Abstract
Background
Pharmacokinetics (PK) and pharmacodynamics of efavirenz and its 8-hydroxy metabolite (8-OH-efavirenz) have not been robustly evaluated in older HIV-infected persons.
Objectives
We investigated relationships between neuropsychological (NP) performance and efavirenz and 8-OH-efavirenz PK in HIV-infected individuals >50 years of age.
Methods
A cross-sectional study of HIV-infected adults on an efavirenz-containing regimen. The 12 and 18 h post-dose plasma efavirenz and 8-OH-efavirenz were quantified. CYP2B6 polymorphisms were investigated. Participants underwent neuropsychological tests; surveys were used for depression, sleep quality and anxiety. We investigated potential correlations of efavirenz and 8-OH-efavirenz plasma concentrations with NP performance, sleep, depression, anxiety and CYP2B6 polymorphisms.
Results
Thirty participants (24 men and 6 women) with mean age 57 years (range 50–68). Plasma efavirenz concentrations did not correlate with NP performance; however, higher plasma 8-OH-efavirenz correlated with better learning (P = 0.002), language (P = 0.002) and total NP z-scores (P = 0.003). No correlation was seen for efavirenz or 8-OH-efavirenz with sleep, anxiety or depression. Median 12 and 18 h efavirenz plasma concentrations were 1967 ng/mL (IQR 1476–2394) and 1676 ng/mL (IQR 1120–2062), respectively. Median 12 and 18 h 8-OH-efavirenz plasma concentrations were 378 ng/mL (IQR 223–589) and 384 ng/mL (IQR 216–621), respectively. CYP2B6 G516T was associated with significantly higher plasma efavirenz at 12 and 18 h (P = 0.02) but not worse NP function.
Conclusions
Better neurocognitive functioning was associated with higher 8-OH-efavirenz but not efavirenz plasma concentrations. No correlation was observed with sleep or depression. These findings point to a need for greater understanding of the metabolic profile of efavirenz and 8-OH-efavirenz in plasma and the CNS and relationships with antiviral effect and neurotoxicity.
Introduction
The US CDC estimates that up to 15% of newly diagnosed cases of HIV infection are among people aged ≥50 years. By 2016, more than one-half of all HIV-infected individuals in the USA will be aged >50 years, not only from new cases but the greatly increased lifespan attributed to ART.1 Assessments of antiretroviral pharmacokinetics (PK) in older HIV-infected patients are sparse and there are no specific dosing guidelines for older patients in contrast to the general geriatric population.2 Studies in older populations demonstrate decrements in liver metabolism and renal clearance, which may require dosage adjustments for drugs eliminated by the kidney. Additionally, decreased bioavailability due to changes in drug transporters alter the PK of many drugs in older populations.3,4 In a relevant study of older HIV-infected patients, trough lopinavir concentrations from 44 subjects showed that older age was associated with higher lopinavir concentrations.3 In another study of 51 patients receiving darunavir, a univariate analysis determined that every 10 years of age lowered the clearance of darunavir by 19%.5 Importantly, efavirenz has been sparsely evaluated in older patients,6 even though this is one of the most commonly prescribed antiretroviral agents.7,8
CNS side effects associated with efavirenz are common and for this reason it is recommended that the drug be taken at bedtime.9 Neuropsychological (NP) performance and symptoms associated with efavirenz use were carefully evaluated in the ACTG study 5097. In this study, individuals on efavirenz experienced an increased rate of bad dreams and had an increased rate of neurological symptoms that correlated with plasma concentrations of efavirenz, but only during the first week of treatment. No differences were seen in depression or anxiety between those on efavirenz and those on other regimens. Six percent of patients discontinued efavirenz as a result of neuropsychiatric side effects.10 Pharmacogenetics (PG) may also play an important role in efavirenz exposure in the older population as previous studies have shown correlations between both efavirenz concentrations and CNS adverse effects and CYP2B6 polymorphisms in adults.11,12
Efavirenz is primarily metabolized in the liver. Clearance of efavirenz occurs predominantly via CYP2B6 to an 8-OH-efavirenz metabolite.13–16 Additionally, there is a subsequent pathway via CYP2A6 to a 7-OH-efavirenz metabolite.13–15 The 8-OH-efavirenz metabolite was found to induce direct neuronal toxicity and death via increased Ca2+ influx in vitro.17 CYP2B6 polymorphisms have been associated with higher plasma concentrations of efavirenz. Subsequently, CYP2B6 polymorphisms have been observed in patients with increased frequency of efavirenz-related side effects and are associated with drug discontinuation.11,12 In addition to CYP2B6, early treatment discontinuation of efavirenz was also associated with a polymorphism in the constitutive androstane receptor.16
The aim of this study was to investigate steady-state PK of efavirenz, in older HIV-infected patients, to correlate efavirenz and 8-OH-efavirenz drug exposure with NP performance and to explore the role of CYP2B6 polymorphisms in efavirenz metabolism in patients >50 years of age.
Patients and methods
A cross-sectional study was conducted at the University of Nebraska Medical Center. Entry criteria were a diagnosis of HIV disease, >50 years of age and on an efavirenz-containing regimen for >12 weeks. Participants were required to be able to provide written informed consent and to complete the questionnaires in English, as not all of the NP screens and questionnaires have been validated in other languages. Exclusion criteria were recent intercurrent acute infection, active psychiatric illness, active neurological disease, current delirium or intoxication and active drug or alcohol abuse.
Participant medical records were accessed to obtain demographic and medical history data, hepatitis C virus (HCV) status, CD4+ T cell count and HIV viral load when available within ≤3 months of study entry. Participants underwent a validated NP battery to evaluate multiple domains most affected by HIV disease including executive function, motor skills, verbal learning, memory and speed of processing. The specific instruments used were: the Wide Range Achievement Test 4 (WRAT-4) Reading Test; Timed Gait; Hopkins Verbal Learning Test—Revised; Trailmaking A and B; Grooved Pegboard; the Wechsler Adult Intelligence Scale (WAIS-3) Digit Symbol; Verbal or Letter Fluency; and the Stroop Interference Task. Normative standards were used, which are corrected for age, education, sex and ethnicity as appropriate. The validated questionnaires administered were the Center for Epidemiologic Studies Depression Scale (CES-D) to assess for depression and the Pittsburgh Sleep Quality Index to assess for sleep.
Participants were asked to record the time of efavirenz dosing the night before and plasma samples were collected 12 and 18 h post-dose for measurement of efavirenz and 8-OH-efavirenz concentrations. The sampling schedule was chosen as efavirenz is typically dosed daily in the evening and PK/pharmacodynamic relationships have been established for mid-dosing interval efavirenz concentrations, including risk of both CNS side effects and virological failure.18 In addition, whole blood was collected for PG analysis. Efavirenz and its 8-OH metabolite concentrations were quantified via LC-MS/MS19,20 in the Antiviral Pharmacology Laboratory of the University of Nebraska Medical Center.
PG analyses were performed at the University of Liverpool. Genotyping was conducted by real-time PCR-based allelic discrimination using standard methodology.16 The genes of interest include genes coding for proteins involved in phase I metabolism (CYP2B6, CYP3A4, CYP2D6 and CYP2A6), phase II metabolism (UGT2B7) and factors of transcriptional regulation of drug disposition (PXR and CAR).
Ethics
The study was approved by the University of Nebraska Institutional Review Board (IRB no. 209-13-FB). All study patients provided signed informed consent.
Statistical analysis
Participant characteristics and outcomes were descriptively summarized using medians, range and proportions. The median and IQR were used to report drug and metabolite concentrations. Raw NP scores were standardized to z-scores (NPZ) in order to adjust for age and education. Pearson's correlation test was used to examine correlations of plasma efavirenz and 8-OH-efavirenz concentrations with NP test results. Bonferroni correction was applied for tests with multiple comparisons. A P value <0.01 (Bonferroni correction) was used to define significance for correlations between efavirenz and 8-OH-efavirenz and NP test results.
Results
Thirty participants were recruited. There were 24 men and 6 women; 22 white and 8 black.
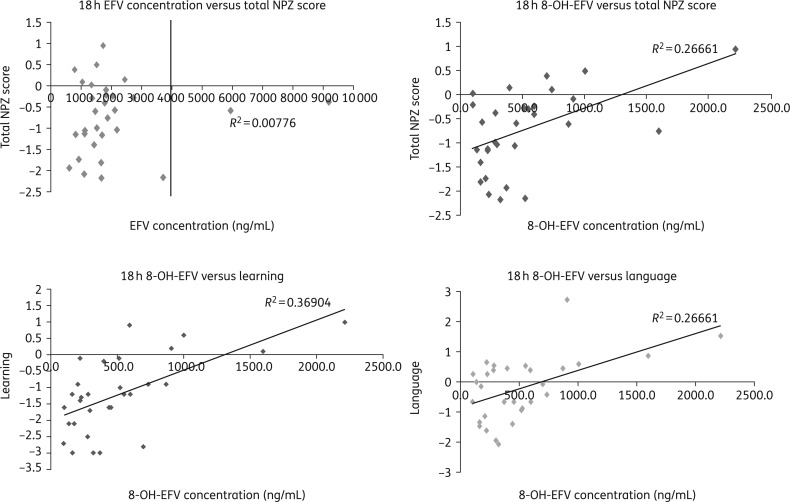
Patient characteristics and median 12 and 18 h efavirenz and 8-OH-efavirenz concentrations are summarized in Table 1. When looking at NP performance, 21 participants scored less than −1 in two domains and 12 overall had composite NPZ scores below −1. Plasma concentrations of efavirenz did not correlate with NP performance (Table 1 and Figure 1). Conversely, higher 8-OH-efavirenz plasma concentrations at 12 and 18 h correlated with better learning (P = 0.002), language (P = 0.002) and total NPZ scores (P = 0.003) (Table 1 and Figure 1).
Table 1.
Patient characteristics, median 12 and 18 h efavirenz and 8-OH-efavirenz concentrations and correlations between efavirenz and 8-OH-efavirenz concentrations and neuropsychological tests
| Variable | Value |
Range (IQR) |
|||
|---|---|---|---|---|---|
| Median age | 57 | 50–68 | |||
| Median time on ART, years | 11.25 | 1–22.8 (10.92) | |||
| Median CD4 cells/mm3 | 657 | 145–2062 (463) | |||
| Nadir CD4 cells/mm3 | 259 | 7–769 (342) | |||
| HIV RNA <20 copies/mL | 30a | — | |||
| Hepatitis C antibody | 3b | — | |||
| PK determination (median), ng/mL | |||||
| 12 h EFV | 1967 | IQR 1476–2394 | |||
| 18 h EFV | 1676 | IQR 1120–2062 | |||
| 12 h 8-OH-EFV | 378 | IQR 223–589 | |||
| 18 h 8-OH-EFV | 384 | IQR 216–621 | |||
| Efavirenz |
8-OH-efavirenz |
||||
| NP test | 12 h post | 18 h post | 12 h post | 18 h post | P valuec |
| Total z | 0.04 | 0.09 | 0.38 | 0.52 | 0.003 |
| Motor | 0.13 | 0.15 | 0.20 | 0.37 | 0.044 |
| Learning | 0.1 | 0.11 | 0.52 | 0.61 | 0.002 |
| Memory | 0.05 | 0.13 | 0.27 | 0.2 | 0.24 |
| Language | 0.05 | 0.08 | 0.5 | 0.53 | 0.002 |
| Executive | −0.03 | −0.05 | 0.16 | 0.29 | 0.12 |
| Speed | 0.11 | −0.02 | 0.31 | 0.42 | 0.02 |
| Fluency | 0.24 | 0.24 | 0.14 | 0.24 | 0.2 |
P values considered significant if <0.01. PK, pharmacokinetics; EFV, efavirenz; 8-OH-EFV, 8-hydroxy efavirenz.
aTwenty-six participants had HIV RNA <20 copies/mL and four had HIV RNA <100 copies/mL, but all four had HIV RNA <20 copies/mL on subsequent measurements.
bThree participants had positive HCV antibodies, all had undetectable HCV viraemia at the time of study. Two participants had been previously treated for HCV and one had spontaneously cleared viraemia.
cAdjusted with Bonferroni correction.
Figure 1.
Correlation of efavirenz (EFV) and 8-OH-EFV concentrations and neuropsychological performance. Not all comparisons are included.
Twenty-one participants had poor sleep quality (score >5 on Pittsburgh Sleep Quality Index) with a median score of 7.5 (range 1–16; IQR = 6). The median CES-D score was 15. Fourteen participants had increased risk of clinical depression (CES-D score >16). There was no significant difference in the median 12 and 18 h efavirenz or 8-OH-efavirenz concentrations between participants with or without depression or between those with poor or better sleep scores. Neither higher or lower efavirenz nor 8-OH-efavirenz plasma concentrations correlated with sleep disturbance, anxiety or depression. No correlation was seen between 8-OH-efavirenz concentrations and age of participants.
Three participants were homozygous for the CYP2B6 G516T polymorphism; two of the three had elevated 12 h (5634 and 8962 ng/mL) and 18 h (5944 and 9194 ng/mL) plasma efavirenz concentrations while the remaining participant had efavirenz concentrations within normal range (12 h = 2562 and 18 h = 2114 ng/mL).
Presence of the CYP2B6 G516T polymorphism was associated with significantly higher concentrations of efavirenz at 12 and 18 h (P = 0.02), but did not correlate with worse NP function.
Discussion
The findings of our study were unexpected. As the 8-OH metabolite of efavirenz has been shown to be neurotoxic in vitro, we expected worse NP function in patients with higher 8-OH-efavirenz concentrations. In contrast, we found that better neurocognitive function was associated with both higher 12 and 18 h 8-OH-efavirenz plasma concentrations. These findings may be explained by previous clinical data that have correlated higher efavirenz concentrations with greater risk of CNS side effects.18 Patients with faster clearance of plasma efavirenz could potentially have higher 8-OH-efavirenz concentrations and lower efavirenz concentrations by nature of clearing efavirenz more quickly, thus leading to lower efavirenz exposure and ultimately less severe CNS adverse effects. Winston et al.21 found a positive correlation between increased frequency of efavirenz-related side effects and CSF 8-OH-efavirenz exposure. Interestingly, there was no correlation between plasma efavirenz or 8-OH-efavirenz and CSF 8-OH-efavirenz concentrations and the authors have proposed that higher exposure to 8-OH-efavirenz in CSF may be related to saturation effects or local efavirenz metabolic pathways in the blood–brain barrier.21 In contrast to the findings by Johnson et al.,22 where abnormal NP performance was found in a group of HIV-negative participants after a single dose of efavirenz, no correlation was found between higher or lower efavirenz concentrations and NP performance in our cohort. Although this can be explained by differences between the population studied by Johnson et al.22 and the patients in our study, who could have self-selected for tolerance of efavirenz CNS effects as they all came into the study with a long history of efavirenz treatment. We were not able to replicate the findings by Gallego et al.,23 where higher efavirenz plasma concentrations were seen in those with insomnia or reduced sleep efficiency, but we must note that mean efavirenz concentrations were higher than in our study. NP performance in our cohort showed a negatively skewed curve; moreover, two-thirds had NPZ scores of less than −1 in two or more domains, which would classify them as being impaired. If we consider total NPZ scores, 12 participants would be classified as impaired.24 This is consistent with NP performance of other chronically HIV-infected patients.6
Limitations of this study include that it was a single-centre study with a small sample size. Moreover, the cohort used in our study had long-standing HIV disease and long history of exposure to efavirenz-containing regimens. These attributes may have rendered our cohort less likely to report NP issues related to efavirenz use. Additionally, we did not have a control group or CSF samples for efavirenz or 8-OH-efavirenz determination.
Although the PK of 8-OH-efavirenz has not been extensively studied in vivo, it is possible that this metabolite does not cross the blood–brain barrier as effectively as efavirenz. 8-OH-efavirenz concentrations in the CSF would be dependent on both 8-OH-efavirenz penetration into the CSF as well as local metabolism of the parent drug in CSF. If this was true, we would expect to find higher CSF concentrations of 8-OH-efavirenz in those with higher concentrations of efavirenz in the CSF only. This hypothesis may in part explain better function related to higher plasma 8-OH-efavirenz, as less efavirenz would be transported in the CSF and the 8-OH metabolite would be compartmentalized in plasma.25
These findings point to a need for greater understanding of the metabolic profile of efavirenz and 8-OH-efavirenz in plasma and the CNS and relationships with antiviral effect and potential neurotoxicity.
Funding
This work was supported by NIH grant P30 MH062261 to Dr Howard Fox.
Transparency declarations
None to declare.
Acknowledgements
This work was partially presented at the 22nd Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 23–26 February 2015 as Oral Abstract no. 84. We thank Dr Howard Fox for his ongoing support, Deanna Hansen for administrative support and we also thank the participants for their invaluable contribution in research studies.
References
- 1.High KP, Brennan-Ing M, Clifford DB et al. . HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012; 60 Suppl 1: S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanlon JT, Aspinall SL, Semla TP et al. . Consensus guidelines for oral dosing of primarily renally cleared medications in older adults. J Am Geriatr Soc 2009; 57: 335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford KW, Spritzler J, Kalayjian RC et al. . Age-related changes in plasma concentrations of the HIV protease inhibitor lopinavir. AIDS Res Hum Retroviruses 2010; 26: 635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilmer SN, McLachlan AJ, Le Couteur DG. Clinical pharmacology in the geriatric patient. Fundam Clin Pharmacol 2007; 21: 217–30. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson LJA, Garvey L, Watson V. et al Population pharmacokinetic modelling of plasma and intracellular once daily ritonavir-boosted darunavir in HIV-infected patients In: Abstracts of the Twelfth International Workshop on Clinical Pharmacology of HIV Therapy, Miami, 2011 Abstract O12. [Google Scholar]
- 6.Winston A, Arenas-Pinto A, Stohr W et al. . Neurocognitive function in HIV infected patients on antiretroviral therapy. PLoS One 2013; 8: e61949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. What's New in HIV Treatment. http://apps.who.int/iris/bitstream/10665/204347/1/WHO_HIV_2015.44_eng.pdf.
- 8.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- 9.Sustiva Package Insert. Bristol-Myers Squibb, Princeton, NJ, USA http://packageinserts.bms.com/pi/pi_sustiva.pdf.
- 10.Clifford DB, Evans S, Yang Y et al. . Long-term impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals (ACTG 5097s). HIV Clin Trials 2009; 10: 343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas DW, Ribaudo HJ, Kim RB et al. . Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS 2004; 18: 2391–400. [PubMed] [Google Scholar]
- 12.Ribaudo HJ, Haas DW, Tierney C et al. . Pharmacogenetics of plasma efavirenz exposure after treatment discontinuation: an Adult AIDS Clinical Trials Group study. Clin Infect Dis 2006; 42: 401–7. [DOI] [PubMed] [Google Scholar]
- 13.Avery LB, VanAusdall JL, Hendrix CW et al. . Compartmentalization and antiviral effect of efavirenz metabolites in blood plasma, seminal plasma, and cerebrospinal fluid. Drug Metab Dispos 2013; 41: 422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.di Iulio J, Fayet A, Arab-Alameddine M et al. . In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet Genomics 2009; 19: 300–9. [DOI] [PubMed] [Google Scholar]
- 15.Markwalder JA, Christ DD, Mutlib A et al. . Synthesis and biological activities of potential metabolites of the non-nucleoside reverse transcriptase inhibitor efavirenz. Bioorg Med Chem Lett 2001; 11: 619–22. [DOI] [PubMed] [Google Scholar]
- 16.Wyen C, Hendra H, Siccardi M et al. . Cytochrome P450 2B6 (CYP2B6) and constitutive androstane receptor (CAR) polymorphisms are associated with early discontinuation of efavirenz-containing regimens. J Antimicrob Chemother 2011; 66: 2092–8. [DOI] [PubMed] [Google Scholar]
- 17.Tovar-y-Romo LB, Bumpus NN, Pomerantz D et al. . Dendritic spine injury induced by the 8-hydroxy metabolite of efavirenz. J Pharmacol Exp Ther 2012; 343: 696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzolini C, Telenti A, Decosterd LA et al. . Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 2001; 15: 71–5. [DOI] [PubMed] [Google Scholar]
- 19.Mutlib AE, Chen H, Nemeth GA et al. . Identification and characterization of efavirenz metabolites by liquid chromatography/mass spectrometry and high field NMR: species differences in the metabolism of efavirenz. Drug Metab Dispos 1999; 27: 1319–33. [PubMed] [Google Scholar]
- 20.Winchester L, Sandkovsky U, Fletcher CV et al. . Quantification of efavirenz and its 7-hydroxy and 8-hydroxy metabolites in human plasma via LC/MS/MS In: Abstracts of the Annual American Association of Pharmaceutical Scientists Meeting, Orlando, FL, 2015. Poster R6190 American Association of Pharmaceutical Scientists, Arlington, VA, USA. [Google Scholar]
- 21.Winston A, Amin J, Clarke A et al. . Cerebrospinal fluid exposure of efavirenz and its major metabolites when dosed at 400 mg and 600 mg once daily: a randomized controlled trial. Clin Infect Dis 2015; 60: 1026–32. [DOI] [PubMed] [Google Scholar]
- 22.Johnson DH, Gebretsadik T, Shintani A et al. . Neuropsychometric correlates of efavirenz pharmacokinetics and pharmacogenetics following a single oral dose. Br J Clin Pharmacol 2013; 75: 997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallego L, Barreiro P, del Rio R et al. . Analyzing sleep abnormalities in HIV-infected patients treated with efavirenz. Clin Infect Dis 2004; 38: 430–2. [DOI] [PubMed] [Google Scholar]
- 24.Arenas-Pinto A, Winston A, Stohr W et al. . Neurocognitive function in HIV-infected patients: comparison of two methods to define impairment. PLoS One 2014; 9: e103498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smurzynski M, Wu K, Letendre S et al. . Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS 2011; 25: 357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]