Abstract
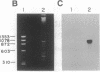
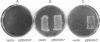
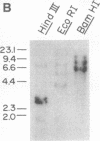
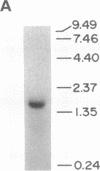
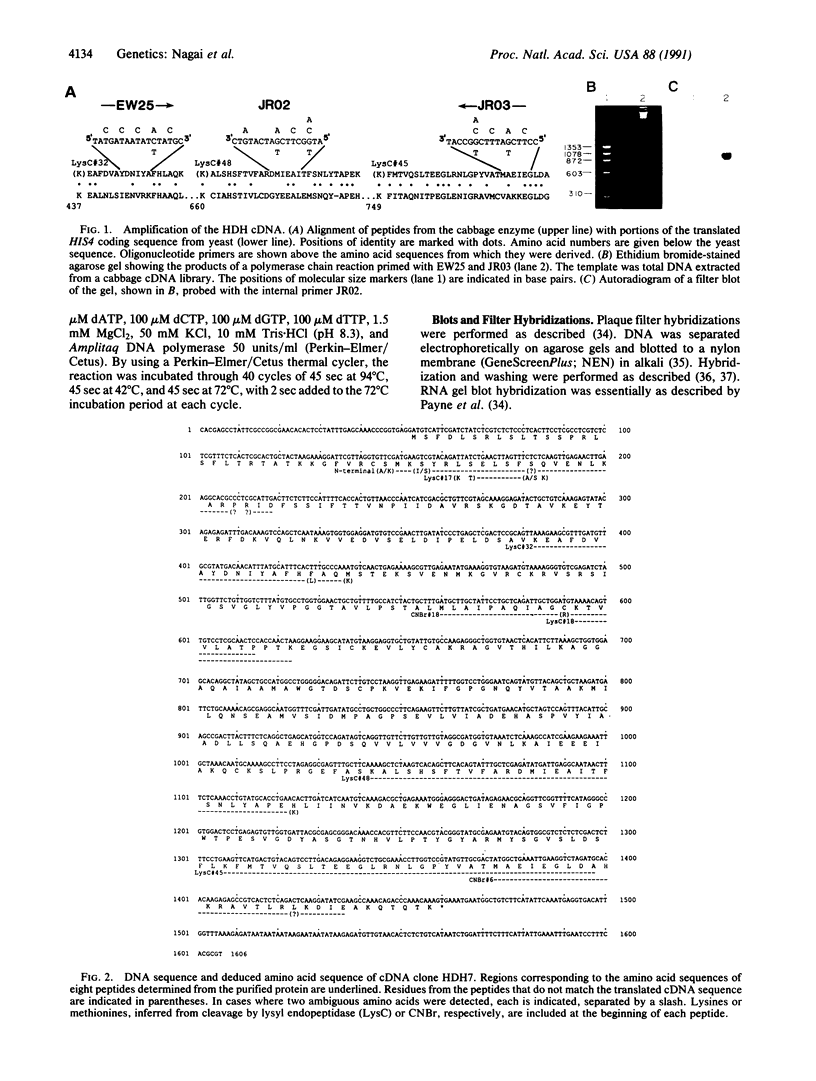
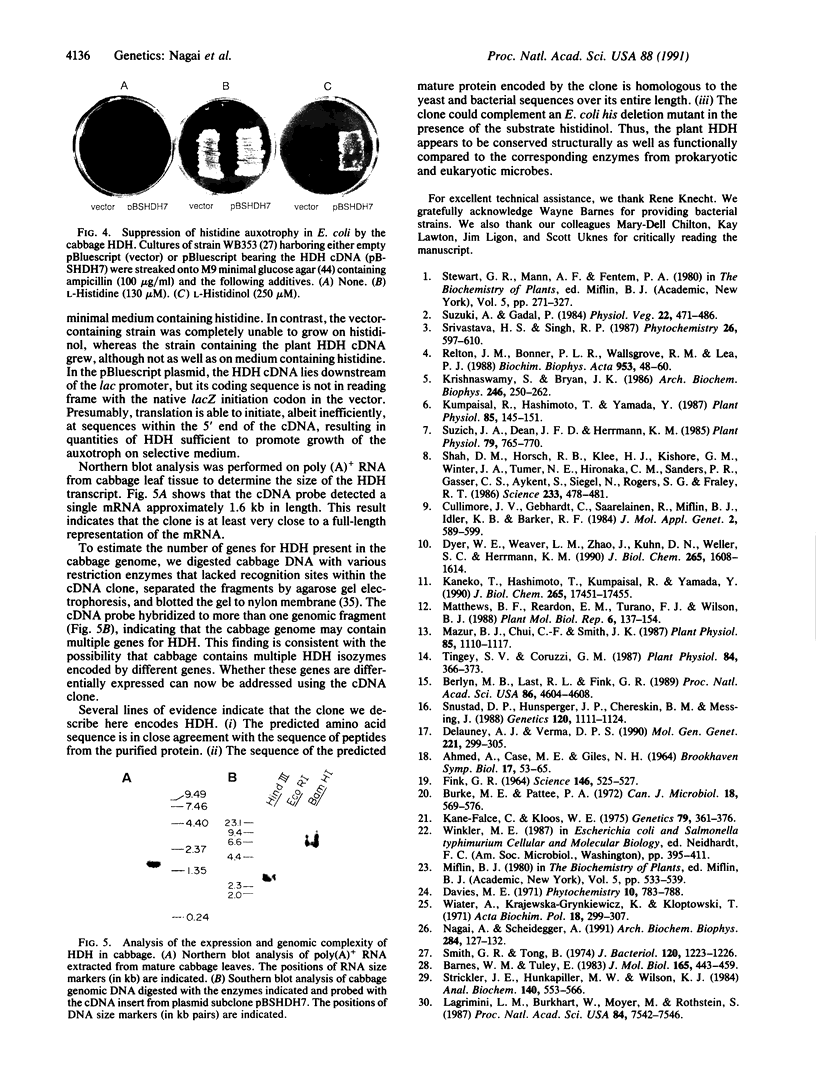
The partial amino acid sequence of histidinol dehydrogenase (L-histidinol:NAD+ oxidoreductase, EC 1.1.1.23) from cabbage was determined from peptide fragments of the purified protein. The relative positions of these peptides were deduced by aligning their sequences with the sequence of the HIS4C gene product of Saccharomyces cerevisiae. cDNA encoding histidinol dehydrogenase was then amplified from a library using a polymerase chain reaction primed with degenerate oligonucleotide pools of known position and orientation. By using this amplified fragment as a probe, an apparently full-length cDNA clone was isolated that is predicted to encode a proenzyme having a putative 31-amino acid chloroplast transit peptide and a mature molecular mass of 47.5 kDa. The predicted protein sequence was 51% identical to the yeast enzyme and 49% identical to the Escherichia coli enzyme. Expression of the cDNA clone in an E. coli his operon deletion strain rendered the mutant able to grow in the presence of histidinol.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHMED A., CASE M. E., GILES N. H. THE NATURE OF COMPLEMENTATION AMONG MUTANTS IN THE HISTIDINE-3 REGION OF NEUROSPORA CRASSA. Brookhaven Symp Biol. 1964 Dec;17:53–65. [PubMed] [Google Scholar]
- Barnes W. M. Cloning and restriction map of the first part of the histidine operon of Salmonella typhimurium. J Bacteriol. 1981 Jul;147(1):124–134. doi: 10.1128/jb.147.1.124-134.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M., Tuley E. DNA sequence changes of mutations in the histidine operon control region that decrease attenuation. J Mol Biol. 1983 Apr 15;165(3):443–459. doi: 10.1016/s0022-2836(83)80212-5. [DOI] [PubMed] [Google Scholar]
- Berlyn M. B., Last R. L., Fink G. R. A gene encoding the tryptophan synthase beta subunit of Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4604–4608. doi: 10.1073/pnas.86.12.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M. E., Pattee P. A. Histidine biosynthetic pathway in Staphylococcus aureus. Can J Microbiol. 1972 May;18(5):569–576. doi: 10.1139/m72-090. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullimore J. V., Gebhardt C., Saarelainen R., Miflin B. J., Idler K. B., Barker R. F. Glutamine synthetase of Phaseolus vulgaris L.: organ-specific expression of a multigene family. J Mol Appl Genet. 1984;2(6):589–599. [PubMed] [Google Scholar]
- Delauney A. J., Verma D. P. A soybean gene encoding delta 1-pyrroline-5-carboxylate reductase was isolated by functional complementation in Escherichia coli and is found to be osmoregulated. Mol Gen Genet. 1990 May;221(3):299–305. doi: 10.1007/BF00259392. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue T. F., Farabaugh P. J., Fink G. R. The nucleotide sequence of the HIS4 region of yeast. Gene. 1982 Apr;18(1):47–59. doi: 10.1016/0378-1119(82)90055-5. [DOI] [PubMed] [Google Scholar]
- Dyer W. E., Weaver L. M., Zhao J. M., Kuhn D. N., Weller S. C., Herrmann K. M. A cDNA encoding 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase from Solanum tuberosum L. J Biol Chem. 1990 Jan 25;265(3):1608–1614. [PubMed] [Google Scholar]
- FINK G. R. GENE-ENZYME RELATIONS IN HISTIDINE BIOSYNTHESIS IN YEAST. Science. 1964 Oct 23;146(3643):525–527. doi: 10.1126/science.146.3643.525. [DOI] [PubMed] [Google Scholar]
- Grubmeyer C. T., Gray W. R. A cysteine residue (cysteine-116) in the histidinol binding site of histidinol dehydrogenase. Biochemistry. 1986 Aug 26;25(17):4778–4784. doi: 10.1021/bi00365a009. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Kane-Falce C., Kloos W. E. A genetic and biochemical study of histidine biosynthesis in Micrococcus luteus. Genetics. 1975 Mar;79(3):361–376. doi: 10.1093/genetics/79.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Hashimoto T., Kumpaisal R., Yamada Y. Molecular cloning of wheat dihydrodipicolinate synthase. J Biol Chem. 1990 Oct 15;265(29):17451–17455. [PubMed] [Google Scholar]
- Krishnaswamy S., Bryan J. K. Use of monoclonal antibodies for the purification and characterization of the threonine-sensitive isozyme of maize homoserine dehydrogenase. Arch Biochem Biophys. 1986 Apr;246(1):250–262. doi: 10.1016/0003-9861(86)90471-6. [DOI] [PubMed] [Google Scholar]
- Kumpaisal R., Hashimoto T., Yamada Y. Purification and characterization of dihydrodipicolinate synthase from wheat suspension cultures. Plant Physiol. 1987 Sep;85(1):145–151. doi: 10.1104/pp.85.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrimini L. M., Burkhart W., Moyer M., Rothstein S. Molecular cloning of complementary DNA encoding the lignin-forming peroxidase from tobacco: Molecular analysis and tissue-specific expression. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7542–7546. doi: 10.1073/pnas.84.21.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni M., Gilbert W. The triosephosphate isomerase gene from maize: introns antedate the plant-animal divergence. Cell. 1986 Jul 4;46(1):133–141. doi: 10.1016/0092-8674(86)90867-6. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F., Smith J. K. Isolation and characterization of plant genes coding for acetolactate synthase, the target enzyme for two classes of herbicides. Plant Physiol. 1987 Dec;85(4):1110–1117. doi: 10.1104/pp.85.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai A., Scheidegger A. Purification and characterization of histidinol dehydrogenase from cabbage. Arch Biochem Biophys. 1991 Jan;284(1):127–132. doi: 10.1016/0003-9861(91)90274-m. [DOI] [PubMed] [Google Scholar]
- Payne G., Ward E., Gaffney T., Goy P. A., Moyer M., Harper A., Meins F., Jr, Ryals J. Evidence for a third structural class of beta-1,3-glucanase in tobacco. Plant Mol Biol. 1990 Dec;15(6):797–808. doi: 10.1007/BF00039420. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. The transport of proteins into chloroplasts. Annu Rev Biochem. 1986;55:879–912. doi: 10.1146/annurev.bi.55.070186.004311. [DOI] [PubMed] [Google Scholar]
- Shah D. M., Horsch R. B., Klee H. J., Kishore G. M., Winter J. A., Tumer N. E., Hironaka C. M., Sanders P. R., Gasser C. S., Aykent S., Siegel N. R., Rogers S. G., Fraley R. T. Engineering herbicide tolerance in transgenic plants. Science. 1986 Jul 25;233(4762):478–481. doi: 10.1126/science.233.4762.478. [DOI] [PubMed] [Google Scholar]
- Smith G. R., Tong B. Construction of phi80 dhis carrying Salmonella typhimurium histidine operon mutations. J Bacteriol. 1974 Dec;120(3):1223–1226. doi: 10.1128/jb.120.3.1223-1226.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snustad D. P., Hunsperger J. P., Chereskin B. M., Messing J. Maize glutamine synthetase cDNAs: isolation by direct genetic selection in Escherichia coli. Genetics. 1988 Dec;120(4):1111–1123. doi: 10.1093/genetics/120.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler J. E., Hunkapiller M. W., Wilson K. J. Utility of the gas-phase sequencer for both liquid- and solid-phase degradation of proteins and peptides at low picomole levels. Anal Biochem. 1984 Aug 1;140(2):553–566. doi: 10.1016/0003-2697(84)90207-0. [DOI] [PubMed] [Google Scholar]
- Suzich J. A., Dean J. F., Herrmann K. M. 3-Deoxy-d-arabino-Heptulosonate 7-Phosphate Synthase from Carrot Root (Daucus carota) Is a Hysteretic Enzyme. Plant Physiol. 1985 Nov;79(3):765–770. doi: 10.1104/pp.79.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey S. V., Coruzzi G. M. Glutamine Synthetase of Nicotiana plumbaginifolia: Cloning and in Vivo Expression. Plant Physiol. 1987 Jun;84(2):366–373. doi: 10.1104/pp.84.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiater A., Krajewska-Grynkiewicz K., Klopotowski T. Histidine biosynthesis and its regulation in higher plants. Acta Biochim Pol. 1971;18(3):299–307. [PubMed] [Google Scholar]