Abstract
DNA double-strand breaks are critical lesions that can lead to chromosomal aberrations and genomic instability. In response to DNA damage, Chk1, a serine/threonine kinase, is responsible for cell cycle arrest to prevent damaged cells from progressing through the cell cycle. Here, we report that the disruption of wat1, a WD repeat-containing protein, leads to the phosphorylation of Chk1. The double-deletion of chk1 and wat1 had a grave effect on the survival of fission yeast cells, and the spontaneous recombination rate was also high upon double-deletion of wat1 and chk1, as compared to the single-mutant. In the absence of wat1, the cells exhibited a high level of nuclear fragmentation that resulted in the accumulation of Rad22 yellow fluorescent protein foci. Furthermore, we show that wat1 is required for the regulation of the oxidative stress response. We observed elevated levels of reactive oxygen species (ROS) generation in wat1-null mutant that led to a high degree of propidium iodide staining at nonpermissive temperature. Based on the results presented here, we hypothesize that ROS production in wat1-null mutant cells generates DNA fragmentation that could trigger a checkpoint response and that, in the absence of checkpoint kinase Chk1, the cells exhibit severe growth defects leading to a synthetic lethal phenotype.
Keywords: Wat1/Pop3, Chk1, reactive oxygen species, cell death, apoptosis
SEVERAL regulatory networks ensure the orderly execution of cell cycle events that increase the fidelity of DNA replication and chromosome segregation (Sclafani and Holzen 2007). A number of proteins have been identified that are involved during the DNA damage checkpoint response. Central to this network are protein kinases of the PIKK (phosphoinositide 3-kinase-related protein kinase) family, like ATM (ataxia-telangiectasia mutated), ATR (ATM and Rad3-related), that work as sensors and transducers. The yeast counterparts of these censor proteins are Tel1/Mec1 and Tel1/Rad3 in budding yeast and fission yeast, respectively (McGowan and Russell 2004). Downstream of ATM and ATR are effector molecules, Chk1 and Chk2, that sense DNA damage and phosphorylate a number of proteins that regulate cell cycle progression (Nyberg et al. 2002; Shiloh 2003). Chk1, an essential component of the DNA damage checkpoint, is an evolutionarily conserved protein kinase (Walworth et al. 1993; Al-Khodairy et al. 1994). Activation of Chk1 in response to DNA damage results in the phosphorylation of Wee1 and Cdc25, which prevents the activation of Cdc2 and hence inhibits mitotic entry (Rhind et al. 1997).
Recently, a genetic screen to identify conditional synthetic lethal mutants with chk1 knockout led to the identification of a novel temperature-sensitive mutant allele of wat1 (Verma et al. 2014). Wat1/Pop3 is a conserved protein composed of WD40 repeats (Neer et al. 1994). The WD40-repeat motif was originally identified in the β-subunit of heterotrimeric G proteins (Fong et al. 1986), and has since been found in a number of regulatory proteins where it mediates protein–protein interaction. WD40 repeat-containing proteins adopt a β-propeller structure, which can use one or two blades to interact with different proteins without affecting the remaining blades (Craig 2003). Wat1/Pop3 is a homolog of Lst8 in budding yeast and is responsible for the delivery of Gap1 protein, and possibly other amino acid permeases, from the Golgi to the cell surface (Roberg et al. 1997). The mutant allele of wat1 (wat1-1) exhibits delocalization of actin patches that leads to the deformation of cell shape in fission yeast (Kemp et al. 1997). Fission yeast Wat1 is also required for mRNA maturation, and its role in the maintenance of genome stability and microtubule integrity has been well-studied (Ochotorena et al. 2001). Mammalian LST8, an ortholog of Wat1/Pop3, interacts with the kinase domain of mTOR that stabilizes its interaction with Raptor and also regulates cell growth through the mTOR-S6K1 signaling pathway (Yang et al. 2013).
TORC2/Ypk1/2 signaling controls the response to oxidative stress and increases the expression of genes that are required for the suppression of reactive oxygen species (ROS) through the oxidative stress-responsive transcription factor Yap1 (Mulet et al. 2006; Niles et al. 2012). ROS such as hydrogen peroxide (H2O2), superoxide, and hydroxyl radical are produced in the cells during normal metabolism and have many beneficial roles. However, unregulated ROS can lead to oxidation of proteins, lipids, and DNA that can result in cellular damage (Farrugia and Balzan 2012). In mammalian and yeast cells, ROS are produced from numerous sources, with a key source being the mitochondrial electron transport chain (Fang and Beattie 2003). Nonmitochondrial sources such as endoplasmic reticulum stress, exposure to heavy metals, and other environmental factors are also responsible for ROS production (Halliwell and Cross 1994; Haynes et al. 2004). More recently, ROS accumulation has also been observed due to the defects in vacuolar acidification, as well as reduced levels of sphingolipids (Milgrom et al. 2007; Kajiwara et al. 2012).
In this study, we show that chk1- and wat1-null mutants exhibit conditional synthetic lethality. We observed that the loss of viability upon wat1 deletion at nonpermissive temperature could be due the resulting DNA damage, which can lead to chromosomal breakage. At permissive temperature, the absence of wat1 leads to the phosphorylation of Chk1. We also shown that, in the absence of wat1, cells spontaneously accumulate Rad22 yellow fluorescent protein (YFP) foci that represent DNA damage sites. Furthermore, the spontaneous recombination rate was much higher upon double-deletion of wat1 and chk1 as compared to each single deletion. We also present evidence that wat1 is required for resistance to oxidative stress. Remarkably, we observed elevated levels of ROS in wat1-null mutants as compared to the wild type, suggesting that, at higher temperature, ROS production might be responsible for DNA damage and chromosomal breakage that leads to cell death in the wat1-deletion background.
Materials and Methods
Strains and growth condition
Schizosaccharomyces pombe strains used in this study are listed in Table 1. Standard genetic methods were utilized for making strains as described earlier (Moreno et al. 1991). For temperature shift experiments, cells were grown to midlog phase at 25° and then shifted to restrictive temperature (36°) in a water bath. To measure camptothecin (CPT) and H2O2 sensitivity, cells were grown at 25° up to midlog phase, following which 1 × 107 cells were serially diluted and spotted on plates containing drugs. To facilitate the detection of Wat1 and Chk1, strains carrying Wat1-FLAG and Chk1-HA were used.
Table 1. Strains used in this study.
| Strain | Genotype |
|---|---|
| SP6 | H− leu1-32 |
| NW158 | H+ leu1-32 ura4D18 chk1::ura4 ade6-216 |
| SH510 | H− leu1-32 ura4+ wat1::kanR |
| SH586 | H− leu1-32 ura4D18 chk1::ura4 wat1::kanR ade6-210 |
| SH669 | H+ leu1-32 wat1-FLAG-kanR |
| SH670 | H+ leu1-32 wat1::kanR |
| EN3222 | H− leu1-32 ura4D18 rad22-YFP-kanR |
| SH706 | H leu1-32 ura4+ wat1::kanR rad22-YFP-kanR ade6-? |
| SH713 | H leu1-32 ura4+ wat1::kanR chk1-ep.ade6–216 |
| E83 | H− ura4D18 his3-D1 ade6-375 int:pUC8/His3/ade6-469 |
| SH942 | H− leu+ ura4D18 his3-D1 wat1::kanR ade6-375 int:pUC8/his3/ade6-469 |
| SH965 | H leu1-32 ura4D18 his3-D1 chk1::ura4 ade6-375 int:pUC8/his3/ade6-469 |
| SH966 | H leu1-32 ura4D18 his3-D1 wat1::kanR chk1::ura4 ade6-375 int:pUC8/his3/ade6-469 |
Gene disruption
For wat1 gene disruption, a two-step gene replacement method was used as previously described (Bahler et al. 1998). In summary, a 1.8-kb fragment containing a kanamycin-resistant gene with wat1 overhangs was transformed in a wild-type strain. Transformants were selected by replica plating on plates containing 100 µg/ml of G418. Homozygous haploid strains with wat1 deletion were isolated. Deletion was confirmed by southern blot hybridization, and by PCR using a primer set encompassing the kanR cassette and upstream of the wat1 gene.
Microscopy and indirect immunofluorescence studies
Cells were grown to midlog phase at 25° then shifted to 36°, samples were collected, fixed with 70% ethanol, and stained with DAPI, then visualized using a fluorescence microscope. Approximately 200 cells were counted for abnormal DAPI-staining bodies and the percentage was calculated. Immunofluorescence studies were performed using exponentially growing cells essentially as described previously (Khan and Ahmed 2015). Rad22-YFP foci were detected using an anti-GFP antibody (1:50 dilution), incubated overnight at room temperature, washed, and detected with secondary antibody coupled to Alexa Fluor 488 (Life Technologies). The cells were analyzed using a fluorescence microscope and processed by using Adobe Photoshop.
Single-cell gel electrophoresis (SCGE) or comet assay
The alkaline comet assay was performed fundamentally as described earlier (Olive et al. 1994; Miloshev et al. 2002). Agarose-precoated slides were prepared by dipping the slides into molten 1% agarose (Promega, Madison, WI) and wiping one side clean, then allowing the agarose to air-dry to form a thin film. Yeast cells were harvested using microcentrifugation at 12,000 rpm for 1 min, cells were washed with water, and resuspended in buffer containing 1 M sorbitol, 25 mM KH2PO4 (pH 6.5). Aliquots of ∼5 × 104 cells were mixed with 1% low-melting agarose containing 2 mg/ml of zymolase 100T (Seikagaku Corp.) and spread over precoated slides. These slides were incubated at 36° for 30 min to obtain spheroplasts. After spheroplasting, the slides were incubated in lysis buffer (30 mM NaOH, 1 M NaCl, 0.1% laurylsarcosine, and 50 mM EDTA, pH 12.3) overnight at 4° to lyse the spheroplasts. The slides were rinsed three times for 20 min each in A2 rinse buffer (30 mM NaOH and 10 mM EDTA, pH 12.4) to unwind DNA and then subjected to electrophoresis in the same buffer. The electrophoresis was carried out for 30 min at 0.6 V/cm, 40 mA. After electrophoresis, the gels were neutralized by submerging the slides in 10 mM Tris-HCl pH 7.5 for 10 min. Finally, the slides were stained with ethidium bromide (1 mg/ml) and visualized under a fluorescence microscope. Individual comet images were analyzed using ImageJ OpenComet analysis software (Gyori et al. 2014). The tail lengths of 50 comets per sample were measured in three independent experiments, and graph was plotted with SD.
Preparation of whole cell lysate and western blot analysis
Cells from midlog phase culture were harvested by centrifugation and lysed using glass beads and a Fast Prep (Bio 101) vortex machine. Lysate was prepared in PBS-containing protease inhibitor cocktail and sodium orthovanadate. Supernatant was collected by centrifugation and protein was estimated using the Bradford assay method. For western blot analysis, 100 mg of total cell lysate was run on 10% SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti-α-tubulin [Sigma (Sigma Chemical), St, Louis, MO, cat. no. T6199] and anti-HA (F7, Santa Cruz) antibodies. A peroxidase-coupled secondary antibody and an ECL detection system (Millipore, Bedford, MA) were used to detect the immune complexes.
ROS and PI-staining assays
ROS and PI-staining was performed essentially as described earlier (Marchetti et al. 2005). In summary, midlog phase cultures were shifted at nonpermissive temperature (36°) for 3 hr. At the end of the incubation, the ROS indicator dye 2′,7′-dichlorodihydrofluorescein diacetate (DCDHFDA; Molecular Probes, Eugene, OR) was added at a final concentration of 10 µg/ml, and samples were further incubated for 80 min. Cells were collected and washed twice with PBS buffer (pH 7.0). The pellets were resuspended in PBS containing propidium iodide (PI) at a final concentration of 10 µg/ml and then analyzed by fluorescence microscopy.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
Chk1 deletion aggravates the growth defect of wat1/pop3 knockout
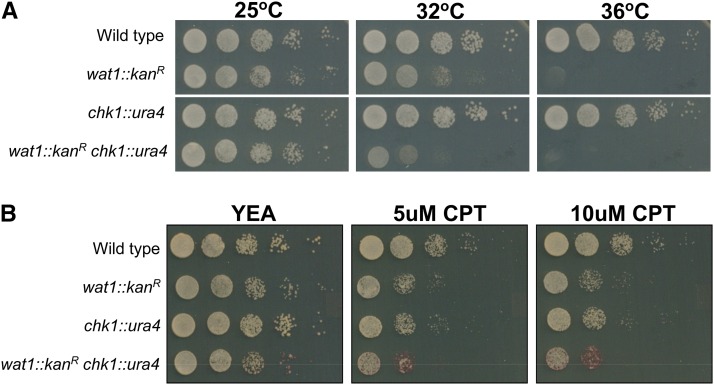
In our earlier studies, we have shown the conditional synthetic lethal genetic interaction between wat1/pop3 mutants with chk1 knockout (Verma et al. 2014). To analyze the phenotypic behavior of wat1 knockout, we deleted the ORF of the wat1 gene with a kanR marker using PCR-based gene targeting (Supplemental Material, Figure S1) (Bahler et al. 1998). The wat1-deleted cells exhibit a normal growth pattern at permissive temperature (25°) but were unable to form colonies at nonpermissive temperature (36°), indicating the temperature-sensitive phenotype of wat1 knockout cells (Figure 1A). The temperature-sensitive phenotype of a wat1 knockout strain has also been reported (Ochotorena et al. 2001). At semipermissive temperature (32°), the wat1-deleted cells were able to form colonies but exhibited reduced growth as compared to wild-type cells (Figure 1A). Interestingly, in a chk1 deletion background, the colony forming ability of the wat1 deletion strain was further reduced at semipermissive temperature (32°) (Figure 1A), indicating a conditional synthetic lethal genetic interaction between these two genes.
Figure 1.
Conditional synthetic lethality between wat1/pop3 knockout and chk1 deletion. (A) Ten-fold serial dilutions of logarithmic growing cells were spotted on YE-rich medium. Plates were incubated at the indicated temperatures for 3–4 days. (B) 10-fold serial dilutions of logarithmic growing cells were spotted on YE-rich medium, containing the indicated concentrations of CPT. Spotting assays were performed in three independent experiments with similar results. CPT, camptothecin; YEA, YE agar.
Further, the wat1-deleted and chk1-deleted cells exhibited sensitivity toward CPT, a topoisomerase I inhibitor that generates DNA damage in fission yeast (Wan et al. 1999). The CPT sensitivity upon double-deletion of wat1 and chk1 was higher as compared to each single-mutant (Figure 1B), suggesting a requirement for these two proteins to overcome the damage induced by CPT.
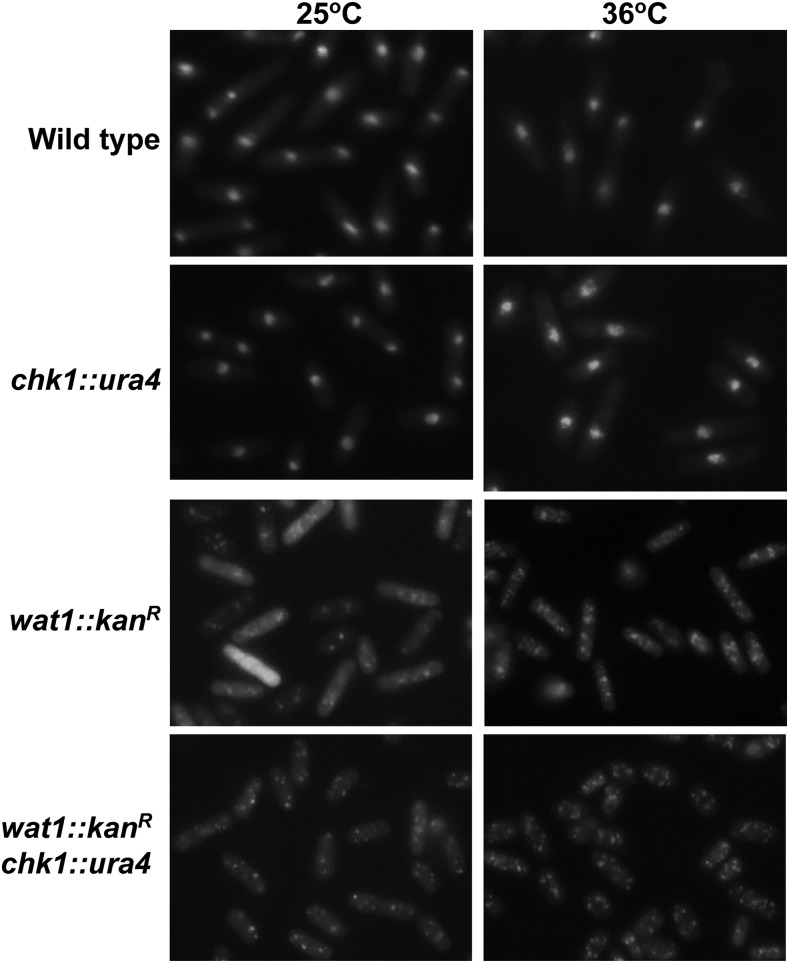
Deletion of wat1 leads to nuclear fragmentation at nonpermissive temperature
To examine the phenotypic behavior, we processed the wat1-deleted cells for DAPI-staining both at permissive and nonpermissive temperature. The wild type and chk1-deleted cells exhibited normal nuclei at permissive as well as nonpermissive temperature (Figure 2). Interestingly, in wat1-deleted cells at non permissive temperature, multiple DAPI-stained nuclei were observed in 98% of the cells (Figure 2) that might have been generated due to the fragmentation of nuclei. At permissive temperature, about 84% cells had punctate nuclear-staining, while 16% of cells exhibited more than two punctate nuclear spots that could also be due to nuclear fragmentation (Figure 2). In chk1-deleted wat1-deleted strains, the cells had high levels of nuclear fragmentation (∼99%) and cell size was also reduced (Figure 2), which could have been due to the loss of checkpoint function. These results indicate that the loss of viability upon wat1-deletion at nonpermissive temperature could be due to DNA damage leading to chromosome breakage.
Figure 2.
Deletion of wat1 leads to nuclear fragmentation at nonpermissive temperature. Indicated strains were grown till midlog phase at 25° and then shifted to 36° for 3 hr, fixed with 70% ethanol, and stained with DAPI. Experiment was performed three times with similar results.
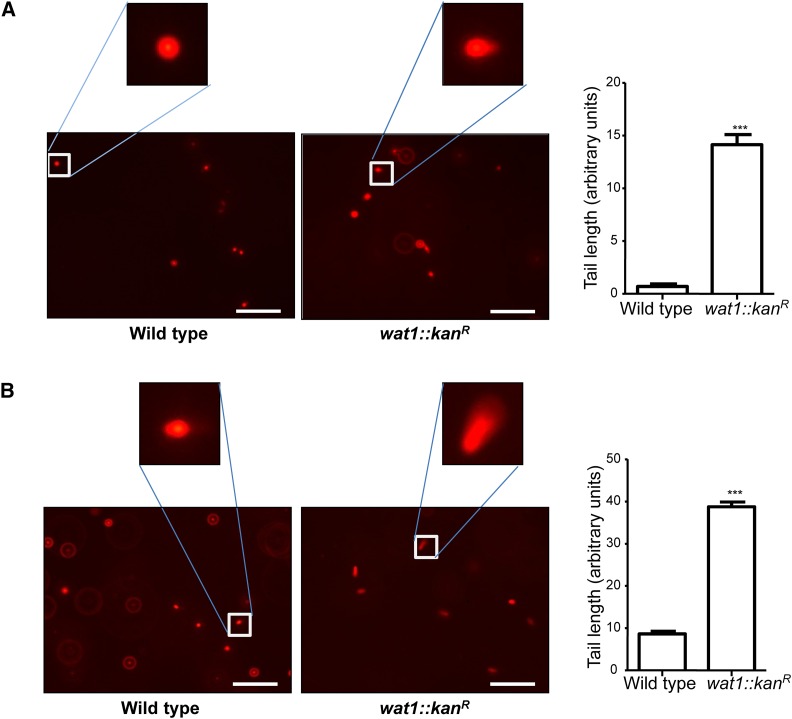
Furthermore, we checked nuclear fragmentation by SCGE or comet assay. Wild-type and wat1-deleted cells were shifted at nonpermissive temperature (36°) for 3 hr and processed for comet assay, as described in Materials and Methods. A characteristic picture of a comet with a small tail was observed in wat1-deleted cells grown at permissive temperature that was absent in wild-type cells (Figure 3A), suggesting partial nuclear fragmentation in wat1-deleted cells. Interestingly, there was threefold increase in the length of comet tail in wat1-deleted cells grown at nonpermissive temperature (Figure 3B), suggesting severe nuclear fragmentation, as was also observed by DAPI-staining (Figure 2).
Figure 3.
Single-cell gel electrophoresis of fission yeast cells. Wild-type and wat1-deleted cells were grown till midlog phase at 25° and then shifted to 36° for 3 hr. Approximately 5 × 104 cells were mixed with 1% low-melting agarose, spheroplasted, and then subjected to comet assay as described in Materials and Methods. Samples grown at 25° (A) and 36° (B) were visualized under a fluorescence microscope. Individual comet images obtained from three independent experiments were analyzed using ImageJ OpenComet analysis software. The tail lengths of 50 comets per sample were measured and the graph was plotted with SD. ***P<0.001, significant.
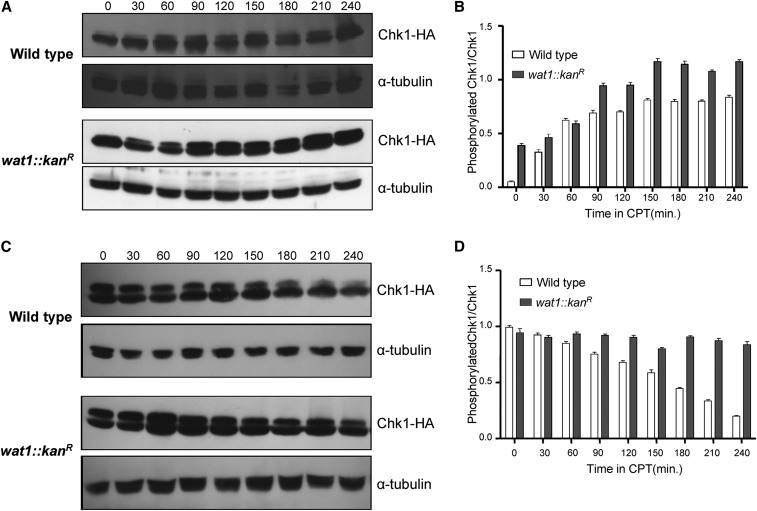
Dephosphorylation of Chk1 after release from CPT was delayed in the wat1 deletion strain
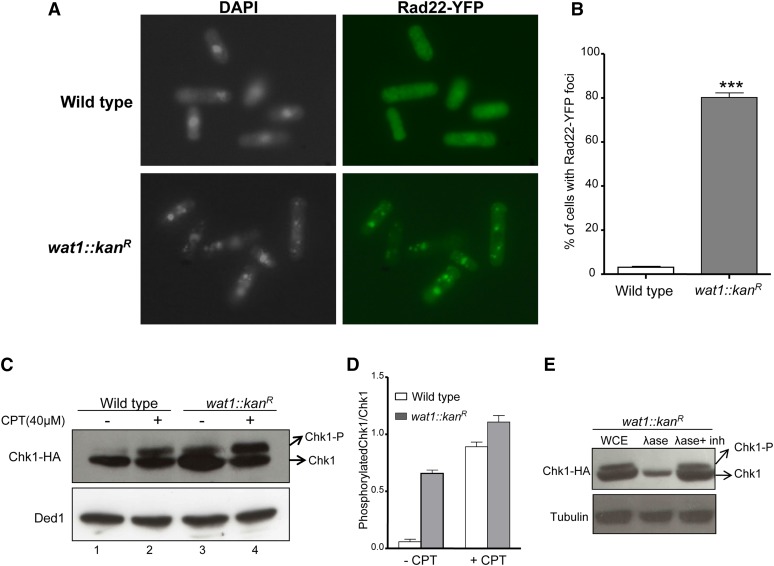
The genetic interaction between wat1 and chk1 prompted us to look for Chk1 phosphorylation in response to the DNA-damaging agent CPT in the wat1 deletion background. Chk1 is phosphorylated in response to DNA damage, resulting in reduced mobility of Chk1 protein on SDS-PAGE (Walworth and Bernards 1996; Wan et al. 1999). To confirm whether the slow-migrating band of Chk1 is a phosphorylated form of Chk1, we treated CPT-induced protein lysate with alkaline phosphatase. The slow-migrating band of Chk1 was absent in the sample treated with alkaline phosphatase (Figure S2, lane 2), but it was clearly visible in the sample treated with alkaline phosphatase along with EDTA (Figure S2, lane 3), suggesting that the slow-migrating band of Chk1 is indeed a phosphorylated form of Chk1. To monitor Chk1 phosphorylation in wild-type and wat1 knockout strains, we first grew cells containing HA-tagged Chk1 in the presence of 40 µM CPT and samples were collected at 30 min time intervals, as described in Materials and Methods. In response to CPT, the wild type and wat1-deleted cells showed phosphorylation of Chk1 (Figure 4A) that kept on increasing until 90 min after initiation, at which point it was saturated (Figure 4B). Interestingly, the Chk1 phosphorylation in wat1-deleted cells at the 0 min time point was greater than that of wild-type cells (Figure 4, A and B). Further, upon removal of CPT, the dephosphorylation of Chk1 was delayed in wat1-deleted cells as compared to wild-type cells (Figure 4, C and D). This delay in the dephosphorylation of Chk1 may suggest that wat1-deleted cells are defective in reentry into the cell cycle. Alternatively, it is also possible that DNA damage generated due to wat1 deletion persists even after removal of CPT, which leads to persistent phosphorylation of Chk1.
Figure 4.
Chk1 dephosphorylation was delayed in wat1 deletion mutants after release from CPT. (A) Wild-type and wat1-deleted cells containing HA-tagged Chk1 were grown to midlog phase in the presence of 40 µM CPT. Protein lysate from each of the indicated time points was analyzed by western blot using anti-HA antibody; α-tubulin antibody was used as loading control. (B) The graph shows average Chk1 phosphorylation relative to Chk1 protein determined from GelQuant Express Image analysis of data obtained in three independent experiments. (C) Wild-type or wat1-deleted cells containing an HA-tagged Chk1 were grown in the presence of 40 µM CPT for 3 hr, cells were washed and resuspended in fresh medium, and allowed to grow for the indicated time. Protein samples from the indicated time points were analyzed by western blot as described above. (D) Quantitation of Chk1 phosphorylation was plotted as described above. CPT, camptothecin.
Abrogation of the wat1 gene leads to DNA damage
To examine the generation of DNA damage in wat1-deleted cells, we checked the accumulation of Rad22-YFP foci. Rad22 is a single-stranded DNA-binding protein that plays an important role during DNA repair by homologous recombination. Quantitative analysis revealed that 5% of wild-type cells contained nuclear Rad22-YFP foci (Figure 5, A and B). In contrast, about 80% of the nuclei in wat1-deleted cells exhibited one or multiple Rad22-YFP foci at permissive temperature (Figure 5, A and B). At nonpermissive temperature, the number of Rad22-YFP foci was much higher, along with fragmented nuclei as described above (data not shown). These data suggest that the wat1-deleted cells accumulate DNA damage at permissive as well as nonpermissive temperature.
Figure 5.
Deletion of wat1 gene leads to DNA damage. (A) Strains were grown in YEA liquid medium at 25° until midlog phase. The cells were processed for indirect immunofluorescence microscopy using anti-GFP antibody as described in Materials and Methods. (B) Approximately 200 cells for each sample in three independent experiments were counted and the average percentage of cells containing Rad22-YFP foci with SD was plotted. (C) Wild-type and wat1-deleted cells containing HA-tagged Chk1 were grown to midlog phase in the presence or absence of 40 µM CPT. Protein lysate was prepared and probed with anti-HA to visualize Chk1. Anti-Ded1 antibody was used as a loading control. (D) The graph shows average Chk1 phosphorylation relative to Chk1 protein determined from GelQuant Express Image analysis of data obtained in three independent experiments. (E) Protein lysate of wat1-deleted cells was treated with alkaline phosphatase or alkaline phosphatase with inhibitor for 30 min, and probed with anti-HA antibody to visualize Chk1. Anti α-tubulin antibody was used as a loading control. YEA, YE agar; YFP, Yellow fluorescent protein.
DNA damage at any stage of the cell cycle activates the DNA damage checkpoint kinase Rad3, which leads to the phosphorylation of the downstream effector kinase Chk1 (Walworth and Bernards 1996). We were intrigued whether the DNA damage generated due to the wat1 deletion could also lead to the phosphorylation of Chk1. Wild-type and wat1-deleted cells showed considerable Chk1 phosphorylation in response to CPT (Figure 5, C and D). Interestingly, we observed a significant increase (∼10-fold) in the intensity of the slow-migrating band of Chk1 in wat1-deleted cells as compared to wild-type cells, even in the absence of any DNA-damaging agent (Figure 5C, compare lane 1 and 3), indicating the phosphorylation of Chk1. To further confirm whether this slow-migrating band of Chk1 is due to phosphorylation, we treated the sample with alkaline phosphatase. As shown in Figure 5E, the slow-migrating band of Chk1 was absent in the sample treated with alkaline phosphatase (Figure 5E, lane 2) but it was visible in the sample treated with alkaline phosphatase along with EDTA (Figure 5E, lane 3), suggesting that the slow-migrating band in the wat1 deletion strain is indeed a phosphorylated form of Chk1. These results clearly indicate that the absence of the wat1 gene results in the generation of DNA damage that might be responsible for the activation of checkpoint kinase Chk1. At nonpermissive temperature, the extent of DNA damage in wat1-deleted cells is significantly higher, leading to chromosomal breakage (as shown in Figure 2 and Figure 3) and the cells being unable to survive.
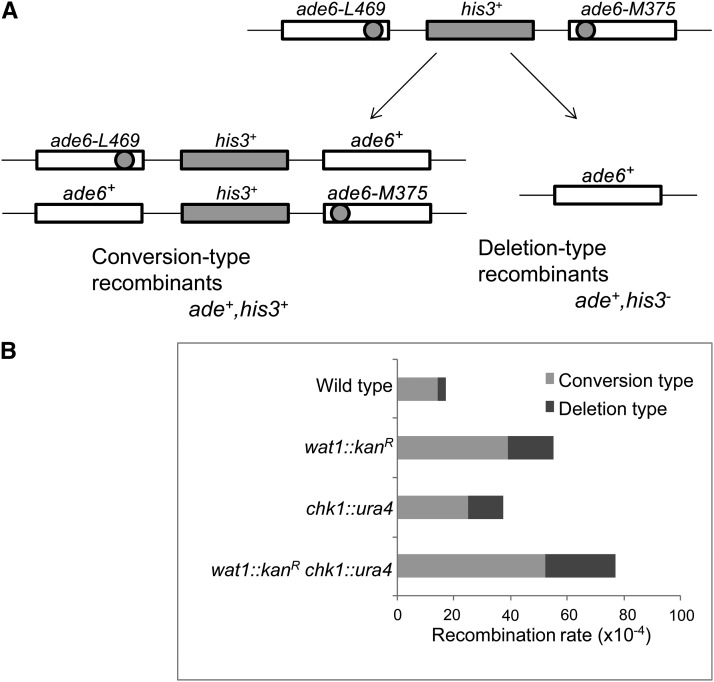
Deletion of wat1 induces mitotic intrachromosomal recombination
To determine the frequency of spontaneous intrachromosomal recombination, we constructed wat1-deleted, chk1-deleted, and double-deletion strains containing nontandem direct repeats of ade6− heteroalleles with a functional his3+ gene between the repeats (Osman et al. 2000). As shown in Figure 6A, the generation of ade+ recombinant can be scored as a spontaneous intrachromosomal recombination event. There are two classes of ade6+ recombinants, Ade+ His− deletion type and Ade+ His+ conversion type recombinants (Figure 6A). We observed a threefold increase in spontaneous recombination rate in wat1-deleted cells as compared to wild-type cells, with 70% of these recombinants being conversion types and the remainder being deletion types (Figure 6B). The chk1-deleted cells exhibited a twofold increase in spontaneous recombination rate as compared to wild-type cells (Figure 6B). Interestingly, in wat1 chk1 double-deletion mutants, the spontaneous recombination rate was 4.5-fold higher in comparison to wild-type cells, with 67% of these recombinants being conversion types and the remainder being deletion types (Figure 6B). The high recombination rate upon double-deletion is in agreement with the synthetic lethal phenotype of the wat1 and chk1 double-deletion.
Figure 6.
Spontaneous recombination rate was increased upon wat1 deletion. (A) Schematic representation of the recombination substrate that gives rise to two types of the Ade+ recombinants. Gene conversion results in Ade+ His+ recombinants, whereas deletion results in the formation of Ade+ His− colonies. (B) Recombination frequencies of the indicated strains (per 1 × 104 cells). Average recombinants of conversion types (gray) or deletion types (black) obtained from three independent experiments were identified by replica plating and plotted.
Wat1 is required for resistance to oxidative stress
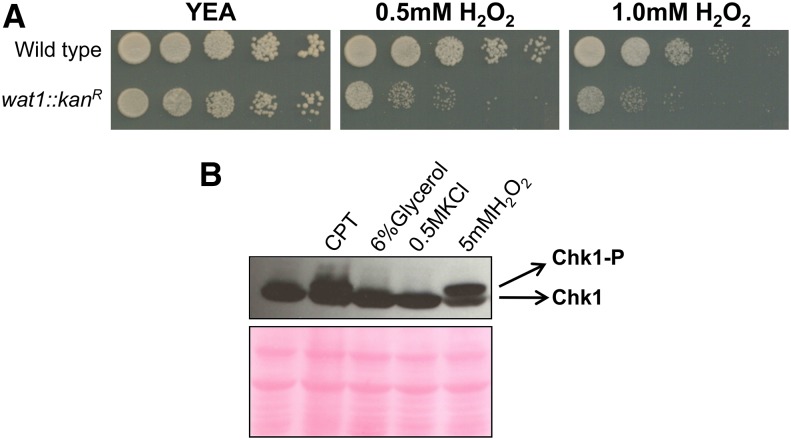
Wat1 is an important component of both the Tor2 and Tor1 complexes, known as TORC1 and TORC2, respectively, in S. pombe (Hayashi et al. 2007). Since TORC2 is responsible for the regulation of the oxidative stress response, we tried to investigate the role of the wat1 gene in the oxidative stress response. The wat1 deletion strain was checked for sensitivity toward H2O2. We observed reduced growth of wat1-deleted strain as compared to the wild type control strain on plates containing 0.5 or 1.0 mM H2O2 (Figure 7A), suggesting that wat1 is required for resistance to oxidative stress.
Figure 7.
Sensitivity of wat1 deletion in response to oxidative stress. (A) Ten-fold serial dilutions of logarithmic growing cells were spotted on YE-rich medium, containing the indicated concentrations of H2O2. Plates were incubated at 25° for 3 days. Spotting assay was performed in three independent experiments with similar results. (B) Wild-type cells containing a HA-tagged Chk1 were grown to log phase and treated with 40 µM CPT, 6% glycerol, 0.5 M KCl, or 5 mM H2O2. Protein lysate was analyzed by western blot using anti-HA antibody; ponceau stained gel is shown as a loading control. Experiment was performed three times with similar results. CPT, camptothecin; H2O2, hydrogen peroxide; YEA, YE agar.
Furthermore, we checked the phosphorylation of Chk1 under different stress conditions like genotoxic (CPT), osmotic (Glycerol and KCl), and oxidative stress (H2O2). Surprisingly, we found that Chk1 is phosphorylated by H2O2 (Figure 7B), suggesting that the ROS generated by H2O2 can lead to DNA damage and hence phosphorylation of Chk1. Similarly, Chk1 phosphorylation was also observed in the wat1 deletion strain (Figure 5C), suggesting that the wat1 deletion might also induce ROS that leads to DNA damage.
Deletion of the wat1 gene results in increased ROS production and cell death
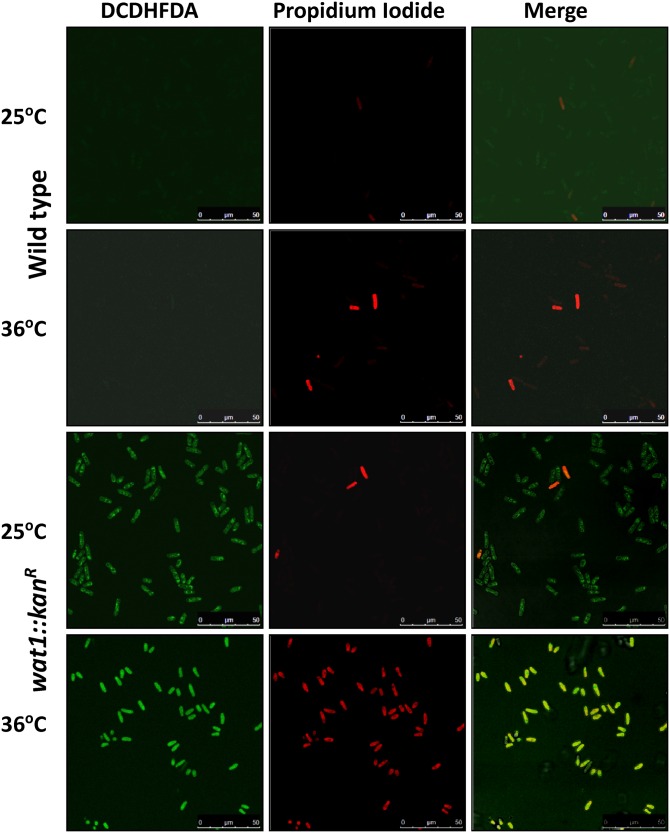
To check ROS production in wat1-deleted, cells we used DCDHFDA dye, which produces green fluorescence in the presence of ROS. The ability of this dye to penetrate the membranes of living cells makes it capable of detecting the ROS in living cells as well as dying cells that lack an intact membrane. To distinguish between living and dead cells, we used PI, which cannot pass through the intact membrane and hence detects only dead cells. As shown in Figure 8, most of the wild type cells displayed insufficient green fluorescence at permissive and nonpermissive temperature, indicating no ROS production. In contrast, 98% of wat1-deleted cells displayed detectable green fluorescence, indicating ROS production at permissive as well as nonpermissive temperature (Figure 8). Furthermore, only 1 and 3% of wild-type cells were stained with PI at permissive and nonpermissive temperature, respectively, indicating dead cells (Figure 8). At permissive temperature, only 3% of wat1-deleted cells stained with PI, suggesting that these cells produce ROS but were able to survive. In contrast, at nonpermissive temperature, most of the wat1-deleted cells (98%) displayed PI-staining indicating dead cells (Figure 8). These results suggest that, at higher temperature, ROS production might be responsible for cell death the in wat1 deletion background.
Figure 8.
Deletion of wat1 gene results in increased ROS production and cell death. Midlog phase culture of wild-type and wat1-deleted cells were incubated for 90 min at 25° in the presence of DCDHFDA to detect ROS. For the 36° experiment, the midlog phase cultures were shifted at 36° for 3 hr, then incubated with media containing DCDHFDA for 90 min at 36°. The cells were harvested, washed, and resuspended in buffer containing PI to detect dead cells. This experiment was performed three times with similar results. DCDHFDA, 2′,7′-dichlorodihydrofluorescein diacetate; H2O2, hydrogen peroxide; PI, propidium iodide; ROS, reactive oxygen species.
Discussion
Disruption of wat1 in fission yeast disables the cells from responding properly to a variety of stress conditions, including genotoxic, osmotic, oxidative, and temperature stresses. In S. pombe, the DNA damage checkpoint is activated by Rad3 (ATR homolog), which in turn activates its downstream protein kinase, Chk1 (Carr 2002). Here, we report that Chk1 phosphorylation in response to CPT was elevated in wat1-null mutants; upon termination of the checkpoint signal, the dephosphorylation of Chk1 was also delayed in wat1-null mutants (Figure 3C). This delay in dephosphorylation of Chk1 may suggest that wat1-deleted cells are defective in reentry into the cell cycle. Alternatively, it is also possible that disruption of wat1 might generate DNA damage leading to persistent phosphorylation of Chk1. In fact, DAPI-staining of wat1-null mutants revealed aberrant nuclei, and at nonpermissive temperatures a fragmented nuclear structure appeared that could have been generated due to extensive DNA damage. The phosphorylation of Chk1 and increased Rad22-YFP foci in wat1-null mutants, even in the absence of genotoxic stimuli, support our hypothesis. Since phosphorylation of Chk1 in response to H2O2 has been reported recently (Willis et al. 2013), and Wat1 is a major component of both the Tor2 and Tor1 complexes (TORC1 and TORC2), we speculated that it plays a role in the regulation of the oxidative stress response. We observed much reduced growth of wat1-deleted cells on plates containing H2O2, indicating its role in the oxidative stress response. Furthermore, we observed that wat1-null mutant cells generate ROS signals at permissive as well as nonpermissive temperature. A high degree of PI-staining (98%) in wat1-deleted cells suggests significant cell death due to ROS production at nonpermissive temperature. Both in yeast and mammalian cells, ROS are generated as a byproduct of mitochondrial and endoplasmic reticular activity, which leads to apoptosis in mammalian cells (Fleury et al. 2002). Fission yeast cells containing conditional mutant alleles of genes encoding DNA replication initiation proteins also stimulate ROS production (Marchetti et al. 2005). ROS production leading to cell death has also been observed in yeast cells bearing temperature-sensitive cdc48 mutation. These cells exhibit many features of apoptosis, including chromatin condensation and nuclear DNA fragmentation at restrictive temperature (Madeo et al. 1999). Programmed cell death induced by DNA damage has also been reported in yeast cells bearing mutation in the cdc6, orc2, cdc18, and dfp1 genes (Blanchard et al. 2002; Qi et al. 2003; Weinberger et al. 2005).
Inhibition of TORC2 kinase and its downstream effector kinase Ypk1/Ypk2 leads to chromosomal fragmentation in the presence of the double-strand break-inducing antibiotic Zeocin (Shimada et al. 2013). Since Ypk1/Ypk2 controls the actin cytoskeleton, it has been suggested that its actin-associated activity impairs genome integrity under enhanced oxidative stress conditions (Shimada et al. 2013). Given that Wat1 is also required for F-actin localization (Kemp et al. 1997), it is possible that this effect could be due to the increase in the ROS activity in wat1-deleted cells. The sensitivity of the actin cytoskeleton in response to an increase in the oxidative status of cells has also been reported (Dalle-Donne et al. 2001). Our results suggest that ROS production in wat1-null mutants could be responsible for chromosome fragmentation that could trigger a checkpoint response and that, in the absence of checkpoint kinase Chk1, the cells exhibit severe growth defects leading to a synthetic lethal phenotype.
Acknowledgments
We acknowledge J. V. Pratap and R. Ravishankar for the critical reading of the manuscript. We also thank members of our lab for helpful discussions and technical support. Our special thanks to Rima Ray Sarkar for the technical help provided in confocal microscopy. Work reported in this project was supported by grants from the Council of Scientific & Industrial Research (CSIR), India (BSC0121 and BSC0103). N.A. acknowledges the Indian Council of Medical Research for providing a research fellowship. The CSIR-Central Drug Research Institute communication number for this manuscript is 9337. The authors declare that they have no conflicts of interest.
Footnotes
Communicating editor: J. A. Nickoloff
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.193896/-/DC1.
Literature Cited
- Al-Khodairy F., Fotou F., Sheldrick K., Griffiths D., Lehmann A., et al. , 1994. Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol. Biol. Cell 5: 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler J., Wu J. Q., Longtine M. S., Shah N. G., McKenzie A., 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943–951. [DOI] [PubMed] [Google Scholar]
- Blanchard F., Rusiniak M. E., Sharma K., Sun X., Todorov I., et al. , 2002. Targeted destruction of DNA replication protein Cdc6 by cell death pathways in mammals and yeast. Mol. Biol. Cell 13: 1536–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr A. M., 2002. DNA structure dependent checkpoints as regulators of DNA repair. DNA Repair (Amst.) 1: 983–994. [DOI] [PubMed] [Google Scholar]
- Craig E. A., 2003. Eukaryotic chaperonins: lubricating the folding of WD-repeat proteins. Curr. Biol. 13(23): R904–R905. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I., Rossi R., Milzani A., Di Simplicio P., Colombo R., 2001. The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic. Biol. Med. 31: 1624–1632. [DOI] [PubMed] [Google Scholar]
- Fang J., Beattie D. S., 2003. External alternative NADH dehydrogenase of Saccharomyces cerevisiae: a potential source of superoxide. Free Radic. Biol. Med. 34: 478–488. [DOI] [PubMed] [Google Scholar]
- Farrugia G., Balzan R., 2012. Oxidative stress and programmed cell death in yeast. Front. Oncol. 2: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury C., Mignotte B., Vayssiere J. L., 2002. Mitochondrial reactive oxygen species in cell death signalling. Biochimie 84: 131–141. [DOI] [PubMed] [Google Scholar]
- Fong H., Hurley J., Hopkins R., Miake-Lye R., Johnson M., et al. , 1986. Repetitive segmental structure of the transducin b subunit: homology with the CDC4 gene identification of related mRNAs. Proc. Natl. Acad. Sci. USA 83: 2162–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyori B. M., Venkatachalam G., Thiagarajan P. S., Hsu D., Clement M., 2014. OpenComet: an automated tool for comet assay image analysis. Redox Biol. 2: 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Cross C. E., 1994. Oxygen-derived species: their relation to human disease and environmental stress. Environ. Health Perspect. 102(Suppl. 10): 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Hatanaka M., Nagao K., Nakaseko Y., Kanoh J., et al. , 2007. Rapamycin sensitivity of the Schizosaccharomyces pombe tor2 mutant and organization of two highly phosphorylated TOR complexes by specific and common subunits. Genes Cells 12: 1357–1370. [DOI] [PubMed] [Google Scholar]
- Haynes V., Elfering S., Traaseth N., Giulivi C., 2004. Mitochondrial nitric-oxide synthase: enzyme expression, characterization, and regulation. J. Bioenerg. Biomembr. 36(4): 341–346. [DOI] [PubMed] [Google Scholar]
- Kajiwara K., Muneoka T., Watanabe Y., Karashima T., Kitagaki H., et al. , 2012. Perturbation of sphingolipid metabolism induces endoplasmic reticulum stress -mediated mitochondrial apoptosis in budding yeast. Mol. Microbiol. 86: 1246–1261. [DOI] [PubMed] [Google Scholar]
- Kemp J., Balasubramanian M., Gould K., 1997. A wat1 mutant of fission yeast is defective in cell morphology. Mol. Gen. Genet. 254: 127–138. [DOI] [PubMed] [Google Scholar]
- Khan S., Ahmed S., 2015. Role of swi7H4 mutant allele of DNA polymerase α in the DNA damage checkpoint response. PLoS One 10: e0124063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F., Frohlich E., Ligr M., Grey M., Sigrist S. J., et al. , 1999. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145: 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti M. A., Weinberger M., Murakami Y., Burhans W. C., Huberman J. A., 2005. Production of reactive oxygen species in response to replication stress and inappropriate mitosis in fission yeast. J. Cell Sci. 119: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan C., Russell P., 2004. The DNA damage response: sensing and signaling. Curr. Opin. Cell Biol. 16: 629–633. [DOI] [PubMed] [Google Scholar]
- Milgrom E., Diab H., Middleton F., Kane P. M., 2007. Loss of vacuolar proton -translocating ATPase activity in yeast results in chronic oxidative stress. J. Biol. Chem. 282: 7125–7136. [DOI] [PubMed] [Google Scholar]
- Miloshev G., Mihaylov I., Anachkova B., 2002. Application of the single cell gel electrophoresis on yeast cells. Mutat. Res. 513: 69–74. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P., 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194: 793–823. [DOI] [PubMed] [Google Scholar]
- Mulet J. M., Martin D. E., Loewith R., Hall M. N., 2006. Mutual antagonism of target of rapamycin and calcineurin signaling. J. Biol. Chem. 281: 33000–33007. [DOI] [PubMed] [Google Scholar]
- Neer E., Schmidt C., Nambudripad R., Smith T., 1994. The ancient regulatory-protein family of WD-repeat proteins. Nature 371: 297–300. [DOI] [PubMed] [Google Scholar]
- Niles B. J., Mogri H., Hill A., Vlahakis A., Powers T., 2012. Plasma membrane recruitment and activation of the AGC kinase Ypk1 is mediated by target of rapamycin complex 2 (TORC2) and its effector proteins Slm1 and Slm2. Proc. Natl. Acad. Sci. USA 109: 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg K., Michelson R., Putnam C., Weinert T., 2002. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 36: 617–656. [DOI] [PubMed] [Google Scholar]
- Ochotorena I., Hirata D., Kominami K., Potashkin J., Sahin F., et al. , 2001. Conserved Wat1/Pop3 WD-repeat protein of fission yeast secures genome stability through microtubule integrity and may be involved in mRNA maturation. J. Cell Sci. 114: 2911–2920. [DOI] [PubMed] [Google Scholar]
- Olive P., Banath J. P., Fjell C. D., 1994. DNA strand breakage and DNA structure influence staining with propidium iodide using the alkaline comet assay. Cytometry 16: 305–312. [DOI] [PubMed] [Google Scholar]
- Osman F., Adriance M., McCready S., 2000. The genetic control of spontaneous and UV induced mitotic intrachromosomal recombination in fission yeast S. pombe. Curr. Genet. 38: 113–125. [DOI] [PubMed] [Google Scholar]
- Qi H., Li T-K., Kuo D., Nur-E-Kamal A., Liu L. F., 2003. Inactivation of Cdc13p triggers MEC1 dependent apoptotic signal in yeast. J. Biol. Chem. 278: 15136–15141. [DOI] [PubMed] [Google Scholar]
- Rhind N., Furnari B., Russell P., 1997. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 11: 504–511. [DOI] [PubMed] [Google Scholar]
- Roberg K., Bickel S., Rowley N., Kaiser C., 1997. Control of amino acid permease sorting in the late secretory pathway of Saccharomyces cerevisiae by SEC13, LST4, LST7 and LST8. Genetics 147: 1569–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani R. A., Holzen T. M., 2007. Cell cycle regulation of DNA replication. Annu. Rev. Genet. 41: 237–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh Y., 2003. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3: 155–168. [DOI] [PubMed] [Google Scholar]
- Shimada K., Filipuzzi I., Stahl M., Helliwell S. B., Studer C., et al. , 2013. TORC2 signaling pathway guarantees genome stability in the face of DNA strand breaks. Mol. Cell 51: 829–839. [DOI] [PubMed] [Google Scholar]
- Verma S. K., Ranjan R., Kumar V., Siddiqi M. I., Ahmed S., 2014. Wat1/pop3, a conserved WD repeat containing protein acts synergistically with checkpoint kinase Chk1 to maintain genome ploidy in fission yeast S. pombe. PLoS One 9(2): e89587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth N. C., Bernards R., 1996. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science 271: 353–356. [DOI] [PubMed] [Google Scholar]
- Walworth N., Davey S., Beach D., 1993. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature 363: 368–371. [DOI] [PubMed] [Google Scholar]
- Wan S., Capasso H., Walworth N., 1999. The topoisomerase I poison camptothecin generates a Chk1-dependent DNA damage checkpoint signal in fission yeast. Yeast 15: 821–828. [DOI] [PubMed] [Google Scholar]
- Weinberger M., Ramachandran I., Feng L., Sharma K., Sun X., et al. , 2005. Apoptosis in budding yeast caused by defects in initiation of DNA replication. J. Cell Sci. 15: 3543–3553. [DOI] [PubMed] [Google Scholar]
- Willis J., Patel Y., Lentz B. L., Yan S., 2013. APE2 is required for ATR-Chk1 checkpoint activation in response to oxidative stress. Proc. Natl. Acad. Sci. USA 110: 10592–10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Rudge D. G., Koos J. D., Vaidialingam B., Yang H., et al. , 2013. mTOR kinase structure, mechanism and regulation. Nature 497: 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.