ABSTRACT
Free fatty acid receptor 1 (FFA1/GPR40) plays a key role in the potentiation of glucose-stimulated insulin secretion by fatty acids in pancreatic β cells. We previously demonstrated that GPR40 signaling leads to cortical actin remodeling and potentiates the second phase of insulin secretion. In this study, we examined the role of p21 activated kinase 4 (PAK4), a known regulator of cytoskeletal dynamics, in GPR40-dependent potentiation of insulin secretion. The fatty acid oleate induced PAK4 phosphorylation in human islets, in isolated mouse islets and in the insulin secreting cell line INS832/13. However, oleate-induced PAK4 phosphorylation was not observed in GPR40-null mouse islets. siRNA-mediated knockdown of PAK4 in INS832/13 cells abrogated the potentiation of insulin secretion by oleate, whereas PAK7 knockdown had no effect. Our results indicate that PAK4 plays an important role in the potentiation of insulin secretion by fatty acids downstream of GPR40.
KEYWORDS: Fatty acids, free fatty acid receptor 1, G protein-coupled receptor 40, Insulin secretion, P21-activated kinase
Introduction
The maintenance of glucose homeostasis relies on the precise regulation of insulin secretion from the pancreatic β cell, alterations of which cause type 2 diabetes. Glucose is the main trigger for insulin secretion and in healthy individuals an elevation of blood glucose levels induces biphasic insulin secretion. Following uptake by the β cell, glucose metabolism leads to closure of ATP-dependent K+ channels, membrane depolarization, opening of voltage-sensitive Ca2+ channels, and insulin exocytosis.1 The fusion of docked and primed insulin secretory granules dominates the acute 1st phase, whereas the 2nd phase is characterized by the priming of pre-docked granules and mobilization of intracellular granule stores to docking sites at the membrane prior to fusion, a process that is facilitated by cortical actin remodeling.2 In addition to glucose, a number of metabolic, hormonal, and neural signals modulate insulin secretion. Among those, medium to long-chain free fatty acids (FFA) potentiate glucose-stimulated insulin secretion (GSIS) in large part by binding to the G protein-coupled receptor free fatty acid receptor 1 (FFA1/GPR40).3,4 Because FFA do not trigger insulin release at low glucose levels but only augment insulin secretion when glucose levels are elevated, therapeutic compounds that activate GPR40 offer the potential benefit of enhancing insulin secretion without the associated risk of iatrogenic hypoglycemia. One such compound, TAK-875, showed promising results in early clinical trials5 providing an impetus for further research into the mechanism of GPR40 signaling.
Activation of GPR40 by FFA potentiates the 2nd phase of insulin secretion.6 GPR40 mainly couples to the Gαq/11 G-protein subunit, which leads to phospholipase C (PLC)-mediated membrane lipid hydrolysis, generation of diacylglycerol (DAG), activation of protein kinase D1 (PKD1), and cortical actin remodeling.6 However, the precise mechanisms by which GPR40-dependent signaling controls 2nd phase insulin secretion remain to be elucidated.
The serine/threonine p21-activated kinase (PAK) family proteins are critical regulators of cell proliferation, cell survival and cytoskeletal reorganization and are divided in 2 groups based on their domain architecture and regulation.7 Group I PAKs includes PAK1, PAK2 and PAK3 and group II PAKs includes PAK4, PAK6 and PAK7 (also known as PAK5). The activity of PAK proteins is regulated by phosphorylation. PAK4 phosphorylation on serine 99 (S99) regulates its localization, protein complex formation and activity in cytoskeletal remodeling.8 On the other hand, S474 in the activation loop of the kinase domain is considered as an auto-phosphorylation site. S474 phosphorylation is associated with translocation of PAK4 from the cytoplasm to the Golgi and correlates with kinase activity.9
Recent studies suggest PAKs are important regulators of insulin secretion. PAK1 is necessary for GSIS in mouse and human islets, regulating glucose-dependent actin dynamics via ERK1/2 and the LIM kinase-ADF/COFILIN pathways.10-13 In vivo, PAK1 deficiency in mice compromises insulin secretion and glucose tolerance under basal and metabolic stress conditions.11,14 Interestingly, PAK1 expression is decreased in islets from diabetic patients.11 In addition, knockdown of PAK7 in insulin-secreting cells impairs GSIS and genome-wide expression analysis identified an inverse correlation between PAK7 expression in islets and HbA1c levels.15 These studies suggest that deregulation of PAK1 or PAK7 may contribute to diabetes. However, despite the compelling data implicating PAKs in GSIS, to our knowledge, a potential role of PAKs as effectors of FFA potentiation of insulin secretion has not been investigated. Given the implication of PAKs in cortical actin remodeling,10-13 we investigated the role of the group II PAK4 in the potentiation of insulin secretion by FFA.
Results and discussion
Oleate induces PAK4 phosphorylation in human islets
We first investigated whether FFA induce PAK4 phosphorylation on S99 by Western blotting of human extracts exposed to the long chain mono-unsaturated FFA oleate, a potent GPR40 agonist.6 As antibodies against total PAK4 protein were unreliable, phospho-PAK4 (S99) levels were normalized to tubulin. Following a 5 min exposure to 0.5 mM oleate, PAK4 phosphorylation was significantly increased compared to 5 mM glucose alone (Fig. 1A and B). These data suggest that PAK4 phosphorylation on S99 is stimulated by FFA in human islets.
Figure 1.
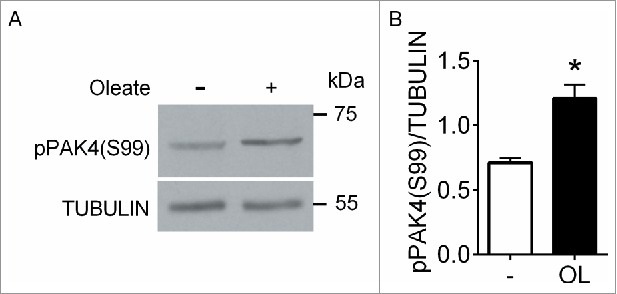
Oleate induces PAK4 phosphorylation in human islets. (A) Protein extracts from human islets stimulated for 5 min with 5 mM glucose with or without 0.5 mM oleate were analyzed by Western blot for phospho-PAK4 (S99) and tubulin. (B) Quantification of phospho-PAK4 (S99) normalized to tubulin. Data are mean ± SEM for 4 independent experiments; *, p < 0 .05 compared to 5 mM glucose.
Oleate and glucose induce PAK4 phosphorylation in INS832/13 cells
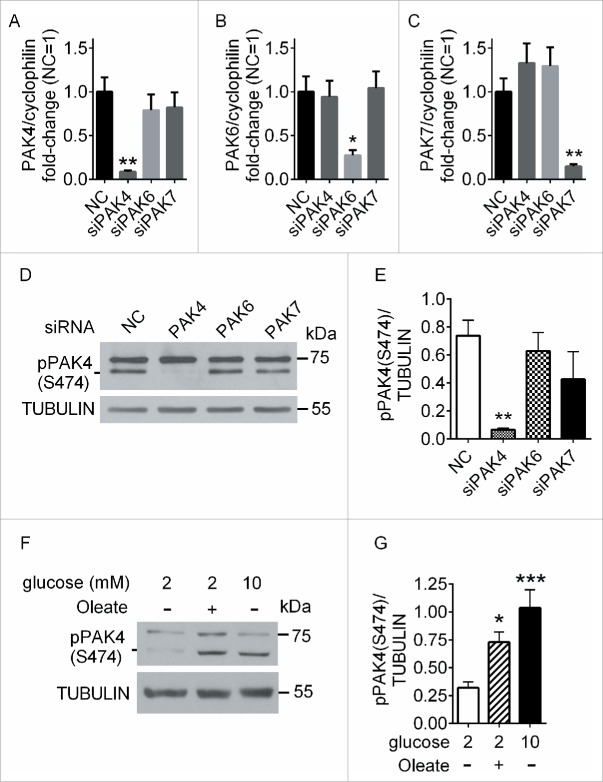
We then studied the role of PAK4 in insulin secretion in INS832/13 cells since they are amenable to siRNA-mediated knockdown and the potentiating effects of FFA on insulin secretion is GPR40-dependent in these cells.16 We first confirmed that PAK4 was phosphorylated in INS832/13 cells in response to oleate. We were unable to detect phospho-PAK4 (S99) by Western blotting (data not shown), although the antibody was expected to react with both rat and human proteins. As an alternative, we used an antibody against phospho-group II PAKs. To confirm phospho-PAK4 (S474) could be detected in Western blots with this phospho-group II PAK antibody, we knocked-down PAK4, PAK6 or PAK7 using siRNAs. Reverse transcriptase-quantitative PCR (RT-qPCR) analyses of INS832/13 cells transfected with siRNA to PAK4, PAK6 or PAK7 revealed a significant and specific knockdown of each PAK (PAK4, 91%; PAK6, 72%; PAK7, 85% reduction compared to control siRNA) (Fig. 2A–C). In Western blots, only PAK4 knockdown lead to the disappearance of an approximately 70 kDa band (the expected molecular weight of PAK4), which therefore corresponds to phospho-PAK4 (S474) (Fig. 2D and E). Treatment of INS832/13 cells with 0.5 mM oleate led to a significant increase in PAK4 phosphorylation on S474 compared to cells exposed to 2 mM glucose alone (Fig. 2F and G). Interestingly, treatment with 10 mM glucose alone also led to a significant increase in PAK4 S474 phosphorylation compare to 2 mM glucose (Fig. 2F and G). Hence, PAK4 is phosphorylated on S474 in response to glucose or oleate in INS832/13 cells.
Figure 2.
Glucose and oleate induces PAK4 phosphorylation in INS832/13 cells. (A-C) INS832/13 cells were electroporated with negative control siRNA (NC) or siRNA targeting PAK4 (A), PAK6 (B) or PAK7 (C). 48 h later RNA was extracted and analyzed by RT-qPCR. Quantification of knockdown efficiency is presented as Ct values normalized to cyclophilin for each PAK compared to the normalized Ct value of the negative control and are mean ± SEM of 5 independent experiments; *, p < 0.05; **, p < 0.01 compared to the negative control. (D) INS832/13 cells electroporated with NC or siRNA targeting PAK4, PAK6 or PAK7 were stimulated for 15 min with 10 mM glucose and 0.5 mM oleate and analyzed by Western blot for phospho-group II PAKs and tubulin. (E) Quantification of phospho-PAK4 (S474) normalized to tubulin. Data are mean ± SEM of 5 individual experiments; **, p < 0.01 compared to the negative control. (F) INS832/13 stimulated for 2 min with 2 mM glucose alone, 2 mM glucose + 0.5 mM oleate or 10 mM glucose alone, were analyzed by Western blot for phospho-group II PAKs and tubulin (G) Quantification of phospho-PAK4 (S474) normalized to tubulin. Data are mean ± SEM for 6 independent experiments; *, p < 0.05; ***, p < 0.001 compared to 2 mM glucose alone.
Oleate-induced PAK4 phosphorylation is GPR40-dependent
To verify whether PAK4 phosphorylation was similarly regulated in primary β cells, we first exposed isolated mouse islets to 2.8 vs 16.7 mM glucose for 5 min (Fig. 3A and B). In contrast to INS832/13 cells, glucose stimulation of islets did not lead to an increase in PAK4 S474 phosphorylation. We then analyzed phospho-PAK4 (S474) levels following oleate stimulation of wild-type (WT) and GPR40-null (GPR40KO) mouse islets. Stimulation of WT islets for 5 min with 0.5 mM oleate in the presence of either 2.8 (Fig. 3C and D) or 16.7 (Fig. 3E and F) mM glucose led to a significant increase in phospho-PAK4 (S474). This increase was not observed in GPR40KO islets (Fig. 3C–F) suggesting that PAK4 S474 phosphorylation in response to oleate is GPR40-dependent in mouse islets.
Figure 3.
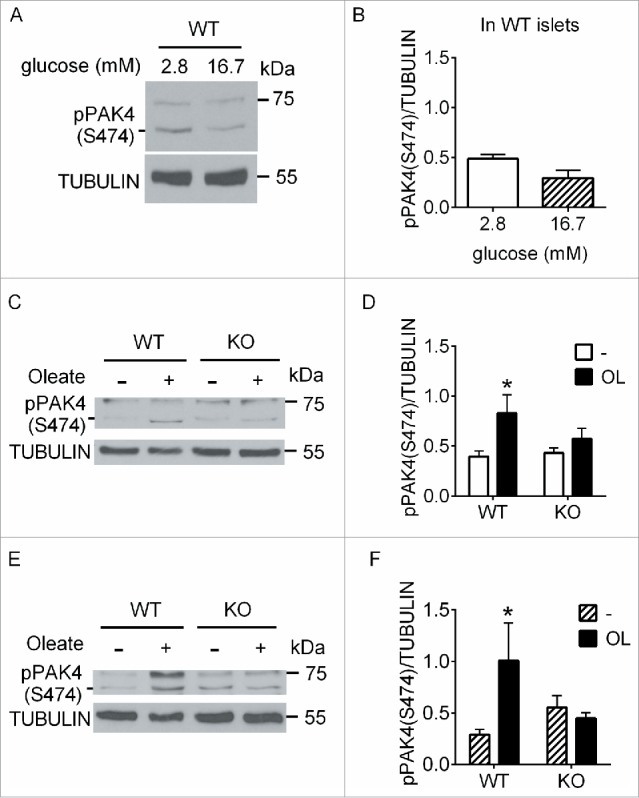
PAK4 phosphorylation in response to oleate is GPR40-dependent. (A) Islets from wild-type (WT) mice stimulated for 5 min with 2.8 mM glucose or 16.7 mM glucose were analyzed by Western blot for phospho-group II PAKs and tubulin. (B) Quantification of phospho-PAK4 (S474) normalized to tubulin. Data are mean ± SEM of 4 independent experiments. (C and E) Islets from wild-type (WT) and GPR40-null (KO) mice stimulated for 5 min with 2.8 mM glucose (C) or 16.7 mM glucose (E) with or without 0.5 mM oleate were analyzed by Western blot for phospho-group II PAKs and tubulin. (D and F) Quantification of phospho-PAK4 (S474) normalized to tubulin. Data are mean ± SEM of 4–6 independent experiments; *, p < 0.05; compared to 2.8 mM (D) or 16.7 mM (F) glucose.
PAK4 is necessary for the potentiation of insulin secretion by oleate
To examine the functional impact of PAK4 phosphorylation in response to oleate we investigated its role in insulin secretion in INS832/13 cells. Despite the increased PAK4 phosphorylation in response to glucose in INS832/13 cells (Fig. 2F), siRNA-mediated PAK4 knockdown had no impact on insulin secretion induced by 10 mM glucose alone. In contrast, PAK4 knockdown completely blocked the potentiation of insulin secretion in response to 0.5 mM oleate (Fig. 4). Conversely, PAK7 knockdown reduced insulin secretion in response to glucose alone as previously reported,15 but did not alter the potentiating effect of oleate (Fig. 4). These results indicate PAK4 is necessary for the potentiation of insulin secretion by FFA.
Figure 4.
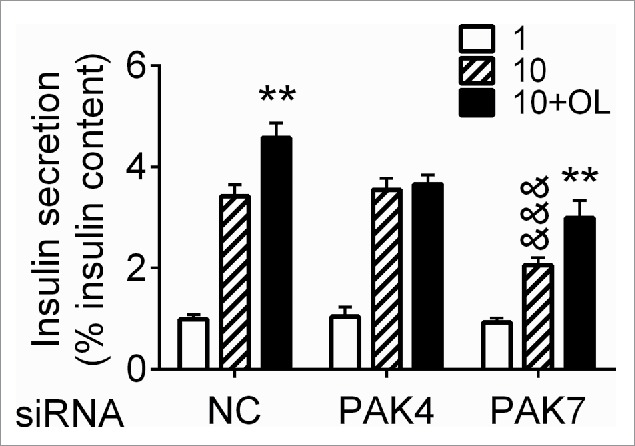
PAK4 is necessary for oleate potentiation of insulin secretion. INS832/13 cells were electroporated with negative control siRNA (NC) or siRNA targeting PAK4 or PAK7. 48 h later, cells were treated for 1 h with 1 mM glucose, 10 mM glucose or 10 mM glucose and 0.5 mM oleate and analyzed for insulin secretion. Insulin secretion is presented as a percentage of insulin content and is the mean ± SEM for 5 independent experiments run in triplicates; &&&, p < 0.001 compared to the 10 mM glucose condition for the negative control, **, p < 0.01 compared to the corresponding 10 mM glucose condition.
Several candidate signaling molecules could be implicated in PAK4 phosphorylation/activation downstream of GPR40. CDC42 participates in PAK4 activation and translocation to actin in fibroblasts and endothelial cells17 and RAC1 activation of ADF/COFILIN is group II PAK-dependent in platelets.18 However, unlike group I PAK members, binding of CDC42/RAC1 to PAK4 is not sufficient for activation19 and whereas CDC42 participates in GSIS via activation of PAK1,10 evidence supporting a role of these GTPases in GPR40 signaling is lacking. In murine mammary gland NMuMG and HeLa cells the activity and subcellular localization of PAK4 is regulated by protein kinase D1 (PKD1) via phosphorylation on S99 and the activation loop S474.8,20,21 Furthermore, activated PKD1 co-localizes with PAK4 on actin filaments in HuMEC cells.20 We previously showed that PKD1 is activated downstream of GPR40 and is necessary for potentiation of insulin secretion by FFA.6 Whether CDC42/RAC1 and/or PKD1 are involved in PAK4 activation downstream of GPR40 in β cells will require further investigation.
Previously we showed that cytoskeletal remodeling is coupled with GPR40-dependent potentiation of insulin secretion.6 Actin remodeling is also a prerequisite for efficient 2nd phase insulin secretion in response to glucose22 and many proteins implicated in GSIS regulate actin dynamics. In light of the known role of PAK4 in cytoskeletal reorganization,9,17,23-25 we speculate that actin remodeling events involved in the potentiation of insulin secretion by FFA are mediated at least in part by PAK4. PAK4 may also be implicated in insulin granule exocytosis via activation of GEF-H124 that interacts with the exocyst complex subunit Sec5,26 an important regulator of docking and fusion of newly recruited insulin granule.27 However, as numerous proteins are involved directly and indirectly in actin remodeling and granule fusion, further investigation will be needed to determine which of these proteins are targeted by PAK4 during the potentiation of insulin secretion by FFA.
In summary, we show here for the first time that the group II PAK family member PAK4 is regulated by FFA signaling via GPR40 and is necessary for the potentiation of GSIS. This study provides valuable mechanistic insight into the mechanism of action of GPR40, a receptor considered as a potential therapeutic target for the treatment of type 2 diabetes.
Experimental procedures
Mice, cell lines, antibodies and reagents
WT and GPR40KO mice were generated as previously described.28 Animals were housed on a 12-h light/dark cycle with free access to water and standard laboratory chow. All procedures were approved by the institutional committee for the protection of animals at the Center Hospitalier de l'Université de Montréal. The rat insulin-secreting INS832/13 cell line (passages 50–56; provided by Dr. Christopher Newgard, Duke University School of Medicine, Durham, NC) was cultured in RPMI 1640 medium (Gibco Life Technologies, 11879-020) supplemented with 11 mM glucose, 10% (wt/vol) FBS (Gibco Life Technologies, 12483-020), 10 mM HEPES (pH 7.4), 2.05 mM L-glutamine, 1 mM sodium pyruvate and 50 μmol/l β-mercaptoethanol. Antibodies against phospho-group II PAKs (Cell Signaling, 3241S), phospho-PAK4 (S99) (Bioss, bs-2270R), total PAK4 (Cell Signaling, 3242S) and total PAK4 and tubulin (Abcam, ab19007 and ab4074) were used in Western blotting. Oleate (Sigma, 07501) was pre-complexed for 1 hour at 37°C with fatty-acid-free BSA (Equitech-Bio, BAH66) to a final molar ratio of 1:5 as described.29 Control conditions contained the same amount of BSA and vehicle (50% (vol/vol) ethanol).
siRNA-mediated knockdown, RNA extraction and RT-qPCR
Electroporation of INS832/13 cells was performed using the Amaxa nucleofector and Ingenio electroporation kits according to the manufacturer's protocol (Lonza, 90 Boroline Road Allendale, NJ, USA). 60 0M of Silencer Select Pre-designed siRNAs targeting PAK4 and PAK7 (Life Technologies, s146689 and s61202) were used to transfect 1×106 cells. 48 h after electroporation, total RNA was extracted and knockdown efficiency was determined by RT-qPCR as described previously30 using primers 5′-CTTGCATCACTTCCATCCAGC-3′ and 5′- GTCAAGCAGCAGTGTGAGAGC-3′ for PAK4; 5′- TGATGGACCTCAGAAAGCAGC-3′ and 5′-ATTGAGGTGCTGGTAGTCACG-3′ for PAK6; 5′-CTCCTGAGGTGATTTCCAGGC-3′ and 5′-GAGGTAAACTGTCCCGGATCC-3′ for PAK7; and 5′-CTTGCTGCAGACATGGTCAAC-3′ and 5′-GCCATTATGGCGTGTGAAGTC-3′ for cyclophilin.
Islet isolation and culture, INS832/13 cell culture and immunoblotting
Upon reception human islets were cultured overnight in Prodo islet media (Prodo Laboratories, PIM-S001GMP) supplemented with 100 U/ml penicillin/streptomycin. The following day, islets were hand-picked and pretreated for 5 h in RPMI 1640 supplemented with 10% (wt/vol) human albumin solution (Grifols Therapeutics Inc., 61953-0002) and 5 mM glucose followed by 1 h in RPMI 1640 supplemented with 0.5% (wt/vol) BSA and 5 mM glucose. Islets were isolated from 12-week old male WT and GPR40KO mice by collagenase digestion as described28 and allowed to recover overnight in RPMI 1640 supplemented with 10% (wt/vol) FBS, 100 U/ml penicillin/streptomycin and 11 mM glucose. The following day islets were hand-picked and pretreated for 4 h in RPMI 1640 supplemented with 10 % (wt/vol) FBS and 2 mM glucose and then for 1 h in RPMI 1640 supplemented with 0.5% (wt/vol) BSA and 2 mM glucose. INS832/13 cells were pretreated overnight with RPMI 1640 supplemented with 5.5 mM glucose and 10%(wt/vol) FBS, and the following day for 5 h in RPMI 1640 medium supplemented with 2 mM glucose, 0.5% (wt/vol) BSA. Human and mouse islets were stimulated for 5 min and INS832/13 cells for 2 min with glucose and oleate in RPMI 1640 as indicated in Figure legends. Protein extracts were subjected to 10% SDS–PAGE, transferred to nitrocellulose membranes and immunoblotted with primary antibodies and horseradish peroxidase-conjugated anti-rabbit IgG secondary antibodies in 5% (wt/vol) fat-free milk and visualized using Western Lighting Plus ECL (Perkin Elmer, NEL104001EA). Band intensity was quantified using Image J software (National Institutes of Health).
Insulin secretion assay
48 h following electroporation, INS832/13 cells were incubated for 2 h in RPMI 1640 medium supplemented with 1 mM glucose, 10% (wt/vol) FBS. Media was then replaced with a Krebs-Ringer Bicarbonate with Hepes (KRBH) solution containing 0.1% (wt/vol) BSA and 1 mM glucose for 1 h, followed by 1-h static incubations in KRBH in the presence of glucose and oleate as described in Figure legends. Each condition was run in triplicate. Secreted insulin was measured in the supernatant and intracellular insulin content was measured after acid–alcohol extraction by radioimmunoassay using a rat insulin RIA kit (Millipore, RI-13K).
Statistics
Data are expressed as mean ± SEM. Significance was tested using Student's paired t-test, or one- or 2-way analysis of variance with Bonferroni post hoc adjustment for multiple comparisons, as appropriate, using GraphPad Software. p < 0.05 was considered significant.
Abbreviations
- FFA
free fatty acids
- FFA1
free fatty acid receptor 1
- GPR40
G protein-coupled receptor 40
- GPR40KO
GPR40-null
- GSIS
glucose-stimulated insulin secretion
- PAK
p21-activated kinase
- PKD1
protein kinase D1
- siRNA
small interfering ribonucleic acid
- RT-qPCR
reverse transcriptase-quantitative polymerase chain reaction
- WT
wild-type
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank G. Dodier, M. Ethier, G. Fergusson and C. Tremblay (CRCHUM) for technical assistance; D. C. Lin and H. Baribault (Amgen, Thousand Oaks, California) for providing GPR40KO breeders; Christopher Newgard (Duke University, Durham, North Carolina) for the INS832/13 cells; and members of the Montreal Diabetes Research Center for fruitful discussions.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR; MOP 86545 to VP). VP holds the Canada Research Chair in Diabetes and Pancreatic Beta-cell Function.
Notes on contributors
VB, JG, VP conceived/designed the study and wrote the manuscript. VB performed experiments and analyzed the data. VB, JG, and VP reviewed/revised the manuscript.
References
- [1].Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000; 49:1751-60; PMID:11078440; http://dx.doi.org/ 10.2337/diabetes.49.11.1751 [DOI] [PubMed] [Google Scholar]
- [2].Kalwat MA, Thurmond DC. Signaling mechanisms of glucose-induced F-actin remodeling in pancreatic islet β cells. Exp Mol Med 2013; 45:e37; PMID:23969997; http://dx.doi.org/ 10.1038/emm.2013.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, et al.. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 2003; 278:11303-11; PMID:12496284; http://dx.doi.org/ 10.1074/jbc.M211495200 [DOI] [PubMed] [Google Scholar]
- [4].Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, et al.. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 2003; 422:173-6; PMID:12629551; http://dx.doi.org/ 10.1038/nature01478 [DOI] [PubMed] [Google Scholar]
- [5].Burant CF, Viswanathan P, Marcinak J, Cao C, Vakilynejad M, Xie B, Leifke E. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2012; 379:1403-11; PMID:22374408; http://dx.doi.org/ 10.1016/S0140-6736(11)61879-5 [DOI] [PubMed] [Google Scholar]
- [6].Ferdaoussi M, Bergeron V, Zarrouki B, Kolic J, Cantley J, Fielitz J, Olson EN, Prentki M, Biden T, MacDonald PE, et al.. G protein-coupled receptor (GPR)40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia 2012; 55:2682-92; PMID:22820510; http://dx.doi.org/ 10.1007/s00125-012-2650-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jaffer ZM, Chernoff J. p21-activated kinases: three more join the Pak. Int J Biochem Cell Biol 2002; 34:713-7; PMID:11950587; http://dx.doi.org/ 10.1016/S1357-2725(01)00158-3 [DOI] [PubMed] [Google Scholar]
- [8].Bastea LI, Doppler H, Pearce SE, Durand N, Spratley SJ, Storz P. Protein kinase D-mediated phosphorylation at Ser99 regulates localization of p21-activated kinase 4. Biochem J 2013; 455:251-60; PMID:23841590; http://dx.doi.org/ 10.1042/BJ20130281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Callow MG, Clairvoyant F, Zhu S, Schryver B, Whyte DB, Bischoff JR, Jallal B, Smeal T. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J Biol Chem 2002; 277:550-8; PMID:11668177; http://dx.doi.org/ 10.1074/jbc.M105732200 [DOI] [PubMed] [Google Scholar]
- [10].Wang Z, Oh E, Thurmond DC. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J Biol Chem 2007; 282:9536-46; PMID:17289663; http://dx.doi.org/ 10.1074/jbc.M610553200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang Z, Oh E, Clapp DW, Chernoff J, Thurmond DC. Inhibition or ablation of p21-activated kinase (PAK1) disrupts glucose homeostatic mechanisms in vivo. J Biol Chem 2011; 286:41359-67; PMID:21969371; http://dx.doi.org/ 10.1074/jbc.M111.291500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kalwat MA, Yoder SM, Wang Z, Thurmond DC. A p21-activated kinase (PAK1) signaling cascade coordinately regulates F-actin remodeling and insulin granule exocytosis in pancreatic β cells. Biochem Pharmacol 2013; 85:808-16; PMID:23246867; http://dx.doi.org/ 10.1016/j.bcp.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Uenishi E, Shibasaki T, Takahashi H, Seki C, Hamaguchi H, Yasuda T, Tatebe M, Oiso Y, Takenawa T, Seino S. Actin dynamics regulated by the balance of neuronal Wiskott-Aldrich syndrome protein (N-WASP) and cofilin activities determines the biphasic response of glucose-induced insulin secretion. J Biol Chem 2013; 288:25851-64; PMID:23867458; http://dx.doi.org/ 10.1074/jbc.M113.464420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ahn M, Yoder SM, Wang Z, Oh E, Ramalingam L, Tunduguru R, Thurmond DC. The p21-activated kinase (PAK1) is involved in diet-induced β cell mass expansion and survival in mice and human islets. Diabetologia 2016; 59:2145-55; PMID:27394663; http://dx.doi.org/ 10.1007/s00125-016-4042-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fadista J, Vikman P, Laakso EO, Mollet IG, Esguerra JL, Taneera J, Storm P, Osmark P, Ladenvall C, Prasad RB, et al.. Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc Natl Acad Sci U S A 2014; 111:13924-9; PMID:25201977; http://dx.doi.org/ 10.1073/pnas.1402665111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mancini AD, Bertrand G, Vivot K, Carpentier E, Tremblay C, Ghislain J, Bouvier M, Poitout V. β-Arrestin Recruitment and Biased Agonism at Free Fatty Acid Receptor 1. J Biol Chem 2015; 290:21131-40; PMID:26157145; http://dx.doi.org/ 10.1074/jbc.M115.644450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Abo A, Qu J, Cammarano MS, Dan C, Fritsch A, Baud V, Belisle B, Minden A. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J 1998; 17:6527-40; PMID:9822598; http://dx.doi.org/ 10.1093/emboj/17.22.6527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pandey D, Goyal P, Dwivedi S, Siess W. Unraveling a novel Rac1-mediated signaling pathway that regulates cofilin dephosphorylation and secretion in thrombin-stimulated platelets. Blood 2009; 114:415-24; PMID:19429871; http://dx.doi.org/ 10.1182/blood-2008-10-183582 [DOI] [PubMed] [Google Scholar]
- [19].Baskaran Y, Ng YW, Selamat W, Ling FT, Manser E. Group I and II mammalian PAKs have different modes of activation by Cdc42. EMBO Rep 2012; 13:653-9; PMID:22653441; http://dx.doi.org/ 10.1038/embor.2012.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Spratley SJ, Bastea LI, Doppler H, Mizuno K, Storz P. Protein kinase D regulates cofilin activity through p21-activated kinase 4. J Biol Chem 2011; 286:34254-61; PMID:21832093; http://dx.doi.org/ 10.1074/jbc.M111.259424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Doppler H, Bastea LI, Borges S, Spratley SJ, Pearce SE, Storz P. Protein kinase d isoforms differentially modulate cofilin-driven directed cell migration. PLoS One 2014; 9:e98090; PMID:24840177; http://dx.doi.org/ 10.1371/journal.pone.0098090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci 2009; 122:893-903; PMID:19295123; http://dx.doi.org/ 10.1242/jcs.034355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dan C, Kelly A, Bernard O, Minden A. Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J Biol Chem 2001; 276:32115-21; PMID:11413130; http://dx.doi.org/ 10.1074/jbc.M100871200 [DOI] [PubMed] [Google Scholar]
- [24].Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci 2005; 118:1861-72; PMID:15827085; http://dx.doi.org/ 10.1242/jcs.02313 [DOI] [PubMed] [Google Scholar]
- [25].Kimmelman AC, Hezel AF, Aguirre AJ, Zheng H, Paik JH, Ying H, Chu GC, Zhang JX, Sahin E, Yeo G, et al.. Genomic alterations link Rho family of GTPases to the highly invasive phenotype of pancreas cancer. Proc Natl Acad Sci U S A 2008; 105:19372-7; PMID:19050074; http://dx.doi.org/ 10.1073/pnas.0809966105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pathak R, Delorme-Walker VD, Howell MC, Anselmo AN, White MA, Bokoch GM, Dermardirossian C. The microtubule-associated Rho activating factor GEF-H1 interacts with exocyst complex to regulate vesicle traffic. Dev Cell 2012; 23:397-411; PMID:22898781; http://dx.doi.org/ 10.1016/j.devcel.2012.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xie L, Zhu D, Kang Y, Liang T, He Y, Gaisano HY. Exocyst sec5 regulates exocytosis of newcomer insulin granules underlying biphasic insulin secretion. PLoS One 2013; 8:e67561; PMID:23844030; http://dx.doi.org/ 10.1371/journal.pone.0067561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Latour MG, Alquier T, Oseid E, Tremblay C, Jetton TL, Luo J, Lin DC, Poitout V. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes 2007; 56:1087-94; PMID:17395749; http://dx.doi.org/ 10.2337/db06-1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Briaud I, Harmon JS, Kelpe CL, Segu VB, Poitout V. Lipotoxicity of the pancreatic β-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes 2001; 50:315-21; PMID:11272142; http://dx.doi.org/ 10.2337/diabetes.50.2.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hagman DK, Latour MG, Chakrabarti SK, Fontes G, Amyot J, Tremblay C, Semache M, Lausier JA, Roskens V, Mirmira RG, et al.. Cyclical and alternating infusions of glucose and intralipid in rats inhibit insulin gene expression and Pdx-1 binding in islets. Diabetes 2008; 57:424-31; PMID:17991758; http://dx.doi.org/ 10.2337/db07-1285 [DOI] [PMC free article] [PubMed] [Google Scholar]