Abstract
Because of its reconstructive nature, autobiographical memory (AM) is subject to a range of distortions. One distortion involves the erroneous incorporation of features from one episodic memory into another, forming what are known as memory conjunction errors. Healthy aging has been associated with an enhanced susceptibility to conjunction errors for laboratory stimuli, yet it is unclear whether these findings translate to the autobiographical domain. We investigated the impact of aging on vulnerability to AM conjunction errors, and explored potential cognitive processes underlying the formation of these errors. An imagination recombination paradigm was used to elicit AM conjunction errors in young and older adults. Participants also completed a battery of neuropsychological tests targeting relational memory and inhibition ability. Consistent with findings using laboratory stimuli, older adults were more susceptible to AM conjunction errors than younger adults. However, older adults were not differentially vulnerable to the inflating effects of imagination. Individual variation in AM conjunction error vulnerability was attributable to inhibitory capacity. An inability to suppress the cumulative familiarity of individual AM details appears to contribute to the heightened formation of AM conjunction errors with age.
Keywords: Autobiographical memory, False memory, Imagination, Aging, Inhibition
Introduction
Episodic memory representations are stored as constituent features distributed widely across the brain, meaning that retrieval of a coherent episode requires these fragments to be relocated, reactivated and reintegrated (Bartlett, 1932; Schacter, Norman, & Koutstaal, 1998). This flexible memory system is generally advantageous (Schacter, Guerin, & St Jacques, 2011), in that it allows us to recombine details to imagine the future (Schacter & Addis, 2007), creatively solve problems (Howe, Garner, Charlesworth, & Knott, 2011; Madore, Addis, & Schacter, 2015), and update memories with recently acquired information (Lee, 2009; St Jacques, Olm, & Schacter, 2013). However, the downside to this constructive setup is that it renders us vulnerable to memory distortions. For instance, erroneous incorporation of features from one memory into another forms what are known as memory conjunction errors. Such errors have previously been shown to occur in autobiographical memory (AM; Burt, Kemp, & Conway, 2004; Devitt, Monk-Fromont, Schacter, & Addis, 2016; Odegard & Lampinen, 2004).
Healthy aging is accompanied by an increased susceptibility to false memory formation (e.g., Jacoby & Rhodes, 2006; Mitchell & Johnson, 2009; Pierce, Simons, & Schacter, 2003), including memory conjunction errors for words (Castel & Craik, 2003; Jones & Jacoby, 2005; Rubin et al., 1999), faces (Kroll, Knight, Metcalfe, Wolf, & Tulving, 1996), and actor-action pairings (Kersten, Earles, Curtayne, & Lane, 2008; Kersten, Earles, & Upshaw, 2013; Kersten & Earles, 2010). Yet it is unknown whether this age-related increase in memory conjunction error susceptibility translates to the autobiographical domain. Laboratory studies have demonstrated that increasing the distinctiveness of stimuli (for example, by presenting stimuli as pictures instead of words) can reduce age differences in source monitoring (Dodson & Schacter, 2002; Ferguson, Hashtroudi, & Johnson, 1992; Johnson, De Leonardis, Hashtroudi, & Ferguson, 1995). Older adults also selectively remember self-relevant information (Castel, Murayama, Friedman, McGillivray, & Link, 2013; Castel, 2007). Given this evidence, aging may not have as large an influence on conjunction error susceptibility when examining more distinctive and self-relevant forms of memory such as AM (McDonough & Gallo, 2008; though see St-Laurent, Abdi, Burianová, & Grady, 2011). Thus, a central aim of this study is to determine whether conjunction errors derived from AM is heightened in healthy aging.
Despite the distinctive nature of autobiographical stimuli, there are still reasons to believe older adults are more susceptible than younger adults to AM conjunction errors. Several cognitive changes accompanying aging may contribute to an increased vulnerability to conjunction errors, including deficits in feature binding mechanisms (Naveh-Benjamin, 2000) and an overreliance on familiarity (e.g., Jones & Jacoby, 2005). These cognitive changes are likely driven by structural and functional dysfunction in brain areas involved in memory encoding, retrieval, and monitoring, particularly the medial temporal lobes and prefrontal cortex (Kroll et al., 1996; McCabe, Roediger, McDaniel, & Balota, 2009; Parkin, Bindschaedler, Harsent, & Metzler, 1996).
Age-related hippocampal dysfunction is associated with deficits in the formation and retrieval of relations between memory components (Hannula, Tranel, & Cohen, 2006; Mitchell, Johnson, Raye, & D’Esposito, 2000; Naveh-Benjamin, 2000; Old & Naveh-Benjamin, 2008; Pertzov et al., 2013), resulting in poorer feature binding and recollection (Chalfonte & Johnson, 1996; Henkel, Johnson, & De Leonardis, 1998; Kessels, Hobbel, & Postma, 2007; Lyle, Bloise, & Johnson, 2006; Naveh-Benjamin, 2000). As such, older adults are reliant on less accurate familiarity processes when making source decisions (Anderson et al., 2008; Craik & McDowd, 1987; Davidson & Glisky, 2002; Dennis, Bowman, & Peterson, 2014; Giovanello, Kensinger, Wong, & Schacter, 2010; Prull, Dawes, Martin III, Rosenberg, & Light, 2006). Consequently, older adults are less efficient at using recall-to-reject mechanisms to identify familiar lures that are semantic associates of studied items (Gallo, Bell, Beier, & Schacter, 2006), and are more susceptible to false alarms based on familiarity with the features comprising a conjunction lure (Fandakova, Shing, & Lindenberger, 2013; Jones & Jacoby, 2005). Consistent with this notion, poorer associative binding ability is linked with an increased conjunction error rate in both younger and older adults (Fandakova et al., 2013).
Age-associated recollective reductions also mean that the enhanced phenomenological quality used to tag a memory as veridical (cf. source monitoring framework; Johnson, Hashtroudi, & Lindsay, 1993) may no longer be available or reliable, the result being that authentic memories and imagined events are less distinguishable (Duarte, Graham, & Henson, 2010; Gallo, Korthauer, McDonough, Teshale, & Johnson, 2011; Johnson & Raye, 1981; Karpel, Hower, & Toglia, 2001). Indeed, the neural signatures associated with veridical and false recognition are more similar as we age (Duarte et al., 2010; Gutchess, Ieuji, & Federmeier, 2007; see also Dennis et al., 2014). Due to this dedifferentiation between internally- and externally-generated memories, imagination inflation is more prominent with age, whereby false memories are more likely to arise following imagination of a corresponding event (Cohen & Faulkner, 1989; Gerlach, Dornblaser, & Schacter, 2014; Lindner & Davidson, 2014; McDaniel, Lyle, Butler, & Dornburg, 2008; Thomas & Bulevich, 2006; though see Pezdek & Eddy, 2001). We have previously demonstrated imagination inflation for AM conjunction errors in younger adults (Devitt et al., 2016); in the current study we examine whether enhanced inflation is observed in older adults.
Age-related medial temporal dysfunction also contributes to the erroneous binding of features across memory representations (Dodson, Bawa, & Krueger, 2007; Fandakova et al., 2013; Kroll et al., 1996). In addition, the ability to inhibit irrelevant stimuli or internally-generated signals is particularly important for accurate memory encoding and retrieval (e.g., Balota et al., 1999, 2000; Budson et al., 2002; Plancher et al., 2009). Reduced inhibition associated with age-related prefrontal changes may mean less efficient suppression of signals derived from the spreading activation of related memory traces (Biss, Campbell, & Hasher, 2013; Campbell, Hasher, & Thomas, 2010; Campbell, Trelle, & Hasher, 2014; Gazzaley, Cooney, Rissman, & D’Esposito, 2005; Hasher, Zacks, & May, 1999). Therefore the tendency to over-bind activated elements pertaining to separate memory traces is magnified (Fandakova et al., 2013; Henkel et al., 1998; Lyle et al., 2006; Lyle & Johnson, 2006). This disinhibition in feature binding could give rise to conjunction errors experienced with a sense of recollection and a high degree of confidence (Dodson, Bawa, & Krueger, 2007; Dodson, Bawa, & Slotnick, 2007; Kroll et al., 1996). Indeed, measures of executive functioning are linked with susceptibility to conjunction errors for laboratory stimuli in older adults (Fandakova et al., 2013; Rubin et al., 1999). Reduced inhibition can also lower suppression of familiarity garnered by the individual memory features comprising a conjunction lure, which may be misattributed as an indicator of event authenticity (Jones & Jacoby, 2001).
Although reduced relational and executive processes are typical in older adults, cognitive aging is a heterogeneous process (Hultsch, Strauss, Hunter, & MacDonald, 2008; Van Petten, 2004), and it is clear that increased susceptibility to false memories is not a universal and inevitable feature of aging (e.g., Intons-Peterson, Rocchi, West, McLellan, & Hackney, 1999; Umanath & Marsh, 2012). Individual variation in cognitive function likely plays a large role in susceptibility to memory errors, with higher-functioning older adults compensating for specific neurocognitive declines by recruiting additional neural resources, particularly prefrontal areas (Berlingeri, Danelli, Bottini, Sberna, & Paulesu, 2013; Cabeza, Anderson, Locantore, & McIntosh, 2002; Cabeza, 2002; Grady, McIntosh, & Craik, 2003, 2005; Gutchess et al., 2005; Maguire & Frith, 2003; Park & Reuter-Lorenz, 2009; Reuter-Lorenz & Cappell, 2008; Rosen et al., 2002).
In line with this idea, older adults who perform at a high level on neuropsychological tests sensitive to medial temporal and prefrontal functioning are no more vulnerable to false memories than younger adults (Butler, McDaniel, Dornburg, Price, & Roediger, 2004; Chan & McDermott, 2007; Fandakova et al., 2013; Glisky, Rubin, & Davidson, 2001; Henkel et al., 1998; McCabe et al., 2009; Rubin et al., 1999; Thomas & McDaniel, 2013). Moreover, individual differences in false memory susceptibility in healthy younger adults are also linked with variation in performance on neuropsychological tests of episodic memory (e.g., Fandakova et al., 2013; Zhu et al., 2010; though see Lepage, Brodeur, & Bourgouin, 2003; McCabe et al., 2009) and executive functioning ability (Chan & McDermott, 2007; Fandakova et al., 2013; Glisky, Polster, & Routhieaux, 1995; McCabe et al., 2009; Plancher et al., 2009; Rhodes & Kelley, 2005). However, the contribution of relational and executive memory processes to memory distortions in AM has not been examined in previous research.
The current study
In the current study we evaluate the influence of aging on the formation and retention of conjunction errors in AM. Younger and older participants completed an AM conjunction error paradigm (Devitt et al., 2016) that involved retrieving AMs and identifying key details, imagining novel past events in response to recombinations of these details, and completing a source recognition test for intact and conjunction detail sets. We collected subjective measures of event phenomenology during imagination and retrieval of conjunction events. Additionally, autobiographical interviews (AI; Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002) of authentic and conjunction memories were coded for event content. Participants also completed a neuropsychological battery of tests probing relational memory and inhibition.
We expected older adults to be more susceptible than younger adults to AM conjunction lures overall, due to an overreliance on familiarity-based recognition, in association with disinhibition of feature binding. We predicted a heightened vulnerability to AM conjunction errors after imagining a conjunction event, compared with a control associative task, particularly when these imaginings were vivid and plausible. Moreover, we hypothesized that if older adults exhibit a dedifferentiation between internally- and externally-derived experiences, imagination inflation should be more prominent in the older group. We also explore the possibility that heterogeneity in conjunction error susceptibility is accountable by individual and age-related differences in relational memory and inhibitory processes vital to episodic memory encoding and retrieval.
Method
Participants
We recruited 30 younger adults and 28 older adults. Data from two younger adults were excluded (both female), one due to task noncompliance in session three, and the other due to abnormalities in a follow-up MRI scan. Data from two older adults were excluded (both female), one due to a history of transient ischemic attack, and the other due to a conjunction error rate more than three standard deviations above the mean, indicating a misunderstanding of session three instructions1. Therefore data from 28 younger adults (5 male) and 26 older adults (9 male) were included in analyses of sessions one-three. Three younger adults (all female), and one older adult (male), did not complete the neuropsychological testing session, thus data from 25 younger and 25 older adults were included in analyses of neuropsychological data.
Power analyses were conducted using G*Power (Faul, Erdfelder, Lang, & Buchner, 2007) on the basis of published data to establish minimum sample sizes needed to achieve 80% power at α = .05. For age differences in conjunction errors (Old & Naveh-Benjamin, 2008), as well as an age by detail type interaction using the AI (Levine et al., 2002), the minimum was 13 participants per group. For a regression including false alarms as the outcome measure and memory and executive measures as predictors (Chan & McDermott, 2007), a minimum of 25 participants per group were needed. Thus our sample sizes satisfy these requirements.
Demographic data are presented in Table 1. All participants were fluent English speakers with no history of learning disabilities, neurological or psychiatric impairments, and had normal or corrected to normal vision. All older adults scored 88 or above on the Addenbrooke’s Cognitive Examination-Third Edition, indicating no early signs of dementia. Participants gave informed consent in a manner approved by the University of Auckland Human Participants Ethics Committee, and were compensated with a $25 supermarket gift certificate for each session completed.
Table 1.
Mean demographics and neuropsychological test scores.
| Measures | Younger adults | Older adults |
|---|---|---|
| Participants completing sessions one-three | ||
| Number of participants | 28 (5 male) | 26 (9 male) |
| Age (years)* | 21.29 (3.54) | 71.85 (4.61) |
| Education (years) | 14.95 (1.90) | 15.80 (3.35) |
| ACE-III | -- | 94.42 (3.35) |
| Participants completing session foura | ||
| Number of participants | 25 (5 male) | 25 (8 male) |
| Age (years)* | 21.44 (3.68) | 72.04 (4.60) |
| Education (years) | 14.94 (1.93) | 15.86 (3.21) |
| ACE-III | -- | 94.64 (3.23) |
| AM conjunction error rate (% trials)* | 2.12 (2.17) | 5.24 (5.06) |
| Memory performance (session four participants) | ||
| CVLT-II: 1–5 free recall total correct* | 60.16 (7.10) | 48.48 (9.64) |
| Visual PA: learning sum* | 15.04 (2.63) | 10.74 (3.43) |
| Verbal PA: learning sum hard pairs* | 9.92 (1.41) | 6.25 (3.63) |
| Memory composite (z-score)* | 0.56 (0.38) | −0.63 (0.82) |
| Inhibition performance (session four participants) | ||
| Trails: ratio number-letter switching to number | 2.19 (0.63) | 2.5 (0.98) |
| Verbal fluency: total letter | 45.8 (8.45) | 41.44 (10.97) |
| Design fluency: switching* | 10.58 (2.75) | 7.58 (2.24) |
| Color-word inhibition: ratio inhibition to color naming* | 1.70 (0.29) | 1.95 (0.31) |
| Tower: achievement score | 18.88 (3.47) | 17.44 (4.59) |
| Inhibition composite (z-score)* | 0.28 (0.43) | −0.29 (0.48) |
Note. Standard deviations are provided in parentheses. PA = paired associates, AM = autobiographical memory, ACE-III = Addenbrooke’s Cognitive Examination, Third Edition.
difference between groups is significant at the relevant Bonferroni-corrected threshold (for demographic measures α = .05, for memory measures α = .01, and for executive functioning measures α = .008).
Note that all participants in session four also completed sessions one-three.
AM Conjunction Error Paradigm
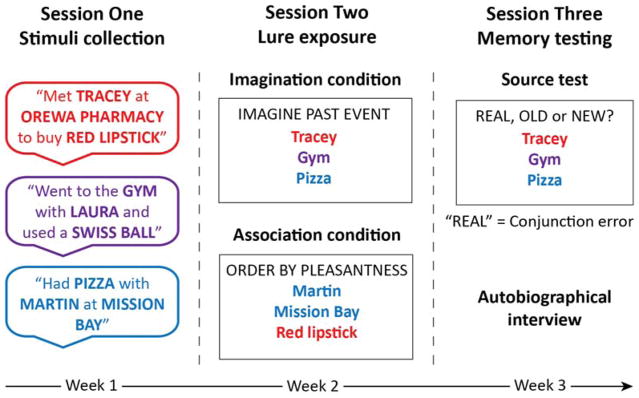
As described in Devitt et al. (2016), the AM conjunction error paradigm involved three sessions, which were modified here for use with older adults. In session one, AM details were collected; in session two, conjunction events were imagined and an associative task completed; in session three, a source test and interview were carried out (see Figure 1). In an additional fourth session, participants completed a battery of neuropsychological tests assessing relational memory and inhibition ability.
Figure 1.
A schematic diagram of the autobiographical memory conjunction error paradigm, depicting example memory components collected during session one, partially and fully recombined detail sets presented in session two, and detail sets presented in the source test of session three. Note colors are used to highlight recombinations; stimuli were presented to participants in black and white.
Session One: Stimuli collection
Participants recalled 40–50 AMs of events (specific in time and place and lasting no longer than a day) from the past 10 years, which typically took around two hours to complete. For each AM, participants wrote a brief description, and specified a person (other than themselves) who was involved, the location where it occurred, and a salient object that was present. We provided participants with an extensive list of event cues to facilitate retrieval, but memories were not limited to these cues. Although older adults produced slightly fewer memories (M = 40.81, SD = 3.10) than younger adults (M = 44.93, SD = 2.14), both groups generated sufficient stimuli for the paradigm. Importantly, the recency of AMs did not differ across the groups (M younger = 2.84 years, SD = 0.99, M older = 2.99 years, SD = 1.31; t(52) = 0.47, p = .64).
Prior to session two, these memories were screened for adherence to the specificity instructions; at least 38 complying memories were required for recombination2. We randomly recombined the memory components to form 78 recombined detail sets (conjunction lures), each consisting of a person, place and object, to be used in sessions two and three. Of these, half were partially recombined (with either the person, place or object component altered), and half fully recombined (where the person, place and object were taken from three different memories). Six additional conjunction lures were created for practice trials in sessions two and three.
Session Two: Conjunction lure exposure
Session two took place approximately 1–2 weeks after session one (M = 10 days, SD = 4), and was two hours in duration. Participants were presented with 60 conjunction lures, split evenly across an imagination and a control associative condition. The order in which participants completed the imagination and associative tasks, as well as the presentation order of person, place and object components, was counterbalanced. Four practice trials were completed to ensure that instructions were understood.
In the imagination condition, participants had 30 seconds for each conjunction lure to silently simulate a novel event occurring within the past 10 years, involving all three memory components. Each imagined scenario was rated by the participant for vividness and plausibility on a 5-point scale (1 = low, 5 = high). These ratings were followed by a written one sentence summary of the imagined event, to verify that a scenario had indeed been generated.
The associative condition was included to disentangle an effect of imagination inflation effect from that of increased retrieval fluency. In this condition participants processed conjunction lures without forming imagined events, by ranking the three components in order of highest to lowest subjective pleasantness (Gaesser, Spreng, McLelland, Addis, & Schacter, 2013). For example, if shown the details “Tracey, Pharmacy, Chocolate” a response might be “I find chocolate more pleasant than Tracey, and Tracey more pleasant than the pharmacy”. Participants completed this task silently, thinking about their decision for 30 seconds. As control ratings for those in the imagination condition, participants rated each pleasantness judgement for difficulty and average pleasantness on a 5-point scale (1 = low, 5 = high), and wrote a sentence indicating the order of pleasantness ranking.
While the conjunction lures were experimentally recombined in a way so as to avoid reconstructing a combination matching an authentic detail set from session one, there was the unavoidable possibility that a conjunction lure may have corresponded to a memory from the individual’s past that was not reported in session one. To eliminate the possibility of inadvertently classifying a true memory as a conjunction error in such cases, for each conjunction lure participants answered whether an event involving the three details together had actually occurred to them before. An average of 3.41 detail sets (M = 5.89%, SD = 4.96) per participant were excluded from subsequent analysis due to a “yes” response to this question. Exclusion of detail recombinations did not differ across imagination and associative conditions (F(1, 52) = 0.17, p = .68), nor across younger and older adults (F(1, 52) = 2.05, p = .16).
Session Three: Source memory recognition test and Autobiographical Interview
The third session was completed approximately one week after session two (M = 9 days, SD = 4), and took two hours to complete. Following nine practice trials, participants were presented with a total of 116 detail sets, corresponding to authentic memories (38), session two conjunction lures (60), and new conjunction lures that were not presented during session two (18). For each detail set, participants had 5 seconds to make a source judgement, deciding whether they believed the detail set belonged to a real event, a recombination seen in session two (regardless of imagination or associative condition), or a new recombination not previously seen. Button press responses were made for this decision, and were followed by a confidence rating on a 5-point scale (1 = low, 5 = high). The critical trials in this source test were those where the participant incorrectly recognized a conjunction lure as belonging to a real event, indicating an AM conjunction error was made.
Following the source test, an adapted version of the AI was conducted (Addis, Wong, & Schacter, 2008). An average of 12 detail sets were randomly selected based on responses in the source test: 5 correctly-identified real sets (real hits), 5 correctly-identified imagined sets (imagined hits), and all AM conjunction errors (M = 2.41, range = 0–7). For each detail set, participants had three minutes to verbally describe what they remembered about the associated event in as much detail as possible while being audio-recorded. Participants then rated the event for vividness, level of emotional response, and personal significance on a 5-point scale (1 = low, 5 = high), indicated what perspective the event was viewed from (first- or third-person), and were asked to again determine the source of the event.
Audio-recordings of event descriptions were later transcribed and scored according to the AI scoring protocol (Addis, Wong, & Schacter, 2008, adapted from Levine et al., 2002). Transcripts were segmented into distinct pieces of information each conveying a unique idea, which were further classified as either internal or external. Internal details were episodic details pertaining directly to the main event, while external details were those not part of, or specific to, the main event being described, such as semantic facts, metacognitive statements or descriptions of episodes outside the main event (for a scoring example, see Levine et al., 2002). Four raters blind to event condition and age group scored the AI transcripts. To establish inter-rater reliability, all raters scored a set of 20 recalled past and imagined future events obtained from a previous study (Addis et al., 2008). These scores were subjected to an intraclass correlation analysis, revealing that reliability across raters was acceptable (two-way mixed model; standardized Cronbach’s α: internal detail score .94; external detail score .86).
Session Four: Neuropsychological testing
Session four took place approximately two months after session three (M = 57 days, SD = 56), and took two hours to complete.
Three measures of relational memory were used; the selection of these tests was guided by the results of a factor analysis of measures in Glisky et al.’s (1995) neuropsychological test battery. For all memory tests the sum of immediate learning scores were used, because they provided the largest amount of variance in the younger adult group, and captured the ability to form associations between items in memory. These tests were: California Verbal Learning Test Second Edition (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2000, trials 1–5 free recall); Visual Paired Associates (PA) and Verbal PA hard list (Wechsler Memory Scale Revised; Wechsler, 1987, trials 1–3).
We selected five subtests from the Delis-Kaplan Executive Function System test battery (D-KEFS; Delis et al., 2001) that load onto an inhibition factor (Floyd, Bergeron, Hamilton, & Parra, 2010; Latzman & Markon, 2010; Li et al., 2015). The tests (and measures) were: Trail Making (ratio of the time to complete the number-letter switching task with completion time for the numbers-only task, where a lower ratio indicates better performance); Verbal Fluency Letter and Design Fluency Switching (number of words or figures generated in accordance with the set rules); Color-Word Interference (ratio of the time taken to name words in the inhibition condition with completion time for the simple color naming condition, where a lower ratio indicates better performance); and Tower Test (total achievement score).
To obtain reliable and stable measures of relational memory and inhibition abilities, raw scores for all neuropsychological tests were converted to z-scores and collapsed across tests to form separate composite scores of memory and inhibition (Glisky & Kong, 2008; Salthouse et al., 2003), whereby a lower score indicates lower functioning (see Table 1).
Statistical analyses
AM conjunction errors were calculated as a percentage of valid conjunction lure trials (valid trials were those given a “no” response to the question “has an event involving all three details occurred to you before?” in session two). The residual errors for conjunction error rates in all conditions (imagined/control/new) were right skewed (skewness < 2.68, kurtosis < 10.71). A log transform was used to normalize the data (Field, 2009), improving skewness (< 0.79) and kurtosis (> −1.30). For data comparing AM conjunction error rates, all statistical tests reported were computed using the transformed data and were analyzed using parametric tests, while means and standard deviations presented in tables and figures are based on the untransformed data3. Subjective ratings were analyzed using appropriate non-parametric tests. Pairwise comparisons (t-tests and Wilcoxon signed-rank tests) were considered significant if they exceeded the stated Bonferroni threshold (corrected for multiple comparisons).
To assess the influence of age and neuropsychological composite scores on memory accuracy and conjunction error rates, path analyses were carried out using Mplus 7.3 (Muthén & Muthén, 2012), with Maximum Likelihood Estimation using 5,000 bootstrapped re-samples. The dependent variable for one model was AM conjunction error rate, and for a second model was overall accuracy rate. Memory and inhibition composite scores were the independent variables of interest. Multiple group modelling was used to determine whether the effects of memory and inhibition on conjunction error rate differed with age. With 25 participants per group, we had over 10 observations for each parameter in our model, satisfying the recommended lower limit for path analysis (Kline, 2011). Diagnostic tests indicated that the models were not unduly biased by multicollinearity, as tolerance statistics for all variables were above the minimum value of .20 as recommended by Menard (1995).
Results
Conjunction error and accuracy rates
Overall, 39 of 54 participants (72.22%) made at least one AM conjunction error during the source test. Participants made, on average, 2.50 (SD = 2.91) conjunction errors, equating to 3.49% of the conjunction lures presented in session three (SD = 4.12). Older adults were more susceptible to AM conjunction errors than younger adults, making nearly three times more conjunction errors on average (t(52) = 2.90, p = .005, d = 0.79; see Table 2 for percentages). Overall, more older than younger adults made conjunction errors (76.92% of older, 67.86% of younger), yet a significant age difference in conjunction error rate still existed when comparing only those individuals who made conjunction errors (t(37) = 3.44, p = .001, d = 1.10). Subjective confidence in these errors did not differ across the age groups (Mdn younger = 3.75, older = 3.57, Mann-Whitney U = 189.00, p = .98, r = 0.00).
Table 2.
Mean percentage of trials resulting in autobiographical memory conjunction errors for younger and older adults, according to exposure condition.
| Exposure Condition | Younger adults | Older adults |
|---|---|---|
| Imagination | 2.66 (4.19) | 6.26 (6.03) |
| Associative | 1.39 (2.20) | 4.92 (6.37) |
| New | 1.43 (2.94) | 4.07 (6.23) |
| Total | 1.89 (2.12) | 5.22 (5.01) |
Note. Standard deviations are provided in parentheses.
The degree of recombination (partial/full) and the type of detail altered (person/place/object) did not differentially influence conjunction error rates of older relative to younger adults. Specifically, a 2×2 mixed analysis of variance (ANOVA) tested the influence of age on conjunction error rates for partially and fully recombined lures. A main effect of recombination degree was found, with more conjunction errors for partially recombined (M = 4.67%, SD = 5.44) compared with fully recombined lures (M = 2.36%, SD = 3.63; F(1, 52) = 15.69, p < .001, η2p = .23), replicating previous findings (Devitt et al., 2016). No interaction of recombination degree with age was found (F(1, 52) = 0.70, p = .41, η2p = .01). A 2×3 mixed ANOVA further revealed no effect of the type of detail altered, or interaction of detail type with age on conjunction error rates (p values > .39). Note that for these and all the following ANOVAs examining conjunction error rates, a significant main effect of age on conjunction error rate was found (p values < .05).
Accuracy on the source test was examined with a 2×2 mixed ANOVA on the percentage of correctly-identified authentic memories (real hits) and correctly-identified conjunction lures (conjunction hits), with age group as the between-subjects variable (younger/older). This analysis showed that older adults had fewer real hits (M younger = 89.57%, SD = 9.34; M older = 73.73%, SD = 15.25) and conjunction hits than younger adults (M younger = 56.79%, SD = 11.51; M older = 45.12%, SD = 11.78; F(1, 52) = 33.70, p < .001, η2p = .39). Both groups had more hits for real memories than conjunction lures (F(1, 52) =180.08, p < .001, η2p = 0.78). No interaction between age and hit type was observed, demonstrating that age differences in correct identification of conjunction lures was not more pronounced relative to authentic memories (F(1, 52) =.83, p = .37, η2p = 0.02). For younger adults, a negative correlation was observed between overall accuracy (percentage of correctly-identified trials) and conjunction error rate (r = −.39, 95%, CI = −.65, −.09, p = .04). No correlation was evident for the older group (r = .01, 95%, CI = −42, .57, p = .98), although the slopes did not significantly differ between the age groups (t(50) = 1.35, p = .18).
In the follow-up AI, approximately one third of the conjunction errors made in the source test were still considered to originate from an authentic memory, and the associated event was described by the participant (M = 32.37%, SD = 33.19). While older adults maintained fewer conjunction errors during the AI than younger adults, this difference was not significant (M younger = 40.79%, SD = 42.39; M older = 24.36%, SD = 19.03; t(24.70) = 1.55, p = .13, d = 0.42). There was, however, a trend for older adults to forget more conjunction error events in the AI than younger adults (i.e., to give an “I don’t remember” response rather than describe an event), suggestive of general age-related recall difficulties (M younger = 13.16%, SD = 28.64; M older = 32.45%, SD = 32.80; t(37) = 1.95, p = .06).
Effect of imagination
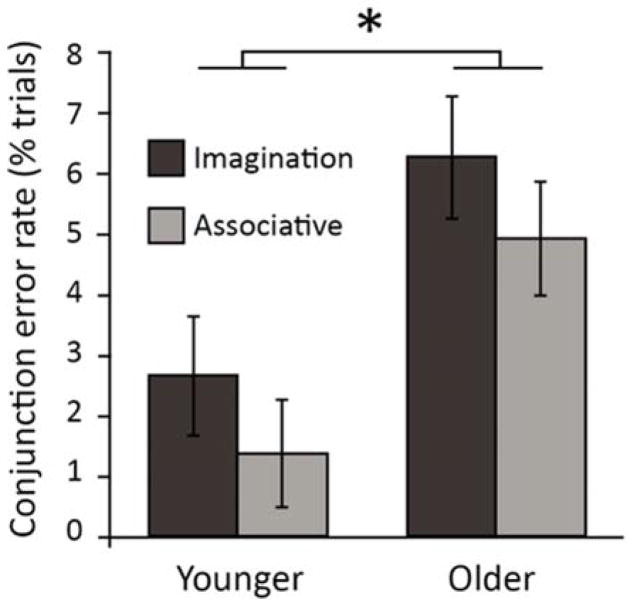
A 2×3 mixed ANOVA explored whether exposure to conjunction lures in session two increased errors above the baseline new condition, with age group as the between-subjects variable, and exposure condition as the within-subjects variable (imagination/associative/new). Because recombinations in the new condition could not be screened prior to the source test for those corresponding to unreported authentic events, comparing the uncorrected error rate of the new condition to the corrected error rate of the imagination and associative conditions (i.e., valid trials only) artificially inflates the number of conjunction errors made for new lures. Therefore, the uncorrected conjunction error rates of all conditions (without removal of invalid trials) was used for this comparison. A main effect of condition was found (F(2, 104) = 13.88, p < .001, η2p = .21). Pairwise comparisons revealed that more conjunction errors were made for lures seen in session two than for new lures, regardless of age, consistent with an effect of familiarity due to prior exposure (M imagination uncorrected = 6.92%, SD = 7.42; M associative uncorrected = 4.86%, SD = 6.52; M new = 2.70%, SD = 4.95; p < .001 and = .004 respectively). No interaction between age and condition was observed (F(1, 104) = .39, p = .68, η2p = .01).4
We then used a 2×2 mixed ANOVA, with age group and exposure condition (imagination/associative), to compare the influence of age on corrected conjunction error rates. Consistent with imagination inflation, more conjunction errors were observed for the imagination condition compared with the associative condition (although this effect was only trending; F(1, 52) = 3.09, p = .09, η2p = .06). Age had no influence on this effect (age x condition interaction F(1, 52) = 0.70, p = .41, η2p = .01; Table 2 and Figure 2).
Figure 2.
Autobiographical memory conjunction error rates (corrected) for younger and older adults, for conjunction lures presented in the imagination and associative conditions. Error bars reflect standard error of the mean. * = p < .05.
Phenomenological characteristics of conjunction errors
Subjective ratings of simulation quality during imagination (session two)
A Friedman’s ANOVA was run separately for each age group to explore differences in plausibility and vividness ratings made during imagination across the subsequent memory classifications of the source test: imagined hits (correctly-identified imagined trials), misses (imagined trials incorrectly judged as “new”) and conjunction errors (imagined events incorrectly judged as “real”). Subjective ratings of imaginations did not appear to strongly influence subsequent memory classification for either age group. For younger adults a main effect of memory condition was found for plausibility ratings (χ2(2) = 7.09, p = .03). Follow-up Wilcoxon signed-rank tests (Bonferroni-adjusted α = .017) indicated that hits were rated as more plausible than misses (Mdn hit = 2.33, Mdn miss = 2.00, T = 53.00, p = .002, r = −0.61), while no difference was found between conjunction errors (Mdn = 2.67) and hits or misses (T <17.00, p > .16). For older adults no difference in plausibility ratings was seen across the memory conditions (Mdn hit = 2.90, Mdn error = 3.84, Mdn miss = 3.21, χ2(2) = 2.09, p = .36). No significant effect of vividness ratings across subsequent memory conditions was evident for either age group (younger adults Mdn hit = 3.33, Mdn error = 3.33, Mdn miss = 3.20; older adults Mdn hit = 3.57, Mdn error = 3.50, Mdn miss = 3.55; χ2(2) < 3.49, p > .18).
Subjective ratings of memory quality during retrieval (session three)
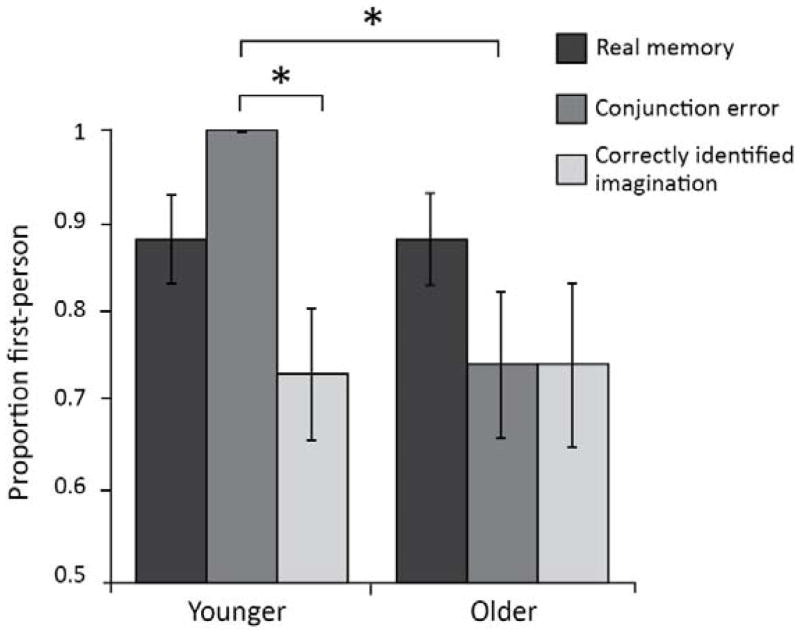
A 2×3 mixed ANOVA explored the influence of age on the proportion of trials viewed from a first-person perceptive during the AI across memory type (real hits/imagined hits/conjunction errors). A main effect of memory type on use of a first-person perspective (F(2, 68) = 4.88, p = .01, η2p = .13), as well as a significant interaction between age and memory condition was found (F(2, 68) = 4.11, p = .02, η2p = .11). Pairwise comparisons demonstrate that younger adults were more likely than older adults to view conjunction errors from a first-person perspective (p = .003). Furthermore, for younger adults only, conjunction errors were viewed from a first-person perspective more often than imagined hits (p = .004; Figure 3).
Figure 3.
Proportion of events recollected from a first-person perspective for younger and older adults, across memory retrieval type. Error bars reflect standard error of the mean. * = p < .05.
Differences in vividness, emotion and personal significance ratings during the AI were tested using a series of Friedman’s ANOVAs for each rating, with follow-up Wilcoxon signed-rank tests for significant effects (Bonferroni-adjusted α = .017). These tests were run separately for each age group, but because the overall pattern of significance did not differ between younger and older adults, collapsed results across age groups are reported here for brevity (see Table 3 for medians). For all three ratings, real hits were rated higher than imagined hits (T < 3.00, p < .001, r > −0.86). For vividness and emotion, real hits were rated higher than conjunction errors (T < 30.50, p < .017, r > −0.55), and no difference was found between conjunction errors and imagined hits (T > 39.00, p > .13, r < −0.35). However, for personal significance, no difference was found between conjunction errors and real (T > 27.00, p > .02, r < −0.55), or imagined hits (T > 20.00, p > .02, r < −0.54).
Table 3.
Mean and median ratings for subjective phenomenological qualities and objective Autobiographical Interview scores during the retrieval of events in session three.
| Event type | Phenomenological quality | |||||
|---|---|---|---|---|---|---|
| Subjective | Objective | |||||
|
|
|
|||||
| Vividnessaª | Emotionaª | Personal significanceaª | First-person perspectiveb | Internal details | External details | |
|
|
|
|||||
| Younger adults | ||||||
| Realc | 4.2 | 3.1 | 2.3 | 0.88 (0.21) | 29.97 (16.24) | 8.67 (5.13) |
| Conjunction errors | 3.83 | 2.00 | 1.00 | 1.00 (0.00) | 20.07 (14.14) | 11.13 (11.16) |
| Imaginedc | 3.00 | 1.73 | 1.37 | 0.73 (0.31) | 14.02 (7.05) | 7.84 (7.96) |
|
| ||||||
| Older adults | ||||||
| Realc | 4.45 | 3.50 | 3.45 | 0.88 (0.22) | 20.96 (7.92) | 19.89 (11.95) |
| Conjunction errors | 3.11 | 2.43 | 2.49 | 0.74 (0.35) | 12.41 (7.58) | 14.72 (10.71) |
| Imaginedc | 2.5 | 2.17 | 2.13 | 0.74 (0.39) | 7.67 (3.19) | 11.30 (5.10) |
Medians, rating scale ranges from 1 (low) to 5 (high).
Proportion events.
Correctly-identified. Standard deviations are provided in parentheses.
Objective scoring of memory quality during retrieval (session three)
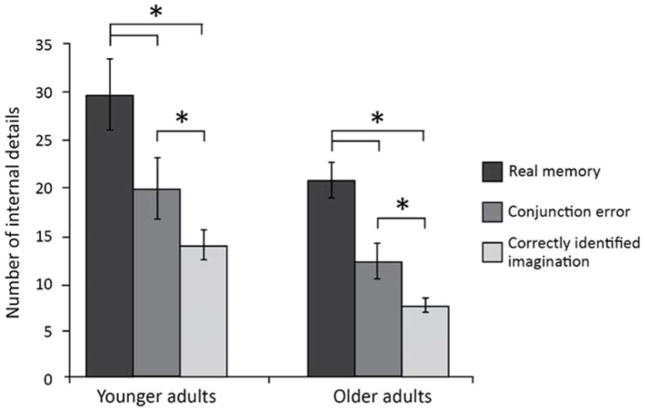
Using a 2×3 mixed ANOVA, we examined the influence of age on mean number of internal details generated in the AI according to memory type (real hits/imagined hits/conjunction errors; Table 3). Younger adults generated more internal details than older adults overall (F(1, 35) = 7.49, p = .01, η2p = .18). A main effect of condition was found (F(2, 70) = 37.24, p < .001, η2p = .52). Pairwise comparisons showed that descriptions of real hits contained more internal details than imagined hits and AM conjunction errors (p values < .001), while conjunction errors contained more details than imagined hits (p = .01; Figure 4). Similarly, a 2×3 mixed ANOVA was run for external details (Table 3); main effects of condition (F(2, 70) = 5.41, p = .01, η2p = .13) and group were found (F(1, 35) = 6.15, p = .02, η2p = .15), as well as a significant group by condition interaction (F(2, 70) = 4.57, p = .01, η2p = .12). Pairwise comparisons showed that whereas younger adults generated a similar amount of external details for real hits, conjunction errors and imagined hits (ps > .26), older adults generated more external details for real compared with imagined hits (p < .001). An age increase in external details was only observed for real hits (p = .001); no difference between younger and older adults was evident for conjunction errors or imagined hits (ps > .13).
Figure 4.
Average number of internal details generated in the adapted Autobiographical Interview for younger and older adults, across memory retrieval type. Error bars represent standard error of the mean. * = p < .05.
Relational memory and inhibition
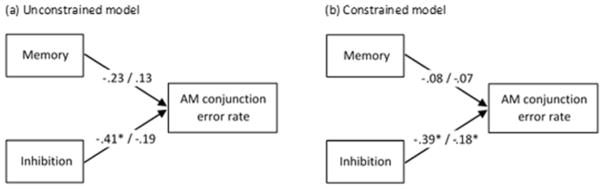
We examined the contributions of neuropsychological measures (relational memory and inhibition composite scores) to AM conjunction error rate using multiple group path analyses. Bivariate correlations of variables entered into the path analyses are presented in Table 4. We first estimated all parameters separately but simultaneously for younger and older adults (Table 5 and Figure 5a). The model was saturated (i.e., all possible relationships were estimated, no degrees of freedom remained), meaning model fit could not be determined. Nonetheless, the model revealed that inhibition ability significantly predicted conjunction error rate for younger, but not older adults, while memory ability did not predict conjunction error rate for either age group. To determine whether age influenced the relationship between the neuropsychological composite scores and conjunction error rate, we examined model fit after constraining the factor loadings to be equal across age groups. The model fit was good, using a minimum criterion of a CFI of .97, and a RMSEA of < .05 (Schermelleh-Engel, Moosbrugger, & Müller, 2003), χ2(2) = 1.68, p = .43, CFI = 1.00, RMSEA = .00. The model fit did not significantly differ from the first model (Δχ2(2) = 1.68, p = .43), indicating that the factor loadings did not differ between younger and older adults. In this constrained model, inhibition ability significantly predicted conjunction error rate for both age groups, while memory ability did not (Table 5 and Figure 5b).
Table 4.
Bivariate correlations for variables entered into path analyses predicting source accuracy and AM conjunction error rate for younger and older adults.
| Younger adults | Older adults | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| 1. AM conjunction error rate | – | – | ||||||
| 2. Source accuracy | −.41* | – | −.01 | – | ||||
| 3. Memory composite score | −.19 | .04 | – | .08 | .12 | – | ||
| 4. Inhibition composite score | −.39 | −.10+ | −.08 | – | −.16 | −.03 | .23 | – |
AM = Autobiographical memory.
p < .05,
p < .06
Table 5.
Unstandardized and standardized beta values for the path analyses predicting source accuracy and AM conjunction error rates.
| Younger adults | Older adults | Constrained model | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||
| B | SE | 95% CI | p | R2 | B | SE | 95% CI | p | R2 | B | SE | 95% CI | p | |
| AM conjunction error rate | .20 | .04 | ||||||||||||
| Memory | −1.29 | 1.17 | −3.43, 0.36 | .27 | 0.78 | 1.43 | −1.27, 3.43 | .59 | −0.44 | 0.80 | −1.70, 0.90 | .58 | ||
| Inhibition | −2.07* | 0.92 | −3.49, −0.48 | .03 | −2.04 | 1.66 | −4.79, 0.60 | .22 | −1.93* | 0.85 | −3.28, −0.53 | .02 | ||
| Source accuracy | .01 | .02 | ||||||||||||
| Memory | 0.79 | 5.26 | −6.31, 11.26 | .88 | 1.43 | 2.40 | −2.10, 5.96 | .55 | 1.37 | 2.12 | −1.81, 5.34 | .52 | ||
| Inhibition | −2.01 | 4.58 | −9.74, 5.11 | .66 | −1.15 | 4.46 | −7.17, 7.75 | .80 | −1.48 | 3.16 | −6.13, 4.24 | .64 | ||
AM = autobiographical memory.
p < .05
Figure 5.
Path analyses predicting autobiographical memory (AM) conjunction error susceptibility. (a) Factor loadings allowed to vary between younger and older adults. (b) Factor loadings constrained to be equal across age groups. The standardized coefficients for younger adults are presented on the left, with those for older adults on the right. * p < .05.
We also examined the contributions of memory and inhibition to accuracy of source judgements in session three (assessed as percentage of correctly-identified trials). The first model estimating all parameters separately for younger and older adults was saturated (i.e., all possible relationships were estimated, no degrees of freedom remained), meaning model fit could not be determined. Neither the memory nor inhibition composite scores contributed significantly to source accuracy for either age group (Table 5). To explore the influence of age on these relationships, factor loadings were constrained to be equal across age groups. The model fit was good, and did not significantly differ from the first model (χ2(2) = 0.04, p = .98, CFI = 1.00, RMSEA = .00; Δχ2(2) = 0.04, p = .98), indicating no difference in factor loadings across age groups. In this constrained model, accuracy was not predicted by either inhibition or memory (Table 5).
Because the path analyses indicated that inhibition predicted AM conjunction error rate, we further determined whether inhibition had a differential influence on error rates across exposure conditions, using a 2×2×3 mixed ANOVA, with inhibition ability (high/low, as determined by a median split within each age group separately) and age group as the between-subjects factors, and exposure condition (imagined/associative/new) as the within-subjects variable5. A main effect of inhibition ability was found, where those with poor inhibition made more conjunction errors overall (M poor = 4.77%, SD = 4.93, M good = 2.50%, SD = 2.78; F(1, 46) = 5.46, p = .02, η2p = .11). The interactions between inhibition, condition and age were not significant (F < 0.90, p > .88), suggesting that individuals with poor inhibition are more susceptible to AM conjunction errors regardless of condition or age.
Discussion
In the current study we evaluated the influence of healthy aging on susceptibility to conjunction errors in AM. In particular, we were interested in whether older adults were differentially vulnerable to the effects of imagination. To further determine the influence of aging on memory phenomenology, we collected measures of memory quality for authentic, conjunction error and correctly-identified imagined events, using subjective rating scales and objective scoring of autobiographical memories. We also explored the role of relational memory and inhibition ability on AM conjunction error susceptibility, as a means of identifying the cognitive mechanisms underpinning these errors.
A number of our findings are consistent with the notion of an age-related overreliance on familiarity in the absence of recollection when making source decisions for conjunction stimuli. Older adults exhibited an increased susceptibility to AM conjunction errors compared with younger adults, extending similar findings for laboratory conjunction stimuli to that for distinctive and personally-relevant autobiographical events (Castel & Craik, 2003; Jones & Jacoby, 2005; Kersten et al., 2008, 2013; Kersten & Earles, 2010; Kroll et al., 1996; Rubin et al., 1999). However, no differential increase in conjunction errors following imagination was observed for older adults, and age did not influence patterns of subjective and objective memory quality across authentic and conjunction events. While overall, the false acceptance rate of AM conjunction lures was higher for older than younger adults, considerable individual variation in error rates was observed within both age groups, which was accountable by differences in inhibitory ability, but not relational memory.
Aging and conjunction error susceptibility
Older adults were more prone than younger adults to making conjunction errors irrespective of prior exposure or imagination of a lure, suggestive of a general increased vulnerability to conjunction errors. An age-difference was also observed for lures presented in the new condition, which could indicate that the increased rate of conjunction error acceptance with age is simply due to a bias towards responding “real” (Gerlach, Dornblaser, & Schacter, 2014; Huh, Kramer, Gazzaley, & Delis, 2006; see also Kapucu, Rotello, Ready, & Seidl, 2008; Koutstaal, Schacter, Galluccio, & Stofer, 1999). However, because conjunction stimuli are being used, it is difficult to tease apart a general response bias from an influence of familiarity. While the recombinations of event components in the new condition are novel, the components themselves are familiar, and relying more on a sense of familiarity than explicit recollection to make source judgements may drive a greater tendency to misattribute previously unseen conjunction lures as corresponding to real events (Jones & Jacoby, 2005).
Regardless of age, more conjunction errors were made in the imagination and associative conditions relative to those in the new condition, consistent with a fluency effect (Garry & Wade, 2005; Jacoby & Dallas, 1981; Kurilla, 2011; Sharman, Garry, & Beuke, 2004; Sharman, Manning, & Garry, 2005). Although only a trend, lures for which an event was imagined were more likely to result in conjunction errors than those presented in the associative condition, in line with imagination inflation (Mazzoni & Memon, 2003; Thomas, Bulevich, & Loftus, 2003) and previous results with AM conjunction errors (Devitt et al., 2016). Yet contrary to our hypothesis, both older and younger adults exhibited the same degree of imagination inflation. Moreover, little evidence was found in the subjective and objective measures of memory quality for an age-related dedifferentiation of authentic and conjunction error memories (for corroborating results, see McGinnis & Roberts, 1996).
It is possible that for older adults, increased difficulties in simulating and retaining imagined events may have dampened the expected increase in imagination inflation. Healthy aging is associated with a reduction in the episodic richness of simulations (Addis, Musicaro, Pan, & Schacter, 2010; Addis et al., 2008), therefore older adults may have had difficulty imagining events that were detailed enough to be mistaken as an authentic memory. Previous studies finding an age difference in imagination inflation used relatively simplistic (and easily imaginable) action scenarios (e.g., McDaniel et al., 2008; Thomas & Bulevich, 2006), in which generation difficulty may have had less of an influence. Declines in the ability to retain simulations between the imagination and test phases may also have limited imagination inflation for older adults. Indeed, those older adults scoring below chance on the source test for the imagination condition, indicating poor memory for imagined events, made fewer conjunction errors to imagined lures (N = 11; M = 3.52%, SD = 4.69) than those above chance (N=15; M = 8.27%, SD = 6.25, t(24) = 2.11, p = .05, d = 0.57), despite subjective ratings of event quality at the time of imagination being equal. Thus deficits in remembering simulated events over time may have precluded the formation of memory conjunction errors in response to imagination for older adults.
Due to recollective declines, and/or time pressures in the source test, it is also likely that older adults based source decisions on feelings of familiarity rather than the phenomenological characteristics elicited by the lures, accounting for the generalized increase in conjunction error rate across both exposure conditions (Anderson et al., 2008; Craik & McDowd, 1987; Davidson & Glisky, 2002; Dennis et al., 2014; Jacoby, 1999; Jones & Jacoby, 2005; Prull et al., 2006). A reliance on familiarity during source decisions with age is further supported by the fact that older adults maintained fewer AM conjunction errors than younger adults during the AI. Those younger adults who were more accurate at determining source overall also made fewer conjunction errors, consistent with a recall-to-reject mechanism of source monitoring (Jones & Atchley, 2006; Jones & Jacoby, 2005; Lampinen, Odegard, & Neuschatz, 2004). Such an approach would allow rejection of a conjunction lure by recalling either the authentic detail combinations or the recombined detail sets encountered in session two. Interestingly, no relationship between overall source accuracy and conjunction error susceptibility was evident for older adults, perhaps indicative of an inability to use recall-to-reject processes (Jones & Jacoby, 2005).
The perspective taken during recall also suggests that older adults have difficulty in retrieving recollective information about conjunction errors. Younger adults were more likely to report viewing conjunction errors from a first-person (as opposed to a third-person) perspective than were older adults. Moreover, only younger adults viewed conjunction errors from a first-person perspective more often than imagined hits. Piolino and colleagues (2006) also report decreased use of a first-person perspective with age, and implicate deficits in memory recall to account for this finding; our results suggest this effect may be more pronounced when retrieving events that did not truly take place. Moreover, an age-related decrease in internal episodic information was found, replicating previous findings (Addis et al., 2010, 2008; Gaesser, Sacchetti, Addis, & Schacter, 2011; Levine et al., 2002) and extending these findings to conjunction memories.
Yet the fact that like younger adults, older adults were able to describe and maintain a proportion of the conjunction errors in the AI suggests at least some of these errors are associated with a sense of phenomenological recollection (see also Dijkstra & Misirlisoy, 2009). These recollected errors may occur due to excessive binding of the independent features across memory traces, as poorer associative binding ability has been previously linked with an increased rate of conjunction errors in both younger and older adults (Fandakova et al., 2013). Conjunction errors were rated as intermediate between authentic and imagined hits in personal significance during recall, suggesting that lures that were falsely recognized as real were more personally salient than those that were correctly rejected, and may also have had stronger associations with stored memory traces (Burt et al., 2004; Heaps & Nash, 2001; Johnson et al., 1988). Regardless of age, descriptions of conjunction error events also contained more internal details than correctly-identified imagined events. These findings implicate increases in conjunction error phenomenology in at least some source misattributions, for both younger and older adults (Johnson et al., 1993).
Relational memory and inhibition ability
We investigated whether individual variation in AM conjunction error rates could be accountable by differences in relational memory and inhibitory ability. Older adults were impaired relative to younger adults on composite measures of memory and inhibition ability, consistent with typical age-related declines (Balota et al., 2000; Buckner, 2004; Hedden & Gabrieli, 2004; Hoyer & Verhaeghen, 2006; Moscovitch & Winocur, 1995; Park et al., 2002; Raz et al., 2005; Tisserand et al., 2004; West, 1996).
For younger adults, only inhibition ability predicted the individual AM conjunction error rate, expanding upon similar findings exploring memory distortions for simple laboratory stimuli (Delbecq-Derouesné, Beauvois, & Shallice, 1990; Fandakova et al., 2013; Garoff-Eaton, Kensinger, & Schacter, 2007; Glisky et al., 1995; McCabe et al., 2009; Parkin et al., 1996; Plancher et al., 2009; Rapcsak, Reminger, Glisky, Kaszniak, & Comer, 1999; Rhodes & Kelley, 2005; Swick & Knight, 1999). The heightened susceptibility to conjunction errors following reductions in inhibition ability may arise due to a difficulty in suppressing the sense of familiarity piqued by the individual components comprising a conjunction lure at retrieval (Arndt & Jones, 2008; Jacoby, 1991; Jones & Jacoby, 2001), compounded by difficulties in recollecting the true source of a lure (Jones & Jacoby, 2005; Leding, 2015). Lowered inhibition ability may also result in deficits in controlling the spreading activation of related – though irrelevant – memory traces, contributing to hyper-binding of disparate memory components (Campbell et al., 2014; Fandakova et al., 2013; Reinitz & Hannigan, 2001). Future research is required to confirm whether one or both of these explanations regarding reduced inhibition are applicable to the formation of AM conjunction errors.
The relationship between inhibition and AM conjunction error rate was not significant when tested on older adults alone, however there was also no difference in this relationship between the age groups, and inhibition remained a significant predictor in the model where group differences were constrained to be equal. Therefore, the results are suggestive of a link between inhibition and conjunction error rates in older as well as younger adults. Previous research points towards a complex relationship between inhibition and memory distortion in older adults (Lövdén, 2003; Sommers & Huff, 2003). Moreover, inhibition is thought to comprise three functions – access, deletion and restraint – which may be reliant on separate mechanisms or neural substrates (Feyereisen & Charlot, 2008; Hasher et al., 1999; Lustig, Hasher, & Zacks, 2007). Accounting for these different aspects of inhibition could provide a clearer picture of the relationship between inhibition and memory distortion in older adults, though a recent attempt at such a fine grained approach did not find an independent contribution of inhibitory functions (Colombel, Tessoulin, Gilet, & Corson, 2016). Future research should disentangle the influence of healthy aging on the role of inhibitory control in memory distortions.
We hypothesized that reductions in relational memory would result in underutilization of recollective strategies and overreliance on familiarity in the source test (Anderson et al., 2008), conferring an increased susceptibility to AM conjunction errors; however, this hypothesis was not borne out. This is not the first report of a lack of relationship between memory functioning and vulnerability to memory distortions (see Balota et al., 1999; Glisky et al., 1995; McCabe et al., 2009). It is possible that the influence of relational memory on AM conjunction error susceptibility is dependent on executive processes. In order to determine whether the influence of memory on AM conjunction error susceptibility was moderated by inhibition ability, we re-ran the path analyses to include a memory by inhibition interaction term. The interaction was not significant, indicating that influence of relational memory abilities on AM conjunction error rates did not differ as a function of inhibition ability for either group (Bs < −0.50, SEs > 2.99, zs < −0.06, ps > .87). Perhaps higher-level cognitive resources (such as those regulated by the prefrontal cortex) are required to make fine-grained distinctions between small content changes in complex AMs, as occurs with AM conjunction errors, and thus relational memory may not have had as noticeable an impact on memory distortions of this type. Moreover, in older adults at least, source monitoring deficits attributable to medial temporal lobe-decline may have been balanced by the recruitment of compensatory frontal mechanisms (see Cabeza, 2002; Grady et al., 2003; Reuter-Lorenz & Cappell, 2008).
Neither relational memory nor inhibitory control were associated with overall source accuracy. It may be that the heterogeneity in the strategies that could be employed to successfully perform the source test masks an influence of these measures on source accuracy. For instance, correct source determination is possible with either a predominately recollective or familiarity strategy: one may explicitly recall a previous experience with the component combination (as self-generated in session one, or presented in session two); alternatively one might rely on familiarity with the detail combination. While a familiarity strategy would inflate rates of false alarms, hit rates would remain fairly comparable as when a recollective strategy is utilized.
Conclusions
In summary, this study adds to the literature of an age-related increase in conjunction errors for simple laboratory stimuli, demonstrating a similar effect for distinctive and personally-relevant AMs. Older adults were not differentially vulnerable to the inflating effects of imagination, suggestive of a generalized susceptibility to conjunction errors with age. Deficits in recollection with increasing age may have necessitated a dependency on error-prone familiarity mechanisms. The findings from the neuropsychological tests reveal that the individual variation in AM conjunction error vulnerability is attributable to declines in inhibitory capabilities, most strongly for younger adults. This lowered inhibition ability may manifest as an inability to suppress the cumulative familiarity of the individual AM components, and the erroneous binding of features due to spreading activation of related memory traces.
These results may have implications for situations in which memory authenticity is of high importance, such as in eyewitness testimony (Loftus, 2003). As many crime cases rely on eyewitness testimony due to a lack of forensic evidence (Zember, Brainerd, Reyna, & Kopko, 2012), it is imperative to an effective justice system to understand the conditions under which memory can become distorted. It has previously been demonstrated that older adults are more likely than young adults to report incorrect information in an eyewitness account (Aizpurua, Garcia-Bojos, & Migueles, 2009; Cohen & Faulkner, 1989). AM conjunction errors can contribute to flawed eyewitness accounts; for instance, spectators can conflate the perpetrator of a crime with innocent bystanders (Buckhout, 1975), a mistake we are more likely to make as we age (Kersten et al., 2008, 2013; Kersten & Earles, 2010). Our results indicate that witnesses with a poor ability to inhibit irrelevant familiarity signals are particularly vulnerable to such errors.
Acknowledgments
We would like to thank Rachael Sumner, Carolin Wickner, Carla Bautista and Reece Roberts for their assistance in scoring memory transcripts. This work was supported by a Rutherford Discovery Fellowship [grant number RDF-10-UOA-024] awarded to DRA and a National Institute on Aging grant [grant number R01 AG08441] awarded to DLS. ALD was supported by The University of Auckland Doctoral Scholarship and the HOPE Selwyn Foundation Scholarship in aging research.
Footnotes
This participant also showed signs of not engaging with the tasks. Removing her from the analyses did not change the pattern of results.
In Session One, one older adult managed to retrieve only 29 valid memories; for this participant the number of recombinations presented in sessions two and three were reduced (48 and 89 respectively). Removing this participant from the dataset did not alter the overall results, thus they were retained.
Analyses on transformed and untransformed data yielded the same pattern of results, so interpretations based on the raw means accurately reflect the findings derived from the transformed data.
For completion, we also ran this analysis on the corrected rate of conjunction errors for the imagination and associative control conditions. Again, a main effect of condition was evident (F(2, 104) = 3.76, p = .03, η2p = .07), and pairwise comparisons revealed that, even at the corrected level, more conjunction errors were made in the imagination condition relative to the new condition (p = .045).
Uncorrected rates of the imagined and associative conditions were used to compare against the new condition, for which invalid trials could not be removed prior.
References
- Addis DR, Musicaro R, Pan L, Schacter DL. Episodic simulation of past and future events in older adults: Evidence from an experimental recombination task. Psychology and Aging. 2010;25(2):369–376. doi: 10.1037/a0017280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Age-related changes in the episodic simulation of future events. Psychological Science. 2008;19:33–41. doi: 10.1111/j.1467-9280.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- Aizpurua A, Garcia-Bojos E, Migueles M. Memory for actions of an event: Older and younger adults compared. Journal of General Psychology. 2009;136:428–441. doi: 10.1080/00221300903269816. [DOI] [PubMed] [Google Scholar]
- Anderson ND, Ebert PL, Jennings JM, Grady CL, Cabeza R, Graham SJ. Recollection- and familiarity-based memory in healthy aging and amnestic mild cognitive impairment. Neuropsychology. 2008;22(2):177–187. doi: 10.1037/0894-4105.22.2.177. [DOI] [PubMed] [Google Scholar]
- Arndt J, Jones TC. Elaborative processing and conjunction errors in recognition memory. Memory & Cognition. 2008;36(5):899–912. doi: 10.3758/mc.36.5.899. [DOI] [PubMed] [Google Scholar]
- Balota DA, Cortese MJ, Duchek JM, Adams D, Roediger HL, McDermott KB, Yerys BE. Veridical and false memories in healthy older adults and in dementia of the Alzheimer’s type. Cognitive Neuropsychology. 1999;16:361–384. [Google Scholar]
- Balota DA, Dolan PO, Duchek JM. Memory changes in healthy older adults. In: Tulving E, editor. The Oxford handbook of memory. New York, NY, US: Oxford University Press; 2000. pp. 395–409. [Google Scholar]
- Bartlett FC. Remembering: A study in experimental and social psychology. Cambridge, England: Cambridge University Press; 1932. [Google Scholar]
- Berlingeri M, Danelli L, Bottini G, Sberna M, Paulesu E. Reassessing the HAROLD model: Is the hemispheric asymmetry reduction in older adults a special case of compensatory-related utilisation of neural circuits? Experimental Brain Research. 2013;224:393–410. doi: 10.1007/s00221-012-3319-x. [DOI] [PubMed] [Google Scholar]
- Biss RK, Campbell KL, Hasher L. Interference from previous distraction disrupts older adults’ memory. Journals of Gerontology - Series B Psychological Sciences and Social Sciences. 2013;68(4):558–561. doi: 10.1093/geronb/gbs074. [DOI] [PubMed] [Google Scholar]
- Buckhout R. Eyewitness testimony. Jurimetrics Journal. 1975;15(3):171–187. [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Budson AE, Sullivan AL, Mayer E, Daffner KR, Black PM, Schacter DL. Suppression of false recognition in Alzheimer’s disease and in patients with frontal lobe lesions. Brain. 2002;125(12):2750–2765. doi: 10.1093/brain/awf277. [DOI] [PubMed] [Google Scholar]
- Burt CDB, Kemp S, Conway MA. Memory for true and false autobiographical event descriptions. Memory. 2004;12:545–552. doi: 10.1080/09658210344000071. [DOI] [PubMed] [Google Scholar]
- Butler KM, McDaniel MA, Dornburg CC, Price AL, Roediger HL. Age differences in veridical and false recall are not inevitable: The role of frontal lobe function. Psychonomic Bulletin & Review. 2004;11(5):921–925. doi: 10.3758/bf03196722. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Hasher L, Thomas RC. Hyper-binding a unique age effect. Psychological Science. 2010;21:399–405. doi: 10.1177/0956797609359910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Trelle A, Hasher L. Hyper-binding across time: Age differences in the effect of temporal proximity on paired-associate learning. Journal of Experimental Psychology: Learning Memory and Cognition. 2014;40(1):300–305. doi: 10.1037/a0034109. [DOI] [PubMed] [Google Scholar]
- Castel AD. The adaptive and strategic use of memory by older adults: Evaluative processing and value-directed remembering. Psychology of Learning and Motivation. 2007;48:225–270. [Google Scholar]
- Castel AD, Craik FIM. The effects of aging and divided attention on memory for item and associative information. Psychology and Aging. 2003;18(4):873–885. doi: 10.1037/0882-7974.18.4.873. [DOI] [PubMed] [Google Scholar]
- Castel AD, Murayama K, Friedman MC, McGillivray S, Link I. Selecting valuable information to remember: Age-related differences and similarities in self-regulated learning. Psychology and Aging. 2013;28(1):232–242. doi: 10.1037/a0030678. [DOI] [PubMed] [Google Scholar]
- Chalfonte B, Johnson MK. Feature memory and binding in young and older adults. Memory & Cognition. 1996;24(4):403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Chan JCK, McDermott KB. The effects of frontal lobe functioning and age on veridical and false recall. Psychonomic Bulletin and Review. 2007;14:606–611. doi: 10.3758/bf03196809. [DOI] [PubMed] [Google Scholar]
- Cohen G, Faulkner D. Age differences in source forgetting: effects on reality monitoring and on eyewitness testimony. Psychology and Aging. 1989;4(1):10. doi: 10.1037//0882-7974.4.1.10. [DOI] [PubMed] [Google Scholar]
- Colombel F, Tessoulin M, Gilet AL, Corson YAI. False memories and normal aging: Links between inhibitory capacities and monitoring processes. Psychology and Aging. 2016;31(3):239–248. doi: 10.1037/pag0000086. [DOI] [PubMed] [Google Scholar]
- Craik FIM, McDowd JM. Age differences in recall and recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13(3):474–479. [Google Scholar]
- Davidson PSR, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cognitive, Affective, & Behavioral Neuroscience. 2002;2(2):174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- Delbecq-Derouesné J, Beauvois MF, Shallice T. Preserved recall versus impaired recognition. Brain. 1990;113(4):1045–1074. doi: 10.1093/brain/113.4.1045. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. CVLT-II. The Psychological Corporation; 2000. [Google Scholar]
- Dennis NA, Bowman CR, Peterson KM. Age-related differences in the neural correlates mediating false recollection. Neurobiology of Aging. 2014;35(2):395–407. doi: 10.1016/j.neurobiolaging.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Devitt AL, Monk-Fromont E, Schacter DL, Addis DR. Factors that influence the generation of autobiographical memory conjunction errors. Memory. 2016;24:204–222. doi: 10.1080/09658211.2014.998680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra K, Misirlisoy M. Recognition accuracy for original and altered verbal memory reports in older adults. The Quarterly Journal of Experimental Psychology. 2009;62:248–256. doi: 10.1080/17470210802303693. [DOI] [PubMed] [Google Scholar]
- Dodson CS, Bawa S, Krueger LE. Aging, metamemory, and high-confidence errors: A misrecollection account. Psychology and Aging. 2007;22:122–133. doi: 10.1037/0882-7974.22.1.122. [DOI] [PubMed] [Google Scholar]
- Dodson CS, Bawa S, Slotnick SD. Journal of Experimental Psychology: Learning, Memory, and Cognition. American Psychological Association; 2007. Aging, source memory, and misrecollections. Dodson, Chad S.: Department of Psychology, University of Virginia, P.O. Box 400400, 102 Gilmer Hall, Charlottesville, VA, US, 22904–4400 cdodson@virginia.edu. [DOI] [PubMed] [Google Scholar]
- Dodson CS, Schacter DL. Aging and strategic retrieval processes: Reducing false memories with a distinctiveness heuristic. Psychology and Aging. 2002;17(3):405–415. doi: 10.1037//0882-7974.17.3.405. [DOI] [PubMed] [Google Scholar]
- Duarte A, Graham KS, Henson RN. Age-related changes in neural activity associated with familiarity, recollection and false recognition. Neurobiology of Aging. 2010;31:1814–1830. doi: 10.1016/j.neurobiolaging.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Fandakova Y, Shing YL, Lindenberger U. High-confidence memory errors in old age: The roles of monitoring and binding processes. Memory. 2013;21:732–750. doi: 10.1080/09658211.2012.756038. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Hashtroudi S, Johnson MK. Age differences in using source-relevant cues. Psychology and Aging. 1992;7(3):443–452. doi: 10.1037//0882-7974.7.3.443. [DOI] [PubMed] [Google Scholar]
- Feyereisen P, Charlot V. Are There Uniform Age-Related Changes Across Tasks Involving Inhibitory Control Through Access, Deletion, and Restraint Functions? A Preliminary Investigation. Experimental Aging Research. 2008;34(4):392–418. doi: 10.1080/03610730802271880. [DOI] [PubMed] [Google Scholar]
- Field A. Discovering statistics using SPSS. Sage publications; 2009. [Google Scholar]
- Floyd RG, Bergeron R, Hamilton G, Parra GR. How do executive functions fit with the Cattell–Horn–Carroll model? Some evidence from a joint factor analysis of the Delis–Kaplan executive function system and the Woodcock–Johnson III tests of cognitive abilities. Psychology in the Schools. 2010;47(7):721–738. [Google Scholar]
- Gaesser B, Sacchetti DC, Addis DR, Schacter DL. Characterizing age-related changes in remembering the past and imagining the future. Psychology and Aging. 2011;26:80–84. doi: 10.1037/a0021054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser B, Spreng RN, McLelland VC, Addis DR, Schacter DL. Imagining the future: Evidence for a hippocampal contribution to constructive processing. Hippocampus. 2013;23(12) doi: 10.1002/hipo.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo DA, Bell D, Beier J, Schacter DL. Two types of recollection-based monitoring in younger and older adults: Recall-to-reject and the distinctiveness heuristic. Memory. 2006;14(6):730–741. doi: 10.1080/09658210600648506. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Korthauer LE, McDonough IM, Teshale S, Johnson EL. Age-related positivity effects and autobiographical memory detail: Evidence from a past/future source memory task. Memory. 2011;19(6):641–652. doi: 10.1080/09658211.2011.595723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff-Eaton RJ, Kensinger EA, Schacter DL. The neural correlates of conceptual and perceptual false recognition. Learning & Memory. 2007;14(10):684–692. doi: 10.1101/lm.695707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry M, Wade KA. Actually, a picture is worth less than 45 words: Narratives produce more false memories than photographs do. Psychonomic Bulletin & Review. 2005;12(2):359–366. doi: 10.3758/bf03196385. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8(10):1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gerlach KD, Dornblaser DW, Schacter DL. Adaptive constructive processes and memory accuracy: Consequences of counterfactual simulations in young and older adults. Memory. 2014;22(1):145–162. doi: 10.1080/09658211.2013.779381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello KS, Kensinger EA, Wong AT, Schacter DL. Age-related neural changes during memory conjunction errors. Journal of Cognitive Neuroscience. 2010;22:1348–1361. doi: 10.1162/jocn.2009.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL, Kong LL. Do young and older adults rely on different processes in source memory tasks? A neuropsychological study. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34(4):809. doi: 10.1037/0278-7393.34.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL, Polster MR, Routhieaux BC. Double dissociation between item and source memory. Neuropsychology. 1995;9:229–235. [Google Scholar]
- Glisky EL, Rubin SR, Davidson PSR. Source memory in older adults: An encoding or retrieval problem? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27(5):1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FIM. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13(5):572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FIM. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43(10):1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Ieuji Y, Federmeier KD. Event-related potentials reveal age differences in the encoding and recognition of scenes. Journal of Cognitive Neuroscience. 2007;19(7):1089–1103. doi: 10.1162/jocn.2007.19.7.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Welsh R, Hedden T, Bangert A, Minear M, Liu L, Park DC. Aging and the neural correlates of successful picture encoding: Frontal activations compensate for decreased medial-temporal activity. Journal of Cognitive Neuroscience. 2005;17(1):84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. The Journal of Neuroscience. 2006;26(32):8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks RT, May CP. Inhibitory control, circadian arousal, and age. In: Gopher D, Koriat A, editors. Attention and performance XVII: Cognitive regulation of performance: Interaction of theory and application. Cambridge, MA, US: The MIT Press; 1999. pp. 653–675. [Google Scholar]
- Heaps CM, Nash M. Comparing recollective experience in true and false autobiographical memories. Journal of Experimental Psychology: Learning, Memory and Cognition. 2001;27:920–930. doi: 10.1037//0278-7393.27.4.920. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Henkel LA, Johnson MK, De Leonardis DM. Aging and source monitoring: Cognitive processes and neuropsychological correlates. Journal of Experimental Psychology: General. 1998;127:251–268. doi: 10.1037//0096-3445.127.3.251. [DOI] [PubMed] [Google Scholar]
- Howe ML, Garner SR, Charlesworth M, Knott L. A brighter side to memory illusions: False memories prime children’s and adult’s insight-based problem solving. Journal of Experimental Child Psychology. 2011;108:383–393. doi: 10.1016/j.jecp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Hoyer WJ, Verhaeghen P. Memory aging. In: Birren J, Schaie W, editors. Handbook of the psychology of aging. Vol. 6. Burlington, MA: Elsevier Academic Press; 2006. pp. 209–232. [Google Scholar]
- Huh TJ, Kramer JH, Gazzaley A, Delis DC. Response bias and aging on a recognition memory task. Journal of the International Neuropsychological Society. 2006;12(1):1–7. doi: 10.1017/S1355617706060024. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Strauss E, Hunter MA, MacDonald SWS. Intraindividual variability, cognition, and aging. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. Vol. 3. New York, NY, US: Psychology Press; 2008. pp. 491–556. [Google Scholar]
- Intons-Peterson MJ, Rocchi P, West T, McLellan K, Hackney A. Age, testing at preferred or nonpreferred times (testing optimality), and false memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25(1):23–40. doi: 10.1037//0278-7393.25.1.23. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30(5):513–541. [Google Scholar]
- Jacoby LL. Ironic effects of repetition: measuring age-related differences in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25(1):3. doi: 10.1037//0278-7393.25.1.3. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Dallas M. On the relationship between autobiographical memory and perceptual learning. Journal of Experimental Psychology: General. 1981;110:306–340. doi: 10.1037//0096-3445.110.3.306. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Rhodes MG. False remembering in the aged. Current Directions in Psychological Science. 2006;15(2):49–53. [Google Scholar]
- Johnson MK, De Leonardis DM, Hashtroudi S, Ferguson SA. Aging and single versus multiple cues in source monitoring. Psychology and Aging. 1995;10(4):507–517. doi: 10.1037//0882-7974.10.4.507. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Foley MA, Suengas AG, Raye CL. Phenomenal characteristics of memories for perceived and imagined autobiographical events. Journal of Experimental Psychology: General. 1988;117(4):371–376. [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychological Bulletin. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL. Reality monitoring. Psychological Review. 1981;94:37–64. [Google Scholar]
- Jones TC, Atchley P. Conjunction errors, recollection-based rejections, and forgetting in a continuous recognition task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32(1):70–78. doi: 10.1037/0278-7393.32.1.70. [DOI] [PubMed] [Google Scholar]
- Jones TC, Jacoby LL. Feature and conjunction errors in recognition memory: Evidence for dual-process theory. Journal of Memory and Language. 2001;45(1):82–102. [Google Scholar]
- Jones TC, Jacoby LL. Conjunction errors in recognition memory: Modality-free errors for older adults but not for young adults. Acta Psychologica. 2005;120:55–73. doi: 10.1016/j.actpsy.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Kapucu A, Rotello CM, Ready RE, Seidl KN. Response bias in “remembering” emotional stimuli: A new perspective on age differences. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34(3):703–711. doi: 10.1037/0278-7393.34.3.703. [DOI] [PubMed] [Google Scholar]
- Karpel ME, Hower WJ, Toglia MP. Accuracy and qualities of real and suggested memories: Nonspecific age differences. Journal of Gerontology: Psychological Science. 2001;56:103–110. doi: 10.1093/geronb/56.2.p103. [DOI] [PubMed] [Google Scholar]
- Kersten AW, Earles JL. Effects of aging, distraction, and response pressure on the binding of actors and actions. Psychology and Aging. 2010;25(3):620–630. doi: 10.1037/a0019131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten AW, Earles JL, Curtayne ES, Lane JC. Adult age differences in binding actors and actions in memory for events. Memory & Cognition. 2008;36(1):119–131. doi: 10.3758/mc.36.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten AW, Earles JL, Upshaw C. False recollection of the role played by an actor in an event. Memory and Cognition. 2013;41:1144–1158. doi: 10.3758/s13421-013-0334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels RPC, Hobbel D, Postma A. Aging, context memory and binding: A comparison of “what, where and when” in young and older adults. International Journal of Neuroscience. 2007;117(6):795–810. doi: 10.1080/00207450600910218. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and Practice of Structural Equation Modeling. New York, NY: Guilford Press; 2011. [Google Scholar]
- Koutstaal W, Schacter DL, Galluccio L, Stofer KA. Reducing gist-based false recognition in older adults: Encoding and retrieval manipulations. Psychology and Aging. 1999;14(2):220–237. doi: 10.1037//0882-7974.14.2.220. [DOI] [PubMed] [Google Scholar]
- Kroll AEA, Knight RT, Metcalfe J, Wolf ES, Tulving E. Cohesion failure as a source of memory illusions. Journal of Memory and Language. 1996;35:176–196. [Google Scholar]
- Kurilla BP. Enhanced processing fluency leads to biases in source memory. The Quarterly Journal of Experimental Psychology. 2011;64:1609–1631. doi: 10.1080/17470218.2011.561866. [DOI] [PubMed] [Google Scholar]
- Lampinen JM, Odegard TN, Neuschatz JS. Robust recollection rejection in the memory conjunction paradigm. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30(2):332–342. doi: 10.1037/0278-7393.30.2.332. [DOI] [PubMed] [Google Scholar]
- Latzman RD, Markon KE. The factor structure and age-related factorial invariance of the Delis-Kaplan Executive Function System (D-KEFS) Assessment. 2010;17(2):172–184. doi: 10.1177/1073191109356254. [DOI] [PubMed] [Google Scholar]
- Leding JK. Memory conjunction clusters: Influence of familiarity and recollection. Memory. 2015:1–9. doi: 10.1080/09658211.2015.1051052. [DOI] [PubMed] [Google Scholar]
- Lee JLC. Reconsolidation: Maintaining memory relevance. Trends in Neuroscience. 2009;32(8):413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Brodeur M, Bourgouin P. Prefrontal cortex contribution to associative recognition memory in humans: an event-related functional magnetic resonance imaging study. Neuroscience Letters. 2003;346(1–2):73–76. doi: 10.1016/s0304-3940(03)00578-0. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay J, Winocur G, Moscovitch M. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology and Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- Li JJ, Chung TA, Vanyukov MM, Wood DS, Ferrell R, Clark DB. A hierarchical factor model of executive functions in adolescents: Evidence of gene-environment interplay. Journal of the International Neuropsychological Society. 2015;21(1):62–73. doi: 10.1017/S1355617714001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner I, Davidson PSR. False action memories in older adults: Relationship with executive functions? Aging, Neuropsychology, and Cognition. 2014;21(5):560–576. doi: 10.1080/13825585.2013.839026. [DOI] [PubMed] [Google Scholar]
- Loftus EF. Our changeable memories: Legal and practical implications. Nature Reviews Neuroscience. 2003;4:231–234. doi: 10.1038/nrn1054. [DOI] [PubMed] [Google Scholar]
- Lövdén M. The episodic memory and inhibition accounts of age-related increases in false memories: A consistency check. Journal of Memory and Language. 2003;49(2):268–283. [Google Scholar]
- Lustig C, Hasher L, Zacks RT. Inhibitory deficit theory: Recent developments in a “new view. Inhibition in Cognition. 2007;17:145–162. [Google Scholar]
- Lyle KB, Bloise SM, Johnson MK. Age-related binding deficits and the content of false memories. Psychology and Aging. 2006;21(1):86–95. doi: 10.1037/0882-7974.21.1.86. [DOI] [PubMed] [Google Scholar]
- Lyle KB, Johnson MK. Importing perceived features into false memories. Memory. 2006;14(2):197–213. doi: 10.1080/09658210544000060. [DOI] [PubMed] [Google Scholar]
- Madore KP, Addis DR, Schacter DL. Creativity and memory: Effects of an episodic-specificity induction on divergent thinking. Psychological Science. 2015;26:1461–1468. doi: 10.1177/0956797615591863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126(7):1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Mazzoni GA, Memon A. Imagination can create false autobiographical memories. Psychological Science. 2003;14:186–188. doi: 10.1046/j.1432-1327.1999.00020.x. [DOI] [PubMed] [Google Scholar]
- McCabe DP, Roediger HL, McDaniel MA, Balota DA. Aging reduced veridical remembering but increases false remembering: Neuropsychological test correlates of remember-know judgements. Neuropsychologia. 2009;47:2164–2173. doi: 10.1016/j.neuropsychologia.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, Lyle KB, Butler KM, Dornburg CC. Age-related deficits in reality monitoring of action memories. Psychology and Aging. 2008;23:646–656. doi: 10.1037/a0013083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough IM, Gallo DA. Autobiographical elaboration reduces memory distortion: Cognitive operations and the distinctiveness heuristic. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34(6):1430–1445. doi: 10.1037/a0013013. [DOI] [PubMed] [Google Scholar]
- McGinnis D, Roberts P. Qualitative characteristics of vivid memories attributed to real and imagined experiences. The American Journal of Psychology. 1996;109(1):59–77. [Google Scholar]
- Menard S. Applied Logistic Regression Analysis. Quantitative Applications in the Social Sciences, No. 106. Sage; London: 1995. [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: What have we learned from fMRI about the neural mechanisms of source memory? Psychological Bulletin. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D’Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Cognitive Brain Research. 2000;10:197–206. doi: 10.1016/s0926-6410(00)00029-x. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. Frontal lobes, memory, and aging. Annals of the New York Academy of Sciences. 1995;769(1):119–150. doi: 10.1111/j.1749-6632.1995.tb38135.x. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus. The Comprehensive Modelling Program for Applied Researchers: User’s Guide. 2012;5 [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(5):1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Odegard TN, Lampinen JM. Memory conjunction errors for autobiographical events: More than just familiarity. Memory. 2004;12:288–300. doi: 10.1080/09658210244000621. [DOI] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: A meta-analysis. Psychology and Aging. 2008;23(1):104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17(2):299–320. [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz PA. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin AJ, Bindschaedler C, Harsent L, Metzler C. Pathological false alarm rates following damage to the left frontal cortex. Brain and Cognition. 1996;32(1):14–27. doi: 10.1006/brcg.1996.0055. [DOI] [PubMed] [Google Scholar]
- Pertzov Y, Miller TD, Gorgoraptis N, Caine D, Schott JM, Butler C, Husain M. Binding deficits in memory following medial temporal lobe damage in patients with voltage-gated potassium channel complex antibody-associated limbic encephalitis. Brain. 2013;136(8):2474–2485. doi: 10.1093/brain/awt129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezdek K, Eddy RM. Imagination inflation: A statistical artifact of regression toward the mean. Memory & Cognition. 2001;29:707–718. doi: 10.3758/bf03200473. [DOI] [PubMed] [Google Scholar]
- Pierce BH, Simons JS, Schacter DL. Aging and the seven sins of memory. Advances in Cell Aging and Gerontology. 2004;15:1–40. [Google Scholar]
- Piolino P, Desgranges B, Clarys D, Guillery-Girard B, Taconnat L, Isingrini M, Eustache F. Autobiographical memory, autonoetic consciousness, and self-perspective in aging. Psychology and Aging. 2006;21(3):510–525. doi: 10.1037/0882-7974.21.3.510. [DOI] [PubMed] [Google Scholar]
- Plancher G, Guyard A, Nicolas S, Piolino P. Mechanisms underlying the production of false memories for famous people’s names in aging and Alzheimer’s disease. Neuropsychologia. 2009;47:2527–2536. doi: 10.1016/j.neuropsychologia.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Prull MW, Dawes LLC, Martin AM, III, Rosenberg HF, Light LL. Recollection and familiarity in recognition memory: Adult age differences and neuropsychological test correlates. Psychology and Aging. 2006;21(1):107–118. doi: 10.1037/0882-7974.21.1.107. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Reminger SL, Glisky EL, Kaszniak AW, Comer JF. Neuropsychological mechanisms of false facial recognition following frontal lobe damage. Cognitive Neuropsychology. 1999;16(3–5):267–292. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, … Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Reinitz MT, Hannigan SL. Effects of simultaneous stimulus presentation and attention switching on memory conjunction errors. Journal of Memory and Language. 2001;44:206–219. [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;17(3):177–182. [Google Scholar]
- Rhodes MG, Kelley CM. Executive processes, memory accuracy, and memory monitoring: An aging and individual difference analysis. Journal of Memory and Language. 2005;52(4):578–594. [Google Scholar]
- Rosen AC, Prull MW, O’Hara R, Race EA, Desmond JE, Glover GH, … Gabrieli JDE. Variable effects of aging on frontal lobe contributions to memory. NeuroReport. 2002;13(18):2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Rubin SR, Petten CV, Glisky EL, Newberg WM. Memory conjunction errors in younger and older adults: Event-related potential and neuropsychological data. Cognitive Neuropsychology. 1999;16:459–488. [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology: General. 2003;132(4):566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transactions of the Royal Society (B) 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Guerin SA, St Jacques PL. Memory distortion: An adaptive perspective. Trends in Cognitive Science. 2011;15:467–474. doi: 10.1016/j.tics.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Norman KA, Koutstaal W. The cognitive neuroscience of constructive memory. Annual Review of Psychology. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. Methods of Psychological Research Online. 2003;8(2):23–74. [Google Scholar]
- Sharman SJ, Garry M, Beuke CJ. Imagination or exposure causes imagination inflation. American Journal of Psychology. 2004;117:157–168. [PubMed] [Google Scholar]
- Sharman SJ, Manning CG, Garry M. Explain this: Explaining childhood events inflates confidence for those events. Applied Cognitive Psychology. 2005;19:67–74. [Google Scholar]
- Sommers MS, Huff LM. The Effects of Age and Dementia of the Alzheimer’s Type on Phonological False Memories. Psychology and Aging. 2003;18(4):791–806. doi: 10.1037/0882-7974.18.4.791. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Olm C, Schacter DL. Neural mechanisms of reactivation-inducing updating that enhance and distort memory. Proceedings of the National Academy of Sciences. 2013;110:19671–19678. doi: 10.1073/pnas.1319630110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Laurent M, Abdi H, Burianová H, Grady CL. Influence of aging on the neural correlates of autobiographical, episodic, and semantic memory retrieval. Journal of Cognitive Neuroscience. 2011;23(12):4150–4163. doi: 10.1162/jocn_a_00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Knight RT. Contributions of prefrontal cortex to recognition memory: Electrophysiological and behavioral evidence. Neuropsychology. 1999;13(2):155–170. doi: 10.1037//0894-4105.13.2.155. [DOI] [PubMed] [Google Scholar]
- Thomas AK, Bulevich JB. Effective cue utilization reduces memory errors in older adults. Psychology and Aging. 2006;21:379–389. doi: 10.1037/0882-7974.21.2.379. [DOI] [PubMed] [Google Scholar]
- Thomas AK, Bulevich JB, Loftus EF. Exploring the role of repetition and sensory elaboration in the imagination inflation effect. Memory & Cognition. 2003;31:630–640. doi: 10.3758/bf03196103. [DOI] [PubMed] [Google Scholar]
- Thomas AK, McDaniel MA. The interaction between frontal functioning and encoding processes in reducing false memories. Aging, Neuropsychology, and Cognition. 2013;20:443–470. doi: 10.1080/13825585.2012.736468. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MPJ, Pruessner JC, Hofman P, Evens AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cerebral Cortex. 2004;14:973–996. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- Umanath S, Marsh EJ. Aging and the memorial consequences of catching contradictions with prior knowledge. Psychology and Aging. 2012;27(4):1033–1038. doi: 10.1037/a0027242. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42(10):1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WMS-R: Wechsler memory scale-revised. Psychological Corporation; 1987. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120(2):272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Zember E, Brainerd CJ, Reyna VF, Kopko KA. The science of law and memory. In: Wethington D, Dunifon RE, editors. Research for the public good: Applying the methods of translational research to improve human health and well-being. Washington, DC: American Psychological Association; US; 2012. pp. 147–167. [Google Scholar]
- Zhu B, Chen C, Loftus EF, Lin C, He Q, Chen C, … Dong Q. Individual differences in false memory from misinformation: Cognitive factors. Memory. 2010;18(5):543–555. doi: 10.1080/09658211.2010.487051. [DOI] [PubMed] [Google Scholar]