Abstract
Of all pathogenic mitochondrial DNA (mtDNA) mutations in humans, ∼25% is de novo, although the occurrence in oocytes has never been directly assessed. We used next-generation sequencing to detect point mutations directly in the mtDNA of 3–15 individual mature oocytes and three somatic tissues from eight zebrafish females. Various statistical and biological filters allowed reliable detection of de novo variants with heteroplasmy ≥1.5%. In total, we detected 38 de novo base substitutions, but no insertions or deletions. These 38 de novo mutations were present in 19 of 103 mature oocytes, indicating that ∼20% of the mature oocytes carry at least one de novo mutation with heteroplasmy ≥1.5%. This frequency of de novo mutations is close to that deducted from the reported error rate of polymerase gamma, the mitochondrial replication enzyme, implying that mtDNA replication errors made during oogenesis are a likely explanation. Substantial variation in the mutation prevalence among mature oocytes can be explained by the highly variable mtDNA copy number, since we previously reported that ∼20% of the primordial germ cells have a mtDNA copy number of ≤73 and would lead to detectable mutation loads. In conclusion, replication errors made during oogenesis are an important source of de novo mtDNA base substitutions and their location and heteroplasmy level determine their significance.
Keywords: mitochondrial DNA, de novo mutations, next-generation sequencing, zebrafish, oogenesis
Comparative sequence analysis of the mtDNA) has revealed a high degree of variability, much higher than its nuclear counterpart (Lynch et al. 2006). This is generally explained by limited recombination and recombination-mediated mtDNA repair events to counteract errors made during mtDNA replication (Barr et al. 2005), and the close proximity of the unprotected mtDNA to the oxidative phosphorylation (OXPHOS) machinery, which produces (potentially) mutagenic reactive oxygen species (ROS) (Brand 2010). As a result, mtDNA mutations are an important cause of a group of devastating inherited diseases (Taylor and Turnbull 2005). To date, over 150 pathogenic mtDNA mutations have been identified, as well as many more polymorphisms of unknown significance (Hellebrekers et al. 2012). As human and animal cells have a high mtDNA copy number, wild-type and variant mtDNA genotypes can coexist, a state referred to as heteroplasmy. The high mtDNA copy number compensates low-level pathogenic mtDNA mutations and avoids disease manifestation. A pathogenic mtDNA mutation will only manifest if its heteroplasmy value exceeds a certain threshold (Hellebrekers et al. 2012). The mtDNA inherits maternally, and a female carrying an mtDNA mutation can transmit this mutation to her offspring through the mtDNA of her oocytes.
Maternal inheritance of a preexisting mtDNA mutation does not explain all patients suffering from mtDNA mutations. In 25% of these patients, the disease-causing mutation cannot be detected in the maternal mtDNA (Sallevelt et al. 2016). Although we cannot exclude the possibility that some of these mutations were present at undetectably low heteroplasmy levels in the maternal mtDNA, this suggests that most of these mutations have occurred de novo during germline development. The inheritance of the mtDNA occurs through a segregational bottleneck; only a limited number of the mtDNA molecules, the so-called bottleneck size, from the oocyte are transmitted to the (primordial) germline cells (PGCs) of the next generation (Cree et al. 2008). We hypothesize that, in cases of constant mutation rate, a low mtDNA copy number at the bottom of the bottleneck could lead to de novo mutations reaching detectable heteroplasmy levels that, dependent on the nature of the mutations, can be of functional and/or pathogenic significance. Experimental evidence for this hypothesis has long been difficult to obtain, because of the low heteroplasmy levels at which these mutations occur, which were generally below the detection level of conventional sequencing techniques. Over the past decade, next-generation sequencing (NGS) technologies have been developed, allowing in-depth, direct determination of the mutations and heteroplasmy levels in the mtDNA of individual oocytes.
An estimation of the prevalence of de novo mtDNA mutation requires a significant number of oocytes to be sequenced. For both biological and ethical reasons, this is difficult to achieve in humans and most animals. In contrast, oocyte collection from zebrafish is relatively easy and efficient; therefore, we used zebrafish mature oocytes to assess the de novo mtDNA mutation risk. We characterized de novo mutations, their location, and their heteroplasmy levels in 103 oocytes and three somatic (maternal) tissues from eight different female zebrafish using NGS with a minimal coverage of 1700. Furthermore, for all oocytes with one or more de novo mutation(s), we estimated the mtDNA copy number at which these mutations arose, based on their heteroplasmy levels. Given the high mtDNA sequence similarity between humans and zebrafish (Broughton et al. 2001) and the conservation of the mtDNA bottleneck within the animal kingdom (Howell et al. 1992; Cree et al. 2008; Wolff et al. 2011; Lee et al. 2012), our findings have insinuations for the occurrence of de novo mtDNA disease in humans.
Materials and Methods
Zebrafish maintenance and sample collection
Wild-type female zebrafish from the AB strain were used. Raising and housing was conducted according to standard procedures at 28° (Kimmel et al. 1995) in the zebrafish facility of Liège University, where local ethical approval by the committee of Animal Research was obtained. Mature unfertilized oocytes were collected by squeezing the abdomen of anesthetized females. The oocytes used were normal in morphological appearance. After oocyte collection, the female fish were killed in ice-cold water, after which biopsies of brain, liver, and muscle were obtained. An overview of the 127 samples is given in Table 1.
Table 1. Overview of samples collected.
| Zebrafish # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Biopsies | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Number of oocytes | 3 | 13 | 15 | 14 | 15 | 14 | 15 | 14 |
Isolation procedure of mtDNA
Oocytes were collected in sterile tubes and lysed for 4 hr at 50° in 500 µl DNA lysis buffer containing 75 mM NaCl, 50 mM EDTA, 20 mM HEPES, 0.4% SDS, and 200 µg proteinase K [Sigma (Sigma Chemical), St. Louis, MO]. Subsequently, isopropanol was added and samples were precipitated overnight at –20°. After thorough centrifugation, the DNA pellet was washed with 70% ethanol and dissolved in TE buffer. The biopsies of brain, liver, and muscle from the adult female fish were collected in sterile tubes containing Nuclei Lysis solution from the Wizard Genomic Purification Kit (Promega, Madison, WI). Subsequently, mtDNA was extracted according to the manufacturer’s instructions and dissolved in FG3-buffer.
mtDNA amplification and sequencing
The mtDNA (reference NCBI: NC002333.2) was amplified in three ∼5.6-kb amplicons (A–C). Fragment A (forward: 5′-CACACCCCTGACTCCCAAAG-3′ and reverse: 5′-GGTCGTTTGTACCCGTCAGT-3′) amplified a target spanning nt 16,594 (gene: trna-pro) to 5952 (nd2); fragment B (forward: 5′-AAATTAACACCCTAACAACGACCTG-3′ and reverse: 5′-GGGGATCAGTACTTTTAGCATTGTAGT-3′) an amplicon from nt 5669 (nd2) to 11,319 (nd4); and fragment C amplified the mtDNA from nt 11,170 (nd4l) to 295 (D-loop). Primers (designed with Primer3) were specific for the mtDNA to avoid the amplification of nuclear-encoded mitochondrial pseudogenes. PCR amplification was performed using Phusion Hot Start II DNA polymerase in GC-buffer (ThermoScientific, Waltham, MA): 30 sec at 98°, followed by 40 cycles of 10 sec at 98° (denaturation), 20 sec at 58° (annealing), and 8 min at 72° (extension), with a final step for 10 min at 72°. The PCR product was checked using electrophoresis on a 1% agarose gel containing ethidium bromide, allowing also the detection of large deletions. Amplicons were purified using the Agencourt AMPure XP system (Beckman, Fullerton, CA), according to the manufacturer’s protocol. Subsequently, the three purified amplicons were (equimolar) mixed and processed using the customized Nextera XT protocol (McElhoe et al. 2014). The library of a random subset of the samples was analyzed using a Bioanalyzer 2100 High Sensitivity DNA chip (Agilent Technologies, Santa Clara, CA) to confirm quantity and size of the library. Libraries were indexed and 18 libraries were pooled per lane and analyzed on the HiSequation 2000 system (Illumina, San Diego, CA), using a read length of 1000 bp. PhiX (1%) was spiked in every lane as an internal control.
Preprocessing of NGS data
As the mtDNA is a multicopy genome (resulting in many biological duplicates), duplicate reads were included in the analysis. Demultiplexing of the data was performed using Illumina CASAVA software (v.1.8.2) and reads were aligned against the mitochondrial reference sequence for the zebrafish (NCBI: NC002333.2) using Burrows–Wheeler Alligner (BWA) software (v.0.5.9) (Li and Durbin 2010). For variant calling, we used Phyton 2.6.6., Phyton Package pysam 0.7.8, and SAMTools 0.1.19. In-house-built Perl tools were used to process the variants. As the prevalence of any of the four nts per position was counted to call a variant, the heteroplasmy value was calculated as the ratio of one of the nt over the coverage, which was defined as the total count of any nt at a certain position.
Identification of heteroplasmic de novo point mutations
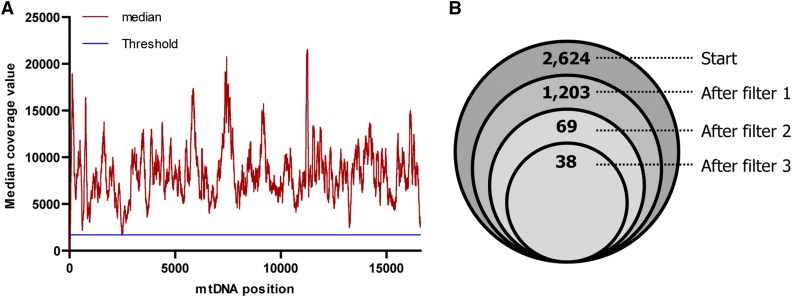
A statistical algorithm was developed to distinguish variant calls from the noise signal, as well as to determine whether point mutations, either a single base substitution or a small insertion or deletion (indel), reported in an oocyte was absent in the corresponding female fish, and thus arose de novo. A call from a sample is included in the analysis if its coverage is above the threshold, which is determined by calculating the median coverage for every position of the mtDNA genome, based on the coverage data of all 127 samples (Figure 1). Assuming sequencing quality is independent of the nt position, the median value is the most robust estimate of the coverage across the entire mtDNA genome. This implies that the lowest median coverage value is a robust estimate of the minimal reliable coverage and of the maximum background (noise) signal. As the lowest median coverage value was ∼1700 (Figure 1A), this was chosen as a cut-off value for a position to be included in the analysis, preventing variants with lower coverage from influencing the statistical calculations we applied to exclude false positives.
Figure 1.
Median reads per position and filtering steps used to determine de novo mutations. (A) Overview of the median reads per position of the mtDNA genome (red line) across all samples. The blue line indicates the threshold value for the coverage value to include samples. (B) Results of filtering steps performed to determine de novo mutations. All reads with coverage above 1700 × (1,592,714 nt) were statistically analyzed, resulting in 2624 variants with a P-value ≤ 0.01. Subsequent filter steps performed: (1) prevalence tissues adult female zebrafish <1.0%, (2) heteroplasmy difference between tissues from adult zebrafish and her oocytes (O-M) ≥1.5%, and (3) mutations not detected in the two nt adjacent (upstream or downstream) of another variant. After filtering, 38 de novo mutations remained. mtDNA, mitochondrial DNA.
For base substitutions, we discriminated false positives in the higher coverage group from true variants by comparing the percentage heteroplasmy of the variant nt of a particular sample with the average percentage heteroplasmy of all other samples (female tissues and oocytes, but without littermate oocytes and tissues from the mother). To this end, for each substitution, a probability distribution of the heteroplasmy values was generated (using all samples) and transformed to a Gaussian distribution using “a rank-transformation.” We assumed this distribution to be a representation of the noise signal for this variant, which is inherent to the NGS procedure (Guo et al. 2013). Hence, we calculated a z-score and P-value (one-tailed) and considered substitutions with a P-value ≤ 0.01 as true variants, being statistically different from the noise signal. The check for de novo substitutions was only performed for variants for which the coverage of the oocyte and of ≥2 tissues of its mother was ≥1700. Finally, a substitution was assumed de novo if the P-value of this variant in the oocyte was ≤0.01, while in the tissues from the corresponding female the P-value was >0.01 (= absent or in noise signal) and if the heteroplasmy percentage was ≥1.0%.
As the sensitivity to identify small indels is at least twofold lower as for single nt substitutions (Krawitz et al. 2010; Neuman et al. 2013; Seneca et al. 2015), we only considered de novo indels with a coverage ≥3400 ×. All suspected de novo indels were inspected manually, as the software tools used are known be less reliable than for the substitutions, using the Integrative Genomics Viewer (IGV) (Thorvaldsdottir et al. 2013).
Estimation of mtDNA copy number and mutation rate
From the average heteroplasmy value (het-%) of all de novo variants within one mature oocyte, we estimated the mtDNA copy number at the time the mutation occurred. Under the assumption that heteroplasmy levels remain stable during oogenesis, as observed in mice (Jenuth et al. 1996) and during stem cell culturing (Yamada et al. 2016), and that only one copy is mutated, the mtDNA copy number (z) at the time the mutation occurred can be calculated using: z = 100/het-%. Subsequently, we aimed to estimate the mutation rate. To do this, we first calculated for every oocyte the total number of detectable nt (y) that were present at the time the mutation arose. Therefore, we multiplied the estimated mtDNA copy number (z) with the number of nt that had coverage >1700: y = z * [#nt > 1700 coverage]. The mutation rate (x) was then calculated by dividing the number of de novo mutations in an oocyte by the total number of analyzable nt: x = [#mutations detected]/y.
Data availability
The authors state that all detected de novo mutations resulting from their statistical analysis are presented within the article.
Results
We studied the occurrence of detectable de novo mutations in 103 mature oocytes derived from eight different female zebrafish. No large deletions were observed, but we did detect significant numbers of point mutations. Of the 1,592,714 different nt positions that had a sequence coverage >1700, we identified 2624 different base substitutions, which were statistically different from the sequencing noise signal and absent in all three maternal tissues, and therefore considered as potential de novo mutations. To increase the reliability of our de novo base substitution detection, we applied three additional biological filter steps (Figure 1). (1) All oocyte variants for which the heteroplasmy level in one of the corresponding maternal tissues was ≥1.0% were considered to be preexisting and were therefore excluded. (2) The heteroplasmy level of the variants was corrected for the noise signal by subtracting the maternal (M) heteroplasmy level (= the average of the heteroplasmy level in the three maternal tissues) from the oocyte (O) heteroplasmy level (O-M), and variants with small O-M values (<1.5%) were excluded (205 variants had an O-M value of 1.0–1.5% and 929 variants of <1.0%). This filter restricted our analysis to de novo point mutations with a mutation load ≥1.5%. (3) A de novo variant was rejected if, within the same oocyte, another variant was detected in the two nt adjacent (upstream or downstream) to this variant, as variants located in close proximity to each other are most likely the result of alignment artifacts (Li and Durbin 2009). By applying these filters, 38 single base substitutions were considered to be de novo (Table 2), of which 36 were unique, as two mutations (m.7247T > G and m.10578C > A) were found in two oocytes isolated from the same zebrafish.
Table 2. Heteroplasmic mtDNA base substitution mutations in all oocytes.
| Oocyte ID | Corr. Het-% (O-M) | Gene Location | Nucleotide Change | Amino Acid Change |
|---|---|---|---|---|
| 1.1 | 2.5 | ND1 | m.4164C > A | Ser > Stop |
| 2.3 | 1.8 | COI | m.6489T > C | Phe > Ser |
| 2.6 | 5.4 | D-loop | m.250C > T | — |
| 2.6 | 5.9 | COII | m.8700G > A | Val > Lys |
| 2.10 | 1.6 | COI | m.6510T > A | Val > Glu |
| 3.2 | 3.3 | D-loop | m.283G > T | — |
| 3.2 | 1.9 | ND5 | m14296T > A | Ile > Asn |
| 3.9 | 4.1 | tRNA-Trp | m.6089G > A | — |
| 3.15 | 1.9 | ND4L/ ND4 | m.11303T > A | Stop > Lys |
| Leu > Gln | ||||
| 4.4 | 3.1 | D-loop | m.1818A > T | — |
| 4.10 | 1.9 | D-loop | m.532G > A | — |
| 4.10 | 2.1 | ND4 | m.11708G > A | Trp > Stop |
| 5.2 | 2.7 | ND1 | m.4077G > A | Trp > Stop |
| 5.2 | 2.2 | COI | m.7112C > T | Leu > Phe |
| 5.2 | 1.6 | COI | m.7247T > G | Trp > Gly |
| 5.2 | 2.2 | COI | m.7574G > T | Gly > Trp |
| 5.2 | 2.2 | COI | m.7580G > A | Val > Met |
| 5.3 | 1.8 | ND6 | m.14894T > A | Leu > Phe |
| 5.10 | 1.6 | 12s rRNA | m.1220A > G | — |
| 5.10 | 2.4 | COI | m.7112C > T | Leu > Phe |
| 5.10 | 2.1 | COI | m.7247T > G | Trp > Gly |
| 5.10 | 1.9 | COI | m.7253A > G | Met > Val |
| 5.10 | 1.8 | COIII | m.9909C > A | Arg > Stop |
| 5.10 | 1.6 | ND4 | m.11500G > C | Val > Leu |
| 5.10 | 1.5 | tRNA-Leu | m.12838T > G | — |
| 5.10 | 1.6 | ND5 | m.13472C > T | Tyr > Tyr |
| 5.13 | 8.2 | ND6 | m.14761G > A | Leu > Leu |
| 7.3 | 1.5 | 16s rRNA | m.2632A > G | — |
| 7.8 | 1.7 | 16s rRNA | m.2537A > G | — |
| 7.9 | 2.5 | 12s rRNA | m.1550G > C | — |
| 7.9 | 1.8 | ND4 | m.12263T > G | Leu > Arg |
| 7.9 | 1.6 | ND5 | m.13205T > G | Phe > Leu |
| 7.9 | 1.6 | CytB | m.16232A > C | Thr > Pro |
| 7.9 | 1.5 | CytB | m.16324A > T | Gly > Gly |
| 8.5 | 2.8 | ND5 | m.14400C > T | Leu > Leu |
| 8.6 | 9.0 | tRNA-Gly | m.10578C > A | — |
| 8.6 | 1.5 | ND4 | m.12464G > T | Trp > Leu |
| 8.10 | 4.6 | tRNA-Gly | m.10578C > A | — |
ID, identifier; Corr. Het-%, oocyte heteroplasmy value (percentage) corrected from noise signal by subtracting the average heteroplasmy of the corresponding female tissues from the heteroplasmy value detected in the oocyte; amino acid change, change in amino acid due to change in the codon sequence; tRNA, transfer RNA; rRNA, ribosomal RNA.
We also checked for the presence of small de novo indels. We did identify three indels with a coverage > 3400: one in oocyte 2.1 (m.7352insC, O-M het: 1.63%, coverage 5868), one in oocyte 5.10 (m.12323del, O-M het: 1.58%, coverage 3848), and one in oocyte 7.15 (m.11305del, O-M het: 2.41%, coverage 4689). Using IGV, we found that the insertion was only detected in ambiguous reads, while both deletions were only detected in duplicate reads. Therefore, these indels were most likely alignment artifacts and excluded from further analysis.
The 38 de novo mutations were detected in only 19 different oocytes (Table 2), indicating that the majority (82%) did not have a detectable de novo mutation. In three oocytes (5.2, 5.10, and 7.9), more than three de novo mutations were found. No de novo mutations were detected in any oocyte from zebrafish 6. The heteroplasmy level of all the de novo point mutations ranged from 1.5 to 9.0% with an average of 2.7%.
For every mature oocyte with at least one de novo mutation, we estimated the mtDNA copy number present at the time the mutation occurred. This number ranged from 18 to 67 (Table 3). Based on this, we calculated the mutation rate for these oocytes (Table 3). For most oocytes, this mutation rate was in the range of 10−6 mutations per nt, while oocytes 4.4, 5.10, and 7.3 had a higher mutation rate. On average, the mutation rate in these 19 oocytes was 4.3 × 10−6 mutations per nt. Strikingly, for oocyte 8.6, the heteroplasmy values of the two reported de novo mutations greatly differed from each other, resulting in two different estimations of the mutation rate.
Table 3. mtDNA copy number and de novo mutation rate ≥1.5% at a given nt for all oocytes in which at least one de novo mutation was detected.
| Oocyte ID | De Novo Mutations | Average Het-% of de Novo Mutation | Copy Number from Het-% | Positions Analyzed (Coverage >1700) | Analyzable Nucleotides When Mutation Occurred | Mutation Rate (>1.5%) per Nucleotide |
|---|---|---|---|---|---|---|
| 1.1 | 1 | 2.5 | 40 | 5,643 | 225,720 | 4.4 × 10−6 |
| 2.3 | 1 | 1.8 | 56 | 16,532 | 925,792 | 1.1 × 10−6 |
| 2.6 | 2 | 5.7 | 18 | 16,580 | 298,440 | 6.7 × 10−6 |
| 2.10 | 1 | 1.6 | 63 | 14,543 | 916,209 | 1.1 × 10−6 |
| 3.2 | 2 | 1.9 | 53 | 16,532 | 876,196 | 2.3 × 10−6 |
| 3.9 | 1 | 4.1 | 24 | 16,555 | 397,320 | 2.5 × 10−6 |
| 3.15 | 1 | 1.9 | 53 | 14,902 | 789,806 | 1.3 × 10−6 |
| 4.4 | 1 | 3.1 | 32 | 2,816 | 90,112 | 1.1 × 10−5 |
| 4.10 | 2 | 2.0 | 50 | 16,583 | 829,150 | 2.4 × 10−6 |
| 5.2 | 5 | 2.2 | 45 | 6,991 | 314,595 | 1.6 × 10−6 |
| 5.3 | 1 | 1.8 | 56 | 12,505 | 700,280 | 1.4 × 10−6 |
| 5.10 | 8 | 1.7 | 59 | 10,740 | 633,660 | 1.3 × 10−5 |
| 5.13 | 1 | 8.2 | 12 | 16,570 | 198,840 | 5.0 × 10−6 |
| 7.3 | 1 | 1.5 | 67 | 16,528 | 1,107,376 | 9.0 × 10−7 |
| 7.8 | 1 | 1.7 | 59 | 16,569 | 977,571 | 1.0 × 10−6 |
| 7.9 | 5 | 1.8 | 56 | 16,426 | 919,856 | 5.4 × 10−6 |
| 8.5 | 1 | 2.8 | 36 | 16,569 | 596,484 | 1.7 × 10−6 |
| 8.6a | 1 | 9.0 | 11 | 16,239 | 178,629 | 5.6 × 10−6 |
| 8.6a | 1 | 1.5 | 67 | 16,239 | 1,088,013 | 9.2 × 10−7 |
| 8.10 | 1 | 4.6 | 22 | 16,533 | 363,726 | 2.7 × 10−6 |
| Average | 2 | 3.2 | 56 | — | — | 4.3 × 10−6 |
ID, identifier; de novo mutations, number of de novo mutations detected in the oocyte; average Het-% of de novo mutation, the average heteroplasmy value for all de novo mutations found in the oocyte; copy number from Het-%, the copy number the oocytes had when the mutation occurred.
The heteroplasmy values of the variants in oocyte 8.6 differed markedly from each other, therefore the calculations were performed for both heteroplasmy values separately.
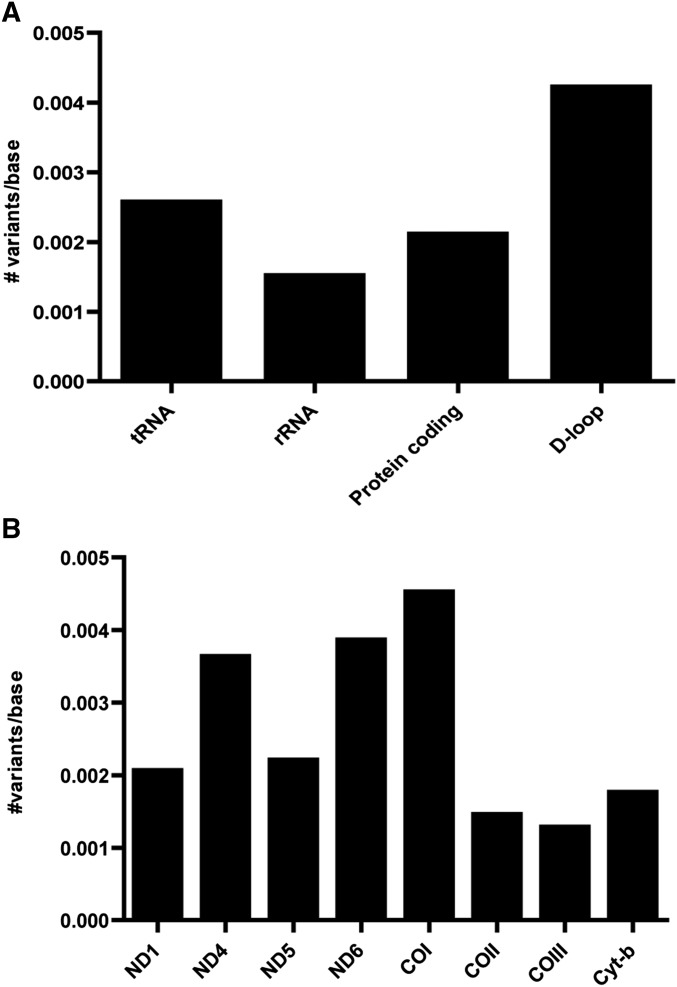
The number of unique de novo mutations per base was assessed for every gene (Figure 2). In case the de novo mutations were classified per gene function (tRNA genes, rRNA genes, protein coding, or D-loop separately), the prevalence of de novo mutations appeared to be slightly higher in the D-loop (four variants per 1000 bases). 26 variants were in protein-coding genes (ND1, ND4, ND5, ND6, COI, COIII, and cyt-b), with little difference in the prevalence among the different protein-coding genes. Four of these mutations were synonymous and 22 nonsynonymous, including four mutations leading to a premature stop codon (Table 2).
Figure 2.
Prevalence of de novo variants. Prevalence (A) per gene type (protein coding genes, tRNA, rRNA genes, and D-loop) and (B) per protein-coding gene. The observed number of variants in all oocytes is expressed as the number of variants per nt. If a de novo mutation occurred multiple times, it is only counted once in the construction of these distributions. rRNA, ribosomal RNA; tRNA, transfer RNA.
Discussion
Robustness of identification of de novo mutations with heteroplasmy levels ≥1.5%
We used the Nextera XT protocol to prepare libraries for sequencing on the HiSeq2000 platform, a system that has been successfully applied before for sequencing of the mitochondrial genome (McElhoe et al. 2014; Rebolledo-Jaramillo et al. 2014). The identification of specific mutations with low heteroplasmy is limited by the noise level of the sequencing procedure (Guo et al. 2013), which is around 1% (Guo et al. 2013; Rebolledo-Jaramillo et al. 2014; Ma et al. 2015). As we cannot be sure that noise levels will be equal among different runs and detection thresholds differ accordingly, we estimated the noise signal of our sequence run using a nonparametric, data-driven approach. Our approach was twofold. First, we estimated the median minimal coverage (Figure 1), which is a robust estimate of the coverage of the entire mitochondrial genome, rendering a threshold coverage above which the calculated heteroplasmy value is reliable. Second, the noise level of the procedure was estimated for every position in the mtDNA based on the heteroplasmy levels reported for this position in all samples. By applying statistics, this allowed the identification of those variants for which the heteroplasmy level was significantly different from the background (or noise) signal (P-value ≤ 0.01), thereby excluding variants with a high occurrence in all samples, something which was not expected. In a previous report, power calculations were used to estimate the reliability of the reads (Rebolledo-Jaramillo et al. 2014). However, power calculations are theoretical and the use of such post hoc calculations for the interpretation of available experimental results is debatable (Hoenig and Heisey 2001). Another step in our analysis involves correction for the noise signal by subtracting the maternal heteroplasmy value from the detected heteroplasmy value. This correction is essential to determine the heteroplasmy value at which the de novo variant arose. A last step involved the exclusion of variants that most likely occurred as a result of alignment artifacts. Based on our and others’ (McElhoe et al. 2014; Rebolledo-Jaramillo et al. 2014) experience with NGS, we excluded variants if they arose in close proximity to each other. Altogether, our data-driven statistical approach allows detection of de novo variants (heteroplasmy levels ≥1.5%) with high reliability, which is corrected for potential differences in quality between sequence runs.
De novo mutations are detected in oocytes with a low mtDNA copy number
After applying statistical and biological filters, we characterized 38 de novo base substitutions with an average heteroplasmy level of 2.7% in ∼19% of the oocytes. No large or small indels were detected in our analysis. Our analysis pipeline allowed the detection of variants with a heteroplasmy value ≥1.5%, which equals detection of a single mutated mtDNA molecule in a population of 65 or less. The estimated mtDNA copy number at which a de novo mutation occurred, ranged, based on the detected heteroplasmy levels of the mutations in the oocytes, from 11 to 67 (Table 2). In our analysis, we only detected an mtDNA mutation in 18.9% of the mature oocytes. The inheritance of the mtDNA through a bottleneck leads to low mtDNA levels in PGCs at the bottom of the bottleneck (Cree et al. 2008), and a mutation originating at this point may lead to higher heteroplasmy levels. In a previous study (Otten et al. 2016), we determined the mtDNA copy number in zebrafish PGCs isolated from several embryonic stages and found, on average, 171 mtDNA molecules at the bottom of the bottleneck, but with high variation in this number (SD = 111). Based on these parameters, we constructed a Gaussian distribution with mean 171 ± 111 (SD). As 18.9% of the mature oocytes harbored a de novo mutation, this distribution allowed us to estimate that the lower 18.9% (left tail of the distribution, z-score –0.88) of the PGCs possess ≤72.8 mtDNA molecules. An mtDNA copy number of 72.8 corresponds to a heteroplasmy level at mutation manifestation of 1.4%, which is close to our detection limit of 1.5%. This means that our pipeline allows detection of de novo mutations in oocytes that were generated from germ cells with the lowest mtDNA content, which includes those germ cells most prone for acquiring a de novo mutation reaching a detectable heteroplasmy of ≥1.5%, after mtDNA replication. Together, this implies that ∼20% of the oocytes had a bottleneck size at which de novo mutations could reach detectable heteroplasmy levels (≥1.5%). This also indicates that the oocytes (the other ∼80%) with a higher mtDNA copy number will equally carry de novo point mutations with a heteroplasmy level ≤1.5%, but the sensitivity at this level was too low for accurate mtDNA heteroplasmy analysis, which was in line with a recent study (Hammond et al. 2016). As the current noise level of sequencing is around 1% (Guo et al. 2013; Rebolledo-Jaramillo et al. 2014; Ma et al. 2015), further improvements of sequencing technologies are needed to detect mtDNA point mutations in all oocytes, including those with a higher mtDNA copy number.
During oogenesis in zebrafish, the mtDNA of the PGC is replicated extensively. mtDNA replication is expected to occur almost exclusively by POLG (Copeland and Longley 2003; Kaguni 2004), which is a two-subunit holoenzyme with high fidelity in nt selection and incorporation, alongside 3′–5′ exonuclease proofreading functionality (Kaguni 2004). For all mature oocytes in which we detected a de novo mutation, the calculated average mutation rate was 4.3 × 10−6 per nt. This is in the same order of the reported POLG error frequencies, which range from 2 × 10−6 to 10 × 10−6 per nt in different animals (Kunkel and Mosbaugh 1989; Longley et al. 2001). Although POLG has a high accuracy, this suggests that errors made by POLG during the extensive replication during oogenesis are the main cause of de novo mutations detected in mature oocytes. The absence of de novo indels is in line with a study in human germline mtDNA (Rebolledo-Jaramillo et al. 2014) and suggests that the error rate of POLG for insertions and deletions is lower than for substitutions, which has been reported previously (Longley et al. 2001).
Although replication errors are made continuously in all oocytes, only replication errors made when mtDNA copy numbers are low (e.g., ≤65) lead to detectable (heteroplasmy levels of ≥1.5%) de novo mutations in mature oocytes, which was likely the case in 19 of the oocytes. Four of these oocytes had a higher mutation rate (range of 10−5). Although this can be a chance event, this could also be a reflection of individual differences in error rates of the mtDNA replicative machinery, or due to another mutagenic source being active in these oocytes. During OXPHOS, which is highly active during oogenesis (Van Blerkom et al. 1995; Dumollard et al. 2007), mutagenic ROS are being produced and this could be a factor contributing to differences in the mutation rate between oocytes. However, based on the mutation rates in most oocytes, errors made by POLG are most likely the dominant source of de novo mtDNA mutations during oogenesis.
Our calculations on the mtDNA copy number and mutation rate are only applicable if random processes prevail and every mtDNA molecule is equally amplified. Studies in mice have suggested that heteroplasmy remains stable during oogenesis (Jenuth et al. 1996). However, due to genetic drift leading to loss or fixation of mutations, especially in small sample sizes, and preferential selection, mutation loads can shift. This could explain the high mtDNA mutation load for one of the two de novo mutations detected in oocyte 8.6, although these mutations could also have manifested during separate replication cycles, as an extremely low mtDNA bottleneck size creates multiple cycles at which the mutation can manifest at heteroplasmy levels ≥1.5%. Negative and positive selection has been demonstrated for some specific mutations (Steffann et al. 2015), further corroborating the possibility that nonrandom processes also influence the heteroplasmy levels of de novo mtDNA mutations. In the case of selection, the physical and effective bottleneck sizes are different. The mtDNA molecules that actively replicate determine the effective bottleneck size, which can be lower than the physical bottleneck size when selective events result in only a subpopulation of mtDNA molecules being more actively replicated. The mtDNA copy number that we have estimated here based on the heteroplasmy levels (11–67) correspond to effective mtDNA copy numbers, and might therefore be an underestimation of the physical bottleneck size.
De novo mutations in oocytes are potentially pathogenic
The 38 de novo mutations with a frequency of >1.5% were randomly distributed over the mtDNA genome. After correction for the size of the gene, the tRNA-Leu, tRNA-Trp, and tRNA-Gly genes had a high number of mutations per nt. However, the numbers were too low to estimate this correctly (only one mutation in the tRNA-Leu and tRNA-Trp genes were observed, and two mutations in the tRNA-Gly gene) and mutations occurred in two oocytes from the same zebrafish. Furthermore, the tRNA genes as a group do not support a higher prevalence of mutations in the tRNA genes. The observed higher prevalence in the three tRNA genes is most likely due to the relatively small group of de novo mutations. On the contrary, a higher prevalence for mutations in the D-loop exists (Figure 2A). This is corroborated by the many variants observed in this mitochondrial control region (Chinnery et al. 1999). Although preferences for the D-loop might exist from an evolutionary perspective, mutations in the mtDNA can arise anywhere in the mtDNA genome. In total, eight protein-coding genes were affected with little differences in the prevalence. Twenty-two mutations were nonsynonymous, including four mutations leading to a premature stop codon. No differences in prevalence in one of the three codon positions were found. This indicates that the effect of a de novo mutation can be of any kind. The nonsynonymous mutations, especially those causing a premature stop codon, are likely pathogenic, implying that these severe mutations, which are rarely found in human patients, can also occur de novo. Most likely in humans, these pathogenic mutations are filtered out by mitophagy (Song et al. 2014) or are at high levels not compatible with embryonic survival and remain unnoticed at low levels.
Given the high sequence homology (72%; NCBI blast performed) between the mtDNA genome of zebrafish and humans and the high evolutionary conservation of the mtDNA bottleneck in animal species (Wolff et al. 2011; Guo et al. 2013; Otten and Smeets 2015; Otten et al. 2016), our results indicate that the de novo risk might be similar among zebrafish and humans. Indeed, a study in 26 human oocytes (Jacobs et al. 2007), seven oocytes (26.9%) were found to harbor de novo variants. This is close to the frequency of 18.9% we report here for zebrafish. This is further corroborated by a similar degree of variation in mtDNA copy number in human oocytes (Otten and Smeets 2015), which suggests variation in the mtDNA bottleneck size and subsequent differences in the de novo risk. In humans, it has been estimated that about 5% of the mutations in the mtDNA alter a conserved nt and are thus potentially pathogenic (Jacobs et al. 2007). As we found de novo mutations in ∼20% of the oocytes, this implies that ∼1% of oocytes will carry a pathogenic de novo mutation (with a heteroplasmy level ≥1.5%). The presence of low-level mtDNA mutations in the oocyte could, after fertilization, lead to mtDNA disease later in life due to genetic drift, which could lead to fixation of the mutation (Greaves et al. 2014; Yin et al. 2015), or in the offspring of the following generation, as inheritance through the mtDNA bottleneck can cause shifts in the heteroplasmy level between mother and child, also leading to fixation of the mutant mtDNA (Blok et al. 1997).
Despite the described similarities between zebrafish and humans, important reproductive and mtDNA differences should be taken into account. Zebrafish oocytes (Otten et al. 2016) possess a much higher absolute mtDNA content compared to human oocytes (Duran et al. 2011; Murakoshi et al. 2013) (factor 100), mostly due to different implantation patterns. In humans, implantation occurs rapidly (Wimsatt 1975), allowing a fast shift to the uterus for energy supply, while in zebrafish implantation is absent and energy must be supplied by the embryo itself. This lower mtDNA copy number in human oocytes might result in lower mtDNA numbers at the bottom of the bottleneck, and the mtDNA genome might even be at higher risk for a de novo mutation to reach detectable heteroplasmy levels. This is supported by the high mutation frequency reported for the mammalian mtDNA compared to other animals, including fish (Lynch 2006). In conclusion, our study in zebrafish has revealed that replication errors made during oogenesis are an important source of de novo mtDNA mutations, and that their location and heteroplasmy determine the eventual significance.
Acknowledgments
We thank Marie Winandy and Hélène Pendeville from the Zebrafish facility of Liège University for their assistance in collecting the zebrafish material. This work was supported by the Interreg IV program of the European Council (the Alma in Silico project to M.M. and H.J.M.S.) and the European Research Area Network for Research Programmes on Rare Diseases 2 project GENOMIT (grant R 50.02.12F to M.M.). Part of this work has been made possible with the support of the Dutch Province of Limburg (M.A., M.G., and H.J.M.S.).
Footnotes
Communicating editor: J. A. Nickoloff
Literature Cited
- Barr C. M., Neiman M., Taylor D. R., 2005. Inheritance and recombination of mitochondrial genomes in plants, fungi and animals. New Phytol. 168: 39–50. [DOI] [PubMed] [Google Scholar]
- Blok R. B., Gook D. A., Thorburn D. R., Dahl H. H., 1997. Skewed segregation of the mtDNA nt 8993 (T→G) mutation in human oocytes. Am. J. Hum. Genet. 60: 1495–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M. D., 2010. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 45: 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton R. E., Milam J. E., Roe B. A., 2001. The complete sequence of the zebrafish (Danio rerio) mitochondrial genome and evolutionary patterns in vertebrate mitochondrial DNA. Genome Res. 11: 1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery P. F., Howell N., Andrews R. M., Turnbull D. M., 1999. Mitochondrial DNA analysis: polymorphisms and pathogenicity. J. Med. Genet. 36: 505–510. [PMC free article] [PubMed] [Google Scholar]
- Copeland W. C., Longley M. J., 2003. DNA polymerase gamma in mitochondrial DNA replication and repair. ScientificWorldJournal 3: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree L. M., Samuels D. C., de Sousa Lopes S. C., Rajasimha H. K., Wonnapinij P., et al. , 2008. A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat. Genet. 40: 249–254. [DOI] [PubMed] [Google Scholar]
- Dumollard R., Duchen M., Carroll J., 2007. The role of mitochondrial function in the oocyte and embryo. Curr. Top. Dev. Biol. 77: 21–49. [DOI] [PubMed] [Google Scholar]
- Duran H. E., Simsek-Duran F., Oehninger S. C., Jones H. W., Jr, Castora F. J., 2011. The association of reproductive senescence with mitochondrial quantity, function, and DNA integrity in human oocytes at different stages of maturation. Fertil. Steril. 96: 384–388. [DOI] [PubMed] [Google Scholar]
- Greaves L. C., Nooteboom M., Elson J. L., Tuppen H. A., Taylor G. A., et al. , 2014. Clonal expansion of early to mid-life mitochondrial DNA point mutations drives mitochondrial dysfunction during human ageing. PLoS Genet. 10: e1004620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Li C. I., Sheng Q., Winther J. F., Cai Q., et al. , 2013. Very low-level heteroplasmy mtDNA variations are inherited in humans. J. Genet. Genomics 40: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond E. R., Green M. P., Shelling A. N., Berg M. C., Peek J. C., et al. , 2016. Oocyte mitochondrial deletions and heteroplasmy in a bovine model of ageing and ovarian stimulation. Mol. Hum. Reprod. 22: 261–271. [DOI] [PubMed] [Google Scholar]
- Hellebrekers D. M., Wolfe R., Hendrickx A. T., de Coo I. F., de Die C. E., et al. , 2012. PGD and heteroplasmic mitochondrial DNA point mutations: a systematic review estimating the chance of healthy offspring. Hum. Reprod. Update 18: 341–349. [DOI] [PubMed] [Google Scholar]
- Hoenig J. M., Heisey D. M., 2001. The abuse of power the pervasive fallacy of power calculations for data analysis. Am. Stat 55: 19–24. [Google Scholar]
- Howell N., Halvorson S., Kubacka I., McCullough D. A., Bindoff L. A., et al. , 1992. Mitochondrial gene segregation in mammals: is the bottleneck always narrow? Hum. Genet. 90: 117–120. [DOI] [PubMed] [Google Scholar]
- Jacobs L., Gerards M., Chinnery P., Dumoulin J., de Coo I., et al. , 2007. mtDNA point mutations are present at various levels of heteroplasmy in human oocytes. Mol. Hum. Reprod. 13: 149–154. [DOI] [PubMed] [Google Scholar]
- Jenuth J. P., Peterson A. C., Fu K., Shoubridge E. A., 1996. Random genetic drift in the female germline explains the rapid segregation of mammalian mitochondrial DNA. Nat. Genet. 14: 146–151. [DOI] [PubMed] [Google Scholar]
- Kaguni L. S., 2004. DNA polymerase gamma, the mitochondrial replicase. Annu. Rev. Biochem. 73: 293–320. [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F., 1995. Stages of embryonic development of the zebrafish. Dev. Dyn. 203: 253–310. [DOI] [PubMed] [Google Scholar]
- Krawitz P., Rodelsperger C., Jager M., Jostins L., Bauer S., et al. , 2010. Microindel detection in short-read sequence data. Bioinformatics 26: 722–729. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Mosbaugh D. W., 1989. Exonucleolytic proofreading by a mammalian DNA polymerase. Biochemistry 28: 988–995. [DOI] [PubMed] [Google Scholar]
- Lee H. S., Ma H., Juanes R. C., Tachibana M., Sparman M., et al. , 2012. Rapid mitochondrial DNA segregation in primate preimplantation embryos precedes somatic and germline bottleneck. Cell Reports 1: 506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R., 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R., 2010. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley M. J., Nguyen D., Kunkel T. A., Copeland W. C., 2001. The fidelity of human DNA polymerase gamma with and without exonucleolytic proofreading and the p55 accessory subunit. J. Biol. Chem. 276: 38555–38562. [DOI] [PubMed] [Google Scholar]
- Lynch M., 2006. The origins of eukaryotic gene structure. Mol. Biol. Evol. 23: 450–468. [DOI] [PubMed] [Google Scholar]
- Lynch M., Koskella B., Schaack S., 2006. Mutation pressure and the evolution of organelle genomic architecture. Science 311: 1727–1730. [DOI] [PubMed] [Google Scholar]
- Ma J., Purcell H., Showalter L., Aagaard K. M., 2015. Mitochondrial DNA sequence variation is largely conserved at birth with rare de novo mutations in neonates. Am. J. Obstet. Gynecol. 212: 530.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhoe J. A., Holland M. M., Makova K. D., Su M. S., Paul I. M., et al. , 2014. Development and assessment of an optimized next-generation DNA sequencing approach for the mtgenome using the Illumina MiSeq. Forensic Sci. Int. Genet. 13: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi Y., Sueoka K., Takahashi K., Sato S., Sakurai T., et al. , 2013. Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. J. Assist. Reprod. Genet. 30: 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman J. A., Isakov O., Shomron N., 2013. Analysis of insertion-deletion from deep-sequencing data: software evaluation for optimal detection. Brief. Bioinform. 14: 46–55. [DOI] [PubMed] [Google Scholar]
- Otten A. B., Smeets H. J., 2015. Evolutionary defined role of the mitochondrial DNA in fertility, disease and ageing. Hum. Reprod. Update 21: 671–689. [DOI] [PubMed] [Google Scholar]
- Otten A. B., Theunissen T. E., Derhaag J. G., Lambrichs E. H., Boesten I. B., et al. , 2016. Differences in strength and timing of the mtDNA bottleneck between zebrafish germline and non-germline cells. Cell Reports 16: 622–630. [DOI] [PubMed] [Google Scholar]
- Rebolledo-Jaramillo B., Su M. S., Stoler N., McElhoe J. A., Dickins B., et al. , 2014. Maternal age effect and severe germ-line bottleneck in the inheritance of human mitochondrial DNA. Proc. Natl. Acad. Sci. USA 111: 15474–15479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallevelt S. C., de Die-Smulders C. E., Hendrickx A. T., Hellebrekers D. M., de Coo I. F., et al. , 2016. De novo mtDNA point mutations are common and have a low recurrence risk. J. Med. Genet. DOI: 10.1136/jmedgenet-2016-103876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneca S., Vancampenhout K., Van Coster R., Smet J., Lissens W., et al. , 2015. Analysis of the whole mitochondrial genome: translation of the Ion Torrent Personal Genome machine system to the diagnostic bench? Eur. J. Hum. Genet. 23: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W. H., Ballard J. W., Yi Y. J., Sutovsky P., 2014. Regulation of mitochondrial genome inheritance by autophagy and ubiquitin-proteasome system: implications for health, fitness, and fertility. BioMed Res. Int. 2014: 981867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffann J., Monnot S., Bonnefont J. P., 2015. mtDNA mutations variously impact mtDNA maintenance throughout the human embryofetal development. Clin. Genet. 88: 416–424. [DOI] [PubMed] [Google Scholar]
- Taylor R. W., Turnbull D. M., 2005. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 6: 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsdottir H., Robinson J. T., Mesirov J. P., 2013. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14: 178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Blerkom J., Davis P. W., Lee J., 1995. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum. Reprod. 10: 415–424. [DOI] [PubMed] [Google Scholar]
- Wimsatt W. A., 1975. Some comparative aspects of implantation. Biol. Reprod. 12: 1–40. [DOI] [PubMed] [Google Scholar]
- Wolff J. N., White D. J., Woodhams M., White H. E., Gemmell N. J., 2011. The strength and timing of the mitochondrial bottleneck in salmon suggests a conserved mechanism in vertebrates. PLoS One 6: e20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Emmanuele V., Sanchez-Quintero M. J., Sun B., Lallos G., et al. , 2016. Genetic drift can compromise mitochondrial replacement by nuclear transfer in human oocytes. Cell Stem Cell 18: 749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin A. H., Peng C. F., Zhao X., Caughey B. A., Yang J. X., et al. , 2015. Noninvasive detection of fetal subchromosomal abnormalities by semiconductor sequencing of maternal plasma DNA. Proc. Natl. Acad. Sci. USA 112: 14670–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all detected de novo mutations resulting from their statistical analysis are presented within the article.