Abstract
Background and study aims: Magnifying narrow-band imaging (NBI) is useful for examination of colorectal lesions, and endocytoscopy (EC) allows diagnostic evaluation of structural atypia, nuclear atypia, and vascular structures of colorectal tumors. The aim of this study was to examine surface microvessels in deep invasive colorectal cancer using EC with a new NBI video processor system.
Patients and methods: We retrospectively assessed 132 colorectal neoplastic lesions: 81 adenomas, 18 intramucosal cancers, 4 submucosal slightly invasive cancers, and 29 submucosal deep invasive cancers. Detailed vascular findings commonly seen in submucosal deep invasive carcinomas included > 2-fold vasodilatation seen in adenomas, abnormal tortuosity and branching, loss of the micro-network pattern, caliber change in > 2 places in a single blood vessel, and blood vessels not visible in a line because they appear like a string of beads (beaded sign).
Results: Univariate analysis revealed 4 vascular findings that were strongly predictive of submucosal deep invasion: vasodilatation (odds ratio [OR] 9.31; 95 % confidence interval [CI] 3.57 – 24.30), loss of the micro-network pattern (OR 61.60; 95 % CI 17.87 – 212.29), caliber change (OR 35.7; 95 % CI 9.16 – 139.14), and the beaded sign (OR 45.90; 95 % CI 5.50 – 382.73).
Conclusions: Detailed assessment of ultra-magnified microvessels could improve the diagnostic performance for submucosal deep invasive cancer.
Study registration: UMIN-CTR000014033
Introduction
The development of endoscopy has greatly benefited diagnosis of colorectal neoplasia. Chromoendoscopy has been used to establish the pit pattern classification, which is effective for differentiation between non-neoplasia and neoplasia as well as between adenoma and cancer 1. Narrow-band imaging (NBI) was developed in 2006; this technique uses superficial tissue structures and emphasizes the imaging of certain features 2, such as vascular and mucosal patterns. Endocytoscopy (EC) was more recently developed and can magnify objects by 380-fold to 450-fold. EC enables on-site observation of structural and nuclear irregularities 3 4, and therefore has the potential to allow for “optical biopsy” 5. The qualitative and quantitative usefulness of superficial microvascular findings in the examination of colorectal lesions with NBI magnification endoscopy (NBI-ME) has been reported by many institutions 6 7 8 9, but the utility of EC with NBI (EC-NBI) has not been demonstrated.
Kudo et al. 10 classified the endocytoscopic vascular pattern (EC-V) into 3 types (Fig. 1): obscure surface microvessels (EC-V1), clearly observed surface microvessels of a uniform caliber and arrangement (EC-V2), and dilated surface microvessels of a non-homogeneous caliber or arrangement (EC-V3). EC-V1 mainly corresponds to non-neoplasia, EC-V2 to neoplasia, and EC-V3 to invasive cancer. However, whether the vascular findings of EC-V3 are irregularities remains unclear.
Fig. 1.
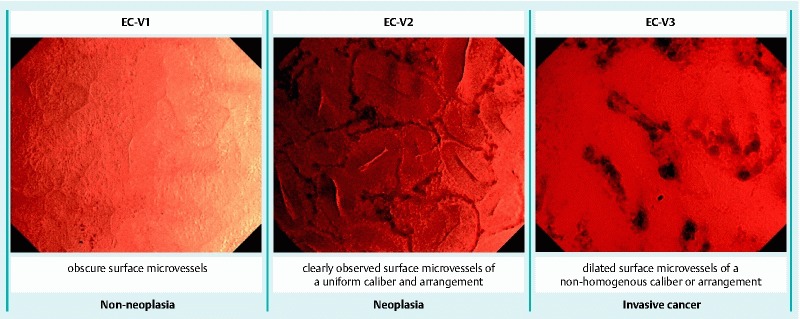
Endocytoscopic vascular pattern (EC-V) classification.
The aims of this study were to examine the vascular findings of submucosal deep invasive cancer by EC-NBI with a new video processor system (EVIS LUCERA ELITE SYSTEM; Olympus, Tokyo, Japan) and to determine the clinical significance of these ultra-high magnification findings.
Patients and methods
Patients
We retrospectively analyzed 98 patients who underwent endoscopic or surgical resection after observation with EC-NBI from May 2013 to December 2014. We excluded patients with inflammatory bowel disease and those with hyperplastic polyps (including sessile serrated adenomas/polyps) and cancers deeper than T2. Before the examination, patients underwent bowel preparation with 2 L to 3 L of polyethylene glycol solution. Diazepam and butylscopolamine were used intravenously for sedation and prevention of peristalsis. The study took place at the Digestive Disease Center of Showa University Northern Yokohama Hospital. The protocol was approved by the Medical Ethics Committee of our hospital (No. 1405-03; approved on June 6, 2014) and registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR000014033; approved on May 25, 2014). All participants gave written informed consent, and the study was conducted according to the Declaration of Helsinki.
EC system
The endocytoscope (CF-Y0020; Olympus) had a magnification range of 80 to 380 × with a standard video processor system (EVIS LUCERA ELITE SYSTEM; CV-290/CLV-290SL) and a digital image filing system (Solemio; Olympus). The EC-NBI was set at enhancement mode A8 and color mode 3. The endocytoscope had a working length of 133 cm, outer diameter of 13.6 mm, and single lens. The instrument allowed gradual magnification at the center of the monitor, thus pinpointing the target area being viewed. Our observation focused on the area of interest, which showed irregularity after conventional NBI magnification before staining. Next, we checked the superficial layers of polyps as thoroughly as possible.
Evaluation of EC-NBI findings
We anticipated 5 different types of vascular findings in light of the detailed vascular findings commonly seen in submucosal deep invasive cancer: 1) caliber dilatation; 2) abnormal tortuosity and branching of microvessels; 3) loss of the micro-network (MN) pattern; 4) caliber change; and 5) beaded sign. First, we defined caliber dilation as vessel thickening > 2-fold (> 30 µm) that seen in adenomas. We reported a mean blood vessel caliber size measured by EC of 19.5 ± 4.2 µm in adenoma and 33.0 ± 7.2 µm in T1 cancer 11. We therefore set a threshold caliber of 30 µm, which was almost twice that for adenoma vessels, to allow us to detect caliber dilation. As for abnormal tortuosity and branching of microvessels, vessels are usually arranged along the crypt opening in adenoma. Abnormal tortuosity and branching was defined as sharply angled microvessels or thick, irregular vessels passing from a deep layer to join a surface vessel. We defined the MN pattern as the presence of fine and tortuous vessels in the intervening part around the crypt opening of adenomas 12 (Fig. 2). However, the MN pattern tended to disappear in invasive carcinomas; thus, we regarded loss of the MN pattern as suggestive of invasive carcinoma. Caliber change was defined as a maximum caliber size of at least double the minimum caliber size in 1 vessel. The vascular thickness is relatively uniform in adenomas, but seemed to become less uniform in cases of submucosal deep invasive cancer. The beaded sign was defined as blood vessels with the appearance of a string of beads (Fig. 3). The blood vessel diameter was measured using Nitenkeisokuki version 1.1.1. and was evaluated relative to an endoscopic screen width of 600 μm. Endoscopic images were randomly allocated to 2 readers (H. N. and M. M.) for evaluation without a final pathologic diagnosis.
Fig. 2.
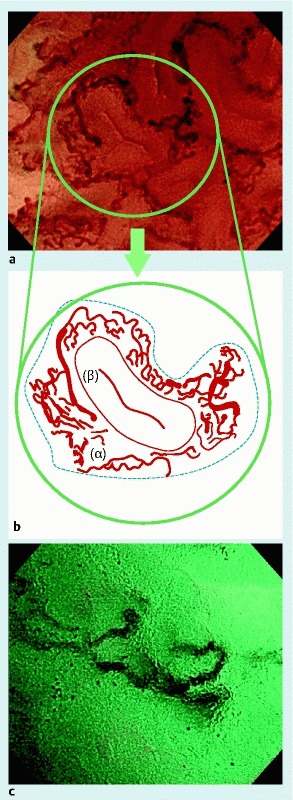
Loss of the micro-network (MN) pattern. a Image of typical adenoma. b Schema of MN pattern; typical adenoma has fine and tortuous vessels in the intervening part (α in dotted line) around the crypt opening (β). We defined these vessels as the MN pattern. c Image of submucosal deep invasive cancer; loss of the MN pattern.
Fig. 3.
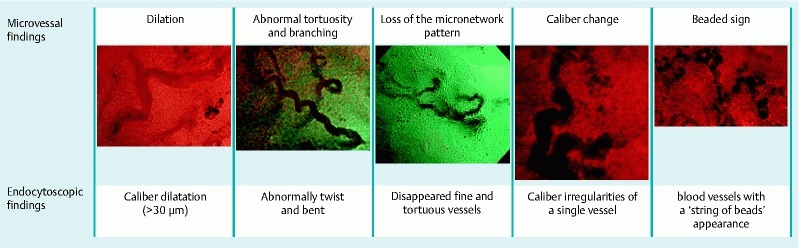
Microvascular findings and schema.
NBI-ME classification
We classified NBI-ME findings into 6 categories according to the vascular pattern classification 9. Sparse and irregular patterns were considered the index of submucosal deep invasive cancer.
Outcome measure and statistical analysis
We calculated the sensitivity, specificity, and overall accuracy of each endoscopic modality. Furthermore, we compared the diagnostic accuracy between the EC-V and NBI-ME. We used SPSS for Windows version 20.0 (IBM Corp., Armonk, NY, USA) for data analysis. Pearson’s chi-square test was applied to calculate the odds ratios (ORs) of the endocytoscopic findings, and McNemar’s test was applied to compare the diagnostic accuracy between EC-V and NBI-ME. κ values were calculated for interobserver agreement. A κ value of 0.00 indicated poor agreement; 0.00 – 0.20, slight agreement; 0.21 – 0.40, fair agreement; 0.41 – 0.60, moderate agreement; 0.61 – 0.80, substantial agreement; and 0.80 – 1.00, almost perfect agreement. P-values of < 0.05 were considered statistically significant.
Pathologic diagnosis
All specimens were fixed in 10 % buffered formalin solution after retrieval. They were stained with conventional hematoxylin and eosin and diagnosed using the JSCCR guideline for the degree of submucosal invasion; each cancer was subclassified accordingly 13.
Results
Overall, 163 lesions were included. Of these, 31 lesions were excluded for various reasons (Fig. 4). We therefore assessed 132 lesions: 81 adenomas, 18 intramucosal cancers, 4 submucosal slightly invasive cancers, and 29 submucosal deep invasive cancers. In total, 959 pictures of these lesions were evaluated. Patient demographics and lesion characteristics are summarized in Table 1. Sensitivity, specificity, and diagnostic accuracy of the caliber dilation for differentiating adenoma/intramucosal cancer/submucosal slightly invasive cancer or submucosal deep invasive cancer were 75.9 %, 74.8 %, and 75.0 %, respectively (P < 0.01; OR 9.31). Furthermore, sensitivity, specificity, and diagnostic accuracy of abnormal tortuosity and branching were 17.2 %, 92.2 %, and 75.8 %, respectively (P = 0.153; OR 2.47). Sensitivity, specificity, and diagnostic accuracy of loss of the MN pattern were 75.9 %, 95.1 %, and 90.9 %, respectively (P < 0.01; OR 61.64). Sensitivity, specificity, and diagnostic accuracy of the caliber change were 35.7 %, 97.1 %, and 87.1 %, respectively (P < 0.01; OR 45.78). Sensitivity, specificity, and diagnostic accuracy of the beaded sign were 31.0 %, 99.0 %, and 84.1 %, respectively (P < 0.01; OR 45.95) (Table 2).
Fig. 4.
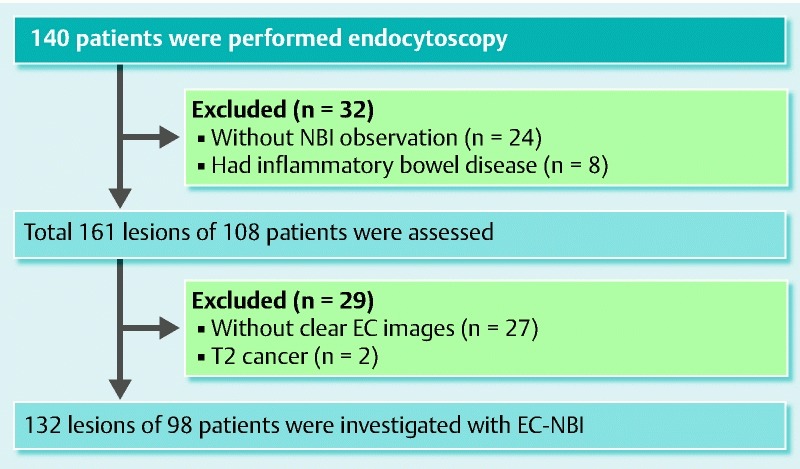
Patient flowchart.
Table 1. Patient and lesion characteristics.
| Adenoma (n = 81) |
Intramucosal carcinoma (n = 18) |
Submucosal slightly invasive carcinoma (n = 4) |
Invasive carcinoma (n = 29) |
|
| Age, y | 63.9 ± 13.1 (32 – 91) | 66.5 ± 8.7 (50 – 81) | 71.8 ± 3.3 (68 – 75) | 63.2 ± 13.5 (36 – 94) |
| Sex | ||||
| Male | 58 | 10 | 4 | 16 |
| Female | 23 | 8 | 0 | 13 |
| Location | ||||
| Left side of colon | 41 | 5 | 1 | 7 |
| Right side of colon | 30 | 8 | 1 | 12 |
| Rectum | 10 | 5 | 2 | 10 |
| Size of lesions, mm | 10.7 ± 6.4 (3 – 35) | 18.7 ± 10.0 (4 – 40) | 18.5 ± 9.9 (4 – 25) | 20.4 ± 7.1 (8 – 35) |
| Protruded and flat-elevated type | 77 | 14 | 2 | 21 |
| Depressed type | 4 | 4 | 2 | 8 |
Data are presented as mean ± standard deviation (range) or n.
Table 2. Diagnostic accuracy of microvascular findings.
| Vascular findings | Sensitivity | Specificity | Accuracy | OR (95 % CI), % |
P value |
| Caliber dilation | 75.9 % (22/29) |
74.8 % (77/103) |
75.0 % (99/132) |
9.31 (3.57 – 24.3) |
< 0.01 |
| Abnormal tortuosity and branching | 17.2 % (5/29) |
92.2 % (95/103) |
75.8 % (100/132) |
2.47 (0.74 – 8.25) |
0.153 |
| Loss of the micro-network pattern | 75.9 % (22/29) |
95.1 % (98/103) |
90.9 % (120/132) |
61.64 (17.87 – 212.29) |
< 0.01 |
| Caliber change | 35.7 % (15/29) |
97.1 % (100/103) |
87.1 % (115/132) |
45.78 (9.16 – 139.14) |
< 0.01 |
| Beaded sign | 31.0 % (9/29) |
99.0 % (102/103) |
84.1 % (111/132) |
45.95 (5.50 – 382.73) |
< 0.01 |
Total number of polyps: 132.
CI, confidence interval; OR, odds ratio.
The interobserver agreement κ value between experienced endoscopists for dilation was 0.67, that for abnormal tortuosity and branching was 0.44, that for loss of the MN pattern was 0.55, that for caliber change was 0.52, and that for the beaded sign was 0.55.
Sensitivity, specificity, and overall accuracy of EC-NBI were 96.3 %, 97.1 %, and 97.0 %, respectively, among the cases included in this study. On the other hand, NBI-ME had a sensitivity of 82.1 %, specificity of 94.2 %, and overall accuracy of 91.7 % (Table 3).
Table 3. Comparison of diagnostic accuracy between EC-V and NBI-ME.
| Predicting invasive cancer (T1b) | EC-V3 | NBI-ME | P value |
| Sensitivity | 96.3 % | 82.1 % | 0.375 |
| Specificity | 97.1 % | 94.2 % | 0.219 |
| Accuracy | 97.0 % | 91.7 % | 0.065 |
McNemar’s test was applied.
EC-V, endocytoscopic vascular pattern; NBI-ME, NBI magnification endoscopy.
Discussion
Our results suggest that caliber dilation, loss of the MN pattern, caliber change, and the beaded sign are highly correlated with submucosal deep invasive cancers. In addition, the endoscopists showed moderate agreement, and the results obtained were validated.
With recent advances in endoscopic techniques such as endoscopic submucosal dissection, even a large lesion requiring endoscopic piecemeal mucosal resection can be resected en bloc. It has also become possible to resect non-lifting lesions other than submucosal deep invasive cancer, because of fibrosis, after biopsy or injection of liquid, and other circumstances. For appropriate treatment, however, it is important to differentiate whether the lesion is submucosal slightly invasive cancer or submucosal deep invasive cancer. Therefore, high specificity is required to prevent excessive surgical treatment. In a comparison of diagnostic accuracy between EC-V and NBI-ME, all of these diagnostic parameters were higher with EC-V than with NBI-ME. However, there were no significant differences between EC-NBI and NBI-ME in terms of these parameters. However, all cases evaluated as EC-V3 and as having an irregular/sparse pattern of NBI-ME were classified as submucosal deep invasive cancer. This finding seems to illustrate an advantage of EC-NBI.
EC-NBI can allow for observation of vascular irregularity in vivo and might facilitate observation of angiogenesis of colorectal lesions 11. Konerding et al. 14 described the normal colonic mucosa as having honeycomb-like vasculature. Colorectal tumor capillaries become thick due to angiogenesis, the caliber changes as the grade of atypia increases, the vessels become blocked, and changes in vascular density occur 15. Ultra-high magnification of EC-NBI may enable the blood vessels to be observed in a manner close to these models. EC-NBI has shown that tumor capillaries are thicker than non-tumor capillaries and that the capillaries are significantly thick in cases of submucosal deep invasive cancer. Loss of the MN pattern is considered to reflect a phenomenon of decreasing vascular density, although severely atypical blood vessels remain as the lesion develops into submucosal deeply invasive cancer. Although significant differences were observed in vascular dilation, the diagnostic accuracy was 75.6 %. This result was lower than that in other cases. A previous study that utilized microvascular corrosion casting revealed that invasive cancer has significantly larger microvessel diameters 14. The vascular dilation could therefore be the most important finding in submucosal deep invasive cancer. Conversely, there were no significant differences in tortuosity. In NBI-ME, tortuosity is an important abnormal vascular finding, and its significance has been confirmed. However, at 380 × magnification with EC, even usual adenomas show mild tortuosity. It becomes difficult to distinguish normal from abnormal findings according to tortuosity.
When we evaluated the detailed vascular findings in this study, high-quality images were needed. We used the EVIS LUCERA SPECTRUM SYSTEM until 2012. With this system, the quality of images was usually poor because sufficient brightness was not obtained with EC-NBI. With the advent of the EVIS LUCERA ELITE SYSTEM in October 2012, the images obtained with EC-NBI were brighter and clearer (Fig. 5). This may have been attributable to increased light intensity, increased exposure time of NBI, and improved noise reduction, thereby allowing clearer observation of the vessels and detailed examination of EC-V. With regard to advantages of EC-NBI over conventional EC, conventional EC always requires staining with crystal violet and methylene blue 16 17. However, EC-NBI does not require staining, and testing with EC-NBI therefore can be performed safely and quickly. We recently reported on use of a computer-aided diagnosis (CAD) system for EC-V 18. The CAD system provided sufficient diagnostic ability for lesion characterization, but it could not predict the invasion depth of colorectal cancer. Therefore, EC-V still has an advantage over the CAD system.
Fig. 5.
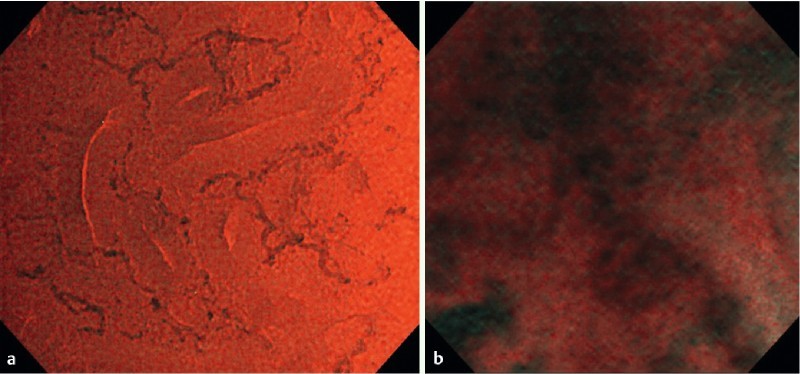
Comparison of endocytoscopy with narrow-band imaging using EVIS LUCERA ELITE SYSTEM and EVIS LUCERA SPECTRUM SYSTEM. The (a) EVIS LUCERA ELITE SYSTEM images became brighter and clearer than the (b) EVIS LUCERA SPECTRUM SYSTEM images.
Future development of quantitative and qualitative diagnostic techniques will use elements different from those of EC classification because EC-NBI does not focus on the nucleus and gland formation of surface colorectal lesions but on the vascular caliber and its irregularity. For example, when cancer is deep-seated and not exposed to the surface, nuclear or structural atypia may not be seen by EC. In these cases, vascular findings might help to diagnose the submucosal deeply invasive area. Furthermore, it might be possible to predict potential metastasis, such as venous invasion, by comparing vascular irregularities and thus select candidates for vascular endothelial growth factor inhibitors (e. g., bevacizumab) or predict treatment efficacy 19 20.
This study had some limitations. First, it was retrospective study and conducted in a single institution. We are planning to increase the number of patients for a further prospective study. Second, although EC enables the living body to be observed, it is difficult to compare the results with pathologic results. We considered that because the focal depth of the endocytoscope is about 50 µm, comparison with dilated vessels on the surface would be possible. However, it was actually difficult to evaluate deformed blood vessels, including changes in vascular caliber, because of dehydration and staining during specimen preparation. Therefore, it may be necessary to consider that pathologic findings are new diagnostic findings in the living body.
Conclusion
In conclusion, dilation, loss of the MN pattern, caliber change, and a beaded sign are important vascular findings observed by EC in patients with colorectal submucosal deep invasion. These results appear to contribute to the definition of irregular vascular findings of EC-V3.
Footnotes
Competing interests: None
References
- 1.Kudo S, Hirota S, Nakajima T. et al. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880–885. doi: 10.1136/jcp.47.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gono K, Obi T, Yamaguchi M. et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed. 2004;9:568–577. doi: 10.1117/1.1695563. [DOI] [PubMed] [Google Scholar]
- 3.Haruhiro I, Kudo S-E, Shiokawa A. et al. Observation of Living Cancer Cells in the Gastrointestinal Tract. Clin Gastroenterol Hepatol. 2005;3565:61–63. doi: 10.1016/s1542-3565(05)00282-x. [DOI] [PubMed] [Google Scholar]
- 4.Kudo S E, Wakamura K, Ikehara N. et al. Diagnosis of colorectal lesions with a novel endocytoscopic classification a pilot study. Endoscopy. 2011;43:869–875. doi: 10.1055/s-0030-1256663. [DOI] [PubMed] [Google Scholar]
- 5.Mori Y, Kudo S, Ikehara N. et al. Comprehensive diagnostic ability of endocytoscopy compared with biopsy for colorectal neoplasms: A prospective randomized noninferiority trial. Endoscopy. 2013;45:98–105. doi: 10.1055/s-0032-1325932. [DOI] [PubMed] [Google Scholar]
- 6.Sano Y, Horimatsu T, Fu K I. et al. Magnifying observation of microvascular architecture of colorectal lesions using a narrow-band imaging system. Dig Endosc. 2006;18:44–51. [Google Scholar]
- 7.Katagiri A, Fu K I, Sano Y. et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther. 2008;27:1269–1274. doi: 10.1111/j.1365-2036.2008.03650.x. [DOI] [PubMed] [Google Scholar]
- 8.Hirata M, Tanaka S, Oka S. et al. Magnifying endoscopy with narrow band imaging for diagnosis of colorectal tumors. Gastrointest Endosc. 2007;65:988–995. doi: 10.1016/j.gie.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 9.Wada Y, Kudo S E, Kashida H. et al. Diagnosis of colorectal lesions with the magnifying narrow-band imaging system. Gastrointest Endosc American Society for Gastrointestinal Endoscopy. 2009;70:522–531. doi: 10.1016/j.gie.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 10.Kudo S, Misawa M, Wada Y. et al. Endocytoscopic microvasculature evaluation is a reliable new diagnostic method for colorectal lesions. Gastrointest Endosc. 2015;82:1–12. doi: 10.1016/j.gie.2015.04.039. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Kudo S E, Misawa M. et al. Comparison of the endocytoscopic and clinico-pathologic features of colorectal neoplasms. EIO. 2016;04:397–402. doi: 10.1055/s-0042-101753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yagi K, Nozawa Y, Endou S. et al. Diagnosis of early gastric cancer by magnifying endoscopy with NBI from viewpoint of histological imaging: Mucosal patterning in terms of white zone visibility and its relationship to histology. Diagn Ther Endosc. 2012;954809:1–7. doi: 10.1155/2012/954809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe T, Itabashi M, Shimada Y, Japanese Society for Cancer of the Colon and Rectum . Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. [DOI] [PMC free article] [PubMed]
- 14.Konerding M, Fait E, Gaumann A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Cancer. 2001;84:1354–1362. doi: 10.1054/bjoc.2001.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skinner S A, Frydman G M, O’Brien P E. Microvascular structure of benign and malignant tumors of the colon in humans. Dig Dis Sci. 1995;40:373–384. doi: 10.1007/BF02065424. [DOI] [PubMed] [Google Scholar]
- 16.Sasajima K, Kudo S E, Inoue H. et al. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest Endosc. 2006;63:1010–1017. doi: 10.1016/j.gie.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Ichimasa K, Kudo S E, Mori Y. et al. Double staining with crystal violet and methylene blue is appropriate for colonic endocytoscopy: An in vivo prospective pilot study. Dig Endosc. 2014;26:403–408. doi: 10.1111/den.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Misawa M, Kudo S, Mori Y. et al. Characterization of Colorectal Lesions Using a Computer-Aided Diagnostic System for Narrow-Band Imaging Endocytoscopy. Gastroenterology. 2016;150:1531–1532. doi: 10.1053/j.gastro.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Goel S, Duda D G, Xu L. et al. Normalization of the Vasculature for Treatment of Cancer and Other Diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain R K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]


