Abstract
An enantioselective, copper-catalyzed alkynylation of cyclic α,α-diaryl ketiminium ions has been developed to deliver isoquinoline products with diaryl, tetrasubstituted stereocenters. The success of this reaction relied on identification of Ph-PyBox as the optimal ligand, i-Pr2NEt as the base, and CHCl3 as the solvent. Broad scope and functional group tolerance was observed. Notably, the use of both aryl and silyl acetylenes result in high yields and enantioselectivities. Mechanistic experiments are consistent with a dimeric or higher order catalyst.
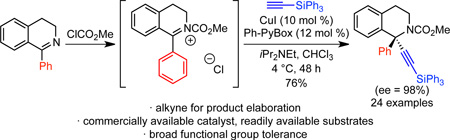
Graphical abstract
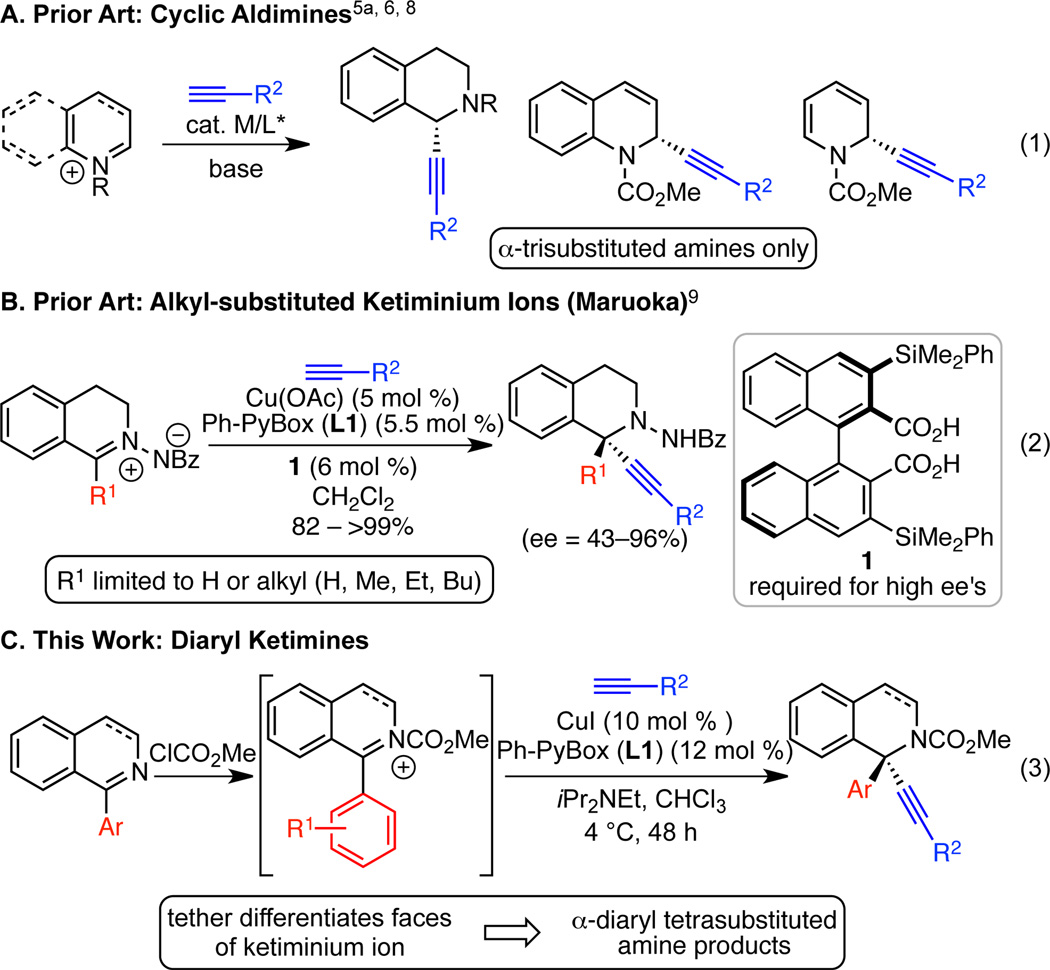
The prevalence of α-chiral cyclic amines in pharmaceuticals, natural products, and other bioactive molecules makes them enticing targets for synthesis.1 In particular, cyclic α-tetrasubstituted amines are found in molecules possessing activity against a range of diseases, including breast cancer, seizures, thrombosis, and human immunodeficiency virus (HIV).2 However, methods to prepare cyclic α-tetrasubstituted amines in high enantiopurity, a requirement for their biomedical use, are limited.3 A powerful approach to these compounds would be enantioselective addition to a ketimine or ketiminium ion. Most substrates for such additions have been limited to those with electronically and/or sterically different substituents on the electrophilic carbon.4 Enantioselective preparation of α-diaryl tetrasubstituted amines remains a challenge; only two classes of carbon nucleophiles (arenes, cyanide) have been delivered in high enantioselectivity to cyclic diaryl ketimines.5,6,7 With an eye towards enantioselective addition of a versatile carbon nucleophile to cyclic diaryl ketimines, we envisioned enantioselective, copper-catalyzed alkynylation may provide a useful solution. Although enantioselective alkynylations of a variety of cyclic aldimines and aldiminium ions to deliver α-trisubstituted amines are known (Scheme 1, eq 1),5a, 6, 8 only a single report of enantioselective alkynylation of a cyclic ketiminium ion exists. Maruoka has impressively shown that a Cu(Ph-PyBox)/Brønsted acid catalyst system enables alkynylation of alkyl-substituted isoquinolinium ions (Scheme 1, eq 2).9,4 However, no methods exist for enantioselective alkynylation of α,α-diaryl ketoimines or iminium ions with either cyclic or acyclic substrates. Given the similarity between Maruoka’s conditions and our enantioselective, copper-catalyzed alkynylation of cyclic diaryl oxocarbenium ions,10 we envisioned that an enantioselective alkynylation of diaryl iminium ions may enable synthesis of the challenging α-diaryl tetrasubstituted amine motif (Scheme 1, eq 3). Herein we report the successful development of this reaction, in which a tether enables differentiation of the faces of a diaryl ketiminium ion to allow synthesis of tetrahydroisoquinolines in excellent enantiomeric enrichment. This reaction relies on a commercially available copper precatalyst and chiral ligand, and boasts broad scope in the ketimine and alkyne. The alkyne provides a versatile functional group for further manipulation.
Scheme 1. Enantioselective, Metal-Catalyzed Alkynylations of Cyclic Iminium Ions.
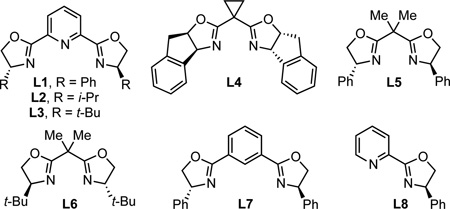
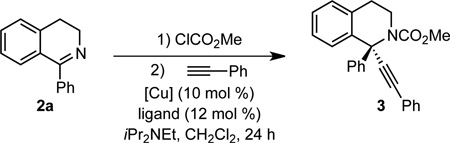
We began by examining the alkynylation of 1-phenyl dihydroisoquinoline 2a with phenyl acetylene. Dihydroisoquinoline 2a is easily prepared via a two-step procedure from commercially available materials.11 Following precedent with unsubstituted pyridines and isoquinolines,8h, 8l–n we acetylated in situ using methyl chloroformate to generate a ketiminium ion. We then added the mixture of ketiminium ion to the other reaction components (alkyne, copper salt, ligand, base). In the presence of an achiral copper(I) catalyst, alkynylation proceeded smoothly to give up to 81% yield (Table 1, entries 1–3). When CuI was stirred with Ph-PyBox L1 for 30 min before addition of the other components, 85% yield and 37% ee were observed (Table 1, entry 4). By lowering the temperature to 4 °C, the ee was increased to 53%, albeit with a drop in yield (Table 1, entry 5). Other pyridine (bis)oxazoline and oxazoline ligands L2–L6 provided no improvement in enantioselectivity (entries 6–10). In addition, the use of alternative bases, including MTBD, the optimal base for the alkynylation of diaryl oxocarbenium ions, also resulted in lower ee (Table 1, entries 11–12). However, by changing the solvent to CHCl3, a dramatic increase in enantioselectivity was observed (Table 1, entry 13).12 The enantioselectivity was increased further by reducing the temperature to −20 °C, but at the cost of yield (Table 1, entry 14). Decreasing the concentration was a more effective improvement, leading to 81% yield and 91% ee (Table, 1 entry 15). Under these conditions, ClCO2Me can be replaced by ClCO2Bn to give product in 64% isolated yield and 95% ee (Table 1, entry 16). By decreasing the concentration further ([2a] = 0.05 M), we achieved high yield and ee without preforming the ketiminium ion in a separate reaction vessel (Table 1, entry 17). Under these conditions, replacing CuI with either CuBr or CuCl resulted in a dramatic decrease in ee (Table 1, entries 18, 19). This result is particularly surprising with CuCl, given that chloride is present due to the acylation with methyl chloroformate.
Table 1.
Optimization of Alkynylation.a
 | |||||
|---|---|---|---|---|---|
| entry | [Cu] | ligand | temp (°C) |
yield (%) |
ee (%) |
| 1 | CuI | - | rt | 81 | - |
| 2 | Cu(MeCN)4PF6 | - | rt | 65 | - |
| 3 | CuSPh | - | rt | 50 | - |
| 4 | CuI | L1 | rt | 85 | 37 |
| 5 | CuI | L1 | 4 | 65 | 53 |
| 6 | CuI | L2 | 4 | 89 | 20 |
| 7 | CuI | L3 | 4 | 75 | 10 |
| 8 | CuI | L4 | 4 | 39 | 11 |
| 9 | CuI | L5 | 4 | 61 | 0 |
| 10 | CuI | L6 | 4 | 76 | 0 |
| 11b | CuI | L1 | 4 | 72 | 19 |
| 12c | CuI | L1 | 4 | 60 | 0 |
| 13d | CuI | L1 | 4 | 80 | 89 |
| 14d | CuI | L1 | −20 | 77 | 92 |
| 15d,e | CuI | L1 | 4 | 81 | 91 |
| 16 d,e,f | CuI | L1 | 4 | (64) | 95 |
| 17d,g | CuI | L1 | 4 | 95 | 91 |
| 18 d,g | CuBr | L1 | 4 | 95 | 35 |
| 19 d,g | CuCl | L1 | 4 | 91 | 33 |
Conditions: Ketimine 2a (0.1 mmol), [Cu] (10 mol %), ligand (12 mol %), phenylacetylene (1.2 equiv), ClCO2Me (1.0 equiv), iPr2NEt (1.5 equiv), CH2Cl2 (0.15 M), 24 h, unless otherwise noted. Yields determined by 1H NMR with 1,3,5-trimethoxybenzene as internal standard. Ee’s determined by HPLC using a chiral stationary phase.
Et3N as base.
MTBD (7-methyl-1,5,7-triazabiocyclo[4.4.0]dec-5-ene) as base.
CHCl3 as solvent.
[2a] = 0.1 M.
ClCO2Bn in place of ClCO2Me. Isolated yield in parentheses.
[2a] = 0.05 M. Iminium ion was not pre-formed; ClCO2Me added last.
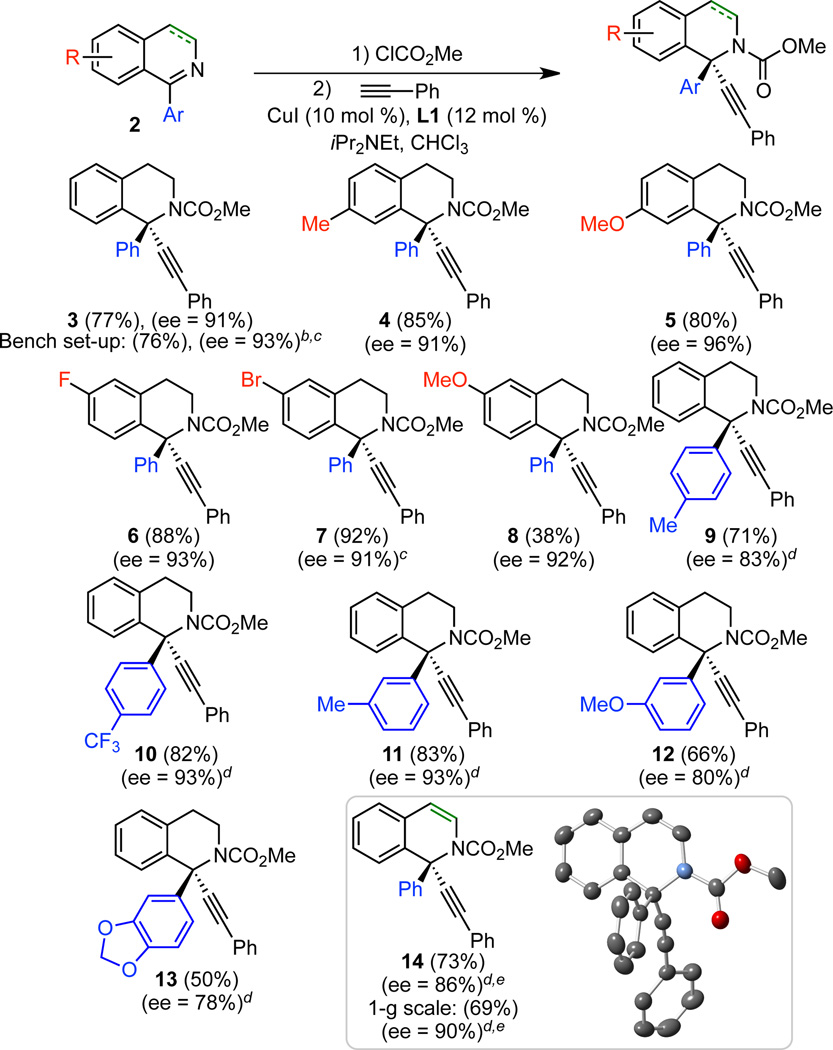
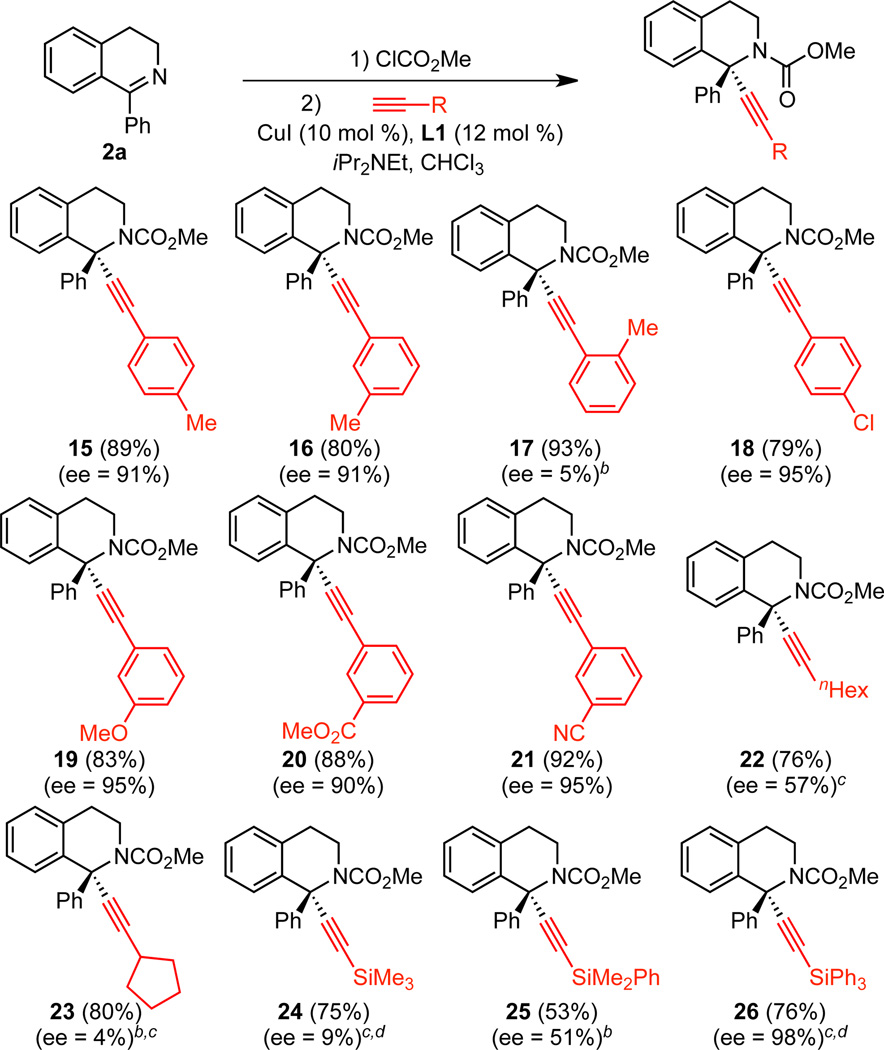
Under the optimized conditions (Table 1, entry 15), a broad range of 1-arylisoquinolines undergo enantioselective alkynylation (Scheme 2). Alkynylation of 2a proceeded effectively, giving 77% isolated yield and 91% ee. If this reaction is set up on the benchtop, instead of the glovebox, similar yield and ee are observed. Both electron-donating and electron-withdrawing groups were tolerated on the isoquinoline backbone (4–8), including an aryl bromide, which offers a useful handle for elaboration (7). The alkynylation also proceeded effectively for substrates with substitution on the 1-phenyl group (9–13). In some cases, reducing the concentration to 0.05 M led to increased enantioselectivity. The aromatic isoquinoline can also be employed to give product 14. As expected, the reversible acylation of isoquinoline was less favorable than for the dihydroisoquinolines, initially leading to lower yields with our original procedure. However, by following a one-pot procedure, in which methyl chloroformate is added last, and by using a reduced concentration ([2] = 0.05 M), 69% yield and 90% ee were obtained in a 1-mmol scale reaction. The crystal structure of 3 indicates that the copper acetylide adds to the re face.13 Interestingly, in the analogous alkynylation of 1-aryl isochroman oxocarbenium ions, addition to the si face is dominant.10 We also examined 1-methyl isoquinoline. However, no reaction was observed, highlighting the benefit of Maruoka’s conditions for 1-alkyl isoquinolines.9a
Scheme 2. Scope in isoqinoline. a.
a Conditions: 2 (0.3 mmol), ClCO2Me (1.0 equiv), alkyne (1.2 equiv), CuI (10 mol %), L1 (12 mol %), iPr2NEt (1.5 equiv), CHCl3 (0.1 M), 4 °C, 48 h. Average isolated yields (±5%) and ee's (±2%) of duplicate experiments, unless noted otherwise. b Set up outside glovebox. c Single experiment. d [2] = 0.05 M. e Iminium ion was not preformed; ClCO2Me added last.
A range of alkynes can also be used (Scheme 3). Both para and meta substitution of aryl acetylenes are tolerated (15, 16). However, o-methyl phenylacetylene resulted in nearly racemic product, albeit excellent yield (17). Alkynes with aryl chloride 18, ether 19, ester 20, and nitrile 21 groups participated effectively. For alkyl acetylenes, high yields, but low enantioselectivities, were observed, with lower ee’s when a branched alkyl group is used (22, 23). We also examined silyl acetylenes. We observed only 9% ee in the addition of trimethylsilyl acetylene 24. However, by increasing the phenyl groups on silicon, steadily higher ee’s were seen. Triphenylsilyl acetylene 26 was formed in good yield and 98% ee. A rationale for the low ee’s observed with alkyl and trimethylsilyl acetylenes currently eludes us. One possibility is that these alkynes or their decomposition products (e.g., allenes for alkyl acetylenes) react with the copper catalyst to generate a less enantioselective catalyst. However, addition of trimethylsilylacetylene, 1-octyne, or 1,2-octadiene to the model reaction (addition of phenyl acetylene to 2a) resulted in only minor decreases in yield and ee.11 This vague outcome suggests further studies are necessary to understand the low enantioselectivity observed with these substrates.
Scheme 3. Scope in alkyne. a.
a Conditions: 2a (0.3 mmol), ClCO2Me (1.0 equiv), alkyne (1.2 equiv), CuI (10 mol %), L1 (12 mol %), iPr2NEt (1.5 equiv), CHCl3 (0.1 M), 4 °C, 48 h. Average isolated yields (±6%) and ee's (± 1%) of duplicate experiments, unless noted otherwise. b Single experiment. c [2a] = 0.05 M. d Iminium ion was not pre-formed; ClCO2Me added last.
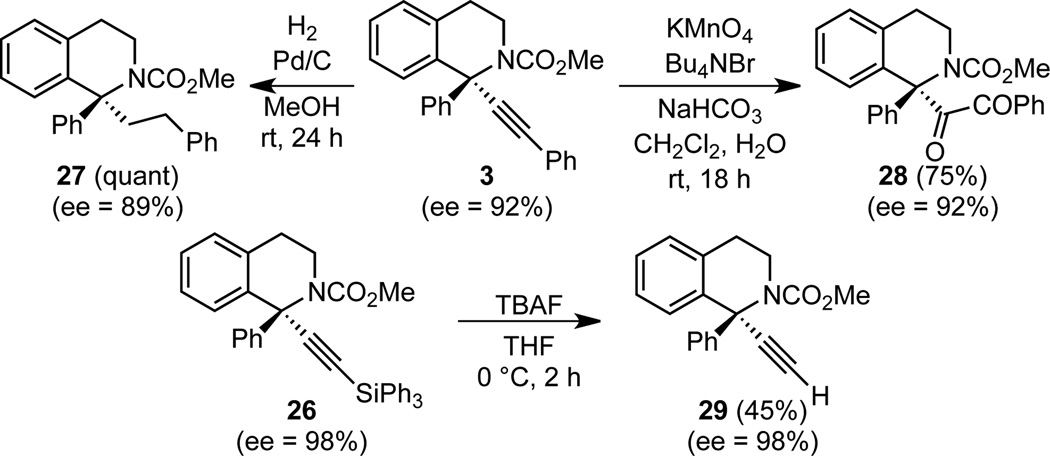
To demonstrate the utility of the alkyne, we performed several elaborations (Scheme 4). Alkyne 3 was reduced to (S)-27 in quantitative yield with only a slight decrease in ee. Hydrogenation of 14 also led to (S)-27, showing that the re face of the iminium ion is attacked for both dihyroisoquinolines and aromatic isoquinolines.11 The alkyne can also be oxidized to diketone 28 in good yield and 100% conservation of ee. Deprotection of triphenylsilane 26 proceeded in an unoptimized 45% yield, again with no loss in enantiomeric enrichment.
Scheme 4. Elaboration of products.
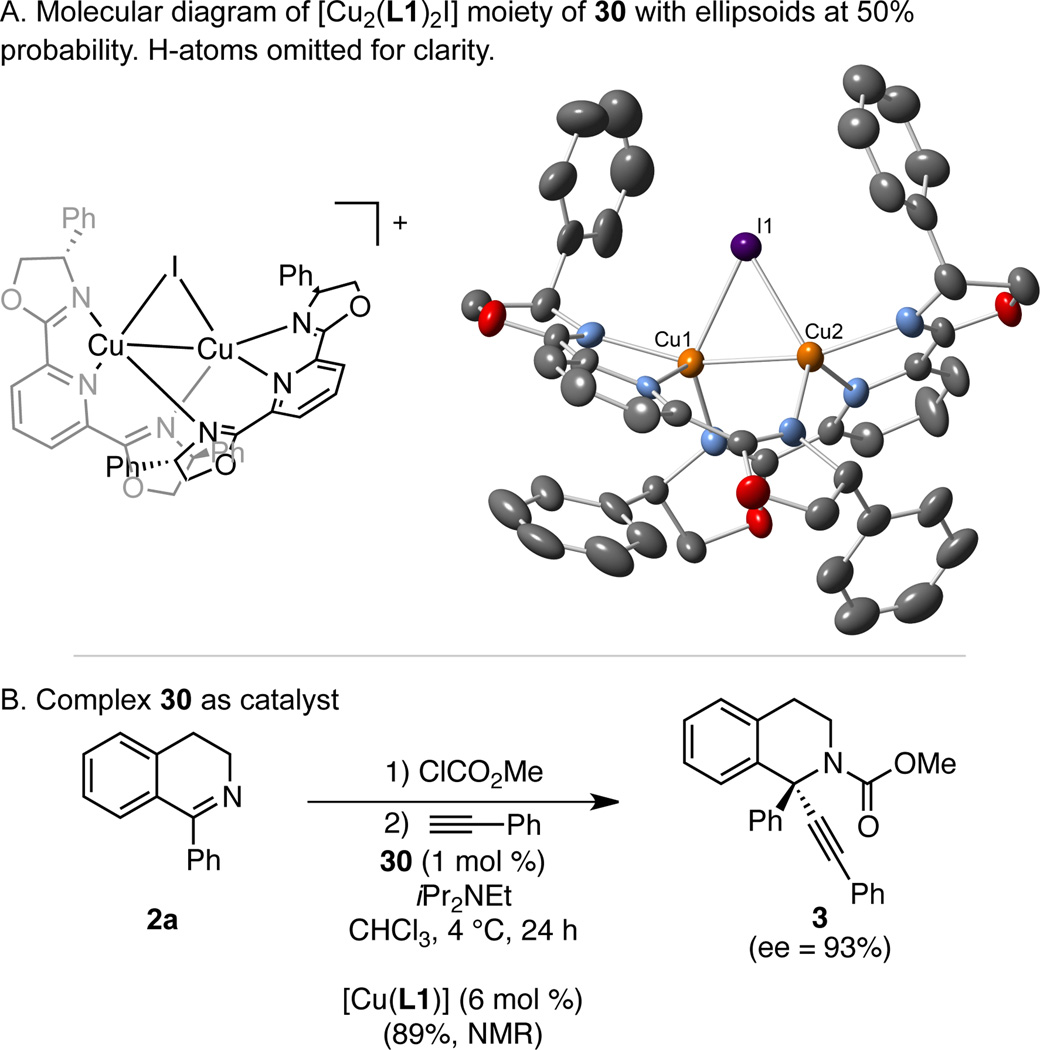
This reaction likely proceeds via a chiral copper acetylide, which adds to the isoquinolinium ion. We are very interested in understanding how the Cu/Ph-PyBox catalyst imparts enantioselectivity. As a first step, we need to understand the structure of the catalyst. Although PyBox ligands often enforce a square geometry on four-coordinate metals, there is no electronic benefit to forcing four ligands into the same plane for Cu(I), making this geometry unlikely. Bidentate coordination of the ligand is possible, but the use of model ligands for bidentate coordination (L7, L8) lead to racemic product 3 (L7: 67% yield by 1H NMR. L8: 58% yield by 1H NMR). In addition, we observe a moderate (+)-nonlinear effect under the optimized two-pot procedure.14,15 These results are similar to our observations in the alkynylation of oxocarbenium ions catalyzed by a Cu/Ph-PyBox catalyst, and suggest that catalyst aggregation may occur.10
In addition, the reaction of CuI (1 equiv) and L1 (1 equiv) in CH2Cl2 resulted in complex 30, [Cu2(L1)2I]3[Cu4I7] which has been characterized by 1H and 13C NMR, as well as X-ray crystallography.12 Notably, [Cu2(PyBox)2X2] complexes with noncoordinating counter-anions (PF6, OTf) have been observed,16 but this is the first crystal structure with a bridging halide counteranion.17 Complex 30 is catalytically competent, delivering high yield and 93% ee (Scheme 5B). Although these results do not exclude the possibility that a higher-order complex is simply an off-cycle reservoir for an active monomeric catalyst, we currently favor a dicopper acetylide as the catalytic intermediate. This mechanism is consistent with the well-recognized importance of such dicopper acetylides in copper acetylide chemistry.16, 18 Ongoing mechanistic studies are directed towards developing a stereochemical model based on such an intermediate, as well as understanding the effects of concentration and base on the enantioselectivity.
Scheme 5. Structure and reactivity of complex 30.
In summary, we developed a copper-catalyzed alkynylation of 1-aryl isoquinolinium ions that delivers cyclic α-diaryl tetrasubstituted amines in good yields and high enantioselectivities. This reaction employs catalyst with commercially available components, and a wide range of isoquinolines and alkynes can be used. Notably, the addition of triphenylsilylacetylene is effective, providing the potential for elaboration of the silyl-protected alkyne. Our mechanistic studies are consistent with an aggregated Cu/L1 complex as the active catalyst, consistent with the recognized importance of dicopper species in copper acetylide chemistry. Efforts are ongoing to expand the scope and more deeply understand the mechanism.
Supplementary Material
Acknowledgments
Acknowledgement is gratefully made to the National Science Foundation (CAREER CHE 1151364). C.A.S. thanks NSF REU 1263018. Data were acquired at UD on instruments obtained with assistance of NSF and NIH funding (NSF CHE0421224, CHE1229234, CHE0840401, and CHE1048367; NIH P20 GM104316, P20 GM103541, and S10 OD016267).
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental details and data (PDF), Crystal structures (CIF)
REFERENCES
- 1.(a) Bentley KW. Nat. Prod. Rep. 2004;21:395. doi: 10.1039/b212259f. [DOI] [PubMed] [Google Scholar]; (b) Chrzanowska M, Rozwadowska MD. Chem. Rev. 2004;104:3341. doi: 10.1021/cr030692k. [DOI] [PubMed] [Google Scholar]; (c) Welsch ME, Snyder SA, Stockwell BR. Curr. Opin. Chem. Biol. 2010;14:347. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Corbett JW, Ko SS, Rodgers JD, Gearhart LA, Magnus NA, Bacheler LT, Diamond S, Jeffrey S, Klabe RM, Cordova BC, Garber S, Logue K, Trainor GL, Anderson PS, Erickson-Viitanen SK. J. Med. Chem. 2000;43:2019. doi: 10.1021/jm990580e. [DOI] [PubMed] [Google Scholar]; (b) Renaud J, Bischoff SF, Buhl T, Floersheim P, Fournier B, Halleux C, Kallen J, Keller H, Schlaeppi J-M, Stark W. J. Med. Chem. 2003;46:2945. doi: 10.1021/jm030086h. [DOI] [PubMed] [Google Scholar]; (c) Stilz HU, Jablonka B, Just M, Knolle J, Paulus EF, Zoller G. J. Med. Chem. 1996;39:2118. doi: 10.1021/jm960210f. [DOI] [PubMed] [Google Scholar]; (d) Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. Proc. Natl. Acad. Sci. USA. 1986;83:7104. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Anderson P, Christy MA, Evans BE. US4399141. 1983
- 3.Riant O, Hannedouche J. Org. Biomol. Chem. 2007;5:873. doi: 10.1039/b617746h. [DOI] [PubMed] [Google Scholar]
- 4.For enantioselective alkynylations of acyclic ketimines with trifluoromethyl, ester, and/or alkyl substituents, see: Huang G, Yang J, Zhang X. Chem. Commun. 2011;47:5587. doi: 10.1039/c1cc10403a. Ma F-GZHMYZJ-A, Ma H, Zheng Y, Ma J-A. Tetrahedron. 2012;68:7663. Huang G, Yin Z, Zhang X. Chem. Eur. J. 2013;19:11992. doi: 10.1002/chem.201301479. Yin L, Otsuka Y, Takada H, Mouri S, Yazaki R, Kumagai N, Shibasaki M. Org. Lett. 2013;15:698. doi: 10.1021/ol3035609.
- 5.(a) Ahamed M, Todd MH. Eur. J. Org. Chem. 2010;31:5935. [Google Scholar]; (b) Funabashi K, Ratni H, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2001;123:10784. doi: 10.1021/ja016935c. [DOI] [PubMed] [Google Scholar]; (c) Nishimura T, Noishiki A, Chit Tsui G, Hayashi T. J. Am. Chem. Soc. 2012;134:5056. doi: 10.1021/ja300697c. [DOI] [PubMed] [Google Scholar]; (d) Nishimura T, Noishiki A, Ebe Y, Hayashi T. Angew. Chem. Int. Ed. 2013;52:1777. doi: 10.1002/anie.201208593. [DOI] [PubMed] [Google Scholar]
- 6.For a single example of alkynlation of a 1-phenylisoquinoline in 75% yield (0% ee) see: Taylor AM, Schreiber SL. Org. Lett. 2006;8:143. doi: 10.1021/ol0526165.
- 7.For enantioselective reduction of diaryl ketimines, see: Nguyen TB, Bousserouel H, Wang Q, Guéritte F. Adv. Synth. Catal. 2011;353:257.
- 8.(a) Cozzi Pier G, Hilgraf R, Zimmermann N. Eur. J. Org. Chem. 2004;2004:4095. [Google Scholar]; (b) Kumagai N, Shibasaki M. Bull. Chem. Soc. Jpn. 2015;88:503. [Google Scholar]; (c) Ohshima T. In: Comprehensive Chirality. Carreira EM, Yamamoto H, editors. Amsterdam: Elsevier; 2012. p. 355. [Google Scholar]; (d) Trost BM, Bartlett MJ. Modern Alkyne Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; 2014. p. 201. [Google Scholar]; (e) Li Z, Li C-J. Org. Lett. 2004;6:4997. doi: 10.1021/ol047814v. [DOI] [PubMed] [Google Scholar]; (f) Li Z, MacLeod PD, Li C-J. Tetrahedron: Asymmetry. 2006;17:590. [Google Scholar]; (g) Lin W, Cao T, Fan W, Han Y, Kuang J, Luo H, Miao B, Tang X, Yu Q, Yuan W, Zhang J, Zhu C, Ma S. Angew. Chem. Int. Ed. 2014;53:277. doi: 10.1002/anie.201308699. [DOI] [PubMed] [Google Scholar]; (h) Pappoppula M, Cardoso FSP, Garrett BO, Aponick A. Angew. Chem. Int. Ed. 2015;54:15202. doi: 10.1002/anie.201507848. [DOI] [PubMed] [Google Scholar]; (i) Perepichka I, Kundu S, Hearne Z, Li C-J. Org. Biomol. Chem. 2015;13:447. doi: 10.1039/c4ob02138j. [DOI] [PubMed] [Google Scholar]; (j) Sun S, Li C, Floreancig PE, Lou H, Liu L. Org. Lett. 2015;17:1684. doi: 10.1021/acs.orglett.5b00447. [DOI] [PubMed] [Google Scholar]; (k) Yu J, Li Z, Jia K, Jiang Z, Liu M, Su W. Tetrahedron Lett. 2013;54:2006. [Google Scholar]; (l) Sun Z, Yu S, Ding Z, Ma D. J. Am. Chem. Soc. 2007;129:9300. doi: 10.1021/ja0734849. [DOI] [PubMed] [Google Scholar]; (m) Black DA, Arndtsen BA. Org. Lett. 2004;6:1107. doi: 10.1021/ol036462+. [DOI] [PubMed] [Google Scholar]; (n) Black DA, Beveridge RE, Arndtsen BA. J. Org. Chem. 2008;73:1906. doi: 10.1021/jo702293h. [DOI] [PubMed] [Google Scholar]
- 9. Hashimoto T, Omote M, Maruoka K. Angew. Chem. Int. Ed. 2011;50:8952. doi: 10.1002/anie.201104017. (b) For enantioselective alkynylation of a trifluoromethyl-substituted cylic ketimine using stoichiometric chiral ligand, see: Jiang B, Si Y-G. Angew. Chem. Int. Ed. 2004;43:216. doi: 10.1002/anie.200352301.
- 10.Dasgupta S, Rivas T, Watson MP. Angew. Chem. Int. Ed. 2015;54:14154. doi: 10.1002/anie.201507373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.See the Supporting Information.
- 12.We do not yet understand this dramatic solvent effect. See the Supporting Information for discussions and further experiments.
- 13.CCDC 1499232 (14) and CCDC 1499233 (30) contain the supplementary crystallographic data for this paper. These can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
- 14.In contrast, no nonlinear effect was observed under the one-pot procedure. See the Supporting Information.
- 15.Girard C, Kagan H. Angew. Chem. Int. Ed. 1998;37:2922. doi: 10.1002/(SICI)1521-3773(19981116)37:21<2922::AID-ANIE2922>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.(a) Díez J, Gamasa MP, Panera Ma. Inorg. Chem. 2006;45:10043. doi: 10.1021/ic061453t. [DOI] [PubMed] [Google Scholar]; (b) Panera Ma, Díez J, Merino I, Rubio E, Gamasa MP. Inorg. Chem. 2009;48:11147. doi: 10.1021/ic901527x. [DOI] [PubMed] [Google Scholar]; (c) Nakajima K, Shibata M, Nishibayashi Y. J. Am. Chem. Soc. 2015;137:2472. doi: 10.1021/jacs.5b00004. [DOI] [PubMed] [Google Scholar]
- 17.For examples of metal/PyBox complexes with bridging anions, see: Cuervo D, Díez J, Gamasa MP, Gimeno J. Organometallics. 2005;24:2224. Paredes P, Díez J, Gamasa MP. Organometallics. 2008;27:2597. Paredes P, Díez J, Gamasa MP. J. Organomet. Chem. 2008;693:3681. Zhu Y-Y, Cui C, Li N, Wang B-W, Wang Z-M, Gao S. Eur. J. Inorg. Chem. 2013;2013:3101. Staples RJ, Aye Y. J. Chem. Crystallogr. 2008;38:49.
- 18.(a) Ahlquist M, Fokin VV. Organometallics. 2007;26:4389. [Google Scholar]; (b) Díez J, Gamasa MP, Gimeno J, Aguirre A, García-Granda S, Holubova J, Falvello LR. Organometallics. 1999;18:662. [Google Scholar]; (c) Mealli C, Godinho SSMC, Calhorda MJ. Organometallics. 2001;20:1734. [Google Scholar]; (d) Rodionov VO, Fokin VV, Finn MG. Angew. Chem. Int. Ed. 2005;44:2210. doi: 10.1002/anie.200461496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.