Abstract
Background
The National Institutes of Health Office of Medical Applications of Research commissioned a structured literature review on the incidence of ductal carcinoma in situ (DCIS) as a background paper for the State of the Science Conference on Diagnosis and Management of DCIS.
Methods
Published studies were abstracted from MEDLINE and other sources. We include articles published through January 31, 2009; 92 publications were abstracted.
Results
DCIS incidence rose from 1.87 per 100 000 in 1973–1975 to 32.5 per 100 000 in 2005. Increases in incidence were greatest in tumors without comedo necrosis. Incidence increased in all ages but more in women older than 50 years. Increased use of mammography explains some but not all of the increased incidence. Risk factors for incident DCIS include older age and positive family history. Whereas tamoxifen prevents both invasive breast cancer and DCIS, raloxifene is associated with decreased invasive breast cancer but not decreased DCIS.
Conclusions
Scientific questions deserving further investigation include the relationship between mammography use and DCIS incidence and the role of chemoprevention for reducing the incidence of DCIS and invasive breast cancer.
Background
Ductal carcinoma in situ (DCIS) is a noninvasive breast cancer that encompasses a wide spectrum of diseases ranging from low-grade lesions that are not life threatening to high-grade lesions that may harbor foci of invasive breast cancer. The epidemiology of DCIS is intertwined with that of invasive breast cancer. This article summarizes information on the incidence and prevalence of DCIS and its specific pathological subtypes, and on how incidence and prevalence are influenced by mode of detection, population characteristics, and other risk factors. This review does not address issues of DCIS incidence in women with a history of DCIS or breast cancer, or predictors of second primaries or recurrence of DCIS.
Methods
Studies were sought from a wide variety of sources, including MEDLINE via PubMed, Scirus, and Cochrane databases; websites of the Sloane Project and of the International Breast Cancer Screening Network; and manual searches of reference lists from systematic reviews and consensus conferences. We include articles published from 1965 through January 31, 2009.
We searched MESH headings, titles, and abstracts for the terms Ductal Carcinoma In Situ, DCIS, noninfiltrating intraductal carcinoma, carcinoma in situ, intraductal carcinoma, localized breast cancer, and stage 0 breast cancer. We did not exclude studies by level of evidence. We reviewed abstracts to confirm eligible target populations of female adults. We excluded studies of invasive breast cancer only, non-breast cancers, and animal or in vitro experiments and analysis of results from other publications, letters, comments, and case reports. We abstracted 92 publications. This article includes a highly abbreviated reference list.
Results
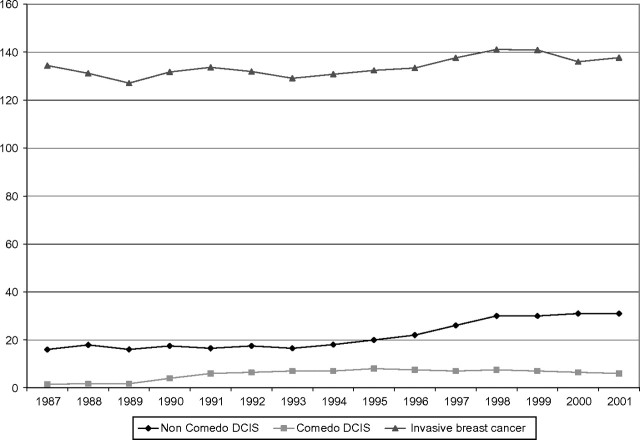
The incidence of DCIS rose from 1.87 per 100 000 women from 1973–1975 to 32.5 per 100 000 in 2005 (1). This increase was observed in all age categories with the greatest rise among those older than 50 years of age. The increase in DCIS has not been uniform across histological types. Comedo histology is associated with a particularly high risk of recurrence and has been stable over recent years, whereas low-grade DCIS, generally considered to be less likely to recur or develop into invasive breast cancer, has accounted for the majority of the recent increase (2) (Figure 1).
Figure 1.
Trends in age-adjusted comedo and non-comedo DCIS and invasive breast cancer per 100 000 women (1,2).
Demographic Variation in DCIS Incidence
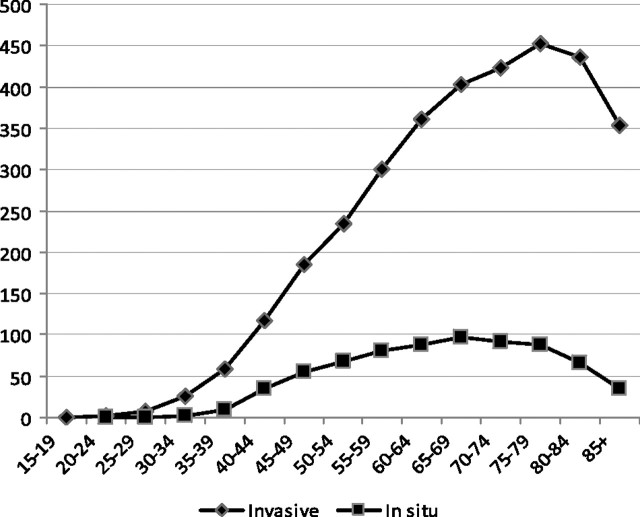
The incidence of DCIS, like invasive breast cancer, is strongly related to age. DCIS is extremely uncommon before age 35–39. After that, the incidence rises steadily to a peak of 96.7 per 100 000 at ages 65–69 and then declines slowly until age 79 and steeply after that. In contrast, invasive breast cancer peaks at age 75–79 with incidence of 453.1 per 100 000 women (Figure 2). At no age is DCIS more common than invasive breast cancer. Between the ages of 40 and 64, between 21% and 22.8% of all breast cancers are DCIS. Before age 40 and after age 64, the proportion of breast cancers that are DCIS drops to as low as 9%. The change in incidence of DCIS over time increases in all age groups but are the greatest among women older than 50 years.
Figure 2.
Incidence of DCIS and invasive breast cancer by age (2002–2006) (1).
The age-adjusted incidence of DCIS was the highest among Caucasian women followed by African American and Asian-Pacific Islanders (3). Hispanic women had the lowest age-adjusted incidence of DCIS. The lower rates of DCIS for African American, Asian, and Hispanic women are coupled with lower rates of invasive cancer. Thus, the evidence does not suggest that lower rates of DCIS in nonwhites should be viewed as indicating a failure to diagnose breast cancer early but could be related to lower underlying risk of breast cancer.
Other DCIS Risk Factors
Several well-designed studies found that women who were older at the time of first birth or had no children had a higher risk of developing DCIS than women who were younger. Similarly, studies generally found that women who had more children had lower risk of DCIS than women who had more (4,5).
The effect of oral contraceptive use and DCIS was examined in five studies (6–10). No study found an association between ever use of oral contraceptive (6–9) or past use (9) and DCIS incidence. Neither of the studies evaluating duration of oral contraceptive use found an association with DCIS incidence (8,9).
The association between hormone replacement therapy (HRT) and DCIS was examined in both observational and randomized studies. The five observational studies using varying definitions of use were generally unable to distinguish between estrogen plus progestin and estrogen alone. A large prospective cohort study from the United Kingdom found a 56% increased risk of DCIS in current users of HRT compared with never users (11). Other US-based studies found that the increased risk of DCIS with HRT varied with duration of use. Current users of HRT for less than 5 years compared with never users had statistically significantly less risk of DCIS than nonusers (pooled relative risk [RR] = 0.78), whereas current users of HRT for more than 5 years had greater risk of DCIS compared with never users (pooled RR = 1.41) (12,13). Two randomized trials of HRT using estrogen plus progestin failed to show any association between HRT and DCIS. The Women’s Health Initiative found no increased risk of DCIS associated with HRT (14). The Million Women Study cohort, failed to comment on whether they observed any increase in DCIS associated with HRT use.
Although a variety of definitions were used, studies consistently found that increased breast density was associated with increased risk of DCIS. For example, women with a mean breast density of more than 45 cm2 also had greater odds of DCIS than women with a low breast density of less than 15 cm2 (odds ratio [OR] 2.59, 95% CI = 1.39 to 4.82) (15).
The association between body composition and body mass index is mixed and not widely studied. For example, the Iowa Women's Health study did not find decreased risk of DCIS to be associated with body mass index. In contrast, Kerlikowske found that heavily obese (body mass index ≥35.0 kg/m2) postmenopausal women not taking HRT had increased odds ratio of DCIS (OR = 1.46) relative to normal weight women after adjustment for race, ethnicity, age, mammography use, and registry (16).
Several studies reported that women with a family history of breast cancer or a first-degree relative with breast cancer had similarly increased odds ratio of DCIS compared with women without a positive family history (pooled OR = 1.97, 95% CI = 1.10 to 3.52) (eg, 5,6,8,17). Likewise, rates of DCIS have been found to be higher among carriers of the BRCA1/2 gene mutation and among those with estimated risk of breast cancer more than 25% (18). A US-based cohort of similarly high-risk women found the cumulative crude incidence of DCIS over 7 years to be 9.1% (95% CI = 2.3 to 30) (19).
Few studies have examined the association between DCIS incidence and behavioral risk factors such as alcohol consumption, smoking, aspirin and nonsteroidal anti-inflammatory drugs, physical activity, dietary beta carotene intake.
Mammography and DCIS
The strongest evidence about the association between DCIS incidence and use of screening mammography are from eight population-based trials of mammography screening (20–27). Although all trials found that mammographic screening was more likely to lead to the diagnosis of invasive breast cancer than that of DCIS, no trial found more than 20% of screen-detected breast cancers to be DCIS. All but the National Breast Cancer Screening trials found mammography to result in significant reductions in breast cancer mortality (20,21). An analysis combining the Gothenburg Trial and the Two-County Trial (28) compared the number of cases of DCIS and invasive cancer in the screened population relative to the control. The authors estimated that 15% of DCIS cases in the Swedish Two-County Trial and 18% of DCIS in the Gothenburg Trial represent overdiagnosis and concluded that overdiagnosed DCIS did not present a major clinical or public health problem.
The conclusions from the randomized trials are supported by a number of population-based studies from the United States and around the world. Namely, although mammography results in increased detection of DCIS, the number of invasive cancers always outnumbers DCIS cases. The effect of screening programs on incidence of DCIS per 1000 screening mammograms was studied using data from the Breast Cancer Surveillance Consortium and the National Breast and Cervical Cancer Early Detection Program (29,30). In all age groups and overall, the incidence of DCIS among screened women (0.78 per 10 000 women) was greater than the incidence of DCIS among women who were not screened (0.13 per 10 000 women). The incidence of DCIS increased over time, even when the rate of mammography was constant.
There is considerable evidence that the detection of DCIS is greatest at baseline screening. An average annual incidence of DCIS per 1000 screening mammograms was greater after the first screening for women 50–59 and 70–84 years of age than for subsequent screens (29). Both screening and population-based studies point to increased detection on baseline screening and decreased rates of DCIS detection on follow-up screens. Though the differences are not large, they do suggest that the greatest increase in incidence will be observed when a population undergoes initial screening and that the increases in incidence based on this initial screen will over estimate population impact for a population undergoing routine screening.
Chemoprevention and Detection of DCIS
Several trials have assessed the value of tamoxifen or raloxifene for preventing DCIS, although the trials, in reality, were designed to assess the value of the agents for preventing breast cancer rather than DCIS. The largest, the National Surgical Adjuvant Breast and Bowel Project P-1 study (31) found statistically significant reductions in both DCIS and invasive breast cancer associated with tamoxifen use among high-risk women. In the International Breast Cancer Intervention Study, more than 7000 high-risk women between the ages of 35 and 70 from the United Kingdom, Australia, and New Zealand were randomized to tamoxifen, 20 mg/day for 5 years, or placebo (32). The tamoxifen group experienced a 69% reduced incidence of DCIS at 50 months (RR = 0.31, 95% CI = 0.12 to 0.82), but the protective effect was not apparent by 4 years after treatment stopped (study month 96), suggesting that the value of tamoxifen for preventing DCIS may not be maintained after treatment ceases. Although, the value of treatment for preventing invasive disease was maintained (33).
The Study of Tamoxifen and Raloxifene trial randomized over 19 000 women to one of two therapies for preventing breast cancer. Women in the tamoxifen group had half the incidence of in situ breast cancer (lobular carcinoma in situ or DCIS) than women in the raloxifene group (57 vs 81 in situ cancers). The study found that both treatments reduced incidence of invasive breast cancer by half (34).
The Continuing Outcomes Relevant to Evista/Multiple Outcomes of Raloxifene Evaluation randomized double-blind trial examined the impact of raloxifene for preventing invasive breast cancer among postmenopausal women with osteoporosis. The study found statistically reduced incidence of invasive breast cancer associated with raloxifene (HR = 0.50) but a nonsignificant increase in the incidence of DCIS among the treated women (HR = 1.78) (35).
Conclusion
There is ample evidence that the incidence of DCIS is increasing and that the increases are largely due to increased use of screening mammography. Several population-based trials along with other population-based registries also support the conclusion that mammography is more effective at identifying invasive breast cancer than DCIS. We were unable to find any study that reported both DCIS and invasive breast cancer that reported detecting more DCIS than invasive breast cancer. Thus, although the increase in DCIS is likely due to screening, the benefits of screening outweigh the increased detection of DCIS.
There is remarkable similarity in risk factors between DCIS and invasive breast cancer with two notable exceptions—first, the age pattern of DCIS and invasive breast cancer are somewhat different. DCIS peaks at a younger age than does invasive cancer. Second, there is no evidence that HRT is associated with increases in DCIS incidence as it is with invasive breast cancer. Other risk factors including breast density, family history, and history of benign breast disease are similar between invasive cancer and DCIS.
Trials of tamoxifen and raloxifene for breast cancer prevention point to both drugs being effective for preventing invasive breast cancer but tamoxifen being more effective for preventing DCIS. Understanding this effect and how best to prevent all forms of breast cancer deserves further attention.
Funding
Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services (290-02-10064-I).
Note
The following is a summary of a report requested by the National Institutes of Health Office of Medical Applications of Research as a background paper for the State of the Science Conference on Diagnosis and Management of Ductal Carcinoma In Situ (DCIS), a noninvasive form of breast cancer. The report is available at http://www.ahrq.gov//clinic/epcix.htm.
References
- 1.Ries LaG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005. Bethesda, MD: National Cancer Institute; 2008. http://seer.cancer.gov/csr/1975_2005/Accessed March 28, 2010. [Google Scholar]
- 2.Li CI, Daling JR, Malone KE. Age-specific incidence rates of in situ breast carcinomas by histologic type, 1980 to 2001. Cancer Epidemiol Biomarkers Prev. 2005;14(4):1008–1011. doi: 10.1158/1055-9965.EPI-04-0849. [DOI] [PubMed] [Google Scholar]
- 3.Innos K, Horn-Ross PL. Recent trends and racial/ethnic differences in the incidence and treatment of ductal carcinoma in situ of the breast in California women. Cancer. 2003;97(4):1099–1106. doi: 10.1002/cncr.11104. [DOI] [PubMed] [Google Scholar]
- 4.Wohlfahrt J, Rank F, Kroman N, et al. A comparison of reproductive risk factors for CIS lesions and invasive breast cancer. Int J Cancer. 2004;108(5):750–753. doi: 10.1002/ijc.11588. [DOI] [PubMed] [Google Scholar]
- 5.Kerlikowske K, Barclay J, Grady D, et al. Comparison of risk factors for ductal carcinoma in situ and invasive breast cancer. J Natl Cancer Inst. 1997;89(1):76–82. doi: 10.1093/jnci/89.1.76. [DOI] [PubMed] [Google Scholar]
- 6.Trentham-Dietz A, Newcomb PA, Storer BE, et al. Risk factors for carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 2000;9(7):697–703. [PubMed] [Google Scholar]
- 7.Vamre TB, Stalsberg H, Thomas DB. Extra-tumoral breast tissue in breast cancer patients: variations with steroid contraceptive use. Int J Cancer. 2006;118(11):2827–2831. doi: 10.1002/ijc.21697. [DOI] [PubMed] [Google Scholar]
- 8.Claus EB, Stowe M, Carter D. Breast carcinoma in situ: risk factors and screening patterns. J Natl Cancer Inst. 2001;93(23):1811–1817. doi: 10.1093/jnci/93.23.1811. [DOI] [PubMed] [Google Scholar]
- 9.Bohlke K, Cramer DW, Trichopoulos D, et al. Insulin-like growth factor-I in relation to premenopausal ductal carcinoma in situ of the breast. Epidemiology. 1998;9(5):570–573. [PubMed] [Google Scholar]
- 10.Claus EB, Stowe M, Carter D. Oral contraceptives and the risk of ductal breast carcinoma in situ. Breast Cancer Res Treat. 2003;81(2):129–136. doi: 10.1023/A:1025728524310. [DOI] [PubMed] [Google Scholar]
- 11.Reeves GK, Beral V, Green J, et al. Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort study and meta-analysis. Lancet Oncol. 2006;7(11):910–918. doi: 10.1016/S1470-2045(06)70911-1. [DOI] [PubMed] [Google Scholar]
- 12.Gapstur SM, Morrow M, Sellers TA. Hormone replacement therapy and risk of breast cancer with a favorable histology: results of the Iowa Women's Health Study. JAMA. 1999;281(22):2091–2097. doi: 10.1001/jama.281.22.2091. [DOI] [PubMed] [Google Scholar]
- 13.Kerlikowske K, Miglioretti DL, Ballard-Barbash R, et al. Prognostic characteristics of breast cancer among postmenopausal hormone users in a screened population. J Clin Oncol. 2003;21(23):4314–4321. doi: 10.1200/JCO.2003.05.151. [DOI] [PubMed] [Google Scholar]
- 14.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA. 2003;289(24):3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 15.Gill JK, Maskarinec G, Pagano I, et al. The association of mammographic density with ductal carcinoma in situ of the breast: the Multiethnic Cohort. Breast Cancer Res. 2006;8(3):R30. doi: 10.1186/bcr1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerlikowske K, Walker R, Miglioretti DL, et al. Obesity, mammography use and accuracy, and advanced breast cancer risk. J Natl Cancer Inst. 2008;100(23):1724–1733. doi: 10.1093/jnci/djn388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trentham-Dietz A, Newcomb PA, Storer BE, et al. Risk factors for carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 2000;9(7):697–703. [PubMed] [Google Scholar]
- 18.Brekelmans CT, Seynaeve C, Bartels CC, et al. Effectiveness of breast cancer surveillance in BRCA1/2 gene mutation carriers and women with high familial risk. J Clin Oncol. 2001;19(4):924–930. doi: 10.1200/JCO.2001.19.4.924. [DOI] [PubMed] [Google Scholar]
- 19.Komenaka IK, Ditkoff BA, Joseph KA, et al. The development of interval breast malignancies in patients with BRCA mutations. Cancer. 2004;100(10):2079–2083. doi: 10.1002/cncr.20221. [DOI] [PubMed] [Google Scholar]
- 20.Miller AB, To T, Baines CJ, et al. Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women aged 50–59 years. J Natl Cancer Inst. 2000;92(18):1490–1499. doi: 10.1093/jnci/92.18.1490. [DOI] [PubMed] [Google Scholar]
- 21.Miller AB, To T, Baines CJ, et al. The Canadian National Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up. A randomized screening trial of mammography in women age 40 to 49 years. Ann Intern Med. 2002;137(5, pt 1):305–312. doi: 10.7326/0003-4819-137-5_part_1-200209030-00005. [DOI] [PubMed] [Google Scholar]
- 22.Bjurstam N, Bjorneld L, Warwick J, et al. The Gothenburg Breast Screening Trial. Cancer. 2003;97(10):2387–2396. doi: 10.1002/cncr.11361. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro S. Periodic screening for breast cancer: the HIP Randomized Controlled Trial. Health Insurance Plan. J Natl Cancer Inst Monogr. 1997;22:27–30. doi: 10.1093/jncimono/1997.22.27. [DOI] [PubMed] [Google Scholar]
- 24.Nystrom L, Andersson I, Bjurstam N, et al. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359(9310):909–919. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- 25.Tabar L, Vitak B, Chen HH, et al. The Swedish Two-County Trial twenty years later. Updated mortality results and new insights from long-term follow-up. Radiol Clin North Am. 2000;38(4):625–651. doi: 10.1016/s0033-8389(05)70191-3. [DOI] [PubMed] [Google Scholar]
- 26.Roberts MM, Alexander FE, Anderson TJ, et al. The Edinburgh randomised trial of screening for breast cancer: description of method. Br J Cancer. 1984;50(1):1–6. doi: 10.1038/bjc.1984.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frisell J, Lidbrink E, Hellstrom L, et al. Followup after 11 years—update of mortality results in the Stockholm mammographic screening trial. Breast Cancer Res Treat. 1997;45(3):263–270. doi: 10.1023/a:1005872617944. [DOI] [PubMed] [Google Scholar]
- 28.Duffy SW, Agbaje O, Tabar L, et al. Overdiagnosis and overtreatment of breast cancer: estimates of overdiagnosis from two trials of mammographic screening for breast cancer. Breast Cancer Res. 2005;7(6):258–265. doi: 10.1186/bcr1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94(20):1546–1554. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 30.Smith-Bindman R, Chu PW, Miglioretti DL, et al. Comparison of screening mammography in the United States and the United Kingdom. JAMA. 2003;290(16):2129–2137. doi: 10.1001/jama.290.16.2129. [DOI] [PubMed] [Google Scholar]
- 31.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 32.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360(9336):817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 33.Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer—96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99(4):272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 34.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 35.Martino S, Cauley JA, Barrett-Connor E, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96(23):1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]