Abstract
Diagnosis of ductal carcinoma in situ (DCIS) has increased dramatically in parallel with the increased use of screening mammography. There are specific mammographic findings, most associated with shapes (amorphous, fine and coarse pleomorphic, and fine linear) and distributions (linear and segmental) of calcifications that permit a reasonable sensitivity for detection of DCIS without an unreasonable decrease in specificity, especially in view of the dramatic decrease in breast cancer mortality associated with early detection. While some DCIS may never progress to invasive disease, at this time, we cannot make that separation. This should be an active area for research.
Ductal carcinoma in situ (DCIS) is a distinct lesion of the breast that is a precursor to development of invasive carcinoma. Before the widespread use of screening mammography, DCIS accounted for less than 5% of breast cancers (1). Currently, this diagnosis is rendered in about 30% of cases. Some of the cases diagnosed as DCIS will not progress to invasive disease, and this has been offered as a risk of screening mammography (2,3). This argument is cogent if we had a means, at the detection phase, to determine which of these in situ malignancies will progress to invasive disease. At this point, it is not possible, with a high degree of certainty, to ascertain which in situ malignancy will progress to invasive disease from those that remain indolent. Certainly, studies aimed at which DCIS may progress and those that may not should be one of the most active areas for research, both at the detection and histology level. The histology diagnosis of DCIS increased with routine use of mammography (4), while the mortality from breast cancer, as verified by many worldwide screening trials, has decreased by at least 30%, in great part due to early nonclinical detection, almost entirely related to mammography (5,6). Thus, it becomes quite clear that until we possess the ability to assign different levels of concern for findings suggestive of DCIS, at both the detection and histology phase, we must continue searching and verifying the presence of in situ disease to help preserve the dramatic decrease in breast cancer mortality we see today.
Materials and Methods/Results
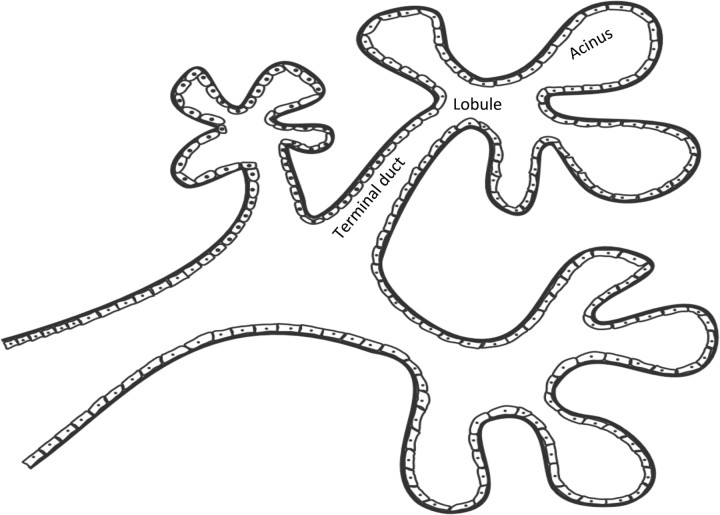
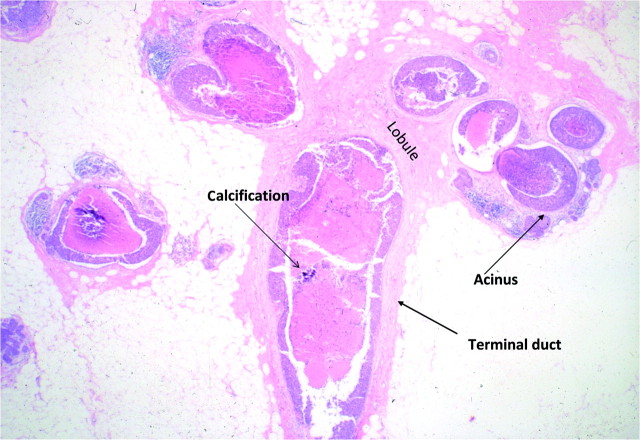
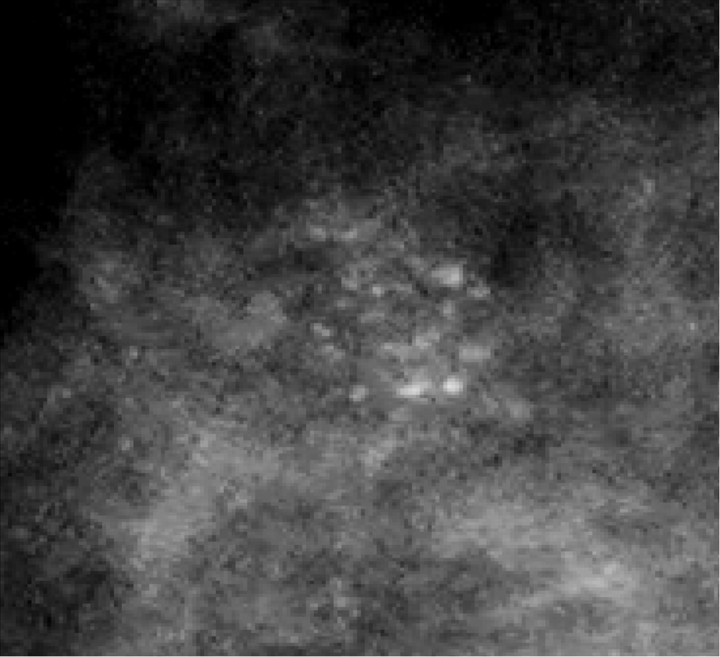
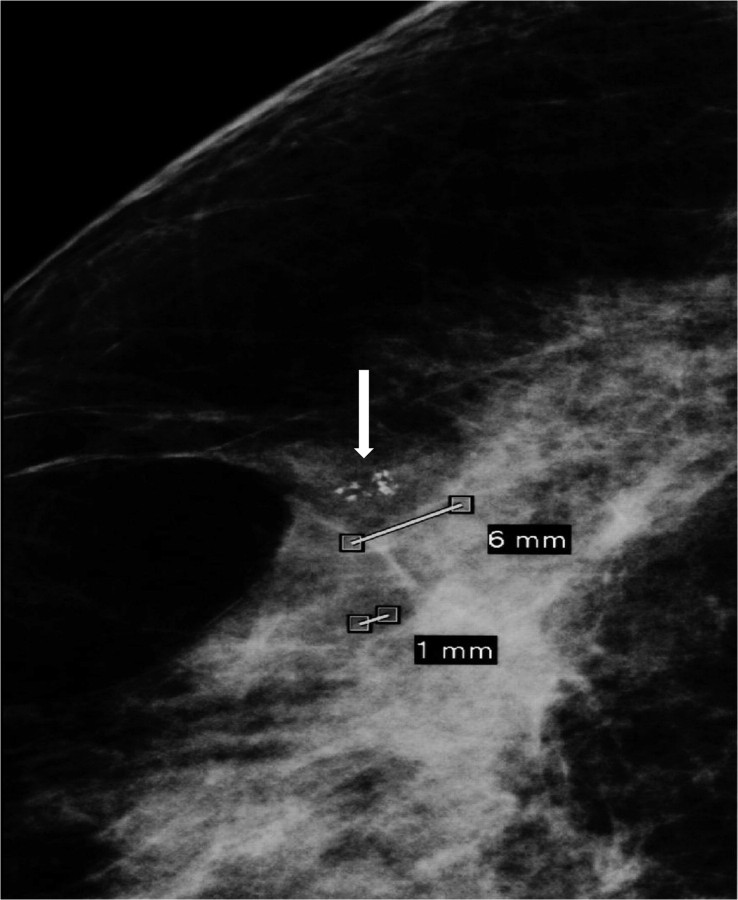
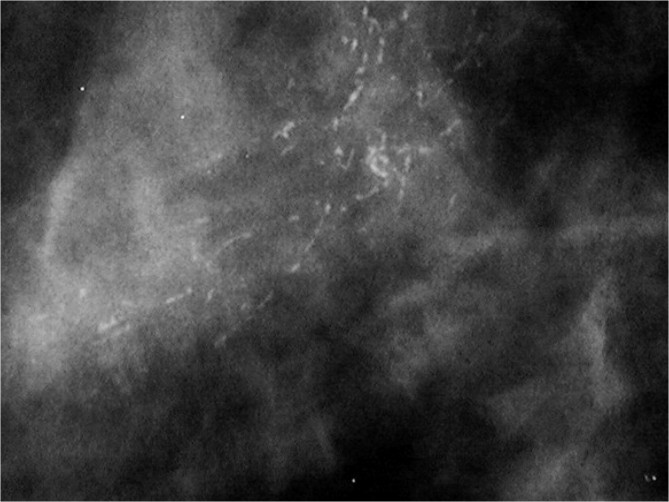
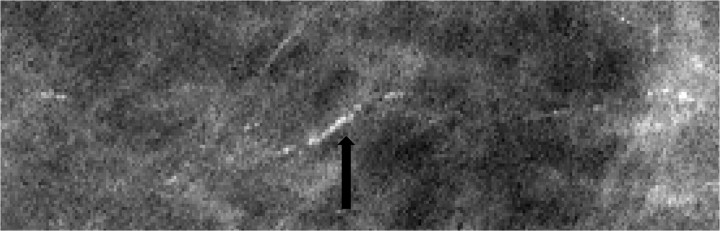
The detection of DCIS involves knowledge of the anatomy of the terminal ductal lobular unit (TDLU) (Figure 1) and the types and distributions of calcifications occurring in the terminal duct portion of the TDLU, which significantly raise the potential for the presence of DCIS (Figures 2 and 3). The specific forms of calcifications related to DCIS are amorphous, coarse and fine pleomorphic, and fine linear. The suspicious distributions are linear and segmental. The amorphous forms are small (2–300 μm) and hazy in appearance (Figure 4). Their association with malignancy, especially DCIS, is as high as 20% (7). Fine pleomorphic calcifications are more conspicuous than the amorphous forms but are also irregular in shape with the same size range as amorphous calcifications (Figure 5, A and B). Coarse pleomorphic calcifications are larger than the fine pleomorphic or amorphous calcifications but do not exceed 1 mm in size (Figure 6). A linear morphology is manifested as thin, irregular and discontinuous calcifications smaller than 0.5 mm (Figure 7). These can be associated with DCIS in up to 80% of cases. The linear and segmental distributions of calcifications are surrogate markers for disease distributed in the duct or ducts of TDLUs. The linear distribution represents calcifications arrayed in a line (Figure 8) suggesting a ductal deposit, while a segmental distribution suggests deposits in a duct and its branches. Distributions are equally as important as the shapes of calcifications and may be also associated with malignancy from 60% to 80% of the time (8).
Figure 1.
Terminal ductal lobular unit. The basic subgross histological unit in the breast is the terminal ductal lobular unit. There is a terminal duct and a lobule. The lobule is subdivided into acini.
Figure 2.
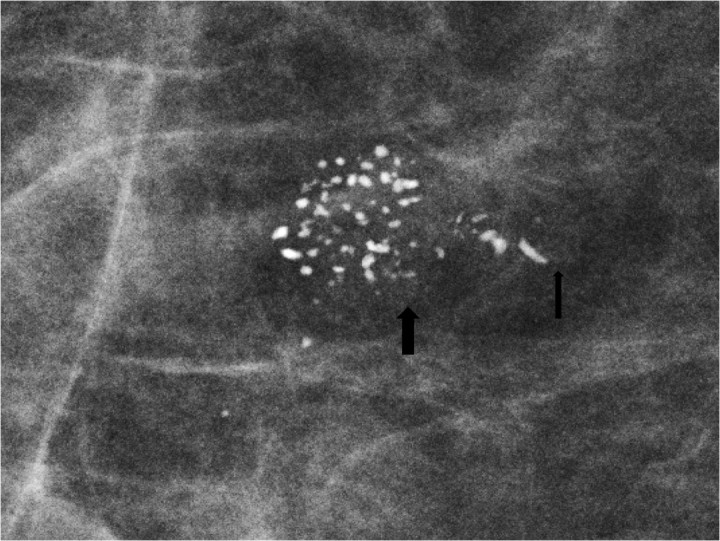
Spot magnification mammogram. The anatomy of the terminal ductal lobular unit is demonstrated on this magnification spot view with the lobule and acini filled with coarse heterogeneous calcifications (large arrow) and the terminal duct filled with linear forms of calcifications (small arrow). Pathology: comedo ductal carcinoma in situ.
Figure 3.
Histology of terminal ductal lobular unit.
Figure 4.
Spot magnification mammogram. Cluster of amorphous calcifications. Note the hazy appearance of these calcifications. Pathology: cribiform ductal carcinoma in situ.
Figure 5.
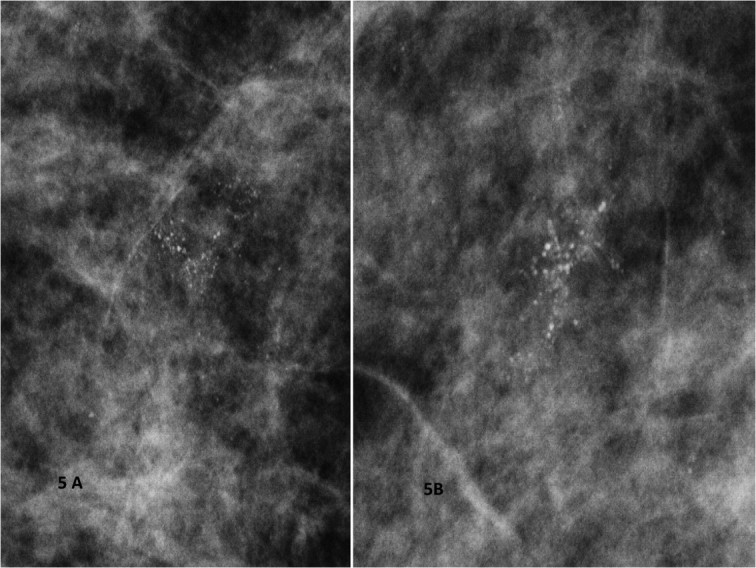
A) and B) Spot magnification mammograms. Two different patients with a segmental arrangement of fine pleomorphic calcifications. Pathology: comedo ductal carcinoma in situ.
Figure 6.
Spot magnification mammography. Cluster of coarse hetereogeneous calcifications. Note size of the individual particles approaching 1 mm (arrow). Pathology: benign fibroadenomatoid change.
Figure 7.
Spot magnification mammogram. A segmental arrangement of fine linear calcifications. Pathology: comedo ductal carcinoma in situ.
Figure 8.
Spot magnification mammogram. Linear arrangement of punctate calcifications. Pathology: cribiform ductal carcinoma in situ.
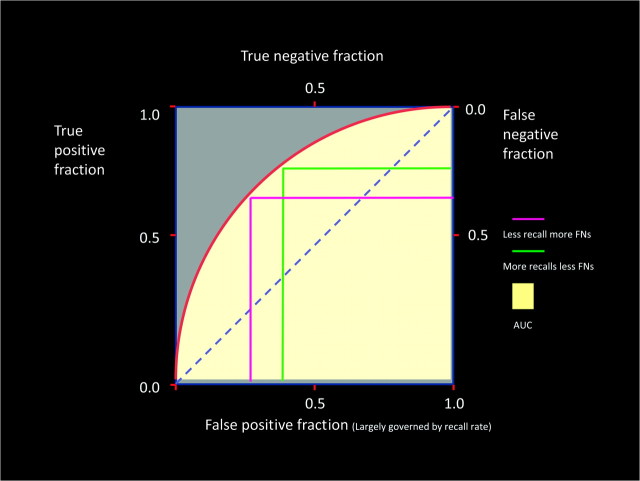
To clearly understand the interplay of risk and benefit related to detection of DCIS, some understanding of the receiver operating characteristic (ROC) curve is warranted. If we define a benefit as detection of histologically proven DCIS and risk as a false-positive benign event, the ROC curve allows us to see how the true-positive and false-positive events interact. As is demonstrated in Figure 9, the combination of sensitivity and specificity is defined as accuracy of the examination and is represented by the area under the curve. Most importantly, as the sensitivity of the examination increases (the ability to identify DCIS in the population tested from all DCIS in that population), the specificity of the examination (the ability of the examination to define those without DCIS from all without DCIS in the population tested) decreases. Put another way, as the false positives increase the false negatives or missed DCIS decrease. As a test improves in accuracy, the apex of the curve will move toward the left increasing both sensitivity and specificity. However, one can maintain an identical accuracy but either increase or decease sensitivity or specificity at the expense of the other. This will indicate movement along an ROC curve but not displacement of that curve upward and to the left. As ones accuracy increases to the 85%–90% level, it will become difficult to further reasonably improve accuracy. If the goal of screening is to detect early breast cancer (DCIS), the false negatives may be decreased and sensitivity for detecting these events increased by increasing the false positives and decreasing the specificity (more benign workups and percutaneous biopsies). This is one definition of the benefit to risk ratio. If we calculate this ratio, defining the benefit of breast cancer detection by annual screening vs the risk of producing a breast cancer by the screening process, that ratio is about 55:1 (9,10). If ones accuracy is suboptimal, education concerning the test (mammography) and features depicted by the test can increase the accuracy and move the curve up and to the left. This underlines the importance of Continuing Medical Education for mammography as well as other breast imaging techniques.
Figure 9.
Receiver operating characteristic curve.
Conclusion
What are the facts related to detection and management of DCIS? The detection of DCIS is a mammographic and almost always not a clinical event. When rates of DCIS were compared from a pre-mammography era with the one in which mammography was in widespread use, the total number of DCIS was 200% higher than expected when these eras were compared (11). The National Surgical Adjuvant Breast and Bowel Project B24 trial (12) demonstrated a 7-year risk of recurrence of 11.1% for DCIS treated with radiation therapy and lumpectomy. Untreated comedo DCIS may lead to invasive disease in 1–5 years, and untreated non-comedo DCIS may lead to invasive disease in 5–15 years. Approximately 25%–30% of DCIS may never progress to invasive malignancy. However, at this point we cannot determine, with any degree of significance, which will and which will not progress. Breast cancer mortality relative risk for eight world trials comparing populations using mammography with those not utilizing mammography ranged from 0.68 to 1.02 (5,6). Breast cancer mortality relative risk comparing the screening epoch with the prescreening epoch ranged from 0.41 to 0.67 (13). There has been a 30% decrease in mortality in the United States beginning at the time of widespread use of screening mammography. These facts compel us to not trivialize DCIS and until there is information that allows one to separate the progressive from nonprogressive DCIS, detection must continue or we potentially place in jeopardy the historic strides made in decreasing mortality from breast cancer.
References
- 1.Rosner D, Bedwani RN, Vana J, et al. Non-invasive breast carcinoma: results of a national survey by the American College of Surgeons. Ann Surg. 1980;192(2):139–147. doi: 10.1097/00000658-198008000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blichert-Toft M, Graversen HP, Andersen J, et al. In sity breast carcinomas: a population-based study on frequency, growth pattern, and clinical aspects. World J Surg. 1988;12:845–851. doi: 10.1007/BF01655494. [DOI] [PubMed] [Google Scholar]
- 3.Ward BA, McKhann CF, Ravikumar TS. Ten-year follow-up of breast carcinoma in situ in Connecticut. Arch Surg. 1992;127(12):1392–1395. doi: 10.1001/archsurg.1992.01420120026004. [DOI] [PubMed] [Google Scholar]
- 4.Barreau B, deMascarel I, Feuga C, et al. Mammography of ductal carcinoma in situ of the breast: review of 909 cases with radiographic-pathologic correlation. Eur J Radiol. 2005;54(1):55–61. doi: 10.1016/j.ejrad.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Tabar L, Duffy SW, Burhenne LW, et al. New Swedish breast cancer detection results for women aged 40-49. Cancer. 1993;72(4 suppl):1437–1448. doi: 10.1002/1097-0142(19930815)72:4+<1437::aid-cncr2820721405>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Tabar L, Yen MF, Vitak B, et al. Mammography service screening and mortality in breast cancer patients: 20 year follow up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410. doi: 10.1016/S0140-6736(03)13143-1. [DOI] [PubMed] [Google Scholar]
- 7.Berg W, Arnoldus CL, Teferra E. Biopsies of amorphous breast calcifications: pathologic outcome and yield at stereotactic biopsy. Radiology. 2001;221(2):495–503. doi: 10.1148/radiol.2212010164. [DOI] [PubMed] [Google Scholar]
- 8.Liberman L, Abramson AF, Squires FB, Glassman JR, Morris EA, Dershaw DD. The breast imaging reporting and data system: positive predictive value of mammographic features and final assessment categories. AJR Am J Roentgenol. 1998;171(1):35–40. doi: 10.2214/ajr.171.1.9648759. [DOI] [PubMed] [Google Scholar]
- 9.Committee to Assess Health Risks from Exposure to Low levels of Ionizing Radiation. Board of Radiation Effects Research, Division on Earth and Life Studies, National Research Council of the National Academies. Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII, Phase 2. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 10.Hendrick RE, Pisano ED, Averbukh A, et al. Comparison of acquisition parameters and breast dose in digital mammography and screen-film mammography in the American College of Radiology Imaging Network (ACRIN) Digital Mammographic Imaging Screening Trial (DMIST) AJR Am J Roentgenol. 2010:194. doi: 10.2214/AJR.08.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernester VL, Barclay J, Kerlikowske K, et al. Incidence and treatment for ductal carcinoma in situ of the breast. JAMA. 1996;275(12):913–918. [PubMed] [Google Scholar]
- 12.Fisher B, Dignam J, Wolmark W, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 13.The Swedish Organised Service Screening Education Group. Reduction in breast cancer mortality from organised service screening with mammography, 1: further confirmation with extended data. Cancer Epdemiol Biomarkers Prev. 2006;15:45–51. doi: 10.1158/1055-9965.EPI-05-0349. [DOI] [PubMed] [Google Scholar]