ABSTRACT
NMDA receptors (NMDARs) have recently been discovered as functional regulators of pancreatic β-cell insulin secretion. While these excitatory receptor channels have been extensively studied in the brain for their role in synaptic plasticity and development, little is known about how they work in β-cells. In neuronal cells, NMDAR activation requires the simultaneous binding of glutamate and a rate-limiting co-agonist, such as D-serine. D-serine levels and availability in most of the brain rely on endogenous synthesis by the enzyme serine racemase (Srr). Srr transcripts have been reported in human and mouse islets but it is not clear whether Srr is functionally expressed in β-cells or what its role in the pancreas might be. In this investigation, we reveal that Srr protein is highly expressed in primary human and mouse β-cells. Mice with whole body deletion of Srr (Srr KO) show improved glucose tolerance through enhanced insulin secretory capacity, possibly through Srr-mediated alterations in islet NMDAR expression and function. We observed elevated insulin sensitivity in some animals, suggesting Srr metabolic regulation in other peripheral organs as well. Srr expression in neonatal and embryonic islets, and adult deficits in Srr KO pancreas weight and islet insulin content, point toward a potential role for Srr in pancreatic development. These data reveal the first evidence that Srr may regulate glucose homeostasis in peripheral tissues and provide circumstantial evidence that D-serine may be an endogenous islet NMDAR co-agonist in β-cells.
KEYWORDS: b-cell, D-serine, islets, insulin secretion, insulin sensitivity, mouse, NMDA receptor, serine racemase
Introduction
Pancreatic β-cells share many common features with neurons,1-3 including membrane ion channels that aid in the generation of action potentials leading to calcium-triggered exocytosis of proteins (i.e. neurotransmitters or insulin).4 Also like neurons, β-cells exhibit adaptive plasticity, changing their sensitivity to glucose or amplitude of insulin release in response to nutrient environment and past activity.5-8 Recently, β-cells were characterized with functional NMDA receptors (NMDARs),9 ionotropic membrane channels that are critical for regulating neuronal plasticity.10 Mice with a β-cell specific deletion of NR1, the requisite NMDAR channel subunit, demonstrated improved glucose tolerance while pharmacological NMDAR antagonists boosted islet insulin secretion and improved diabetic symptoms in both mice and humans.9 Despite this evidence of functional expression, the native activity and regulatory physiology of β-cell NMDARs is largely unknown.
In the central nervous system, a significant subset of NMDARs are regulated by D-serine, a non-proteinogenic amino acid that acts as a trigger co-agonist, along with the agonist glutamate, to activate NMDARs.11,12 In addition to acute co-agonism, repetitive D-serine binding can stimulate changes in NMDAR expression and cellular plasticity.13,14 D-serine may also play a role in tissue development as it is notably elevated in fetal serum and perinatal brain tissues, where it has been suggested to guide cell migration, synaptogenesis and to set expression levels of NMDARs in adult mice.11,15,16 In addition to NMDARs, the murine pancreas contains a basal level of D-serine17-19 and islets express almost all of the proteins necessary to support D-serine signaling (e.g. ASCTII, Asc-1, GlyT1, DAO).20 However, it is not known whether the pancreas can synthesize D-serine endogenously, via the serine racemase (Srr) enzyme, a critical regulator of local D-serine concentration and NMDAR activation in the brain.21 β-cell Srr transcripts have been identified in both a mouse and human transcriptomics screens.22,23 Interestingly, Srr polymorphisms have also been linked to diabetes susceptibility24,25 and metformin efficacy26 in GWAS studies among the Chinese Han population. These data suggest that Srr could play a meaningful role in glucose homeostasis and β-cell physiology but no direct examination of this topic has been conducted. Therefore, in the present study, we sought to investigate whether Srr is functionally present in pancreatic islets, and β-cells specifically, and whether it contributes to the regulation of glucose metabolism.
Our results show that Srr is highly expressed in human and mouse islets, the latter from a very early stage of development. We characterized a metabolic phenotype in the whole body Srr knockout (KO) mouse, revealing improvements in glucose tolerance, insulin sensitivity and insulin secretion as well as deficits in pancreas weight and islet insulin content. Finally, we show that Srr KO islets express fewer NMDA receptors (NR1 subunit transcripts) and do not respond to the NMDAR antagonist MK-801, which suggests a functional link between Srr activity and NMDA receptor modulation in the pancreas.
Materials and methods
Experimental mice
Serine Racemase −/− (Srr KO) mice and their wildtype controls (Srr WT) were generated in a C57/Bl6 background, originally from Joe Coyle of Harvard University.27 Mice were conventionally housed with ad libitum access to food and water on a 14:10 light cycle. The age range of mice tested was 2–5 months old. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
In vivo mouse procedures
All mice were weighed in both a random (non-fasted) state and after a 14-hour overnight fast. Blood glucose was assessed by tail vein using a handheld glucometer (Bayer CONTOUR). For plasma insulin, a facial vein blood sample was spun through an EDTA-coated tube and the plasma stored at −20°C until insulin assessment using an Ultrasensitive mouse insulin ELISA kit (ALPCO). Glucose and insulin tolerance tests were performed in overnight fasted animals with glucose levels assessed just before and up to 2 h after an intraperitoneal injection of 2 g/kg glucose (Hospira, Pfizer) or 0.75 U/kg insulin (Humalog, Eli Lilly). For in vivo glucose-stimulated insulin secretion (GSIS), plasma samples were assayed from facial vein blood collected from overnight fasted mice before and 5–7 min after a 3 g/kg injection of glucose. Data are presented as a stimulation index - insulin level post-glucose over fasted insulin level.
Tissue collection
Mice were euthanized by CO2 asphyxiation followed by cervical dislocation. Following a mid-line incision, animals were exsanguinated by clipping the aorta and the internal organs were washed in cold PBS. Tissue samples (liver, visceral fat) were then collected and frozen in foil on dry ice. Pancreata were perfused in situ for islet isolation or dissected out and fixed in 3.7% formalin overnight (adult) or for 6 h (neonatal, embryonic) then transferred into 70% ethanol in preparation for sectioning. Human pancreata were provided by the University of Minnesota Medical School Anatomy Bequest Program. Human pancreas tissue procurement quality was assessed independently by Drs. Robert Sorenson and Cyprian Weaver.
Immunostaining of pancreatic sections
Using standard methods, formalin-fixed pancreatic tissues were embedded in paraffin for sectioning. After deparaffinization and rehydration, 5-μm sections were treated with a citrate buffer (10 mM Sodium citrate, 0.05% Tween 20, pH 6.0) for antigen retrieval then incubated overnight at 4°C in primary antibodies against insulin (IS002, Dako), serine racemase (H-150, Santa Cruz) or E-Cadherin (610181, BD). For immunofluorescent imaging, sections were stained with secondary antibodies conjugated to FITC or Cy3 (Jackson Immunoresearch) and coverslipped with DAPI-containing mounting media (Vector Laboratories). These were imaged with a Zeiss Axio Observer Z.1 microscope, equipped with a motorized stage capable of whole pancreas scanning. To assess β-cell mass, FIJI software was used to assess the ratio of insulin-positive area over total pancreas area (β-cell area) and multiplied by the pancreas weight (β-cell mass) for 5 insulin-stained sections taken 200 uM apart through the depth of the pancreas.7 For immunochemical imaging, Srr-stained sections were incubated with secondary antibodies conjugated to biotin and then Vectastain ABC reagent (from Vectastain Elite HRP kit, Vector Laboratories). Sections were then stained with DAB peroxidase substrate (Vector Labs) and counterstained with Mayer's Hematoxylin (Sigma) before imaging on a Leica MC 120 HD microscope.
Islet isolation
To isolate islets, pancreata were perfused through the common bile duct with a 1 mg/mL cold solution of Collagenase P (Roche) in Mg2+/Ca2+-free HBSS (Gibco, Life Technologies) as previously described.7,28 Digestion was achieved by incubation at 37°C for 15–17 minutes and stopped by washing 3 times with cold cation-supplemented HBSS with 2% FBS (EmbryoMax, EMD Millipore) and passed through a 70-μm strainer (Fisher Scientific). Islets were washed off the strainer and handpicked into warm RPMI 1640 media with L-glutamine (Corning), 5 mM glucose, 10% FBS, 100 IU/ml penicillin and 100 g/ml streptomycin.
In vitro islet insulin secretion and insulin content
Clean picked islets were allowed to rest overnight at 37°C, 5% CO2 in a humidified incubator and then incubated for 2 hours in sterile Krebs buffer containing (in mM): 114.6 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.1 MgSO4–7H2O, 8 HEPES, 1 CaCl2–2H2O, 10 NaHCO3 and 0.08% BSA w/v. 8–10 size-matched islets were then transferred into 8-μm cell culture inserts (Millicell) and successively incubated for 30 minutes in 2 mM low glucose (LG) then 16.7 mM high glucose (HG). After a 15-minute low glucose rest, islet inserts were finally incubated for 15-minutes in 30 mM KCl. In one experiment, 10 uM (+) MK-801 (M107, Sigma) was added to the pre-incubation solution (last 30 minutes before LG) and into the LG and HG solutions for some islets. Collected supernatant was analyzed for insulin concentration by ELISA kit (ALPCO) and normalized to insulin content. Insert screens were punched out into lysis buffer (1x RIPA + protease inhibitor), sonicated and assayed for insulin content by ELISA and for DNA content by Quant-iT PicoGreen dsDNA Assay Kit (Molecular Probes, Life Technologies). Insulin content of naïve and post-GSIS islets was normalized to DNA.
Protein and RNA isolation
Liver tissue was frozen with liquid nitrogen and ground with a mortar and pestle before suspension in lysis buffer. Suspended tissue was then sonicated to lyse cells and total protein quantified by Pierce BCA Protein Assay Kit (Thermo Scientific) according to the manufacturer's instructions. For RNA, islets were washed in PBS and snap-frozen dry until RNA isolation using the Quick-RNA MiniPrep kit (Zymo Research), according to kit instructions. RNA concentration was assessed on a BioTek Synergy H1 microplate spectrophotometer using Gen5 software. All samples were stored at −80°C when not in use.
Western blotting
Protein lysates were resolved on an 8% gel, transferred to a PVDF membrane, blocked with TBS blocking solution (LI-COR Biosciences) and immunoblotted with various primary antibodies, Phospho-Akt (Ser473, Cell Signaling) or β-Actin (Cell Signaling), overnight at 4°C. After washing, blots were incubated in 800CW or 680RD infrared fluorescent IgG secondary antibodies (IRDye, LI-COR Biosciences). Following an additional wash step, membranes were imaged on a LI-COR Odyssey 9120 infrared imager. Densitometry analysis of bands was done with Odyssey software v.3.0, normalizing to β-Actin as loading control.
Gene expression analysis
cDNA was synthesized from islet RNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems). Relative gene expression was assessed on an Applied Sciences 7900HT Real-Time PCR system using SYBR green (Applied Biosciences), according to the ΔΔCT method, normalized to β-actin. Primer sequences are listed in supplementary Table 1.
Statistical analysis
All data are presented as mean +/− SEM. Single outcome data was assessed by unpaired 2-tailed Student t-test. Multiple outcome data was assessed using repeated measures 2-way ANOVA followed by Sidak multiple comparisons test. Statistical analyses were performed in GraphPad Prism version 7 with a significance threshold of p < 0.05.
Results
Serine racemase is expressed in pancreatic islets
Using immunofluorescence (IF) and immunohistochemistry imaging of whole pancreatic sections, we found that Srr was highly expressed in adult mouse islets where it co-localizes with insulin (Fig. 1A), which confirms and extends previously published findings on β-cell Srr transcript expression.22 Our antibody failed to detect Srr in islets from Srr KO mice (Figure 1A, C), validating our model and approach, while the intensity of IF insulin staining appeared lighter in female KO islets, suggesting deficits in insulin content supported by subsequent data (see Fig. 4B). Furthermore, we now report for the first time the presence of Srr protein in human insulin-positive islet cells (Fig. 1A), complementing a just-published report showing human Srr gene expression in multiple pancreatic cell types, including β-cells.23 In addition to adult protein expression, we also made the novel discovery of robust Srr in insulin-producing cells during early pancreatic development, both in newborn mouse (Fig. 1B) and embryonic day 14.5 pancreatic tissue (data not shown).
Figure 1.
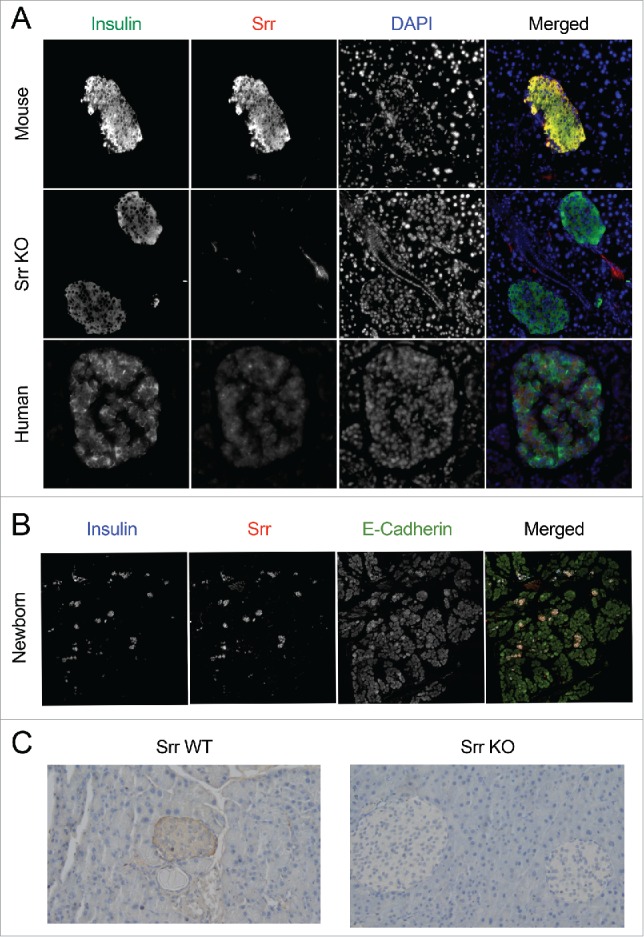
Serine racemase is expressed in human and mouse islets. (A) Immunofluorescence staining of insulin (green), serine racemase (Srr, red) and nuclei (DAPI, blue) in adult mouse (WT, Srr KO) and adult human pancreatic sections (pseudocolor added to merged image only). 20X magnification. (B) Immunofluorescence staining of insulin (blue), serine racemase (Srr, red) and E-Cadherin (green) in mouse neonatal pancreatic sections (pseudocolor added to merged image only). 40X magnification, composite scan. (C) Immunohistochemical staining of Srr in pancreas sections from adult WT and Srr KO mice. 20X magnification.
Figure 4.
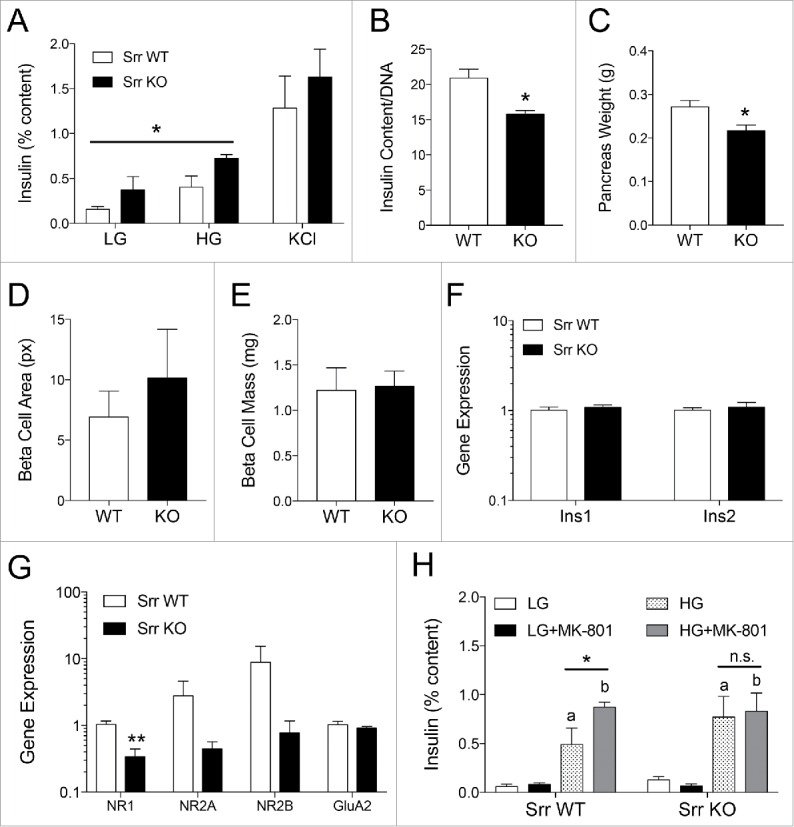
Srr KO islets have potentiated insulin secretion but lower content and diminished NMDAR expression and function. (A) In vitro GSIS of female Srr KO (black) vs. WT (white) islets showing insulin release as a percentage of total insulin content in response to low glucose (LG, 2 mM), high glucose (HG, 16.7 mM) and potassium chloride (KCl, 30 mM) (n = 3). (B) Islet insulin content normalized to DNA (n = 5–6). Pancreas weight (C), β-cell area (pixel2) (D) and β-cell mass (E) from a cohort of Srr WT and KO female mice (n = 5). Gene expression of insulin genes (F) and ionotropic glutamate receptor subunits (G), assessed by RTqPCR relative to actin (n = 5–6), logarithmic scale. (H) In vitro GSIS +/− the NMDAR open channel blocker MK-801 (10 uM) (n = 4). *p < 0.05, **p < 0.01 for comparisons between adjacent data or as indicated. In panel H, a and b indicate significance (p < 0.05) for LG vs. HG for glucose only and glucose+MK-801 conditions, respectively. Statistics assessed by Student t-test (B-G) or 2-way ANOVA (A, genotype x glucose; H, glucose x MK-801 for each genotype).
Srr KO mice display improved glucose tolerance
To test the hypothesis that islet Srr may be functionally relevant to glucose homeostasis, we tested both male and female WT mice against mice with a constitutive whole-body loss of Srr (Srr−/−, Srr KO).16,29 Srr KO mice showed no gross changes in random or overnight fasted body weight (p > 0.16) (Fig. 2A and B). Random blood glucose was significantly lower in Srr KOs during random tail vein tests (p < 0.01 female, p < 0.001 male) (Fig. 2C) but not for fasted blood glucose (p > 0.43) (Fig. 2D). On the other hand, random plasma insulin (Fig. 2D) was unaffected by genotype (p > 0.47) while fasted plasma insulin was reduced by 50% in the Srr KO females (p < 0.05) and trended lower in the males (Fig. 2F), suggesting improved insulin efficiency in the Srr KO mice.
Figure 2.
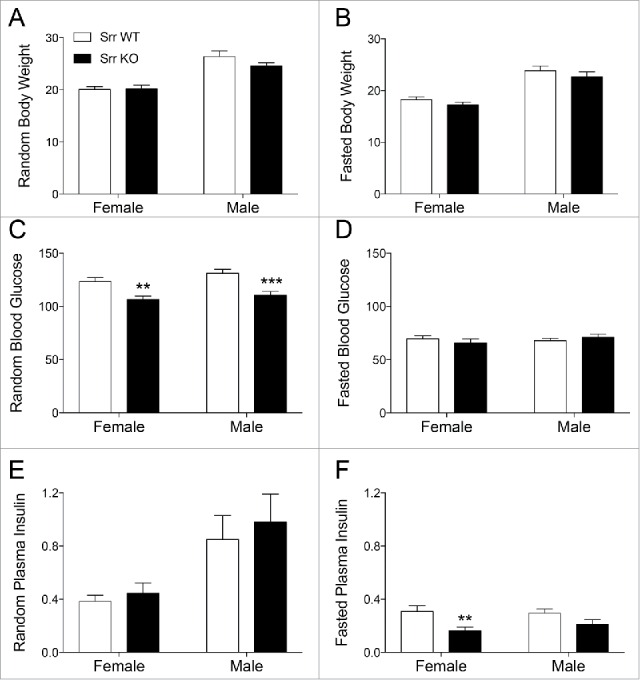
Random blood glucose and fasted plasma insulin are reduced in Srr KO mice. (A-B) Random (A) and 14-hour fasted (B) body weight (g) of adult Srr WT (white) and KO (black) female and male mice (n = 11–14). (C) Random blood glucose (mg/dL) from tail vein, sampled in the morning (n = 19–21). D: 14-hour fasted blood glucose (n = 11–13). (E) Random plasma insulin (ng/mL) from facial vein, determined by ELISA (n = 11–14). (E) 14-hour fasted plasma insulin showing a significant decrease in female Srr KO mice (n = 5–6). Mice were age-matched between genotypes across a range of 2–4 months. *p < 0.05, **p < 0.01, ***p < 0.001 by unpaired Student t-test.
Next, we assessed how Srr KO mice would respond to a glucose or insulin challenge following an overnight fast. Glucose tolerance (2 g/kg ip) was significantly improved in both Srr KO female (p < 0.01) and male (p < 0.05) mice (Fig. 3A and B). Insulin sensitivity was improved but only in male Srr KO mice (p < 0.05) compare with WT controls (Fig. 3C and D). Next we tested whether this increase in insulin sensitivity could also be detected at the tissue level. However, the weights of insulin sensitive peripheral tissues (visceral fat and liver) were not impacted by genotype (p > 0.6) (Supplemental Fig. 1A and B) nor were the basal or insulin-stimulated concentrations of liver phospho-Akt (p = 0.36), a marker for insulin receptor activation (Supplemental Fig. 1C and D). Together, these data show that Srr KO mice have lower levels of random blood glucose and fasting insulin with concomitant improvements in glucose tolerance and insulin sensitivity.
Figure 3.
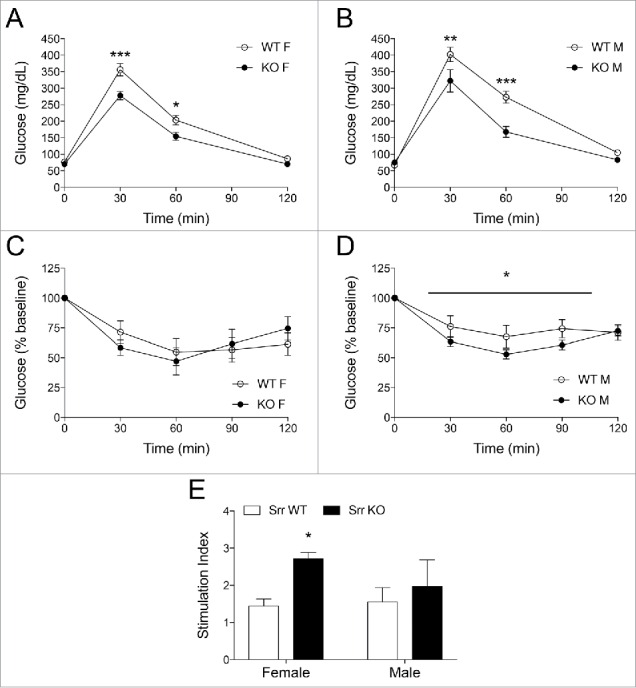
Srr KO mice showed improved glucose tolerance, insulin sensitivity and in vivo glucose-stimulated insulin secretion. (A-B) IPGTT for Srr KO (black) vs. Srr WT (white) in female (A) and male (B) mice (n = 6–8). C-D: ITT in female (C) and male (D) mice, normalized to baseline. (E) In vivo GSIS stimulation index (post-glucose insulin/fasted insulin) (n = 4–5). All tests performed after a 14-hour fast. *p < 0.05, **p < 0.01, ***p < 0.001 by 2-way ANOVA (A-D, genotype × time) or Student t-test (E).
Srr KO mice display improved insulin secretion capacity
Because we observed lower fasting insulin levels in Srr KO mice compare with control, we hypothesized that Srr KO mice might have an altered response during glucose-stimulated insulin secretion (GSIS). Surprisingly, in vivo GSIS testing revealed a higher stimulation index in Srr KO females (p < 0.01), as calculated by the amount of insulin released after a 3 g/kg glucose injection normalized to the fasting insulin level, although the male stimulation index was normal (p = 0.70) (Fig. 3E). To remove the impact of neural and hormonal stimuli, we isolated islets from Srr KO mice and controls in order to perform an in vitro GSIS. In agreement with the in vivo data, Srr KO islets from female mice secreted more insulin during glucose incubations (2-way ANOVA glucose x genotype, both significant at p < 0.05) (Fig. 4A). Despite the secretory potentiation, female KOs also showed a loss of islet insulin content (p < 0.01) (Fig. 4B) and decreased pancreas weight (p < 0.05) (Fig. 4C). We assessed β-cell area, mass and insulin gene expression (Fig. 4D–F) but found no changes that might explain the deficits. Male islet GSIS and insulin content were largely unaffected by genotype (p > 0.5) (Supplemental Fig. 2A-C). The data here suggest a complicated but significant relationship between Srr loss and insulin secretory capacity, including a sexually dimorphic phenotype, which has been previously observed in this model27 (see discussion for details).
Srr KO islets have reduced NMDA receptor expression and function
Seeking an explanation behind the potentiated secretory phenotype, we investigated islet expression of NMDA receptors, which have been linked to both insulin secretory regulation and Srr activity in published literature. Previous investigations into the Srr KO mouse phenotype have revealed alterations in ionotropic glutamate receptor expression (NMDA, AMPA), specifically the ratio of NMDA/AMPA, in various tissues.29,30 Given that expression of these receptors may modulate β-cell secretory function,9,31 we assessed the expression profile of NMDAR subunits (NR1, NR2A, NR2B) and AMPA receptor subunits (GluA1, GluA2) in Srr KO or WT islets by analyzing their gene expression using qPCR on islet mRNA. The expression of all NMDA receptor subunits appeared lower in the Srr KO lysates, but this was only significant for NR1 (p < 0.01) (Fig. 4E). GluA1 was not reliably detected in samples from either WT or Srr KO mice but GluA2 showed no change in mRNA transcripts (p = 0.35). These data suggest that loss of Srr led to a reduction in NR1 expression level in islets. To test whether NMDAR-mediated functional responses were altered in the Srr KO mice, we exposed Srr WT and KO islets to 10 uM MK-801, a non-competitive NMDAR antagonist that was previously shown to potentiate islet insulin secretion,9 during the course of an in vitro GSIS. As expected, WT islets secreted more insulin in response to high glucose (HG) + MK-801 compare with HG alone (p < 0.05) while KO islets demonstrated no drug response (p > 0.99) (Fig. 4H). Together, these data suggest that NMDAR signaling is altered in Srr KO mice and suggests a functional link between Srr activity and NMDA receptor modulation in islets.
Discussion
In this investigation, we show for the first time that the enzyme serine racemase is robustly expressed in both human and mouse β-cells, confirming and extending an earlier report of mouse islet Srr transcripts, expressed preferentially in β-cells vs. α cells.22 We also showed that lack of Srr induces alterations in glucose homeostasis, specifically improving glucose tolerance by enhancing insulin sensitivity and insulin secretion capacity, despite concomitant losses in fasting and islet insulin content. Finally, we show that Srr KO islets express fewer NMDAR transcripts, notably a significant loss of the NR1 subunit, and a reduced response to MK-801 antagonism that suggests a functional link between Srr activity and NMDA receptor modulation in the pancreas.
Srr was first discovered in 1999 from rat brain lysates32 and has been studied primarily in the central nervous system. Srr catalyzes 2 potential reactions to either break L-serine down into ammonia and pyruvate (α, β elimination) or convert it to D-serine (isomerization). Although it's possible that pyruvate production could drive cellular metabolism by providing a TCA substrate, the magnitude of this contribution is thought to be insignificant compare with glycolytically-derived pyruvate.21 On the other hand, Srr is known to play a large role in regulating D-serine availability in the forebrain11 and, although less well characterized, in some peripheral tissues including the kidney and bone.33 In the present study, we now report that Srr co-localizes with insulin within β-cells, implicating a potential novel production and endogenous function for D-serine in these cells as well. In the brain, Srr-derived D-serine is released from neurons (>80%) or glia (10–20%)34 in a non-vesicular manner, through neutral amino acid transporters (asc-1, ASCT) or through other depolarization-triggered methods, and is then available to bind NMDA receptors on the neural membrane until it is re-absorbed into neighboring glia for degradation.34 Asc-1, ASCT and NMDA receptors have been identified in islets previously,9,20,35 suggesting that a D-serine shuttle, similar to the central nervous system model, might exist in islets to regulate β-cell insulin secretion.
The Srr KO mouse has been used extensively to study the role of Srr and D-serine in vivo but has not been previously characterized for parameters of glucose homeostasis. Our results showed that whole body Srr KO mice have improved glucose tolerance, a novel finding indicating a functional role for Srr in regulating systemic metabolism. The improvement in glucose tolerance is explained, in part, by increased insulin sensitivity. This suggests increased insulin responsiveness in peripheral tissues, some of which are known to express Srr (e.g., liver, kidney). Srr's functional role in the liver is not clear, aside from a potential role in promoting hepatic cancer growth.33 Interestingly, equivalent hepatic D-serine concentrations were found in Srr WT and KO livers,18 suggesting Srr-independent source of D-serine. We examined insulin receptor activation (i.e., phosphorylation of Akt at Ser473) in WT and Srr KO liver tissue but observed no differences in basal or insulin-stimulated conditions. However, a potentiation of Akt-independent insulin signaling cannot be ruled out. It is also possible that the source of improved insulin sensitivity lies in other metabolic tissues, such as fat and skeletal muscle, which are common sites of insulin-stimulated glucose uptake. However, Srr expression has only been shown in the latter tissue36 and no functional studies have been undertaken on Srr's role in muscle. Srr is highly expressed in the kidney, an organ that plays a role in glucose reabsorption/clearance, where Srr-derived D-serine has been linked to NMDAR-mediated constriction of renal perfusion in rats.37 More studies are clearly needed to understand the full extent of Srr's function in peripheral tissues.
We also uncovered in vivo and in vitro evidence that female Srr KO mice have a stronger glucose-stimulated insulin release capacity. These data were surprising given that Srr KO mice are generally considered a model of NMDAR hypofunction.38 In the hippocampus, for example, Srr loss leads to suppression of long-term potentiation,38 a physiological correlate of β-cell stimulus-secretion coupling. In most regions of the brain, D-serine activation of NMDA receptors promotes vesicular release and synaptic remodeling such that its acute absence leads to a weaker immediate and adaptive stimulus response.11,27 However, this physiological model is predicated on NMDAR activation leading to an excitation of the cell. By contrast, a recent study by et al. suggests that the net effect of β-cell NMDAR activation is to hyperpolarize the cell and decrease insulin secretion.9 Furthermore, they showed that inhibiting NMDARs, through pharmacological blockers or transgenic deletion of β-cell NR1, potentiates insulin secretion,9 a finding we re-iterate in part here with our MK-801 study. Therefore, islets from Srr KO mice may secrete more insulin because a loss of synthesized D-serine reduces the activation of β-cell NMDARs. It is important to note that a previous report found no changes in pancreatic D-serine concentration between Srr WT and KO animals, however.18 This may be because localized changes in D-serine production are not detectable in a gross tissue assay (D-serine can also arise from dietary or bacterial sources) but a measurement of islet-specific D-serine concentrations would be critical to any future investigation of this hypothesis. Another possibility for Srr KO secretory potentiation arises from previous KO studies reporting tissue-dependent changes in NMDAR expression, suggesting that Srr activity may play a role in regulating dynamic30 or developmentally-driven15,39 levels of NMDARs. We found a reduction in NR1 mRNA in Srr KO islets, as well as a trend toward decreased NR2A, while expression levels of the D-serine independent AMPA receptor subunit GluA2 were unchanged. Thus, the enhanced insulin secretory capacity we observed in our data may be due to loss of NMDAR (NR1) expression, similar to the phenotype of the β-cell Grin1 (NR1) KO mouse.9 Further supporting this hypothesis, Srr KO islets were resistant to the secretion-potentiating effects of an NMDAR antagonist, MK-801, consistent with the idea of diminished expression or activation of β-cell NMDARs.
A potential role for Srr in pancreas development may explain why we observed a mild but significant reduction in pancreatic weight and insulin content in Srr KO mice. Srr is expressed in the placenta15 and has been implicated in the differentiation of multiple cell types.33 In the brain, Srr-derived D-serine has been linked to neural migration and synaptogenesis11 with Srr KO mice showing reduced hippocampal tissue volume and neural complexity.38 Therefore, it seems possible that Srr activity may also play a role in pancreatic development and endocrine cell differentiation. In fact, mTORC1 signaling is reduced in the Srr KO hippocampus, a well-known regulatory complex that promotes protein synthesis, cell growth and differentiation in many tissues, including pancreatic β-cells.38,40 Nevertheless, we did not see a change in gross β-cell area or mass, despite the 20% decrease in pancreas weight. While this does not negate the possibility of an Srr influence on endocrine cell fate and gene expression, it is also possible that the change in pancreatic mass arises from deficits in exocrine tissue development, which cells were recently found to express Srr transcripts in humans.23 Thus it would be interesting in the future to explore the potential role of Srr in pancreas development.
Another notable aspect of the Srr KO metabolic phenotype was the disparity between the sexes on some parameters. We observed enhanced secretory potency primarily in female Srr KO mice while males showed more prominent increases in insulin sensitivity. It is possible that in these males, any mechanisms of enhanced secretion are counterbalanced by the reduced secretory demand of the more insulin-sensitive peripheral tissues. Although it is beyond the original scope of the current manuscript, it is important to point out differences in insulin sensitivity in peripheral tissue and insulin secretion capacity and insulin content, between female and male Srr KO. Indeed, sexually dimorphic characteristics have been observed in previous studies on this mouse model. Notably, Srr KO males have been characterized with hyperactivity and spatial learning deficits while female mice, lacking these traits, instead showed mild anxiety and an enhanced startle response.27 The fact that D-serine levels are similarly effected by Srr KO in either sex suggests a yet unknown relationship between sex and either NMDAR function27 or adaptive responses to Srr loss that extend beyond the central nervous system. Recognizing the limitations of the current manuscript, future studies could focus on in dissecting the mechanisms behind the divergence of male and female Srr KO phenotype.
In the current study, we have shown that the enzyme serine racemase is robustly expressed in islet β-cells and that its systemic loss leads to changes in multiple aspects of glucose homeostasis. Our data implicate Srr activity in peripheral insulin sensitivity, β-cell secretory capacity and pancreatic development, identifying it as a novel and potentially broad-ranging regulator of metabolic function.
Supplementary Material
Abbreviations
- GSIS
Glucose-Stimulated Insulin Secretion
- IF
Immunofluorescence.
- NMDAR
NMDA receptor
- Srr
Serine Racemase
- Srr KO
Serine Racemase −/−
- Srr WT
Serine Racemase +/+
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Ms. Nathalia Torres Jimenez for her assistance with husbandry and Mr. Seokwon Jo for technical assistance as well as Dr. Thomas Pengo for instruction in β-cell mass analysis techniques. We thank Dr. Anthony Weinhaus for providing human pancreas tissue and Dr. Robert Sorenson and Dr. Cyprian Weaver for their guidance and assessment of tissue procurement quality. We thank Drs. Alessandro Bartolomucci and David Bernlohr for discussions.
Funding
This work was supported by National Institutes of Health Grant NIDDK (K01-DK-103823 to EUA), Committee for Pharmaceutical Development of the Minnesota Clinical and Translational Science Institute (5UL1TR000114-05) and the Department of Integrated Biology and Physiology. RFM is supported through NIH (R21 EY025027).
ORCID
Brian Akhaphong http://orcid.org/0000-0002-1268-6623
Alleah Abrenica http://orcid.org/0000-0001-7299-206X
References
- [1].Atouf F, Czernichow P, Scharfmann R. Expression of neuronal traits in pancreatic beta cells. Implication of neuron-restrictive silencing factor/repressor element silencing transcription factor, a neuron-restrictive silencer. J Biol Chem 1997; 272:1929-34; PMID:8999882; http://dx.doi.org/ 10.1074/jbc.272.3.1929 [DOI] [PubMed] [Google Scholar]
- [2].Martens GA, Jiang L, Hellemans KH, Stange G, Heimberg H, Nielsen FC, Sand O, Van Helden J, Van Lommel L, Schuit F, et al.. Clusters of conserved beta cell marker genes for assessment of beta cell phenotype. PLoS One 2011; 6:e24134; PMID:21912665; http://dx.doi.org/ 10.1371/journal.pone.0024134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Eberhard D. Neuron and beta-cell evolution: learning about neurons is learning about beta-cells. Bioessays 2013; 35:584; PMID:23575922; http://dx.doi.org/ 10.1002/bies.201300035 [DOI] [PubMed] [Google Scholar]
- [4].Yang SN, Shi Y, Yang G, Li Y, Yu J, Berggren PO. Ionic mechanisms in pancreatic beta cell signaling. Cell Mol Life Sci 2014; 71:4149-77; PMID:25052376; http://dx.doi.org/ 10.1007/s00018-014-1680-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kebede M, Ferdaoussi M, Mancini A, Alquier T, Kulkarni RN, Walker MD, Poitout V. Glucose activates free fatty acid receptor 1 gene transcription via phosphatidylinositol-3-kinase-dependent O-GlcNAcylation of pancreas-duodenum homeobox-1. Proc Natl Acad Sci U S A 2012; 109:2376-81; PMID:22308370; http://dx.doi.org/ 10.1073/pnas.1114350109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].GJd Santos, Ferreira SM, Ortis F, Rezende LF, Li C, Naji A, Carneiro EM, Kaestner KH, Boschero AC. Metabolic memory of ß-cells controls insulin secretion and is mediated by CaMKII. Mol Metabol 2014; 3:484-9; PMID:24944908; http://dx.doi.org/ 10.1016/j.molmet.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alejandro EU, Gregg B, Wallen T, Kumusoglu D, Meister D, Chen A, Merrins MJ, Satin LS, Liu M, Arvan P, et al.. Maternal diet-induced microRNAs and mTOR underlie beta cell dysfunction in offspring. J Clin Invest 2014; 124:4395-410; PMID:25180600; http://dx.doi.org/ 10.1172/JCI74237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alarcon C, Boland BB, Uchizono Y, Moore PC, Peterson B, Rajan S, Rhodes OS, Noske AB, Haataja L, Arvan P, et al.. Pancreatic beta-Cell Adaptive Plasticity in Obesity Increases Insulin Production but Adversely Affects Secretory Function. Diabetes 2016; 65:438-50; PMID:26307586; http://dx.doi.org/ 10.2337/db15-0792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Marquard J, Otter S, Welters A, Stirban A, Fischer A, Eglinger J, Herebian D, Kletke O, Klemen MS, Stozer A, et al.. Characterization of pancreatic NMDA receptors as possible drug targets for diabetes treatment. Nat Med 2015; 21:363-72; PMID:25774850; http://dx.doi.org/ 10.1038/nm.3822 [DOI] [PubMed] [Google Scholar]
- [10].Volianskis A, France G, Jensen MS, Bortolotto ZA, Jane DE, Collingridge GL. Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Res 2015; 1621:5-16; PMID:25619552; http://dx.doi.org/ 10.1016/j.brainres.2015.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bardaweel SK, Alzweiri M, Ishaqat AA. D-Serine in neurobiology: CNS neurotransmission and neuromodulation. Can J Neurol Sci 2014; 41:164-76; PMID:24534026; http://dx.doi.org/ 10.1017/S031716710001653X [DOI] [PubMed] [Google Scholar]
- [12].Mothet JP, Le Bail M, Billard JM. Time and space profiling of NMDA receptor co-agonist functions. J Neurochem 2015; 135:210-25; PMID:26088787; http://dx.doi.org/ 10.1111/jnc.13204 [DOI] [PubMed] [Google Scholar]
- [13].Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW. Glycine binding primes NMDA receptor internalization. Nature 2003; 422:302-7; PMID:12646920; http://dx.doi.org/ 10.1038/nature01497 [DOI] [PubMed] [Google Scholar]
- [14].Nong Y, Huang YQ, Salter MW. NMDA receptors are movin' in. Curr Opin Neurobiol 2004; 14:353-61; PMID:15194116; http://dx.doi.org/ 10.1016/j.conb.2004.05.001 [DOI] [PubMed] [Google Scholar]
- [15].Chen Z, Huang W, Srinivas SR, Jones CR, Ganapathy V, Prasad PD. Serine racemase and D-serine transport in human placenta and evidence for a transplacental gradient for D-serine in humans. J Soc Gynecol Investig 2004; 11:294-303; PMID:15219883; http://dx.doi.org/ 10.1016/j.jsgi.2004.02.003 [DOI] [PubMed] [Google Scholar]
- [16].Romero GE, Lockridge AD, Morgans CW, Bandyopadhyay D, Miller RF. The postnatal development of D-serine in the retinas of two mouse strains, including a mutant mouse with a deficiency in D-amino acid oxidase and a serine racemase knockout mouse. ACS Chem Neurosci 2014; 5:848-54; PMID:25083578; http://dx.doi.org/ 10.1021/cn5000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Imai K, Fukushima T, Santa T, Homma H, Huang Y, Sakai K, Kato M. Distribution of free D-amino acids in tissues and body fluids of vertebrates. Enantiomer 1997; 2:143-5; PMID:9676266 [PubMed] [Google Scholar]
- [18].Horio M, Kohno M, Fujita Y, Ishima T, Inoue R, Mori H, Hashimoto K. Levels of D-serine in the brain and peripheral organs of serine racemase (Srr) knock-out mice. Neurochem Int 2011; 59:853-9; PMID:21906644; http://dx.doi.org/ 10.1016/j.neuint.2011.08.017 [DOI] [PubMed] [Google Scholar]
- [19].Miyoshi Y, Hamase K, Okamura T, Konno R, Kasai N, Tojo Y, Zaitsu K. Simultaneous two-dimensional HPLC determination of free D-serine and D-alanine in the brain and periphery of mutant rats lacking D-amino-acid oxidase. J Chromatogr B Analyt Technol Biomed Life Sci 2011; 879:3184-9; PMID:20851062; http://dx.doi.org/ 10.1016/j.jchromb.2010.08.024 [DOI] [PubMed] [Google Scholar]
- [20].Zhou Y, Waanders LF, Holmseth S, Guo C, Berger UV, Li Y, Lehre AC, Lehre KP, Danbolt NC. Proteome analysis and conditional deletion of the EAAT2 glutamate transporter provide evidence against a role of EAAT2 in pancreatic insulin secretion in mice. J Biol Chem 2014; 289:1329-44; PMID:24280215; http://dx.doi.org/ 10.1074/jbc.M113.529065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wolosker H, Mori H. Serine racemase: an unconventional enzyme for an unconventional transmitter. Amino Acids 2012; 43:1895-904; PMID:22847782; http://dx.doi.org/ 10.1007/s00726-012-1370-3 [DOI] [PubMed] [Google Scholar]
- [22].Benner C, van der Meulen T, Caceres E, Tigyi K, Donaldson CJ, Huising MO. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics 2014; 15:620; PMID:25051960; http://dx.doi.org/ 10.1186/1471-2164-15-620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Segerstolpe A, Palasantza A, Eliasson P, Andersson EM, Andreasson AC, Sun X, Picelli S, Sabirsh A, Clausen M, Bjursell MK, et al.. Single-Cell Transcriptome Profiling of Human Pancreatic Islets in Health and Type 2 Diabetes. Cell Metab 2016; PMID:27667667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tsai FJ, Yang CF, Chen CC, Chuang LM, Lu CH, Chang CT, Wang TY, Chen RH, Shiu CF, Liu YM, et al.. A genome-wide association study identifies susceptibility variants for type 2 diabetes in Han Chinese. PLoS Genet 2010; 6:e1000847; PMID:20174558; http://dx.doi.org/ 10.1371/journal.pgen.1000847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Y, Nie M, Li W, Ping F, Hu Y, Ma L, Gao J, Liu J. Association of six single nucleotide polymorphisms with gestational diabetes mellitus in a Chinese population. PLoS One 2011; 6:e26953; PMID:22096510; http://dx.doi.org/ 10.1371/journal.pone.0026953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dong M, Gong ZC, Dai XP, Lei GH, Lu HB, Fan L, Qu J, Zhou HH, Liu ZQ. Serine racemase rs391300 G/A polymorphism influences the therapeutic efficacy of metformin in Chinese patients with diabetes mellitus type 2. Clin Exp Pharmacol Physiol 2011; 38:824-9; PMID:21933224; http://dx.doi.org/ 10.1111/j.1440-1681.2011.05610.x [DOI] [PubMed] [Google Scholar]
- [27].Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, et al.. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry 2009; 14:719-27; PMID:19065142; http://dx.doi.org/ 10.1038/mp.2008.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Alejandro EU, Bozadjieva N, Kumusoglu D, Abdulhamid S, Levine H, Haataja L, Vadrevu S, Satin LS, Arvan P, Bernal-Mizrachi E. Disruption of O-linked N-Acetylglucosamine Signaling Induces ER Stress and beta Cell Failure. Cell Rep 2015; 13:2527-38; PMID:26673325; http://dx.doi.org/ 10.1016/j.celrep.2015.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sullivan SJ, Esguerra M, Wickham RJ, Romero GE, Coyle JT, Miller RF. Serine racemase deletion abolishes light-evoked NMDA receptor currents in retinal ganglion cells. J Physiol 2011; 589:5997-6006; PMID:22041185; http://dx.doi.org/ 10.1113/jphysiol.2011.217059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Balu DT, Coyle JT. Glutamate receptor composition of the post-synaptic density is altered in genetic mouse models of NMDA receptor hypo- and hyperfunction. Brain Res 2011; 1392:1-7; PMID:21443867; http://dx.doi.org/ 10.1016/j.brainres.2011.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jayanarayanan S, Anju TR, Smijin S, Paulose CS. Vitamin D3 supplementation increases insulin level by regulating altered IP3 and AMPA receptor expression in the pancreatic islets of streptozotocin-induced diabetic rat. J Nutr Biochem 2015; 26:1041-9; PMID:26054778; http://dx.doi.org/ 10.1016/j.jnutbio.2015.04.011 [DOI] [PubMed] [Google Scholar]
- [32].Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci U S A 1999; 96:13409-14; PMID:10557334; http://dx.doi.org/ 10.1073/pnas.96.23.13409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Montesinos Guevara C, Mani AR. The role of D-serine in peripheral tissues. Eur J Pharmacol 2016; 780:216-23; PMID:27038518; http://dx.doi.org/ 10.1016/j.ejphar.2016.03.054 [DOI] [PubMed] [Google Scholar]
- [34].Wolosker H, Radzishevsky I. The serine shuttle between glia and neurons: implications for neurotransmission and neurodegeneration. Biochem Soc Trans 2013; 41:1546-50; PMID:24256252; http://dx.doi.org/ 10.1042/BST20130220 [DOI] [PubMed] [Google Scholar]
- [35].Molnar E, Varadi A, McIlhinney RA, Ashcroft SJ. Identification of functional ionotropic glutamate receptor proteins in pancreatic beta-cells and in islets of Langerhans. FEBS Lett 1995; 371:253-7; PMID:7556603; http://dx.doi.org/ 10.1016/0014-5793(95)00890-L [DOI] [PubMed] [Google Scholar]
- [36].Xia M, Liu Y, Figueroa DJ, Chiu CS, Wei N, Lawlor AM, Lu P, Sur C, Koblan KS, Connolly TM. Characterization and localization of a human serine racemase. Brain Res Mol Brain Res 2004; 125:96-104; PMID:15193426; http://dx.doi.org/ 10.1016/j.molbrainres.2004.03.007 [DOI] [PubMed] [Google Scholar]
- [37].Lin CS, Hung SF, Huang HS, Ma MC. Blockade of the N-Methyl-D-Aspartate Glutamate Receptor Ameliorates Lipopolysaccharide-Induced Renal Insufficiency. PLoS One 2015; 10:e0132204; PMID:26133372; http://dx.doi.org/ 10.1371/journal.pone.0132204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Balu DT, Li Y, Puhl MD, Benneyworth MA, Basu AC, Takagi S, Bolshakov VY, Coyle JT. Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc Natl Acad Sci U S A 2013; 110:E2400-9; PMID:23729812; http://dx.doi.org/ 10.1073/pnas.1304308110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gustafson EC, Morgans CW, Tekmen M, Sullivan SJ, Esguerra M, Konno R, Miller RF. Retinal NMDA receptor function and expression are altered in a mouse lacking D-amino acid oxidase. J Neurophysiol 2013; 110:2718-26; PMID:24068757; http://dx.doi.org/ 10.1152/jn.00310.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Blandino-Rosano M, Chen AY, Scheys JO, Alejandro EU, Gould AP, Taranukha T, Elghazi L, Cras-Meneur C, Bernal-Mizrachi E. mTORC1 signaling and regulation of pancreatic beta-cell mass. Cell Cycle 2012; 11:1892-902; PMID:22544327; http://dx.doi.org/ 10.4161/cc.20036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Takahata Y, Takarada T, Osawa M, Hinoi E, Nakamura Y, Yoneda Y. Differential regulation of cellular maturation in chondrocytes and osteoblasts by glycine. Cell Tissue Res 2008; 333:91-103; PMID:18427837; http://dx.doi.org/ 10.1007/s00441-008-0607-7 [DOI] [PubMed] [Google Scholar]
- [42].Dzamba D, Honsa P, Valny M, Kriska J, Valihrach L, Novosadova V, Kubista M, Anderova M. Quantitative Analysis of Glutamate Receptors in Glial Cells from the Cortex of GFAP/EGFP Mice Following Ischemic Injury: Focus on NMDA Receptors. Cell Mol Neurobiol 2015; 35:1187-202; PMID:25994914; http://dx.doi.org/ 10.1007/s10571-015-0212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


