Abstract
Objectives
To characterize the effects of CYP2B6 polymorphisms, diurnal variation and demographic factors on nevirapine pharmacokinetics in African children.
Methods
Non-linear mixed-effects modelling conducted in NONMEM 7.3 described nevirapine plasma concentration–time data from 414 children aged 0.3–15 years.
Results
Nevirapine pharmacokinetics was best described using a one-compartment disposition model with elimination through a well-stirred liver model accounting for a first-pass effect and transit-compartment absorption. Intrinsic clearance was affected by diurnal variation (characterized using a cosine function with peak amplitude 29% at 12 noon) and CYP2B6 metabolizer status [extensive metabolizer (EM) 516GG|983TT, reference; intermediate metabolizer (IM) 516GT|983TT or 516GG|983TC, 17% lower; slow metabolizer (SM) 516TT|983TT or 516GT|983TC, 50% lower; ultra-slow metabolizer (USM) 516GG|983CC, 68% lower]. Age was found to affect pre-hepatic bioavailability: 31.7% lower at birth and increasing exponentially. Median (90% CI) evening Cmin values in the different metabolizer groups were 5.01 (3.01–7.47), 6.55 (3.65–13.32), 11.59 (5.44–22.71) and 12.32 (12.32–27.25) mg/L, respectively. Evening Cmin values were <3 mg/L in 43% of EM weighing <6 kg and 26% of IM weighing <6 kg, while 73% of SM and 88% of USM in all weight-bands had evening Cmin values >8 mg/L. Cmin was not markedly affected by administration time, but was altered by unequal splitting of the daily dose.
Conclusions
Diurnal variation does not greatly affect nevirapine exposure. However, when daily doses cannot be split equally, the larger dose should be given in the morning. To achieve homogeneous exposures, nevirapine doses for SM and USM should be reduced by 50%, and children weighing <6 kg with EM or IM metabolizer status should receive the same dose as children weighing 6–10 kg.
Introduction
Nevirapine was the first NNRTI available in low-income countries in a generic paediatric fixed-dose combination (FDC) tablet. This contributed to substantial cost reductions and improved the feasibility of treating HIV-infected children, and nevirapine is still widely used in resource-limited settings.1–4 Nevirapine has several advantageous characteristics: it has fewer drug interactions than PIs, it does not cause adverse CNS events when compared with efavirenz, and its bioavailability is not affected by food.5
Despite its high potency, nevirapine has a low genetic barrier for mutations and suboptimal drug exposures increase the risks of developing drug resistance and treatment failure.6,7 Several studies have reported highly variable nevirapine concentrations, with levels <3 mg/L among children in the lower paediatric weight-bands when dosed according to WHO guidelines, increasing the risk of virological failure.1,2,8–12 Nevirapine concentrations >8 mg/L, on the other hand, were associated with an increased risk of treatment discontinuation due to adverse events among adults.7 However, paediatric studies quantifying nevirapine pharmacokinetic variability due to different sources and suggesting optimization of current dosing remain limited.8,13,14
Nevirapine has a complex metabolism mediated mainly by CYP3A4- and CYP2B6-coded enzymes.15 SNPs present in CYP2B6 (516G > T and 983T > C) were identified as the main source of nevirapine variability in adults,16–18 as for efavirenz.4,18,19 The prevalence of 516G > T loss of function (LOF) polymorphisms differs between populations and is particularly high in black Africans, whereas 983T > C variants are not observed among Caucasians.4,18,19 In our previous investigation of efavirenz pharmacokinetics in African children, we showed that extensive metabolizers (EM; CYP2B6 516GG|983TT genotype) are at higher risk of developing subtherapeutic efavirenz concentrations.20 A similar investigation of differences in nevirapine exposures between various metabolizer groups when dosed by weight-band according to current WHO guidelines has not yet been conducted in children. CYP2B6 expression may be further modified by polymorphisms in genes coding nuclear receptors CAR (NR1|3) and PXR (NR1|2),21,22 although this has not been proved for nevirapine.23
The effect of the CYP3A4 pathway on nevirapine pharmacokinetics is less studied. Although not confirmed for nevirapine, systemic exposures of CYP3A substrates have been shown to be altered by SNPs rs35599367 (CYP3A4*22)24,25 and rs776746 (CYP3A5*1).26,27 Additionally, CYP3A activity exhibits diurnal variation, with nevirapine clearance rates increasing during the day and reducing at night.28,29 Differences between morning (AM) and evening (PM) nevirapine trough concentrations (Cmin) have been previously reported30 and may relate to diurnal variation in the CYP3A-mediated effects on pharmacokinetics.
The aim of this analysis was: (i) to model the steady-state population pharmacokinetics of nevirapine in the largest cohort of African children studied so far; (ii) to quantify demographic and genotypic effects on nevirapine disposition; (iii) to characterize the effect of diurnal variation on nevirapine exposures under various dosing scenarios; and (iv) to propose optimal dosing strategies for this population.
Methods.
In this analysis, sparsely sampled data from the CHAPAS-3 trial (Children with HIV in Africa—Pharmacokinetics and Adherence of Simple Antiretroviral Regimens)31 was enriched with intensive data from an earlier pharmacokinetic sub-study1 (part of CHAPAS-1).32 Both studies were conducted in African children from Uganda and Zambia, as briefly described below.
CHAPAS-1
The trial evaluated dosing of, and adherence to, new paediatric FDC tablets: Triomune Baby (50 mg nevirapine, 6 mg stavudine and 30 mg lamivudine) and Junior (100 mg nevirapine, 12 mg stavudine and 60 mg lamivudine) in children <14 years dosed twice daily according to WHO 2006 guidelines.33 When the daily dose could not be split equally, the larger dose was given at night.
Children in the pharmacokinetic sub-study were sampled on one occasion at least 4 weeks after starting treatment. Samples were taken immediately prior to giving the morning dose and 1, 2, 4, 6, 8 and 12 h afterwards. The time of the preceding evening dose was assumed to be 12 h before the morning dose. Samples were stored and assayed using ultra HPLC with UV detection at the Department of Pharmacy of the Radboud University Medical Centre, Nijmegen, The Netherlands. The method was linear over the range of 0.1–10 mg/L. The average intra-assay and inter-assay coefficients of variation (CV) and relative error (RE) were 2.9%, 2.4% and 97%, respectively.34
CHAPAS-3
Pharmacokinetics, toxicity, acceptability, adherence and virological efficacy were compared between three first-line antiretroviral regimens in children 13 years or younger.31 Depending on treatment allocation, patients received: Triomune Baby, Triomune Junior, Duovir-N Baby (50 mg nevirapine, 60 mg zidovudine and 30 mg lamivudine) or nevirapine (100 mg)—all paediatric formulations; or Duovir-N (200 mg nevirapine, 300 mg zidovudine and 200 mg lamivudine) or Triomune30 (200 mg nevirapine, 30 mg stavudine and 150 mg lamivudine), formulated for adults. Nevirapine-based regimens were dosed twice daily according to WHO 2010 guidelines.35 When the daily dose could not be split equally, the larger dose was given in the morning.
Children on nevirapine were sampled during clinic visits at week 6, week 36 and every 24 weeks thereafter until the end of the study; at each visit two samples were taken at least 2 h apart. The self-reported times of the morning and penultimate doses were recorded. Samples were stored and analysed by LC-tandem MS at the Division of Clinical Pharmacology, University of Cape Town, South Africa. The method was linear over the range of 0.0195–20 mg/L. The average intra-assay and inter-assay CV and RE were 2.9%, 2.4% and 97%, respectively.
Genotyping
Genotyping was performed only on patients from CHAPAS-3 by allelic discrimination real-time PCR assay on a DNA Engine Chromo4 system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The PCR protocol involved an initial denaturation step at 95°C for 15 min, followed by 50 cycles of amplification at 95°C for 15 s and final annealing at 60°C for 1 min. TaqMan® Genotyping Master Mix and assays for CYP2B6 516G > T (rs3745274; ID: C_7817765_60), CYP2B6 983T > C (rs28399499; ID: C_60732328_20), CYP2B6 15582C > T (rs4803419; ID: C_7817764_10), CYP3A4*22 (rs35599367, C__59013445_10), CYP3A5 6986G > A (rs776746, C__59013445_10), NR1I3 (rs3003596, C__16194070_10 and rs2307424, C__25746794_20), NR1I2 63396C > T (rs2472677, C__26079845_10), and ABCC10 (rs2125739, C__16173668_10) were obtained from Life Technologies Ltd (Paisley, UK). Opticon Monitor® version 3.1 (Bio-Rad Laboratories) was used to obtain allelic discrimination plots and make allele calls.
The distribution of the genotypes was tested for Hardy–Weinberg equilibrium using the exact test in the R ‘genetics’ package.
Population pharmacokinetic analysis
Model building
The steady-state pharmacokinetics of nevirapine was analysed using non-linear mixed-effects modelling with NONMEM 7.336 and the first-order conditional estimation method with interaction. PsN 4.4.0, Pirana and Xpose were used to facilitate modelling and for model diagnostics.37 Model building was conducted starting with intensive pharmacokinetic data from CHAPAS-1 followed by sparse data from CHAPAS-3.38 The stepwise process was guided by differences in the NONMEM objective function value (OFV; proportional to −2 log-likelihood), inspection of goodness-of-fit (GOF) plots and visual predictive checks (VPCs), biological plausibility and clinical relevance. OFV drops >3.84 between two hierarchical models after adding one parameter were considered a significant improvement (P ≤ 0.05, χ2-distribution, df = 1). The stability and robustness of the final model, together with the precision of parameter estimates, was evaluated using non-parametric bootstrap (n = 50, due to long model run times).
The model-derived empirical Bayesian estimates for the individual parameters were used to predict morning and evening Cmin and AUC0–12 (area under the concentration–time curve between dosing events) at steady state for each sampling occasion and patient.
Structural model
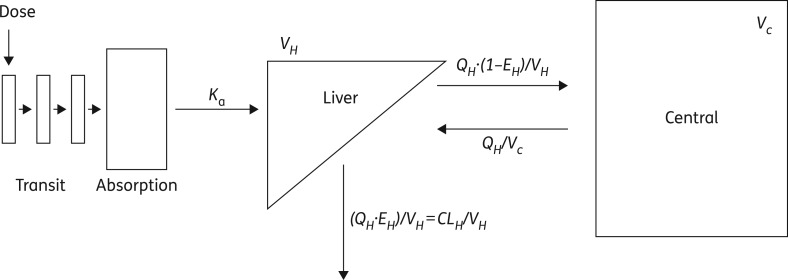
One-, two- and three-compartment disposition models with first-order absorption and elimination were tested, as well as delayed and transit-compartment39 absorption. A semi-mechanistic well-stirred hepatic extraction model was tested for elimination, as in Gordi et al.40 This hepatic model assumed the following parameters: nevirapine fraction unbound in plasma (fu) 40%,41 hepatic plasma flow (QH) 50 L/h42 and liver volume (VH) 1 L40 for a typical 70 kg individual (allometrically scaled).
Between-subject variability (BSV) and between-occasion variability (BOV) were tested on all pharmacokinetic parameters assuming log-normal distribution. Residual unexplained variability (RUV) was described using a combined proportional and additive structure. We excluded from the analysis data with uncertain dosage history and nevirapine concentrations below the limit of quantification (BLQ), presumed to be due to non-compliance38 (confirmed by undetectable concentrations of the companion antiretroviral drugs). Further implausible outliers were identified using visual checks and excluded based on conditional weighted residuals (|CWRESI| > 3).
Covariate effects
Allometric scaling was added to the model at an early stage (before covariate testing), as suggested by Anderson and Holford,43 and applied to all clearance and volume parameters. For intrinsic clearance (CLint) and pre-hepatic bioavailability (FpreH) we tested the effect of age using a power, hockey-stick, exponential or sigmoidal function with/without Hill coefficient models.43 The effect of diurnal variations was investigated using step or cosine functions.29 Besides weight and age, the other covariates tested were: study site, NRTI treatment backbone, sex, weight-for-age Z-score (WAZ), height-for-age Z-score (HAZ) and formulation. Pharmacogenetic effects were tested as individual SNPs (rs3745274, rs28399499, rs4803419, rs35599367, rs776746, rs3003596, rs2307424, rs2472677, rs2125739) and as metabolizer status determined by SNPs 516G > T and 983T > C [EM, genotype 516GG|983TT; intermediate metabolizer (IM), single variant allele (516GT|983TT or 516GG|983TC); slow metabolizer (SM), two variant alleles (516TT|983CC or 516GT|983TC); ultra-slow metabolizer (USM), 983CC irrespective of 516G > T genotype].
Mixture modelling with frequencies fixed to those observed in the study population was used to impute missing genotypes (predominantly in CHAPAS-1).44 Proportionality and correction factors were applied on RUV to test for differences between the assays and laboratories used.
Simulations
For the simulation (conducted with NONMEM 7.3), the demographics of the 414 patients (weight 3.5–29.6 kg) from the original analysis were used and enriched with 116 records of individuals weighing 20–35 kg from CDC Growth Charts (age and corresponding median weight used).45 The final model was used to simulate exposures after nevirapine administration under various dosing scenarios and assuming 3–8 mg/L as the therapeutic range for nevirapine.46 Each in silico patient was resimulated 100 times, changing their metabolizer status according to the proportions in the study population, which ensured the same distribution in each weight-band. The effect of drug intake time (6:00, 7:00, 8:00, 9:00 AM/PM) and dose-splitting strategies (AM/PM D1:100/50 mg, D2:75/75 mg, D3:50/100 mg) was studied in a single patient (0.44 years, 7.2 kg, IM) simulated 1000 times. To avoid generating implausibly extreme values, the maximum variability for each random effect was limited to 3 standard deviations. Data analysis and plot generation was performed using R.47
Results
Demographic characteristics and samples
This analysis included 3305 samples (539 in intensive and 2766 in sparse pharmacokinetic profiles) from 414 African children (78 CHAPAS-1, 330 CHAPAS-3, 6 in both). Baseline demographic characteristics are presented in Table 1; 246 samples were excluded from the analysis (111 due to unclear dosage history, 87 outliers and 48 BLQ). Genotypes were available for 324 children (Table S1, available as Supplementary data at JAC Online); CYP2B6 metabolizer groups were 33.1% EM, 44.6% IM, 21.7% SM and 0.6% USM (Table 2); the mixture-model allocation for the remaining 96 individuals was 41.7% EM, 49.0% IM and 9.4% SM. All tested genotypes were in Hardy–Weinberg equilibrium (Table S1).
Table 1.
Demographic characteristics
| Characteristic | CHAPAS-1 | CHAPAS-3 | Combined |
|---|---|---|---|
| No. of children | 84 | 336 | 414 |
| No. of samples included | 539 | 2766 | 3305 |
| No. of samples excluded (BLQ) | 8 (0) | 238 (48) | 246a |
| No. of sampling occasions, n or median (range) | 1 | 3 (1–7) | 3 (1–8) |
| Age (years)b, median (range) | 6.2 (0.4–15.0) | 2.6 (0.3–12.2) | 2.92 (0.3–15.0) |
| Weight (kg)b, median (range) | 15.75 (3.5–29.0) | 11.5 (4.9–29.6) | 12.2 (3.5–29.6) |
| WAZ, median (range) | −1.1 (−4.2–2.0) | −1.7 (−7.2–1.2) | −1.5 (−7.2–2.0) |
| Male/female, n/n | 52/32 | 177/159 | 80/89 |
| NRTI, n | |||
| abacavir | 0 | 115 | 115 |
| stavudine | 84 | 107 | 191 |
| zidovudine | 0 | 114 | 114 |
Six patients rolled over from CHAPAS-1 to CHAPAS-3; all patients were black Africans.
aSamples excluded from the analysis: unclear dosage history, 111; implausible (visual check confirmed by |CWRES| > 3), 87; and BLQ confirmed by undetectable levels of the companion drugs, 48.
bBaseline values.
Table 2.
Exposures of different metabolic subgroups determined by 516G>T|983T>C SNP vector
| Metabolizer status | Patients, n (%) | CminAM (mg/L), median (5th–95th percentile) | CminPM (mg/L), median (5th–95th percentile) | CminPM <3 mg/La, n (%) | CminPM 3–8 mg/La, n (%) | CminPM >8 mg/La, n (%) | AUCAM (mg · h/L), median (5th–95th percentile) | AUCPM (mg · h/L), median (5th–95th percentile) |
|---|---|---|---|---|---|---|---|---|
| EM | 106 (33.3) | 5.01 (3.01–7.47) | 4.58 (2.53–7.03) | 77 (16.6) | 361 (77.6) | 27 (5.8) | 68.51 (39.42–104.16) | 69.34 (38.65–104.42) |
| IM | 141 (44.2) | 6.55 (3.65–13.32) | 6.08 (3.25–12.93) | 33 (5.8) | 378 (66.8) | 155 (27.4) | 88.93 (50.06–173.72) | 88.60 (50.06–173.72) |
| SM | 70 (21.9) | 11.59 (5.44–22.71) | 10.91 (5.06–22.44) | 4 (1.3) | 78 (25.7) | 222 (73.0) | 152.07 (72.42–270.46) | 151.27 (71.54–287.46) |
| USM | 2 (0.6) | 12.32 (12.32–27.25) | 11.71 (11.71–26.43) | 0 (0) | 1 (12.5) | 7 (87.5) | 170.81 (170.81–362.97) | 152.12 (152.12–337.26) |
EM, 516GG|983TT; IM, 516GG|983TC or 516GT|983TT; SM, 516TT|983TT or 516GT|983TC; USM, 516GG|983CC.
Data for 319 individuals from the CHAPAS-3 trial with available genotype dosed according to WHO 2010 guidelines35 corresponding to 1343 pharmacokinetic visits. When multiple pharmacokinetic visits were available, measurements were used to calculate the geometric mean for every patient, which were then used to calculate median and percentiles in each subgroup.
aNumber of Cmin below, within and above the therapeutic range of 3–8 mg/L.45
Population pharmacokinetics
Nevirapine pharmacokinetics were best described using one-compartment disposition, absorption through transit compartments and elimination using the semi-physiological model with first-pass hepatic extraction (Figure 1 and Appendix S1). The final model parameters were estimated relative to pre-hepatic bioavailability (FpreH, with typical value fixed to 1) and are presented in Table 3. All parameter estimates were found to be reasonably robust and adequate model fit was confirmed through GOF and VPC plots, which showed adequate fit of our model to the analysed data (Figures S1 and S2).
Figure 1.
Compartmental structure of the nevirapine pharmacokinetic model. CLH, hepatic clearance; EH, hepatic extraction; Ka, absorption rate constant; QH, hepatic plasma flow; VH, volume of the liver; VC, volume of the central compartment. The model parameters and presented relations are explained in detail in Appendix S1.
Table 3.
Final parameter estimates (5th–95th percentile)a
| Parameter | Typical values | Variability (%)b |
|---|---|---|
| CLint | ||
| EM (L/h) | 3.27 (3.00–3.69) | BSV CLint: 21.40 (20.08–32.46) |
| IM (L/h) | 2.72 (2.27–2.94) | |
| SM (L/h) | 1.65 (1.47–1.89) | |
| USM (L/h) | 1.04 (0.87–1.38) | |
| AMP (%) | 29.2 (27.7–45.2) | |
| SHIFT (h) | −12.30 (−13.32 to −10.38) | |
| VC (L) | 21.92 (20.24–26.23) | |
| FpreH | ||
| older childrenc | 1 (fixed) | BSV FpreH: 18.72 (6.59–20.66) |
| at birth (%) | 58.30 (50.48–68.24) | BOV FpreH: 17.02 (16.12–20.87) |
| t1/2 (years) | 1.54 (1.47–2.58) | |
| Increased BOV FpreH for unobserved intake | 1.54 (1.20–1.65) | |
| MTT (h) | 0.56 (0.49–0.70) | BOV MTT: 199.73 (177.23–217.70) |
| Ka (1/h) | 0.84 (0.67–1.12) | BOV Ka: 44.91 (31.32–50.46) |
| NTRANS (number) | 3 (fixed) | |
| Additive error (mg/L) | 0.32 (0.21–0.38) | |
| Proportional error (%) | 5.26 (4.26–6.18) | |
| Increased error for sparse data | 1.56 (1.49–1.81) | |
CLint, intrinsic clearance; AMP, amplitude of cosine function; SHIFT, shift in the zenith of cosine function from midnight; VC, volume of central compartment; FpreH, pre-hepatic bioavailability; NTRANS, number of transit compartments (in the implementation of Savic et al.39 this would be NN = 2); MTT, absorption mean transit time; Ka, absorption rate constant; BSV, between-subject variability; BOV, between-occasion variability.
Final parameter estimates are typical population values estimated by the model. All clearance and volume parameters scaled allometrically to the median weight of 14.5 kg.
The number of transit compartments was first estimated and then fixed during the covariate analysis in order to improve model stability. The number was then re-estimated in the final model and proved not to be different from that previously fixed. The equations explaining the relation between presented parameters can be found in Appendix S1.
aEstimated from non-parametric bootstrap (n = 50) of the final model.
bExpressed as approximate %CV on SD scale (√ETA×100).
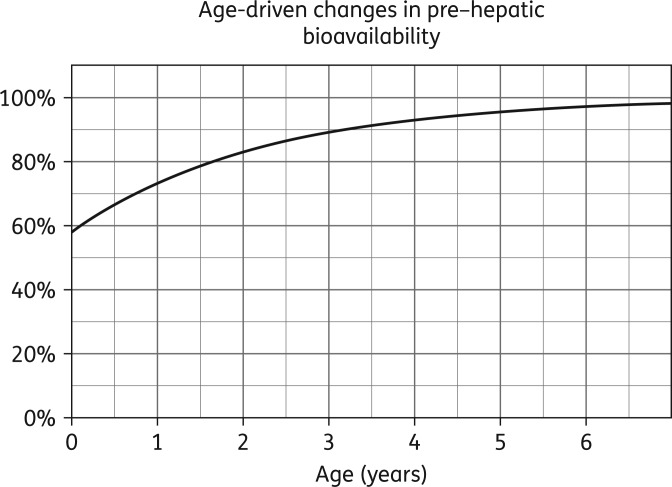
cOlder children refers to individuals where no further age-driven increase in bioavailability can be observed (Figure 3).
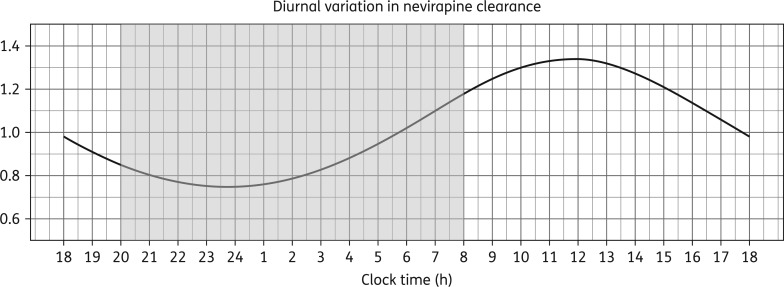
Implementing the well-stirred liver model decreased OFV by 42, without adding extra parameters. The model was parameterized with CLint following a circadian rhythm expressed through oscillations of the cosine function with zenith around 12 noon and amplitude of ∼29% (ΔOFV = −91, df = 2, P < 0.001) (Figure 2). The model identified distinct pre-hepatic (FpreH) and hepatic components (FH) of bioavailability, since changes in liver activity mechanistically affected also FH. The reference value of FpreH was fixed to 1, and BSV and BOV were estimated. Including the diurnal effect reduced BSV in CLint by 34% and BOV in FpreH by 41%. More details on the model implementation, including formulae explaining the relationship between model parameters, are presented in Appendix S1.
Figure 2.
Diurnal variation in nevirapine intrinsic clearance detected by the model, presented over 24 h. The shaded area corresponds to night-time (20.00–08.00 h).
After applying allometric scaling to account for the effect of body size, and including diurnal effects and first-pass metabolism, the most significant covariate was the metabolizer status on CLint determined by CYP2B6 516G > T|983T > C genotype (ΔOFV = −217, df = 3, P < 0.001), explaining 85% of remaining BSV in CLint. Using six rather than four 516G > T|983T > C SNP-vector metabolizer groups20 reduced OFV by only 5 points (df = 2, P = 0.08) and was therefore not used.
Our data did not support a maturation effect on CLint, but we identified age-driven differences in FpreH, which were described using an exponential model [Equation (7) in Appendix S1]. FpreH at birth was estimated as 58.3% of the value in older children (reference fixed to 100%), 90% of FpreH was reached by age of ∼3.3 years and the half-life of the process was 1.55 years (Figure 3).
Figure 3.
Change in nevirapine pre-hepatic bioavailability with age.
The model estimated that an average child weighing 14.5 kg and aged 4.1 years would have FpreH = 93% and their values of oral clearance (CLoral, see Appendix S1 and Table S2) were 1.31 L/h EM (reference), 1.09 L/h IM (17% lower), 0.66 L/h SM (50% lower) and 0.42 L/h USM (68% lower). A summary of the individual exposures in children from the CHAPAS-3 trial dosed according to WHO 2010 guidelines35 is presented in Table 2, split by metabolizer genotype.
Higher uncertainty related to unobserved intake time (for all sparse data and pre-dose samples in intensive data) was accounted for by scaling factors (proportional model) on RUV and BOV FpreH, which were found to be respectively 1.56 and 1.54 times larger than in pharmacokinetic samples after observed intake.
No other covariates were identified as significant. The remaining stochastic variability in clearance and bioavailability was low (BSV CLint 21.4%, BSV FpreH 18.7% and BOV FpreH 17%), but absorption parameters (where no covariates improved model fit) remained highly variable [BOV absorption rate constant (Ka) 44.9%, BOV absorption mean transit time (MTT) 199.7%].
Simulations
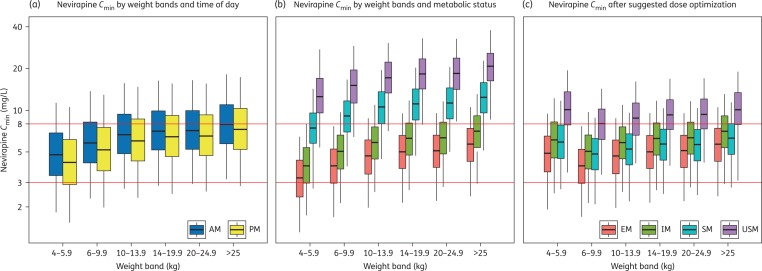
Simulations were conducted to compare average CminAM and CminPM in weight-bands of African children divided into metabolizer groups and dosed following WHO 2010 recommendations.35 Average CminAM and CminPM in weight-bands >6 kg were >3 mg/L for most simulated individuals regardless of metabolizer status (Figure 4a). In contrast, >25% of children in the lowest weight-band (4–6 kg) had CminPM below the efficacy threshold (Figure 4b); this effect was driven mostly by EM and IM (43% and 26% <3 mg/L, respectively).
Figure 4.
Model-simulated exposures shown by dosing weight-bands. (a) Difference between morning and evening Cmin when dosed according to current WHO recommendations.35 (b) Difference in Cmin between metabolic groups when dosed according to current WHO recommendations (evening Cmin is shown). (c) Difference in Cmin between metabolic groups when dosed according to the proposed dose optimization strategy (evening Cmin is shown). Red horizontal lines correspond to the nevirapine therapeutic range, from 3 to 8 mg/L. The boxes in the percentile plots show the 25th percentiles, medians and 75th percentiles, while the whiskers correspond to the 5th and 95th percentiles of the simulated data. EM, 516GG|983TT; IM, 516GG|983TC or 516GT|983TT; SM, 516TT|983TT or 516GT|983TC; USM, 516GG|983CC. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Given the detected diurnal variation in nevirapine CLint, we evaluated the effect of administration time (see the Methods section) on average morning and evening exposures. The changes in median concentration depending on administration time and differences in systemic drug exposures are presented in Figure S3. Depending on administration time, the ratios of morning/evening exposures varied between 1.09–1.15 for Cmin and 1.03–1.07 for AUC0–12, differences that are unlikely to be clinically significant.
Use of some nevirapine FDCs can lead to unequal splitting of the advised daily dose between morning and evening intakes. Simulation results showed that ratios between simulated median CminAM/PM for tested dose-splitting strategies (see the Methods section) were: D1 (larger morning) 0.93, D2 (equal) 1.13 and D3 (larger evening) 1.41; and AUC0–12 0.90, 1.04 and 1.22, respectively (Figure S4).
Discussion
We present the largest investigation to date of nevirapine pharmacogenetics, the first report of the effect of 983CC homozygosity on nevirapine pharmacokinetics and the first study in children to quantify the combined effect of CYP2B6 516G > T|983T > C. Our analysis is also the first to date to characterize the diurnal variation in nevirapine clearance through population pharmacokinetic modelling and to evaluate the effect of this phenomenon on systemic drug exposures through simulations.
The main predictor of nevirapine clearance in our cohort of African children was the combined effect of the CYP2B6 516G > T|983T > C genotype. Oral clearance estimated by our model before adjusting for the CYP2B6-SNPs was 3.8 L/h, comparable to the 3.93 L/h reported previously in children13 (both scaled up to 70 kg) and the 2.82–3.97 L/h found in adults.16,17,38,48–53 Comparing the CYP2B6 516G > T|983T > C effect with other reports is problematic, since our study is the first to use this categorization with four metabolizer subgroups for nevirapine, although it has been extensively applied to efavirenz.20,54 The 50% lower nevirapine clearance we detected for SM is greater than the 30%–37% drop previously reported for 516TT versus 516GG.8,14,16,17,55,56 Similar to efavirenz,18,20 the effect of CYP2B6 983CC (recessive homozygosity) on nevirapine pharmacokinetics is of greater magnitude than that of 516TT (68% versus 50% drop). Unsurprisingly, nevirapine clearance is affected to a lesser degree by CYP2B6 polymorphisms than efavirenz in the same population.20 This can be explained by a different contribution of the CYP3A4 pathway to the metabolism of these drugs.57
Diurnal variation has been previously documented for several CYP3A4 substrates,58,59 consistently revealing increased clearance rates during the day as compared with during the night.28,60,61 Our study replicated those findings and detected significantly higher nevirapine clearance during the day, with a maximum at midday. The estimated amplitude of the diurnal variation is somewhat larger than previous reports in CYP3A4 probes.28,60 This could be due to the considerable contribution of CYP2B6 enzymes to nevirapine clearance. Although little is known about the chrono-pharmacokinetics of this pathway, our hypothesis is supported by the fact that CAR, which regulates expression of CYP2B6,21 exhibits a circadian rhythm linked to a 1.7-fold magnitude induction of CYP2B mRNA.62
Despite the 29% amplitude of diurnal variation in nevirapine clearance, the simulated difference between morning and evening trough exposures was <15%. This lack of effect is due to nevirapine's relatively long half-life (25–30 h at steady-state)41 in comparison with, for example, PIs, where the reported median difference in troughs is almost 60%.61 Simulations revealed only a marginal effect of intake time on exposures, but showed that the diurnal variation should be considered when the daily dose of nevirapine cannot be split equally, since 50% difference in the ratio of median Cmin AM/PM was found depending on whether the larger dose is given in the morning or evening. To minimize this effect and the risk of suboptimal exposures, uneven splitting should be implemented with the larger dose given in the morning, which is currently not specified in the WHO guidelines.35
A further innovation of our study was the use of a semi-physiological well-stirred liver model, allowing the effect of hepatic clearance (expressed as intrinsic clearance) on both systemic clearance and first-pass hepatic extraction to be captured, so that clearance and its covariates affect bioavailability. This model allowed us to separate the pre-hepatic and hepatic components of bioavailability.
A significant degree of variability in nevirapine pharmacokinetics was explained in our model by age-driven differences in pre-hepatic bioavailability, which possibly overshadowed the expected effect of maturation of the metabolic pathways. A similar effect was found for nevirapine by Foissac et al.13 and reported for other antiretroviral drugs63 and could hypothetically be caused by reduced drug absorption in neonates and younger children. This may be due to more rapid gastric emptying, smaller gastric volume, higher gastric pH, smaller gastrointestinal absorption area, as well as adherence and palatability issues.64 This phenomenon could explain the subtherapeutic concentrations seen in the youngest age groups in other paediatric studies.1,2,8–12 Our simulations show in particular that individuals in the <6 kg weight-band who are EM and IM are at risk of suboptimal exposures (observed evening Cmin <3 mg/L in 43% and 26% of individuals, respectively).
Despite significant differences in nevirapine pharmacokinetics determined by CYP2B6 genotype, a genotype-driven dose optimization strategy has not been previously suggested. This could be due to the fact that, unlike efavirenz, the relationship between high exposures and toxicity is not strongly apparent.7,16,65 Nonetheless, suboptimal concentrations are of concern, as they could lead to virological failure.7–9,13 To prevent suboptimal exposures we suggest the dose for EM and IM in the lowest weight-band be increased from 100 to 150 mg. Further harmonization of exposures across metabolizer groups could be achieved by 50% reduction of nevirapine dose for SM and USM in all other weight-bands, as >75% of those children had evening Cmin above the 8 mg/L therapeutic upper limit, although this might be of limited clinical relevance. When the daily dose cannot be split equally, larger doses should be given in the morning. The simulated Cmin based on this dose-optimization approach are presented in Figure 4(c). We acknowledge, however, that practical implementation of such a strategy in resource-limited settings would be hindered by restricted access to genotyping and current use of FDCs.
Our study has several limitations. The therapeutic range for nevirapine used in our analysis has not been previously evaluated in children or in African populations. The intake time for the sparse pharmacokinetic data was self-reported and might be inaccurate, given the large variability in absorption parameters, and could inflate the magnitude of the detected diurnal variation. We tried to minimize this effect by excluding samples with uncertain dosage information and BLQ. The detected diurnal effect could hypothetically be further affected by food intake, which was not recorded in our study. However, food has been previously reported not to modify nevirapine bioavailability or clearance.5 Additionally, the analysed trials differed in the morning/evening dose-splitting strategy (see the Methods section), but the model-based approach we employed accounts for this difference.
Conclusions
This is the first study quantifying the combined effect of CYP2B6 516G>T|983T>C on nevirapine clearance in children and classifying metabolizers into four metabolic groups (EM, IM, SM and USM). To prevent subtherapeutic exposures, EM and IM children weighing <6 kg should receive same the dose as those in the 6–10 kg weight-band. Further homogenization of exposures can be achieved by reducing the current recommended dose for SM and USM by 50% in other weight-bands. Additionally, we characterized the effect of diurnal variation on nevirapine pharmacokinetics, and found that it is of limited clinical relevance, possibly due to nevirapine's long half-life. However, this phenomenon should be taken into consideration when daily doses cannot be split equally and larger doses should be given in the morning.
Funding
CHAPAS-1 was supported by the European and Developing Countries Clinical Trials Partnership (EDCTP, 2004.01.H.d2.33011). Cipla Ltd donated first-line antiretrovirals.
CHAPAS-3 was supported by EDCTP (IP.2007.33011.006), Medical Research Council UK, Department for International Development UK and Ministerio de Sanidad y Consumo Spain. Cipla Ltd donated first-line antiretrovirals.
The drug assays were supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (UM1 AI068634, UM1 AI068636 and UM1 AI106701, U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (AI068632).
Transparency declarations
A. B., A. C., V. M., C. K., A. K., A. S. W., D. M. G., D. B. and H. M. have received support through grants from the European Developing Countries Clinical Trials Partnership (EDCTP). A. C., A. K., A. S. W. and D. M. G. have additionally received grants from the Medical Research Council (MRC) UK. H. M. additionally declares support in part by the National Research Foundation of South Africa, grant 90729. A. O. has received support in form of grants from Janssen, ViiV and Tandem Nano, as well as personal fees from Merck and was issued a patent ‘Compositions of efavirenz’. L. W. and P. D.: none to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of any funders.
Supplementary data
Acknowledgements
We thank all the patients and the staff from all the centres involved in the CHAPAS-1 and CHAPAS-3 studies. The studies were a joint collaboration of the following institutions: JCRC, Kampala, Uganda; Baylor, Uganda; JCRC, Gulu, Uganda; University Teaching Hospital, School of Medicine, Lusaka, Zambia; MRC CTU, London, UK; Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands; and University of Cape Town, Cape Town, South Africa.
The Division of Clinical Pharmacology at the University of Cape Town would like to acknowledge Novartis Pharma for their support of the development of pharmacometric skills in Africa.
References
- 1.L'homme RF, Kabamba D, Ewings FM et al. Nevirapine, stavudine and lamivudine pharmacokinetics in African children on paediatric fixed-dose combination tablets. AIDS 2008; 22: 557–65. [DOI] [PubMed] [Google Scholar]
- 2.Poerksen G, Pollock L, Moons P et al. Steady-state nevirapine, lamivudine and stavudine levels in Malawian HIV-infected children on antiretroviral therapy using split Triomune 30 tablets. Antivir Ther 2010; 15: 343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanprapar N, Cressey TR, Chokephaibulkit KPM et al. A chewable pediatric fixed-dose combination tablet of lamivudine, and nevirapine: pharmacokinetics and safety compared with the individual liquid formulations in human immunodeficiency virus-infected children. Pediatr Infect Dis J 2010; 29: 940–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Čolić A, Alessandrini M, Pepper MS. Pharmacogenetics of CYP2B6, CYP2A6 and UGT2B7 in HIV treatment in African populations: focus on efavirenz and nevirapine. Drug Metab Rev 2015; 47: 111–23. [DOI] [PubMed] [Google Scholar]
- 5.Milinkovic A, Martínez E. Nevirapine in the treatment of HIV. Expert Rev Anti Infect Ther 2004; 2: 367–73. [DOI] [PubMed] [Google Scholar]
- 6.Veldkamp AI, van Heeswijk RP, Mulder JW et al. Limited sampling strategies for the estimation of the systemic exposure to the HIV-1 nonnucleoside reverse transcriptase inhibitor nevirapine. Ther Drug Monit 2001; 23: 606–11. [DOI] [PubMed] [Google Scholar]
- 7.de Vries-Sluijs TEMS, Dieleman JP, Arts D et al. Low nevirapine plasma concentrations predict virological failure in an unselected HIV-1-infected population. Clin Pharmacokinet 2003; 42: 599–605. [DOI] [PubMed] [Google Scholar]
- 8.Nikanjam M, Kabamba D, Cressey TR et al. Nevirapine exposure with WHO pediatric weight band dosing: enhanced therapeutic concentrations predicted based on extensive international pharmacokinetic experience. Antimicrob Agents Chemother 2012; 56: 5374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fillekes Q, Mulenga V, Kabamba D et al. Pharmacokinetics of nevirapine in HIV-infected infants weighing 3 kg to less than 6 kg taking paediatric fixed dose combination tablets. AIDS 2012; 26: 1795–800. [DOI] [PubMed] [Google Scholar]
- 10.Pollock L, Else L, Poerksen G et al. Pharmacokinetics of nevirapine in HIV-infected children with and without malnutrition receiving divided adult fixed-dose combination tablets. J Antimicrob Chemother 2009; 64: 1251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swaminathan S, Ramachandran G, Kupparam HKA et al. Factors influencing plasma nevirapine levels: a study in HIV-infected children on generic antiretroviral treatment in India. J Antimicrob Chemother 2011; 66: 1354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis JC, L'homme RF, Ewings FM et al. Nevirapine concentrations in HIV-infected children treated with divided fixed-dose combination antiretroviral tablets in Malawi and Zambia. Antivir Ther 2007; 12: 253–60. [PubMed] [Google Scholar]
- 13.Foissac F, Bouazza N, Frange P et al. Evaluation of nevirapine dosing recommendations in HIV-infected children. Br J Clin Pharmacol 2012; 76: 137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saitoh A, Sarles E, Capparelli E et al. CYP2B6 genetic variants are associated with nevirapine pharmacokinetics and clinical response in HIV-1-infected children. AIDS 2007; 21: 2191–9. [DOI] [PubMed] [Google Scholar]
- 15.Erickson DA, Mather G, Trager WF et al. Characterization of the in vitro biotransformation of the HIV-1 reverse transcriptase inhibitor nevirapine by human hepatic cytochromes P-450. Drug Metab Dispos 1999; 27: 1488–95. [PubMed] [Google Scholar]
- 16.Dickinson L, Chaponda M, Carr DF et al. Population pharmacokinetic and pharmacogenetic analysis of nevirapine in hypersensitive and tolerant HIV-infected patients from Malawi. Antimicrob Agents Chemother 2014; 58: 706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schipani A, Wyen C, Mahungu T et al. Integration of population pharmacokinetics and pharmacogenetics: an aid to optimal nevirapine dose selection in HIV-infected individuals. J Antimicrob Chemother 2011; 66: 1332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyen C, Hendra H, Vogel M et al. Impact of CYP2B6 983T>C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients. J Antimicrob Chemother 2008; 61: 914–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas DW, Gebretsadik T, Mayo G et al. Associations between CYP2B6 polymorphisms and pharmacokinetics after a single dose of nevirapine or efavirenz in African americans. J Infect Dis 2009; 199: 872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bienczak A, Cook A, Wiesner L et al. The impact of genetic polymorphisms on the pharmacokinetics of efavirenz in African children. Br J Clin Pharmacol 2016; 82: 185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.di Masi A, De Marinis E, Ascenzi P et al. Nuclear receptors CAR and PXR: molecular, functional, and biomedical aspects. Mol Aspects Med 2009; 30: 297–343. [DOI] [PubMed] [Google Scholar]
- 22.Wyen C, Hendra H, Siccardi M et al. Cytochrome P450 2B6 (CYP2B6) and constitutive androstane receptor (CAR) polymorphisms are associated with early discontinuation of efavirenz-containing regimens. J Antimicrob Chemother 2011; 66: 2092–8. [DOI] [PubMed] [Google Scholar]
- 23.Vardhanabhuti S, Acosta EP, Ribaudo HJ et al. Clinical and genetic determinants of plasma nevirapine exposure following an intrapartum dose to prevent mother-to-child HIV transmission. J Infect Dis 2013; 208: 662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Guo Y, Wrighton SA et al. Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharmacogenomics J 2011; 11: 274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elens L, Bouamar R, Hesselink DA et al. The new CYP3A4 intron 6 C>T polymorphism (CYP3A4*22) is associated with an increased risk of delayed graft function and worse renal function in cyclosporine-treated kidney transplant patients. Pharmacogenet Genomics 2012; 22: 373–80. [DOI] [PubMed] [Google Scholar]
- 26.Kuypers DRJ, de Loor H, Naesens M et al. Combined effects of CYP3A5*1, POR*28, and CYP3A4*22 single nucleotide polymorphisms on early concentration-controlled tacrolimus exposure in de-novo renal recipients. Pharmacogenet Genomics 2014; 24: 597–606. [DOI] [PubMed] [Google Scholar]
- 27.Monostory K, Tóth K, Kiss Á et al. Personalizing initial calcineurin inhibitor dosing by adjusting to donor CYP3A-status in liver transplant patients. Br J Clin Pharmacol 2015; 80: 1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomalik-Scharte D, Suleiman AA, Frechen S et al. Population pharmacokinetic analysis of circadian rhythms in hepatic CYP3A activity using midazolam. J Clin Pharmacol 2014; 54: 1162–9. [DOI] [PubMed] [Google Scholar]
- 29.van Rongen A, Kervezee L, Brill M et al. Population pharmacokinetic model characterizing 24-hour variation in the pharmacokinetics of oral and intravenous midazolam in healthy volunteers. CPT Pharmacometrics Syst Pharmacol 2015; 4: 454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Heeswijk RP, Veldkamp AI, Mulder JW et al. The steady-state pharmacokinetics of nevirapine during once daily and twice daily dosing in HIV-1-infected individuals. AIDS 2000; 14: F77–82. [DOI] [PubMed] [Google Scholar]
- 31.Mulenga V, Musiime V, Kekitiinwa A et al. Abacavir, zidovudine, or stavudine as paediatric tablets for African HIV-infected children (CHAPAS-3): an open-label, parallel-group, randomised controlled trial. Lancet Infect Dis 2016; 16: 169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulenga V, Cook A, Walker AS et al. Strategies for nevirapine initiation in HIV-infected children taking pediatric fixed-dose combination “baby pills” in Zambia: a randomized controlled trial. Clin Infect Dis 2010; 51: 1081–9. [DOI] [PubMed] [Google Scholar]
- 33.WHO. WHO Recommendations on ARV Medicines for Treating and Preventing HIV Infections in Younger Children, Technical Summary 2006. http://www.who.int/hiv/paediatric/technicalsummary113006.pdf.
- 34.Hollanders RM, van Ewijk-Beneken Kolmer EW, Burger D et al. Determination of nevirapine, an HIV-1 non-nucleoside reverse transcriptase inhibitor, in human plasma by reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 2000; 744: 65–71. [DOI] [PubMed] [Google Scholar]
- 35.WHO. Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access 2010. http://www.who.int/hiv/pub/paediatric/infants2010/en/.
- 36.Beal S, Sheiner L, Boeckmann A et al. NONMEM User Guides (1989–2009). Ellicott City, MD, USA: ICON Development Solutions, 2009. [Google Scholar]
- 37.Keizer RJ, Karlsson MO, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol 2013; 2: e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svensson E, van Der Walt JS, Barnes KI et al. Integration of data from multiple sources for simultaneous modelling analysis: experience from nevirapine population pharmacokinetics. Br J Clin Pharmacol 2012; 74: 465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savic RM, Jonker DM, Kerbusch T et al. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 2007; 34: 711–26. [DOI] [PubMed] [Google Scholar]
- 40.Gordi T, Xie R, Huong NV et al. A semiphysiological pharmacokinetic model for artemisinin in healthy subjects incorporating autoinduction of metabolism and saturable first-pass hepatic extraction. Br J Clin Pharmacol 2005; 59: 189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boehringer Ingelheim International GmbH. Summary of Product Characteristics - Viramune http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000183/WC500051481.pdf.
- 42.Bradley S, Ingelfinger F, Bradley G et al. The estimation of hepatic blood flow in man. J Clin Invest 1945; 24: 890–7. [PubMed] [Google Scholar]
- 43.Anderson BJ, Holford NHG. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 2008; 48: 303–32. [DOI] [PubMed] [Google Scholar]
- 44.Keizer RJ, Zandvliet AS, Beijnen JH et al. Performance of methods for handling missing categorical covariate data in population pharmacokinetic analyses. AAPS J 2012; 14: 601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.CDC. Growth Charts http://www.cdc.gov/growthcharts/percentile_data_files.htm.
- 46.La Porte CJL, Back D, Blaschke T et al. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev Antivir Ther 2006; 3: 5–14. [Google Scholar]
- 47.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2015. http://www.R-project.org/. [Google Scholar]
- 48.Capparelli EV, Aweeka F, Hitti J et al. Chronic administration of nevirapine during pregnancy: impact of pregnancy on pharmacokinetics. HIV Med 2008; 9: 214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chou M, Bertrand J, Segeral O et al. Population pharmacokinetic-pharmacogenetic study of nevirapine in HIV-infected Cambodian patients. Antimicrob Agents Chemother 2010; 54: 4432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elsherbiny D, Cohen K, Jansson B et al. Population pharmacokinetics of nevirapine in combination with rifampicin-based short course chemotherapy in HIV- and tuberculosis-infected South African patients. Br J Clin Pharmacol 2009; 65: 71–80. [DOI] [PubMed] [Google Scholar]
- 51.Kappelhoff BS, van Leth F, MacGregor TR et al. Nevirapine and efavirenz pharmacokinetics and covariate analysis in the 2NN study. Antivir Ther 2005; 10: 145–55. [PubMed] [Google Scholar]
- 52.Moltó J, Valle M, Miranda C et al. Once- or twice-daily dosing of nevirapine in HIV-infected adults: a population pharmacokinetics approach. J Antimicrobal Chemother 2008; 62: 784–92. [DOI] [PubMed] [Google Scholar]
- 53.Zhou X, Sheiner LB, Aquila RTD et al. Population pharmacokinetics of nevirapine, zidovudine, and didanosine in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 1999; 43: 121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dooley KE, Denti P, Martinson N et al. Pharmacokinetics of efavirenz and treatment of HIV-1 among pregnant women with and without tuberculosis co-infection. J Infect Dis 2015; 211: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kappelhoff BS, van Leth F, Robinson PA et al. Are adverse events of nevirapine and efavirenz related to plasma concentrations? Antivir Ther 2005; 10: 489–98. [PubMed] [Google Scholar]
- 56.Lehr T, Yuan J, Hall D et al. Integration of absorption, distribution, metabolism, and elimination genotyping data into a population pharmacokinetic analysis of nevirapine. Pharmacogenet Genomics 2011; 21: 721–30. [DOI] [PubMed] [Google Scholar]
- 57.Haas DW, Kwara A, Richardson DM et al. Secondary metabolism pathway polymorphisms and plasma efavirenz concentrations in HIV-infected adults with CYP2B6 slow metabolizer genotypes. J Antimicrob Chemother 2014; 69: 2175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eagling VA, Wiltshire H, Whitcombe IWA et al. CYP3A4-mediated hepatic metabolism of the HIV-1 protease inhibitor saquinavir in vitro. Xenobiotica 2002; 32: 1–17. [DOI] [PubMed] [Google Scholar]
- 59.Patki KC, Von Moltke LL, Greenblatt DJ. In vitro metabolism of midazolam, triazolam, nifedipine, and testosterone by human liver microsomes and recombinant cytochromes P450: role of CYP3A4 and CYP3A5. Drug Metab Dispos 2003; 31: 938–44. [DOI] [PubMed] [Google Scholar]
- 60.Gupta SK, Lindemulder EA, Sathyan G. Modeling of circadian testosterone in healthy men and hypogonadal men. J Clin Pharmacol 2000; 40: 731–8. [DOI] [PubMed] [Google Scholar]
- 61.Justesen US, Pedersen C. Diurnal variation of plasma protease inhibitor concentrations. AIDS 2002; 16: 2487–9. [DOI] [PubMed] [Google Scholar]
- 62.Kanno Y, Otsuka S, Hiromasa T et al. Diurnal difference in CAR mRNA expression. Nucl Recept 2004; 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Best B, Capparelli E, Diep H. Pharmacokinetics of lopinavir/ritonavir crushed versus whole tablets in children. J Acquir Immune Defic Syndr 2011; 58: 385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.L'homme R, Warris A, Gibb D et al. Children with HIV are not small adults: what is different in pharmacology? Curr Opin HIV AIDS 2007; 2: 405–9. [DOI] [PubMed] [Google Scholar]
- 65.Van Leth F, Kappelhoff BS, Johnson D et al. Pharmacokinetic parameters of nevirapine and efavirenz in relation to antiretroviral efficacy. AIDS Res Hum Retroviruses 2006; 22: 232–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.