Abstract
Left ventricular hypertrophy (LVH) predicts cardiovascular risk in hypertensive patients. We analyzed baseline/follow-up ECGs in 26,376 ALLHAT participants randomized to amlodipine (A), lisinopril (L), or chlorthalidone (C). Prevalent/incident LVH were examined using continuous and categorical classifications of Cornell voltage. At 2- and 4-years, prevalence of LVH in the C-group (5.57%; 6.14%) was not statistically different from A-group (2-years: 5.47%; p=0.806, 4-years: 6.54%; p=0.857), or L-group (2-years: 5.64%; p=0.857, 4-years: 6.50%; p=0.430). Incident LVH followed similarly, with no difference at 2-years for C (2.99%) compared to A (2.57%; p=0.173) or L (3.16%; p=0.605), and at 4-years (C=3.52%, A=3.29%, L=3.71%; p=0.521 C vs A, p=0.618 C vs L). Mean Cornell voltage decreased comparably across treatment groups (Δ baseline, 2-years = +3 to -27 μV, ANOVA p=0.8612; 4-years = +10 to -17 μV, ANOVA p=0.9692). We conclude that risk reductions associated with C treatment in secondary endpoints of ALLHAT cannot be attributed to differential improvements in ECG LVH.
Keywords: electrocardiography, left ventricular hypertrophy, amlodipine, lisinopril, chlorthalidone
Introduction
Electrocardiography (ECG) is a useful modality to identify the presence of left ventricular hypertrophy (LVH), a common manifestation of preclinical cardiovascular disease that predicts cardiovascular morbidity and mortality.1,2 ECG LVH is correlated with blood pressure control, and regression of LVH is associated with a reduction in the risk for cardiovascular events.3-5
Chlorthalidone, a long-acting thiazide diuretic, has demonstrated benefit in reducing cardiovascular events compared with other drugs in several major clinical trials, including the secondary endpoints of the Antihypertensive and Lipid-Lowering to Prevent Heart Attack Trial (ALLHAT).6 These findings occurred in spite of small differences in blood pressure reduction between groups which may not fully account for differences observed in clinical endpoints. In a retrospective analysis from another large study, the Multiple Risk Factor Intervention Trial (MRFIT), favorable reductions in ECG measures of LVH were observed in men prescribed chlorthalidone compared to those prescribed hydrochlorothiazide.7
The purpose of this study was to examine the prevalence and incidence of ECG LVH over time in ALLHAT participants and to determine whether these findings paralleled the blood pressure changes and major clinical endpoints reported in the main analysis of the trial. Further, we sought to determine whether the lower risk with chlorthalidone found in secondary endpoints of ALLHAT could be related to differential effects of the three treatment groups on ECG LVH.
Methods
Study Population
Details about ALLHAT and its principal findings have been extensively published and disseminated.6,8 Briefly, ALLHAT was a multicenter randomized, controlled trial in 42,418 high-risk hypertensive individuals aged 55 and older comparing the risk for cardiovascular and renal events (primary endpoint: fatal coronary heart disease or nonfatal myocardial infarction) with amlodipine, lisinopril, or doxazosin-based treatments, compared to chlorthalidone. Follow-up visits were conducted at intervals of 1, 3, 6, 9, and 12 months, followed by every 4 months thereafter.
For this analysis, all participants randomized to amlodipine, lisinopril, or chlorthalidone and with ECG data available at baseline, and either 2-, and/or 4-years of follow-up were included. Participants with LVH at baseline were included to allow for examination of regression of LVH over time.
ECGs from individuals randomized to doxazosin were excluded due to shorter follow-up since this arm of the study was terminated early after the finding of an increased risk of cardiovascular events, particularly heart failure.9 ECGs were also excluded if they had one of the following Minnesota codes (Online Supplemental Appendix Table 1): 7.1.× (complete left bundle branch block), 7.2.× (complete right bundle branch block), 7.4 (non-specific intraventricular conduction delay QRS ≥ 120ms), 7.8 (concomitant presence of 7.2 and 7.7 [left anterior fascicular block]), 6.6 (aberrant AV conduction which includes QRS ≥ 120ms as part of the definition), 6.4× (Wolff-Parkinson-White Syndrome), or 6.8 (pacemaker). These exclusions are based on ECG interpretation guidelines which recommend caution in interpreting ECG in the presence of major intraventricular conduction defects;10 in addition, LVH detection is suppressed in the presence of the exclusion codes listed.
ECG Coding and Ascertainment of LVH
The ALLHAT protocol called for standard 12-lead ECG measurements to be conducted at baseline, 2, and 4 year follow-up visits, recorded at clinical sites using standardized procedures. Individual ECG tracings were forwarded to the core ECG Reading Center (University of Minnesota, Minneapolis), where cross-sectional and serial coding of multiple variables was performed manually by reviewers blinded to treatment assignment. These readings were obtained from 1994-2002.
Current ECG interpretation guidelines do not advocate a preferred criteria set for assessing LVH, as long as an established criteria set is used and named explicitly.10 We determined LVH using Cornell voltage, defined as the sum of the voltages of the R wave in lead aVL and the S wave in lead V3 (i.e. RaVL + SV3 = Cornell Voltage, in μV). LVH was considered present when Cornell voltage exceeded 2200 μV (22 mm) in women and 2800 μV (28 mm) in men (where 1 mm = 100 μV).11,12 Cornell product, another common criteria set for examining LVH, was not calculated because the ALLHAT ECG dataset does not include other variables related to left ventricular mass such as QRS duration, QRS axis, PR-interval, and QT interval. Furthermore, Cornell voltage has shown higher diagnostic performance and similar prognostic significance as Cornell product,13 and since we excluded those with major conduction defects, the impact of QRS as part of the LVH (the advantage of Cornell product, if used) in the remaining sample would be minimal.
Given differences noted in prevalence of ECG LVH in women compared to men when using different ECG cutpoints, we ran a sensitivity analysis examining the prevalence of LVH at baseline in men compared to women, and using the original ALLHAT cutpoints and alternate cutpoints of 2000 μV (20 mm) in women and 2400 μV (24mm) in men, to determine whether sex-specific differences persisted. While prevalence of LVH was changed using the alternate cutpoints, the actual sex-specific differences remained similar regardless of cutpoint used. Therefore, our main results are reported using the original ALLHAT-defined cutpoints of 2200 μV (22 mm) in women and 2800 μV (28 mm) in men to remain consistent with previous ALLHAT publications, and findings using the alternate cutpoints are found in Supplemental Appendix Tables 3a-3c.
LVH incidence was defined as new LVH at 2 and/or 4 years in participants without evidence of LVH at baseline, while prevalence at baseline, 2 and 4 years included all cases with LVH at those time points. Regression of LVH occurred when reduction or disappearance of LVH was observed in those with evidence of LVH at baseline. In addition to a categorical/binary classification of LVH, Cornell voltage was also examined as a continuous variable which abrogates its dependence on the cut points selected to define LVH, and enhances its predictive ability for future cardiovascular mortality.3
Statistical Analysis
Baseline characteristics between the treatment groups were compared using chi-square and t-tests, for categorical and continuous variables, respectively. In keeping with the original a priori analysis plan of ALLHAT, the comparisons for LVH measures among treatment groups were initially pairwise (amlodipine vs chlorthalidone; lisinopril vs chlorthalidone).
Prevalence and incidence of LVH as a categorical variable at specific time points were compared across treatment groups using logistic regression, unadjusted and then adjusted for relevant variables found to predict Cornell voltage such as age, race, sex, systolic blood pressure, diastolic blood pressure, history of diabetes, current smoking, history of coronary heart disease, body mass index, estimated glomerular filtration rate, low density lipoprotein, high density lipoprotein, and triglycerides (a complete listing of variables is found in the Online Supplemental Appendix, Table 2).
No change in Cornell voltage was defined as within +/- 400 μV, as outlined by the Minnesota Code Manual of Electrocardiographic Findings.12 The proportion of participants, by treatment group, experiencing a decrease, no change, or increase in Cornell voltage over time was examined using chi-square. Paired t-tests were used to compare the mean Cornell voltage between baseline, year 2, and year 4 within treatment groups, while unpaired t-tests were used for the comparisons between groups. Linear regression, unadjusted and adjusted for the variables noted above, was used to compare changes in Cornell voltage across treatment groups over time. ANOVA for multiple groups was also performed.
Mean Cornell voltage was examined across subgroups of gender, age, race, body mass index, systolic blood pressure, smoking, ECG evidence of myocardial infarction, diabetes mellitus, and chronic kidney disease to examine for any subgroup heterogeneity. Additionally, a sensitivity analysis was performed on a common cohort (individuals with ECG data and no exclusion codes at all three time periods – baseline, 2-year, and 4-years).
Results
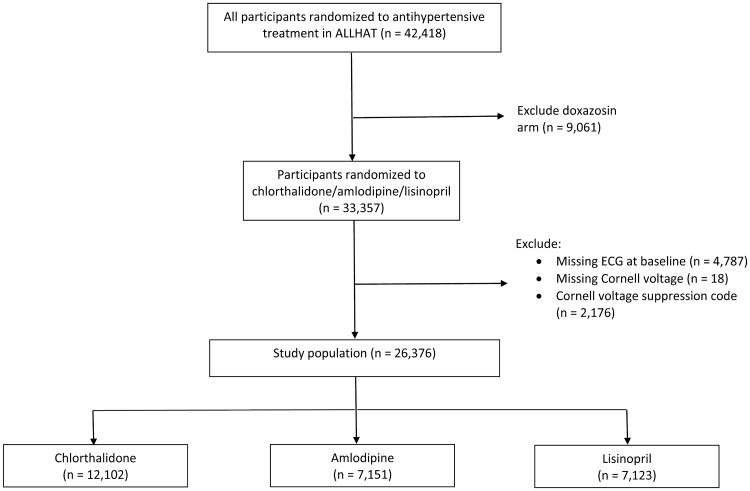
A total of 26,376 participants from ALLHAT were included in the analysis (Figure 1). The mean age of participants was approximately 67 years, and 34% reported black race. Most were receiving antihypertensives at baseline; systolic blood pressure averaged 146 mmHg across groups (145 mmHg for those treated and 156 mmHg for those untreated at baseline). Approximately 51% of participants had a past history of atherosclerotic cardiovascular disease. Eligibility risk factors and baseline characteristics were well matched across treatment groups, with no significant differences observed between treatment groups (Table 1).
Figure 1.
CONSORT diagram for study participants at baseline.
Table 1. Demographics of ALLHAT participants with ECG at baselinea.
| Characteristic | Chlorthalidone (n = 12,102) | Amlodipine (n = 7,151) | P (A vs C) | Lisinopril (n = 7,123) | P (L vs C) |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 66.71 (7.57) | 66.65 (7.58) | 0.5942 | 66.66 (7.60) | 0.6231 |
| Age range, years, mean (SD) | 0.638 (chi-square for all categories) | ||||
| 55-64 | 5,238 (43.28) | 3,120 (43.63) | 3,113 (43.70) | 0.569 | |
| ≥ 65 | 6,864 (56.72) | 4,031 (56.37) | 4,010 (56.30) | ||
| Race, n (%) | 0.729 (chi-square for all categories) | 0.724 | |||
| White, non-Hispanic | 6,018 (49.73) | 3,564 (49.84) | 3,540 (49.70) | ||
| Black, non-Hispanic | 3,772 (31.17) | 2,253 (31.51) | 2,262 (31.76) | ||
| White Hispanic | 1,383 (11.43) | 805 (11.26) | 785 (11.02) | ||
| Black Hispanic | 327 (2.70) | 202 (2.82) | 201 (2.82) | ||
| Other | 602 (4.97) | 327 (4.57) | 335 (4.70) | ||
| Women, n (%) | 5,578 (46.09) | 3,354 (46.90) | 0.276 | 3,272 (45.94) | 0.834 |
| Education, years, mean (SD) | 11.10 (4.02) | 11.09 (3.89) | 0.8615 | 11.09 (4.03) | 0.9069 |
| Receiving antihypertensive treatment, n (%) | 10,903 (90.09) | 6,443 (90.10) | 0.988 | 6,410 (89.99) | 0.819 |
| Blood pressure, mm Hg, mean (SD) | |||||
| Systolic | 146.21 (15.67) | 146.24 (15.59) | 0.8931 | 146.30 (15.54) | 0.7022 |
| Diastolic | 84.14 (10.03) | 84.16 (10.12) | 0.8888 | 84.22 (10.01) | 0.5903 |
| Treated at baseline | |||||
| Systolic | 145.13 (15.66) | 145.11 (15.61) | 0.9207 | 145.21 (15.50) | 0.7379 |
| Diastolic | 83.55 (9.96) | 83.53 (10.00) | 0.8933 | 83.67 (9.94) | 0.4421 |
| Untreated at baseline | |||||
| Systolic | 155.98 (12.04) | 156.52 (12.22) | 0.3401 | 156.02 (12.20) | 0.9417 |
| Diastolic | 89.49 (9.00) | 89.90 (9.35) | 0.3468 | 89.16 (9.28) | 0.4327 |
| Eligibility risk factors, n (%) | |||||
| Cigarette smoker | 2,713 (22.42) | 1,580 (22.09) | 0.603 | 1,572 (22.07) | 0.575 |
| Atherosclerotic CVD | 6,224 (51.43) | 3,625 (50.69) | 0.323 | 3,632 (50.99) | 0.556 |
| History of MI or stroke | 2,841 (23.48) | 1,641 (22.95) | 0.403 | 1,608 (22.57) | 0.153 |
| History of coronary revascularization | 1,587 (13.11) | 876 (12.25) | 0.083 | 936 (13.14) | 0.957 |
| Other atherosclerotic CVD | 2,753 (22.75) | 1,643 (22.98) | 0.716 | 1,628 (22.86) | 0.864 |
| Major ST depression or T-wave inversion | 1,285 (10.72) | 737 (10.41) | 0.496 | 756 (10.73) | 0.991 |
| Type 2 diabetes | 4,264 (35.23) | 2,542 (35.55) | 0.660 | 2,450 (34.40) | 0.239 |
| HDL-C < 35 mg/dl | 1,458 (12.05) | 842 (11.77) | 0.573 | 872 (12.24) | 0.690 |
| History of CHD at baseline, n (%) | 3,115 (25.95) | 1,718 (24.21) | 0.008 | 1,736 (24.58) | 0.036 |
| Body mass index, mean (SD) | 29.64 (6.01) | 29.89 (6.31) | 0.0065 | 29.77 (6.15) | 0.1539 |
| Current medication use, n (%) | |||||
| Aspirin | 4,374 (36.14) | 2,638 (36.89) | 0.376 | 2,609 (36.63) | 0.683 |
| Estrogen supplementation (women only) | 1,067 (19.13) | 628 (18.73) | 0.463 | 591 (18.06) | 0.296 |
| Lipid trial participants, n (%) | 3,093 (25.56) | 1,839 (25.72) | 0.807 | 1,786 (25.07) | 0.456 |
Included individuals must have the following: a baseline ECG, a valid Cornell voltage on baseline ECG, and no Cornell voltage suppression codes at baseline.
About 84-90% of participants randomized to chlorthalidone, amlodipine, or lisinopril and with ECG available at baseline and no exclusion codes, completed follow-up visits at 2-, and 4-years, with a majority also completing ECG at those visits (Table 2). The percentage of patients taking the blinded study drug (or the same class of drug) to which they were initially randomized remained at about 82%, 82%, and 76% for chlorthalidone, amlodipine, and lisinopril, respectively, at 4-years. Systolic blood pressure was slightly lower in the chlorthalidone group compared to the amlodipine and lisinopril groups, by about 1.1 and 2.7 mmHg at 2-years, and about 0.8 and 1.6 mmHg at 4-years, respectively.
Table 2. Expected and completed visits and antihypertensive medication use at annual visits in individuals with ECGs at baselinea.
| At 2 years | At 4 years | |
|---|---|---|
| Chlorthalidone, n (%) | 12,102 | 12,102 |
| Expected visits | 11,711 (96.8) | 10,049 (83.0) |
| Completed visits | 10,512 (89.8) | 8,729 (86.9) |
| ECG completed at visit | 8,739 (72.2) | 7,482 (61.8) |
| Receiving blinded study drug | 8,452 (80.4) | 6,745 (74.0) |
| Receiving blinded study drug or same class | 8,941 (85.1) | 7,432 (81.6) |
| Full crossovers | 682 (6.5) | 747 (8.2) |
| Partial crossovers | 606 (5.8) | 963 (10.6) |
| Receiving step 2 or 3 | 3,476 (33.1) | 3,541 (38.9) |
| Other antihypertensive medication | 472 (4.5) | 453 (5.0) |
| Number of antihypertensive medications, mean (SD) | 1.5 (0.8) | 1.7 (1.0) |
| Systolic blood pressure, mm Hg, mean (SD) | 135.83 (15.90) | 133.96 (15.71) |
| Diastolic blood pressure, mm Hg, mean (SD) | 78.42 (9.55) | 76.54 (9.62) |
| Amlodipine, n (%) | 7151 | 7151 |
| Expected visits | 6,918 (96.7) | 5,999 (83.9) |
| Completed visits | 6,149 (88.9) | 5,209 (86.8) |
| ECG completed at visit | 5,145 (71.9) | 4,413 (61.7) |
| Receiving blinded study drug | 4,965 (80.8) | 4,051 (74.6) |
| Receiving blinded study drug or same class | 5,301 (86.2) | 4,433 (81.6) |
| Full crossovers | 267 (4.3) | 312 (5.7) |
| Partial crossovers | 545 (8.9) | 729 (13.4) |
| Receiving step 2 or 3 | 2,036 (33.1) | 2,074 (38.2) |
| Other antihypertensive medication | 367 (6.0) | 433 (8.0) |
| Number of antihypertensive medications, mean (SD) | 1.6 (0.8) | 1.8 (1.0) |
| Systolic blood pressure, mm Hg, mean (SD) | 136.96 (14.79) | 134.73 (14.79) |
| Diastolic blood pressure, mm Hg, mean (SD) | 77.68 (9.59) | 75.74 (9.52) |
| Lisinopril, n (%) | 7123 | 7123 |
| Expected visits | 6,900 (96.9) | 5,916 (83.1) |
| Completed visits | 6,077 (88.1) | 4,994 (84.4) |
| ECG completed at visit | 4,932 (69.2) | 4,165 (58.5) |
| Receiving blinded study drug | 4,381 (72.1) | 3,409 (65.4) |
| Receiving blinded study drug or same class | 4,796 (78.9) | 3,937 (75.5) |
| Full crossovers | 318 (5.2) | 369 (7.1) |
| Partial crossovers | 531 (8.7) | 691 (13.3) |
| Receiving step 2 or 3 | 2,283 (37.6) | 2,180 (41.8) |
| Other antihypertensive medication | 682 (11.2) | 640 (12.3) |
| Number of antihypertensive medications, mean (SD) | 1.7 (1.0) | 1.9 (1.1) |
| Systolic blood pressure, mm Hg, mean (SD) | 138.53 (17.80) | 135.52 (17.20) |
| Diastolic blood pressure, mm Hg, mean (SD) | 78.76 (10.28) | 76.58 (10.33) |
Included individuals must have the following: a baseline ECG, a valid Cornell voltage on baseline ECG, and no Cornell voltage suppression codes at baseline
The prevalence of Cornell voltage-defined LVH, approximately 6.5%, was similar across the three treatment groups at baseline (Table 3a). At 2-, and 4-years of follow-up, the prevalence of LVH in the chlorthalidone group was 5.57% and 6.14%, and was not statistically different than the prevalence observed in amlodipine (2-years: 5.47%, p=0.806; 4-years: 6.54%, p=0.366) or lisinopril groups (2-years: 5.64%, p=0.857; 4-years: 6.5%, p=0.430). For those individuals with LVH at baseline, approximately half in each treatment group experienced regression of their LVH at 2-years (C: 52.69%, A: 51.90%, L: 51.04%; p>0.05 for all comparisons). These percentages were also similar at 4-years of follow-up (C: 52.96%, A: 50.21%, L: 49.27%; p>0.05 for all comparisons).
Table 3a. Prevalence of LVH by Cornell voltage at baseline, 2-years and 4-years a.
| Treatment group | Baseline, n (%) | 2-years, n (%) | 4-years, n (%) |
|---|---|---|---|
| Chlorthalidone | 783/12,102 (6.47) | 526/9,450 (5.57) | 488/7,947 (6.14) |
| Amlodipine | 482/7,151 (6.74) | 308/5,629 (5.47) | 311/4,753 (6.54) |
| Lisinopril | 473/7,123 (6.64) | 305/5,411 (5.64) | 292/4,494 (6.50) |
Included individuals must have the following: a valid Cornell voltage on ECG and no Cornell voltage suppression codes at the specific time point referenced
P=NS for comparisons of A vs C and L vs C at all time points
Incident LVH (Table 3b) followed similar trends and was not statistically different in those receiving chlorthalidone compared to amlodipine or lisinopril at 2-years (2.99%, 2.57%, and 3.16%, respectively; p=0.173 for C vs A and p=0.605 for C vs L) or at 4-years (3.52%, 3.29%, and 3.71%, respectively; p=0.521 for C vs A and p=0.618 for C vs L). After adjustment for multiple variables, the odds ratios for incident LVH by Cornell voltage at 2-years and 4-years were not statistically different for amlodipine or lisinopril, when compared to chlorthalidone as the reference group (Online Supplemental Appendix, Table 4).
Table 3b. Incidence of LVH by Cornell voltage at 2-years and 4-yearsb.
| Treatment Group | 2-years, n (%) | 4-years, n (%) |
|---|---|---|
| Chlorthalidone | 238/7957 (2.99) | 237/6731 (3.52) |
| Amlodipine | 120/4663 (2.57) | 130/3955 (3.29) |
| Lisinopril | 143/4530 (3.16) | 139/3743 (3.71) |
Included individuals must have the following: a valid Cornell voltage on ECG and no Cornell voltage suppression codes at baseline and at the specific time point referenced, and no LVH at baseline
P=NS for comparisons of A vs C and L vs C at all time points
Examining LVH using continuous measures in a common cohort (individuals with valid measurements for both time points), the mean Cornell voltage for the different treatment arms at baseline and follow-up is shown in Table 4. At baseline, the three treatment groups had similar values (ANOVA P=0.6647). At 2-years of follow-up, there were small changes in mean Cornell voltage in the three treatment groups (Δ from baseline = +3 to -28 μV; ANOVA P=0.8612) which remained not significantly different at 4 years of follow-up as well (ANOVA P=0.9692).
Table 4. Mean Cornell voltage for all treatment arms, by comparison group*.
| LVH by Cornell voltage, mean (SD) in μV, N | ||||
|---|---|---|---|---|
|
|
||||
| Treatment Group | Baseline | 2-years | Baseline | 4-years |
| All treatment arms combined | 1447 (632), 18,126 | 1432 (627), 18,126 | 1446 (625), 15,241 | 1444 (643), 15,241 |
| Chlorthalidone | 1448 (640), 8,403 | 1429 (628), 8,403 | 1446 (629), 7,103 | 1445 (642), 7,103 |
| Amlodipine | 1456 (632), 4,952 | 1428 (618), 4,952 | 1455 (625), 4,190 | 1438 (644), 4,190 |
| Lisinopril | 1437 (619), 4,771 | 1440 (634), 4,771 | 1437 (619), 3,948 | 1447 (643), 3,948 |
Included individuals must have the following: a valid Cornell voltage on ECG, no Cornell voltage suppression codes at the specific time point(s) referenced, and have these measurements for both time point(s) referenced i.e. the intersection of individuals at both time points 1 mm = 100 μV
When mean Cornell voltage was examined within individual treatment arms, amlodipine was associated with significant reductions from baseline to 2-years (-28 μV; p<0.001), and 4 years (-17 μV; p=0.02); chlorthalidone at 2-years (-19 μV; p=0.0002) but not 4-years (-1 μV; p=0.81), and lisinopril at neither time point (+3 μV; p=0.71, and +10 μV; p=0.18). Comparisons overall and within treatment groups for Cornell voltage measures demonstrated consistency across the various subgroupings of the relevant variables that predicted Cornell voltage, including gender, race, age group, BMI, systolic blood pressure, smoking status, ECG evidence of MI, diabetes, and chronic kidney disease (Online Supplemental Appendix, Tables 5a-5d).
In a further exploratory analysis, we observed that in each of the three randomized groups, the percentage of individuals who experienced a decrease in Cornell voltage progressively increased throughout the follow-up time period (Online Supplemental Appendix, Tables 6a and 6b). Examining this further in between-group comparisons revealed there was a significantly greater percentage with decreased Cornell voltage measurements from baseline to 2- and 4-years for participants prescribed amlodipine, compared to those prescribed lisinopril (2-years: 13.87% vs 12.91%, p=0.032; 4-years: 15.11% vs 14.03%, p=0.016). This same finding was noted for amlodipine compared to chlorthalidone at 4-years, but not 2-years (p=0.045 and 0.760, respectively). No differences in this pattern were observed between chlorthalidone and lisinopril at 2- or 4-years (p=0.07 and 0.646, respectively).
Discussion
In this analysis of ALLHAT participants, treatment with chlorthalidone, amlodipine, or lisinopril led to similar prevalence and incidence of Cornell voltage ECG LVH through 4-years of follow-up. This finding is consistent with the observed benefits of lowering blood pressure on LVH and the primary endpoint of ALLHAT (non-fatal MI or CHD death). It is also expected, given that all three antihypertensive treatments have each shown improvement in ECG LVH measures in other studies.1,7,14-17
Chlorthalidone, amlodipine, and lisinopril were all associated with small reductions in mean Cornell voltage over time that did not differ statistically between treatment groups. Given the secondary endpoints of ALLHAT which found that chlorthalidone-treated participants had lower risks of heart failure (vs. amlodipine, or lisinopril), stroke (vs. lisinopril, in blacks only), and combined cardiovascular disease (vs. lisinopril), we assumed the effect would be paralleled in ECG LVH measures favoring chlorthalidone over other treatments during the 4-year follow-up period. However, we largely observed no differences in the treatments, with the exception of a slightly more sustained reduction in mean Cornell voltage and a slightly greater percentage of participants experiencing decreases in Cornell voltage with amlodipine-based treatment. The clinical significance of these small changes in Cornell voltage may be questionable as they are within the reproducibility of measurements of Cornell voltage. Furthermore, Cornell voltage was only minimally reduced on average at 2- and 4-years of follow-up which does not seem to argue strongly for LVH as the mechanism driving the observed secondary clinical outcomes of ALLHAT.
Although mean clinic blood pressure differences between amlodipine and chlorthalidone-treated participants during follow-up in this analysis were small and nonsignificant, an alternate explanation for the minor trend favoring amlodipine in LVH measures may be in the variability of blood pressures across the groups. This potential variability would not have been detected in the absolute blood pressure differences measured clinically at office study visits. In a recent patient-level analysis of ALLHAT blood pressure data, visit-to-visit variability of systolic blood pressure was reportedly lower in amlodipine-treated subjects than other treatment groups.18 Compared to chlorthalidone-treated participants, those randomized to amlodipine had a 0.36 lower standard deviation of systolic blood pressure, and participants randomized to lisinopril had a 0.77 higher standard deviation. Both amlodipine and chlorthalidone have remarkably long half-lives, allowing them to maintain blood pressure lowering throughout 24-hour period as well as increase arterial compliance through vasodilation;19-21 pharmacologic properties which may facilitate their ability to lower systolic blood pressure variability relative to other antihypertensive classes such as angiotensin converting enzyme inhibitors which have been found to increase systolic blood pressure variability.22 Higher visit-to-visit variability in blood pressure is strongly associated with increased risk for cardiovascular events,23-25 suggesting the possibility that small differences in systolic blood pressure variability favoring amlodipine in ALLHAT may have led to the finding of a greater percentage of those participants experiencing decreases in Cornell voltage. Further investigation may help more fully explain the interrelationship between LVH, office blood pressure, visit-to-visit blood pressure variability, and clinical outcomes in ALLHAT.
Study Strengths and Limitations
Our analysis has strengths that include: the randomized study design employed in ALLHAT using three commonly used classes of antihypertensive medications, the large sample size and use of individual patient-level data, the inclusion of a high number of ethnic minorities, centralized coding of ECGs obtained across a large number of sites, and a very high completion rate of ECGs through 4-years of follow-up.
Limitations include that it is a secondary analysis of the data; given the many multivariate, subgroup, and interaction analyses performed, statistical significance at the 0.05 level should be interpreted with caution. While our final sample size remained large, the inclusion of ECGs missing at 2- and/or 4-years of follow-up in participants who had a baseline ECG could have altered the results. Another common debate is the use of ECG rather than echocardiogram to evaluate LVH. Although echocardiogram is a more sensitive measure of anatomical LVH, both methods predict mortality independently of each other and other cardiovascular risk factors.26 Furthermore, LVH detected by ECG has been shown to predict poor ocutome similar to LVH detected by imaging, and regression of LVH defined by ECG is associated with better prognosis.5,13,27-29 These findings, coupled with the wide availability of ECG, makes ECG-LVH a very practical tool to identify advanced clinical states, and predict improvement with therapy in patients with hypertension.
Supplementary Material
Highlights.
The comparative effects of chlorthalidone and other antihypertensives on ECG LVH are unknown.
ALLHAT data were analyzed to determine the changes in ECG LVH through 2 and 4-years of follow-up.
Chlorthalidone led to similar overall change in ECG Cornell voltage as amlodipine and lisinopril.
Chlorthalidone's benefit in ALLHAT cannot be attributed to a greater reduction in ECG LVH.
Acknowledgments
Sources of Funding: This study was supported by contracts NO1-HC-35130 and HHSN268201100036C with the National Heart, Lung, and Blood Institute. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial investigators acknowledge study medications contributed by Pfizer, Inc (amlodipine and doxazosin), AstraZeneca (atenolol and lisinopril), and Bristol-Myers Squibb (pravastatin), and financial support provided by Pfizer, Inc.
Footnotes
Conflict of Interest Statement: This study was supported by contracts NO1-HC-35130 and HHSN268201100036C with the National Heart, Lung, and Blood Institute. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial investigators acknowledge study medications contributed by Pfizer, Inc (amlodipine and doxazosin), AstraZeneca (atenolol and lisinopril), and Bristol-Myers Squibb (pravastatin), and financial support provided by Pfizer, Inc. William Cushman has received honoraria from Takeda. Dr. Oparil has received honoraria from Daiichi Sankyo and Novartis.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, National Institutes of Health, or Department of Health and Human Services.
Financial conflict of interest statement: Dr. Cushman has received honoraria from Takeda. Dr. Oparil has received honoraria from Daiichi Sankyo and Novartis. All other authors have no financial interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MacMahon S, Collins G, Rautaharju P, Cutler J, Neaton J, Prineas R, Crow R, Stamler J. Left ventricular hypertrophy and effects of antihypertensive drug therapy in hypertensive participants in the Multiple Risk Factor Intervention Trial. Am J Cardiol. 1989 Jan 15;63:202–10. doi: 10.1016/0002-9149(89)90286-5. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Salomon M, D'Agostino RB, et al. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation. 1994;90:1786–93. doi: 10.1161/01.cir.90.4.1786. [DOI] [PubMed] [Google Scholar]
- 3.Prineas RJ, Rautaharju PM, Grandits G, Crow R for the MRFIT Research Group. Independent risk for cardiovascular disease predicted by modified continuous score electrocardiographic criteria for 6-year incidence and regression of left ventricular hypertrophy among clinically disease free men: 16-year follow-up for the Multiple Risk Factor Intervention Trial. J Electrocardiol. 2001;34:91–101. doi: 10.1054/jelc.2001.23360. [DOI] [PubMed] [Google Scholar]
- 4.Okin PM, Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]
- 5.Verdecchia P, Staessen JA, Angeli F, et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomized trial. Lancet. 2009;374:525–533. doi: 10.1016/S0140-6736(09)61340-4. [DOI] [PubMed] [Google Scholar]
- 6.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 7.Ernst ME, Neaton JD, Grimm RH, Jr, et al. Long-term effects of chlorthalidone versus hydrochlorothiazide on electrocardiographic left ventricular hypertrophy in the Multiple Risk Factor Intervention Trial. Hypertension. 2011;58:1001–1007. doi: 10.1161/HYPERTENSIONAHA.111.181248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis BR, Cutler JA, Gordon DJ, et al. Rationale and design for the Antihypertensive and Lipid Lowering to Prevent Heart Attack Trial (ALLHAT) Am J Hypertens. 1996;9(Part 1):342–360. doi: 10.1016/0895-7061(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 9.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering to prevent heart attack trial (ALLHAT) JAMA. 2000;283:1967–1975. [PubMed] [Google Scholar]
- 10.Hancock EW, Deal BJ, Mirvis DM, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:992–1002. doi: 10.1016/j.jacc.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Casale PN, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 12.Prineas RJ, Crow RS, Zhang ZM. The Minnesota Code Manual of Electrocardiographic Findings. Second. Published by Springer; London: 2010. [Google Scholar]
- 13.Jain A, Tandri H, Dalal D, et al. Diagnostic and prognostic utility of electrocardiography for left ventricular hypertrophy defined by magnetic resonance imaging in relationship to ethnicity: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2010;159:652–658. doi: 10.1016/j.ahj.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the Hypertension Detection and Follow-up Program. Prevention and reversal of left ventricular hypertrophy with antihypertensive drug therapy. Hypertension. 1985;7:105–112. [PubMed] [Google Scholar]
- 15.Ofili EO, Cohen JD, St Vrain JA, et al. Effect of treatment of isolated systolic hypertension on left ventricular mass. JAMA. 1998;279:778–780. doi: 10.1001/jama.279.10.778. [DOI] [PubMed] [Google Scholar]
- 16.Skoularigis J, Strugo V, Weinberg J, Chopamba A, Chautsane Z, Lee A, Reddy K, Sareli P. Effects of amlodipine on 24-hour ambulatory blood pressure profiles, electrocardiographic monitoring, and left ventricular mass and function in black patients with very severe hypertension. J Clin Pharmacol. 1995;35:1052–1059. doi: 10.1002/j.1552-4604.1995.tb04025.x. [DOI] [PubMed] [Google Scholar]
- 17.Schmieder RE, Martus P, Klingbeil A. Reversal of left ventricular hypertrophy in essential hypertension. A meta-analysis of randomized double-blind studies. JAMA. 1996;275:1507–1513. [PubMed] [Google Scholar]
- 18.Muntner P, Levitan EB, Lynch AI, Simpson LM, Whittle J, Davis BR, Kostis JB, Whelton PK, Oparil S. Effect of chlorthalidone, amlodipine, and lisinopril on visit-to-visit variability of blood pressure: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. J Clin Hypertens (Greenwich) 2014;16:323–330. doi: 10.1111/jch.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernst ME, Moser M. Use of diuretics in patients with hypertension. N Engl J Med. 2009;361:2153–2164. doi: 10.1056/NEJMra0907219. [DOI] [PubMed] [Google Scholar]
- 20.Ichihara A, Kaneshiro Y, Sakoda M, et al. Add-on amlodipine improves arterial function and structure in hypertensive patients treated with an angiotensin receptor blocker. J Cardiovasc Pharmacol. 2007;49:161–166. doi: 10.1097/FJC.0b013e31803104e5. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Z, Zhu S, Liu D, et al. Thiazide-like diuretics attenuate agonist-induced vasoconstriction by calcium desensitization linked to Rho kinase. Hypertension. 2005;45:233–239. doi: 10.1161/01.HYP.0000152701.97426.5f. [DOI] [PubMed] [Google Scholar]
- 22.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 23.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 24.Muntner P, Shimbo D, Tonelli M, et al. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1998-1994. Hypertension. 2011;57:160–166. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 25.Muntner P, Whittle J, Lynch Al, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med. 2015;163:329–338. doi: 10.7326/M14-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundström J, Lind L, Ārnlöv J, Zethelius B, Andrén B, Lithell HO. Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001;103:2346–2351. doi: 10.1161/01.cir.103.19.2346. [DOI] [PubMed] [Google Scholar]
- 27.Rautaharju PM, LaCroix AZ, Savage DD, et al. Electrocardiographic estimate of left ventricular mass versus radiographic cardiac size and the risk of cardiovascular disease mortality in the epidemiologic follow-up study of the First National Health and Nutrition Examination Survey. Am J Cardiol. 1988;62:59–66. doi: 10.1016/0002-9149(88)91365-3. [DOI] [PubMed] [Google Scholar]
- 28.Havranek EP, Emsermann CD, Froshaug DN, et al. Thresholds in the relationship between mortality and left ventricular hypertrophy defined by electrocardiography. J Electrocardiol. 2008;41:342–350. doi: 10.1016/j.jelectrocard.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bacharova L, Estes H, Bang L, et al. The first statement of the Working Group on Electrocardiographic Diagnosis of Left Ventricular Hypertrophy. J Electrocardiol. 2010;43:197–199. doi: 10.1016/j.jelectrocard.2010.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.