Abstract
Background
Although studies of melphalan-based isolated limb infusion (ILI) combine data from upper extremity (UE) treatments with those from lower extremity (LE) treatments, differences between the 2 may be clinically important.
Methods
Candidates for UE ILI (n = 51) and LE ILI (n = 192) were identified from prospective databases at 2 institutions. The response Evaluation Criteria in Solid Tumors and Wieberdink toxicity scale were used as appropriate.
Results
The following patients had indications for UE ILI: melanoma, 36 of 47 patients (77%); sarcoma, 5 of 47 patients (11%); Merkel cell sarcoma, 3 of 47 patients (6%), and squamous cell carcinoma, 3 of 47 patients (6%). The patients who underwent UE ILI, as expected, had lower limb volumes (mean, 2.5 L vs 8.6 L; P < .001) and lower mean melphalan doses (20.7 mg vs 49.5 mg; P < .001). On perfusate blood gas analysis, the mean base excess at 30 minutes (−13.9 vs −9.1; P < .001) and the mean pH at 30 minutes (7.06 vs 7.15; P < .001) were lower for UE procedures than for LE procedures, although the mean ischemic time was longer in LE procedures (67.2 minutes) than in UE procedures (61.6 minutes; P = .03). The rate of regional toxicity grade ≥3 for UE ILI was 7% compared with 24% (P = .005) for LE ILI. There was no difference in the complete response rate for melanoma UE procedures (28%; 95% confidence interval, 16%–44%) compared with LE ILI procedures (32%; 95% confidence interval, 25%–39%).
Conclusions
ILI for UE disease was associated with similar complete response rates but lower toxicity than ILI for LE disease and with different physiologic sequelae despite comparable methods. The UE appears relatively resistant to toxic effects of melphalan-based ILI as currently performed, which suggests a potential for further optimization of drug dosing for UE ILI.
Keywords: melanoma, limb infusion, melphalan, in-transit disease, tumor hypoxia
INTRODUCTION
Isolated limb infusion (ILI) is a well-tolerated, minimally invasive technique for delivering high-dose regional chemotherapy. Although the procedure is used primarily as a modality to treat unresectable in-transit melanoma, other indications include unresectable, limb-threatening extremity sarcoma and other tumor types that recur in a local, multifocal manner. For advanced extremity melanoma, single-institution complete response (CR) rates after ILI with melphalan with or without dactinomycin range from 25% to 38% with a median durability of approximately 12 months.1–3 Differences in treated patient populations, minor variations in performing the technique, and nonuniform response criteria account for most of the variability among centers. A recent, retrospective, US multicenter study of ILI for melanoma reported a CR rate of 29% (n = 68) in patients who had a melphalan dose corrected for ideal body weight (IBW), a method of dosing that reportedly decreases toxicity from ILI while not altering CR rates.4 For advanced soft tissue sarcoma of the extremity, the largest case series reported a CR rate of 57% and an overall limb salvage rate of 76% in 21 patients.5 Thus, ILI can achieve durable CR rates that are higher than other forms of treatment for advanced extremity malignancies. The toxicity profile of the procedure also is acceptable, with catastrophic toxicities (limb loss) nearly 10-fold less than such toxicities that result from the maximally invasive counterpart to ILI, hyperthermic isolated limb perfusion (HILP).6 Therefore, ILI has become a popular first-line procedure of choice at many institutions for patients who present with advanced extremity tumors that are not amenable to surgical resection.
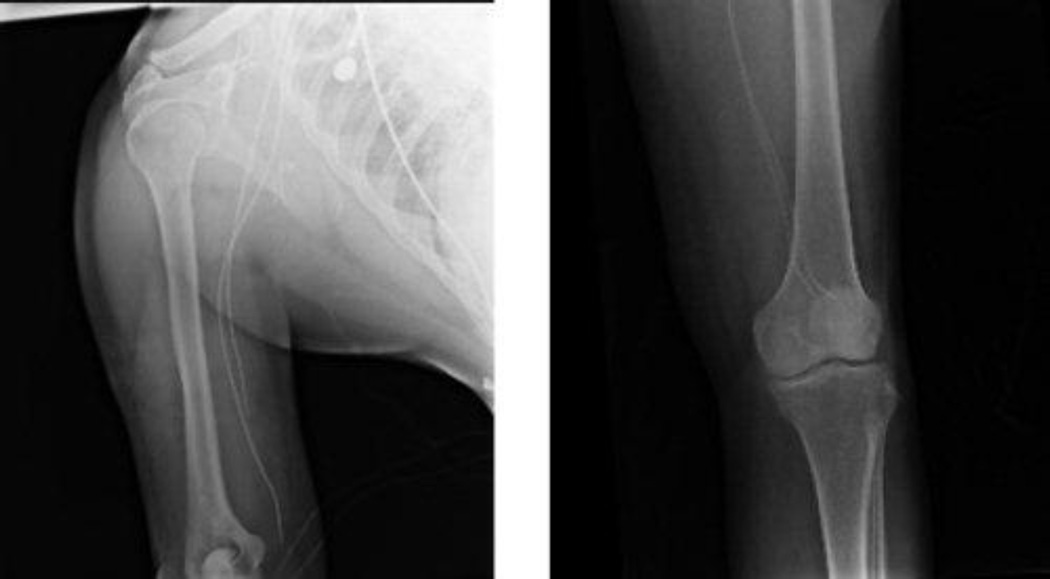
In the 2 largest single-center case series of ILI for melanoma, upper extremity (UE) procedures accounted for only 7% (n = 13) and 14% (n = 21) of total procedures performed.3,6 In the case series of advanced extremity sarcoma, only 3 patients (14%) received treatment for UE disease. The exact reason why UE in-transit disease appears to have a lower incidence than LE in-transit disease is unclear but cannot be explained fully by the larger surface area of the LE. UE ILI may be more challenging from a technical standpoint than LE ILI, because catheters typically are placed percutaneously through the femoral artery and vein and must be positioned near the elbow joint of the affected UE. Thus, the catheter tips are much farther from the access site compared with the LE (Fig. 1), and the caliber of vessels (brachial artery and vein compared with femoral artery and vein) is much smaller in the UE; both factors may contribute to lower flow rates achieved during ILI of the UE. It is believed that maximizing the flow rate during ILI is critical for optimal drug exposure.7
FIGURE 1.
These radiographic images show catheter tips (Left) in the upper extremity near the elbow joint and (Right) in the lower extremity near the knee joint.
Despite the lower flow rates typically achieved with UE in contrast to LE ILI, drug distribution may be better achieved in smaller limb volumes as opposed to larger limbs. In the multicenter US study of 75 ILI procedures, patients who had an overall response to treatment (partial responses [PRs] + CRs) had significantly smaller limb volume (6.45 ± 2.1 L) than patients who did not respond (8.01 ± 347 L; P = .021).4 This observation further suggests that drug distribution and delivery may be important in determining response. In the largest case series of ILI reported by the Sydney Melanoma Unit at Royal Prince Alfred Hospital (Camperdown, New South Wales, Australia), a higher melphalan concentration in the isolated circuit at the end of the procedure appeared to be significant for both CR (P = .013) and overall response (P = .022).3 Because drug delivery appears to have an impact on response and drug delivery may vary between UE and LE ILI procedures, we sought to review our experience of UE and LE ILI procedures to determine whether any important differences in response rates or toxicity were present.
MATERIALS AND METHODS
Prospective databases from 2 centers were used to identify 51 patients who were candidates for UE ILI and 192 patients who were candidates for LE ILI from 2004 to 2011. Toxicity after all completed procedures (n = 47) was evaluated using the Wieberdink toxicity scale.7 Grade 3 toxicities included considerable edema and blistering, whereas grade 4 toxicities were compartment syndromes. Treatment response to ILI performed for melanoma only (n = 36) was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) 3 months after the procedure. This study was reviewed and approved by the Institutional Review Board of Duke University Medical Center and Moffitt Cancer Center.
Intervention
ILI procedures were performed as previously described.1 Percutaneous arterial and venous catheters were placed by vascular surgery or interventional radiology. The catheters were inserted using the Seldinger technique in the ipsilateral (only for UE procedures) or contralateral (for UE or LE procedures) femoral artery and vein; and, using fluoroscopic guidance, the tips were positioned near the involved elbow or knee joint (Fig. 1). After the extremity had been warmed to at least 37°C, chemotherapy was rapidly infused (2–5 minutes) into the arterial line. Chemotherapy was circulated for 30 minutes followed by a wash-out. Circuit (perfusate) blood gases were taken at 25 minutes and 30 minutes after the initial infusion of chemotherapy to document the degree of hypoxia and acidosis. The dose of melphalan was 10 mg/L for the UE and 7.5 mg/L for the LE at both centers (Duke University Medical Center and Moffitt Cancer Center). The dose of dactinomycin was 100 µg/L for the UE and 75 µg/L for the LE at Duke University Medical Center and 100 µg/L for the UE and LE at Moffitt Cancer Center. Chemotherapy doses were corrected for ideal body weight (IBW) based on evidence that this dosing modification significantly reduced grade 3 and 4 toxicities by 27% (P = .027) without altering the CR rate.8,9 The melphalan concentration in plasma from the infusion circuit (UE ILI, n = 9; LE ILI, n = 70) was measured at 5 minutes, 10 minutes, 15 minutes, 20 minutes, 25 minutes, and 30 minutes by an improved assay based on a high-performance liquid chromatography-fluorescence method published by Ehrsson et al.10
Statistics
Summary statistics were derived using established methods and are presented either as percentages for categorical values or as means with ranges for continuous variables. Variables were compared using the Student t test, the Fisher exact test, and r × c contingency tables, as appropriate. Multiple logistic regression analyses were performed to identify predictors of toxicity. Given our effective sample size of 37 patients, the model included only 2 covariates with a C statistic of 0.64. A 2-sided α value of .05 was used for all tests. All statistical analyses were performed using Enterprise Guide version 4.2 (SAS Institute Inc., Cary, NC). The association of response (CR vs all others) with overall survival was assessed with the log-rank test, and Kaplan-Meier curves were used display the results of these tests.
RESULTS
In total, 51 UE and 192 LE planned procedures were analyzed from 2 centers. One planned UE procedure was unable to be performed because of small vessel size. One other UE procedure was aborted because of intimal flap creation during catheter insertion, and 2 additional procedures were aborted because of difficulty obtaining adequate flow. One LE procedure was aborted because of inadequate flow. Thus, 47 UE procedures and 191 LE procedures were available for analysis. Patient variables for all procedures are listed in Tables 1 and 2. Age and body mass index were similar between groups. The majority of patients with UE tumors had melanoma (n = 36 of 47; 77%). The remaining patients had sarcoma (n = 5), Merkel cell carcinoma (n = 3), and squamous cell carcinoma (n = 3). LE procedures were performed for similar indications (Table 2). Some patients (n = 56) were treated as part of clinical trials involving systemic administration or ADH-1 (an N-cadherin antagonist) or sorafenib in combination with melphalan by ILI. In particular, 4 patients who underwent UE ILI received systemic ADH-1; whereas 34 patients who underwent LE ILI received systemic ADH-1, and 18 patients who underwent LE ILI received systemic sorafenib. Because it has not been demonstrated that the administration of these agents increases overall response rates to ILI with melphalan alone, these patients were included in the current study.11,12 Finally, there was no significant difference in the percentage of patients who had their melphalan doses corrected for IBW between LE ILI procedures (89%) and UE ILI procedures (83%).
TABLE 1.
Patient Continuous Variables
| Upper Extremity (n = 47) | Lower Extremity (n = 191) | |||
|---|---|---|---|---|
| Variable | Median | Range | Median | Range |
| Age, y | 70 | 33–92 | 66 | 19–94 |
| Body Mass index (Duke only), kg/m2 |
28.8 | 20.5–37.8 | 29.7 | 19.2–49.9 |
| LOS, d | 6 | 3 to 9 | 7 | 4 to 30 |
Abbreviations: Duke, Duke University Medical Center; LOS, length of stay
TABLE 2.
Patient Categorical Variables
| Upper Extremity | Lower Extremity | |||
|---|---|---|---|---|
| Variable | No. | % Total (n = 47) | No. | % Total (n = 191) |
| Men | 28 | 59 | 74 | 38 |
| Diagnosis | ||||
| Melanoma | 36 | 77 | 173 | 91 |
| Sarcoma | 5 | 11 | 16 | 8 |
| Merkel cell carcinoma | 3 | 6 | 2 | 1 |
| Squamous cell carcinoma | 3 | 6 | 0 | 0 |
| Corrected for ideal body weight | 39 | 83 | 170 | 89 |
| Papaverine use | 32 | 67 | 130 | 68 |
| ADH-1 triala | 4 | 8 | 34 | 22.4 |
| Sorafenib trialb | 0 | 0 | 18 | 13.4 |
Part of the trial in which patients received systemic ADH-1 plus melphalan by isolated limb infusion.
Part of the trial in which patients received systemic sorafenib before and after isolated limb infusion with melphalan.
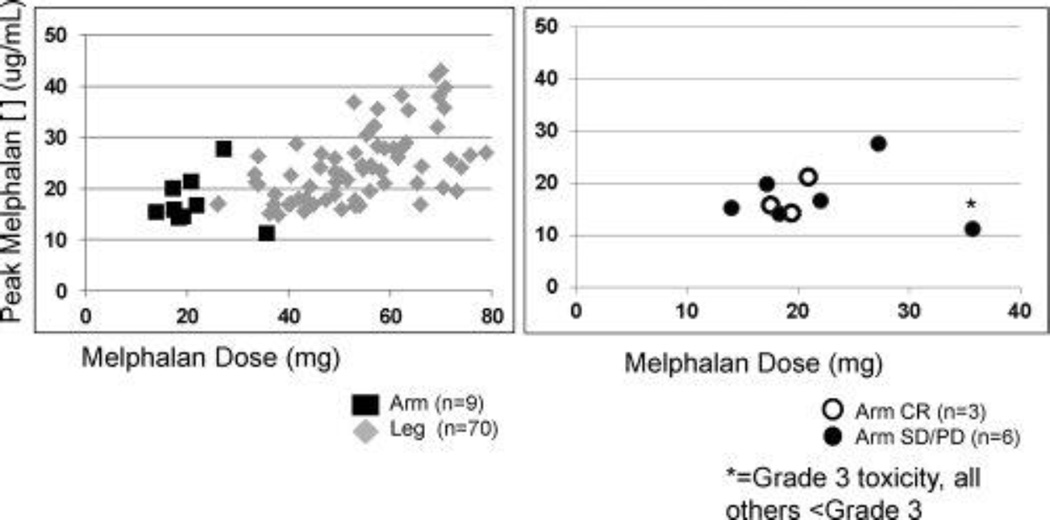
Procedural variables are listed in Table 3. Peak temperatures were similar between groups (mean, 38.6°C for UE vs 38.9°C for LE). The mean volume circulated during the 30-minute infusion, which was calculated only in patients at Duke University Medical Center, was lower for the UE group (1250 mL; n = 11) than for the LE group (1867 mL; n = 73; P < .001). The mean ischemic time was longer in the LE group (67.2 for LE vs 61.6 for UE; P = .03). The difference in ischemic time did not reflect any planned procedural differences between the UE and LE groups but may have been caused in part by the more rapid achievement of mild hyperthermia (37°C) in the UE group compared with the LE group. The mean limb volume was significantly lower in the UE group (2.5 L) compared with the LE group (8.6 L; P < .001), resulting in a mean melphalan dose that was significantly lower for the UE group (20.7 mg) compared with that for the LE group (49.65 mg; P < .001). The melphalan dose was volume-based, as discussed above (see Materials and Methods), although the dose for UE ILI (10 mg/L) was higher than that for LE ILI (7.5 mg/L). We examined the peak plasma concentration of melphalan in a subset of patients who underwent UE ILI (n = 9) and LE ILI (n = 70) whose melphalan doses were corrected for IBW (Fig. 2, left). Although dosing was 10 mg/L for the UE group and 7.5 mg/L for the LE group and was corrected for IBW in all procedures in this subset, there was a wide range of peak plasma concentrations (11.2–27.63 µg/mL for UE and 14.8–40.06 µg/mL for LE). Because of the small number of UE melphalan levels, a formal statistical analysis was not performed comparing peak plasma concentration of melphalan in the UE group with that in the LE group. However, there was a trend toward a higher peak concentration in the LE group in this sample population. Next, we examined the correlations of response and toxicity with plasma melphalan concentrations for UE procedures only (Fig. 2, right). There appeared to be no correlation between peak plasma concentration and either clinical response or toxicity. The patient who had grade 3 toxicity in this subset of the UE group had received the highest melphalan dose but did not have the highest peak plasma concentration (Fig. 2, right).
TABLE 3.
Procedure Variables
| Upper Extremity | Lower Extremity | ||||
|---|---|---|---|---|---|
| Variable | No. | Mean ± SD | No. | Mean ± SD | Pa |
| Melphalan dose, mg | 47 | 20.7 ± 5.2 | 188 | 49.5 ± 13 | <.001 |
| Limb volume, L | 47 | 2.5 ± 0.8 | 185 | 8.6 ± 2.8 | <.001 |
| pH at 30 min | 43 | 7.06 ± 0.07 | 184 | 7.15 ± 0.09 | <.001 |
| Base excess at 30 min, mmol/L | 43 | −13.9 ± 9.1 | 184 | −9.1 ± 5.8 | <.001 |
| pO2 at 25 min, mm Hg | 42 | 11.7 ± 3.2 | 182 | 7.9 ± 3.3 | .013 |
| Ischemic time, min | 47 | 61.6 ± 15.4 | 188 | 67.2 ± 16.1 | .03 |
| Volume re-circulated, MI | 11 | 1250 ± 251 | 73 | 1867 ± 321 | <.001 |
| Peak temperature, °C | 47 | 38.6 ± 0.8 | 186 | 38.9 ± 0.8 | .089 |
| Peak CPK, U/L | 47 | 918 ± 1853 | 188 | 1673 ± 2712 | .021 |
Abbreviations: CPK, creatine phosphokinase; pO2, oxygen partial pressure; SD, standard deviation.
P values were calculated for differences in the mean.
FIGURE 2.
These charts illustrate the melphalan dose (in mg) plotted against the peak plasma melphalan concentration (in µg/mL). (Left) Nine upper extremity isolated limb infusion (ILI) procedures and 70 lower extremity ILI procedures are plotted. (Right) Only upper extremity procedures are plotted, and the patients who had a complete response (CR) and grade 3 toxicity are identified. SD/PD indicates stable disease/progressive disease.
It is noteworthy that there were differences in blood perfusate gases that suggested a greater degree of acidosis in the UE group versus the LE group during ILI (Table 3). The mean pH at 30 minutes was lower in the UE group (7.06 vs 7.15; P < .001), and there was also a greater mean base deficit at 30 minutes in the UE group (−13.9 mmol/L vs −9.1 mmol/L; P < .001). These differences were observed despite a mean ischemic time that was 6 minutes longer in the LE group (67.2 minutes vs 61.6 minutes in the UE group; P = .03). It is noteworthy that the longer ischemic time was associated with a greater degree of hypoxia in the LE group (mean oxygen partial pressure [pO2] at 30 minutes, 7.9 mm Hg) than in the UE group (mean pO2 at 30 minutes, 11.7 mm Hg; P = .013). However, greater hypoxia in the LE group did not lead to greater acidosis as measured by pH in the LE group. In summary, patients in the UE group appeared to become more acidotic but less hypoxic than the patients in the LE group. The rate of grade ≥3 Wieberdink toxicity for those undergoing UE ILI for any indication was 6% (n = 3 of 47), and no grade 4 toxicities were observed. For LE procedures, the rate of grade ≥3 toxicities was 24% (n = 46 of 190), and 5% of patients (n = 10) experienced grade 4 toxicity (excluding 1 LE patient whose toxicity assessment had not been recorded). The percentage of patients with grade ≥3 toxicity was significantly higher for those undergoing LE ILI (24% LE vs 6% UE; P = .005). It was reported previously that the peak creatine kinase (CK) level is a strong predictor of severe toxicity.13 In our study, the mean peak CK was higher in patients who underwent LE ILI compared with those who underwent UE ILI (1673 U/L vs 918 U/L). The Student t test could not be used to compare UE and LE groups, because the variances in CK were not similar between the groups. Therefore, we compared the 2 groups with respect to the proportion of patients who had a CK level >1000 U/L, because, historically, 1000 U/L has been the value at which concern for severe toxicity arises and typically prompts the administration of high-dose steroid treatment.1,14 The proportion of patients who had peak CK levels >1000 U/L after LE ILI (33%; 95% confidence interval, 27%–41%) was similar to the proportion after UE ILI (19%; 95% confidence interval, 17%–42%). Furthermore, even when controlling for the peak CK level, UE procedures still produced fewer toxicities compared with LE procedures (P = .019). It is noteworthy that correcting the melphalan dose for IBW is associated with less toxicity. However, the proportion of patients who had their melphalan doses corrected for IBW was similar for both groups (81% for UE, 88% for LE).
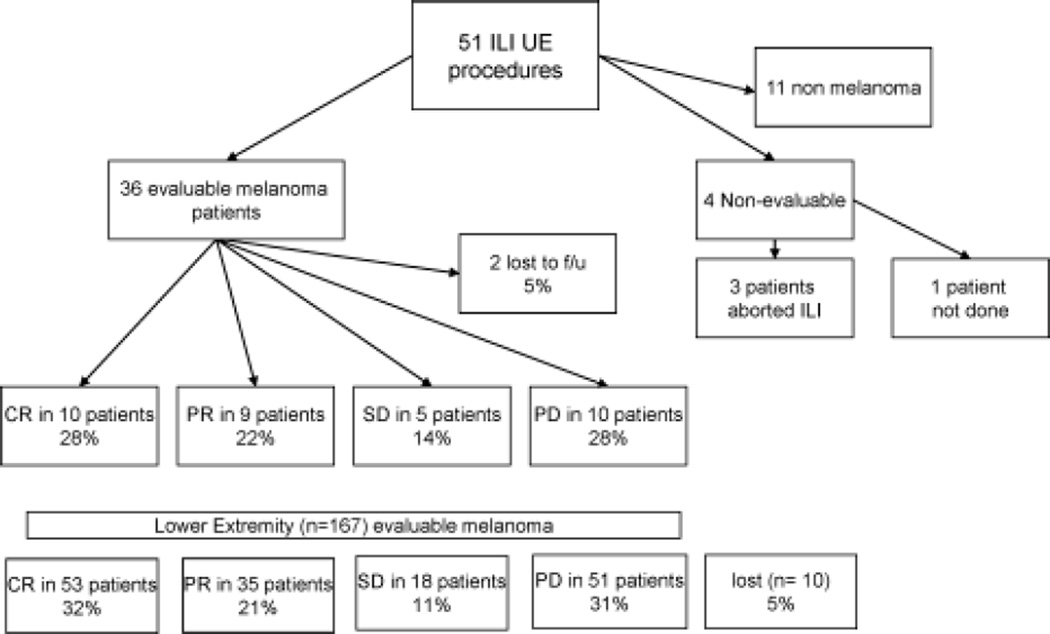
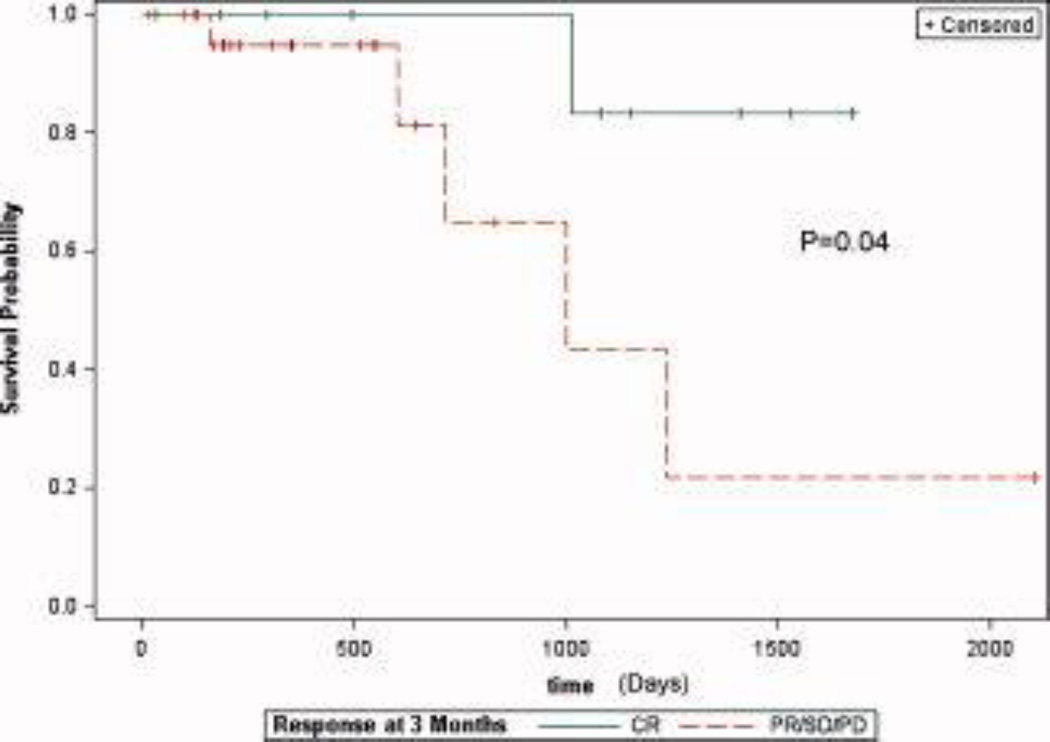
Next, we examined the clinical response rate for the group of patients who underwent ILI for melanoma (n = 36). Four patients had received previous melphalan-based regional therapies but were included in the response rates. As outlined in Figure 3, 10 of 36 patients (28%) had a CR, 9 of 36 patients (22%) had a PR, 5 of 36 patients (14%) had stable disease (SD), 10 of 36 patients (28%) had progressive disease (PD), and 2 of 36 patients (5%) were lost to follow-up. By comparison, 173 patients underwent LE ILI procedures for melanoma, and 167 were evaluable for response. Two patients underwent LE ILI procedures for prophylactic indications, and 4 patients had not yet reached the 3-month time point and, thus, were excluded from the analysis. Of the remaining 167 patients, 53 of 157 patients (32%) who underwent LE ILI had a CR, 35 of 157 patients (21%) had a PR, 18 of 156 patients (11%) had SD, 51 of 157 patients (31%) had PD, and 10 of 167 patients (6%) were lost to follow-up. A similar percentage of patients were lost to follow-up in both groups. Overall, there was no statistically significant difference in the CR rate between the UE and LE groups (28% vs 32%, respectively; P = .58). We also examined overall survival for the patients who underwent UE ILI for melanoma (Fig. 4). Those who achieved a CR had a significant improvement in survival compared with those who had SD, a PR, or PD (P = .04).
FIGURE 3.
This is a flow diagram of upper extremity (UE) isolated limb infusion (ILI) procedures. Responses for lower extremity (LE) ILI procedures also are illustrated. CR indicate complete response; PR, partial response; SD, stable disease, PD, progressive disease; f/u, follow-up.
FIGURE 4.
These Kaplan-Meier curves illustrate survival after upper extremity isolated limb infusion procedures only for those patients who achieved a complete response (CR) (green line; n = 36) compared with those who did not achieve a CR (orange line). PR/SD/PD indicates partial response/stable disease/progressive disease.
DISCUSSION
Optimizing drug delivery to tumor cells is essential to maximizing response. It has been suggested that the relatively low flow system of ILI compared with HILP leads to lower levels of melphalan uptake by tumor cells and, subsequently, a lower response, although this finding has not been examined thoroughly. Consequently, to maximize ILI flow rates, 1 center has described placing a shorter venous catheter below the tourniquet on the ipsilateral side to improve blood flow and, thus, the total volume of blood circulated through the ILI circuit.7 Because ILI procedures of the UE are associated with lower flow rates, we sought to examine whether there were differences in response or toxicity between UE ILI and LE ILI. Furthermore, most studies combine the results from both UE and LE procedures without separate examination, although the patients are dosed differently for melphalan. In the current study, there were no differences in the CR rate (28% UE vs 32% LE), but the frequency of grade ≥3 toxicities was significantly lower in UE procedures (6% vs 24%).
Although the volume circulated over a 30-minute period is lower for UE ILI, limb volumes are much smaller in the arm, and the melphalan dose is higher for UE procedures (10 mg/L for UE vs 7.5 mg/L for LE). Peak plasma concentrations actually appear to be higher in patients undergoing LE ILI, although this finding was limited by a small sample size. Ultimately, it is believed that the concentration of drug in tumor tissue is the most important factor in ILI, and this issue has been studied by Thompson and colleagues using a microdialysis technique. Those authors observed a significant association between tumor response and melphalan concentrations measured over time in subcutaneous microdialysate (P < .01).14 Enhanced uptake of melphalan into tumor cells also may be enhanced by hypoxia and reduced pH, as suggested in animal models.3,13 In our current study, the differences in melphalan dose between the UE (10 mg/L) and LE (7.5 mg/L) groups, the 6-minute difference in ischemic times, and the differences in physiologic sequelae between UE and LE procedures did not lead to differences in CR rates between UE and LE procedures. However, UE procedures appear to be resistant to some of the toxic effects of the procedure as currently performed despite differences in physiologic sequelae. Although our study was limited by a heterogenous and small group of patients, the results suggest that further optimization of drug dosing could enhance the efficacy of ILI for the treatment of UE tumors.
These results have important implications with regard to the management of patients who have unresectable, in-transit extremity melanoma. ILI with melphalan previously produced CR rates ranging from 25% to 38% in single-center studies and 29% in a multicenter, retrospective study.1,2,4 In addition, having a clinical response to regional therapy (including ILI) has been associated with improved survival compared with the survival of those who do not respond to therapy.3,6 The results from this study, in which we examined data from 2 large-volume ILI centers, include response rates for UE and LE procedures that were similar to the rates reported previously and the finding that patients who attained a CR to UE ILI had a significant survival benefit over those who did not achieve a CR (Fig. 4). Despite a potential for less effective drug delivery in the UE group, the CR rate was are similar to that in the LE group, suggesting that UE ILI for unresectable, in-transit melanoma is an important therapeutic option for these patients.
Although the current study was focused on ILI, we certainly advocate the role of HILP in the appropriate patient and clinical setting. HILP may be particularly valuable in the setting of documented regional lymph node disease or after ILI failure. However, ILI has an important role in the management of advanced extremity neoplasms; therefore, it is critical to identify strategies for improving efficacy and minimizing toxicity to ILI. Such strategies may include novel therapies. Although melphalan-based regional therapies currently are used, we are conducting a phase 1 trial of temozolomide using ILI. With the addition of new targeted agents, potential also exists to further improve response rates with novel combination therapies that may include regional therapy. We also are starting to examine the tumor microenvironment using the hypoxia marker EF5 (2-[2 nitro-1H-imidazol-1-yl]-N-[2,2,3,3,3-pentafluoropropyl]) before ILI to determine how hypoxia and acidosis may affect treatment response. For example, animal studies suggest that the magnitude and direction of the pH gradient (intracellular pH and extracellular pH) can be exploited for optimal intracellular drug delivery.15 Given the significant differences observed in the physiologic parameters in UE procedures versus LE procedures, future studies will need to analyze UE ILI and LE ILI separately in an attempt to best optimize the therapeutic potential of this form of regional treatment.
Acknowledgments
FUNDING SOURCES
The ADH-1 trial was supported by a grant from Adherex Technologies, Inc.
CONFLICT OF INTEREST DISCLOSURES
Dr. Tyler received honorarium as a conference attendee from Adherex Technologies, Inc., and Bayer Healthcare Pharmaceuticals provided study drug (sorafenib, Nexavar) for the phase 1 trial of systemic sorafenib and regional melphalan. Dr. Tyler also received honorarium from Bayer Healthcare Pharmaceuticals for participation in an investigator's conference.
REFERENCES
- 1.Beasley GM, Petersen RP, Yoo YS. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15:2195–2205. doi: 10.1245/s10434-008-9988-9. [DOI] [PubMed] [Google Scholar]
- 2.Brady MS, Brown K, Patel A, et al. Isolated limb infusion with melphalan and dactinomycin for regional melanoma and soft-tissue sarcoma of the extremity: final report of a phase II clinical trial. Melanoma Res. 2009;19:106–111. doi: 10.1097/CMR.0b013e32832985e3. [DOI] [PubMed] [Google Scholar]
- 3.Kroon HM, Moncrieff M, Kam PCA, et al. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol. 2008;15:3003–3013. doi: 10.1245/s10434-008-9954-6. [DOI] [PubMed] [Google Scholar]
- 4.Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208:706–715. doi: 10.1016/j.jamcollsurg.2008.12.019. discussion 715–717. [DOI] [PubMed] [Google Scholar]
- 5.Moncrieff M, Kroon H, Kam PCA. Isolated limb infusion for advanced soft tissue sarcoma of the extremity. Ann Surg Oncol. 2008;15:2749–2756. doi: 10.1245/s10434-008-0045-5. [DOI] [PubMed] [Google Scholar]
- 6.Raymond AK, Beasley GM, Broadwater G, et al. Current trends in regional therapy for melanoma: lessons learned from 225 regional chemotherapy treatments between 1995 and 2010 at a single institution. J Am Coll Surg. 2011;213:306–316. doi: 10.1016/j.jamcollsurg.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vyas A, Avritscher R, Ensor J, Ross M, Wallace MJ. Isolated limb infusion with cytotoxic agents: a simplified approach for venous access. Cancer. 2010;116:459–464. doi: 10.1002/cncr.24736. [DOI] [PubMed] [Google Scholar]
- 8.McMahon N, Cheng TY, Beasley GM, et al. Optimizing melphalan pharmacokinetics in regional melanoma therapy: does correcting for ideal body weight alter regional response or toxicity? Ann Surg Oncol. 2009;16:953–961. doi: 10.1245/s10434-008-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng TY, Gruubs E, Abduke-Wahab O, et al. Marked variability of melphalan plasma drug levels during regional hyperthermic isolated limb perfusion. Am J Surg. 2003;186:460–467. doi: 10.1016/j.amjsurg.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Ehrsson H, Eksborg S, Lindfors A. Quantitative determination of melphalan in plasma by liquid chromatography after derivatization with N-acetylcysteine. J Chromatogr. 1986;380:222–229. doi: 10.1016/s0378-4347(00)83648-8. [DOI] [PubMed] [Google Scholar]
- 11.Beasley GM, Riboh J, Augustine CK, et al. Prospective multicenter phase II trial of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with advanced extremity melanoma. J Clin Oncol. 2011;29:1210–1215. doi: 10.1200/JCO.2010.32.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMahon N, Beasley G, Sanders G, et al. A phase I study of systemic sorafenib in combination with isolated limb infusion with melphalan (ILI-M) in patients (pts) with locally advanced in-transit melanoma [abstract] J Clin Oncol. 2009;27(15S) doi: 10.1002/cncr.24509. Abstract 9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skarsgard LD, Skwarchuk MW, Vinczan A, et al. The cytotoxicity of melphalan and its relationship to pH, hypoxia, and drug uptake. Anticancer Res. 1995;15:219–223. [PubMed] [Google Scholar]
- 14.Thompson JF, Anisimov YG, Smithers BM, et al. Microdialysis and response during regional chemotherapy by isolated limb infusion of melphalan for limb malignancies. Br J Cancer. 2001;85:157–165. doi: 10.1054/bjoc.2001.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prescott DM, Charles HC, Poulson JM, et al. The relationship between intracellular and extracellular pH in the spontaneous canine tumors. Clin Cancer Res. 2000;6:2501–2505. [PubMed] [Google Scholar]