.24–2.42 (m, 4H), 1.13–1.14 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 162.7, 137.4, 135.1, 134.8, 134.7, 130.4, 130.1, 130.0, 129.1, 129.0, 128.7, 128.1, 127.1, 68.1, 65.4, 62.4, 53.2, 53.1, 52.8, 52.7, 52.6, 46.4, 46.2, 42.0, 19.9; Anal. (C22H26Cl2N2O3S · C2H2O4) C, H, N.
Radioligand Binding Assays
DAT Binding Assay
Striata were dissected from male Sprague-Dawley rat brains (supplied on ice from Bioreclamation (Hicksville, NY) and prepared by homogenizing tissues in 20 volumes (w/v) of ice cold modified sucrose phosphate buffer (0.32 M sucrose, 7.74 mM Na2HPO4, 2.26 mM NaH2PO4, pH adjusted to 7.4) using a Brinkman Polytron (Setting 6 for 20 sec) and centrifuged at 20,000 RPM for 10 min at 4 °C. The resulting pellet was re-suspended in buffer, re-centrifuged and suspended in buffer again to a concentration of 10 mg/mL, original wet weight (OWW). Experiments were conducted in assay tubes containing 0.5 mL sucrose phosphate buffer, 0.5 nM [3H]WIN 35,42854 (Kd value = 5.53, specific activity = 84 ci/mmol; Perkin Elmer Life Sciences, Waltham, MA), 1.0 mg of tissue OWW, and various concentrations of inhibitor. The reaction was started with the addition of tissue and tubes were incubated for 120 min on ice. Nonspecific binding was determined using 100 µM cocaine HCl.
SERT Binding Assay
Membranes from frozen brain stem dissected from male Sprague-Dawley rat brains (supplied on ice from Bioreclamation, Hicksville, NY) were homogenized in 20 volumes (w/v) of 50 mM Tris buffer (120 mM NaCl and 5 mM KCl, adjusted to pH 7.4) at 25 °C using a Brinkman Polytron (at setting 6 for 20 sec). The tissue was centrifuged at 20,000 RPM for 10 min at 4 °C. The resulting pellet was suspended in buffer and centrifuged again. The final pellet was resuspended in cold buffer to a concentration of 15 mg/mL OWW. Experiments were conducted in assay tubes containing 0.5 mL buffer, 1.4 nM [3H]citalopram (Kd value = 1.94 nM, specific activity = 83 ci/mmol; Perkin Elmer Life Sciences, Waltham, MA), 1.5 mg brain stem tissue, and various concentrations of inhibitor. The reaction was started with the addition of the tissue and the tubes were incubated for 60 min at room temperature. Nonspecific binding was determined using 10 µM fluoxetine.
NET Binding Assay
Membranes from frozen frontal cortex dissected from male Sprague-Dawley rat brains (supplied on ice from Bioreclamation, Hicksville, NY) were homogenized in 20 volumes (w/v) of 50 mM Tris buffer (120 mM NaCl and 5 mM KCl, adjusted to pH 7.4) at 25 °C using a Brinkman Polytron (at setting 6 for 20 sec). The tissue was centrifuged at 20,000 RPM for 10 min at 4 °C. The resulting pellet was suspended in buffer and centrifuged again. The final pellet was resuspended in cold buffer to a concentration of 80 mg/mL OWW. Experiments were conducted in assay tubes containing 0.5 mL buffer, 0.5 nM [3H]nisoxetine (Kd value = 1.0 nM, specific activity = 82 ci/mmol; Perkin Elmer Life Sciences, Waltham, MA), 8 mg frontal cortex tissue, and various concentrations of inhibitor. The reaction was started with the addition of the tissue and the tubes were incubated for 180 min at 0–4 °C. Nonspecific binding was determined using 1 µM desipramine.
The solvent used to dissolve the various analogs of modafinil was typically methanol and was present at a final concentration of 5%. Extensive studies previously in this and other laboratories determined that methanol has no effect on binding at the DAT and SERT. However there is an effect of methanol on binding at the NET and therefore methanol concentration was controlled in all tubes in that assay. When compounds were not soluble in methanol we used either ethanol or DMSO at final concentrations of no greater than 5 or 6%, respectively. Previous studies found no effect of either of these solvents at these concentrations on binding at any of the sites. For all three MAT binding assays, incubations were terminated by rapid filtration through Whatman GF/B filters, presoaked in 0.3% (SERT) or 0.05% (DAT, NET) polyethylenimine, using a Brandel R48 filtering manifold (Brandel Instruments Gaithersburg, Maryland). The filters were washed twice with 5 mL cold buffer and transferred to scintillation vials. Cytoscint (MP Biomedicals, OH) (3.0 mL) was added and the vials were counted the next day using a Beckman 6000 liquid scintillation counter (Beckman Coulter Instruments, Fullerton, California) or a Tri-Carb 2910-B liquid scintillation counter (Perkin Elmer Life Sciences, MA). The Ki values for the modafinil derivatives were obtained using nonlinear least-squares regression (using GraphPad Prism Software, San Diego, CA) of the displacement data giving IC50 values, from which affinities (Ki values) were calculated using the Cheng-Prusoff equation.55
σ1 Receptor Binding Assay
σ1 receptor binding was performed as previously reported56 Briefly, frozen whole-guinea pig brains (minus cerebellum) were thawed on ice, weighed and homogenized (with a glass and Teflon homogenizer) in 10 mM Tris-HCl with 0.32 M sucrose at pH 7.4 (10 mL/g tissue). The homogenate was centrifuged at 1000 g for 10 minutes at 4 °C. The supernatant was collected into a clean centrifuge tube, and the remaining pellet was resuspended by vortex in 10 mL buffer (tissue) and centrifuged again at 50,000 g for 15 minutes at 4 °C. The resulting pellet was resuspended in experimental buffer to 80 mg/mL original wet weight (OWW).
Ligand binding experiments were conducted in polypropylene assay tubes containing 0.5 mL of 50 mM Tris-HCl buffer at pH 8.0. Each tube contained 3 nM [3H](+)-pentazocine (PerkinElmer Life and Analytical Sciences, Waltham, MA) and 8.0 mg tissue OWW. Nonspecific binding was determined using 10 mM haloperidol. The reaction was started with the addition of tissue and the tubes were incubated for 120 minutes at room temperature.
Incubations for all binding assays were terminated by rapid filtration through Whatman GF/B filters, presoaked in polyethylenimine, using a Brandel R48 filtering manifold (Brandel Instruments, Gaithersburg, MD). The filters were washed twice with 5 mL of ice-cold buffer and transferred to scintillation vials. Beckman Ready Safe (3.0 mL) was added, and the vials were counted the next day using a Beckman 6000 liquid scintillation counter (Beckman Coulter Instruments, Fullerton, CA) at 50% efficiency. Assays were typically conducted in at least three independent experiments, each performed in triplicate.
For the displacement of radioligand binding, IC50 values were computed using a nonlinear, least-squares regression analysis (Prism; GraphPad Software Inc., San Diego, CA). Affinities (Ki values) were calculated using the concentration of radioligand used in the assay.
D2-like Binding Assay
Binding at dopamine D2-like receptors was determined using previously described methods.57 Membranes were prepared from HEK293 cells stably expressing human D2R, D3R, or D4R, grown in a 50:50 mix of DMEM and Ham’s F12 culture media, supplemented with 20 mM HEPES, 2 mM L-glutamine, 0.1 mM non-essential amino acids, 1× antibiotic/antimycotic, 10% heat-inactivated fetal bovine serum, and 200 µg/mL hygromycin (Life Technologies, Grand Island, NY) and kept in an incubator at 37°C and 5% CO2. Upon reaching 80–90% confluence, cells were harvested using pre-mixed Earle’s Balanced Salt Solution (EBSS) with 5 mM EDTA (Life Technologies) and centrifuged at 3000 rpm for 10 min at 21 °C. The supernatant was removed and the pellet was resuspended in 10 mL hypotonic lysis buffer (5 mM MgCl2 · 6 H2O, 5 mM Tris, pH 7.4 at 4 °C) and centrifuged at 20,000 rpm for 30 min at 4 °C. The pellet was then resuspended in fresh EBSS buffer made from 8.7 g/L Earle’s Balanced Salts without phenol red (US Biological, Salem, MA), 2.2 g/L sodium bicarbonate, pH to 7.4. A Bradford protein assay (Bio-Rad, Hercules, CA) was used to determine the protein concentration and membranes were diluted to 500 µg/mL and stored in a −80 °C freezer for later use. Radioligand competition binding experiments were conducted using thawed membranes. Test compounds were freshly dissolved in 30% DMSO and 70% H2O to a stock concentration of 1 mM or 100 µM. To assist the solubilization of free-base compounds, 10 µl of glacial acetic acid was added along with the DMSO. Each test compound was then diluted into 11 half-log serial dilutions using 30% DMSO vehicle; final test concentrations ranged from 100 µM to 100 pM or from 10 µM to 10 pM. Previously frozen membranes were diluted in fresh EBSS to a 200 µg/mL (for hD2R or hD3R) or 300 µg/mL (hD4R) stock for binding. Radioligand competition experiments were conducted in 96-well plates containing 300 µl fresh EBSS buffer with 0.2 mM sodium metabisulfite, 50 µl of diluted test compound, 100 µl of membranes (20 µg total protein for hD2R or hD3R, 30 µg total protein for hD4R), and 50 µl of [3H]N-methylspiperone (0.4 nM final concentration; Perkin Elmer). Nonspecific binding was determined using 10 µM butaclamol (Sigma-Aldrich, St. Louis, MO) and total binding was determined with 30% DMSO vehicle. The reaction was incubated for one hour at room temperature and then terminated by filtration through Perkin Elmer UniFilter-96 GF/B filters, presoaked for one hour in 0.5% polyethylenimine, using a Brandel 96-Well Plates Harvester Manifold (Brandel Instruments, Gaithersburg, MD). The filters were washed 3 times with 3 mL (3 × 1 mL/well) of ice cold EBSS buffer. 65 µL Perkin Elmer MicroScint 20 Scintillation Cocktail was added to each well and filters were counted using a Perkin Elmer MicroBeta Microplate Counter. IC50 values for each compound were determined from dose-response curves and Ki values were calculated using the Cheng-Prusoff equation.55 These analyses were performed using GraphPad Prism version 6.00 for Macintosh (GraphPad Software, San Diego, CA). Reported Ki values were determined from at least three independent experiments, each with duplicate determinations.
Bioluminescence Resonance Energy Transfer (BRET) Assay
The Gα-γ protein activation assay uses RLuc8-fused Gα protein subunit and GFP10-fused Gγ protein for a resonance energy transfer pair. Flag-tagged receptor and untagged Gβ constructs were co-transfected. The BRET assays were performed as described previously.58 Briefly, human embryonic kidney cells (HEK-293T) were transfected, using a constant amount of plasmid DNA but various ratios of plasmids encoding the fusion protein partners. Expression of GFP10 fusion proteins was estimated by measuring fluorescence at 515 nm following excitation at 405 nm. Expression of RLuc8 fusion proteins was estimated by measuring the luminescence of the cells after incubation with 5 µM coelenterazine 400a. In parallel, BRET was measured as the fluorescence of the cells at 535 nm at the same time points using a Mithras LB940 reader (Berthold). In order to measure the antagonist activity, 10 min preincubation of 1 µM quinpirole precedes the 10 min incubation of tested compounds before the sample reading.
Molecular Pharmacology
Site-directed mutagenesis
Synthetic cDNA encoding the human DAT (synDAT) were subcloned into pcDNA3 (Invitrogen, Carlsbad, CA). The Y156F mutation was introduced using QuickChange (adapted from Stratagene, La Jolla, CA) and confirmed by restriction enzyme mapping and DNA sequencing. DAT WT and Y156F cDNA containing plasmids were amplified by transformation into XL1 blue competent cells (Stratagene) and grown in LB media over night at 37 °C in an orbital incubator (Infors) @ 200 rpm. Plasmids were harvested using the maxi prep kit (Qiagen) according to the manufacturer’s manual.
Cell Culture and Transfection
COS7 cells were grown in Dulbecco’s modified Eagle’s medium 041 01885 supplemented with 10% fetal calf serum, 2 mM L-glutamine and 0.01 mg/mL gentamicin at 37 °C in 10% CO2. DAT WT and Y156F were transiently transfected into COS7 cells with Lipo2000 (Invitrogen) according to manufacturer’s manual using a cDNA:Lipo2000 ratio of 3:6.
[3H]DA uptake experiments
Uptake assays were performed on intact COS7 cells essentially as described59 using 3,4-[Ring-2,5,6-3H]-dihydroxyphenylethylamine ([3H]DA) (30–60 Ci/mmol) (Perkin Elmer). Briefly, transfected COS7 cells were plated in either 24-well dishes (105 cells/well) coated with poly-ornithine (Sigma) to achieve an uptake level of no more than 10% of total added [3H]DA. The uptake assays were carried out 2 days after transfection in uptake buffer (UB) (25 mM HEPES, 130 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, 1 mM L-ascorbic acid, 5 mM D-glucose, and 1 µM of the catechol-O-methyltransferase inhibitor Ro 41-0960 (Sigma), pH 7.4). Prior to the experiment, the cells were washed once in 500 µl of UB and the non-labeled compound was added to the cells in the indicated concentrations in a total volume of 500 µl. The assay was initiated by the addition of 6–10 nM [3H]DA. Nonspecific uptake was determined with 1 µM nomifensine (Sigma-Aldrich). After 5 min of incubation at room temp, the cells were washed twice with 500 µL of ice cold UB, lysed in 250 µL (24 well) 1% SDS and left for >30 min at 37 °C on gentle shaking. All samples were transferred to 24-well counting plates (Perkin Elmer, Waltham, MA), 500 µL (24 well) of Opti-phase Hi Safe 3 scintillation fluid (Perkin Elmer) was added followed by counting of the plates in a Wallac Tri-Lux β-scintillation counter (Perkin Elmer). All experiments were carried out with 12 determinations of DA or inhibitor concentrations ranging from 1 nM to 1 mM performed in triplicates.
[3H]WIN35,428 binding experiments
Binding assays were carried out essentially as described for the uptake experiments on whole cells only using [3H]2β-carbomethoxy-3β-(4-fluorophenyl)tropane ([3H]WIN35,428) (76–87 Ci/mmol) (Perkin Elmer). Previous to the binding experiment, cells were washed once in ice cold UB and, after the addition of unlabeled compound in the indicated concentrations and [3H]WIN35,428, the reactions were incubated at 5°C until equilibrium were obtained (>90 min). All experiments were carried out with 12 determinations with inhibitor concentration range from 1 nM to 1 mM, performed in triplicates.
Mouse microsomal stability assay
The phase I metabolic stability assay was conducted in mouse liver microsomes as described previously47 with minor modifications. Briefly, the reaction was carried out using potassium phosphate buffer (100 mM, pH 7.4), in the presence of NADPH regenerating system, (compound final concentration was 10 µM; and 0.5 mg/mL microsomes). Testosterone was used as a positive control. Compound disappearance was monitored over time using a liquid chromatography and tandem mass spectrometry (LC/MS/MS) method.
Chromatographic analysis was performed using an Accela™ ultra high-performance system consisting of an analytical pump and an autosampler coupled with a TSQ Vantage mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA). Separation of analyte was achieved at ambient temperature using Agilent Eclipse Plus column (100 × 2.1mm i.d.) packed with a 1.8 µm C18 stationary phase. The mobile phase used was composed of 0.1% formic acid in acetonitrile and 0.1% formic acid in H2O with gradient elution, starting with 10% (organic) linearly increasing to 99% up to 2 min, maintaining at 99% (2–2.5min) and reequlibrating to 10%. The total run time 4.5 min. The mass transitions used for compounds for LC/MS/MS analysis are given in Table S1 (Supporting Information).
Locomotor Activity Studies in Mice
Thirty-two male mice with a C57BL/6J genetic background and body weight 22–25 g were purchased from the Charles River Laboratories (Raleigh, NC). After arrival, they were group housed (4 per cage) in the animal facility under a reversed 12 h light-dark cycle (light on at 7:00 PM) with free access to food and water, and allowed to acclimate to the new environment for 7 days prior to initiating the experiment. All procedures were in accordance with the “Principles of Laboratory Animal Care” outlined by National Institute of Health (NIH publication 86-23, 1996).
Locomotor activity tests were conducted in open-field chambers (43 × 43 × 30 cm) (Accuscan Instruments, Inc., Columbus, OH, USA). Before testing, the mice were habituated to the locomotor chamber (2 h/day for 3 consecutive days), and then were randomly divided into 4 groups (n=8 per group) to examine the effects of cocaine, (R)-modafinil [(R)-2], compounds 4 and and 11b on locomotor activity, respectively. On habituation days, the animals were moved to the test room, acclimated there for about 30 min, and then placed in their assigned locomotor chambers. On test day 1, each group of mice was first placed in the locomotor chambers for 1 h habituation, and then they received either vehicle (25% of 2-hydroxypropyl-β-cyclodextrin) or one dose of the corresponding test compound. The animals were then immediately placed into the test chambers. Their locomotor activities were recorded every 10 min for 2 h. Each animal was tested 3 times with 3 different doses, with the inter-test interval of 3–5 days. The order of the drug doses was counter-balanced.
The locomotor behavioral data are expressed as means (±S.E.M.). The traveled distance (cm) every 10 min (Fig. 5) was used to evaluate the locomotor effects of each test compound in mice. Two-way ANOVA with repeated measures over time was used to evaluate the statistical significance of the changes in locomotor activity after each drug administration. Post-hoc Fisher’s least significant difference was used for multiple comparisons. p<0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
Support for this research was provided to AHN, JC, RDS OMB, CB, TK, AB, MPE, HY, YH, G-HB, and Z-XX by the National Institute on Drug Abuse - Intramural Research Program. CJL is supported by the Lundbeck Foundation and the Danish Council for Independent Research Sapere Aude programme.
ABBREVIATIONS USED
- DA
dopamine
- DAT
dopamine transporter
- SERT
serotonin transporter
- NET
norepinephrine transporter
- CDI
N,N’-carbonyldiimidazole
- IA
Inactive
- ND
not determined
- NADPH
Nicotinamide adenine dinucleotide phosphate
Footnotes
ASSOCIATED CONTENT
Supporting information. Elemental analysis results. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Keck TM, John WS, Czoty PW, Nader MA, Newman AH. Identifying Medication Targets for Psychostimulant Addiction: Unraveling the Dopamine D3 Receptor Hypothesis. J. Med. Chem. 2015;58:5361–5380. doi: 10.1021/jm501512b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Czoty PW, Stoops WW, Rush CR. Evaluation of the “Pipeline” for Development of Medications for Cocaine Use Disorder: A Review of Translational Preclinical, Human Laboratory, and Clinical Trial Research. Pharmacol. Rev. 2016;68:533–562. doi: 10.1124/pr.115.011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster CR, Kuhar MJ, Balster RL, editors. Handbook of Experimental Pharmacology. Vol. 118. New York, USA: Springer; 1996. Pharmacological Aspects of Drug Dependence: Toward an Integrated Neurobehavioral Approach. [Google Scholar]
- 4.Schmitt KC, Reith MEA. Regulation of the Dopamine Transporter. Ann. N.Y. Acad. Sci. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- 5.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New Insights into the Mechanism of Action of Amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 6.Wood S, Sage JR, Shuman T, Anagnostaras SG. Psychostimulants and Cognition: A Continuum of Behavioral and Cognitive Activation. Pharmacol. Rev. 2014;66:193–221. doi: 10.1124/pr.112.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trifilieff P, Martinez D. Chapter Five – Cocaine: Mechanism and Effects in the Human Brain. In: Madras B, Kuhar K, editors. The Effects of Drug Abuse on the Human Nervous System. Oxford, United Kingdom: Elsevier Inc.; 2014. pp. 103–133. [Google Scholar]
- 8.Stoops WW, Rush CR. Agonist Replacement for Stimulant Dependence: A Review of Clinical Research. Curr. Pharm. Des. 2013;19:7026–7035. doi: 10.2174/138161281940131209142843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preti A. Review: New Developments in the Pharmacotherapy of Cocaine Abuse. Addict. Biol. 2007;12:133–151. doi: 10.1111/j.1369-1600.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- 10.Gowrishankar R, Hahn MK. Good Riddance to Dopamine: Roles for the Dopamine Transporter in Synaptic Function and Dopamine-Associated Brain Disorders. Neurochem. Int. 2014;73:42–48. doi: 10.1016/j.neuint.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Heal DJ, Cheetham SC, Smith SL. The Neuropharmacology of ADHD Drugs In Vivo: Insights on Efficacy and Safety. Neuropharmacology. 2009;57:608–618. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Mereu M, Bonci A, Newman AH, Tanda G. The Neurobiology of Modafinil as an Enhancer of Cognitive Performance and a Potential Treatment for Substance Use Disorders. Psychopharmacology. 2013;229:415–434. doi: 10.1007/s00213-013-3232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loland CJ, Mereu M, Okunola OM, Cao J, Prisinzano TE, Mazier S, Kopajtic T, Shi L, Katz JL, Tanda G, Newman AH. R-Modafinil (Armodafinil): A Unique Dopamine Uptake Inhibitor and Potential Medication for Psychostimulant Abuse. Biol. Psychiatry. 2012;72:405–413. doi: 10.1016/j.biopsych.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, Rowan A, Poole S, White L, O’Brien CP. Modafinil and Cocaine: A Double-Blind, Placebo-Controlled Drug Interaction Study. Drug Alcohol Depend. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- 15.Malcolm R, Swayngim K, Donovan JL, DeVane CL, Elkashef A, Chiang N, Khan R, Mojsiak J, Myrick DL, Hedden S, Cochran K, Woolson RF. Modafinil and Cocaine Interactions. Am. J. Drug Alcohol Abuse. 2006;32:577–587. doi: 10.1080/00952990600920425. [DOI] [PubMed] [Google Scholar]
- 16.Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked Cocaine Self-Administration is Decreased by Modafinil. Neuropsychopharmacology. 2008;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- 17.Newman JL, Negus SS, Lozama A, Prisinzano TE, Mello NK. Behavioral Evaluation of Modafinil and the Abuse-Related Effects of Cocaine in Rhesus Monkeys. Exp. Clin. Psychopharmacol. 2010;18:395–408. doi: 10.1037/a0021042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwin MR, Bjurstrom MF, Olmstead R. Polysomnographic Measures of Sleep in Cocaine Dependence and Alcohol Dependence: Implications for Age-Related Loss of Slow Wave, Stage 3 Sleep. Addiction. 2016;111:1084–1092. doi: 10.1111/add.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irwin MR, Opp MR. Sleep-Health: Reciprocal Regulation of Sleep and Innate Immunity. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.148. Online Early Access] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Raga J, Cepeda CK, Modafinil S. A Useful Medication for Cocaine Addiction? Review of the Evidence from Neuropharmacological, Experimental and Clinical Studies. Curr. Drug Abuse Rev. 2008;1:213–221. doi: 10.2174/1874473710801020213. [DOI] [PubMed] [Google Scholar]
- 21.Anderson AL, Reid MS, Li S-H, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, III, Elkashef AM. Modafinil for the Treatment of Cocaine Dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kampman KM, Lynch KG, Pettinati HM, Spratt K, Wierzbicki MR, Dackis C, O’Brien CP. A Double Blind, Placebo Controlled Trial of Modafinil for the Treatment of Cocaine Dependence without Co-Morbid Alcohol Dependence. Drug Alcohol Depend. 2015;155:105–110. doi: 10.1016/j.drugalcdep.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH, Newman AH. Novel N-Substituted 3α-[Bis(4‘-fluorophenyl)methoxy]tropane Analogues: Selective Ligands for the Dopamine Transporter. J. Med. Chem. 1997;40:4329–4339. doi: 10.1021/jm970525a. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt KC, Reith MEA. The Atypical Stimulant and Nootropic Modafinil Interacts with the Dopamine Transporter in a Different Manner than Classical Cocaine-Like Inhibitors. PLoS One. 2011;6:e25790. doi: 10.1371/journal.pone.0025790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X-F, Bi G-H, He Y, Yang H-J, Gao J-T, Okunola-Bakare OM, Slack RD, Gardner EL, Xi Z-X, Newman AH. R-Modafinil Attenuates Nicotine-Taking and Nicotine-Seeking Behavior in Alcohol-Preferring Rats. Neuropsychopharmacology. 2015;40:1762–1771. doi: 10.1038/npp.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao J, Prisinzano TE, Okunola OM, Kopajtic T, Shook M, Katz JL, Newman AH. SARs at the Monoamine Transporters for a Novel Series of Modafinil Analogues. ACS Med. Chem. Lett. 2011;2:48–52. doi: 10.1021/ml1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okunola-Bakare OM, Cao J, Kopajtic T, Katz JL, Loland CJ, Shi L, Newman AH. Elucidation of Structural Elements for Selectivity across Monoamine Transporters: Novel 2-[(Diphenylmethyl)sulfinyl]acetamide (Modafinil) Analogues. J. Med. Chem. 2014;57:1000–1013. doi: 10.1021/jm401754x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis D, Zhang Y, Prisinzano T, Dersch CM, Rothman RB, Jacobson AE, Rice KC. Further Exploration of 1-{2-[Bis-(4-fluorophenyl)methoxy]ethyl}piperazine (GBR 12909): role of N-Aromatic, N-Heteroaromatic, and 3-Oxygenated N-Phenylpropyl Substituents on Affinity for the Dopamine and Serotonin Transporter. Bioorg. Med. Chem. Lett. 2003;13:1385–1389. doi: 10.1016/s0960-894x(03)00108-2. [DOI] [PubMed] [Google Scholar]
- 29.Hsin L-W, Dersch CM, Baumann MH, Stafford D, Glowa JR, Rothman RB, Jacobson AE, Rice KC. Development of Long-Acting Dopamine Transporter Ligands as Potential Cocaine-Abuse Therapeutic Agents: Chiral Hydroxyl-Containing Derivatives of 1-[2-[Bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine and 1-[2-(Diphenylmethoxy)ethyl]-4-(3-phenylpropyl)piperazine. J. Med. Chem. 2002;45:1321–1329. doi: 10.1021/jm010430f. [DOI] [PubMed] [Google Scholar]
- 30.Matecka D, Lewis D, Rothman RB, Dersch CM, Wojnicki FHE, Glowa JR, DeVries AC, Pert A, Rice KC. Heteroaromatic Analogs of 1-[2-(Diphenylmethoxy)ethyl]- and 1-[2-[Bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazines (GBR 12935 and GBR 12909) as High-Affinity Dopamine Reuptake Inhibitors. J. Med. Chem. 1997;40:705–716. doi: 10.1021/jm9606599. [DOI] [PubMed] [Google Scholar]
- 31.Matecka D, Rothman RB, Radesca L, de Costa BR, Dersch CM, Partilla JS, Pert A, Glowa JR, Wojnicki FHE, Rice KC. Development of Novel, Potent, and Selective Dopamine Reuptake Inhibitors through Alteration of the Piperazine Ring of 1-[2-(Diphenylmethoxy)ethyl]- and 1-[2-[Bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazines (GBR 12935 and GBR 12909) J. Med. Chem. 1996;39:4704–4716. doi: 10.1021/jm960305h. [DOI] [PubMed] [Google Scholar]
- 32.Lewis DB, Matecka D, Zhang Y, Development of Long-Acting Dopamine Transporter Ligands as Potential Cocaine-Abuse Therapeutic, L.-W. Dersch CM, Stafford D, Glowa JR, Rothman RB, Rice KC. Oxygenated Analogues of 1-[2-(Diphenylmethoxy)ethyl]- and 1-[2-[Bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazines (GBR 12935 and GBR 12909) as Potential Extended-Action Cocaine-Abuse Therapeutic Agents. J. Med. Chem. 1999;42:5029–5042. doi: 10.1021/jm990291q. [DOI] [PubMed] [Google Scholar]
- 33.Baumann MH, Char GU, De Costa BR, Rice KC, Rothman RB. GBR12909 Attenuates Cocaine-Induced Activation of Mesolimbic Dopamine Neurons in the Rat. J. Pharmacol. Exp. Ther. 1994;271:1216–1222. [PubMed] [Google Scholar]
- 34.Rothman RB, Mele A, Reid AA, Akunne HC, Greig N, Thurkauf A, de Costa BR, Rice KC, Pert A. GBR12909 Antagonizes the Ability of Cocaine to Elevate Extracellular Levels of Dopamine. Pharmacol. Biochem. Behav. 1991;40:387–397. doi: 10.1016/0091-3057(91)90570-r. [DOI] [PubMed] [Google Scholar]
- 35.Cao J, Kopajtic T, Katz JL, Newman AH. Dual DAT/σ1 Receptor Ligands Based on 3-(4-(3-(Bis(4-fluorophenyl)amino)propyl)piperazin-1-yl)-1-phenylpropan-1-ol. Bioorg. Med. Chem. Lett. 2008;18:5238–5241. doi: 10.1016/j.bmcl.2008.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao J, Kulkarni SS, Husbands SM, Bowen WD, Williams W, Kopajtic T, Katz JL, George C, Newman AH. Dual Probes for the Dopamine Transporter and σ1 Receptors: Novel Piperazinyl Alkyl-bis(4’-fluorophenyl)amine Analogues as Potential Cocaine-Abuse Therapeutic Agents. J. Med. Chem. 2003;46:2589–2598. doi: 10.1021/jm030008u. [DOI] [PubMed] [Google Scholar]
- 37.Husbands SM, Izenwasser S, Kopajtic T, Bowen WD, Vilner BJ, Katz JL, Newman AH. Structure–Activity Relationships at the Monoamine Transporters and σ Receptors for a Novel Series of 9-[3-(cis-3,5-Dimethyl-1-piperazinyl)-propyl]carbazole (Rimcazole) Analogues. J. Med. Chem. 1999;42:4446–4455. doi: 10.1021/jm9902943. [DOI] [PubMed] [Google Scholar]
- 38.Husbands SM, Izenwasser S, Loeloff RJ, Katz JL, Bowen WD, Vilner BJ, Newman AH. Isothiocyanate Derivatives of 9-[3-(cis-3,5-Dimethyl-1-piperazinyl)propyl]-carbazole (Rimcazole): Irreversible Ligands for the Dopamine Transporter. J. Med. Chem. 1997;40:4340–4346. doi: 10.1021/jm9705519. [DOI] [PubMed] [Google Scholar]
- 39.Hiranita T, Soto PL, Kohut SJ, Kopajtic T, Cao J, Newman AH, Tanda G, Katz JL. Decreases in Cocaine Self-Administration with Dual Inhibition of the Dopamine Transporter and σ Receptors. J. Pharmacol. Exp. Ther. 2011;339:662–677. doi: 10.1124/jpet.111.185025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katz JL, Hiranita T, Kopajtic TA, Rice KC, Mesangeau C, Narayanan S, Abdelazeem AH, McCurdy CR. Blockade of Cocaine or σ Receptor Agonist Self Administration by Subtype-Selective σ Receptor Antagonists. J. Pharmacol. Exp. Ther. 2016;358:109–124. doi: 10.1124/jpet.116.232728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katz JL, Hong WC, Hiranita T, Su T-P. A Role for Sigma Receptors in Stimulant Self-Administration and Addiction. Behav. Pharmacol. 2016;27:100–115. doi: 10.1097/FBP.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothman RB, Baumann MH, Prisinzano TE, Newman AH. Dopamine Transport Inhibitors Based on GBR12909 and Benztropine as Potential Medications to Treat Cocaine Addiction. Biochem. Pharmacol. 2008;75:2–16. doi: 10.1016/j.bcp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loland CJ, Desai RI, Zou M-F, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. Relationship between Conformational Changes in the Dopamine Transporter and Cocaine-Like Subjective Effects of Uptake Inhibitors. Mol. Pharmacol. 2008;73:813–823. doi: 10.1124/mol.107.039800. [DOI] [PubMed] [Google Scholar]
- 44.Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, Loland CJ. The Binding Sites for Cocaine and Dopamine in the Dopamine Transporter Overlap. Nat. Neurosci. 2008;11:780–789. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang KH, Penmatsa A, Gouaux E. Neurotransmitter and psychostimulant Recognition by the Dopamine Transporter. Nature. 2015;521:322–327. doi: 10.1038/nature14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bisgaard H, Larsen MAB, Mazier S, Beuming T, Newman AH, Weinstein H, Shi L, Loland CJ, Gether U. The Binding Sites for Benztropines and Dopamine in the Dopamine Transporter Overlap. Neuropharmacology. 2011;60:182–190. doi: 10.1016/j.neuropharm.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rais R, Thomas AG, Wozniak K, Wu Y, Jaaro-Peled H, Sawa A, Strick CA, Engle SJ, Brandon NJ, Rojas C, Slusher BS, Tsukamoto T. Pharmacokinetics of Oral D-Serine in D-Amino Acid Oxidase Knockout Mice. Drug Metab. Dispos. 2012;40:2067–2073. doi: 10.1124/dmd.112.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. Evidence for the Involvement of Dopamine Transporters in Behavioral Stimulant Effects of Modafinil. J. Pharmacol. Exp. Ther. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newman AH, Allen AC, Izenwasser S, Katz JL. Novel 3-α-(Diphenylmethoxy)tropane Analogs: Potent Dopamine Uptake Inhibitors without Cocaine-like Behavioral Profiles. J. Med. Chem. 1994;37:2258–2261. doi: 10.1021/jm00041a002. [DOI] [PubMed] [Google Scholar]
- 50.Katz JL, Izenwasser S, Kline RH, Allen AC, Newman AH. Novel 3α-Diphenylmethoxytropane Analogs: Selective Dopamine Uptake Inhibitors with Behavioral Effects Distinct from Those of Cocaine. J. Pharmacol. Exp. Ther. 1999;288:302–315. [PubMed] [Google Scholar]
- 51.Desai RI, Kopajtic TA, French D, Newman AH, Katz JL. Relationship between In Vivo Occupancy at the Dopamine Transporter and Behavioral Effects of Cocaine, GBR 12909 [1-{2-[Bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine], and Benztropine Analogs. J. Pharmacol. Exp. Ther. 2005;315:397–404. doi: 10.1124/jpet.105.091231. [DOI] [PubMed] [Google Scholar]
- 52.Hiranita T, Wilkinson DS, Hong WC, Zou M-F, Kopajtic TA, Soto PL, Lupica CR, Newman AH, Katz JL. 2-Isoxazol-3-phenyltropane Derivatives of Cocaine: Molecular and Atypical System Effects at the Dopamine Transporter. J. Pharmacol. Exp. Ther. 2014;349:297–309. doi: 10.1124/jpet.113.212738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong WC, Kopajtic TA, Xu L, Lomenzo SA, Jean B, Madura JD, Surratt CK, Trudell ML, Katz JL. 2-Substituted 3β-Aryltropane Cocaine Analogs Produce Atypical Effects without Inducing Inward-Facing Dopamine Transporter Conformations. J. Pharmacol. Exp. Ther. 2016;356:624–634. doi: 10.1124/jpet.115.230722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheffel U, Boja JW, Kuhar MJ. Cocaine receptors: in vivo labeling with 3H-(−)cocaine, 3H-WIN 35,065-2, and 3H-WIN 35,428. Synapse. 1989;4:390–392. doi: 10.1002/syn.890040415. [DOI] [PubMed] [Google Scholar]
- 55.Cheng Y-C, Prusoff WH. Relationship between the Inhibition Constant (Ki) and the Concentration of Inhibitor which causes 50 Percent Inhibition (I50) of an Enzymatic Reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 56.Hiranita T, Soto PL, Tanda G, Kopajtic TA, Katz JL. Stimulants as Specific Inducers of Dopamine-Independent σ Agonist Self-Administration in Rats. J. Pharmacol. Exp. Ther. 2013;347:20–29. doi: 10.1124/jpet.113.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen J, Levant B, Jiang C, Keck TM, Newman AH, Wang S. Tranylcypromine Substituted cis-Hydroxycyclobutylnaphthamides as Potent and Selective Dopamine D3 Receptor Antagonists. J. Med. Chem. 2014;57:4962–4968. doi: 10.1021/jm401798r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urizar E, Yano H, Kolster R, Galés C, Lambert N, Javitch JA. CODA-RET Reveals Functional Selectivity as a Result of GPCR Heteromerization. Nat. Chem. Biol. 2011;7:624–630. doi: 10.1038/nchembio.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pedersen AV, Andreassen TF, Loland CJ. A Conserved Salt Bridge between Transmembrane Segments 1 and 10 Constitutes an Extracellular Gate in the Dopamine Transporter. J. Biol. Chem. 2014;289:35003–35014. doi: 10.1074/jbc.M114.586982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.