Abstract
The University of Southern California/Van Nuys Prognostic Index is an algorithm that quantifies five measurable prognostic factors known to be important in predicting local recurrence in conservatively treated patients with ductal carcinoma in situ (tumor size, margin width, nuclear grade, age, and comedonecrosis). With three times as many patients since originally developed, sufficient numbers now exist for analysis by individual scores rather than groups of scores. To achieve a local recurrence rate of less than 20% at 12 years, these data support excision alone for all patients scoring 4, 5, or 6 and patients who score 7 but have margin widths ≥3 mm. Excision plus RT achieves the less than 20% local recurrence requirement at 12 years for patients who score 7 and have margins <3 mm, patients who score 8 and have margins ≥ 3 mm, and for patients who score 9 and have margins ≥5 mm. Mastectomy is required for patients who score 8 and have margins <3 mm, who score 9 and have margins <5 mm, and for all patients who score 10, 11, or 12 to keep the local recurrence rate less than 20% at 12 years. These recommendations in this article represent substantial changes from those previously published.
In 2008, the National Comprehensive Cancer Network included excision alone as an acceptable treatment alternative for patients with ductal carcinoma in situ (DCIS), but they did not define the subset of patients in which excision without radiation therapy was appropriate (1). Researchers have attempted to accomplish this for years but only with marginal success.
Multivariate analysis has shown that six factors are independent predictors of local recurrence in patients with DCIS treated with breast conservation: treatment (radiation therapy yields a lower local recurrence rate than excision alone), age (older age is better), size (smaller size is better), nuclear grade (lower grade is better), margin width (wider margins are better), and comedonecrosis (no necrosis is better) (2–4).
In 1995, the Van Nuys Classification predicted local recurrence using a combination of nuclear grade and necrosis (5). In 1996, the Van Nuys Prognostic Index (VNPI) added size and margin width to the numerical algorithm (6), and in 2002, the University of Southern California/VNPI (USC/VNPI) added age at diagnosis to the algorithm (4,7). These studies collected all pathological features in a prospective fashion but treatment (excision alone vsversus excision plus radiation therapy) was not randomized.
The USC/VNPI was devised by combining four statistically significant independent prognostic factors for local tumor recurrence (tumor size, margin width, pathological classification [determined by nuclear grade and the presence or absence of comedo-type necrosis] and age). A score, ranging from 1 for lesions with the best prognosis to 3 for lesions with the worst prognosis, was given for each of the four prognostic predictors. Table 1 details the scoring system. Scores range from a low of 4 (least likely to recur) to a high of 12 (most likely to recur).
Table 1.
Scoring system for University of Southern California/Van Nuys Prognostic Index
| Score | 1 | 2 | 3 |
| Size | ≤15 mm | 16–40 | >40 |
| Margin | ≥10 mm | 1–9 | <1 |
| Class | Grade 1/2 without necrosis | Grade 1/2 with necrosis | Grade 3 |
| Age | >60 | 40–60 | <40 |
Current recommendations are as follows:
Excision alone for those who score 4, 5, or 6.
Excision plus radiation therapy for those who score 7, 8, or 9.
Mastectomy for those who scored 10, 11, or 12.
This article will use the USC/VNPI to analyze local recurrence rates and to update and refine treatment recommendations in a large series of patients with pure DCIS in whom all histopathological factors were collected within a prospective database. When originally published in 1996, the Index was based on 333 patients (6). With three times as many patients accrued since originally described, sufficient numbers of patients now exist for analysis by individual score rather than groups of scores.
Methods
Through April 2009, 1437 patients with pure DCIS were treated. No patients with invasive cancer, no matter how small the invasive focus, were included. A total of 488 patients were treated with mastectomy and, therefore, did not have the ipsilateral breast at risk for local recurrence. They were excluded in this analysis.
The subjects of this article are 949 patients treated with breast conservation, 345 with excision and radiation therapy, and 604 with excision alone. No patient received any form of chemotherapy or endocrine therapy. Treatment was not randomized. Patient preference, after full disclosure and discussion of available data, was the major factor in the treatment decision-making process.
Every effort was made to excise all lesions completely and to examine microscopically all excised tissue. Localization by needle, intraoperative radiography of the specimen, and correlation with the preoperative mammogram were performed in every case involving a nonpalpable tumor. Margins were marked with ink or dye, and the specimens were serially sectioned.
Pathological Evaluation
Tissue sections were arranged and prepared for evaluation in sequence. Pathological evaluation included determination of the histological subtype, nuclear grade, the presence or absence of comedonecrosis, the maximal extent of the lesion, and margin width. The size of small lesions was determined by direct measurement (ocular micrometry) of stained slides. The size of large lesions was determined by a combination of direct measurement and calculation according to three-dimensional reconstruction with a sequential series of slides. This approach is now the recommended protocol of the College of American Pathologists (8,9).
Margin width was determined by direct measurement (ocular micrometry). The smallest single distance between the edge of the tumor and an inked line delineating the margin of normal tissue was reported. Margins in patients who underwent re-excision and in whom no additional DCIS was found were reported as being equivalent to10 mm in width.
Tumors were divided into three groups by using the Van Nuys DCIS Classification: group 1 = low or intermediate nuclear grade without necrosis; group 2 = low or intermediate nuclear grade with necrosis; and group 3 = high nuclear grade with or without necrosis.
Statistical Analysis
The outcome measure used was time to local recurrence, calculated as the time from tumor excision to the date of local recurrence. All ipsilateral tumor events, regardless of in which quadrant they occurred, were scored as local recurrences. Data from patients who did not have a local recurrence were censored at the date of last follow-up. Kaplan–Meier plots were used to estimate the probability of remaining free of local recurrence at 12 years. The statistical significance between survival curves was determined by the log-rank test.
In previous articles, patients were grouped by USC/VNPI scores of 4, 5, and 6; 7, 8, and 9; and 10, 11, and 12. In this study, all analyses were done by individual scores from 4 to 12. The goal was to define the parameters necessary to allow a local recurrence rate of less than 20% at 12 years for each individual score. Less than 20% local recurrence rate at 12 years was an arbitrary choice but seem reasonable based on previously reported prospective randomized data. Although mastectomy patients were excluded from the analysis, when a local recurrence of less than 20% could not be achieved by varying margin width and radiation therapy for a given USC/VNPI score, the default treatment was mastectomy since that procedure always achieves a local recurrence rate of less than 5% (Table 2).
Table 2.
New treatment recommendations to achieve a local recurrence rate of less than 20% at 12 years using the University of Southern California/Van Nuys Prognostic Index (USC/VNPI)
| USC/VNPI | Treatment | 12-yr recur, % |
| 4, 5 or 6 | Excision alone | ≤6 |
| 7, margins ≥3 mm | Excision alone | 16 |
| 7, margins <3 mm | Radiation | 14 |
| 8, margins ≥3 mm | Radiation | 15 |
| 8, margins <3 mm | Mastectomy | 1 |
| 9, margins ≥5 mm | Radiation | 19 |
| 9, margins <5 mm | Mastectomy | 1 |
| 10, 11, or 12 | Mastectomy | 4 |
Local recurrence rates, regardless of treatment were so low for all patients who scored 4, 5, or 6 (<6%) that they were grouped together in the final analysis. Local recurrence rates, regardless of treatment, were so high for patients who scored 10, 11, or 12 (>40%) that they were also grouped together in the final analysis. Patients who scored 7, 8, or 9 are shown by individual score.
Results
A total of 949 patients were treated with breast conservation; 345 with excision and postoperative radiation therapy and 604 with excision alone. There were 165 local recurrences: 103 among patients who underwent excision only (37 invasive carcinoma and 66 DCIS) and 62 among patients treated with excision plus postoperative radiation therapy (34 invasive carcinoma and 28 DCIS). The median follow-up was 86 months for all patients, 109 months for patients who received radiation therapy and 75 months for patients treated with excision only. 142 of 165 local recurrences (86%) were at or near the site of the original lesion and were probably true local recurrences or persistences of original disease. Eight patients developed metastatic breast cancer after local invasive recurrence, seven of whom died from breast cancer. Sixty patients died from causes not related to breast cancer.
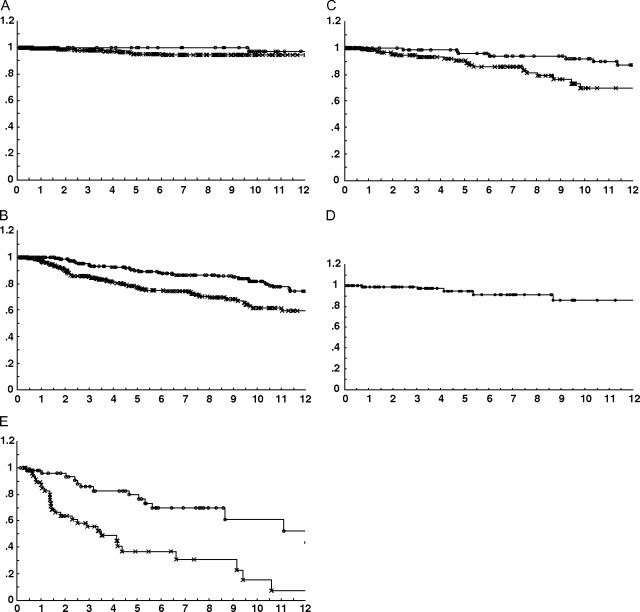
Figure 1, A shows 320 patients with scores of 4, 5, or 6 analyzed by treatment (excision alone vs excision plus radiation therapy). The local recurrence rate at 12 years for those who received radiation therapy was 2.5%. For those treated with excision alone, it was 5.4% (P = NS). When analyzed by individual score, those who scored 4, 5, or 6, regardless of treatment (excision alone or excision plus radiation therapy), had a local recurrence rate of 6% or less at 12 years.
Figure 1.
A) A total of 320 patients with scores of 4, 5, or 6 analyzed by treatment (excision alone vs excision plus radiation therapy); B) 529 patients who scored 7, 8, or 9; C) 219 patients who scored 7; D) 86 patients who scored 7 and who had margin widths of 3 mm or more; E) 98 patients who scored 10, 11, or 12.
Figure 1, B shows 529 patients who scored 7, 8, or 9. Neither treatment line meets the less than 20% local recurrence guideline at 12 years, and so each score was analyzed individually.
Figure 1, C shows 219 patients who scored 7. The local recurrence rate at 12 years for those who scored 7 and received radiation therapy was 13%, but for those treated with excision alone, it was 30%. The next step was to analyze various margin widths for those who scored 7 and were treated with excision alone to find the margin width necessary to lower the local recurrence rate to less than 20% at 12 years.
Figure 1, D shows 86 patients who scored 7 and who had margin widths of 3 mm or more. This subgroup had a local recurrence rate of 16% at 12 years, meeting the requirement of less than 20% at 12 years.
This process was repeated for patients who scored 8–12. The treatment necessary to achieve a local recurrence rate less than 20% at 12 years is given in Table 2.
Figure 1, E shows 98 patients who scored 10, 11, or 12. Regardless of margin width, no group could achieve a local recurrence rate less than 40% with radiation therapy.
Discussion
With almost three times as many patients as originally published, the USC/VNPI can be more finely tuned to aid in the treatment decision-making process. To achieve a local recurrence rate of less than 20% at 12 years, these data support excision alone for all patients scoring 4, 5, or 6 and patients who score 7 but have margin widths ≥3 mm.
Excision plus radiation therapy achieves the less than 20% local recurrence requirement at 12 years for patients who score 7 and have margins <3 mm, patients who score 8 and have margins ≥3 mm, and for patients who score 9 and have margins ≥5 mm.
Mastectomy is required for patients who score 8 and have margins <3 mm, who score 9 and have margins <5 mm, and for all patients who score 10, 11, or 12 to keep the local recurrence rate less than 20% at 12 years.
If the closest margin width is less than 10 mm and the patient is amenable to re-excision, the USC/VNPI can theoretically be lowered by 1–2 points. Margin width is the only variable under surgical control. Grade cannot be lowered and size cannot be reduced by re-excision.
The recommendations in this article represent substantial changes from those previously published.
References
- 1.Carlson RW, Allred DC, Anderson BO, et al. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Fort Washington, PA: National Comprehensive Cancer Network; 2008. www.nccn.org. 2008. Accessed September 15, 2010. [Google Scholar]
- 2.Silverstein MJ. Predicting local recurrence in patients with ductal carcinoma in situ. In: Silverstein MJ, editor. Ductal Carcinoma In Situ of the Breast. 1st ed. Baltimore, MD: Williams and Wilkins; 1997. pp. 271–284. [Google Scholar]
- 3.Silverstein MJ. Prognostic factors and local recurrence in patient with ductal carcinoma in situ of the Breast. Breast J. 1998;4:349–362. [Google Scholar]
- 4.Silverstein MJ. The University of Southern California/Van Nuys prognostic index. In: Silverstein MJ, Recht A, Lagios M, editors. Ductal Carcinoma In Situ of the Breast. 2nd ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2002. pp. 459–473. [Google Scholar]
- 5.Silverstein MJ, Poller D, Waisman J, et al. Prognostic classification of breast ductal carcinoma-in-situ. Lancet. 1995;345(8950):1154–1157. doi: 10.1016/s0140-6736(95)90982-6. [DOI] [PubMed] [Google Scholar]
- 6.Silverstein MJ, Poller D, Craig P, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer. 1996;77(11):2267–2274. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2267::AID-CNCR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 7.Silverstein MJ. The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am J Surg. 2003;186(4):337–343. doi: 10.1016/s0002-9610(03)00265-4. [DOI] [PubMed] [Google Scholar]
- 8.Lester S, Bose S, Chen Y-Y, et al. Protocol for the Examination of Specimens From Patients With Ductal Carcinoma In Situ (DCIS) of the Breast. Northfield, IL: American College of Pathologists; 2009. http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2009/BreastDCIS_09protocol.pdf. Accessed January 6, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Lester S, Bose S, Chen Y-Y, et al. Protocol for the examination of specimens from patients with ductal carcinoma in situ (DCIS) of the Breast. Arch Pathol Lab Med. 2009;133(1):15–25. doi: 10.5858/133.1.15. [DOI] [PubMed] [Google Scholar]