Summary
In animals, networks of clock neurons containing molecular clocks orchestrate daily rhythms in physiology and behavior. How various types of clock neurons communicate and coordinate with one another to produce coherent circadian rhythms is not well understood. Here, we investigate clock neuron coupling in the brain of Drosophila and demonstrate that the fly’s various groups of clock neurons display unique and complex coupling relationships to core pacemaker neurons. Furthermore, we find that coordinated free-running rhythms require molecular clock synchrony not only within the well-characterized lateral clock neuron classes, but also between lateral clock neurons and dorsal clock neurons. These results uncover unexpected patterns of coupling in the clock neuron network and reveal that robust free-running behavioral rhythms require a coherence of molecular oscillations across most of the fly’s clock neuron network.
Keywords: Circadian, Brain, Network, Coupling
Graphical Abstract
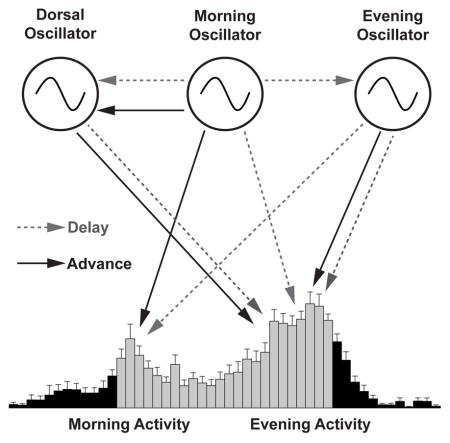
Introduction
Circadian clocks orchestrate daily rhythms of physiology and behavior. In animals, the master clock consists of a network of so-called “clock neurons,” each containing a molecular clock that generates molecular oscillations with periods of approximately 24 hours (Herzog, 2007). Clock neurons coordinate their molecular clocks via inter-neuronal signaling to form a coherent clock network producing robust circadian rhythms (Welsh et al., 2010). How this coordination occurs is not well understood.
Drosophila melanogaster is a valuable model system in which to investigate clock neuron communication and coordination. The Drosophila clock network consists of approximately 150 clock neurons, several orders of magnitude fewer than those of mammals, yet it shares both anatomical and functional similarities with the mammalian clock network (Nitabach and Taghert, 2008; Vansteensel et al., 2008). Studies of the Drosophila clock network suggest that it is organized into multiple oscillatory units that are differentially coupled to one another (Hermann-Luibl and Helfrich-Forster, 2015; Yao and Shafer, 2014). At the heart of the clock neuron network rest two critical groups of neurons: (i) the ventral lateral neurons (LNvs), consisting of four pairs of large LNvs (l-LNvs) and four pairs of small LNvs (s-LNvs), both of which express the neuropeptide pigment-dispersing factor (PDF) and (ii) six pairs of dorsal lateral neurons (LNds) along with a pair of PDF-negative s-LNvs, also called 5th s-LNvs. The LNvs are required for the fly’s morning peak of activity, which commences in anticipation of lights-on, and are therefore considered to be the “Morning Oscillator” (Grima et al., 2004; Stoleru et al., 2004). The LNvs are also critical pacemakers that help maintain free-running rhythms in constant environments (Renn et al., 1999) and the PDF they express is important for inter-clock-neuron coordination (Lin et al., 2004; Peng et al., 2003). The LNds and 5th s-LNv are important for the evening peak of activity, which commences in anticipation of lights-off, and as a group are considered the “Evening Oscillator” (Grima et al., 2004) (Figure S1).
The development of specific genetic drivers for a third group of clock neurons, the DN1ps (Figure S1), has recently made possible the experimental investigation of their function (Cavanaugh et al., 2014; Fujii and Amrein, 2010; Zhang et al., 2010a; Zhang et al., 2010b). Though there is some evidence that the DN1ps contribute to free-running circadian rhythms (Zhang et al., 2010a) most manipulations of these neurons suggest that they modulate circadian timekeeping rather than driving it. For example, recent work using specific genetic drivers to manipulate the DN1ps indicates they drive plasticity within the clock neuron network in the face of changing light and temperature conditions (Zhang et al., 2010b), act as conduits of circadian output in the brain (Cavanaugh et al., 2014; Fujii and Amrein, 2010; Zhang et al., 2010a), and shape daily activity profiles through promotion of sleep via the inhibition of lateral neurons (Guo et al., 2016). Are the DN1ps simply mediators and modulators of circadian outputs or are they, like the morning and evening oscillators, central to the production of coherent circadian rhythmicity?
Here we address the role that the DN1ps play in influencing the timing of the morning and evening peaks of activity through the creation of temporally mosaic brains, (e.g., (Smyllie et al., 2016; Stoleru et al., 2005)) and find that they influence the timing of locomotor activity peaks and are critical for the production of coherent free-running rhythms. Our results indicate that these neurons, in addition to their recently established modulatory and output functions, rest at the heart of circadian pacemaking, working in concert with the morning and evening oscillators of the central clock neuron network to create a coherent, free-running circadian rhythm. In addition, our results reveal an unexpected influence of morning and evening oscillators on evening and morning peaks of activity, respectively, and establish that the evening oscillator and the DN1ps while both coupled to the morning oscillator, display unexpectedly distinct coupling relationships to the morning oscillator.
Results and Discussion
A quantitative approach to the measurement of activity peak phase in individual flies
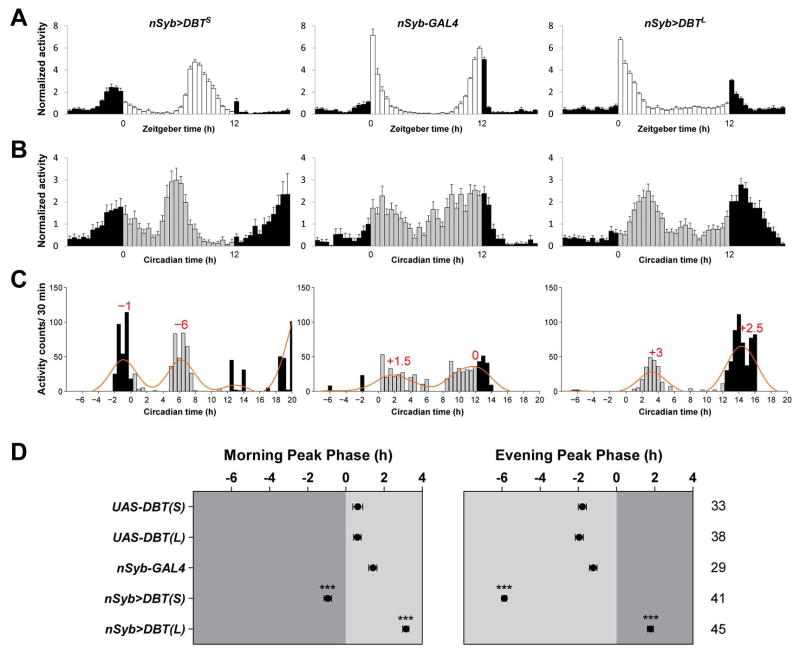
Drosophila are crepuscular animals: they display a bout of activity around dawn (morning activity) and another major bout of activity around dusk (evening activity), the timing of which is thought to be controlled by a morning oscillator and an evening oscillator, respectively (Grima et al., 2004; Stoleru et al., 2004). Under 12hr:12hr light:dark cycles (LD) the morning and evening peaks of activity anticipate lights-on and lights-off, respectively (e.g., Figure 1A, middle panel). Speeding-up all the neuronal clocks by about five hours per cycle through the overexpression of a mutant form of the Doubletime (DBT) kinase, DBTShort (DBTS) (Muskus et al., 2007) using the pan-neuronal driver neuronal synaptobrevin-GAL4 (nSyb-GAL4) (Table S1) resulted in a slightly advanced morning peak and a more substantially advanced evening peak (Figure 1A, compare left to middle). When all the neuronal clocks were slowed-down by about three hours per cycle through the overexpression of DBTLong (DBTL) (Muskus et al., 2007), the expected delays of morning and evening peaks were not observed (Figure 1A, compare right to middle). This was likely due to the masking effects of the light transitions, which induce an immediate increase of activity that is independent of the circadian clock (Wheeler et al., 1993). Indeed, when these flies were transferred to constant darkness (DD), both the morning and evening peaks of activity were observed to be delayed on the first day of DD (DD1) (Figure 1B). Thanks to the absence of masking effects, the DD1 activity profile reveals the endogenous timing of morning and evening peaks of activity. Highly similar results were observed using the widespread clock cell drivers timeless-GAL4 and Clock(856)-GAL4 to drive mutant forms of DBT.
Figure 1. Quantitative measurement of morning and evening activity peak phase.
(A–B) Population averaged activity profiles of nSyb>DBTS, nSyb-GAL4, and nSyb>DBTL flies in LD (A) and in DD1 (B). The white bars in (A) indicate the light period and the gray bars in (B) indicate the subjective light period.
(C) Raw activity profiles of single representative nSyb>DBTS, nSyb-GAL4, and nSyb>DBTL flies are plotted as bar graphs. Orange lines are activity profiles smoothed by a low-pass Butterworth filter. The morning and evening peaks are identified as local maxima of the filtered activity profile and their phases are indicated on the graphs (in hours) relative to the lights-on time for the morning peak and lights-off time for the evening peak. See Experimental Procedures for details.
(D) The average phases of morning and evening activity peaks in DD1 of the indicated genotypes. “0” marks the time of subjective lights-on for the left panel and the time of subjective lights-off for the right panel, times in which the light transitions would have occurred had the LD cycle continued. Dark gray indicates the subjective dark period and light gray indicates the subjective light period. The numbers of flies analyzed are indicated on the right of the phase panels. *** P < 0.001. Details of statistical analysis are described in Experimental Procedures. For all the plots, lines represent mean ± SEM (standard error of the mean).
The phases of morning and evening activity peaks are often inferred from the averaged activity profile of a population of flies (Zhang et al., 2010a; Zhang et al., 2010b). We sought to quantitatively measure the phases of morning and evening peaks for individual flies so that phases could be compared objectively between genotypes (e.g.,(Schlichting et al., 2016)). By applying a zero-phase low-pass Butterworth filter to individual fly activity profiles we diminished random noise with periods of less than 14 hours (see Experimental Procedures for details), thereby allowing for the determination of activity peaks (Figure 1C). Using this approach, we determined the phases of morning and evening peaks on DD1 for single flies. Such single fly peak calculations were consistent with the overall population average activity profiles and allowed us to address variability within genotypes (compare Figure 1C–D to Figure 1B).
Morning and evening oscillators each influence both morning and evening peak phase
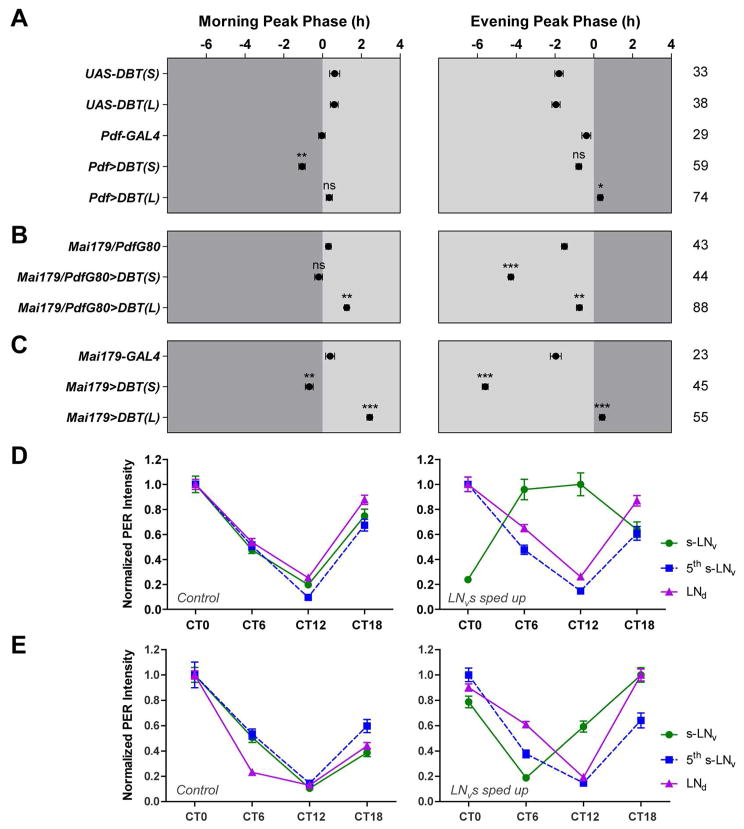
A functional molecular clock only in the morning oscillator supports the generation of a morning peak of activity while a functional molecular clock only in the evening oscillator supports the generation of an evening peak (Grima et al., 2004; Stoleru et al., 2004). When the morning oscillator was specifically sped-up through DBTS overexpression, the morning peak but not the evening peak was advanced (Figure 2A and Figure S2), consistent with the prediction of the dual-oscillator model (Stoleru et al., 2005). In contrast to the predictions of this model, when we slowed-down the morning oscillator through DBTL overexpression, the evening peak but not the morning peak was significantly delayed (Figure 2A and Figure S2). Here we used a conservative statistical analysis by comparing the experimental genotype to both GAL4 and UAS controls, using only the larger p value for our interpretation (see Experimental Procedures for details).
Figure 2. Morning and evening oscillators collaborate to control the timing of daily activity peaks.
(A–C) The average phases of morning and evening activity peaks in DD1 of the indicated genotypes. Genetic manipulations were targeted to the morning oscillator in (A), the 22 evening oscillator in (B), and both morning and evening oscillators in (C). See Table S1 for expression pattern of each genetic driver. “0” marks the time of subjective lights-on for the left panels and the time of subjective lights-off for the right panels. Dark gray indicates the subjective dark period and light gray indicates the subjective light period. The numbers of flies analyzed are indicated on the right of the phase panels. * P < 0.05; ** P < 0.01; *** P < 0.001; ns, not significant. See Experimental Procedures for details of the statistics.
(D) Normalized PER immunostaining intensity of the s-LNvs (morning oscillator), the 5th s-LNv (evening oscillator), and the LNds (evening oscillator) of UAS-DBTS control flies (left) and Pdf>DBTS flies (right).
(E) Normalized PER immunostaining intensity of different clock neuron classes of UAS-SGGHypo control flies (left) and Pdf>SGGHypo flies (right). Note that for UAS>SGGHypo flies (left), the troughs of PER oscillations in s-LNv, 5th s-LNv, and LNd are all at CT12, whereas for Pdf>SGGHypo flies (right), the trough of PER oscillation in s-LNv is at CT6 while those of 5th s-LNv and LNd remain at CT12. All the data in this figure are presented as mean ± SEM.
When the evening oscillator was specifically sped-up or slowed-down, the evening peak was significantly advanced and delayed respectively, but with smaller shifts compared to the clock manipulations in all neurons (compare Figure 2B to Figure 1D). Unexpectedly, the morning peak was also significantly delayed when the evening oscillator was slowed-down (Figure 2B and Figure S2). Finally, when the morning and evening oscillators were simultaneously sped-up or slowed-down, both the morning and evening peaks were shifted with magnitudes comparable to the shifts caused by manipulations in all neurons (compare Figure 2C to Figure 1D, and Figure S2). These results reveal that, in the context of a fully functional clock network, the morning and evening oscillators collaborate to control the timing of morning and evening activity peaks, with each oscillator imposing some control over both daily peaks of activity. Interestingly, the morning oscillator could advance but not delay the morning peak (Figure 2A and Figure S2). In contrast, the evening oscillator could direct moderate advances and delays of the evening peak and could delay but not advance the morning peak (Figure 2B and Figure S2). Only when morning and evening oscillators were sped-up and slowed down in unison was there complete, bi-directional (i.e., advancing and delaying) control of both morning and evening activity peaks (Figure 2C). These results suggest that the control of circadian rhythms depends on a neural network that is more distributed than previously appreciated.
The morning oscillator can delay but not advance the evening oscillator
Previous work has yielded conflicting views of the coupling of the molecular clocks between the morning and evening oscillators. Using in situ hybridization to timeless mRNA, Stoleru and colleagues (2005) found that evening oscillator clock cycling was tightly coupled to the morning oscillator whose clocks were sped-up through the expression of the kinase Shaggy. In contrast, when Zhang and colleagues (2010a) slowed-down the morning oscillator through the knockdown of the casein kinase II (CK2) or via the down regulation of synaptic signaling, they observed little or no effect on the clocks within the evening cells. Using higher resolution imaging, Yao and Shafer (2014) found that the clocks of only two evening cells were coherently reset by slow-running (DBTL expressing) morning oscillator, revealing that a subset of the evening cells was tightly coupled to the decelerated morning oscillator. The mode of coupling between the morning and evening oscillators is therefore an important and still unsettled question.
In light of our previous finding that morning oscillator could delay a subset of evening cells (Yao and Shafer, 2014), we turned our attention to the apparent delay-specific influence of the morning oscillator on the evening peak phase of activity – the morning oscillator could delay but not advance the evening peak (Figure 2A). Our finding that the morning oscillator is not capable of advancing the evening peak (Figure 2A) leads to the prediction that the morning oscillator cannot phase-advance the clocks of any of the evening cells. To test this prediction, we specifically sped-up the morning oscillator through DBTS overexpression. On the third day under DD, the molecular rhythm of the morning cells was phase-shifted by about 12 hours when compared to controls (Figure 2D), consistent with the shortening the clock period by about five to six hours per (Muskus et al., 2007). As predicted, none of the evening cells were phase-shifted in their molecular rhythms when compared to controls (Figure 2D), indicating that none were phase-advanced by the morning oscillator.
To be sure that a failure to reset evening oscillator clocks was not due simply to morning oscillator period being outside the entrainment limits of the evening oscillator, we repeated this experiment but only sped-up the morning oscillator by about two hours per cycle through the overexpression of a hypomorphic allele of the Shaggy kinase (SGGHypo) (Martinek et al., 2001; Yao and Shafer, 2014). Even with this smaller period discrepancy, the morning oscillator failed to phase-advance any of the evening cells (Figure 2E). Therefore, while a subset of evening cells is tightly coupled to slowed morning cells (Yao and Shafer, 2014), all evening cells fail to couple to sped-up morning cells. These results reveal that the morning oscillator exerts only a delaying influence on evening oscillator clocks and likely explain why the morning oscillator can delay but not advance the evening peak of activity.
The morning oscillator can both advance and delay the molecular clocks of CRY-expressing DN1ps
In contrast to the relative independence of the evening oscillator from the morning oscillator ((Yao and Shafer, 2014; Zhang et al., 2010a); Figure 2D–E), there is evidence that DN1p clocks might be more sensitive to changes in morning oscillator speed. Zhang and colleagues (2010a) reported that PER rhythms of the DN1ps dampened when the morning oscillator was slowed-down by about six hours per cycle through the RNAi knockdown of casein kinase II β subunit (CK2β-RNAi) but were coherently phase-delayed when the morning oscillator was slowed-down by about two hours per cycle through the overexpression of the temperature-sensitive dominant-negative dynamin shibire (shits). It is unclear why these two manipulations of slowing-down the morning oscillator had different effects on the DN1p clocks, and it is unknown how the DN1p PER rhythm would respond if the morning oscillator was sped-up, although previous work using in situ hybridization to timeless mRNA to track clock cycling, found that DN1 clock cycling was tightly coupled to the morning oscillator accelerated through the expression of the kinase Shaggy. Are DN1ps coherently reset by the morning oscillator or do their clocks dampen in the face of period discrepancies?
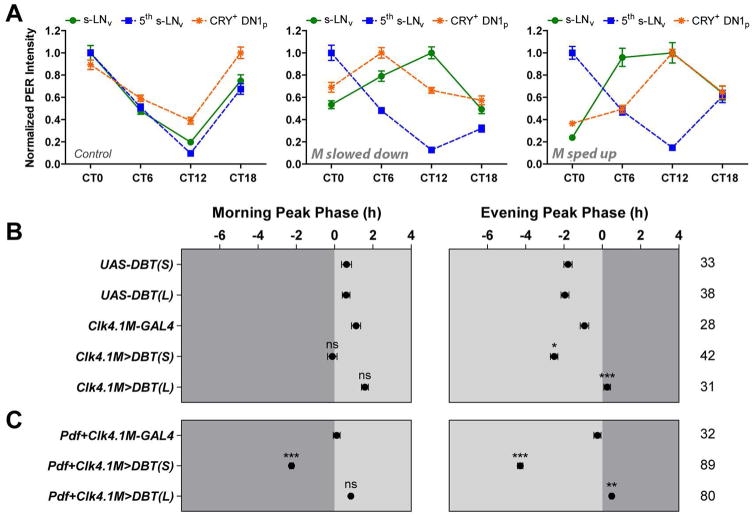
Using CRY immunostaining to distinguish the CRY+ and CRY− subsets of the DN1ps, we found that the CRY− DN1ps expressed very low levels of PER and did not show strong PER rhythms under DD. In contrast, the CRY+ DN1ps expressed much higher levels of PER and displayed PER rhythms of a much higher amplitude, in both un-manipulated control flies and flies with altered morning oscillator speeds (Figure S3). These results are in agreement with previous work showing that the CRY− DN1ps do not maintain Timeless protein rhythms in DD whereas the CRY+ DN1ps support high amplitude Timeless cycling (Yoshii et al., 2009). Focusing on the CRY+ DN1ps, we found that their PER rhythms maintained high-amplitude PER cycling and were phase-delayed when the morning oscillator was slowed down via DBTL overexpression (Figure 3A, compare middle to left). Furthermore, in contrast to our results for the evening oscillator, CRY+ DN1p clocks were advanced by the morning oscillator even when it was sped-up by five to six hours per cycle through DBTS overexpression (Figure 3A, compare right to left). This result is consistent with the finding that DN1 tim mRNA rhythms (a group that included DN1as as well as both CRY+ and CRY− DN1ps) were phase-advanced when the morning oscillator was sped-up by about three hours per cycle through SGG overexpression (Stoleru et al., 2005). Thus, the molecular rhythms of CRY+ DN1ps are strongly coupled to those of the morning oscillator which can both advance and delay the molecular clocks of the CRY+ DN1ps.
Figure 3. The CRY+ DN1p clocks are tightly and bidirectionally phased-coupled to those of the morning oscillator.
(A) Normalized PER immunostaining intensity of the CRY+ DN1ps of Pdf-GAL4 flies (left), Pdf>DBTL flies (middle), and Pdf>DBTS flies (right). The PER rhythms of the s-LNvs (morning oscillator) and the 5th s-LNv (evening oscillator) are also shown for comparison.
(B–C) The average phases of morning and evening activity peaks in DD1 of the indicated genotypes. Genetic manipulations were targeted to the DN1ps in (B), and the DN1ps along with the morning oscillator in (C). “0” marks the time of subjective lights-on for the left panels and the time of subjective lights-off for the right panels. Dark gray indicates the subjective dark period and light gray indicates the subjective light period. The numbers of 23 flies analyzed are indicated on the right of the phase panels. * P < 0.05; ** P < 0.01; *** P < 0.001; ns, not significant. See Experimental Procedures for details of the statistics. All the data are presented as mean ± SEM.
We previously established that the delaying of molecular clocks in a specific subset of evening cells by the morning oscillator is mediated by PDF signaling (Yao and Shafer, 2014). The coupling of CRY+ DN1ps to the morning oscillator is also likely to depend on PDF signaling, as the CRY+ DN1ps express PDF receptor (Im et al., 2011)and respond physiologically to synthetic PDF peptide (Seluzicki et al., 2014; Shafer et al., 2008). Given that PDF is required for molecular clock oscillations in the CRY+ DN1ps under constant conditions (Yoshii et al., 2009), we could not experimentally address this possibility. It is clear from our results that the DN1ps and the evening oscillator display fundamentally different modes of coupling to the morning oscillator. The mechanistic basis of such differential coupling will be the focus of future investigation. One possibility is that the evening oscillator suppresses its receptivity to PDF specifically during times that would otherwise produce PDF-induced advances, whereas the DN1ps may not. Recent work has revealed a daily rhythm in the autocrine response of the morning oscillator to PDF (Klose et al., 2016). Similar mechanisms may shape the mode of PDF dependent coupling between the morning oscillator and its targets.
Speeding-up and slowing-down the CRY+ DN1p clocks modestly advanced and delayed the evening peak on DD1, respectively, whereas it had no significant effect on the morning peak (Figure 3B). Previous work on these neurons revealed that they could, when overexpressing DBTS, advance the morning peak but not the evening peak of activity under LD conditions (Zhang et al., 2010b). Under DD conditions however, these neurons drove evening peaks of activity when the remaining network lacked functional molecular clocks (Zhang et al., 2010b). These results suggested that the contribution of the DN1ps to daily activity peaks depends on environmental light, driving morning activity in the light and evening activity in the dark. Thus, our failure to see morning peak effects was likely due to the absence of light. Our results reveal that the DN1ps can influence the evening peak of activity under constant darkness even when the remaining network supports normal timekeeping, further extending our understanding of the contributions these neurons make under constant darkness.
Simultaneously changing the molecular clock speed of the CRY+ DN1ps and the morning oscillator using a combination of Clk4.1M-GAL4 and Pdf-GAL4 revealed an even larger effect on the timing of morning and evening peaks by these two groups of clock neurons (compare Figure 3C to Figure 2A and Figure 3B). However, the CRY+ DN1ps and the morning oscillator together were still not capable of fully controlling the timing of daily activity peaks: the morning peak was not significantly delayed when the two groups of neurons were simultaneously slowed-down through DBTL overexpression (Figure 3C, especially when compared to the UAS-DBTL control shown in Figure 3B) and the magnitudes of evening peak advances and delays were smaller than those of whole-neuronal-network manipulation (compare Figure 3C to Figure 1D). Thus, the morning oscillator and the CRY+ DN1ps together cannot determine morning and evening peak timing when the remaining network functions normally, whereas the morning and evening oscillators together are sufficient for such control.
Coherent free-running activity rhythms require molecular clock synchrony between the lateral and dorsal clock neurons
The Drosophila clock neuron network has long been modeled as a hierarchical network, with the morning oscillator functioning as a master pacemaker that dictates the pace of free-running rhythms (Stoleru et al., 2005). This view was recently challenged, as several groups showed that the morning oscillator is not capable of coherently resetting free-running rhythms when its clock speed is genetically altered and revealed that clock neurons other than the morning oscillator (e.g., the evening oscillator) have independent control over free-running rhythms (Beckwith and Ceriani, 2015; Guo et al., 2014; Yao and Shafer, 2014). It remains unaddressed whether there is a minimal set of clock neurons that can function as a master pacemaker for the entire clock network, driving behavioral rhythms with their endogenous rhythm, despite discrepant timekeeping in the remaining neuronal oscillators. Given previous work (Zhang et al., 2010a) and our results above indicating that the evening oscillator is relatively independent from the morning oscillator in its molecular rhythms, and that the two sets of oscillators together can fully override the remaining network to set the phases of both morning and evening peaks, we hypothesized that the morning and evening oscillators could collectively constitute a master pacemaker for the entire clock network. We therefore predicted that changing molecular clock speed within these two oscillators simultaneously would result in a coherent resetting of behavioral rhythms under free-running conditions.
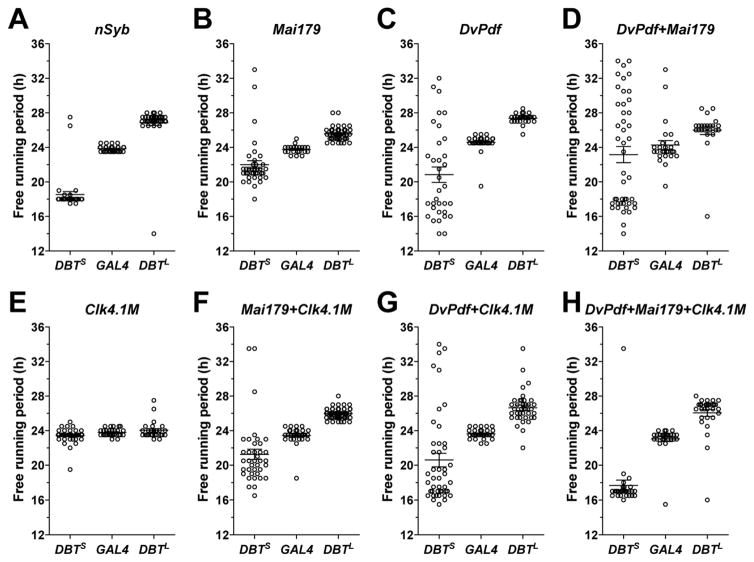
Unexpectedly, the overexpression of DBTS and DBTL in both the morning and evening oscillators using the Mai179-GAL4 driver (Table S1) failed to coherently reset the free-running periods of locomotor rhythms when compared to whole-neuronal-network manipulations (compare Figure 4B to Figure 4A, Table S2). We observed the same failure to fully reset rhythms with a second lateral neuron driver, DvPdf-GAL4 (Table S1) (Figure 4C and Table S2). Mai179-GAL4 and DvPdf-GAL4 drive expression in slightly different subsets of the lateral clock neurons (see Table S1 for details). A combined use of the two drivers should drive expression in all neurons of the morning and evening oscillators. However, even when every morning and evening oscillator neuron expressed DBTS and DBTL, free-running periods were not coherently reset (Figure 4D, Figure S3, Figure S4, and Table S2). Therefore, while the morning and evening oscillators together can fully control the timing of daily activity peaks, they are not sufficient for setting the pace for the entire clock network under free-running conditions.
Figure 4. Coherent free-running rhythms require molecular clock synchrony in every neuron of the morning and evening oscillators as well as the CRY+ DN1ps.
(A–H) Scatter plots of the predominant free-running periods of rhythmic GAL4 control flies and flies overexpressing DBTS and DBTL under the control of the indicated drivers. See Table S1 for details of the expression pattern of each GAL4 driver. For all the plots, lines represent mean ± SEM.
As discussed above, the effects of DN1p manipulation have largely suggested that they act as accessory neurons within the clock neuron network, refining the timing of sleep and activity and mediating circadian outputs. Do DN1ps contribute to coherent free-running timekeeping, or do they modulate and mediate the output of a separate master clock? Although our results indicate that the morning oscillator can both advance and delay CRY+ DN1p clocks (Figure 3A), we suspect that such resetting was not always complete, especially when a large clock speed discrepancy exists between the DN1ps and the morning oscillator. The failure of the morning and evening oscillators alone to coherently reset free-running locomotor rhythms led us to ask if the free-running period of the locomotor rhythm would be more coherently reset when the DN1p clock speed is altered simultaneously with those of the morning and evening oscillators. Changing the clock speed of only the CRY+ DN1ps had no clear effects on free-running period (Figure 4E and Table S2). Changing the clock speed in the CRY+ DN1ps and in large subsets of the morning and evening oscillator neurons simultaneously using a combination of Clk4.1M-GAL4 and Mai179-GAL4 drivers, or a combination of Clk4.1M-GAL4 and DvPdf-GAL4 drivers (see Table S1 for details) was still not sufficient to coherently reset free-running periods, especially when DBTS expression was driven by these GAL4s (Figure 4F–G, Figure S3, Figure S4, and Table S2). When the CRY+ DN1ps and every neuron of the morning and evening oscillators were simultaneously sped-up or slowed-down, through the combined use of Clk4.1M-GAL4, Mai179-GAL4, and DvPdf-GAL4 drivers, free-running rhythms were greatly improved (Figure 4H, Figure S3, Figure S4, and Table S2) but still not completely and coherently reset, as even these flies displayed a higher incidence of arrhythmicity, a higher variability in period, and weaker free-running rhythms relative to network-wide manipulations, especially for the DBTS experiments (Figure S3, Figure S4 and Table S2). This incomplete rescue of free-running rhythms indicates that strong free-running rhythms require coherence across a much larger network of neurons than previously appreciated. Nevertheless, given the improved rhythms attained through the inclusion of the CRY+ DN1ps along with the morning and evening oscillators, these results reveal that, in addition to their recently established roles as conduits and modulators of clock network output, the DN1ps make significant contributions to the establishment of coherent circadian rhythms.
The creation of temporal mosaics in mammals has revealed neuronal subsets in the suprachiasmatic nucleus (SCN, the central clock of the mammalian brain), that are capable of driving coherent free-running rhythms despite the presence of period discrepancies among SCN neurons. The expression of a dominant-negative form of Clock in the neuromedin S expressing neurons of the SCN produced coherent free-running locomotor rhythm with a period extended by one hour (Lee et al., 2015). Similarly, increasing or decreasing CK1 expression in the arginine vasopressin (AVP) expressing neurons of the SCN produced free-running rhythms that were 50 minutes longer and 30 minutes shorter respectively (Mieda et al.) However, the induction of SCN period discrepancies of a larger magnitude in approximately two-thirds of the SCN, via the CRE-mediated excision of the Tau CK1 mutation in dopamine 1a receptors expressing neurons, resulted in variable behavioral phenotypes, with ~60% of mice coherently displaying the induced 24-hour period, ~30% displaying coherent 20-hour mutant periods, and ~10% displaying unstable rhythms (Smyllie et al., 2016). These results were highly reminiscent of the effects of creating large period discrepancies between the morning oscillator and the remaining clock neuron network in Drosophila (Yao and Shafer, 2014). It appears therefore that the induction of large period discrepancies is required to fully gauge the extent to which coherence is required in the network for strong behavioral rhythms, which is likely the reason why coherent rhythms required larger subsets of the network for our DBTS manipulations compared to those of DBTL (Figure 4). Thus, in both flies and mammals, the determination of free-running period is a distributed function of clock neuron networks and when temporal discrepancies exist between oscillators the determination of period is remarkably stochastic (Smyllie et al., 2016; Yao and Shafer, 2014; Figure S4). Our results indicate that the neuronal subsets capable of setting the phase of activity under entrained conditions are not capable of coherently determining free-running period. Indeed, the determination of both the daily phase of behavioral outputs and the free-running circadian period is not centered on a few key oscillators. Rather, these critical functions are distributed over a remarkably large proportion of the clock neuron network.
Experimental Procedures
Fly strains
Flies were reared on cornmeal-sucrose-yeast media at 25 °C under 12-hour light: 12-hour dark (LD) cycles, or at room temperature under the quasi-diurnal conditions of the lab. All of the fly strains used in this study have been described previously and are described in Supplemental Experimental Procedures.
Locomotor activity rhythm recording and analysis
Locomotor activity rhythms of adult male flies were recorded using the TriKinetics DAM2 Drosophila Activity Monitors (Waltham, MA) as described previously (Pfeiffenberger et al., 2010; Yao and Shafer, 2014). For the determination of morning and evening peak phases, DD1 activity profile of individual flies was filtered with a zero-phase low-pass Butterworth filter to diminish oscillations with periods of less than 14 hours (Levine et al., 2002). The morning and evening peaks were identified as local maxima of the filtered activity profile. An experimenter who was blind to the genotypes manually confirmed the accuracy of morning and evening peaks. The phases of morning and evening peaks of an experimental genotype were compared to those of the corresponding GAL4 and UAS controls using one-way ANOVA and a Tukey’s multiple comparison test. Significance was only reported if the experimental genotypes differed significantly from both controls in the same direction. Only the larger p value was reported in the figures. The analysis of free-running activity rhythms was done using the ClockLab software from Actimetrics (Wilmette, IL) as previously described (Yao and Shafer, 2014). Detailed methods are described in Supplemental Experimental Procedures.
Immunocytochemistry
Immunostaining of whole-mount Drosophila brains was done as previously described (Yao and Shafer, 2014). Flies were entrained to LD cycles for a minimum of three days and then released into DD. Flies were collected every six hours for four time points on the third day under DD for DBTS overexpressing flies, and on the fourth day under DD for the other manipulations. Please see Supplemental Experimental Procedures for details.
Supplementary Material
Acknowledgments
This work was supported by the NIH (NINDS) grant R01NS077933 to O.T.S. Z.Y. was further supported by the Rackham Predoctoral Fellowship (University of Michigan). We thank J. L. Price, P. H. Taghert, M. Rosbash, F. Rouyer, P. E. Hardin, P. Emery, J. H. Park, and the Bloomington Drosophila Stock Center for providing fly stocks. We thank M. Rosbash for the PER antisera, C. Helfrich-Forster for the CRY antisera, and the Developmental Studies Hybridoma Bank for the monoclonal PDF antibodies. We thank J. D. Levine for providing Matlab codes for signal analysis, and R. Allada for providing the Counting Macro. Finally, we thank Maria de la Paz Fernandez and Paul Taghert for reading and discussing a draft of this manuscript.
Footnotes
Author Contributions
Z.Y. and O.T.S. designed the study. Z.Y. conducted all the experiments. A.J.B. developed the method for analyzing the phase of activity peaks for single flies; J.L.C. quantified the PER immunostaining intensity of the DN1ps; Z.Y. performed the remaining analyses. Z.Y. and O.T.S. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bahn JH, Lee G, Park JH. Comparative analysis of Pdf-mediated circadian behaviors between Drosophila melanogaster and D. virilis. Genetics. 2009;181:965–975. doi: 10.1534/genetics.108.099069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith EJ, Ceriani MF. Communication between circadian clusters: The key to a plastic network. FEBS Lett. 2015;589:3336–3342. doi: 10.1016/j.febslet.2015.08.017. [DOI] [PubMed] [Google Scholar]
- Cavanaugh Daniel J, Geratowski Jill D, Wooltorton Julian RA, Spaethling Jennifer M, Hector Clare E, Zheng X, Johnson Erik C, Eberwine James H, Sehgal A. Identification of a Circadian Output Circuit for Rest:Activity Rhythms in Drosophila. Cell. 2014;157:689–701. doi: 10.1016/j.cell.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Amrein H. Ventral lateral and DN1 clock neurons mediate distinct properties of male sex drive rhythm in Drosophila. Proceedings of the National Academy of Sciences. 2010;107:10590–10595. doi: 10.1073/pnas.0912457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- Guo F, Cerullo I, Chen X, Rosbash M. PDF neuron firing phase-shifts key circadian activity neurons in Drosophila. Elife. 2014:3. doi: 10.7554/eLife.02780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Yu J, Jung HJ, Abruzzi KC, Luo W, Griffith LC, Rosbash M. Circadian neuron feedback controls the Drosophila sleep–activity profile. Nature. 2016;536:292–297. doi: 10.1038/nature19097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann-Luibl C, Helfrich-Forster C. Clock network in Drosophila. Current Opinion in Insect Science. 2015;7:65–70. doi: 10.1016/j.cois.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Herzog ED. Neurons and networks in daily rhythms. Nat Rev Neurosci. 2007;8:790–802. doi: 10.1038/nrn2215. [DOI] [PubMed] [Google Scholar]
- Im SH, Li W, Taghert PH. PDFR and CRY Signaling Converge in a Subset of Clock Neurons to Modulate the Amplitude and Phase of Circadian Behavior in Drosophila. PLoS ONE. 2011;6:e18974. doi: 10.1371/journal.pone.0018974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose M, Duvall Laura B, Li W, Liang X, Ren C, Steinbach Joe H, Taghert Paul H. Functional PDF Signaling in the Drosophila Circadian Neural Circuit Is Gated by Ral A-Dependent Modulation. Neuron. 2016;90:781–794. doi: 10.1016/j.neuron.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Ivan T, Chang Alexander S, Manandhar M, Shan Y, Fan J, Izumo M, Ikeda Y, Motoike T, Dixon S, Seinfeld Jeffrey E, et al. Neuromedin S-Producing Neurons Act as Essential Pacemakers in the Suprachiasmatic Nucleus to Couple Clock Neurons and Dictate Circadian Rhythms. Neuron. 2015;85:1086–1102. doi: 10.1016/j.neuron.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Funes P, Dowse H, Hall J. Advanced analysis of a cryptochrome mutation’s effects on the robustness and phase of molecular cycles in isolated peripheral tissues of Drosophila. BMC Neuroscience. 2002;3:5. doi: 10.1186/1471-2202-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. The Neuropeptide Pigment-Dispersing Factor Coordinates Pacemaker Interactions in the Drosophila Circadian System. J Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinek S, Inonog S, Manoukian AS, Young MW. A Role for the Segment Polarity Gene shaggy/GSK-3 in the Drosophila Circadian Clock. Cell. 2001;105:769–779. doi: 10.1016/s0092-8674(01)00383-x. [DOI] [PubMed] [Google Scholar]
- Mieda M, Okamoto H, Sakurai T. Manipulating the Cellular Circadian Period of Arginine Vasopressin Neurons Alters the Behavioral Circadian Period. Current Biology. 2016;26:2535–2542. doi: 10.1016/j.cub.2016.07.022. [DOI] [PubMed] [Google Scholar]
- Muskus MJ, Preuss F, Fan JY, Bjes ES, Price JL. Drosophila DBT Lacking Protein Kinase Activity Produces Long-Period and Arrhythmic Circadian Behavioral and Molecular Rhythms. Molecular and Cellular Biology. 2007;27:8049–8064. doi: 10.1128/MCB.00680-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila Circadian Control Circuit. Current Biology. 2008;18:R84–R93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Park JH, Helfrich-Forster C, Lee G, Liu L, Rosbash M, Hall JC. Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc Natl Acad Sci U S A. 2000;97:3608–3613. doi: 10.1073/pnas.070036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli A, Althoff F, Oliveira RA, Heidmann S, Schuldiner O, Lehner CF, Dickson BJ, Nasmyth K. Cell-type-specific TEV protease cleavage reveals cohesin functions in Drosophila neurons. Dev Cell. 2008;14:239–251. doi: 10.1016/j.devcel.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M. Drosophila Free-Running Rhythms Require Intercellular Communication. PLoS Biol. 2003;1:e13. doi: 10.1371/journal.pbio.0000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Lear BC, Keegan KP, Allada R. Processing Circadian Data Collected from the Drosophila Activity Monitoring (DAM) System. Cold Spring Harbor Protocols. 2010;2010 doi: 10.1101/pdb.prot5519. pdb.prot5519. [DOI] [PubMed] [Google Scholar]
- Picot M, Cusumano P, Klarsfeld A, Ueda R, Rouyer F. Light Activates Output from Evening Neurons and Inhibits Output from Morning Neurons in the Drosophila Circadian Clock. PLoS Biol. 2007;5:e315. doi: 10.1371/journal.pbio.0050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf Neuropeptide Gene Mutation and Ablation of PDF Neurons Each Cause Severe Abnormalities of Behavioral Circadian Rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Schlichting M, Menegazzi P, Lelito KR, Yao Z, Buhl E, Dalla Benetta E, Bahle A, Denike J, Hodge JJ, Helfrich-Forster C, et al. A Neural Network Underlying Circadian Entrainment and Photoperiodic Adjustment of Sleep and Activity in Drosophila. The Journal of Neuroscience. 2016;36:9084–9096. doi: 10.1523/JNEUROSCI.0992-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seluzicki A, Flourakis M, Kula-Eversole E, Zhang L, Kilman V, Allada R. Dual PDF Signaling Pathways Reset Clocks Via TIMELESS and Acutely Excite Target Neurons to Control Circadian Behavior. PLoS Biol. 2014;12:e1001810. doi: 10.1371/journal.pbio.1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread Receptivity to Neuropeptide PDF throughout the Neuronal Circadian Clock Network of Drosophila Revealed by Real-Time Cyclic AMP Imaging. 2008;58:223–237. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Taghert PH. RNA-Interference Knockdown of Drosophila Pigment Dispersing Factor in Neuronal Subsets: The Anatomical Basis of a Neuropeptide’s Circadian Functions. PLoS ONE. 2009;4:e8298. doi: 10.1371/journal.pone.0008298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyllie NJ, Chesham JE, Hamnett R, Maywood ES, Hastings MH. Temporally chimeric mice reveal flexibility of circadian period-setting in the suprachiasmatic nucleus. Proceedings of the National Academy of Sciences. 2016;113:3657–3662. doi: 10.1073/pnas.1511351113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- Vansteensel MJ, Michel S, Meijer JH. Organization of cell and tissue circadian pacemakers: A comparison among species. Brain Research Reviews. 2008;58:18–47. doi: 10.1016/j.brainresrev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties. Annual Review of Physiology. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DA, Hamblen-Coyle MJ, Dushay MS, Hall JC. Behavior in Light-Dark Cycles of Drosophila Mutants That Are Arrhythmic, Blind, or Both. Journal of Biological Rhythms. 1993;8:67–94. doi: 10.1177/074873049300800106. [DOI] [PubMed] [Google Scholar]
- Yao Z, Shafer OT. The Drosophila Circadian Clock Is a Variably Coupled Network of Multiple Peptidergic Units. Science. 2014;343:1516–1520. doi: 10.1126/science.1251285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii T, Todo T, Wulbeck C, Stanewsky R, Helfrich-Forster C. Cryptochrome is present in the compound eyes and a subset of Drosophila’s clock neurons. The Journal of Comparative Neurology. 2008;508:952–966. doi: 10.1002/cne.21702. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Wulbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, Helfrich-Forster C. The Neuropeptide Pigment-Dispersing Factor Adjusts Period and Phase of Drosophila’s Clock. The Journal of Neuroscience. 2009;29:2597–2610. doi: 10.1523/JNEUROSCI.5439-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chung BY, Lear BC, Kilman VL, Liu Y, Mahesh G, Meissner RA, Hardin PE, Allada R. DN1p Circadian Neurons Coordinate Acute Light and PDF Inputs to Produce Robust Daily Behavior in Drosophila. Current Biology. 2010a;20:591–599. doi: 10.1016/j.cub.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Bilodeau-Wentworth D, Hardin PE, Emery P. Light and Temperature Control the Contribution of Specific DN1 Neurons to Drosophila Circadian Behavior. Current Biology. 2010b;20:600–605. doi: 10.1016/j.cub.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.