Abstract
Inconsistent associations between smoking and telomere length (TL) have been reported in epidemiologic studies, perhaps because of the time-varying nature of smoking behaviors. We estimated the associations of TL, which was measured by quantitative polymerase chain reaction using saliva DNA, with concurrent and past smoking status reported biennially for up to 16 years before TL measurement in 5,624 participants in the Health and Retirement Study (1992–2008). Smoking was associated with reduced TL when we used prospective data on smoking statuses among men and women, but the association was strongly attenuated among men in cross-sectional analyses. This attenuation was largely due to a higher rate of smoking cessation during the study period among men with shorter TL than among men with longer TL. Short TL was also associated with poorer overall health in men, which suggests that male smokers with short TL were more likely to quit smoking because of poor health. Analyses of years since cessation, smoking duration, and pack-years of smoking all support the hypothesis that increased cigarette use shortens TL. Our results provide a potential explanation for the inconsistent associations between smoking and TL reported in previous cross-sectional studies. Time-varying associations should be considered in future studies of smoking behavior, TL, aging, and disease risk.
Keywords: aging, Health and Retirement Study, sex, smoking, smoking cessation, telomere length
Telomeres are DNA-protein complexes at chromosome ends that protect DNA from damage. Shortening of the DNA component of telomeres occurs with cell division, with average leukocyte telomere length (TL) decreasing by approximately 25 to 50 base pairs per year in adults (1). TL has been studied extensively as a biomarker of aging and susceptibility to age-related diseases, including cardiovascular diseases, cancers, and neurocognitive diseases (2–5).
Cigarette smoking results in exposure to free radicals and reactive oxygen species, which leads to increased oxidative stress and inflammation (6). Both processes have also been linked to TL shortening (7–9), leading to the hypothesis that smoking promotes TL shortening (10). However, epidemiologic reports of smoking and TL have been inconsistent; in some studies, investigators reported shorter TL associated with smoking (11–20), whereas others found no association (21–26). This inconsistency has also been seen in studies of smoking and longitudinal measures of TL attrition, with associations between smoking and TL shortening seen in some studies (17, 18) but not in others (19, 20). Furthermore, a study of older men in Finland (27) found that smoking during midlife was associated with short TL measured later in life but that smoking was not associated with TL in cross-sectional analyses conducted in later life, which suggests that the timing of smoking behavior assessment may be important.
In the present study, we examined the associations of TL measured in 2008 with both concurrent smoking and prior smoking reported every 2 years for up to 16 years before TL measurement among 5,624 participants in the Health and Retirement Study (HRS), a population-based study of subjects 50 years of age or older in the United States. Because both TL (28–30) and smoking behavior (31, 32) vary by sex, we further examined whether the association between smoking and TL also varied by sex.
METHODS
Study participants
The HRS is a nationally representative longitudinal study of more than 26,000 subjects who are 50 years of age or older (33). The study is sponsored by the National Institute on Aging and conducted by the University of Michigan (34). Investigators collect individual-level information on social, economic, and physical health characteristics every 2 years, beginning in 1992. At wave 9 (2008), saliva was collected from a subset of individuals to obtain DNA using an Oragene self-collection kit (DNA Genotek, Kanata, Ontario, Canada).
TL measurement
TL was measured using quantitative polymerase chain reaction in saliva samples (n = 5,808) from which DNA was extracted. In brief, TL was measured by comparing telomere sequence copy number to a single-copy gene copy number, with the resulting ratio (hereafter referred to as the T/S ratio) being proportional to the mean TL of a sample. Assays were run either in duplicate or triplicate, with a coefficient of variation of 2.1% between the 2 procedures. Sample replicates with a coefficient of variation greater than 12.5% were re-assayed. Control DNA from 3 cancer cell lines were included on each plate, resulting in interplate assay coefficients of variation ranging from 3.5% to 6.3%. Additional details are available in the 2008 Telomere Length Data Documentation (34).
To reduce skewness, TL was (natural) log transformed. Subjects with log-transformed TLs that were more than 3 standard deviations from the mean (n = 66) and subjects younger than age 50 years at the time of enrollment (partners of original subjects; n = 118) were excluded before the analyses, resulting in 5,624 remaining subjects. Because the log-transformed TL distribution was still not normally distributed (skewness = −0.50, kurtosis = 6.69), we repeated the analyses using a normal-quantile transformation of TL.
Smoking status and covariates
At each wave, data were collected on smoking status (never, former, or current smoker) and number of cigarettes smoked per day for current smokers (<10, 10–19, or ≥20). Also collected at least once during the study for each participant were data on years of smoking initiation and smoking cessation (if applicable). From the available data, we constructed 4 variables: 1) For subjects who were smokers in their first wave, we created a binary variable for quitting during the observation period, to which we refer as “recent smoking cessation”; 2) for all individuals who were former smokers at wave 9, we created a “years since smoking cessation” variable that represented years since quitting relative to wave 9 (<20, 20–29, or ≥30) and captured historical smoking cessation across the lifetime; 3) for ever smokers, we created a “smoking duration” variable in years (<20, 20–39, or ≥40) using the reported year of smoking initiation and year of smoking cessation (or 2008 for those who still smoked in wave 9); and 4) although data on smoking intensity prior to the study was not collected, for individuals who smoked during the study, we calculated “on-study” pack-years of smoking (<5, 5–9, or ≥10). We imputed missing numbers of cigarettes smoked per day for each wave by carrying forward the last observation. Estimated lifetime pack-years of smoking, which were determined by assuming the smoking intensity before enrollment was the same as the lifetime maximum smoking intensity, were also investigated.
Other covariates were age, sex, race, educational level, income-to-poverty ratio (i.e., ratio of household income to the poverty threshold for household size), body mass index, alcohol consumption (0, 1–6, or ≥7 drinks per week), and daily physical activity level (none, light, medium, or vigorous). Covariates were either provided by or derived from RAND HRS, version M (35).
Health conditions related to smoking may also be predictors of TL or consequences of variation in TL. We used 2 variables as indicators of individuals’ overall health status. The first was a comorbidity index that sums indicators for whether respondents reported that a doctor had ever told them they had any of 8 conditions: high blood pressure, diabetes, cancer, lung disease, heart disease, stroke, psychiatric problems, or arthritis. The second was a self-reported general health status, with 1 being excellent and 5 being poor.
Statistical analysis
To assess the cross-sectional association between current smoking status and TL in wave 9, we used a linear regression model with TL as the outcome. Additive interactions of smoking with age and sex were tested. Sex- and age-stratified analyses (<65 years vs. ≥65 years) were also performed to assess potential differences in associations by sex and age. Analyses were repeated using prospective measures of smoking status reported at waves 1–8 rather than smoking status at wave 9. This analysis was restricted to subjects with TL data who entered in wave 1 (n = 2,369).
Additional smoking characteristics (number of cigarettes smoked per day in wave 9, recent smoking cessation, years since cessation, smoking duration, and on-study pack-years of smoking) were also tested in covariate-adjusted models. To assess the association between TL and overall health, models were fit containing each of the health measures as additional covariates. We also assessed potential residual confounding due to experimental/batch variation in TL measurement by further adjusting the statistical model for DNA sample batch (HRS-provided plate numbers). In sensitivity analyses, we used the post-hoc sampling weights provided by the HRS (for each wave based on post-stratification of the sample to the Current Population Survey or American Community Survey (36) for the survey year).
Additional stratification by age, adjustment for survey weights, modeling using normal-quantile transformed TL as the outcome, and adjustment for sample batch were also performed. All analyses were conducted using Stata, version 14.0 (StataCorp LP, College Station, Texas).
Simulations to assess bias due to loss to follow-up
To assess potential bias arising from differential loss to follow-up with respect to smoking and short TL, we conducted analyses of simulated data sets (Web Appendix 1, available at http://aje.oxfordjournals.org/). The simulations were conducted under the assumption that both smoking and short TL increase the probability of being lost to follow-up.
RESULTS
Cross-sectional analysis
The characteristics of the 5,624 HRS subjects at the time of TL measurement (wave 9), as well as means and standard deviations of TL within strata of participant characteristics, are shown in Table 1. A total of 5,517 subjects had nonmissing data for all demographic and lifestyle covariates included in the cross-sectional regression analysis. Table 2 displays the regression coefficients and confidence intervals for all variables included in the multivariate model. β coefficients are interpreted as the per-unit difference in ln(T/S) for continuous variables or the difference in ln(T/S) compared with reference category for categorical variables after adjustment for all other covariates. TL was shorter with increasing age (P = 4.0 × 10−17), longer among women (P = 5.8 × 10−3), and longer among black and Hispanic subjects than among white subjects (P = 1.7 × 10−11 and P = 3.5 × 10−3, respectively). TL was also positively associated with increasing educational level, income, body mass index, alcohol consumption, and physical activity level. Estimates were similar when stratified by age (<65 years vs. ≥65 years) (Web Table 1), adjusted for survey weights (Web Table 2), modeled using normal-quantile transformed TL as the outcome (Web Table 3), and adjusted for sample batch (Web Table 4).
Table 1.
Mean Telomere Length Within Strata of Subject Characteristics, Health and Retirement Study, 2008
| Characteristica | Men | Women | All | |||
|---|---|---|---|---|---|---|
| Telomere Length, mean (SD) | No. | Telomere Length, mean (SD) | No. | Telomere Length, mean (SD) | No. | |
| Age, years | ||||||
| 50–54 | 1.46 (0.35) | 66 | 1.42 (0.30) | 209 | 1.43 (0.31) | 275 |
| 55–59 | 1.38 (0.36) | 355 | 1.41 (0.33) | 483 | 1.39 (0.34) | 838 |
| 60–64 | 1.34 (0.35) | 270 | 1.38 (0.33) | 462 | 1.37 (0.33) | 732 |
| 65–69 | 1.29 (0.34) | 447 | 1.34 (0.33) | 613 | 1.32 (0.34) | 1,060 |
| 70–74 | 1.30 (0.32) | 435 | 1.30 (0.35) | 601 | 1.30 (0.34) | 1,036 |
| 75–79 | 1.28 (0.35) | 361 | 1.29 (0.35) | 401 | 1.28 (0.35) | 762 |
| 80–84 | 1.25 (0.33) | 223 | 1.28 (0.31) | 278 | 1.27 (0.31) | 501 |
| ≥85 | 1.23 (0.39) | 153 | 1.28 (0.34) | 267 | 1.26 (0.36) | 420 |
| Race | ||||||
| White | 1.29 (0.34) | 1,781 | 1.32 (0.33) | 2,430 | 1.30 (0.33) | 4,211 |
| Black | 1.40 (0.41) | 266 | 1.42 (0.37) | 466 | 1.42 (0.38) | 732 |
| Hispanic | 1.33 (0.34) | 213 | 1.38 (0.33) | 344 | 1.36 (0.33) | 557 |
| Other | 1.35 (0.38) | 50 | 1.33 (0.36) | 74 | 1.34 (0.37) | 124 |
| Educational level | ||||||
| Less than high school | 1.31 (0.34) | 464 | 1.31 (0.34) | 700 | 1.31 (0.34) | 1,164 |
| GED | 1.23 (0.30) | 103 | 1.36 (0.36) | 142 | 1.31 (0.34) | 245 |
| High school graduate | 1.28 (0.37) | 616 | 1.32 (0.33) | 1,134 | 1.31 (0.34) | 1,750 |
| Some college | 1.34 (0.37) | 501 | 1.37 (0.35) | 769 | 1.36 (0.36) | 1,270 |
| College degree or more | 1.31 (0.32) | 624 | 1.36 (0.32) | 566 | 1.33 (0.32) | 1,190 |
| Income to poverty ratio | ||||||
| <1.0 | 1.32 (0.32) | 129 | 1.35 (0.38) | 370 | 1.34 (0.37) | 499 |
| 1.0–1.9 | 1.30 (0.36) | 359 | 1.29 (0.34) | 629 | 1.29 (0.35) | 988 |
| 2.0–3.9 | 1.28 (0.34) | 735 | 1.34 (0.33) | 1,066 | 1.32 (0.34) | 1,801 |
| ≥4.0 | 1.32 (0.35) | 1,087 | 1.36 (0.32) | 1,249 | 1.34 (0.34) | 2,336 |
| Body mass indexb | ||||||
| <25.0 | 1.26 (0.33) | 595 | 1.31 (0.33) | 1,083 | 1.29 (0.33) | 1,678 |
| 25.0–29.9 | 1.31 (0.36) | 1,015 | 1.34 (0.34) | 1,087 | 1.33 (0.35) | 2,102 |
| 30.0–34.9 | 1.33 (0.33) | 489 | 1.35 (0.33) | 636 | 1.34 (0.33) | 1,125 |
| 35.0–39.9 | 1.35 (0.39) | 151 | 1.35 (0.32) | 292 | 1.35 (0.35) | 443 |
| ≥40.0 | 1.36 (0.29) | 60 | 1.40 (0.38) | 216 | 1.39 (0.36) | 276 |
| Smoking status | ||||||
| Never smoker | 1.33 (0.34) | 727 | 1.37 (0.33) | 1,687 | 1.36 (0.33) | 2,414 |
| Former smoker | 1.30 (0.36) | 1,248 | 1.31 (0.35) | 1,194 | 1.30 (0.35) | 2,442 |
| Current smoker | 1.31 (0.33) | 320 | 1.29 (0.33) | 417 | 1.30 (0.33) | 737 |
| Alcohol consumption, drinks per week | ||||||
| 0 | 1.29 (0.34) | 1,307 | 1.33 (0.34) | 2,422 | 1.32 (0.34) | 3,729 |
| 1–6 | 1.34 (0.36) | 550 | 1.35 (0.34) | 622 | 1.35 (0.35) | 1,172 |
| ≥7 | 1.32 (0.35) | 448 | 1.35 (0.33) | 266 | 1.33 (0.34) | 714 |
| Daily activity level | ||||||
| None | 1.30 (0.35) | 1,913 | 1.34 (0.34) | 2,618 | 1.32 (0.34) | 4,531 |
| Light | 1.37 (0.32) | 100 | 1.30 (0.34) | 306 | 1.31 (0.34) | 406 |
| Medium | 1.32 (0.33) | 213 | 1.32 (0.32) | 304 | 1.32 (0.33) | 517 |
| Vigorous | 1.36 (0.34) | 82 | 1.41 (0.33) | 80 | 1.38 (0.34) | 162 |
| Total | 1.31 (0.35) | 2,310 | 1.34 (0.34) | 3,314 | 1.32 (0.34) | 5,624 |
Abbreviations: GED, general education diploma; SD, standard deviation.
a Note that some variables have missing values, with a maximum of 31 subjects missing any 1 variable.
b Weight (kg)/height (m)2.
Table 2.
Sex-Stratified and Overall Associationsa of Telomere Length With Demographic and Lifestyle Variables, Health and Retirement Study, 2008
| Characteristic | Men (n = 2,286) | Women (n = 3,231) | All (n = 5,517) | |||
|---|---|---|---|---|---|---|
| βb | P Value | βb | P Value | βb | P Value | |
| Age | −0.003 | 5.0 × 10−6 | −0.003 | 3.0 × 10−12 | −0.003 | 4.0 × 10−17 |
| Female sex | 0.020 | 5.8 × 10−3 | ||||
| Race | ||||||
| White | 0 | Referent | 0 | Referent | 0 | Referent |
| Black | 0.074 | 3.3 × 10−5 | 0.072 | 8.3 × 10−8 | 0.072 | 1.7 × 10−11 |
| Hispanic | 0.018 | 0.37 | 0.048 | 2.6 × 10−3 | 0.037 | 3.5 × 10−3 |
| Other | 0.018 | 0.63 | −0.023 | 0.44 | −0.003 | 0.91 |
| Educational level | ||||||
| Less than high school | 0.005 | 0.80 | −0.025 | 0.12 | −0.011 | 0.37 |
| GED | −0.060 | 0.04 | 0.015 | 0.55 | −0.018 | 0.33 |
| High school graduate | −0.030 | 0.05 | −0.009 | 0.52 | −0.016 | 0.10 |
| Some college | 0.005 | 0.74 | 0.014 | 0.31 | 0.012 | 0.26 |
| College degree or more | 0 | Referent | 0 | Referent | 0 | Referent |
| Income to poverty ratio | ||||||
| <1.0 | −0.015 | 0.56 | −0.012 | 0.46 | −0.016 | 0.27 |
| 1.0–1.9 | −0.009 | 0.63 | −0.033 | 0.01 | −0.024 | 0.02 |
| 2.0–3.9 | −0.017 | 0.21 | −0.003 | 0.76 | −0.008 | 0.34 |
| ≥4.0 | 0 | Referent | 0 | Referent | 0 | Referent |
| Body mass indexc | 0.004 | 1.5 × 10−4 | 0.001 | 0.53 | 0.002 | 6.6 × 10−3 |
| Smoking status | ||||||
| Never smoker | 0 | Referent | 0 | Referent | 0 | Referent |
| Former smoker | −0.023 | 0.07 | −0.049 | 2.6 × 10−7 | −0.039 | 2.1 × 10−7 |
| Current smoker | −0.019 | 0.30 | −0.078 | 6.3 × 10−8 | −0.056 | 7.0 × 10−7 |
| Alcohol consumption, drinks per week | ||||||
| 0 | 0 | Referent | 0 | Referent | 0 | Referent |
| 1–6 | 0.029 | 0.03 | 0.012 | 0.33 | 0.020 | 0.02 |
| ≥7 | 0.027 | 0.06 | 0.029 | 0.08 | 0.026 | 0.02 |
| Daily activity level | ||||||
| None | 0 | Referent | 0 | Referent | 0 | Referent |
| Light | 0.052 | 0.05 | −0.046 | 2.5 × 10−3 | −0.018 | 0.17 |
| Medium | 0.024 | 0.20 | −0.008 | 0.59 | 0.007 | 0.58 |
| Vigorous | 0.050 | 0.10 | 0.033 | 0.24 | 0.039 | 0.05 |
Abbreviations: GED, general education diploma; SD, standard deviation.
a We adjusted for all variables as covariates.
b β coefficients represent the per-unit difference in telomere length for continuous variables or difference in telomere length compared with reference category for categorical variables.
c Weight (kg)/height (m)2.
Among women, we observed shorter TLs for both former smokers (P = 2.6 × 10−7) and current smokers (P = 6.3 × 10−8) compared with never smokers (Table 2); however, these associations were not observed among men, and a test for interaction indicated that these cross-sectional associations differed by sex (for former smokers, P for interaction = 0.05; for current smokers, P for interaction = 0.03). Similarly, smoking more cigarettes per day was associated with shorter TL during wave 9 in women but not men (Table 3).
Table 3.
Sex-Stratified and Overall Associations Between Telomere Length Measured at Wave 9 and Smoking-Related Characteristics, Health and Retirement Study, 2008
| Characteristic | Men | Women | Alla | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | βb | P Value | P for Trend | No. | βb | P Value | P for Trend | No. | βb | P Value | P for Trend | |
| Cigarettes smoked per day in wave 9 | ||||||||||||
| 0 | 724 | 0 | Referent | 1,661 | 0 | Referent | 2,385 | 0 | Referent | |||
| <10 | 80 | −0.057 | 0.07 | 127 | −0.040 | 0.08 | 207 | −0.049 | 6.4 × 10−3 | |||
| 10–19 | 85 | 0.006 | 0.84 | 140 | −0.095 | 1.2 × 10−5 | 225 | −0.056 | 1.3 × 10−3 | |||
| ≥20 | 148 | −0.014 | 0.58 | 0.64 | 136 | −0.092 | 2.9 × 10−3 | 1.9 × 10−8 | 284 | −0.058 | 2.9 × 10−4 | 4.8 × 10−6 |
| Recent smoking cessation after entering study as a smoker | ||||||||||||
| Continuing smoker | 320 | 0 | Referent | 404 | 0 | Referent | 724 | 0 | Referent | |||
| Quitterc | 152 | −0.087 | 4.1 × 10−3 | 169 | −0.031 | 0.27 | 321 | −0.048 | 0.02 | |||
| Years since smoking cessation among former smokers at wave 9 | ||||||||||||
| 0 | 724 | 0 | Referent | 1,661 | 0 | Referent | 2,385 | 0 | Referent | |||
| ≥30 | 519 | 0.007 | 0.64 | 394 | −0.020 | 0.15 | 913 | −0.007 | 0.48 | |||
| 20–29 | 284 | −0.032 | 0.08 | 251 | −0.037 | 0.03 | 535 | −0.037 | 2.2 × 10−3 | |||
| <20 | 284 | −0.055 | 2.9 × 10−3 | 1.3 × 10−3 | 319 | −0.076 | 4.8 × 10−7 | 2.0 × 10−7 | 603 | −0.067 | 8.0 × 10−9 | 2.5 × 10−9 |
| Duration of smoking, years | ||||||||||||
| 0 | 724 | 0 | Referent | 1,661 | 0 | Referent | 2,385 | 0 | Referent | |||
| <20 | 413 | 0.003 | 0.87 | 386 | −0.010 | 0.46 | 799 | −0.007 | 0.53 | |||
| 20–39 | 541 | −0.029 | 0.05 | 492 | −0.060 | 2.7 × 10−6 | 1033 | −0.049 | 3.8 × 10−7 | |||
| ≥40 | 344 | −0.033 | 0.06 | 0.018 | 364 | −0.088 | 1.8 × 10−9 | 6.2 × 10−12 | 708 | −0.064 | 1.1 × 10−8 | 1.9 × 10−11 |
| Pack-years during the study (sum of packs/day times years smoked per wave)d | ||||||||||||
| 0 | 724 | 0 | Referent | 1,661 | 0 | Referent | 2,385 | 0 | Referent | |||
| <5 | 233 | −0.039 | 0.06 | 323 | −0.081 | 1.6 × 10−7 | 556 | −0.064 | 1.9 × 10−7 | |||
| 5–9 | 124 | −0.058 | 0.02 | 168 | −0.080 | 1.1 × 10−4 | 292 | −0.070 | 1.2 × 10−5 | |||
| ≥10 | 182 | −0.060 | 8.6 × 10−3 | 2.8 × 10−3 | 166 | −0.080 | 1.1 × 10−4 | 7.5 × 10−10 | 348 | −0.074 | 1.2 × 10−6 | 3.4 × 10−11 |
a The percentage of missing data among subjects with telomere length measurement was <1% (31 of 5,554) for wave 9 smoking status, 1% (8 of 724) for wave 9 smoking rate, 15% (366 of 2,414) for years since smoking cessation, 18% (565 of 3,138) for smoking duration, and 2% (28 of 1,243) for “on-study” pack-years. Telomere length did not differ by missing status of years since smoking cessation (P = 0.41) or smoking duration (P = 0.69).
b Estimates are from a linear regression of telomere length on smoking characteristics adjusted for sex (when appropriate), age, race, educational level, income, body mass index, alcohol consumption, and daily physical activity level.
c Subjects who quit smoking between wave of entry and wave 9.
d Estimates are further adjusted for wave of entry into study.
Analysis of prospective measures of smoking
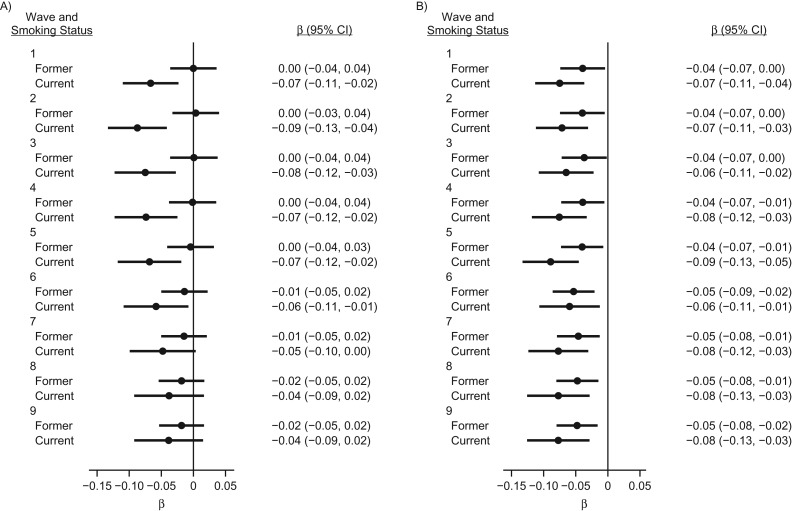
We examined the associations between wave 9 TL and smoking status in earlier waves (Figure 1 and Web Table 5). Associations between smoking measured in early waves and subsequent TL were similar for men (Figure 1A) and women (Figure 1B). Among men, we observed a gradual attenuation of the association in later waves. The point estimate decreased in magnitude by more than 50%, with P values ranging from 2 × 10−4 to 0.17, which was consistent with a weaker association for smoking reported in more recent waves. For women, the associations of shorter TL with both current and former smoking status was consistent across all waves.
Figure 1.
β coefficients and 95% confidence intervals for the associations between telomere length measured at wave 9 and smoking status measured across multiple waves among men (A) and women (B), Health and Retirement Study, 1992–2008. Values were adjusted for age, race, educational level, income, body mass index, alcohol consumption, and daily physical activity. Samples were restricted to subjects who entered the study in wave 1 and had telomere length measured at wave 9 (1,092 men and 1,277 women). β coefficients represent the difference in telomere length compared with never smokers.
Additional characteristics of smoking
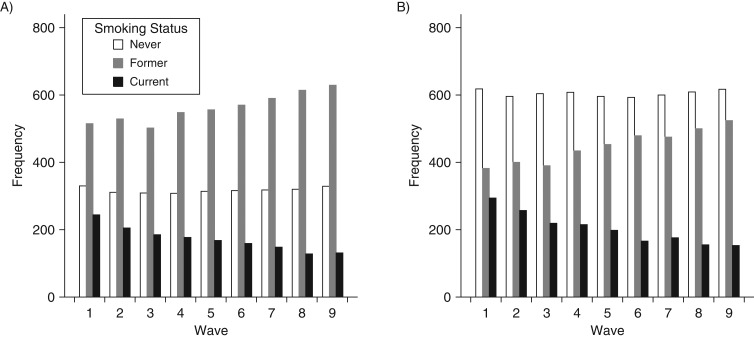
In order to determine why the association between smoking and TL among men was weaker for more recent measures of smoking, we first examined the change in distribution of smoking status over time. We observed that almost half of those in the current smoker category in wave 1 moved to the former smoker category by wave 9; this was true for both men (Figure 2A) and women (Figure 2B). However, despite the similar rates of smoking cessation, we observed a key difference between men and women who quit. Male smokers who quit during the study had shorter TL than did those who continued to smoke (P = 4.1 × 10−3) (Table 3); this differential quitting by TL was not observed among female smokers (P = 0.27). Conversely, when assessing other characteristics of smoking behavior, such as years since cessation (among former smokers), duration, and on-study pack-years of smoking, we observed consistent evidence that greater smoking exposure was associated with shorter TL in both men and women (Table 3). Analyses of estimates of lifetime pack-years of smoking showed similar results (Web Table 6).
Figure 2.
Distribution of smoking statuses among men (A) and women (B) across waves 1–9, Health and Retirement Study, 1992–2008. Samples were restricted to subjects who entered the study in wave 1 and had telomere length measured at wave 9 (1,095 men and 1,301 women).
Analysis of TL and overall health status
In order to further understand the link between smoking cessation and short TL in men, we examined the association between TL and health status. In the analyses of TL and measures of health status, we observed shorter TL among men who reported poorer health based on self-reported general health status (P = 0.02) and the comorbidity index (P = 0.05); however, these associations were not observed among women (Table 4).
Table 4.
Associations Between Telomere Length and Health Conditions, Stratified by Sex, Health and Retirement Study, 2008
| Health Status Measure | Men (n = 2,284) | Women (n = 3,232) | All (n = 5,516) | |||
|---|---|---|---|---|---|---|
| βa | P Value | βa | P Value | βa | P Value | |
| Self-reported healthb | −0.009 | 0.02 | 0.002 | 0.53 | −0.003 | 0.28 |
| Sum of conditionsc | −0.011 | 0.05 | 0.003 | 0.50 | −0.002 | 0.48 |
a Estimates are from a linear regression adjusted for sex (when appropriate), age, race, educational level, income, body mass index, smoking status, alcohol consumption, and daily physical activity level.
b Self-reported general health status, with 1 being excellent and 5 being poor.
c Sum of indicators for whether respondent reported a doctor had ever told them they had any of 8 conditions: high blood pressure, diabetes, cancer, lung disease, heart disease, stroke, psychiatric problems, or arthritis.
Simulations to assess bias due to loss to follow-up
Simulations demonstrated that when there was a higher risk of loss to follow-up for both smokers and individuals with shorter TL, the association between smoking and TL was biased in a positive direction (Web Table 7). Therefore, under the assumption that both smoking and short TL increase the probability of being lost to follow-up before wave 9 (presumably through increased morbidity and mortality), the associations between smoking and short TL observed in this study would be biased towards the null.
DISCUSSION
In the present study of older men and women in the United States, we observed that smoking was associated with TL when using prospective measures of smoking status determined up to 16 years before TL measurement, which supports the hypothesis that smoking reduces TL. However, this association was strongly attenuated among men but not women in cross-sectional analyses. Our analyses indicated that the attenuation was driven by the fact that more male smokers with shorter TL quit smoking during the course of the study than did male smokers with longer TL. The movement of men with shorter TL out of the current smoking category and into the former smoking category led to an overrepresentation of men with longer TL in the current smoking category. This phenomenon resulted in very weak associations between TL and smoking status in later waves, with those associations likely being biased estimates of the true effect of smoking on TL.
Male smokers with shorter TL may have been more likely to quit because short TL was correlated with poorer health among men in the HRS; furthermore, previous studies from the HRS showed that smokers who experience acute and chronic health events are more likely to quit smoking than are individuals who do not (37, 38). This may be an example of the “healthy smoker” phenomenon, in which individuals who are more resistant to the adverse effects of smoking continue to smoke, whereas those who are more susceptible quit, resulting in underestimation of the effect of smoking (39). This adverse health–driven smoking cessation may be particularly relevant to men in the HRS, because they are more likely to experience major and chronic health events (37), and to older individuals, who are more likely than younger individuals to experience adverse health events and to quit smoking in response (40).
Our hypothesized relationships among smoking, TL, and health for men are shown in Figure 3. The bias towards the null observed in cross-sectional analyses of men can be conceptualized in several ways. First, assuming TL affects health, this bias could be attributed to reverse causation, whereby TL influences smoking behavior through its effects on health (Figure 3). Second, we could view this bias as confounding of the smoking-TL association by factors that influence both TL and health (and health-related smoking behaviors) in later life (Figure 3). Third, the bias could be considered self-selection bias, in which individuals select themselves into a group (e.g., former smokers) in a nonrandom way (e.g., based on TL and health). Fourth, we could consider current smoking to be an error-prone measure of the true exposure of interest (e.g., lifetime exposure or exposure in early to mid-life), thus producing bias due to differential measurement error (differential with respect to TL).
Figure 3.
Causal model showing the hypothesized relationships among smoking, telomere length, health outcomes, and unmeasured determinants of telomere length in male participants in the Health and Retirement Study. Solid lines depict associations that are likely to represent causal relationships. The dashed line depicts a potential effect.
In contrast to what was seen among men, the association between smoking status and TL did not vary across waves among women, and female smokers who quit during the study did not exhibit shorter TL than did smokers who did not quit. This could be due to different motivations for quitting smoking between men and women (41); that is, women are less likely to quit in response to an adverse health event than are men (42). Also, women tend to be less successful than men with regard to smoking cessation (43, 44) and therefore may be less able to maintain cessation in response to an adverse health event. Finally, sex differences in the accuracy of self-reported smoking behavior, exposure to second-hand smoking, duration and intensity of smoking, and biological effects of smoking on TL could all contribute to the differences we observed.
We utilized 2 different categories of data to study smoking cessation in our analysis: whether or not a subject quit during the study period and the reported time since quitting during the individual's lifetime. The former represents recent quitting among individuals who smoked in later life (which may correlate with concurrent disease status and health-seeking behavior), whereas the latter captures smoking cessation events relevant to cumulative lifetime exposure. The association observed between greater time since smoking cessation and longer TL was consistent with the results from the prospective analysis, as well as analyses of 2 other characteristics of smoking exposure (i.e., duration and pack-years of smoking), which supports the hypothesis that smoking shortens telomeres.
To our knowledge, the present study is the first in which the association between smoking and subsequent TL has been characterized while treating smoking as a time-varying exposure. The differences in this association that we observed across time and between sexes potentially explain conflicting findings in prior cross-sectional, longitudinal, and prospective studies of smoking and TL. Most previous cross-sectional studies in which an association between smoking and short TL was reported were studies of female subjects only (11–14, 16), whereas those in which no association was reported were mostly studies of men or of older subjects (21, 23–26), among whom estimates may be more likely to be biased because of higher rates of smoking cessation among older men with short TL. Previous longitudinal studies in which associations between smoking and TL attrition were reported involved younger subjects than did studies in which such an association was not reported (17–20), further suggesting that estimates obtained from studies of older individuals could be biased because of adverse health–driven smoking cessation. In the Helsinki Businessmen Study, Strandberg et al. (27) compared prospective and cross-sectional measures of smoking status and TL among men and reported findings consistent with those from our study. However, they assessed smoking at only 2 time points and did not consider smoking cessation as an explanation for the phenomenon; instead, they posited that unmeasured factors that contribute to TL attrition in old age, such as frailty, telomerase insufficiency, and oxidative stress, were possible explanations.
Our study has a number of strengths. A large sample size and rich covariate data allowed for comprehensive adjustments for demographic and lifestyle variables. The study was conducted within a nationally representative cohort of older subjects—an important age group in which to study these associations, given the tremendous interest in TL as a biomarker of aging-related diseases and mortality. The use of repeated measures of smoking over 16 years shed light on the dynamics of the association between smoking and TL over time and demonstrated the importance of evaluating both recent and historical exposure to smoking in order to understand its association with TL over the life course.
Our study is limited by the possible issue of bias due to loss to follow-up. In other words, subjects who were not lost to follow-up (including death) prior to wave 9 (which allowed for TL measurement) may have been systematically different from those who were lost to follow-up by wave 9 with respect to the variables analyzed, and this selection could bias the observed association between smoking and TL. This phenomenon has been previously described as a type of collider bias resulting from selection into a study conditional on a variable (e.g., survival or health) that is influenced by other variables under analysis (e.g., smoking and TL) (45). We conducted simulation analyses in which we demonstrated that greater loss to follow-up among male smokers and among men with short TL would create a positive bias that would increase the β coefficient for the association between smoking and TL. Such a bias would therefore attenuate the association between smoking and short TL and could contribute, in part, to the lack of association between smoking and TL observed in cross-sectional analyses among men. However, our analyses of individuals present in both wave 1 and wave 9 (1,095 men who were not lost to follow-up) showed significant associations between prior smoking (at wave 1) and short TL (at wave 9), despite the presence of any such attenuating bias. Cross-sectional analyses of these same 1,095 male participants showed no association between smoking and TL, but that attenuation was apparently due to a higher rate of quitting among individuals with short TL. Taken together, these findings indicated that 1) the association between prospectively measured smoking and subsequent short TL was observed despite potential bias due to loss to follow-up and 2) the cross-sectional null association was not primarily driven by bias due to loss to follow-up.
Another limitation is that it was necessary to assume that the available TL measure at wave 9 was relatively constant within the time scale of this study and therefore an appropriate proxy for TL across the earlier waves during which cessation occurred. This assumption is likely valid because prior findings indicated that there is a weak association between recent (≈ 12 years) changes in smoking behavior and TL attrition (46). Finally, we note that the source of DNA in the present study, saliva, is different from that in most previous studies of TL, which used leukocyte DNA. Although up to 74% of saliva DNA originates from white blood cells, the composition of leukocytes in normal saliva samples will differ from the composition of blood samples (47). However, previous studies have shown that the quality of saliva genomic DNA is comparable with blood DNA in terms of purity, genotyping quality, and polymerase chain reaction amplification analyses (48, 49), and TL measured from saliva DNA has been found to be highly correlated with TL measured from leukocyte DNA (r = 0.72) (50). Additionally, there is no a priori reason to favor leukocyte DNA over saliva DNA as a source to assess the association between smoking and TL in human cells in the absence of a specific target tissue of interest for health outcome assessment.
In summary, we used longitudinal data on a representative sample of older adults in the United States to demonstrate that smoking is associated with reduced TL among both men and women when using repeated measures of smoking status over time. However, in cross-sectional analyses, smoking status was not associated with TL among men, and this apparent discrepancy was likely driven by a higher propensity for smoking cessation among men with short TL, a condition that appears to be accompanied by poorer overall health. These findings provide a framework for understanding conflicting reports of smoking and TL in previous studies and highlight the need to further investigate the time-varying and sex-specific associations among smoking behavior, TL, and health.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Public Health Sciences, The University of Chicago, Chicago, Illinois (Chenan Zhang, Diane S. Lauderdale, Brandon L. Pierce); Department of Human Genetics, The University of Chicago, Chicago, Illinois (Brandon L. Pierce); and Comprehensive Cancer Center, The University of Chicago, Chicago, Illinois (Brandon L. Pierce).
This work was supported by the National Institute on Aging (grants T32AG000243 and P30AG012857), the National Institute of Environmental Health (grant R01 ES020506), and the National Human Genome Research Institute (grant U01 HG007601) at the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1.Müezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev. 2013;12(2):509–519. [DOI] [PubMed] [Google Scholar]
- 2.Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither. Epidemiol Rev. 2013;35:112–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serrano AL, Andrés V. Telomeres and cardiovascular disease: does size matter. Circ Res. 2004;94(5):575–584. [DOI] [PubMed] [Google Scholar]
- 4.Willeit P, Willeit J, Mayr A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69–75. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Ruiz C, Dickinson HO, Keys B, et al. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60(2):174–180. [DOI] [PubMed] [Google Scholar]
- 6.van der Vaart H, Postma DS, Timens W, et al. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax. 2004;59(8):713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. [DOI] [PubMed] [Google Scholar]
- 8.O'Donovan A, Pantell MS, Puterman E, et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS One. 2011;6(5):e19687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenny NS. Inflammation in aging: cause, effect, or both. Discov Med. 2012;13(73):451–460. [PubMed] [Google Scholar]
- 10.Babizhayev MA, Yegorov YE. Smoking and health: association between telomere length and factors impacting on human disease, quality of life and life span in a large population-based cohort under the effect of smoking duration. Fundam Clin Pharmacol. 2011;25(4):425–442. [DOI] [PubMed] [Google Scholar]
- 11.Carty CL, Kooperberg C, Liu J, et al. Leukocyte telomere length and risks of incident coronary heart disease and mortality in a racially diverse population of postmenopausal women. Arterioscler Thromb Vasc Biol. 2015;35(10):2225–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aviv A, Valdes A, Gardner JP, et al. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J Clin Endocrinol Metab. 2006;91(2):635–640. [DOI] [PubMed] [Google Scholar]
- 13.Valdes A, Andrew T, Gardner J, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. [DOI] [PubMed] [Google Scholar]
- 14.Parks CG, Miller DB, McCanlies EC, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18(2):551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Donnell CJ, Demissie S, Kimura M, et al. Leukocyte telomere length and carotid artery intimal medial thickness: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2008;28(6):1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Q, Shi L, Prescott J, et al. Healthy lifestyle and leukocyte telomere length in US women. PLoS One. 2012;7(5):e38374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huzen J, Wong LSM, van Veldhuisen DJ, et al. Telomere length loss due to smoking and metabolic traits. J Intern Med. 2014;275(2):155–163. [DOI] [PubMed] [Google Scholar]
- 18.Bendix L, Thinggaard M, Fenger M, et al. Longitudinal changes in leukocyte telomere length and mortality in humans. J Gerontol A Biol Sci Med Sci. 2014;69(2):231–239. [DOI] [PubMed] [Google Scholar]
- 19.Weischer M, Bojesen SE, Nordestgaard BG. Telomere shortening unrelated to smoking, body weight, physical activity, and alcohol intake: 4,576 general population individuals with repeat measurements 10 years apart. PLoS Genet. 2014;10(3):e1004191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müezzinler A, Mons U, Dieffenbach AK, et al. Smoking habits and leukocyte telomere length dynamics among older adults: results from the ESTHER cohort. Exp Gerontol. 2015;70:18–25. [DOI] [PubMed] [Google Scholar]
- 21.Bischoff C, Graakjaer J, Petersen HC, et al. The heritability of telomere length among the elderly and oldest-old. Twin Res Hum Genet. 2005;8(5):433–439. [DOI] [PubMed] [Google Scholar]
- 22.Bekaert S, De Meyer T, Rietzschel ER, et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6(5):639–647. [DOI] [PubMed] [Google Scholar]
- 23.Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidemiol. 2007;165(1):14–21. [DOI] [PubMed] [Google Scholar]
- 24.Nordfjäll K, Eliasson M, Stegmayr B, et al. Telomere length is associated with obesity parameters but with a gender difference. Obesity (Silver Spring). 2008;16(12):2682–2689. [DOI] [PubMed] [Google Scholar]
- 25.Batty GD, Wang Y, Brouilette SW, et al. Socioeconomic status and telomere length: the West of Scotland Coronary Prevention Study. J Epidemiol Community Health. 2009;63(10):839–841. [DOI] [PubMed] [Google Scholar]
- 26.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369(9556):107–114. [DOI] [PubMed] [Google Scholar]
- 27.Strandberg TE, Saijonmaa O, Tilvis RS, et al. Association of telomere length in older men with mortality and midlife body mass index and smoking. J Gerontol A Biol Sci Med Sci. 2011;66(7):815–820. [DOI] [PubMed] [Google Scholar]
- 28.Barrett EL, Richardson DS. Sex differences in telomeres and lifespan. Aging Cell. 2011;10(6):913–921. [DOI] [PubMed] [Google Scholar]
- 29.Aviv A, Shay J, Christensen K, et al. The longevity gender gap: are telomeres the explanation. Sci Aging Knowledge Environ. 2005;2005(23):pe16. [DOI] [PubMed] [Google Scholar]
- 30.Aviv A. Telomeres, sex, reactive oxygen species, and human cardiovascular aging. J Mol Med (Berl). 2002;80(11):689–695. [DOI] [PubMed] [Google Scholar]
- 31.Bolego C, Poli A, Paoletti R. Smoking and gender. Cardiovasc Res. 2002;53(3):568–576. [DOI] [PubMed] [Google Scholar]
- 32.Bauer T, Göhlmann S, Sinning M. Gender differences in smoking behavior. Health Econ. 2007;16(9):895–909. [DOI] [PubMed] [Google Scholar]
- 33.Sonnega A, Faul JD, Ofstedal MB, et al. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43(2):576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.University of Michigan Health and Retirement Study: 2008 Telomere Length Data. Ann Arbor, MI: University of Michigan; 2013. http://hrsonline.isr.umich.edu/modules/meta/telo2008/desc/Telomere08DD.pdf Accessed February 16, 2016. [Google Scholar]
- 35.RAND Center for the Study of Aging RAND HRS Data Documentation, Version M. Arbor, MI: University of Michigan; 2013. http://hrsonline.isr.umich.edu/modules/meta/rand/randhrsm/randhrsM.pdf. [Google Scholar]
- 36.Ofstedal MB, Weir DR, Chen KT, et al. HRS Documentation Report: Updates to HRS Sample Weights. Arbor, MI: University of Michigan; 2011. [Google Scholar]
- 37.Falba T. Health events and the smoking cessation of middle aged americans. J Behav Med. 2005;28(1):21–33. [DOI] [PubMed] [Google Scholar]
- 38.Keenan PS. Smoking and weight change after new health diagnoses in older adults. Arch Intern Med. 2009;169(3):237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becklake MR, Lalloo U. The “healthy smoker”: a phenomenon of health selection. Respiration. 1990;57(3):137–144. [DOI] [PubMed] [Google Scholar]
- 40.Grøtvedt L, Stavem K. Association between age, gender and reasons for smoking cessation. Scand J Public Health. 2005;33(1):72–76. [DOI] [PubMed] [Google Scholar]
- 41.McKee SA, O'Malley SS, Salovey P, et al. Perceived risks and benefits of smoking cessation: gender-specific predictors of motivation and treatment outcome. Addict Behav. 2005;30(3):423–435. [DOI] [PubMed] [Google Scholar]
- 42.McKee SA, Maciejewski PK, Falba T, et al. Sex differences in the effects of stressful life events on changes in smoking status. Addiction. 2003;98(6):847–855. [DOI] [PubMed] [Google Scholar]
- 43.Wetter DW, Kenford SL, Smith SS, et al. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67(4):555–562. [DOI] [PubMed] [Google Scholar]
- 44.Piper ME, Cook JW, Schlam TR, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;12(6):647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearce N, Richiardi L. Commentary: three worlds collide: Berkson's bias, selection bias and collider bias. Int J Epidemiol. 2014;43(2):521–524. [DOI] [PubMed] [Google Scholar]
- 46.Benetos A, Kark JD, Susser E, et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. 2013;12(4):615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thiede C, Prange-Krex G, Freiberg-Richter J, et al. Buccal swabs but not mouthwash samples can be used to obtain pretransplant DNA fingerprints from recipients of allogeneic bone marrow transplants. Bone Marrow Transplant. 2000;25(5):575–577. [DOI] [PubMed] [Google Scholar]
- 48.Rylander-Rudqvist T, Håkansson N, Tybring G, et al. Quality and quantity of saliva DNA obtained from the self-administrated oragene method--a pilot study on the cohort of Swedish men. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1742–1745. [DOI] [PubMed] [Google Scholar]
- 49.Hansen TV, Simonsen MK, Nielsen FC, et al. Collection of blood, saliva, and buccal cell samples in a pilot study on the Danish nurse cohort: comparison of the response rate and quality of genomic DNA. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2072–2076. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell C, Hobcraft J, McLanahan SS, et al. Social disadvantage, genetic sensitivity, and children's telomere length. Proc Natl Acad Sci USA. 2014;111(16):5944–5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.