Abstract
Background
Isolated limb infusion (ILI) has been associated with persistent edema, numbness, pain, and functional impairment of the treated limb. However, health-related quality of life (HRQOL) has not yet been assessed using a validated questionnaire.
Methods
Functional Assessment of Cancer Therapy-Melanoma (FACT-M) questionnaires were collected from subjects enrolled a phase I ILI trial with temozolomide at baseline and 2, 6 weeks, and 3 months post-ILI. Of 28 enrolled patients, 19 patients received maximum tolerated dose of temozolomide and are included in the HRQOL analysis. Clinical and operative variables and treatment response data also were collected.
Results
HRQOL scores showed a trend of improvement from baseline through 3-months post-ILI as measured by FACT-M and the melanoma surgery scores. There were no differences in HRQOL when patients were stratified by disease burden, clinical toxicity level, and 3-month disease response. Additionally, fewer patients complained of pain, numbness, and swelling of the affected limb at 3 months post-ILI compared to baseline, and also these symptoms were improved at the immediate postoperative visit compared with baseline.
Conclusions
Despite the known morbidity of ILI, we have demonstrated with a validated HRQOL questionnaire that HRQOL is not adversely impacted at therapeutic doses of temozolomide delivered intra-arterially from baseline through 3 months posttreatment. Patient centered-outcomes should be evaluated as a standard part of all future regional therapy trials using standardized melanoma-specific HRQOL questionnaires to more completely evaluate the utility of this type of treatment strategy.
In-transit extremity melanoma, defined as recurrent deposits of tumor in dermal and subcutaneous tissue between the primary site and regional lymph node basin, can be treated with isolated limb infusion (ILI) in which high-dose chemotherapy is infused into the affected limb via percutaneous catheters. The typical complete response rate is 31 % and partial response rate is 33 % using standard-of-care melphalan chemotherapy.1 Although less invasive than hyperthermic isolated limb perfusion (HILP), ILI nonetheless carries substantial postprocedural morbidity, including pain, edema, erythema, numbness, and functional impairment of the treated limb. However, the impact of ILI on overall health-related quality of life (HRQOL) has not yet been evaluated using a validated questionnaire.
Melphalan is currently the standard-of-care chemotherapy used during ILI. Melphalan-based ILI leads to grade 1 clinical toxicities, such as limb swelling, erythema, pain, and hyperpigmentation, in nearly all patients; 18 % of those treated will experience grade 3 or greater clinical toxicity.2
Patients undergoing ILI experience a multitude of issues that may affect HRQOL, including drug toxicities, problems related to the survey and infusion, and disease-related symptoms. Furthermore, the medical, psychological, social, financial, and existential stress of having advanced melanoma and undergoing ILI can be substantial. Personal HRQOL of people undergoing ILI needs to be understood in order to optimize this approach as a meaningful treatment for in-transit melanoma. This was the first study of the impact of ILI on HRQOL assessed using a validated HRQOL questionnaire as conducted as a part of a Phase I trial.
PATIENTS AND METHODS
Study and Patient Characteristics
Twenty-eight patients were enrolled in a phase I, dose escalation study of intra-arterial temozolomide during ILI. This trial was approved by the Duke University Internal Review Board. Temozolomide is a DNA-methylating agent that has recently been reformulated into an intravenous version. Early animal models of extremity melanoma demonstrated that temozolomide had greater efficacy as a regional agent than either systemic administration or regional melphalan therapy.3 Eligible patients for this phase I trial had histologically proven primary or recurrent extremity melanoma (stage IIIB, IIIC, or resected stage IV by American Joint Commission on Cancer classification) distal to the site of planned tourniquet.4 The first 25 patients had their melanoma progress despite prior ILI treatment, whereas the next 3 patients were naïve to regional treatment. Patients who had received prior regional treatment must have recovered from the relevant toxicity of that procedure. One patient each was enrolled at the first four dose levels (200, 400, 800, and 1,600 mg per m2 of body surface area). Nineteen patients were enrolled at maximum tolerated dose level (MTD, 3,200 mg/m2). Five patients were enrolled at greater than MTD (3,600 mg/m2).
Lesion measurements were taken at baseline and posttreatment clinic visits and visible disease was photo-documented. Response was determined at 3 months post-ILI based on RECIST criteria modified for cutaneous lesions.2 Disease burden was measured before ILI, with high disease burden defined as having 10 or more lesions or any one lesion greater than 3 cm. Clinical toxicity was defined by common terminology for adverse events (CTCAE) criteria and was collected in the postoperative period as well as at every follow-up visit.2
ILI Procedure
ILIs were performed as previously described in the literature.2,5 Postoperatively, patients were admitted for a minimum of 3 days, as peak limb toxicity was expected at the first or second postoperative day. The limb was monitored for toxicity in the immediate postoperative period as well as at follow-up visits.
HRQOL Questionnaire
The Functional Assessment of Cancer Therapy-Melanoma (FACT-M) questionnaire is a 43-item, self-administered questionnaire comprised of the FACT-G and a melanoma subscale (MS).6,7 The FACT-G is further divided in four subscales that address issues related to cancer diagnosis: physical well-being (PWB), social and family well-being (SWB), emotional well-being (EWB), and functional well-being (FWB). The MS addresses issues specific to a melanoma diagnosis, whereas an additional subscale addresses concerns following melanoma surgery subscale (MSS). Each of these subscales has been shown to have high levels of internal consistency (Cronbach’s alpha 0.71–0.95) and high test–retest reliability (r 0.71–0.90).7 We also have calculated the trial outcome index (TOI) subscale from the PWB, FWB, and MS components, as the TOI has been demonstrated to be the most reliable and sensitive indicator of HRQOL in versions of FACT for other cancers.8 Patients completed the FACT-M and MSS questionnaires at four time points: baseline (immediately before ILI), 2 weeks post-ILI, 6 weeks post-ILI, and 3 months post-ILI. Changes of greater than 4–6 points in total FACT-M score are generally considered to represent meaningful differences.9
Treatment-Associated Symptoms
Pain, numbness, and swelling severity ratings were taken from items M1, M16, and M10, respectively, of the FACT-M questionnaire. Presence of each of these symptoms was rated from a score of 0 (not at all) to 4 (very much).
Statistical Analysis
Nineteen patients administered MTD of temozolomide (3,200 mg/m2) were included in this patient-centered outcomes analysis. To descriptively compare the mean scores between subgroups, the 95 % confidence intervals around the mean were estimated and examined for overlap. Nonoverlapping confidence intervals may indicate meaningful differences between subgroups and should be corroborated with larger sample sizes. This was done at each time point for FACT-M, its subcomponent scores, and the melanoma surgery score, and as broken down by disease burden (high vs. low), clinical toxicity level (0/1 vs. 2), and response (progressive disease vs. complete response/partial response/stable disease).
All statistical analyses were performed using R version 0.96.331 (Vienna, Austria). Significance for all statistical testing was set at p < 0.05.
RESULTS
Patient and Procedure Characteristics
Nineteen patients received the MTD of temozolomide for ILI (3,200 mg/m2) and were included in the HRQOL study. Of these, 8 were male and 11 were female, with a mean age of 69.6 years. Four ILIs were performed on the upper extremity and 15 on the lower extremity. Eight of the patients were stage IIIB and 11 were stage IIIC at time of ILI. Eleven patients had high disease burden, whereas eight patients had low burden. The majority of patients had a baseline ECOG score of 0 (13 patients), with 4 patients having an ECOG score of 1 and 2 patients with an ECOG score of 2. Sixteen patients had undergone previous ILI with melphalan. Thirteen patients had undergone previous lymph node dissection prior to the current ILI. Postprocedurally, median CPK was 582 (range 73–23,739), and occurred between postoperative days 0 to 2. CPK levels exceeded 1,000 during the immediate postoperative period in 6 patients and per protocol these patients were administered dexamethasone. No patients required fasciotomy or amputation in this cohort. Highest clinical toxicity level during the follow-up period was CTCAE grade 0 in 1 patient, grade 1 in 13 patients, and grade 2 in 5 patients (Table 1).
TABLE 1.
Patient and treatment characteristics for patients treated at MTD (3200 mg/m2) of temozolomide.
| Characteristic | Value |
|---|---|
| Total patients | 19 |
| Sex, M:F | 8:11 |
| Age, mean (SD), years | 69.6 ± 9.1 |
| BMI, mean (SD), kg/m2 | 28.2 ± 5.5 |
| Site, n (%) | |
| Upper extremity | 4 (21 %) |
| Lower extremity | 15 (79 %) |
| Stage of disease, n (%) | |
| IIIB | 8 (42 %) |
| IIIC | 11 (58 %) |
| IV | 0 |
| Disease burden, n (%) | |
| High | 11 (58 %) |
| Low | 8 (42 %) |
| ECOG at Pre-ILI, n (%) | |
| 0 | 13 (68 %) |
| 1 | 4 (21 %) |
| 2 | 2 (11 %) |
| Prior therapy | |
| Lymph node dissection | 13 |
| Interferon | 5 |
| Radiation | 2 |
| Previous ILI | 16 |
| Temozolamide dose, mg/m2 | 3,200 |
| Actual dose, upper extremity, mean (SD), mg | 566.66 ± 17.3 |
| Actual dose, lower extremity, mean (SD), mg | 1087.3 ± 154.4 |
| Infusion time, minutes | 30 |
| Tourniquet time, mean (SD), minutes | 60.8 ± 10.6 |
| Volume recirculated, mean (SD), mL | 1513.6 ± 571.3 |
| Upper Extremity, means (SD), mL | 1186.7 ± 167.7 |
| Lower extremity, mean (SD), mL | 1589.1 ± 608.6 |
| Peak limb temperature, mean (SD), Celsius | 39.1 ± 0.99 |
| Peak CPK, median (range) | 582 (73-23739) |
| Greatest clinical toxicity | |
| 0 | 1 |
| 1 | 13 |
| 2 | 5 |
| Fasciotomy | 0 patients |
| Amputation | 0 patients |
In this MTD cohort, 2 patients had a complete response (10.5 %), 1 patient had a partial response (5.3 %), 4 patients had stable disease (21.1 %), and 12 patients had progressive disease (63.2 %) at the 3 month post-ILI visit.
Health-Related Quality of Life
Of the 19 patients included in the study over all 4 time points, there were 52 complete FACT-M surveys out of 76 possible. Nine patients had complete data from all visits, 14 patients had baseline surveys available, and 12 patients had 2 or more postprocedure assessments. There were three reasons for missing surveys: first, several patients who enrolled earlier in the trial who had taken their surveys on tablet computers had files that could not be retrieved. This represented 18 of the 24 data points missing. Second, 4 patients left the trial early due to disease progression, representing 5 data points lost. Finally, there was 1 survey from a baseline visit that was not mailed to our center from an external study site. Two of the 4 patients who left the trial early reported HRQOL scores below average at each visit before their departure.
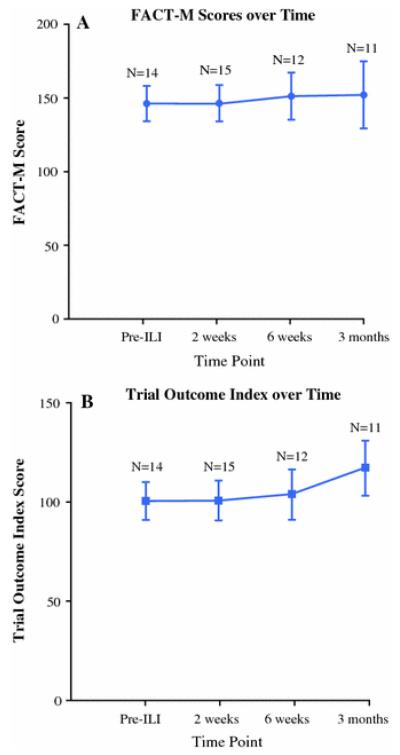
FACT-M scores and its subcomponent scores (FACT-G, TOI, PWB, SWB, EWB, FWB, melanoma, and melanoma surgery scores), and melanoma surgery scores showed a trend of improving HRQOL (Fig.1). In addition, HRQOL between baseline and the immediate posttreatment visit did not decrease (Fig.1; Table 2). These patterns are consistent with our expectations, for example the MSS score improves as the patients convalesce, and HRQOL scores do not decrease posttreatment as patients are extensively counseled on the anticipated post-ILI morbidities. While these HRQOL trends were promising, the 95 % confidence intervals between time points overlapped, likely due to our small sample size.
FIGURE 1.
HRQOL scores over time, with number of surveys available at each time point (a FACT-M score over time; b Trial outcome index score over time). Mean of the score and 95 % confidence interval are presented for each time point.
TABLE 2.
Subscale and total scores at the pre-ILI and 2 weeks post-ILI visits
| Time point |
|||||
|---|---|---|---|---|---|
| Possible score range |
Pre-ILI | 2 Weeks | p | ||
| Scores, Median (95 % CI) |
SWB | 0-28 | 25.335 (21.92, 26.55) |
25 (22.17, 26.4) |
0.078 |
| EWB | 0-24 | 22 (18.53, 22.58) |
20 (18.6, 22.27) |
0.797 | |
| PWB | 0-28 | 26 (22.45, 27.33) |
27 (21.85, 26.82) |
0.555 | |
| FWB | 0-28 | 24 (19.04, 25.64) |
24 (20.47, 25.13) |
0.57 | |
| MS | 0-64 | 51 (44, 56.51) |
57.5 (46.28, 58.85) |
0.296 | |
| MSS | 0-32 | 26.5 (19.58, 27.28) |
28.5 (19.08, 28.79) |
0.484 | |
| FACTG | 0-108 | 95 (83.79, 100.28) |
97 (85.57, 98.14) |
0.625 | |
| TOI | 0-120 | 98.335 (89.44, 108.73) |
104 (89.17, 109.36) |
0.329 | |
| FACTM | 0-172 | 140.5 (131.45, 156.31) |
149 (131.55, 156.43) |
0.169 | |
Higher scores indicate higher health-related quality of life. SWB social and family well-being, EWB emotional well-being, PWB physical well-being, FWB functional well-being, MS melanoma subscale, MSS melanoma surgery subscale, FACT-G Functional Assessment of Cancer Therapy-General, TOI trial outcome index, FACT-M Functional Assessment of Cancer Therapy-Melanoma
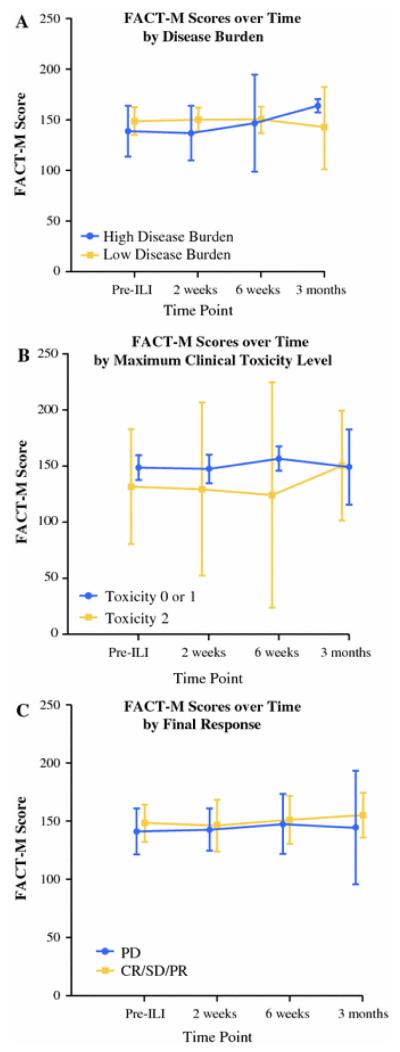
Finally, exploration of patient and disease factors does not clearly demarcate groups by HRQOL. When high versus low disease burden groups were visualized over time as well as compared directly between the perioperative visits, there were no differences in HRQOL between groups as visualized by overlapping 95 % confidence intervals. Similarly, when divided into groups according to final responses (PD vs. CR/PR/SD) or when grouped by maximum clinical toxicity level (0/1 vs. 2), we saw that HRQOL scores were not different between groups over time (Fig.2).
FIGURE 2.
FACT-M score over time by clinical characteristics (a by disease burden; b by greatest clinical toxicity level; c by 3-month final response). Mean of the score and 95 % confidence interval are presented for each time point.
Symptoms
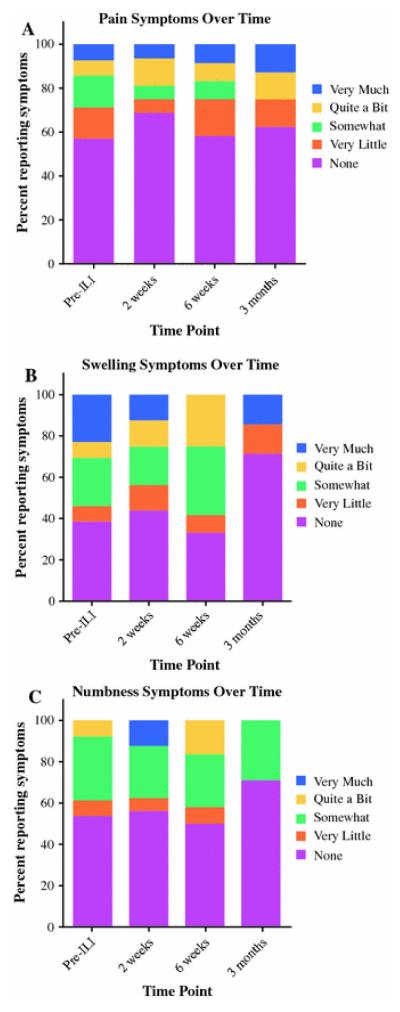
Treatment-site pain, swelling, and numbness showed a trend of improvement over time, with a greater percentage of patients reporting no or very little of each symptom at the immediate postoperative visit compared with baseline. There also was a trend of improvement through the 3-month visits with more patients reporting “None” or “very little” presence of each symptom, although swelling and numbness showed dips at 6 weeks with a greater percentage of patients reporting being at least “somewhat” symptomatic compared with the 2-week visit (Fig.3).
FIGURE 3.
Percentage of patients reporting pain (a), numbness (b), or swelling (c) over time.
DISCUSSION
Numerous studies have examined quality of life in patients with distantly metastatic melanoma, but there is limited insight into the experience of those with locally advanced extremity melanoma. In contrast to patients with advanced metastatic melanoma, these patients often are able to maintain their usual routines and responsibilities. HRQOL in this population may be affected by frequent doctors’ visits, anxiety about new lesions, treatment of new lesions by resection, radiation, or other therapies, the aesthetic appearance of scars, and concern about the future course of their disease, and the available treatment options. Patients who undergo regional chemotherapy for advanced extremity melanoma are further faced with another set of known complications that can affect HRQOL, most commonly pain, swelling, numbness, and functional impairment of the treated limb. How these factors impact HRQOL in patients with locally advanced extremity melanoma following treatment with regional chemotherapy has not been established with a validated questionnaire.
Patient demographics, including disease burden, for our MTD cohort were similar to those reported elsewhere in the literature for melphalan-based ILI.1 However, there were differences in clinical toxicity and response in the temozolomide cohort compared to previously described ILI procedures. In our MTD cohort for temozolomide, all patients had clinical toxicity grade 2 or lower, which is in contrast melphalan-based ILI in which 35 % of patients experience grade 3 or higher clinical toxicity.1,10 Furthermore, temozolomide was less efficacious than melphalan. Compared with an expected complete response rate with melphalan-based ILI of 30 %, patients in our temozolomide MTD cohort had a complete response rate only of 10.5 %.11 This, however, is difficult to precisely interpret in light of the fact that the majority of patients treated with temozolomide had already failed a previous melphalan ILI.
HRQOL scores, which include FACT-M and its components as well as the MSS, demonstrated no statistically significant changes in score over time. However, we observed a trend of improving scores from baseline through 3 months. In contrast to our expectations, we failed to observe a transient decrease in HRQOL immediately posttreatment compared with baseline. Rather, scores were equal to baseline and, in fact, most subscale scores were slightly higher posttreatment than baseline.
The effect of increased HRQOL following treatment is consistent with theoretical frameworks of coping with cancer. According to Lazarus and Folkman, in the coping process, patients choose to employ either problem-focused coping in which they gather information and mobilize a plan to maintain control over their environment, or emotion-focused approach in which they attempt to regulate emotions linked to the stressor.12,13 The problem-focused approach is adaptive and associated with higher emotional states and may be the reason our patients experience increased HRQOL scores after treatment despite experiencing the known morbidities associated with ILI.14 HRQOL also may be modulated by patients’ expectation versus actual experience of posttreatment symptoms; our patients are preoperatively counseled to expect pain, numbness, and swelling after treatment, but actual patient complaints of pain, numbness, and swelling are equal to or lesser than baseline values at the 2-week post-ILI visit. Furthermore, there was no association of HRQOL with disease burden, clinical toxicity, or final response.
In a previous study examining HRQOL among patients treated with ILI, the authors reported increased symptoms of pain, numbness, edema, and stiffness immediately following treatment, with most adverse symptoms decreasing over time, although a portion of patients developed persistent pain and neuropathy.15 Similarly, we also observed improvements in self-reported swelling and numbness after treatment over time. In contrast to the previous study, however, a greater proportion of patients experienced “none” or “very little” pain, numbness, and swelling immediately after treatment compared to baseline. We believe our findings are, again, consistent with literature on the psychological benefit of the decision-to-treat, as well as literature on patients’ expectations of posttreatment symptoms modulating their actual experiences. The differences in findings between our study and the previous author’s may be in part due to methodology; our quality of life assessments were conducted prospectively while the previous study was done retrospectively, relying on patient’s and family members’ recollections of pre- and postprocedure experiences. Although we did not have objective measurements of these symptoms (for example, limb volume measurements postoperatively or mapping of areas of numbness), our experience is that these physical symptoms are present in the vast majority of our patients, and that they do improve over time. The self-reported symptoms we measured in this study appear to track with our observations. Furthermore, we find it encouraging that our patients tend to rate their symptoms on the lower end of the severity scale. Finally, we observe that there does not seem to be an impact on overall HRQOL despite patient reports of symptoms.
Our study is limited in several ways. First, this HRQOL study was conducted as part of a clinical trial using temozolomide as the chemotherapeutic agent for ILI. Standard of care ILI for extremity of melanoma uses melphalan, an alkylating agent with a greater toxicity profile. We expect that melphalan-treated patients may experience a more prominent perioperative dip in HRQOL. In addition, melphalan appears to be more efficacious in treating in-transit melanoma, which may affect HRQOL scores when patients are surveyed at 3 months. Furthermore, we had a limited number of patients who were surveyed, with some drop-out at the later visits, and as a result this study was not powered to assess other clinical or treatment variables that may better predict HRQOL. Finally, the FACT-M questionnaire did not assess patient expectations about the ILI procedure, which would have helped us elucidate why HRQOL improved immediately posttreatment. It also does not address other comorbidities, health care utilization, or psychological factors that affect coping.
In summary, using a validated HRQOL measure, quality of life was not impacted by ILI for advanced extremity melanoma. Paradoxically, fewer patients complain of pain, numbness, and swelling immediately after treatment compared to baseline despite the side effects of surgery, and this is reflected in increased HRQOL scores. The psychological benefit of choosing to pursue treatment should be acknowledged in this population of patients. Furthermore, in addition to response and treatment expectations, HRQOL issues should be discussed preoperatively with patients. We recommend that HRQOL be assessed in all future regional therapy trials for melanoma.
Footnotes
DISCLOSURES None
REFERENCES
- 1.Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208(5):706–15. doi: 10.1016/j.jamcollsurg.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15(8):2195–205. doi: 10.1245/s10434-008-9988-9. [DOI] [PubMed] [Google Scholar]
- 3.Ueno T, Ko SH, Grubbs E, Pruitt SK, Friedman HS, Tyler DS. Temozolomide is a novel regional infusion agent for the treatment of advanced extremity melanoma. Am J Surg. 2004;188(5):532–7. doi: 10.1016/j.amjsurg.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Balch CM, Gershenwald JE, Soong S-j, et al. Final Version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol. 1998;14(3):238–47. doi: 10.1002/(sici)1098-2388(199804/05)14:3<238::aid-ssu8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Cormier JN, Davidson L, Xing Y, Webster K, Cella D. Measuring quality of life in patients with melanoma: development of the FACT-melanoma subscale. J Support Oncol. 2005;3(2):139–45. [PubMed] [Google Scholar]
- 7.Cormier JN, Ross MI, Gershenwald JE, et al. Prospective assessment of the reliability, validity, and sensitivity to change of the Functional Assessment of Cancer Therapy-Melanoma questionnaire. Cancer. 2008;112(10):2249–57. doi: 10.1002/cncr.23424. [DOI] [PubMed] [Google Scholar]
- 8.Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12(3):199–220. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- 9.Askew RL, Xing Y, Palmer JL, Cella D, Moye LA, Cormier JN. Evaluating minimal important differences for the FACT-Melanoma quality of life questionnaire. Value Health. 2009;12(8):1144–50. doi: 10.1111/j.1524-4733.2009.00570.x. [DOI] [PubMed] [Google Scholar]
- 10.Santillan AA, Delman KA, Beasley GM, et al. Predictive factors of regional toxicity and serum creatine phosphokinase levels after isolated limb infusion for melanoma: a multi-institutional analysis. Ann Surg Oncol. 2009;16(9):2570–8. doi: 10.1245/s10434-009-0563-9. [DOI] [PubMed] [Google Scholar]
- 11.Raymond AK, Beasley GM, Broadwater G, et al. Current trends in regional therapy for melanoma: lessons learned from 225 regional chemotherapy treatments between 1995 and 2010 at a single institution. J Am Coll Surg. 2011;213(2):306–16. doi: 10.1016/j.jamcollsurg.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazarus RS, Folkman S. Stress, appraisal, and coping. Springer; 1984. [Google Scholar]
- 13.Thomsen TG, Rydahl-Hansen S, Wagner L. A review of potential factors relevant to coping in patients with advanced cancer. J Clin Nurs. 2010;19(23-24):3410–26. doi: 10.1111/j.1365-2702.2009.03154.x. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien CW, Moorey S. Outlook and adaptation in advanced cancer: a systematic review. Psycho-Oncology. 2010;19(12):1239–49. doi: 10.1002/pon.1704. [DOI] [PubMed] [Google Scholar]
- 15.McClaine RJ, Giglia JS, Ahmad SA, McCoy SJ, Sussman JJ. Quality of life outcomes after isolated limb infusion. Ann Surg Oncol. 2012;19(5):1373–8. doi: 10.1245/s10434-012-2239-0. [DOI] [PubMed] [Google Scholar]