Abstract
Individual patient data were available for all four of the randomized trials that began before 1995, and that compared adjuvant radiotherapy vs no radiotherapy following breast-conserving surgery for ductal carcinoma in situ (DCIS). A total of 3729 women were eligible for analysis. Radiotherapy reduced the absolute 10-year risk of any ipsilateral breast event (ie, either recurrent DCIS or invasive cancer) by 15.2% (SE 1.6%, 12.9% vs 28.1% 2 P <.00001), and it was effective regardless of the age at diagnosis, extent of breast-conserving surgery, use of tamoxifen, method of DCIS detection, margin status, focality, grade, comedonecrosis, architecture, or tumor size. The proportional reduction in ipsilateral breast events was greater in older than in younger women (2P < .0004 for difference between proportional reductions; 10-year absolute risks: 18.5% vs 29.1% at ages <50 years, 10.8% vs 27.8% at ages ≥50 years) but did not differ significantly according to any other available factor. Even for women with negative margins and small low-grade tumors, the absolute reduction in the 10-year risk of ipsilateral breast events was 18.0% (SE 5.5, 12.1% vs 30.1%, 2P = .002). After 10 years of follow-up, there was, however, no significant effect on breast cancer mortality, mortality from causes other than breast cancer, or all-cause mortality.
Until the 1980s, ductal carcinoma in situ (DCIS) of the breast was usually treated by mastectomy. However, following the introduction of breast-conserving therapy for the treatment of early-stage invasive breast cancer, local excision of DCIS with or without radiotherapy to the conserved breast began to be used and, from 1985 to 1990, four randomized trials comparing adjuvant radiotherapy vs no radiotherapy following local excision for DCIS were initiated. We report here an overview of their results based on individual patient data.
Methods
Every 5 years since 1985, evidence from the randomized trials in early breast cancer has been reviewed centrally in a worldwide collaboration between the individuals responsible for them (the Early Breast Cancer Trialists’ Collaborative Group [EBCTCG]). Two 2005 EBCTCG reports gave the results up to the year 2000 from the trials that began recruitment by 1995 of adjuvant systemic treatments (studying various types of chemotherapy or hormonal therapy) (1) and of local treatments (studying various types of surgery and/or adjuvant radiotherapy) (2). The present report uses similar methods and gives the results up to September 2006 of the trials that began by 1995 of adjuvant radiotherapy vs no radiotherapy following local excision for DCIS.
Trial Identification and Main Outcomes
Trial identification procedures were as in previous EBCTCG reports. Only unconfounded trials were sought (ie, trials in which there was to be no difference between the treatment groups in the extent of surgery or in the use of systemic therapy). Five trials were identified that began by 2000, and brief design details are given in Table 1. One trial (Radiation Therapy Oncology Group 9804) began only in 1999 and is not yet available. The remaining four began in 1985–1990 and have provided information for each patient on characteristics at diagnosis, allocated treatment, time to first event and whether the event was ipsilateral recurrence of DCIS, ipsilateral occurrence of invasive breast cancer, occurrence of contralateral DCIS or contralateral invasive breast cancer, or regional or distant metastasis of breast cancer. Information was also provided on cause-specific mortality and incident non-breast primary cancers. It was assumed that any death attributed to breast cancer had been preceded by metastatic breast cancer.
Table 1.
Randomized trials comparing radiotherapy vs the same management without radiotherapy following breast-conserving surgery for ductal carcinoma in situ (DCIS) of the breast*
| Year code, study name (reference) | Entry dates | No. of women randomized | No. of women eligible for analysis† | Median follow-up (yr) | Mammo-graphic detection (%) | Breast and axillary surgery | Negative surgical margins required | Central pathological review | Breast radiotherapy |
| Data available for overview | |||||||||
| NSABP B-17 (3, 4, 5) | 1985–1990 | 818 | 798 | 16.5 | 80 | Local excision (37% axillary dissection) | Yes (13% involved or unknown)‡ | 623 (76%) | 50 Gy (2 Gy/f) 9% with boost |
| EORTC 10853 (6, 7, 8, 9) | 1986–1996 | 1010 | 918 | 10.4 | 72 | Local excision (20% axillary dissection) | Yes (16% “not free,” <1mm, involved or unknown)‡ | 824 (82%) | 50 Gy (2 Gy/f) 5% with boost |
| SweDCIS(10, 11, 12) | 1987–1999 | 1067 | 1011 | 8.4 | 79 | Sector resection (17% axillary dissection) | No (11% positive, 9% unknown)‡ | 271 (25%) | 50 Gy (2 Gy/f) (80%) or 48 Gy (2.4 Gy/f) (13%) or 54 Gy (2 Gy/f) then 2 wk gap (7%) Boost not recommended |
| UK/ANZ DCIS§(13) | 1990–1998 | 1030 | 1002 | 4.8 | 100 | Local excision (No axillary dissection) | Yes | 0 (0%) | 50 Gy (2 Gy/f) Boost not recommended |
| Data not yet available | |||||||||
| RTOG 9804 | 1999–2006 | 636 | – | – | ns | Local excision (No axillary dissection) | Yes | 0 (0%) | 50.4 Gy (1.8 Gy/f) or 50 Gy (2 Gy/f) or 42.5 Gy (2.7 Gy/f) Boost not recommended |
EORTC = European Organisation for Research and Treatment of Cancer; f = fraction; Gy = Gray; ns = not specified; NSABP = National Surgical Adjuvant Breast and Bowel Project; RTOG = Radiation Therapy Oncology Group.
After exclusion of women with a benign lesion only or with microinvasion, invasion, Paget’s disease, other cancer present at randomization, or other study-specific protocol violation.
Including information from later pathological reviews, provided that that it was based on material obtained at the time of initial diagnosis or treatment and provided that samples had not been selected for pathological review according to allocated treatment or outcome.
Microinvasion (<1 mm) allowed, present in 0.3%. A total of 1694 women were randomized in this trial. However, 664 were women randomized in a comparison of tamoxifen vs not. They have therefore been excluded from the present overview. A total of 540 of the 1002 women included in the overview were randomized to radiotherapy and tamoxifen vs tamoxifen alone, while the remainder were randomized to radiotherapy vs not.
Data Management
Data management procedures were as in recent EBCTCG reports (1,2) except that for each woman additional clinical and pathological details were sought about her disease. This information could have been gathered during later pathological review, provided that it was based on material obtained at the time of initial diagnosis or treatment, and provided that samples had not been selected for pathological review according to allocated treatment or outcome.
Statistical Analysis
Analyses were based on allocated treatment and were stratified by trial and time since randomization in single years. The analyses of breast events, breast cancer mortality, heart disease mortality, mortality without a breast event, non-breast primary cancer incidence, and any death were also stratified by age at randomization in five groups (<40, 40–49, 50–59, 60–69, ≥70 years). Only two age groups [<50 and ≥50 years, as in previous analyses (2)] were used, however, for analyses that were also subdivided by other characteristics. Unless otherwise indicated, other aspects of the statistical methods and the formats of the figures are as before (1,2) and are described on the EBCTCG website (www.ctsu.ox.ac.uk/projects/ebctcg).
Collaborative Review
A preliminary meta-analysis of these trials was presented and discussed at a meeting of collaborators in September 2006, after which much additional information was sought about clinical and pathological details and about outcomes. Revised meta-analyses were presented at the National Institutes of Health State-of-the-Science Conference on DCIS in September 2009 and were circulated for comment by collaborating EBCTCG trialists. A draft of the present report was circulated for comment to the trialists in December 2009, and the manuscript was revised in the light of comments received.
Results
A total of 3925 women were randomized and, after exclusion of those who had only a benign lesion at the time of randomization, or who already had microinvasion, invasion, Paget's disease or another cancer present at the time of initial diagnosis, or who had another study-specific protocol violation, a total of 3729 remained eligible for the analysis. A total of 21% of them were randomized during 1985–1989, 46% during 1990–1995, and 32% during 1995–2000. Median follow-up was 8.9 woman-years. A total of 924 women were reported as having experienced a breast event after randomization, and 74% of the first events were in the ipsilateral breast (Table 2).
Table 2.
Numbers of women for whom a breast event during follow-up was reported*
| Years since randomization | Allocated BCS+RT (n = 1878) | Allocated BCS (n = 1851) | Total (n = 3729) |
| Any breast event | |||
| 0–4 | 196 | 359 | 555 |
| 5–9 | 116 | 141 | 257 |
| ≥10 | 55 | 57 | 112 |
| Total | 367 | 557 | 924 |
| Any ipsilateral breast event as first event | |||
| 0–4 | 131 | 311 | 442 |
| 5–9 | 61 | 111 | 172 |
| ≥10 | 37 | 33 | 70 |
| Total | 229 | 455 | 684 |
| Any contralateral breast event as first event† | |||
| 0–4 | 47 | 35 | 82 |
| 5–9 | 42 | 24 | 66 |
| ≥10 | 16 | 22 | 38 |
| Total | 105‡ | 81‡ | 186 |
| Regional or distant event as first event | |||
| 0–4 | 18 | 13 | 31 |
| 5–9 | 13 | 6 | 19 |
| ≥10 | 2 | 2 | 4 |
| Total | 33 | 21 | 54 |
| Woman-years until first breast event, or end of follow-up if no event | |||
| 0–4 | 8199 | 7662 | 15 861 |
| 5–9 | 4785 | 4150 | 8935 |
| ≥10 | 2457 | 2080 | 4537 |
| Total | 15 441 | 13 892 | 29 333 |
BCS = breast-conserving surgery; RT = radiotherapy.
Includes four RT and six No RT where a contralateral and ipsilateral event occurred within 7 days of each other.
77 RT and 56 No RT events were due to invasive cancer.
Ipsilateral Breast Events
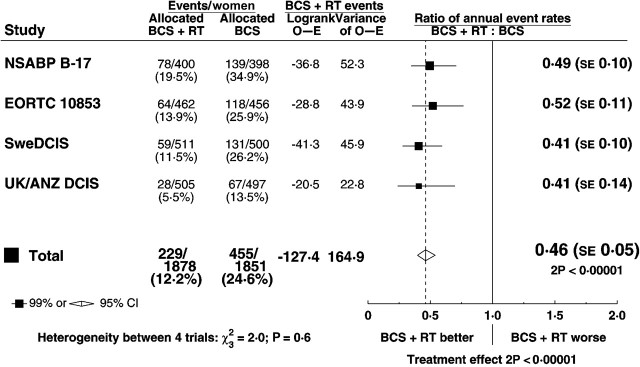
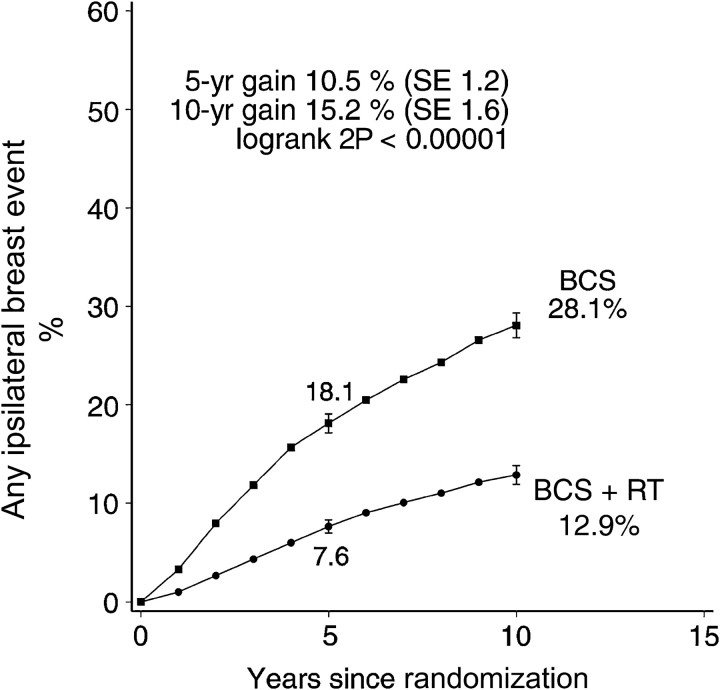
Radiotherapy approximately halved the rate of ipsilateral breast events (rate ratio 0.46, standard error [SE] 0.05, 2 P < .00001). At 5 years after randomization the absolute reduction in risk was 10.5% (SE 1.2%, 7.6% vs 18.1%) while at 10 years it was 15.2% (SE 1.6%, 12.9% vs 28.1%) (Figure 1). By 10 years after randomization 192 of the women allocated to BCS+RT had had an ipsilateral breast event, and for 100 of them it was a recurrence of DCIS while for the remaining 92 women it was an invasive cancer. The corresponding numbers for the women allocated to BCS were 218 with a recurrence of DCIS and 204 with invasive cancer. Thus for both endpoints the number of events observed was approximately halved. The rate of ipsilateral breast events was approximately halved in all four trials, with no evidence of heterogeneity between the trials in the proportional reduction (Figure 2).
Figure 2.
Effect of radiotherapy (RT) after breast-conserving surgery (BCS): ratio of annual event rates of any ipsilateral breast event by trial.
SE = standard error; CI = confidence interval.
Figure 1.
Effect of radiotherapy (RT) after breast-conserving surgery (BCS) (four trials, start dates 1985–1990, 3729 women): 10-year cumulative risks of any ipsilateral breast event (ie recurrent DCIS or invasive cancer).
Vertical lines indicate 1 SE above or below the 5 and 10 year percentages.
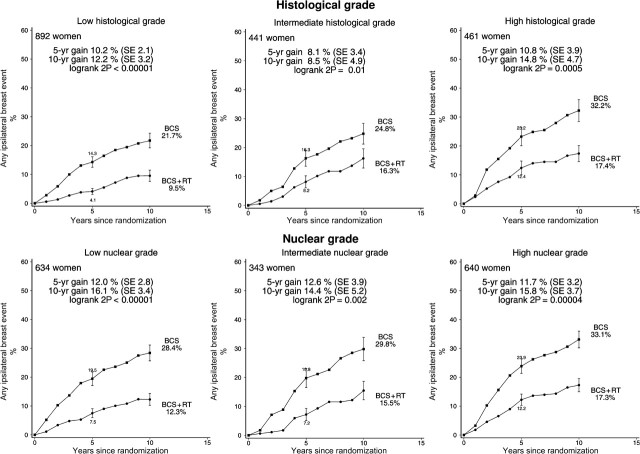
Radiotherapy was effective in reducing ipsilateral breast events regardless of whether the woman was aged younger than or older than 50 years at diagnosis, whether local excision or sector resection had been performed, and whether or not tamoxifen was to be given to both treatment arms or to neither (Figure 3). For each other characteristic, information was unavailable for many women. Nevertheless, the information that was available sufficed to show that radiotherapy was effective in reducing ipsilateral breast events regardless of whether the original tumor was detected by mammography only or by clinical symptoms, whether the excised lesion had negative margins, and whether the tumor was unifocal (Figure 4). Radiotherapy was also effective in reducing ipsilateral breast events irrespective of histological or nuclear grade (Figure 5), of whether there was comedonecrosis or comedo/solid architecture (Figure 6), and of clinical or pathological tumor size (Figure 7).
Figure 3.
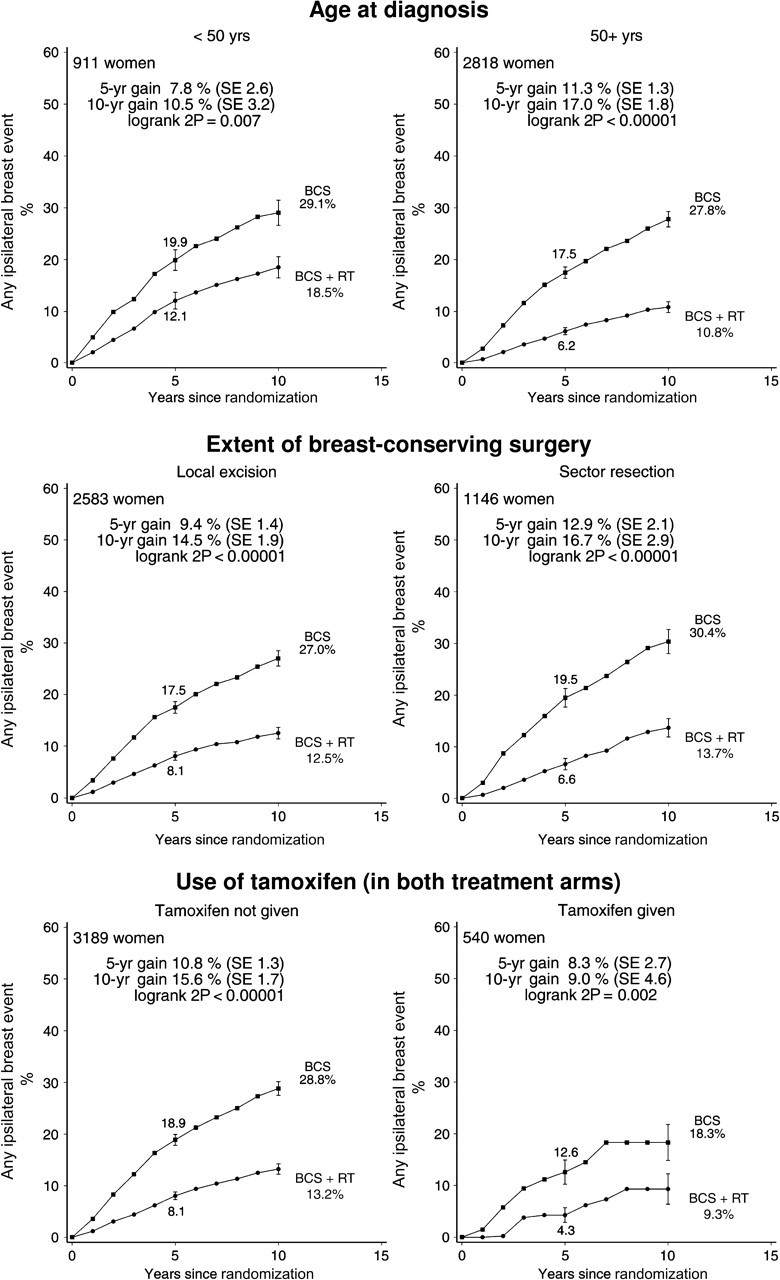
Effect of radiotherapy (RT) after breast-conserving surgery (BCS): 10-year cumulative risks of any ipsilateral breast event by age at diagnosis, extent of surgery, and use of tamoxifen (3729 women). Women given sector resection were from either the SweDCIS trial (1011 women) or the EORTC 10853 trial (135 women), and women using tamoxifen were all in the UK/ANZ DCIS trial. Information was not available on estrogen or progesterone receptor status.
Vertical lines indicate 1 SE above or below the 5 and 10 year percentages.
Figure 4.
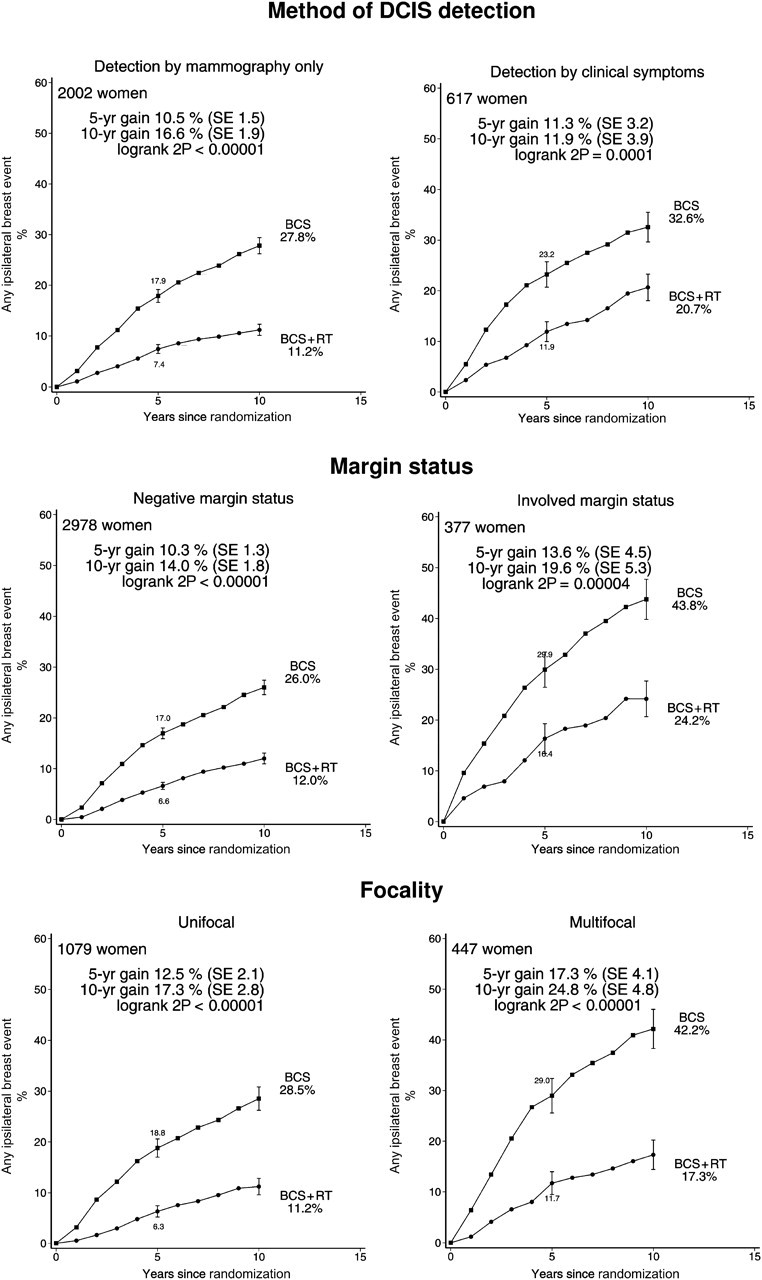
Effect of radiotherapy (RT) after breast-conserving surgery (BCS): 10-year cumulative risks of any ipsilateral breast event by detection method (2619 women), margin status (3355 women) and focality (1526 women). Women for whom the surgical margins were close (<2 mm) were classified as having negative surgical margins.
Vertical lines indicate 1 SE above or below the 5 and 10 year percentages.
Figure 5.
Effect of radiotherapy (RT) after breast-conserving surgery (BCS): 10-year cumulative risks of any ipsilateral breast event by histological grade (1794 women) and nuclear grade (1617 women).
Vertical lines indicate 1 SE above or below the 5 and 10 year percentages.
Figure 6.
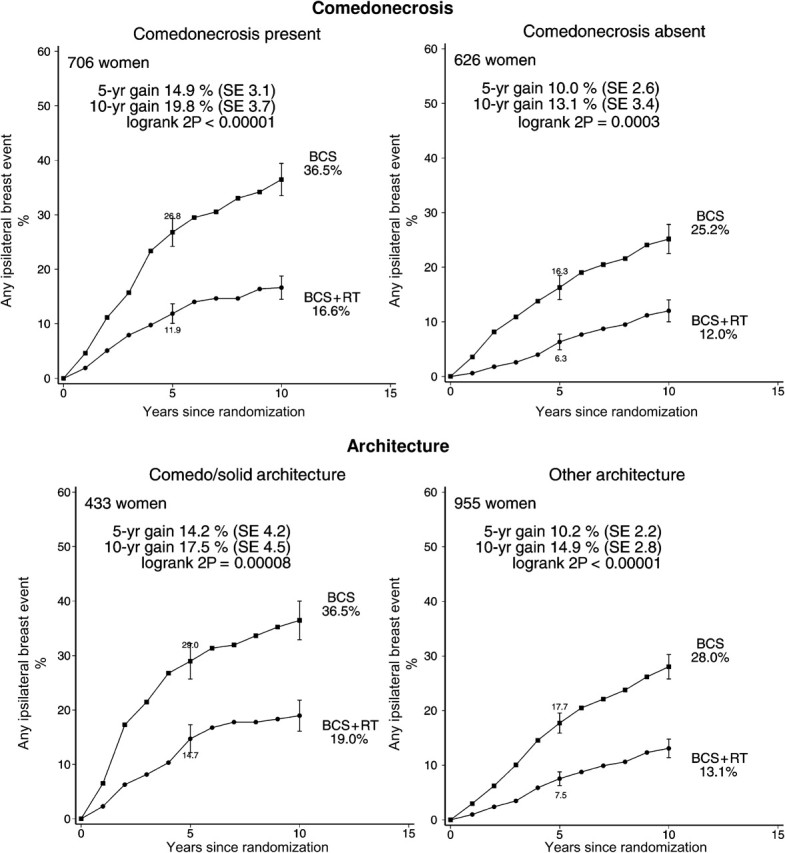
Effect of radiotherapy (RT) after breast-conserving surgery (BCS): 10-year cumulative risks of any ipsilateral breast event by comedonecrosis (1332 women) and architecture (1388 women).
Vertical lines indicate 1 SE above or below the 5 and 10 year percentages.
Figure 7.
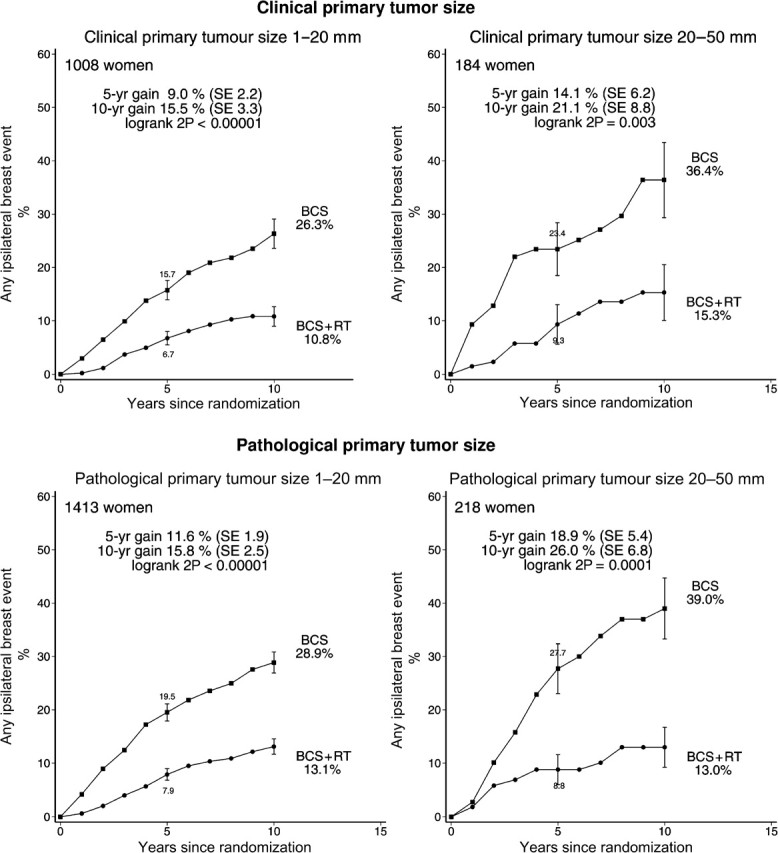
Effect of radiotherapy (RT) after breast-conserving surgery (BCS): 10-year cumulative risks of any ipsilateral breast event by clinical tumor size (1192 women) and pathological tumor size (1631 women).
Vertical lines indicate 1 SE above or below the 5 and 10 year percentages.
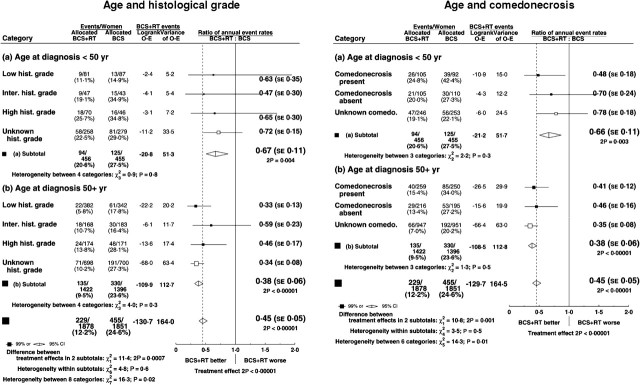
Radiotherapy resulted in a larger proportional reduction in the rate of ipsilateral breast recurrence for women aged more than 50 years than for younger women (rate ratios: age <50 years 0.69, SE 0.12; ≥50 years 0.38, SE 0.06, 2P = .0004 for the difference between these proportional reductions), but the proportional reduction did not differ significantly according to any other factor (Figure 8). When the data were subdivided into five groups according to age (<40, 40–49, 50–59, 60–69, ≥70), the trend in the proportional reduction with age was significant (P = .02). The difference between the proportional reductions in younger and older women did not appear to be accounted for by differences in histological grade or comedonecrosis (Figure 9) or by differences in nuclear grade or architecture (data not shown).
Figure 8.
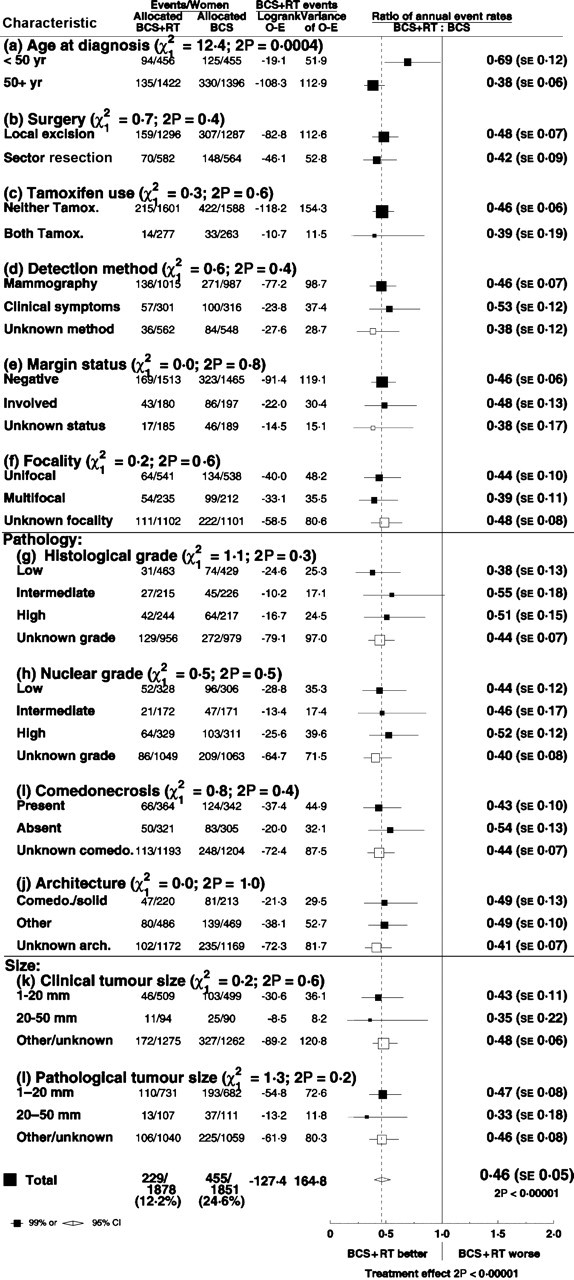
Effect of radiotherapy (RT) after breast-conserving surgery (BCS): Ratio of annual event rates of any ipsilateral breast event by various patient and tumor characteristics.
SE = standard error; CI = confidence interval.
Figure 9.
Effect of radiotherapy (RT) after breast-conserving surgery (BCS): ratio of annual event rates of any ipsilateral breast event by age and histological grade and age and comedonecrosis.
SE = standard error; CI = confidence interval.
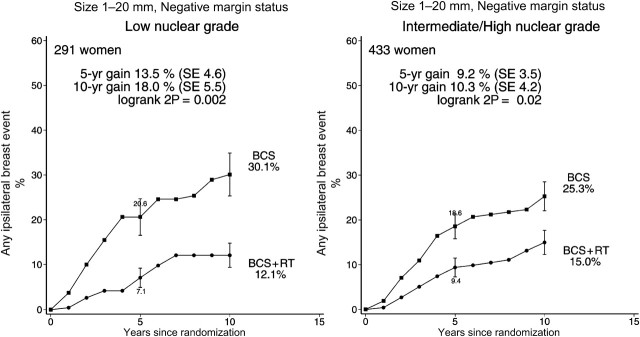
Women with negative margins and small low-grade tumors were identified a priori as a group expected to be at low absolute risk of ipsilateral breast events, for whom radiotherapy might therefore provide little absolute gain. However, information was often unavailable on one or more of these factors, so only 291 such women could be studied. Among them, the 10-year risk of an ipsilateral event in those allocated not to receive radiotherapy was, however, substantial at 30.1%, and even with this relatively small number of women the effect of radiotherapy was highly significant (rate ratio 0.48, SE 0.17 2P = .002), with a 10-year absolute gain of 18.0% (SE 5.5%) (Figure 10, left-hand panel).
Figure 10.
Effect of radiotherapy (RT) after breast-conserving surgery (BCS) on 724 women with negative margin status and pathological tumor size 1–20 mm according to nuclear grade: 10-year cumulative risks of any ipsilateral breast event.
Vertical lines indicate 1 SE above or below the 5 and 10 year percentages.
Other End-Points
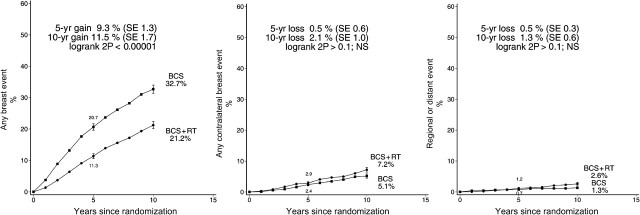
Radiotherapy reduced the risk of any breast event (ie ipsilateral recurrence of DCIS, ipsilateral occurrence of invasive breast cancer, contralateral occurrence of DCIS or invasive breast cancer, or regional or distant metastasis of breast cancer) (rate ratio 0.59, SE 0.05, P < .00001), and at 5 years after randomization the absolute reduction was 9.3% (SE 1.3%, 11.3% vs 20.7%), while at 10 years it was 11.5% (SE 1.7%, 21.2% vs 32.7%) (Figure 11). In this analysis, which considered first events only, women who were allocated to radiotherapy experienced higher risks compared with those allocated to no radiotherapy for both contralateral and regional or distant events but neither difference was significant (contralateral rate ratio 1.16, SE 0.16 2P > .1; regional or distant rate ratio 1.51, SE 0.34, 2P > .1).
Figure 11.
Effect of radiotherapy (RT) after breast-conserving surgery (BCS) on 3729 women: 10-year cumulative risks of any breast event, any contralateral breast event and any regional or distant event.
NS = not statistically significant.
Vertical lines indicate 1 SE above or below the 5 and 10 year percentages.
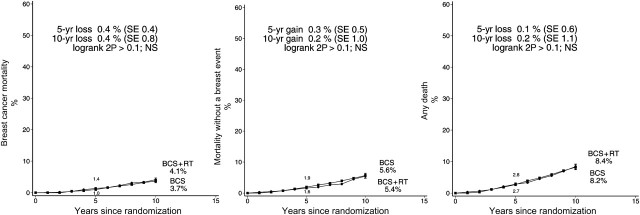
A total of 353 women were known to have died during follow-up, 96 from breast cancer, 217 from other causes (including 55 from heart disease), and 40 for whom the cause of death was unknown (Table 3). For breast cancer mortality and for mortality from all causes, women who were allocated to radiotherapy experienced slightly higher risks compared with those allocated to no radiotherapy (breast cancer mortality rate ratio 1.22, SE 0.18, 2P > .1; all-cause mortality rate ratio 1.11, SE 0.11, 2P > .1) (Figure 12). Mortality from causes other than breast cancer in the period before a breast event and mortality from heart disease were also slightly higher among women allocated to radiotherapy, but the increases were not significant (all-cause mortality rate ratio 1.04 SE 0.15, 2P > .1; heart disease rate ratio: 1.11, SE 0.33, 2P > .1). A total of 74 non-breast primary cancers were reported during follow-up, but there was no evidence that radiotherapy had any net effect on the incidence of such cancers (rate ratio 0.99, SE 0.20, 2P > .1).
Table 3.
Total numbers of deaths and non-breast primary cancers reported during follow-up (including events reported after a breast event)*
| Years since randomization | Allocated BCS + RT (n = 1878) | Allocated BCS (n = 1851) | Total (n = 3729) |
| All causes of death | |||
| 0–4 | 48 | 45 | 93 |
| 5–9 | 63 | 65 | 128 |
| ≥10 | 75 | 57 | 132 |
| Total | 186 | 167 | 353 |
| Breast cancer death | |||
| 0–4 | 14 | 16 | 30 |
| 5–9 | 21 | 17 | 38 |
| ≥10 | 17 | 11 | 28 |
| Total | 52 | 44 | 96 |
| Heart disease death | |||
| 0–4 | 9 | 10 | 19 |
| 5–9 | 7 | 11 | 18 |
| ≥10 | 10 | 8 | 18 |
| Total | 26† | 29† | 55 |
| All other known causes of death | |||
| 0–4 | 18 | 17 | 35 |
| 5–9 | 30 | 32 | 62 |
| ≥10 | 37 | 28 | 65 |
| Total | 85 | 77 | 162 |
| Unknown cause of death | |||
| 0–4 | 7 | 2 | 9 |
| 5–9 | 5 | 5 | 10 |
| ≥10 | 11 | 10 | 21 |
| Total | 23 | 17 | 40 |
| Non-breast primary cancers | |||
| 0–4 | 11 | 10 | 21 |
| 5–9 | 10 | 13 | 23 |
| ≥10 | 17 | 13 | 30 |
| Total | 38 | 36 | 74 |
| Woman-years until death or end of follow-up | |||
| 0–4 | 8600 | 8520 | 17 120 |
| 5–9 | 5634 | 5631 | 11 265 |
| ≥10 | 3329 | 3413 | 6742 |
| Total | 17 563 | 17 564 | 35 127 |
BCS = breast-conserving surgery; RT = radiotherapy.
Left-sided breast cancer, 8 BCS + RT vs 10 BCS; right-sided breast cancer 10 vs 6; unknown side 8 vs 13.
Figure 12.
Effect of radiotherapy (RT) after breast-conserving surgery (BCS) on 3729 women: 10-year cumulative risks of breast cancer mortality, mortality without a breast event (ie, mortality from causes other than breast cancer in the period before a breast event), and any death. (Analysis based on first events only.)
NS = not statistically significant.
Vertical lines indicate 1 SE above or below the 5 and 10 year percentages.
Discussion
These randomized trials provide strong and consistent evidence that, in the populations studied, radiotherapy after breast-conserving surgery for DCIS approximately halved the rate of ipsilateral breast events during the subsequent decade with little effect on contralateral or distant events. The absolute magnitude of the10-year risk reduction was 15%, and in the irradiated group both the number of women with recurrent DCIS and the number of women with invasive breast cancer in the conserved breast were substantially reduced. The proportional reduction in the rate of ipsilateral breast events achieved with radiotherapy was greater in older than in younger women but did not differ significantly according to any other factor. The age effect did not appear to be accounted for by younger women being more likely to have high-grade lesions or comedonecrosis, and the explanation for it is unknown. A radiotherapy boost was rarely used (Table 1), and so the impact of a boost in DCIS could not be assessed.
In these trials, in most of which tamoxifen was not given, 12.9% of women allocated to radiotherapy had an ipsilateral breast event within the first decade. This risk is similar to that in a large multi-institutional series of women diagnosed up to 1995 and given breast-conserving surgery with radiotherapy (14), suggesting that the women in these trials were reasonably typical of women diagnosed with DCIS during that era. Since then, breast screening has become more common, so in recent years women diagnosed with DCIS tend to have smaller lesions. In addition, greater attention is now paid to achieving negative surgical margins. Both factors are associated with a lower rate of ipsilateral breast events in the absence of radiotherapy. Therefore, there has been considerable interest in identifying patients with favorable features for whom the rate of ipsilateral breast events in the absence of radiotherapy is so low that radiotherapy can reasonably safely be omitted (15–17).
In the data available from these trials, it was not possible to subdivide the women with negative margin status according to margin width, so women with close (<2 mm) surgical margins had to be included with other women who had wider margins. Nor was it possible to subdivide women with tumors smaller than 20 mm according to tumor size. Therefore, all women with negative margin status and low-grade tumors smaller than 20 mm were combined in our “low-risk” group. These criteria are less stringent than those used in recent nonrandomized studies (15–17) to define low risk, which could well explain the higher risk of ipsilateral breast events in the “low-risk” women in these trials. The trial results suggest, however, that no matter what the underlying rate of ipsilateral breast events may be for particular categories of women, it will be approximately halved by radiotherapy.
The risks of a contralateral breast event and of a regional or distant breast event both appeared to be somewhat larger among the women allocated to radiotherapy than among the controls. Neither of these increases was statistically significant and so chance may be the explanation for them. However, the analyses presented in this article consider only first events and make the assumption that women who experience an ispilateral breast event are no more or less likely than other women to experience a regional, distant, or contralateral event. This independence assumption cannot be verified from the data. If women with more aggressive disease are at greater risk of all three types of event, then, as the predominant effect of the radiotherapy is on ipsilateral events, the apparent slight increase in the risk of contralateral and regional or distant events may be an artifact accounted for by events that would, in the absence of radiotherapy, have occurred after an ipsilateral breast event. The analysis of any breast event does not depend on the independence assumption and confirms the efficacy of radiotherapy in reducing breast events overall.
In these randomized trials, the risk of death from breast cancer was non-significantly greater in the women allocated to radiotherapy than in the women allocated to breast-conserving surgery only, as was the risk of death from all causes. Breast cancer mortality is unlikely to be affected by the issues referred to in the previous paragraph, while mortality from all causes cannot be affected by it. Therefore, as the differences are not significant, chance seems a likely explanation for them.
Among the much larger numbers of women in the trials of radiotherapy following breast-conserving surgery for early invasive breast cancer, radiotherapy had little effect on breast cancer mortality during the first few years of follow-up but, by 15 years, about one breast cancer death was avoided for every four local recurrences avoided in the first 5 years (2). Theoretically, if about the same 1:4 ratio applied to ipsilateral invasive cancers following breast-conserving therapy for DCIS, then radiotherapy might be expected to reduce breast cancer mortality by an absolute amount of about 1% or 2% by year 15 or 20, which the present trials can neither exclude nor confirm.
Attendees at EBCTCG Steering Committee Meetings
K. Albain, S. Anderson, R. Arriagada, W. Barlow, J. Bergh, J. Bliss, *M. Buyse, D. Cameron, *†M. Clarke, A. Coates, *†R. Collins, J. Costantino, J. Cuzick, *†S. Darby, N. Davidson, *†C. Davies, A. Di Leo, M. Dowsett, *M. Ewertz, R. Gelber, C. Geyer, †J. Godwin, A. Goldhirsch, †R. Gray, D. Hayes, C. Hill, J. Ingle, R. Jakesz, M. Kaufmann, †P. McGale, L. Norton, Y. Ohashi, S. Paik, E. Perez, *†R. Peto, *M. Piccart (co-chair), L. Pierce, G. Pruneri, *K. Pritchard (co-chair), V. Raina, P. Ravdin, J. Robertson, E. Rutgers, Y. F. Shao, S. Swain, †C. Taylor, P. Valagussa, G. Viale, T. Whelan, *E. Winer, †Y. Wang, *W. Wood.
*Executive Group, †Secretariat
Appendix 1: EBCTCG Collaborators, Listed Alphabetically by Institution and Then Alphabetically by Name
ACETBC, Tokyo, Japan—O. Abe, R. Abe, K. Enomoto, K. Kikuchi, H. Koyama, H. Masuda, Y. Nomura, Y. Ohashi, K. Sakai, K. Sugimachi, M. Toi, T. Tominaga, J. Uchino, M. Yoshida
Addenbrooke's Hospital, Cambridge, UK—J. L. Haybittle.
Anglo-Celtic Cooperative Oncology Group, UK—C. F. Leonard.
ARCOSEIN Group, France—G. Calais, P. Geraud.
ATLAS Trial Collaborative Study Group, Oxford, UK—V. Collett, C. Davies, A. Delmestri, J. Sayer.
Auckland Breast Cancer Study Group, New Zealand—V. J. Harvey, T. M. Holdaway, R. G. Kay, B. H. Mason.
Australian-New Zealand Breast Cancer Trials Group, Sydney, Australia—J. F. Forbes, N. Wilcken.
Austrian Breast Cancer Study Group, Vienna, Austria—T. Bauernhofer, P. Dubsky, C. Fesl, H. Fohler, L. Filipcic, M. Filipits, M. Fridrik, M. Gnant, R. Greil, K. Hegenbarth, R. Jakesz, W. Kwasny, A. Lang, G. Luschin-Ebengreuth, C. Marth, C. Menzel, B. Mlineritsch, H. Samonigg, M. Seifert, P. Sevelda, C. Singer, G. G. Steger, H. Stöger, J. Thaler, J. Tschmelitsch, C. Zielinski.
Beatson Oncology Centre, Glasgow, UK—P. Canney, H. M. A. Yosef.
Belgian Adjuvant Breast Cancer Project, Liège, Belgium—C. Focan.
Berlin-Buch Akademie der Wissenschaften, Germany—U. Peek.
Birmingham General Hospital, UK—G. D. Oates, J. Powell.
Bordeaux Institut Bergonié, France—M. Durand, L. Mauriac.
Bordet Institute, Brussels, Belgium—A. Di Leo, S. Dolci, M. J. Piccart.
Bradford Royal Infirmary, UK—M. B. Masood, D. Parker, J. J. Price.
Breast Cancer International Research Group (BCIRG)—M. A. Lindsay, J. Mackey, M. Martin.
Breast Cancer Study Group of the Comprehensive Cancer Centre, Limburg, the Netherlands—P. S. G. J. Hupperets.
British Association of Surgical Oncology BASO II Trialists, London, UK—T. Bates, R. W. Blamey, U. Chetty, I. O. Ellis, E. Mallon, D. A. L. Morgan, J. Patnick, S. Pinder.
British Columbia Cancer Agency, Vancouver, Canada—S. Jackson, J. Ragaz.
Cancer and Leukemia Group B, Washington DC, USA—D. Berry, G. Broadwater, C. Cirrincione, H. Muss, L. Norton, R. B. Weiss.
Cancer Care Ontario, Canada—H. T. Abu-Zahra.
Cancer Research Centre of the Russian Academy of Medical Sciences, Moscow, Russia—S. M. Portnoj.
Cancer Research UK, London, UK—M. Baum, J. Cuzick, M. Dowsett, J. Houghton, J. Ledermann, D. Riley.
Cancer Research UK Clinical Trials Unit (CRCTU), NCRI, Birmingham, UK—S. Bowdon, C. Brookes, I. Fernando, D. Rea, D. Spooner.
Cardiff Trialists Group, UK—R. E. Mansel.
Case Western Reserve University, Cleveland, OH, USA—N. H. Gordon.
Central Oncology Group, Milwaukee, WI, USA—H. L. Davis.
Centre Léon-Bérard, Lyon, France—Y. Lehingue, P. Romestaing.
Centre Paul Lamarque, Montpellier, France—J. B. Dubois.
Centre Regional François Baclesse, Caen, France—T. Delozier, B. Griffon, J. Mace Lesec’h.
Centre René Huguenin, Paris, St Cloud, France—P. Rambert.
Centro Oncologico, Trieste, Italy—G. Mustacchi.
Charles University, Prague, Czech Republic—L. Petruzelka, O. Pribylova.
Cheltenham General Hospital, UK—J. R. Owen.
Chemo N0 Trial Group, Germany —N. Harbeck, F. Jänicke, C. Meisner, M. Schmitt, C. Thomssen.
Chicago University, IL, USA—P. Meier.
Christie Hospital and Holt Radium Institute, Manchester, UK—A. Howell, R. Swindell.
Clinical Trial Service Unit, Oxford, UK (ie, EBCTCG Secretariat)—J. Burrett, M. Clarke, R. Collins, C. Correa, D. Cutter, S. Darby, C. Davies, K. Davies, A. Delmestri, P. Elphinstone, V. Evans, L. Gettins, J. Godwin, R. Gray, C. Gregory, D. Hermans, C. Hicks, S. James, A. Kerr, E. MacKinnon, M. Lay, P. McGale, T. McHugh, R. Peto, J. Sayer, C. Taylor, Y. Wang.
Coimbra Instituto de Oncologia, Portugal—J. Albano, C. F. de Oliveira, H. Gervásio, J. Gordilho.
Copenhagen Radium Centre, Denmark—H. Johansen, H. T. Mouridsen.
Dana-Farber Cancer Institute, Boston, MA, USA—R. S. Gelman, J. R. Harris, D. Hayes, I. C. Henderson, C. L. Shapiro, E. Winer.
Danish Breast Cancer Cooperative Group, Copenhagen, Denmark— P. Christiansen, B. Ejlertsen, M. Ewertz, H. T. Mouridsen, S. Møller, M. Overgaard.
Danish Cancer Registry, Copenhagen, Denmark—B. Carstensen, T. Palshof.
Düsseldorf University, Germany—H. J. Trampisch.
Dutch Working Party for Autologous Bone Marrow Transplant in Solid Tumours, Amsterdam and Groningen, the Netherlands—O. Dalesio, E. G. E. de Vries, S. Rodenhuis, H. van Tinteren.
Eastern Cooperative Oncology Group, Boston, MA, USA—R. L. Comis, N. E. Davidson, R. Gray, N. Robert, G. Sledge, L.J. Solin, D. C. Tormey, W. Wood.
Edinburgh Breast Unit, UK—D. Cameron, U. Chetty, P. Forrest, W. Jack.
Elim Hospital, Hamburg, Germany—J. Rossbach.
Erasmus MC/Daniel den Hoed Cancer Center, Rotterdam, the Netherlands— J. G. M. Klijn, A. D. Treurniet-Donker, W. L. J. van Putten.
European Institute of Oncology, Milan, Italy—A. Costa, U. Veronesi, G. Viale.
European Organization for Research and Treatment of Cancer, Brussels, Belgium—H. Bartelink, J. Bogaerts, N. Bijker, J. P. Julien, C. Legrand, E. Rutgers, R. Sylvester, C. J. H. van de Velde, J. G. H. van Nes.
Evanston Hospital, IL, USA—M. P. Cunningham.
Finnish Breast Cancer Group, Finland—R. Huovinen, H. Joensuu.
Fondazione Maugeri Pavia, Italy—A. Costa, C. Tinterri, P. Valagussa.
Fondazione Michelangelo, Milan, Italy—P. Valagussa.
Fox Chase Cancer Center, Philadelphia, PA, USA—L. J. Goldstein.
French Adjuvant Study Group (GFEA), Guyancourt, France—J. Bonneterre, P. Fargeot, P. Fumoleau, P. Kerbrat, E. Luporsi, M. Namer.
German Adjuvant Breast Group (GABG), Frankfurt, Germany—W. Eiermann, J. Hilfrich, W. Jonat, M. Kaufmann, R. Kreienberg, M. Schumacher.
German Breast Cancer Study Group (BMFT), Freiburg, Germany—G. Bastert, H. Rauschecker, R. Sauer, W. Sauerbrei, A. Schauer, M. Schumacher.
German Breast Group (GBG), Neu-Isenburg, Germany—J. U. Blohmer, S. D. Costa, H. Eidtmann, B. Gerber, C. Jackisch, S. Loibl, G. von Minckwitz.
Ghent University Hospital, Belgium—A. de Schryver, L. Vakaet.
GIVIO Interdisciplinary Group for Cancer Care Evaluation, Chieti, Italy—M. Belfiglio, A. Nicolucci, F. Pellegrini, M. Sacco, M. Valentini.
Glasgow Victoria Infirmary, UK—C. S. McArdle, D. C. Smith, S. Stallard.
Gruppo Oncologico Clinico Cooperativo del Nord Est, Aviano, Italy—E. Galligioni.
Gruppo Oncologico Dell’Italia Meridionale (GOIM), Rome, Italy—M. Lopez.
Gruppo Ricerca Ormono Chemio Terapia Adiuvante (GROCTA), Genova, Italy—F. Boccardo, A. Rubagotti.
Groote Schuur Hospital, Cape Town, South Africa—D. M. Dent, C. A. Gudgeon, A. Hacking, E. Murray, E. Panieri.
Grupo Español de Investigación en Cáncer de Mama (GEICAM), Spain—L. Briones, E. Carrasco, M. Martin.
Guadalajara Hospital de 20 Noviembre, Mexico—A. Erazo, J. Y. Medina.
Gunma University, Japan—J. Horiguchi, H. Takei.
Guy's Hospital, London, UK—I. S. Fentiman, J. L. Hayward, R. D. Rubens, D. Skilton.
Heidelberg University I, Germany—H. Scheurlen.
Heidelberg University II, Germany—M. Kaufmann, H. C. Sohn.
Helios Klinikum Berlin-Buch, Germany—M. Untch.
Hellenic Breast Surgeons Society, Greece—U. Dafni, C. Markopoulos.
Hellenic Cooperative Oncology Group, Athens, Greece—U. Dafni, G. Fountzilas.
Hellenic Oncology Research Group, Greece—D. Mavroudis.
Helsinki Deaconess Medical Centre, Finland—P. Klefstrom.
Helsinki University, Finland—C. Blomqvist, T. Saarto.
Hospital del Mar, Barcelona, Spain—M. Gallen.
Innsbruck University, Austria—R. Margreiter.
Institut Claudius Regaud, Toulouse, France—B. de Lafontan, J. Mihura, H. Roché.
Institut Curie, Paris, France—B. Asselain, R. J. Salmon, J. R. Vilcoq.
Institut Gustave-Roussy, Paris, France—R. Arriagada, C. Hill, A. Laplanche, M. G Lê, M. Spielmann.
Institute of Cancer Research Clinical Trials and Statistics Unit (ICR-CTSU, NCRI), UK—R. A’Hern, P. Barrett-Lee, J. Bliss, P. Ellis, L. Kilburn, J. R. Yarnold.
Instituto Nazionale per la Ricerca sul Cancro, Genova, Italy—P. Bruzzi, L. Del Mastro, P. Pronzato, M. R. Sertoli, M. Venturini.
Instituto Oncologico Romagnolo, Forli, Italy—D. Amadori.
Integraal Kankercentrum, Amsterdam, the Netherlands—J. Benraadt, M. Kooi, A. O van de Velde, J. A. van Dongen, J. B. Vermorken.
International Breast Cancer Study Group (Ludwig), Bern, Switzerland—M. Castiglione, F. Cavalli, A. Coates, J. Collins, J. Forbes, R. D. Gelber, A. Goldhirsch, J. Lindtner, K. N. Price, V. Raina, C. M. Rudenstam, H. J. Senn.
International Collaborative Cancer Group, Charing Cross Hospital, London, UK—J. M. Bliss, C. E. D. Chilvers, R. C. Coombes, E. Hall, M. Marty.
International Drug Development Institute, Louvain-la-Neuve, Belgium—M. Buyse.
International TABLE Study Group, Berlin, Germany—K. Possinger, P. Schmid, M. Untch, D. Wallwiener.
Israel NSABC, Tel Aviv, Israel—R. Borovik, G. Brufman, H. Hayat, E. Robinson, N. Yaal-Hahoshen.
Istituto Nazionale per lo Studio e la Cura dei Tumori, Milan, Italy—G. Bonadonna, T. Camerini, G. De Palo, M. G. Di Mauro, F. Formelli, P. Valagussa.
Italian Cooperative Chemo-Radio-Surgical Group, Bologna, Italy—A. Martoni, F. Pannuti.
Italian Oncology Group for Clinical Research, Parma, Italy—G. Cocconi, A. Colozza, R. Camisa, S. Gori.
Japan Clinical Oncology Group–Breast Cancer Study Group, Matsuyama, Japan—K. Aogi, S. Takashima.
Japanese Foundation for Multidisciplinary Treatment of Cancer, Tokyo, Japan—O. Abe, T. Ikeda, K. Inokuchi, K. Kikuchi, K. Sawa.
Kawasaki Medical School, Japan—H. Sonoo.
Krakow Institute of Oncology, Poland—S. Korzeniowski, J. Skolyszewski.
Kumamoto University Group, Japan—M. Ogawa, J. Yamashita.
Leuven Akademisch Ziekenhuis, Gasthuisberg, Belgium—R. Christiaens, P. Neven, R. Paridaens, W. Van den Bogaert.
Ludwig-Maximilians University, Munich, Germany—S. Braun, W. Janni.
Marseille Laboratoire de Cancérologie Biologique APM, France—P. Martin, S. Romain.
Memorial Sloan-Kettering Cancer Center, New York, NY, USA—T. Hakes, C. A. Hudis, L. Norton, R. Wittes.
Metaxas Memorial Cancer Hospital, Athens, Greece—G. Giokas, D. Kondylis, B. Lissaios.
Mexican National Medical Centre, Mexico City, Mexico—R. de la Huerta, M. G. Sainz.
National Cancer Institute, Bethesda, MD, USA—R. Altemus, K. Camphausen, K. Cowan, D. Danforth, A. Lichter, M. Lippman, J. O’Shaughnessy, L. J. Pierce, S. Steinberg, D. Venzon, J. A. Zujewski.
National Cancer Institute of Bari, Italy—C. D’Amico, M. Lioce, A. Paradiso.
NCIC Clinical Trials Group, Kingston, Ontario, Canada—J. W. Chapman, P. E. Goss, M. N. Levine, J. D. Myles, J. L. Pater, K. I. Pritchard, L. E. Shepherd, D. Tu, T. Whelan, B. Zee.
National Kyushu Cancer Center, Japan—Y. Nomura.
National Surgical Adjuvant Breast and Bowel Project (NSABP), Pittsburgh, PA, USA—S. Anderson, G. Bass, A. Brown, J. Bryant (deceased), J. Costantino, B. Fisher, C. Geyer, S. Paik, C. Redmond, L. Wickerham, N. Wolmark.
Nolvadex Adjuvant Trial Organisation, London, UK—M. Baum, I. M. Jackson (deceased), M. K. Palmer.
North Central Cancer Treatment Group, Mayo Clinic, Rochester, MN, USA—E. Perez, J. N. Ingle, V. J. Suman.
North Sweden Breast Cancer Group, Umea, Sweden—N. O. Bengtsson, S. Emdin, B. Granstrand, H. Jonsson.
North-West Oncology Group (GONO), Italy—L. Del Mastro, M. Venturini.
North-Western British Surgeons, Manchester, UK—J. P. Lythgoe, R. Swindell.
Northwick Park Hospital, London, UK—M. Kissin.
Norwegian Breast Cancer Group, Oslo, Norway—B. Erikstein, E. Hannisdal, A. B. Jacobsen, J. E. Varhaug.
Norwegian Radium Hospital, Oslo, Norway—B. Erikstein, S. Gundersen, M. Hauer-Jensen, H. Høst, A. B. Jacobsen, R Nissen-Meyer.
Nottingham City Hospital, UK—R. W. Blamey, A. K. Mitchell, D. A. L. Morgan, J. F. R. Robertson.
Oncofrance, Paris, France—M. Di Palma, G. Mathé, J. L. Misset.
Ontario Clinical Oncology Group, Hamilton, Canada—R. M. Clark, M. Levine, K. I. Pritchard, T. Whelan.
Osaka City University, Japan—K. Morimoto.
Osaka National Hospital, Japan—K. Sawa, Y. Takatsuka.
Oxford Radcliffe Hospitals NHS Trust, Churchill Hospital, UK—E. Crossley, A. Harris, D. Talbot, M. Taylor.
PACS Adjuvant Study Group, France—A. L. Martin, H. Roché.
Parma Hospital, Italy—G. Cocconi, B. di Blasio.
Petrov Research Institute of Oncology, St Petersburg, Russia—V. Ivanov, V. Semiglazov.
Piedmont Oncology Association, Winston-Salem, NC, USA—J. Brockschmidt, M. R. Cooper.
Prefectural Hospital, Oita, Japan—H. Ueo.
Pretoria University, South Africa—C. I. Falkson.
Royal Marsden Hospital, Institute of Cancer Research, London, UK—R. A’Hern, S. Ashley, A. Makris, T. J. Powles, I. E. Smith, J. R. Yarnold.
St George's Hospital, London, UK—J. C. Gazet.
St George's Hospital, Sydney, Australia—L. Browne, P. Graham.
St Luke's Hospital, Dublin, Ireland—N. Corcoran.
Sardinia Oncology Hospital A Businico, Cagliari, Sardinia—N. Deshpande, L. di Martino.
SASIB International Trialists, Cape Town, South Africa—P. Douglas, A. Hacking, H. Høst, A. Lindtner, G. Notter.
Saskatchewan Cancer Foundation, Regina, Canada—A. J. S. Bryant, G. H. Ewing, L. A. Firth, J. L. Krushen-Kosloski.
Scandinavian Adjuvant Chemotherapy Study Group, Oslo, Norway—R. Nissen-Meyer.
Scottish Cancer Therapy Network, Edinburgh, UK—L. Foster, W. D. George, H. J. Stewart, P. Stroner.
South Sweden Breast Cancer Group, Lund, Sweden—H. Anderson, P. Malmström, T. R. Möller, A. Ringberg, L. Rydén, I. Tengrup, L. Tennvall-Nittby.
South-East Sweden Breast Cancer Group, Linköping, Sweden—L.-G. Arnesson, J. Carstensen, M. Dufmats, B. Nordenskjöld, M. Söderberg.
South-Eastern Cancer Study Group and Alabama Breast Cancer Project, Birmingham, AL, USA—J. T. Carpenter.
Southwest Oncology Group, San Antonio, TX, USA—K. Albain, W. Barlow, J. Crowley, D. Hayes, J. Gralow, S. Green, G. Hortobagyi, R. Livingston, S. Martino, C. K. Osborne, P. M. Ravdin.
Southampton Oncology Centre, UK—N. Murray, G. T. Royle, P. D. Simmonds.
Stockholm Breast Cancer Study Group, Sweden—J. Askergren, M. Bäckdahl, J. Bergh, R. Fernstad, T. Fornander, J. Frisell, U. Glas, T. Hatschek, K. Ideström U. Johansson, L. Perbeck, S. Rotstein, L. E. Rutqvist, K. Sandelin, T. Singnomklao, L. Skoog, A. Somell, A. Wallgren, N. Wilking.
Swiss Group for Clinical Cancer Research (SAKK), Bern, and OSAKO, St Gallen, Switzerland—M. Castiglione, A. Goldhirsch, R. Maibach, H. J. Senn, B. Thürlimann.
Tampere University Hospital, Finland—K. Holli, K. Rouhento.
Tel Aviv University, Israel—H. Brenner, A. Hercbergs.
The High-Dose Chemotherapy for Breast Cancer Study Group (PEGASE), France—A. L. Martin, H. Roché.
Tokyo Cancer Institute Hospital, Japan—M. Yoshimoto.
Toronto-Edmonton Breast Cancer Study Group, Canada—G. DeBoer, A. H. G. Paterson, K. I. Pritchard.
Toronto Princess Margaret Hospital, Canada—A. Fyles, J. W. Meakin, T. Panzarella, K. I. Pritchard.
Tumour Hospital, Chinese Academy of Medical Sciences, Beijing, People's Republic of China (in collaboration with the Oxford CTSU)—Y. Shan, Y. F. Shao, X. Wang, D. B. Zhao (CTSU: Z. M. Chen, H. C. Pan).
Tunis Institut Salah Azaiz, Tunisia—J. Bahi.
UK Multicentre Cancer Chemotherapy Study Group, London, UK—M. Reid, M. Spittle.
UK/ANZ DCIS Trial in Women with Locally Excised DCIS—H. Bishop, N. J. Bundred, J. Cuzick, I. O. Ellis, I. S. Fentiman, J. F. Forbes, S. Forsyth, W. D. George, V. R. Pai, S. E. Pinder, I. Sestak.
UK/Asia Collaborative Breast Cancer Group, London, UK—G. P. Deutsch, D. L. W. Kwong, V. R. Pai, F. Senanayake.
University Federico II, Naples, Italy—A. R. Bianco, C. Carlomagno, M. De Laurentiis, S. De Placido.
University of Texas MD Anderson Cancer Center, Houston, TX, USA—K. Broglio, A. U. Buzdar.
University of Wisconsin—R. R. Love.
Uppsala-Örebro Breast Cancer Study Group, Sweden—J. Bergh, H. Garmo, L. Holmberg, G. Liljegren, J. Nilsson.
US Oncology, Houston, TX, USA—S. E. Jones, D. M. Loesch.
Vienna University Hospital 1st Department of Gynaecology, Austria—M. Janauer, M. Seifert, P. Sevelda, C. C. Zielinski.
West German Study Group (WSG), Germany—O. Gluz, U. Nitz.
West Midlands Oncology Association, Birmingham, UK—J. A. Dunn, R. K. Hills, M. Lee, J. M. Morrison, C. Poole, D. Rea, D. Spooner.
West of Scotland Breast Trial Group, Glasgow, UK—A. Litton.
West Sweden Breast Cancer Study Group, Gothenburg, Sweden—A. Wallgren, P. Karlsson.
Western Cancer Study Group, Torrance, CA, USA—R. T. Chlebowski.
Würzburg University, Germany—H. Caffier.
Footnotes
The writing committee for this paper were: C. Correa, P. McGale, C. Taylor, Y. Wang, M. Clarke, C. Davies, R. Peto, N. Bijker, L. Solin, and S. Darby. The EBCTCG secretariat in the Clinical Trial Service Unit, University of Oxford (listed above) accept full responsibility for the overall content of this report.
The writing committee and secretariat declare that they have no conflict of interest.
The main acknowledgment is to the thousands of women who took part in the trials reviewed here. Funding for the EBCTCG secretariat is from the general long-term financial support from the Cancer Research UK and the UK Medical Research Council to the Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), University of Oxford. These organisations had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Costantino J, Redmond C, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328(22):1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16(2):441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 5.Fisher ER, Dignam J, Tan-Chiu E, et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) eight-year update of Protocol B-17. Intraductal carcinoma. Cancer. 1999;86(3):429–438. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Julien JP, Bijker N, Fentiman IS, et al. Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the EORTC randomised phase III trial 10853. EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. Lancet. 2000;355(9203):528–533. doi: 10.1016/s0140-6736(99)06341-2. [DOI] [PubMed] [Google Scholar]
- 7.Bijker N, Peterse JL, Duchateau L, et al. Histological type and marker expression of the primary tumour compared with its local recurrence after breast-conserving therapy for ductal carcinoma in situ. Br J Cancer. 2001;84(4):539–544. doi: 10.1054/bjoc.2000.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bijker N, Peterse JL, Duchateau L, et al. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ: analysis of European Organization for Research and Treatment of Cancer Trial 10853. J Clin Oncol. 2001;19(8):2263–2271. doi: 10.1200/JCO.2001.19.8.2263. [DOI] [PubMed] [Google Scholar]
- 9.Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24(21):3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 10.Emdin SO, Granstrand B, Ringberg A, et al. SweDCIS: radiotherapy after sector resection for ductal carcinoma in situ of the breast. Results of a randomised trial in a population offered mammography screening. Acta Oncol. 2006;45(5):536–543. doi: 10.1080/02841860600681569. [DOI] [PubMed] [Google Scholar]
- 11.Ringberg A, Nordgren H, Thorstensson S, et al. Histopathological risk factors for ipsilateral breast events after breast conserving treatment for ductal carcinoma in situ of the breast—results from the Swedish randomised trial. Eur J Cancer. 2007;43(2):291–298. doi: 10.1016/j.ejca.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol. 2008;26(8):1247–1252. doi: 10.1200/JCO.2007.12.7969. [DOI] [PubMed] [Google Scholar]
- 13.Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362(9378):95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 14.Solin LJ, Fourquet A, Vicini FA, et al. Long-term outcome after breast-conservation treatment with radiation for mammographically detected ductal carcinoma in situ of the breast. Cancer. 2005;103(6):1137–1146. doi: 10.1002/cncr.20886. [DOI] [PubMed] [Google Scholar]
- 15.Wong JS, Kaelin CM, Troyan SL, et al. Prospective study of wide excision alone for ductal carcinoma in situ of the breast. J Clin Oncol. 2006;24(7):1031–1036. doi: 10.1200/JCO.2005.02.9975. [DOI] [PubMed] [Google Scholar]
- 16.Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27(32):5319–5324. doi: 10.1200/JCO.2009.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverstein MJ, Lagios MD, Groshen S, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med. 1999;340(19):1455–1461. doi: 10.1056/NEJM199905133401902. [DOI] [PubMed] [Google Scholar]