Abstract
The incidence of Clostridium difficile infection (CDI) in Europe has increased markedly since 2000. Previous meta-analyses have suggested a strong association between cephalosporin use and CDI, and many national programmes on CDI control have focused on reducing cephalosporin usage. Despite reductions in cephalosporin use, however, rates of CDI have continued to rise. This review examines the potential association of CDI with cephalosporins, and considers other factors that influence CDI risk. EUCLID (the EUropean, multicentre, prospective biannual point prevalence study of CLostridium difficile Infection in hospitalized patients with Diarrhoea) reported an increase in the annual incidence of CDI from 6.6 to 7.3 cases per 10 000 patient bed-days from 2011–12 to 2012–13, respectively. While CDI incidence and cephalosporin usage varied widely across countries studied, there was no clear association between overall cephalosporin prescribing (or the use of any particular cephalosporin) and CDI incidence. Moreover, variations in the pharmacokinetic and pharmacodynamic properties of cephalosporins of the same generation make categorization by generation insufficient for predicting impact on gut microbiota. A multitude of additional factors can affect the risk of CDI. Antibiotic choice is an important consideration; however, CDI risk is associated with a range of antibiotic classes. Prescription of multiple antibiotics and a long duration of treatment are key risk factors for CDI, and risk also differs across patient populations. We propose that all of these are factors that should be taken into account when selecting an antibiotic, rather than focusing on the exclusion of individual drug classes.
Introduction
The incidence of Clostridium difficile infection (CDI) has been increasing markedly across Europe, North America and Asia since 2000.1 Over 14 000 cases of CDI were reported across all National Health Service (NHS) hospitals in England between April 2014 and March 2015, with a CDI rate of 4.1 per 10 000 patient bed-days, an increase of 6% from the previous financial year.2 Between 2001 and 2011, the rate of C. difficile hospitalizations in the USA increased nearly 3-fold, from 5.6 per 1000 discharges in 2001 to 12.7 per 1000 discharges in 2011.3 According to data from a surveillance study conducted by the US CDC, the estimated number of incident cases of CDI in the USA in 2011 was 453 000, approximately two-thirds of which were healthcare-associated infections.4 Increases in CDI have also been observed outside the healthcare setting, with the proportion of CDI attributed to community-associated infections ranging from 10% to 42%.5 The primary symptom of CDI is diarrhoea, although many patients will also have clinical features of colitis, including abdominal cramps, fever and leucocytosis.6 CDI can vary in severity from mild diarrhoea to pseudomembranous colitis. Country-specific, 30 day mortality estimates range from 2.8% to 29.8%.7 In a prospective, multicentre study in 6522 patients from the UK, 30 day crude mortality (during a non-endemic period) was 16.6%, about half of which was directly attributable to CDI.8
Preventing C. difficile transmission in hospitals and community settings is clearly a key priority in the prevention of CDI; however, it is equally important that we achieve a better understanding of the factors influencing the risk of developing CDI, including host factors and antibiotic prescribing behaviour.9 CDI characteristically occurs in elderly patients with comorbidities in whom the intestinal microbiota is disrupted due to antibiotic exposure.1
Three recent meta-analyses have evaluated the association between antibiotic use and CDI.10–12 They reported that cephalosporins and clindamycin were most strongly associated with hospital-associated CDI,10 while for community-associated infection, the strongest association was seen with clindamycin, cephalosporins and quinolones.11,12 These analyses may, however, be subject to several potential sources of confounding and bias from the included studies, and so reported associations between CDI and specific antibiotics should be interpreted with caution.13 Possible confounding factors that could affect the analyses include the presence of comorbidities, polypharmacy, dose and duration of antibiotic treatment, and the use of multiple antibiotics.13 Additional potential sources of bias include sampling bias (meaning that commonly prescribed antibiotics will be more often reported as being associated with cases), selection of inappropriate controls and misclassification of C. difficile. In addition, studies may be open to clinical susceptibility bias, whereby patients with illnesses requiring antibiotics may have inherent increased risks of developing CDI, and cases may therefore be falsely attributed solely to the clinically indicated use of antibiotics.13 Furthermore, there were between-study differences in patient populations which, importantly, may have included different levels of exposure to C. difficile.13 Notably, most of the data on CDI have been collected from observational studies in the context of outbreaks,14 and therefore may not reflect the risk of CDI in the non-epidemic setting. Finally, the assumption that all antibiotics within a given class are equally associated with CDI risk is not well founded. Notably, differences in pharmacokinetics among cephalosporins, particularly the route of excretion, can mean that exposures of the gut microbiome and C. difficile vary markedly.
Antibiotic stewardship programmes have been established in an attempt to optimize and sustain the utility of antibiotics; this includes reducing the rates of resistance and hospital-associated CDI. Some policies are focused on the restriction of cephalosporin prescribing.15 For example, in 2008, the UK Department of Health and Public Health England recommended that NHS hospitals should develop restrictive antibiotic guidelines specifying the use of narrow-spectrum agents alone or as combination therapy.16 The guidelines specifically highlighted that the use of clindamycin and second- and third-generation cephalosporins should be avoided, especially in the elderly; reduced use of fluoroquinolones and carbapenems was also advocated.16
As data accumulate linking other broad-spectrum antibiotics to CDI, we consider it timely to reassess the evidence for the potential association of CDI with cephalosporins in Europe, to explore whether cephalosporins still have a role in the era of CDI.
Pattern of cephalosporin use and incidence of CDI across Europe
EUCLID (the EUropean, multicentre, prospective biannual point prevalence study of CLostridium difficile Infection in hospitalized patients with Diarrhoea) is the largest and most comprehensive study of CDI epidemiology ever performed in Europe.17 The study involved a total of 482 hospitals in 20 European countries. Hospitals provided details on local policies for CDI testing and reporting, and the laboratory methods used for CDI diagnosis, together with local testing rates and CDI rates.17 Data were collected from participating hospitals for the periods September 2011–August 2012 and September 2012–August 2013. In addition, on two sampling days (one day in winter 2012–13 and one day in summer 2013), hospitals sent all diarrhoeal samples submitted to their microbiology laboratory for standardized CDI testing at national coordinating laboratories.17 The results obtained by optimized testing were compared with local data.
EUCLID documented an increase in the reported annual incidence of CDI from 6.6 cases per 10 000 patient bed-days in 2011–12 to 7.3 cases per 10 000 patient bed-days in 2012–13.17 Furthermore, analysis of data from the two sampling days revealed that 23% of CDI cases were missed owing to lack of clinical suspicion [i.e. samples that were not originally tested by the participating hospital tested positive for CDI (defined as testing positive for both glutamate dehydrogenase and C. difficile toxin) at the national coordinating laboratory]. Overall, and taking into account false negatives from local hospitals, each hospital missed an average of 82 cases per year. Across the 482 participating hospitals, there could be as many as 40 000 inpatients per year not diagnosed with CDI as a result of suboptimal testing or lack of clinical suspicion.17
Cephalosporin use and incidence of CDI in individual European countries
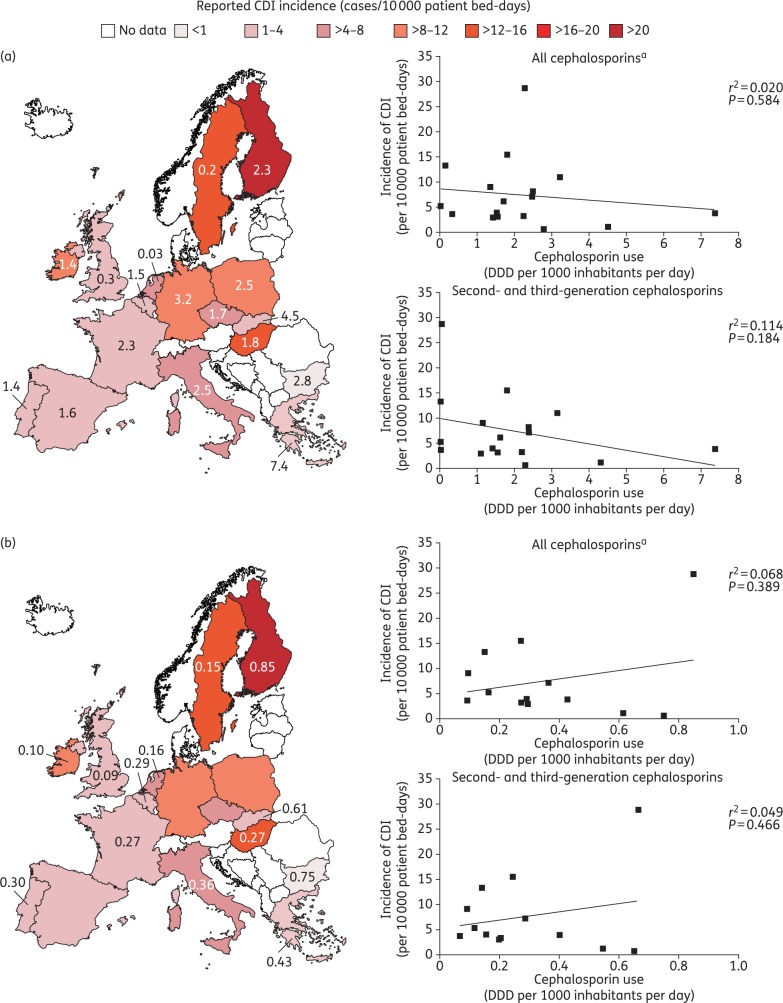
Data on the reported incidence of CDI by country across Europe for 2012–13 are presented in Figure 1. CDI incidence (given in cases per 10 000 patient bed-days) varied widely across Europe, ranging from <1 in Bulgaria to >20 in Finland.17 When the EUCLID CDI rates are assessed in relation to data for overall cephalosporin usage across Europe (in both hospital and community settings), there is no clear association between cephalosporin prescribing and incidence of CDI (Figure 1). Antibiotic surveillance data from the ECDC show that although the use of any cephalosporin in the community setting varied widely across countries, from a defined daily dose (DDD) per 1000 inhabitants per day of 0.03 in the Netherlands to 7.4 in Greece,18 there is no apparent association with CDI incidence [r2 = 0.020 (P = 0.584); Figure 1a]; in fact, there is a weak inverse relationship, i.e. CDI incidence decreases as cephalosporin use increases. For example, cephalosporin usage in Sweden was among the lowest in Europe (0.2 DDD per 1000 inhabitants per day),18 while the reported CDI incidence was among the highest (13.3 cases/10 000 patient bed-days).17 In addition, considerable variation in cephalosporin usage was observed across countries with similar reported CDI incidence, such as the UK and France (0.3 and 2.3 DDD per 1000 inhabitants per day, respectively). Confining the analysis to second- and third-generation cephalosporins (the use of which should be restricted, according to UK guidelines16) produces similar results, with no apparent association observed between cephalosporin use and CDI incidence [r2 = 0.114 (P = 0.184); Figure 1a]. Similarly, there is no significant correlation between cephalosporin use and CDI incidence in the hospital setting [r2 = 0.068 (P = 0.389); Figure 1b]; apart from one country, as seen for community data (Figure 1a), there is a weak inverse relationship between CDI incidence and cephalosporin prescribing. For example, cephalosporin usage in the hospital setting in Bulgaria was among the highest in Europe (0.75 DDD per 1000 inhabitants per day), while reported CDI incidence was among the lowest (0.7 cases/10 000 patient bed-days).17,18 The lack of correlation between cephalosporin use and CDI incidence is also apparent when the analysis is confined to second- and third-generation cephalosporins [r2 = 0.049 (P = 0.466); Figure 1b]. Under-testing/reporting and variations in the reporting systems used in the different European countries will clearly affect country-specific CDI rates, while the methods employed to capture antibiotic use may also vary between countries. Despite the limitations inherent in this type of analysis, however, it seems unlikely that ‘corrected’ incidence data would reveal a correlation with cephalosporin prescribing, given the existent data show a lack of correlation.
Figure 1.
Incidence of CDI and overall cephalosporin use in (a) the community and (b) hospital settings during 2012–13. The text overlay reports usage of first-, second-, third- and fourth-generation cephalosporins in EU/EEA countries in 2013, expressed as DDD per 1000 inhabitants and per day, if available. Community/hospital usage of second- and third-generation cephalosporins (as a percentage of first-, second-, third- and fourth-generation usage) is: Belgium, 92.8/53.6; Bulgaria, 82.1/87.0; Czech Republic, 94.5/NA; Finland, 2.6/77.9; France, 97.7/75.0; Germany, 97.8/NA; Greece, 100/94.1; Hungary, 99.4/90.1; Ireland, 85.3/95.8; Italy, 96.4/78.8; Netherlands, 100/71.3; Poland, 95.2/NA; Portugal, 77.6/67.4; Slovakia, 95.6/89.2; Spain, 99.4/NA; Sweden, 18.8/94.0; UK, 11.8/73.1. Data are from the ECDC.18 Regression analyses are based on least-squares means. CDI incidence data for 2012–13 are from Davies et al.17 aIncludes data for first-, second-, third- and fourth-generation cephalosporins.
Use of different cephalosporins and incidence of CDI in Europe
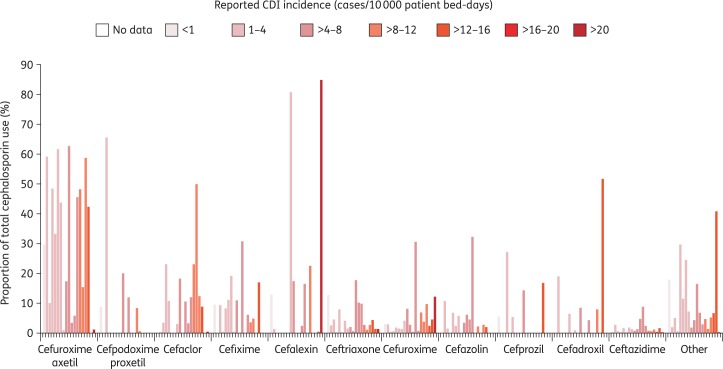
Table 1 shows the usage of specific cephalosporin antibiotics in different European countries. These data also revealed no clear associations between the reported CDI incidence from EUCLID17 and the use of any particular cephalosporin (Figure 2). There were considerable variations in the use of particular drugs (as a proportion of total cephalosporin use) across countries with similar CDI incidence. For example, the use of cefuroxime axetil differed markedly in France and Belgium (10.0% and 59.2%, respectively, of cephalosporin prescriptions), although reported rates for CDI were in the range of 1–4 cases/10 000 patient bed-days in the two countries. Similarly, ceftriaxone use differed in Italy (17.7% of cephalosporin prescriptions) and Austria (2.0%), although CDI incidence was similar (4–8 cases/10 000 patient bed-days). Furthermore, similar levels of use for some cephalosporins were seen in countries with differing CDI incidence. For example, cefuroxime axetil accounted for 42%–46% of cephalosporin prescriptions in Spain, Romania and Hungary, but CDI incidence differed across these countries (1–4, 4–8 and 12–16 cases/10 000 patient bed-days, respectively). Similarly, use of ceftriaxone was similar in Romania (9.9%) and Bulgaria (12.7%), although CDI incidence differed (4–8 and <1 cases/10 000 patient bed-days, respectively). In Slovakia and the Czech Republic, the overall profiles of cephalosporin use were similar, despite the differing incidence of CDI (1–4 and 4–8 cases/10 000 patient bed-days, respectively).17
Table 1.
Cephalosporins most commonly used across Europe in the year ending August 2013
| Country | CDI incidence, cases/10 000 patient bed-days | Total cephalosporin use, SUs, 1000s | Cephalosporin use, SUs, % (1000s) of total cephalosporin use in that country |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cefuroxime axetil | cefpodoxime proxetil | cefaclor | cefixime | cefalexin | ceftriaxone | cefuroxime | cefazolin | cefprozil | cefadroxil | ceftazidime | other | |||
| Austria | 4.1 | 13 059 | 17.4 (2271) | 20.0 (2609) | 18.3 (2386) | 10.9 (1422) | 17.4 (2279) | 2.0 (265) | 8.1 (1058) | 3.4 (444) | — | — | 0.7 (88) | 1.8 (237) |
| Belgium | 4.0 | 10 996 | 59.2 (6507) | — | <0.05 (0.2) | — | 1.2 (129) | 2.5 (275) | 2.8 (306) | 10.7 (1176) | — | 19.0 (2084) | 2.7 (302) | 2.0 (216) |
| Bulgaria | 0.7 | 16 702 | 29.7 (4958) | 8.8 (1465) | — | 9.5 (1582) | 12.8 (2145) | 12.7 (2116) | 3.0 (502) | — | 5.6 (929) | — | 0.2 (32) | 17.8 (2972) |
| Czech Rep. | 6.2 | 11 048 | 62.7 (6928) | — | — | — | — | 0.6 (64) | 2.6 (287) | 6.0 (668) | 14.3 (1580) | 8.4 (923) | 1.2 (131) | 4.2 (467) |
| Finland | 28.7 | 19 486 | 1.1 (211) | — | 0.3 (59) | — | 84.9 (16 540) | 1.3 (255) | 12.2 (2381) | — | — | — | 0.2 (40) | <0.05 (0.6) |
| France | 3.3 | 236 373 | 10.0 (23 708) | 65.6 (155 043) | 3.3 (7878) | 9.2 (21 721) | — | 4.4 (10 341) | 0.4 (1043) | 1.4 (3310) | — | — | 0.6 (1432) | 5.0 (11 897) |
| Germany | 11.0 | 204 103 | 48.2 (98 358) | 8.4 (17 056) | 23.0 (46 928) | 3.6 (7328) | — | 2.7 (5531) | 6.8 (13 796) | 2.1 (4208) | — | — | 0.7 (1399) | 4.7 (9500) |
| Greece | 3.9 | 44 798 | 48.4 (21 680) | — | 23.0 (10 300) | 0.4 (199) | — | <0.05 (20) | 0.5 (221) | — | 27.1 (12 162) | — | <0.05 (12) | 0.5 (204) |
| Hungary | 15.5 | 14 361 | 42.3 (6072) | — | 8.7 (1250) | 16.9 (2420) | — | 4.3 (615) | 2.4 (345) | 1.9 (269) | 16.8 (2411) | — | 0.3 (37) | 6.6 (941) |
| Ireland | 9.1 | 8492 | 15.4 (1306) | 0.6 (51) | 49.9 (4240) | 4.9 (414) | 22.6 (1917) | 1.0 (83) | 3.7 (314) | 0.05 (4) | — | — | 0.6 (51) | 1.3 (113) |
| Italy | 7.2 | 127 431 | 3.4 (4285) | 11.9 (15 123) | 10.6 (13 507) | 30.7 (39 125) | — | 17.7 (22 527) | 0.3 (418) | 4.5 (5787) | — | — | 4.6 (5822) | 16.4 (20 850) |
| Netherlands | 5.3 | 2913 | 5.9 (173) | — | 3.2 (94) | — | 2.3 (67) | 10.2 (296) | 30.6 (892) | 32.2 (938) | — | — | 8.8 (257) | 6.8 (197) |
| Poland | 8.2 | 74 377 | 58.8 (43 701) | — | 12.3 (9132) | — | — | 2.6 (1923) | 9.7 (7250) | 2.6 (1952) | — | 7.8 (5770) | 1.1 (787) | 5.2 (3901) |
| Portugal | 3.0 | 11 544 | 33.2 (3837) | — | 10.8 (1246) | 8.2 (952) | — | 7.9 (911) | 1.8 (208) | 6.8 (782) | — | — | 1.6 (180) | 29.7 (3427) |
| Romania | 7.4 | 69 306 | 45.6 (31 592) | — | 11.9 (8227) | 6.0 (4181) | 16.4 (11 376) | 9.9 (6847) | 0.7 (456) | — | — | 4.3 (2997) | 2.3 (1616) | 2.9 (2014) |
| Slovakia | 1.2 | 13 901 | 61.7 (8575) | — | — | 11.0 (1523) | — | 0.2 (24) | 1.5 (202) | 2.3 (315) | 5.4 (746) | 6.5 (904) | 0.1 (14) | 11.5 (1598) |
| Spain | 3.2 | 58 333 | 43.8 (25 568) | — | — | 19.1 (11 130) | — | 4.1 (2403) | 1.2 (676) | 5.7 (3299) | — | — | 1.7 (966) | 24.5 (14 293) |
| Sweden | 13.3 | 3210 | 0 (0) | — | — | — | 0.4 (14) | 1.2 (37) | 4.5 (144) | — | — | 51.6 (1657) | 1.6 (52) | 40.7 (1306) |
| UK | 3.7 | 52 992 | 0.9 (458) | — | 3.1 (1664) | 0.4 (232) | 80.8 (42 842) | 1.5 (802) | 4.1 (2191) | — | — | 0.8 (415) | 1.2 (618) | 7.1 (3769) |
SU, standard unit.
CDI incidence data for September 2012 to August 2013 from Davies et al.17 Prescription data from IMS Health.
Figure 2.
Use of the most common cephalosporins across Europe, as a proportion of total cephalosporin use in each country, in the year ending August 2013. Countries (from left to right for each agent, arranged by CDI incidence): <1: Bulgaria; 1–4: Belgium, France, Greece, Portugal, Slovakia, Spain, UK; >4–8: Austria, Czech Republic, Italy, Netherlands, Romania; >8–12: Germany, Ireland, Poland; >12–16: Hungary, Sweden; >20: Finland. CDI incidence data from September 2012 to August 2013 from Davies et al.17 Prescription data from IMS Health.
Although confounding factors, such as the use of other antibiotics, could affect CDI incidence, the data do not suggest a close association between increased use of oral cephalosporins and CDI incidence. Oral agents comprised approximately 80%–90% of total cephalosporin use in more than half of the countries studied, and CDI incidence ranged from 1–4 to >20 cases per 10 000 patient bed-days in these countries. In countries where oral cephalosporin use was less widespread (52%–66% overall), CDI incidence also varied markedly (from <1 to 12–16 cases per 10 000 patient bed-days). Thus, these data suggest that determining the association between CDI risk and antibiotic usage is more complicated than simply correlating the risk with the type of drug, highlighting the need for more detailed analysis.
Principles underlying CDI risk
The risk of CDI is not uniform across all patient populations, but is dependent on a number of issues, notably age, comorbidities and exposure to C. difficile. If C. difficile is not epidemic or has low endemicity, then the risk of CDI is likely to be lower than in settings in which bacterial transmission is high. Acquisition of C. difficile is associated primarily with healthcare facilities, although community-acquired severe disease has been reported among individuals previously thought to be at low risk of infection.5 A study using whole-genome sequencing has shown that, in an endemic CDI setting, the majority of CDI cases are not closely linked to previous cases.19 Furthermore, the rate of appearance of new distinct C. difficile strains in the study population was constant over a 3 year period, suggesting a close interplay between strains found in the community and those found in hospitals.
Previous antimicrobial use is considered a key risk factor for CDI among hospitalized patients20 and those in the community.21 A systematic review showed that the incidence of CDI was associated with the use of clindamycin, cephalosporins and penicillins, and with the number of antibiotics a patient received, although the authors expressed concerns about weaknesses with most of the reported studies.20 A study of community-associated CDI showed that exposure to antibiotic therapy in the previous 4 weeks, particularly multiple agents and oral cephalosporins, was associated with a significantly increased risk of CDI, as was hospitalization in the previous 6 months.21 However, approximately half the cases had not received antibiotic therapy in the month before C. difficile detection, and approximately one-third had neither exposure to antibiotics nor recent hospitalization. These data have been corroborated in the Netherlands.22
Gut microbiota provides an important host defence against C. difficile by inhibiting its establishment or proliferation.23 Studies in patients with CDI have reported that CDI is associated with significant changes in the composition of faecal microbiota, including, in some cases, the depletion of Gram-negative Bacteroides spp., and reductions in normally abundant butyrate-producing anaerobic bacteria in the Ruminococcaceae and Lachnospiraceae families (part of the Clostridia class), suggesting that they may also be involved in the defence against infection.24 Disruption of gut microbiota during antimicrobial use helps to create conditions favourable for C. difficile expansion.25,26 Long or repeated courses of antimicrobial therapy and the use of multiple antimicrobials can increase the risk of CDI.27 Some broad-spectrum antimicrobials have been implicated in CDI owing to their wide-ranging effects on the microbiota. Importantly, the impact of an antimicrobial on gut microbiota will depend on the drug's pharmacokinetic distribution and the concentration achieved in the gut, as well as its antimicrobial activity.28
Are all cephalosporins the same with regard to CDI risk?
Categorization of cephalosporins according to ‘generation’ is insufficient for predicting impact on gut microbiota. Differences in both pharmacokinetics (Table 2) and pharmacodynamic properties (Table 3) are apparent between different cephalosporins of the same generation, as well as different generations. For the majority of cephalosporins, excretion occurs mainly via the kidney. Most are excreted by glomerular filtration and this is particularly pronounced for agents such as cefadroxil, cefalexin, cefuroxime, ceftazidime and ceftobiprole. Biliary excretion is the main alternative route (Tables 2 and 3).29 In general, orally administered cephalosporins are absorbed rapidly. Cefalexin, cefadroxil, cefradine and cefaclor show almost complete absorption, whereas absorption of cefixime and cefuroxime axetil is in the region of 40%–50%.30 These agents are acid stable,28 and they achieve therapeutic concentrations in most tissues, including the gut.30 Therefore, it is reasonable to assume that these active compounds in the gut may well influence the gut microbiota and so affect the risk of CDI. Following parenteral administration, cephalosporins are distributed to the tissues, including bone and fluids, including the pleural, synovial and cerebrospinal fluids.30 Many cephalosporins are excreted in the bile, and although concentrations tend to be relatively low (indicating that gut exposure will be less than that achieved with orally administered cephalosporins), therapeutic concentrations of the drug are generally achieved.30 For a few agents, such as cefoperazone and ceftriaxone, elimination occurs primarily or substantially via the biliary system, and so gut exposure is likely to be higher than with other parenteral cephalosporins (Table 2).29,30 Indeed, bile concentrations of ceftriaxone reported in two studies31,32 were substantially higher than those seen with other cephalosporins in other studies (Table 2).
Table 2.
Summary of pharmacokinetic parameters for cephalosporins commonly used in Europe
| Agent | Serum t½, h | Protein binding, % | Urinary excretion, % | Biliary excretion, % | Bile concentration, mean ± SD (range), mg/L | Dose (number of doses)a | Administration | Citation |
|---|---|---|---|---|---|---|---|---|
| Cefadroxil | 1.3–1.6 | 20 | 90 | 2 | 9.9b | 1000 mg | oral | Karachalios and Charalabopoulos 200229 |
| Cefalexin | 0.8–1.0 | 10 | 90 | 0.5 | (14.4–92)c [G] | 500 mg every 6 h (×5) | oral | Sales et al. 197267 |
| Cefazolin | 1.8 | 80 | 65 | 0.2 | 17.1 ± 8.5d | 500 mg | iv | Brogard et al. 197568 |
| 14.0 ± 4.7 [T] | 500 mg | iv | Brogard et al. 197568 | |||||
| (0.85–21) [T] | 1000 mg | iv | Nishida et al. 197669 | |||||
| 46 [T] | 1000 mg | iv | Ratzan et al. 197870 | |||||
| 32.8 [G] | 500 mg | im | Ram et al. 197371 | |||||
| 92.1 [G] | 500 mg every 6 h (×4) | im | Ram et al. 197371 | |||||
| Cefaclor | 0.6 | 25 | 50–60 | 0.05 | 7.6 ± 2.4 [T] | 1000 mg | oral | Brogard et al. 198272 |
| Cefprozil | 1.45 | 40 | 76 | — | — | — | — | — |
| Cefuroxime axetil | 1.3 | 33–50 | 90 | — | — | — | — | — |
| Cefuroxime | 1.3 | 35 | 95 | 0.5 | 10.3 ± 2.4 [T] | 500 mg | iv | Brogard et al. 198173 |
| 5.4 [G]/42.8 [BD] | 1500 mg | iv | Thomas et al. 198174 | |||||
| 4.8 [G]/9.0 [BD] | 750 mg | iv | Severn and Powis 197975 | |||||
| Cefpodoxime proxetil | 2.0–3.6 | 20 | 80 | — | no data available | — | — | — |
| Cefixime | 3.0–4.0 | 65 | 50 | 10 | 56.9 ± 70.9 [T] | 200 mg | oral | Westphal et al. 199376 |
| 199.3 (8.8–1163.8) | 200 mg twice daily (×4) | oral | Moorthi et al. 199077 | |||||
| Ceftriaxone | 8.5 | 83–96 | 65 | 30–40 | 1078 ± 158 [T]e | 2 g every 12 h (×5) | iv | Brogard et al. 198831 |
| 4730 (2970–5880) [G] | 2 g every 12 h (×5)f | iv | Hayton et al. 198632 | |||||
| Ceftazidime | 1.8 | 17 | 80–90 | 3 | 21.2 ± 9.2d,e | 2000 mg | iv | Brogard et al. 198778 |
| 36.3 ± 4.0 [T]e | 2000 mg | iv | Brogard et al. 198778 | |||||
| 34.1 ± 24.8 [T]g | 2000 mg | iv | Bouza et al. 198379 | |||||
| 46.7 [T] | 1000 mg | iv | Tanimura et al. 198380 | |||||
| 3.9 ± 1.1 [G]/31.8 ± 3.7 [BD]e | 1000 mg | iv | Shirmatsu et al. 198881 | |||||
| 18.5 [G]/26.6 [BD]c | 1000 mg | iv | Walstad et al. 198682 | |||||
| Ceftobiprole | 3–4 | 16 | 80–90 | — | — | — | — | — |
| Ceftaroline | 2.5 | 20 | 88 | — | — | — | — | — |
BD, concentration in common bile duct; G, concentration in gall bladder bile; im, intramuscular; iv, intravenous; T, concentration in bile obtained from a T-tube or drain tube; t½, terminal elimination half-life.
Bile concentration data are shown for patients either undergoing or following cholecystectomy or with cholecystolithiasis, unless otherwise indicated. Concentrations in bile obtained from a T-tube or drain tube (indicated by T) are peak concentrations unless otherwise stated. Bile concentration data and associated population/dosing information are from the references indicated; data for the other parameters are from Marshall and Blair 199983 except for ceftobiprole (Murthy et al. 200884) and ceftaroline (Summary of Product Characteristics85).
aSingle dose, unless otherwise indicated.
bAt 6–8 h after dosing.
cPatients with functioning gall bladder.
dPeak concentration in normal individuals, obtained by duodenal tubing.
eMean ± SEM.
fTwo (of seven) patients received only three doses.
gAt 1 h after infusion.
Table 3.
Pharmacodynamic properties of cephalosporins commonly used in Europe
| Generation | Name | Route of administration | Route of elimination | Distribution | Effect on intestinal microbiota | Faecal concentration |
|---|---|---|---|---|---|---|
| First | cefadroxil/cefadroxyl (Duricef®) | oral | almost completely absorbed from the Gl tract, and not metabolized | largest concentrations observed in the duodenum, with lower levels in the stomach and jejunum, and very low levels in the ileum and colon when sampled 20 min after oral dosing in mice86 | administration to 20 healthy individuals did not cause measurable disturbance to the colonic ecology, when evaluating the effect of cefadroxil 500 mg taken for 10 days. | not detected (<0.125 mg/L) following administration (500 mg twice daily for 10 days) in healthy volunteers36 |
| excreted unchanged in the urine by renal glomerular filtration, active tubular secretion and active tubular reabsorption87 | cefadroxil is present in the gallbladder and bile duct, as well as at a high concentration in bile29 | effect on the intestinal microbiota was minor and the microbiota was normal 2 weeks after withdrawal of the drug36 | ||||
| First | cefalexin/cefalexin (Keflex®) | oral | almost completely absorbed from the GI tract and not metabolized | absorbed in the upper intestine88 | CDAD has been reported with use of nearly all antibacterial agents, including cefalexin (SPC) | — |
| excreted in the urine unchanged by renal glomerular filtration and active tubular secretion (SPC) | cefalexin excreted in bile accounts for 0.29% of the administered dose29 | 7/12 patients with urinary tract infection treated with oral cefalexin became faecal carriers of Pseudomonas aeruginosa; this acquisition rate was significantly higher than in patients who received no antibiotics89 | ||||
| First | cefazolin | parenteral | not metabolized | biliary excretion is low and amounted to 0.03% of the administered dose; the concentration is about the same or slightly in excess of the simultaneous serum level, provided that the biliary tract is not obstructed29 | in patients undergoing a gastrectomy, prophylactic cefazolin caused a significant decrease in the numbers of Bifidobacterium, Lactobacillus and Eubacterium spp.; there was a significant suppression of streptococci and an increase in enterococci90 | — |
| excreted in a microbiologically active form in the urine, mainly by renal glomerular filtration (SPC) | ||||||
| Second | cefaclor (Ceclor®; Distaclor®; Keflor®; Raniclor®) | oral | almost completely absorbed from the GI tract and not metabolized | actively excreted in bile of dogs at a concentration more than sufficient to be effective against susceptible pathogens29 | aerobic intestinal microbiota was unchanged during and after cefaclor administration, while a minor impact on the anaerobic intestinal microbiota was observed; the anaerobic intestinal flora returned to its normal state within 1 week | not detected following administration (250 mg every 8 h for 7 days) in healthy volunteers35 |
| excreted in the urine unchanged by renal glomerular filtration, active tubular secretion and active tubular reabsorption (SPC) | no new colonization with cefaclor-resistant microorganisms was observed and no side effects were registered during the investigation period35 | |||||
| Second | cefprozil | oral | elimination is predominantly renal by glomerular filtration and tubular secretion; about 10% of the drug is eliminated by extrarenal mechanisms91 | cefprozil shows penetration into tonsillar and adenoidal tissue at concentrations equivalent to nearly 40% of those in the plasma at ∼3 h after oral dosing92 | there was a moderate decrease in Enterobacteriaceae and a slight increase in enterococci, staphylococci and bacteroides during cefprozil administration in healthy volunteers (500 mg twice daily for 8 days)91 | — |
| no metabolites were detected in the urine91 | penetration of cefprozil into blister (interstitial) fluid (simulating penetration into skin and soft tissues) was similar to cefaclor, although the time during which cefprozil concentration exceeded MIC90 was usually at least two times greater than cefaclor for common pathogens, including Streptococcus pneumoniae and Staphylococcus aureus92 | numbers of bacteria returned to normal 4 days after the last study day91 | ||||
| Second | cefuroxime axetil (Zinat®) | oral | absorbed from the GI tract and rapidly hydrolysed in the intestinal mucosa and blood to release cefuroxime into the circulation | concentrations of cefuroxime in excess of the MIC for common pathogens can be achieved in the tonsilla, sinus tissues, bronchial mucosa, bone, pleural fluid, joint fluid, synovial fluid, interstitial fluid, bile, sputum and aqueous humour (SPC) | in healthy volunteers, numbers of enterococci increased while the levels of Enterobacteriaceae remained stable during cefuroxime axetil administration (250 mg twice daily for 10 days); the numbers of clostridia were slightly decreased, whereas other anaerobes were unaffected34 | detected in the faeces of all 10 healthy volunteers following administration (250 mg twice daily for 10 days), but on only one or two sampling occasions in three individuals; on day 7, mean concentration was 0.57 mg/kg (range: <0.125–0.84), with detectable levels in nine individuals34 |
| not metabolized, and excreted via the kidneys by glomerular filtration and tubular secretion (SPC) | intestinal microbiota had returned to normal 2 weeks after stopping treatment34 | |||||
| cefuroxime axetil (250 mg twice daily for 10 days) significantly decreased staphylococci, Enterobacteriaceae and clostridia in patients suffering from acute exacerbation of chronic bronchitis93 | ||||||
| Second | cefuroxime (Zefu®; Zinacef®; Ceftin®; Biofuroksym®; Xorimax®) | parenteral | metabolically stable and eliminated primarily via the kidneys by glomerular filtration and tubular secretion94 | biliary levels are lower than simultaneous serum levels, but at levels that exceeded the MIC for many common gallbladder pathogens, including Escherichia coli and salmonellae29 | — | — |
| Third | cefpodoxime proxetil | oral | a prodrug that is absorbed from the GI tract and de-esterified to its active metabolite, cefpodoxime | body tissue and fluid distribution of cefpodoxime is extensive after administration of cefpodoxime proxetil95 | cefpodoxime proxetil administration (200 mg twice daily for 7 days) strongly reduced the numbers of streptococci, Enterobacteriaceae and clostridia in 10 healthy volunteers, while there was a marked increase in enterococci96 | not detected in faeces of seven healthy volunteers following oral administration (200 mg twice daily for 7 days), but high concentrations were found in three individuals on days 4, 7 and 9, when mean concentrations were 220, 430 and 140 mg/kg, respectively34 |
| approximately 50% of the administered dose is absorbed systemically | 2/10 individuals became colonized by high levels of staphylococci and yeasts during cefpodoxime proxetil administration, and five volunteers were colonized by Clostridium difficile after the end of administration96 | |||||
| undergoes minimal metabolism, and is eliminated primarily by renal excretion. Any unabsorbed drug is degraded in the GI tract and excreted in the faeces95 | 2 weeks after cefpodoxime withdrawal, intestinal microbiota had returned to normal (except for two subjects with C. difficile)96 | |||||
| Third | cefixime (Fixx®; Zifi®; Suprax®) | oral | almost completely absorbed from the GI tract, and not metabolized | after administration of cefixime, high antibiotic levels were achieved in bile and gallbladder tissue, even 13–17 h after the last application77 | there was a marked decrease in the numbers of streptococci and E. coli, and an increase in the numbers of enterococci during the administration of cefixime; in the anaerobic microbiota, the numbers of cocci, clostridia and bacteroides were suppressed, while there were minor changes in the numbers of bifidobacteria. C. difficile was isolated in five individuals on day 7, but cytotoxin was only detected in one person33 | concentrations in faeces increased during administration (200 mg twice daily for 7 days) in 10 healthy volunteers; one individual had detectable concentrations on day 2, three on day 4, and eight on day 7, which were in the range 237–912 mg/kg33 |
| excreted in the urine unchanged by renal glomerular filtration (SPC) | the intestinal microbiota was normalized within 2 weeks after treatment cessation33 | |||||
| Third | ceftriaxone (Rocephin®) | parenteral | eliminated mainly as unchanged drug, approximately 60% of the dose being excreted in the urine (almost exclusively by glomerular filtration) and the remainder via the biliary and intestinal tracts (SPC) | average fraction of a dose of ceftriaxone excreted in bile is estimated as 15%29 | had a profound effect on the faecal flora; none of the Gram-negative bacilli, only 24% of aerobic Gram-positive organisms and only 10% of anaerobes persisted during ceftriaxone administration97 | mean concentrations 152 mg/kg (range, 0–657) and 258 mg/kg (0–806) on days 4 and 8, respectively, following iv infusion (2000 mg once daily) for 7 days in healthy volunteers37 |
| Third | ceftazidime (Meezat®; Fortum®; Fortaz®) | parenteral | excreted unchanged in the urine by glomerular filtration (SPC) | concentrations in excess of the MIC for common pathogens can be achieved in tissues such as bone, heart, bile, sputum, aqueous humour, and synovial, pleural and peritoneal fluids (SPC) | in volunteers who received iv ceftazidime at a dose of 4000 mg for 1 day, Enterobacteriaceae and lactobacilli decreased considerably, while no effect on other microorganisms in the flora could be observed98 | — |
| biliary excretion accounts for <1% of non-renal excretion of ceftazidime in healthy individuals29 | ||||||
| Fifth | ceftobiprole (Zevtera®; Mabelio®) | parenteral | primarily excreted via the kidneys, resulting in relatively low levels of intestinal exposure and only minor disruption of intestinal anaerobes38,84 | binds minimally (16%) to plasma proteins, and binding is independent of the drug and protein concentrations84 | in comparison with other cephalosporins, ceftobiprole demonstrates relatively good activity against clostridia, including some strains of C. difficile41,99 | not detected following iv administration (500 mg every 8 h for 7 days) in healthy volunteers38 |
| undergoes minimal hepatic metabolism84 | in healthy volunteers, ceftobiprole had no significant ecological impact on the human intestinal microbiota38 | |||||
| ceftobiprole and ceftobiprole medocaril did not promote growth of or toxin production by C. difficile in mouse caecal contents, whereas ceftazidime, cefoxitin, ceftriaxone, cefotaxime and ertapenem did53 | ||||||
| this was attributable to inhibitory activity against C. difficile and sparing of anaerobic microbiota | ||||||
| Fifth | ceftaroline (Zinforo™) | parenteral | primarily eliminated by the kidneys | after 12 healthy subjects received 600 mg ceftaroline iv twice daily for 7 days, no measurable concentrations of drug were found in faeces on days 1, 2, 5, 7, 9, 14 or 2139 | there was a minor impact on the numbers of E. coli strains, while the numbers of enterococci and Candida albicans strains were not affected39 | not detected following iv administration (600 mg every 12 h for 7 days) in healthy volunteers39 |
| following a single 600 mg iv dose of radiolabelled ceftaroline in healthy adult men, 6% of radioactivity was recovered in faeces within 48 h; however, the lack of active drug in faeces, reported by Panagiotidis et al.,39 suggests that the majority of recovered label may be from inactive metabolites40 | there were moderate decreases in the numbers of bifidobacteria and lactobacilli during the first 7 days, while the numbers of clostridia increased during the same period39 | |||||
| no impact on the numbers of bacteroides bacteria was noticed; no new colonizing aerobic or anaerobic bacteria resistant to ceftaroline were found39 |
CDAD, C. difficile-associated diarrhoea; GI, gastrointestinal; iv, intravenous; SPC, Summary of Product Characteristics.
Some studies have evaluated the concentrations of cephalosporins in the faeces, and shown differences between the various agents (Table 3). In healthy volunteers, both cefixime and cefuroxime axetil were detected in faecal samples after being taken orally, although marked differences in concentrations were reported for the two drugs.33,34 Cefadroxil and cefaclor were not detectable in faeces following oral administration.35,36 These differences probably reflect variations in intestinal absorption observed between these agents. Marked differences in faecal concentrations between individuals were observed following oral administration of cefpodoxime proxetil.34 High concentrations were reported in three volunteers, but cefpodoxime was not detected in the faeces of the other seven, suggesting that intestinal absorption and/or degradation of the drug varies between individuals. The presence of cephalosporins in faeces has also been detected following parenteral administration, with ceftriaxone reported in faecal samples from healthy volunteers following intravenous infusion.37 By contrast, ceftobiprole and ceftaroline achieve low levels of gut exposure, with only minor effects on gut microbiota.38,39 Indeed, no measurable concentrations of either drug were detectable in faeces following intravenous administration in healthy volunteers.38,39 Careful selection of particular cephalosporins, considering relevant gut pharmacokinetic parameters, may therefore theoretically avoid disruption of the normal gut microbiota and help to manage the risk of patients developing CDI.
Effects of cephalosporins on C. difficile
The ability of a cephalosporin to inhibit C. difficile growth and toxin production may reduce the risk of CDI, while also preventing the emergence of resistance and recurrence. Currently, however, there are comparatively few data available on the susceptibility of C. difficile to cephalosporins. In general, cephalosporins have poor in vitro activity against C. difficile (Table 4).40–52 These studies also showed that Gram-negative anaerobic bacteria, such as Bacteroides spp., which make up a substantial proportion of the gastrointestinal microbiota, typically had low susceptibility to cephalosporins. Ceftaroline and ceftobiprole showed the greatest activity against C. difficile isolates, with an MIC50 of 2–4 mg/L for both agents;40,41,52 one review also reported good activity of cefprozil.42 As noted earlier, however, most cephalosporins are excreted primarily by the kidney, and thus antimicrobial activity against C. difficile may be of limited clinical relevance for drugs that do not penetrate the gut at therapeutic levels (Table 3).
Table 4.
In vitro susceptibility of Clostridium difficile to cephalosporins commonly used in Europe
| Agent | Isolates tested, n | MIC, mg/L |
Citation | ||
|---|---|---|---|---|---|
| range | MIC50 | MIC90 | |||
| Cefadroxil | — | no data | no data | no data | — |
| Cefalexin | 36 | — | 64a | 128a | Thornsberry 199242 |
| Cefazolin | 26 | ≤0.5–>128 | 16 | 32 | Pierard et al. 198943 |
| 17 | — | 25.0 | — | Simon et al. 198844 | |
| Cefaclor | 10 | 16–>32 | >32 | >32 | Spangler et al. 199445 |
| 12 | 32–>64 | 64 | >64 | Bauernfeind 199146 | |
| 36 | — | 32–128 | 32–>100 | Thornsberry 199242 | |
| Cefprozil | 36 | — | 4a | 4–8a | Thornsberry 199242 |
| 12 | 64–>64 | 64 | >64 | Bauernfeind 199146 | |
| Cefuroxime | 26 | 2–>128 | >128 | >128 | Pierard et al. 198943 |
| 10 | 16–>32 | >32 | >32 | Spangler et al. 199445 | |
| 12 | 64–>64 | >64 | >64 | Bauernfeind 199146 | |
| 73 | 64–≥256 | ≥256 | ≥256 | Chow et al. 198547 | |
| 401 | >256b | — | — | Noren et al. 201048 | |
| 51 | — | 512 | 512 | Freeman and Wilcox 200149 | |
| Cefpodoxime | 10 | 16–>32 | >32 | >32 | Spangler et al. 199445 |
| 12 | 64–>64 | >64 | >64 | Bauernfeind 199146 | |
| Cefixime | 12 | >64 | >64 | >64 | Bauernfeind 199146 |
| Ceftriaxone | 26 | ≤0.015–>64 | 32 | 64 | Snydman et al. 201152 |
| 42 | 2–64 | 32 | 32 | Chow et al. 198547 | |
| 60 | 8–128 | 32 | 64 | Baines et al. 201340 | |
| 86 | 8–256 | 48 | 256 | Buchler et al. 201450 | |
| Ceftazidime | 73 | 16–≥256 | 32 | 64 | Chow et al. 198547 |
| NR | 32–256 | 64 | 128 | Rolfe and Finegold 198151 | |
| Ceftobiprole | 30 | 1–8 | 4 | 8 | Ednie et al. 200741 |
| Ceftaroline | 26 | ≤0.015–8 | 2 | 8 | Snydman et al. 201152 |
| 60 | 0.125–16 | 4 | 4 | Baines et al. 201340 | |
NR, not reported.
aMode values from several studies.
bAll isolates.
The impact of different cephalosporins on C. difficile growth and toxin production in the gut has also been investigated using animal and in vitro models.40,53,54 Nerandzic and Donskey53 showed that neither ceftobiprole nor its prodrug ceftobiprole medocaril promoted the growth of C. difficile or the production of C. difficile toxin in a mouse model of caecal C. difficile colonization. By contrast, ceftazidime, cefotaxime and ceftriaxone were pro-C. difficile. In an in vitro model of the human gut, exposure to cefotaxime, with or without its active metabolite desacetylcefotaxime, led to C. difficile proliferation and increased levels of cytotoxin.54 Reductions in gut bacteria were also observed, particularly in Bifidobacterium and Bacteroides spp., suggesting that these genera may play a role in colonization resistance.54 A more recent study using the in vitro human gut model showed that both ceftaroline and ceftriaxone induced C. difficile spore germination, proliferation and toxin production.40 Both spore germination and growth of C. difficile were delayed with ceftaroline compared with ceftriaxone, although the reasons for this are unclear. The production and release of C. difficile toxin was also delayed with ceftaroline, probably reflecting differences in the balance between antibiotic-mediated effects on the gut microbiota and on C. difficile for the two agents.40
The concentrations and activity of cephalosporins in the gut could also be affected by the presence of β-lactamases expressed by commensal gut bacteria, such as Bacteroides fragilis, although the clinical effect of such activity is unclear.55 Combining cephalosporins with β-lactamase inhibitors in the context of active CDI is intended to overcome this and to broaden the spectrum of activity of the drug.56–60 For example, the combination of ceftazidime with the non-β-lactam, β-lactamase inhibitor avibactam significantly improved the in vitro activity of ceftazidime against anaerobic bacteria, such as C. difficile and B. fragilis.59,60 In a small study in 12 healthy volunteers, ceftazidime/avibactam (2000 g/500 g every 8 h on days 1–6) was shown to have a significant effect on the intestinal microbiota, with reductions in the numbers of Enterobacteriaceae, lactobacilli and bacteroides in the faeces.56 Notably, toxigenic strains of C. difficile were reported in five volunteers, with four reporting loose stools. A similar study of ceftaroline/avibactam (600 mg/600 mg every 8 h on days 1–6) in 12 healthy volunteers found that while numbers of Escherichia coli and lactobacilli in the faeces were reduced, there was no notable effect on bacteroides. A toxigenic C. difficile strain was reported in one patient, but this was not associated with adverse events.57
Taken together, these differences likely mean that some cephalosporins present a lower CDI risk than others. Agents that are primarily excreted via the kidneys result in relatively low levels of intestinal exposure, and only minor disruption of intestinal microbiota, especially anaerobes. Moreover, although many cephalosporins have poor activity against C. difficile, some agents display relatively high activity and are able to inhibit the growth of C. difficile, thus minimizing the likelihood of CDI.40,53
All of the above factors should be taken into consideration when assessing the risk associated with CDI from cephalosporin use. It is important to note that the risk of CDI is not the same for all patients. For example, in a CDC surveillance study, the risk of CDI was markedly greater in patients aged 65 years and over than in those younger than 65 years [rate ratio = 8.65 (95% CI = 8.16–9.31)].4 Moreover, elderly individuals, patients with severe or multiple comorbidities (modified Horn index score of 3 or 4) and those receiving additional antibiotics are at an increased risk of recurrent CDI.61 Thus, using a cephalosporin in a 25 year old patient with pneumonia, with no other risk factors for CDI, in a low-endemic CDI incidence country or setting is likely to carry considerably less risk than using a cephalosporin in an 80 year old patient with multiple comorbidities; in a hospital setting where the background incidence of CDI is high, such risk may be even greater. The risk of CDI may be further mitigated by careful selection from the array of cephalosporins available, noting their pharmacokinetic parameters (such as the achieved gut levels), effects on microbiota and impact on C. difficile growth and toxin production.
Antibiotic selection pressure for C. difficile
New evidence from detailed molecular epidemiological studies of over 3000 C. difficile isolates from the UK and other countries suggests that fluoroquinolones have provided a key selection pressure for epidemic clones. Compelling antibiotic prescribing data help to explain the rise and fall of CDI incidence in the UK. In response to UK guidance recommending restriction of cephalosporin and fluoroquinolone use,16 marked changes occurred in antibiotic prescribing. During 1998–2014, fluoroquinolones (but not total antibiotic prescribing) correlated strongly with the incidence of CDI.62 Coincident with these declines, the types of prevalent C. difficile strains also changed markedly. Of particular note is that the decrease in CDI incidence was due to substantial reductions in C. difficile clones that were resistant to fluoroquinolones; the prevalence of fluoroquinolone-resistant clones declined from 67% to 3%, but fluoroquinolone-susceptible clones persisted. Although reductions in cephalosporin prescribing also correlated with CDI incidence, the clone-specific effects cannot sensibly be explained by changes in cephalosporin use, because C. difficile is generally resistant to these antibiotics. Thus, if cephalosporin prescribing imparted a selection pressure on C. difficile, then decreases in all strain types would have been expected to occur. The importance of fluoroquinolone restriction as a potential control measure was also manifested by significant decreases (P < 0.001) in the incidence of CDIs caused by fluoroquinolone-resistant strains for the subgroups of patients with and without a likely hospital donor. No such effect was seen in respect of fluoroquinolone-susceptible CDIs. These compelling data emphasize the potential value of fluoroquinolone restriction as a key component of antimicrobial stewardship in controlling CDI.62
Clinical evidence of CDI risk with cephalosporins
Recent meta-analyses have sought to establish the strength of association between the use of broad-spectrum antibiotics and CDI.10–12 Overall, findings from the three analyses were similar, with clindamycin showing the strongest association with CDI in both hospital and community settings.10–12 The risk of CDI with cephalosporins was similar to that observed with other classes of antibiotics, such as quinolones/fluoroquinolones,10,11 carbapenems10 and penicillins.11 Slimings and Riley10 assessed the association between antibiotic use and hospital-acquired CDI. The meta-analysis involved one cohort and 13 case–control studies, of which all except one were of high or moderate quality. Overall, the risk of CDI with cephalosporins (OR = 1.97; 95% CI = 1.21–3.23) was lower than with clindamycin (OR = 2.86; 95% CI = 2.04–4.02) and similar to that with carbapenems (OR = 1.84; 95% CI = 1.26–2.68) and quinolones (OR = 1.66; 95% CI = 1.17–2.35).10 Analysis of cephalosporins by generation showed that the risk of CDI was greatest with third-generation agents (OR = 3.20; 95% CI = 1.80–5.71), and lower with second-generation (OR = 2.23; 95% CI = 1.47–3.37) and fourth-generation drugs (OR = 2.14; 95% CI = 1.30–3.52). In addition, the analysis showed that penicillin combination antibiotics, such as piperacillin/tazobactam, were associated with an increased risk of hospital-associated CDI (OR = 1.54; 95% CI = 1.05–2.24).10
The other two meta-analyses evaluated the association between community-associated CDI and antibiotic use.11,12 All of the studies used a case–control design, except for one cohort study, and there was some overlap of studies between the two reports. Deshpande et al.11 reported that the risk of CDI with cephalosporins (OR = 4.47; 95% CI = 1.60–12.50) was less than with clindamycin (OR = 20.43; 95% CI = 8.50–49.09) and similar to that with fluoroquinolones (OR = 5.50; 95% CI = 4.26–7.11) and penicillins (OR = 3.25; 95% CI = 1.89–5.57). The meta-analysis did, however, show a high degree of heterogeneity among the included studies, particularly those in the analyses of the antibiotics cephalosporins, clindamycin and penicillins.11 In the other meta-analysis, the risk of community-associated CDI with cephalosporins, monobactams and carbapenems (OR = 5.68; 95% CI = 2.12–15.23) was less than with clindamycin (OR = 16.80; 95% CI = 7.48–37.76) and similar to that observed with fluoroquinolones (OR = 5.50; 95% CI = 4.26–7.11).12
In all cases, analysis of the association between cephalosporin use and CDI has been based on the inclusion of all cephalosporins as a single group, or analysing by generation; however, as discussed above, this can be misleading, given the marked variations observed between different cephalosporins, including those of the same generation. Unfortunately, CDI data for individual cephalosporins are largely absent from the literature. Furthermore, the studies included in the three meta-analyses were all observational studies and were therefore prone to confounding and bias. Heterogeneity was commonly observed, with all three meta-analyses reporting substantial heterogeneity between studies in most of the antibiotic subclass analyses. Between-study heterogeneity was particularly marked for cephalosporins in both the hospital-based10 and community-based11 analyses, and was still present when cephalosporins were analysed by generation.10 Notwithstanding the differences among cephalosporins noted in this review, variations in study populations and methodologies, case definitions and C. difficile strains may all contribute to the between-study heterogeneity.13
One major limitation of previous studies is the failure to account for the propensity of clinicians to prescribe specific antibiotics for certain conditions, such as the use of cephalosporins and macrolides for pneumonia. It is therefore useful for analyses to focus on a single disease. A prospective study in 107 patients with community-acquired pneumonia (CAP) found that while the choice of antimicrobial therapy was not associated with acquisition of C. difficile, length of treatment and previous hospitalization were risk factors; however, it should be noted that this study examined C. difficile colonization and there were no reports of active CDI in this study.63 A further prospective, observational cohort study of 1883 patients with CAP from Edinburgh, UK, used Cox proportional hazards regression analysis to assess risk factors for the development of CDI. Age, duration of hospitalization, total number of antibiotics and duration of antibiotic therapy were shown to be major risk factors for CDI. Consistent with the previous study, however, antibiotic class was not an independent predictor of CDI when adjusted for these risk factors.64
Antibiotic strategies to reduce CDI risk
The points explored in this review raise the concern that attempts to reduce CDI risk by restricting the use of a small number of antibiotic classes (such as cephalosporins and clindamycin) may fail to reduce the overall incidence of CDI, because those agents may be replaced by antibiotics with a similar risk of CDI (such as fluoroquinolones and β-lactam/β-lactamase inhibitors). Thus, a balanced approach to antibiotic stewardship may be more beneficial. This should include reducing unnecessary antibiotic use, reducing prolonged antibiotic duration, avoiding the use of multiple antibiotic classes and promoting de-escalation of broad-spectrum therapy as soon as possible. Such an approach would promote the use of antibiotic agents carrying the lowest risk of CDI whenever possible, but without mandating a homogeneous approach to prescribing based on a simplistic classification of ‘good’ or ‘bad’ antibiotics. Moreover, increasing the heterogeneity of antibiotic prescribing is associated with reduced selection pressure and the emergence of resistance.65,66 A study conducted in a single intensive care unit showed that antibiotic prescribing protocols for ventilator-associated pneumonia that led to highly homogeneous prescribing were associated with marked increases in carbapenem-resistant Acinetobacter baumannii and extended-spectrum β-lactamase-producing Enterobacteriaceae.65 A meta-analysis showed that increased heterogeneity of prescribing was beneficial in reducing the incidence of all hospital-acquired infections and resistant infections.66 Positive effects were also observed for most pathogens, and effects were particularly pronounced when baseline levels of resistance were low.66 Therefore, selective use of cephalosporins, as part of a stewardship programme that delivers antibiotic diversity, could be an effective and well-tolerated therapeutic option.
Summary
Reducing the incidence of CDI presents an important challenge, given the multitude of factors that can affect the risk of CDI. Choice of antibiotic treatment is an important consideration when it comes to reducing risk; however, CDI risk is associated with a range of antibiotic classes, and is clearly not specific to cephalosporins. Indeed, there is evidence that use of fluoroquinolones, rather than of cephalosporins, has provided a much more profound selection pressure for particular epidemic C. difficile clones. In addition, the prescription of multiple antibiotics and an inappropriate length of treatment should be considered key risk factors for CDI. Furthermore, the risk is not the same across all patient populations, and is likely to differ at the national, local and care centre levels. All of these are factors that should be taken into account when selecting an antibiotic. The assessment of CDI risk simply based only on drug class is uninformative, because each drug (even within the same class) may have distinct pharmacokinetic and pharmacodynamic properties, which should be given the appropriate weighting in clinical decision-making. For instance, a broad-spectrum antibiotic with an appropriate pharmacokinetic profile (e.g. one that is eliminated predominantly by the kidneys and hence may limit exposure in the gut) may be a suitable choice for urgent empirical therapy. Reducing the incidence of CDI is best achieved by concentrating on rational prescribing, reducing the duration of antibiotic use and adhering to good infection control practices, rather than by focusing on the exclusion of individual drug classes. Indeed, antibiotic class exclusion will likely lead to reduced prescribing diversity, which in turn may drive resistance.
Funding
Medical writing and editorial support for the development of this review was funded by Basilea Pharmaceutica International Ltd (Basel, Switzerland).
Transparency declarations
M. H. W. reports receiving: consulting fees from Abbott Laboratories, Actelion, Astellas, AstraZeneca, Basilea Pharmaceutica International Ltd, Bayer, bioMérieux, Cerexa, Cubist, Durata, European Tissue Symposium, The Medicines Company, MedImmune, Merck, Motif Biosciences, Nabriva, Optimer, Paratek, Pfizer, Roche, Sanofi-Pasteur, Seres, Summit and Synthetic Biologics; lecture fees from Abbott, Alere, Astellas, AstraZeneca, Merck, Pfizer and Roche; and grant support from Abbott, Actelion, Astellas, bioMérieux, Cubist, Da Volterra, European Tissue Symposium, Merck and Summit. J. D. C. reports providing consultancy for Basilea Pharmaceutica International Ltd. J. F. reports research grant support from Astellas, Melinta Therapeutics and Morphochem AG. C. E. N. and E. B.: none to declare.
The authors take full responsibility for the content of the article.
Oxford PharmaGenesis (Oxford, UK) provided medical writing and editorial support.
Acknowledgements
We thank Oxford PharmaGenesis (Oxford, UK) for providing medical writing and editorial support.
References
- 1.Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359: 1932–40. [DOI] [PubMed] [Google Scholar]
- 2.PHE. Clostridium difficile: Guidance, Data and Analysis. Annual Counts and Rates of Clostridium difficile (C. difficile) Infections by Acute Trust and Clinical Commissioning Group (CCG) in Patients Aged 2 years and Over. https://www.gov.uk/government/statistics/clostridium-difficile-infection-annual-data.
- 3.Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis 2015; 60 Suppl 2: S66–71. [DOI] [PubMed] [Google Scholar]
- 4.Lessa FC, Mu Y, Bamberg WM et al. . Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372: 825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol 2010; 4: 409–16. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis 2008; 46 Suppl 1: S12–18. [DOI] [PubMed] [Google Scholar]
- 7.Wiegand PN, Nathwani D, Wilcox MH et al. . Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect 2012; 81: 1–14. [DOI] [PubMed] [Google Scholar]
- 8.Planche TD, Davies KA, Coen PG et al. . Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C. difficile infection. Lancet Infect Dis 2013; 13: 936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015; 372: 1539–48. [DOI] [PubMed] [Google Scholar]
- 10.Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother 2014; 69: 881–91. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande A, Pasupuleti V, Thota P et al. . Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother 2013; 68: 1951–61. [DOI] [PubMed] [Google Scholar]
- 12.Brown KA, Khanafer N, Daneman N et al. . Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother 2013; 57: 2326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence. Evidence Summary: Medicines and Prescribing Briefing. Clostridium difficile Infection: Risk with Broad-Spectrum Antibiotics. 2015. https://www.nice.org.uk/advice/esmpb1/chapter/key-points-from-the-evidence.
- 14.Freeman J, Bauer MP, Baines SD et al. . The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev 2010; 23: 529–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davey P, Brown E, Charani E et al. . Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2013; issue 4: CD003543. [DOI] [PubMed] [Google Scholar]
- 16.PHE and Department of Health. Clostridium difficile Infection: How to Deal with the Problem. http://www.gov.uk/government/publications/clostridium-difficile-infection-how-to-deal-with-the-problem.
- 17.Davies KA, Longshaw CM, Davis GL et al. . Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect Dis 2014; 14: 1208–19. [DOI] [PubMed] [Google Scholar]
- 18.ECDC. Antimicrobial Consumption Interactive Database (ESAC-Net). http://ecdc.europa.eu/en/healthtopics/antimicrobial_resistance/esac-net-database/Pages/database.aspx#sthash.H18pL8La.dpuf.
- 19.Eyre DW, Cule ML, Wilson DJ et al. . Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med 2013; 369: 1195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas C, Stevenson M, Riley TV. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review. J Antimicrob Chemother 2003; 51: 1339–50. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox MH, Mooney L, Bendall R et al. . A case–control study of community-associated Clostridium difficile infection. J Antimicrob Chemother 2008; 62: 388–96. [DOI] [PubMed] [Google Scholar]
- 22.Hensgens MP, Goorhuis A, van Kinschot CM et al. . Clostridium difficile infection in an endemic setting in the Netherlands. Eur J Clin Microbiol Infect Dis 2011; 30: 587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borriello SP. The influence of the normal flora on Clostridium difficile colonisation of the gut. Ann Med 1990; 22: 61–7. [DOI] [PubMed] [Google Scholar]
- 24.Antharam VC, Li EC, Ishmael A et al. . Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol 2013; 51: 2884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edlund C, Nord CE. A model of bacterial-antimicrobial interactions: the case of oropharyngeal and gastrointestinal microflora. J Chemother 1991; 3 Suppl 1: 196–200. [PubMed] [Google Scholar]
- 26.Spencer RC. The role of antimicrobial agents in the aetiology of Clostridium difficile-associated disease. J Antimicrob Chemother 1998; 41 Suppl C: 21–7. [DOI] [PubMed] [Google Scholar]
- 27.Stevens V, Dumyati G, Fine LS et al. . Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis 2011; 53: 42–8. [DOI] [PubMed] [Google Scholar]
- 28.Levison ME, Levison JH. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect Dis Clin North Am 2009; 23: 791–815, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karachalios G, Charalabopoulos K. Biliary excretion of antimicrobial drugs. Chemotherapy 2002; 48: 280–97. [DOI] [PubMed] [Google Scholar]
- 30.Kalman D, Barriere SL. Review of the pharmacology, pharmacokinetics, and clinical use of cephalosporins. Tex Heart Inst J 1990; 17: 203–15. [PMC free article] [PubMed] [Google Scholar]
- 31.Brogard JM, Blickle JF, Jehl F et al. . High biliary elimination of ceftriaxone in man. Int J Clin Pharmacol Ther Toxicol 1988; 26: 167–72. [PubMed] [Google Scholar]
- 32.Hayton WL, Schandlik R, Stoeckel K. Biliary excretion and pharmacokinetics of ceftriaxone after cholecystectomy. Eur J Clin Pharmacol 1986; 30: 445–51. [DOI] [PubMed] [Google Scholar]
- 33.Nord CE, Movin G, Stalberg D. Impact of cefixime on the normal intestinal microflora. Scand J Infect Dis 1988; 20: 547–52. [DOI] [PubMed] [Google Scholar]
- 34.Edlund C, Stark C, Nord CE. The relationship between an increase in β-lactamase activity after oral administration of three new cephalosporins and protection against intestinal ecological disturbances. J Antimicrob Chemother 1994; 34: 127–38. [DOI] [PubMed] [Google Scholar]
- 35.Nord CE, Heimdahl A, Lundberg C et al. . Impact of cefaclor on the normal human oropharyngeal and intestinal microflora. Scand J Infect Dis 1987; 19: 681–5. [DOI] [PubMed] [Google Scholar]
- 36.Adamsson I, Edlund C, Sjostedt S et al. . Comparative effects of cefadroxil and phenoxymethylpenicillin on the normal oropharyngeal and intestinal microflora. Infection 1997; 25: 154–8. [DOI] [PubMed] [Google Scholar]
- 37.Pletz MW, Rau M, Bulitta J et al. . Ertapenem pharmacokinetics and impact on intestinal microflora, in comparison to those of ceftriaxone, after multiple dosing in male and female volunteers. Antimicrob Agents Chemother 2004; 48: 3765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Backstrom T, Panagiotidis G, Beck O et al. . Effect of ceftobiprole on the normal human intestinal microflora. Int J Antimicrob Agents 2010; 36: 537–41. [DOI] [PubMed] [Google Scholar]
- 39.Panagiotidis G, Backstrom T, Asker-Hagelberg C et al. . Effect of ceftaroline on normal human intestinal microflora. Antimicrob Agents Chemother 2010; 54: 1811–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baines SD, Chilton CH, Crowther GS et al. . Evaluation of antimicrobial activity of ceftaroline against Clostridium difficile and propensity to induce C. difficile infection in an in vitro human gut model. J Antimicrob Chemother 2013; 68: 1842–9. [DOI] [PubMed] [Google Scholar]
- 41.Ednie L, Shapiro S, Appelbaum PC. Antianaerobe activity of ceftobiprole, a new broad-spectrum cephalosporin. Diagn Microbiol Infect Dis 2007; 58: 133–6. [DOI] [PubMed] [Google Scholar]
- 42.Thornsberry C. Review of the in vitro antibacterial activity of cefprozil, a new oral cephalosporin. Clin Infect Dis 1992; 14 Suppl 2: S189–94; discussion S95–6. [DOI] [PubMed] [Google Scholar]
- 43.Pierard D, De Meyer A, Rosseel P et al. . In vitro activity of amoxycillin plus clavulanic acid and ticarcillin plus clavulanic acid compared with that of other antibiotics against anaerobic bacteria. Acta Clin Belg 1989; 44: 228–36. [DOI] [PubMed] [Google Scholar]
- 44.Simon C, Simon M, Plieth C. In vitro activity of flomoxef in comparison to other cephalosporins. Infection 1988; 16: 131–4. [DOI] [PubMed] [Google Scholar]
- 45.Spangler SK, Jacobs MR, Appelbaum PC. Activity of WY-49605 compared with those of amoxicillin, amoxicillin-clavulanate, imipenem, ciprofloxacin, cefaclor, cefpodoxime, cefuroxime, clindamycin, and metronidazole against 384 anaerobic bacteria. Antimicrob Agents Chemother 1994; 38: 2599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauernfeind A. Comparative antimicrobial spectrum and activity of ceftibuten against clinical isolates from West Germany. Diagn Microbiol Infect Dis 1991; 14: 63–74. [DOI] [PubMed] [Google Scholar]
- 47.Chow AW, Cheng N, Bartlett KH. In vitro susceptibility of Clostridium difficile to new β-lactam and quinolone antibiotics. Antimicrob Agents Chemother 1985; 28: 842–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noren T, Alriksson I, Akerlund T et al. . In vitro susceptibility to 17 antimicrobials of clinical Clostridium difficile isolates collected in 1993-2007 in Sweden. Clin Microbiol Infect 2010; 16: 1104–10. [DOI] [PubMed] [Google Scholar]
- 49.Freeman J, Wilcox MH. Antibiotic activity against genotypically distinct and indistinguishable Clostridium difficile isolates. J Antimicrob Chemother 2001; 47: 244–6. [DOI] [PubMed] [Google Scholar]
- 50.Buchler AC, Rampini SK, Stelling S et al. . Antibiotic susceptibility of Clostridium difficile is similar worldwide over two decades despite widespread use of broad-spectrum antibiotics: an analysis done at the University Hospital of Zurich. BMC Infect Dis 2014; 14: 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rolfe RD, Finegold SM. Comparative in vitro activity of new β-lactam antibiotics against anaerobic bacteria. Antimicrob Agents Chemother 1981; 20: 600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snydman DR, Jacobus NV, McDermott LA. In vitro activity of ceftaroline against a broad spectrum of recent clinical anaerobic isolates. Antimicrob Agents Chemother 2011; 55: 421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nerandzic MM, Donskey CJ. Effect of ceftobiprole treatment on growth of and toxin production by Clostridium difficile in cecal contents of mice. Antimicrob Agents Chemother 2011; 55: 2174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freeman J, O'Neill FJ, Wilcox MH. Effects of cefotaxime and desacetylcefotaxime upon Clostridium difficile proliferation and toxin production in a triple-stage chemostat model of the human gut. J Antimicrob Chemother 2003; 52: 96–102. [DOI] [PubMed] [Google Scholar]
- 55.Nord CE, Hedberg M. Resistance to β-lactam antibiotics in anaerobic bacteria. Rev Infect Dis 1990; 12 Suppl 2: S231–4. [DOI] [PubMed] [Google Scholar]
- 56.Rashid MU, Rosenborg S, Panagiotidis G et al. . Ecological effect of ceftazidime/avibactam on the normal human intestinal microbiota. Int J Antimicrob Agents 2015; 46: 60–5. [DOI] [PubMed] [Google Scholar]
- 57.Rashid MU, Rosenborg S, Panagiotidis G et al. . Ecological effect of ceftaroline-avibactam on the normal human intestinal microbiota. Antimicrob Agents Chemother 2015; 59: 4504–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhanel GG, Lawson CD, Adam H et al. . Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Drugs 2013; 73: 159–77. [DOI] [PubMed] [Google Scholar]
- 59.Citron DM, Tyrrell KL, Merriam V et al. . In vitro activity of ceftazidime-NXL104 against 396 strains of β-lactamase-producing anaerobes. Antimicrob Agents Chemother 2011; 55: 3616–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dubreuil LJ, Mahieux S, Neut C et al. . Anti-anaerobic activity of a new β-lactamase inhibitor NXL104 in combination with β-lactams and metronidazole. Int J Antimicrob Agents 2012; 39: 500–4. [DOI] [PubMed] [Google Scholar]
- 61.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect 2012; 18 Suppl 6: 21–7. [DOI] [PubMed] [Google Scholar]
- 62.Dingle KE, Didelot X, Quan P et al. . Elimination of healthcare associated fluoroquinolone-resistant, but not fluoroquinolone-susceptible Clostridium difficile. Lancet Infect Dis 2016. In press. [Google Scholar]
- 63.Bruns AH, Oosterheert JJ, Kuijper EJ et al. . Impact of different empirical antibiotic treatment regimens for community-acquired pneumonia on the emergence of Clostridium difficile. J Antimicrob Chemother 2010; 65: 2464–71. [DOI] [PubMed] [Google Scholar]
- 64.Chalmers JD, Akram AR, Singanayagam A et al. . Risk factors for Clostridium difficile infection in hospitalized patients with community-acquired pneumonia. J Infect 2016; 73: 45–53. [DOI] [PubMed] [Google Scholar]
- 65.Sandiumenge A, Diaz E, Rodriguez A et al. . Impact of diversity of antibiotic use on the development of antimicrobial resistance. J Antimicrob Chemother 2006; 57: 1197–204. [DOI] [PubMed] [Google Scholar]
- 66.Abel zur Wiesch P, Kouyos R, Abel S et al. . Cycling empirical antibiotic therapy in hospitals: meta-analysis and models. PLoS Pathog 2014; 10: e1004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sales JE, Sutcliffe M, O'Grady F. Cephalexin levels in human bile in presence of biliary tract disease. Br Med J 1972; 3: 441–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brogard JM, Dorner M, Pinget M et al. . The biliary excretion of cefazolin. J Infect Dis 1975; 131: 625–33. [DOI] [PubMed] [Google Scholar]
- 69.Nishida M, Murakawa T, Matsubara T et al. . Characteristics of biliary excretion of cefazolin and other cephalosporins with reference to the relationship between serum levels and administration conditions. Chemotherapy 1976; 22: 30–6. [DOI] [PubMed] [Google Scholar]
- 70.Ratzan KR, Baker HB, Lauredo I. Excretion of cefamandole, cefazolin, and cephalothin into T-tube bile. Antimicrob Agents Chemother 1978; 13: 985–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ram MD, Watanatittan S. Levels of cefazolin in human bile. J Infect Dis 1973; 128: S361–3. [DOI] [PubMed] [Google Scholar]
- 72.Brogard JM, Pinget M, Comte F et al. . Biliary excretion of cefaclor. Experimental and clinical study. Chemotherapy 1982; 28: 189–99. [DOI] [PubMed] [Google Scholar]
- 73.Brogard JM, Pinget M, Arnaud JP et al. . Biliary excretion of cefuroxime. Experimental and human study. Chemotherapy 1981; 27: 18–28. [DOI] [PubMed] [Google Scholar]
- 74.Thomas MH, Dash CH, Burnand KG et al. . The excretion of cefuroxime in human bile. Br J Surg 1981; 68: 290–1. [DOI] [PubMed] [Google Scholar]
- 75.Severn M, Powis SJ. Biliary excretion and tissue levels of cefuroxime. A study in eleven patients undergoing cholecystectomy. J Antimicrob Chemother 1979; 5: 183–8. [DOI] [PubMed] [Google Scholar]
- 76.Westphal JF, Jehl F, Schloegel M et al. . Biliary excretion of cefixime: assessment in patients provided with T-tube drainage. Antimicrob Agents Chemother 1993; 37: 1488–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moorthi K, Fleckenstein G, Nies B. Concentration of cefixime in bile, gallbladder wall and serum after preoperative administration in patients undergoing cholecystectomy. Methods Find Exp Clin Pharmacol 1990; 12: 287–90. [PubMed] [Google Scholar]
- 78.Brogard JM, Jehl F, Paris-Bockel D et al. . Biliary elimination of ceftazidime. J Antimicrob Chemother 1987; 19: 671–8. [DOI] [PubMed] [Google Scholar]
- 79.Bouza E, Hellin T, Rodriguez-Creixems M et al. . Comparison of ceftazidime concentrations in bile and serum. Antimicrob Agents Chemother 1983; 24: 104–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanimura H, Kobayashi N, Miki K et al. . Chemotherapy in biliary tract infections (XVIII) with special reference to the concentration of ceftazidime in gallbladder tissue, the secretion in bile and ascitic fluid, and its clinical efficacy. Chemotherapy (Tokyo) 1983; 31 Suppl 3: 717–38. [Google Scholar]
- 81.Shiramatsu K, Hirata K, Yamada T et al. . Ceftazidime concentration in gallbladder tissue and excretion in bile. Antimicrob Agents Chemother 1988; 32: 1588–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walstad RA, Wiig JN, Thurmann-Nielsen E et al. . Pharmacokinetics of ceftazidime in patients with biliary tract disease. Eur J Clin Pharmacol 1986; 31: 327–31. [DOI] [PubMed] [Google Scholar]
- 83.Marshall WF, Blair JE. The cephalosporins. Mayo Clin Proc 1999; 74: 187–95. [DOI] [PubMed] [Google Scholar]
- 84.Murthy B, Schmitt-Hoffmann A. Pharmacokinetics and pharmacodynamics of ceftobiprole, an anti-MRSA cephalosporin with broad-spectrum activity. Clin Pharmacokinet 2008; 47: 21–33. [DOI] [PubMed] [Google Scholar]
- 85.AstraZeneca UK Limited. Zinforo 600 mg Powder for Concentrate for Solution for Infusion—Summary of Product Characteristics. https://www.medicines.org.uk/emc/medicine/26988.
- 86.Posada MM, Smith DE. In vivo absorption and disposition of cefadroxil after escalating oral doses in wild-type and PepT1 knockout mice. Pharm Res 2013; 30: 2931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marino EL, Dominguez-Gil A, Muriel C. Influence of dosage form and administration route on the pharmacokinetic parameters of cefadroxil. Int J Clin Pharmacol Ther Toxicol 1982; 20: 73–7. [PubMed] [Google Scholar]
- 88.Griffith RS. The pharmacology of cephalexin. Postgrad Med J 1983; 59 Suppl 5: 16–27. [PubMed] [Google Scholar]
- 89.Gaya H, Adnitt PI, Turner P. Changes in gut flora after cephalexin treatment. Br Med J 1970; 3: 624–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takesue Y, Yokoyama T, Akagi S et al. . Changes in the intestinal flora after the administration of prophylactic antibiotics to patients undergoing a gastrectomy. Surg Today 2002; 32: 581–6. [DOI] [PubMed] [Google Scholar]
- 91.Lode H, Muller C, Borner K et al. . Multiple-dose pharmacokinetics of cefprozil and its impact on intestinal flora of volunteers. Antimicrob Agents Chemother 1992; 36: 144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barriere SL. Review of in vitro activity, pharmacokinetic characteristics, safety, and clinical efficacy of cefprozil, a new oral cephalosporin. Ann Pharmacother 1993; 27: 1082–9. [DOI] [PubMed] [Google Scholar]
- 93.Novelli A, Mazzei T, Fallani S et al. . Betalactam therapy and intestinal flora. J Chemother 1995; 7 Suppl 1: 25–31. [PubMed] [Google Scholar]
- 94.Foord RD. Cefuroxime: human pharmacokinetics. Antimicrob Agents Chemother 1976; 9: 741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Borin MT. A review of the pharmacokinetics of cefpodoxime proxetil. Drugs 1991; 42 Suppl 3: 13–21. [DOI] [PubMed] [Google Scholar]
- 96.Brismar B, Edlund C, Nord CE. Impact of cefpodoxime proxetil and amoxicillin on the normal oral and intestinal microflora. Eur J Clin Microbiol Infect Dis 1993; 12: 714–9. [DOI] [PubMed] [Google Scholar]
- 97.Bodey GP, Fainstein V, Garcia I et al. . Effect of broad-spectrum cephalosporins on the microbial flora of recipients. J Infect Dis 1983; 148: 892–7. [DOI] [PubMed] [Google Scholar]
- 98.Edlund C, Nord CE. Ecological impact of antimicrobial agents on human intestinal microflora. In: Heidt P, Rusch V, van der Waaij D, eds. Old Herborn University Seminar Monograph 7: Immune System and Microflora. Herborn-Dill: Herborn Litterae, 2003; 37–65. [Google Scholar]
- 99.Goldstein EJ, Citron DM, Merriam CV et al. . In vitro activity of ceftobiprole against aerobic and anaerobic strains isolated from diabetic foot infections. Antimicrob Agents Chemother 2006; 50: 3959–62. [DOI] [PMC free article] [PubMed] [Google Scholar]