Abstract
The Saccharomyces cerevisiae transcription factor Gcn4 is expressed during amino acid starvation, and its abundance is controlled by ubiquitin-mediated proteolysis. Cdk8, a kinase component of the RNA polymerase II Mediator complex, phosphorylates Gcn4, which triggers its ubiquitination/proteolysis, and is thought to link Gcn4 degradation with transcription of target genes. In addition to phosphorylation and ubiquitination, we previously showed that Gcn4 becomes sumoylated in a DNA-binding dependent manner, while a nonsumoylatable form of Gcn4 showed increased chromatin occupancy, but only if Cdk8 was present. To further investigate how the association of Gcn4 with chromatin is regulated, here we examine determinants for Gcn4 sumoylation, and how its post-translational modifications are coordinated. Remarkably, artificially targeting Gcn4 that lacks its DNA binding domain to a heterologous DNA site restores sumoylation at its natural modification sites, indicating that DNA binding is sufficient for the modification to occur in vivo. Indeed, we find that neither transcription of target genes nor phosphorylation are required for Gcn4 sumoylation, but blocking its sumoylation alters its phosphorylation and ubiquitination patterns, placing Gcn4 sumoylation upstream of these Cdk8-mediated modifications. Strongly supporting a role for sumoylation in limiting its association with chromatin, a hyper-sumoylated form of Gcn4 shows dramatically reduced DNA occupancy and expression of target genes. Importantly, we find that Cdk8 is at least partly responsible for clearing hyper-sumoylated Gcn4 from DNA, further implicating sumoylation as a stimulus for Cdk8-mediated phosphorylation and degradation. These results support a novel function for SUMO in marking the DNA-bound form of a transcription factor, which triggers downstream processes that limit its association with chromatin, thus preventing uncontrolled expression of target genes.
Keywords: Gcn4, sumoylation, Cdk8, transcription, gene activation
THE expression of numerous genes is controlled by gene-specific transcription factors (TFs), which bind to target DNA sequences and activate transcription. TFs contain DNA binding domains that recognize DNA elements referred to as upstream activator sequences (UAS) in budding yeast, located proximal to their cognate promoters, or enhancers in higher eukaryotes, which can be situated several 100 kb away from the genes that they regulate (Hahn and Young 2011; Shlyueva et al. 2014; Vernimmen and Bickmore 2015). Once bound, TFs trigger the ordered assembly of the general transcription factors (GTFs) on target gene promoters, and the recruitment of RNA polymerase II (RNAP II), to form the transcriptional preinitiation complex (PIC). To promote PIC formation, many DNA-bound TFs make physical contact between their activation domains and specific GTF components, either directly, through coactivators, or through the Mediator complex (Thomas and Chiang 2006; Hahn and Young 2011). DNA binding is critical for TF function, as unbound activation domains lack functionality, and hybrid TFs, generated by fusing activation domains with heterologous DNA binding domains, are capable of activating transcription of targeted genes (Keaveney and Struhl 1998). Because they play a major role in gene expression, TFs are highly regulated in their subcellular location, abundance, and access to DNA, particularly by post-translational modifications (PTMs) (Filtz et al. 2014). However, little is known about how cells regulate TFs once they are bound to DNA.
The rate at which TFs occupy target DNA sequences during gene activation, the duration of their occupancy, and the rate at which they are cleared from DNA, varies not only among TFs, but also for different genes targeted by the same TF (Ni et al. 2009; Charoensawan et al. 2015). Patterns of TF occupancy can be examined by time-course chromatin immunoprecipitation (ChIP), which determines the levels of TFs bound to specific DNA sequences within a population of cells at various times during gene induction (Ni et al. 2009). However, the residence time of individual TF molecules on target DNA can also be determined, and such analyses have indicated that the TF-DNA interaction is highly dynamic, and often short-lived. For example, fluorescence microscopy photobleaching experiments demonstrated that there is a continuous rapid exchange of hormone-stimulated glucocorticoid receptor molecules on target DNA sites in living cells, with an average residence time of 10 sec per molecule (McNally et al. 2000; De Angelis et al. 2015). Competition ChIP experiments in yeast showed that Rap1, a multiple-target TF, has long residence times on highly active genes, but displays rapid binding turnover on genes with low transcriptional output (Lickwar et al. 2012). TBP, a GTF component involved in transcription of all classes of eukaryotic genes, shows rapid turnover at RNAP II and III promoters, but binds stably to the RNAP I promoter, and on DNA templates in in vitro experiments (Hoopes et al. 1992; van Werven et al. 2009; Grimaldi et al. 2014). These observations suggest that cells control DNA binding dynamics of TFs as an important step in regulating gene expression.
The abundance of many TFs is limited by their ubiquitin-mediated proteolysis in the 26S proteasome (Lipford and Deshaies 2003; Geng et al. 2012). Some TFs, however, are marked for proteolysis specifically when they are associated with activated target genes, which reflects an unexpected relationship between TF function and stability (Chi et al. 2001; Sundqvist and Ericsson 2003; Lipford et al. 2005; Muratani et al. 2005; Chymkowitch et al. 2011). It was noticed several years ago that the activation domains of many TFs (∼30 known to date) overlap with regions targeted by ubiquitination for proteolysis, and gene activation by these transcription factors is dependent on their ability to be degraded (Lipford et al. 2005; Muratani et al. 2005; Wang et al. 2010; Geng et al. 2012). These observations have led to a model in which proteolysis of some DNA-bound TFs clears them from targeted genes after recruitment of RNAP II, which allows further TF molecules to bind and drive subsequent rounds of transcription (Lipford et al. 2005; Geng et al. 2012). Whereas long TF residence times can result in several rounds of RNAP II recruitment and efficient transcription in some situations (e.g., Rap1, as noted above), by this model, rapid turnover might be necessary for continued gene activation in other cases (e.g., ERα; Reid et al. 2003), particularly if each TF molecule is capable of driving only a limited number of rounds of transcription. Regulating ubiquitin-mediated degradation of DNA-bound TFs, then, is a potentially important yet largely unexplored method of controlling the duration of gene activation.
Gcn4 is an example of a yeast TF whose activation domain overlaps with its degradation domain (Kornitzer et al. 1994; Chi et al. 2001; Tansey 2001; Geng et al. 2012). Gcn4 is expressed under conditions of amino acid starvation, and when amino acid levels are restored it is phosphorylated by the kinase Pho85, which triggers its ubiquitination and subsequent proteolysis through the 26S proteasome (Meimoun et al. 2000). Independent of amino acid levels, Cdk8 (previously known as Srb10) phosphorylates Gcn4, also causing its ubiquitin-mediated proteolysis (Chi et al. 2001). However, Cdk8 is a component of the Mediator complex, which is recruited to promoters by Gcn4, suggesting that this kinase specifically targets promoter-bound Gcn4 (Rawal et al. 2014). As such, it is thought that Cdk8 acts to remove Gcn4 from DNA by stimulating its degradation after successful recruitment of RNAP II (Lipford and Deshaies 2003; Lipford et al. 2005; Geng et al. 2012). Previously, we demonstrated that Gcn4 becomes sumoylated at two specific Lys residues (K50 and K58), and a SUMO-deficient form of Gcn4 (Gcn4-K50,58R) showed increased occupancy on target DNA, as determined by time-course ChIP (Rosonina et al. 2012). This increased occupancy was dependent on the presence of Cdk8, suggesting that Gcn4 sumoylation represents a mechanism of regulating the Cdk8-mediated clearance of the activator from DNA. However, the determinants for Gcn4 sumoylation and whether Gcn4 sumoylation, phosphorylation, and ubiquitination are coordinated to control its Cdk8-mediated clearance, are not known.
Here, we demonstrate that DNA binding alone is sufficient for Gcn4 SUMO modification in vivo, which limits its occupancy on DNA, and, consequently, the expression of its target genes. Gcn4 lacking a DNA binding domain is not sumoylated, as we have previously shown, but artificially targeting Gcn4 to a heterologous UAS restores its sumoylation at its natural modification sites. We find that neither prior phosphorylation or ubiquitination, nor recruitment of RNAP II to activated genes are required for Gcn4 sumoylation. However, blocking Gcn4 sumoylation prevents further modifications that likely represent Cdk8 phosphorylated and associated ubiquitinated Gcn4 isoforms, specifically. We then demonstrate that, compared with normal Gcn4, a hyper-sumoylated form of Gcn4 shows dramatically less occupancy on a target DNA sequence, with a concomitant reduction in expression of target genes. Finally, we demonstrate that deletion of CDK8 partially restores DNA occupancy and target gene expression levels in a strain expressing hyper-sumoylated Gcn4. Together, our data indicate that DNA binding is the critical determinant for Gcn4 sumoylation, which then stimulates a Cdk8-dependent pathway that clears Gcn4 from target DNA. We propose that this pathway is necessary for restricting the activity of DNA-bound molecules of Gcn4, thereby supporting appropriate levels of target gene expression.
Materials and Methods
Yeast strains and plasmids
Yeast strains and plasmids used in this study are listed in Supplemental Material, Table S1 and Table S2, respectively. Unless otherwise indicated, yeast were grown using standard conditions, namely growth in synthetic complete (SC) medium, lacking appropriate amino acids for selection, at 30° to midlog phase. Derived yeast strains were generated by the gene replacement method using PCR products that included appropriate marker genes (Knop et al. 1999), or by transformation with expression plasmids. The Smt3-Gcn4-6HA fusion strain was generated by fusion PCR followed by gene replacement transformation (based on Kitazono et al. 2002). Further cloning details and oligonucleotide primer sequences used for cloning are indicated in Table S1 and Table S2.
Yeast growth assay (spot assay)
Yeast cultures were grown in appropriate liquid medium overnight, and culture densities were determined on the following morning. Approximately 10,000 cells of each strain were then spotted side-by-side in the first position on the indicated solid medium plates, and serial fivefold dilutions were spotted in the adjacent positions. All plates were incubated at 30°, and images were recorded after the indicated number of days.
Preparation of yeast lysates and immunoprecipitation (IP)
Yeast cultures (10–40 ml) were grow to midlog phase, then induced for 20 min with 0.5 µg/ml sulfometuron methyl (SM) where indicated. For inhibition of the 26S proteasome, the method of Liu et al. (2007) was used to increase cell permeability of MG132. Cells were harvested by centrifugation and resuspended in IP buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl) plus 0.1% Nonidet P-40 (NP40), 1× yeast protease inhibitor cocktail (BioShop), 0.1 mM dithiothreitol, and 2.5 mg/ml N-ethylmaleimide (NEM), followed by glass bead vortex homogenization for 30 min, then removal of insoluble material by two rounds of centrifugation at 3000 × g for 5 min each. Samples were then analyzed by immunoblot, or, for IP, an aliquot was retained as input sample, and the remainder was incubated with Protein G agarose beads and 1 µg of HA epitope tag primary antibody (NEB) overnight at 4°. IPs were washed three times with ice-cold IP buffer plus 0.1% NP40, then samples were boiled in SDS sample buffer for 3 min prior to analysis by immunoblot. For phosphatase treatment, lysates were incubated with 400 U Lambda protein phosphatase (NEB) per 50 μl lysate for 15 min at 30° prior to IP. Densitometry was performed using a MicroChemi chemiluminescence imager (DNR), and ImageJ quantification software. Antibodies used include rabbit and mouse HA (NEB), Smt3/SUMO (Santa Cruz), and FK1/Ub (Cayman Chemical).
ChIP
Yeast cultures (50 ml) were grown as indicated for IP, followed by cross-linking with 1.1% formaldehyde for 20 min, before quenching with 450 mM of glycine for 5 min. Samples were pelleted by centrifugation, and washed with ice-cold TBS (20 mM Tris-HCl, pH 7.5, and 150 mM NaCl), then in ChIP buffer (50 mM HEPES-KOH, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, and 0.1% SDS). Pelleted samples were resuspended in ChIP buffer and subjected to bead beating with glass beads followed by sonication to shear chromatin to fragments of ∼500 bp in length. Samples were then centrifuged for 5 min, and additional NaCl was added to the isolated supernatants to a final concentration of 212.5 mM. Aliquots of the supernatants (40 µl) were retained as input samples, and the remainders of the salt-adjusted supernatants were incubated overnight at 4° with washed Protein G agarose beads plus 1 µg of the appropriate antibody for IP. On the following day, beads were washed first in ChIP buffer with 275 mM NaCl, then in ChIP buffer with 500 mM NaCl, followed by an additional washing buffer (10 mM Tris-HCl, pH 8, 0.25 M LiCl, 1 mM EDTA, 0.5% NP-40, and 0.5% sodium deoxycholate), and finally with Tris-EDTA buffer (10 mM Tris-HCl, pH 8, and 1 mM EDTA). Beads were then incubated in ChIP elution buffer (50 mM Tris-HCl, pH 7.5, 10 mM EDTA, and 1% SDS) for 10 min at 65°. Samples were then centrifuged at 8000 × g, and supernatants were treated with proteinase K at 42° for 1 hr, then transferred to 65° for 4 hr to overnight to reverse cross-links. The following day, LiCl was added to each sample to a final concentration of 0.4 M, and DNA was recovered by phenol–chloroform extraction, and ethanol precipitation. ChIP experiments were performed at least three times each, and the average of quantitative PCR (qPCR) analyses are presented, using the percent input method, with SD shown as error bars. qPCR primers used are listed in Table S3.
Isolation of RNA and reverse transcription (RT)
Yeast cultures (10 ml) were grown and induced as indicated above, then RNA was prepared as previously reported (Amberg et al. 2006). For RT, 12 μg samples of RNA were first treated with DNase I (NEB) as indicated by the supplier, then 1 µg of DNA-free-RNA was used for cDNA synthesis using the iScript cDNA synthesis kit (Bio-Rad) as per the manufacturer’s instructions. For qualitative analysis, 26 cycles of standard PCR was used, and products were resolved on 2% agarose gels. For quantitative analysis, qPCR was performed and values were normalized to 25S rRNA levels. All experiments were performed at least three times, and average values are presented with SD shown as error bars. Primer sequences are listed in Table S3. For qPCR analysis of both ChIP and RT-PCR samples, where statistical comparisons were performed, a Student’s t-test was applied with P-values <0.05 indicated as asterisks between relevant samples in figures.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. All yeast strains and plasmids are available upon request.
Results
DNA binding is sufficient for Gcn4 sumoylation
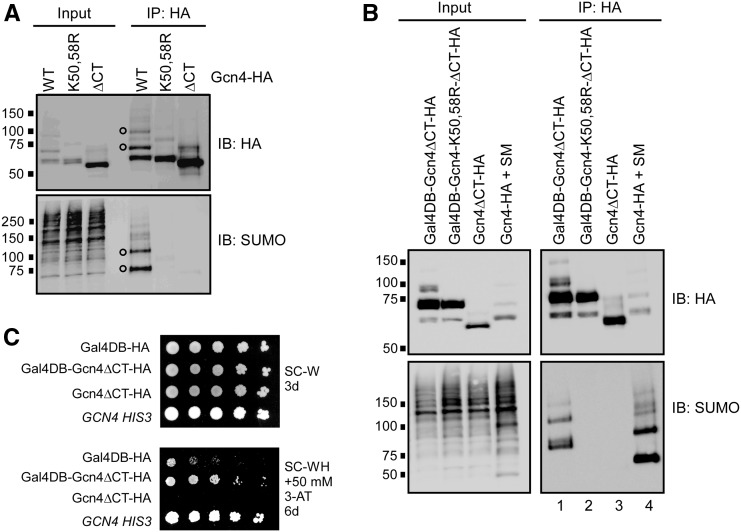
We previously demonstrated that Gcn4 is sumoylated at two lysine residues, K50 and K58, and that deleting its 40 C-terminal residues (ΔCT mutant), which are not needed for nuclear import but are necessary for DNA binding (Hope and Struhl 1985; Pries et al. 2002), abolished its sumoylation (Rosonina et al. 2012). This suggests that Gcn4 sumoylation occurs after it binds to the UAS of its target genes. We wished to further examine the requirement for DNA binding on Gcn4 sumoylation. Sumoylation of Gcn4 was examined by IP followed by SUMO immunoblot analysis from yeast that express Gcn4 with a 6× HA C-terminal epitope tag from its natural chromosomal locus, or from an expression plasmid (Rosonina et al. 2012). Gcn4 expression is induced by treating cells with SM, which triggers amino acid starvation (Falco and Dumas 1985). Lysates prepared in the presence of NEM, which impairs SUMO proteases and is critical for detection of modified forms of Gcn4 (Figure S1A; Patterson and Cyr 2005), are then subjected to IP with an HA antibody, followed by immunoblot analysis. Two prominent modified forms of Gcn4 are detectable in both HA and SUMO immunoblots following IP, which we previously attributed to mono- and di-sumoylated Gcn4 (open circles in Figure 1A; Rosonina et al. 2012). As expected, these forms are absent in IPs from strains expressing Gcn4 with Lys-to-Arg mutations at K50 and K58 (Gcn4-K50,58R), and in the Gcn4-ΔCT mutant.
Figure 1.
DNA binding is sufficient for Gcn4 sumoylation on Lys 50 and 58. (A) HA and SUMO immunoblot analysis of HA IPs from strains expressing plasmid-derived WT, SUMO-deficient (K50,58R), or DNA-binding-deficient (ΔCT) forms of Gcn4, all of which contain a 6× HA C-terminal epitope tag. Open circles indicate position of the two major sumoylated forms of Gcn4, detectable in both HA and SUMO blots, as previously reported (Rosonina et al. 2012). Expression of Gcn4 was induced by addition of SM for 20 min to synthetic complete growth medium lacking Val and Ile to generate amino acid starvation conditions, and lysates were prepared with NEM to impair SUMO proteases and deubiquitinating enzymes. Inputs represent ∼2.5–5% of immunoprecipitated material analyzed in the immunoblot. Strains analyzed are ERYM663, ERYM664F, and ERYM709. (B) IP-immunoblot analysis, as in (A), from strains expressing indicated HA-tagged proteins. All proteins, except Gcn4-HA, were generated from plasmids containing the constitutive ADH1 promoter, in the HF7c strain grown under standard conditions (see Materials and Methods). Gcn4-HA was expressed from the GCN4 chromosomal locus in the BY4741 strain to which SM was added, and was included in this analysis for comparison. Strains analyzed are YER026, YER028, YER029, and ERYM663. (C) Yeast spot assay comparing growth of HF7c yeast expressing indicated proteins, as in (B), on minimal medium selective for yeast containing expression plasmids (SC-W; lacking Trp), or the same lacking His (SC-WH) plus 50 mM 3-AT. HF7c contains a HIS3 reporter gene controlled by the GAL4 UAS, to which the Gal4 DB binds. A control HIS+ strain (with WT GCN4) is included for comparison. Plates were photographed after incubation for the number of days indicated. Strains analyzed are YER027, YER026, YER029, and YAA020A.
To determine whether targeting Gcn4 to a heterologous UAS could also result in its sumoylation, we transformed the HF7c yeast strain with a plasmid that expresses a fusion protein (Gal4DB-Gcn4ΔCT) consisting of the ΔCT truncated form of Gcn4 fused with the Gal4 DNA-binding domain (Gal4DB), which binds the GAL4 UAS. IP-immunoblot analysis indicated that the fusion protein is indeed sumoylated, showing at least two prominent sumoylated forms in a SUMO immunoblot (lane 1 in Figure 1B). Sumoylation was dependent on the presence of Gal4DB (cf. lanes 1 and 3), and, importantly, mutation of Lys residues in the Gal4DB-Gcn4ΔCT fusion that correspond to Gcn4 K50 and K58 abolished sumoylation (cf. lanes 1 and 2), suggesting that Gcn4 becomes sumoylated at these specific residues whenever it can bind DNA. To determine whether DNA-bound Gal4DB-Gcn4ΔCT is functional as a transcriptional activator, we examined growth of the HF7c strain expressing the fusion protein on medium lacking His, and supplemented with 3-aminotriazole (3AT), which prevents growth unless the HIS3 gene is highly expressed. In the HF7c strain, the HIS3 gene is under control of the GAL4 UAS, and, whereas expression of Gcn4ΔCT alone did not allow growth on –His/3AT medium, cells expressing the Gal4DB-Gcn4ΔCT fusion protein grew significantly better than cells expressing the Gal4-DB alone (Figure 1C). This indicates that Gcn4 that is targeted to the heterologous GAL4 UAS becomes sumoylated at its natural target Lys residues, and is functional as a TF. Furthermore, the Gal4DB-Gcn4ΔCT fusion and derivatives examined in Figure 1B were constitutively expressed from the ADH1 promoter in HF7c cells grown in normal medium, meaning that Gcn4 sumoylation is not dependent on amino acid starvation, or on the presence of SM. Instead, these data demonstrate that binding to DNA is necessary and sufficient for Gcn4 to become sumoylated at K50 and K58, at least in the context of a functional UAS.
Gcn4 sumoylation can occur in the absence of its phosphorylation
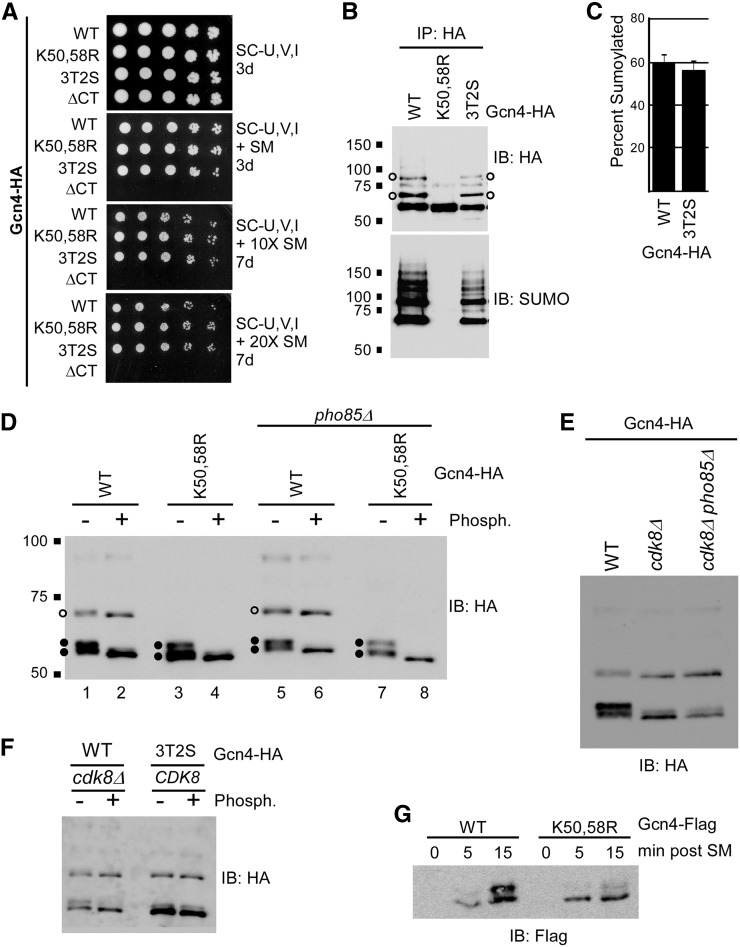
For some SUMO targets, sumoylation requires prior phosphorylation, while in other cases, sumoylation regulates subsequent protein phosphorylation (Hietakangas et al. 2006; Yao et al. 2011). To identify additional signals that trigger Gcn4 sumoylation, and to explore how sumoylation and phosphorylation of Gcn4 are coordinated, we examined mutant yeast strains deficient in either sumoylation or phosphorylation of Gcn4. Pho85 and Cdk8 phosphorylation-deficient Gcn4 was generated by mutating all five possible cyclin-dependent kinase (CDK) targeted Thr or Ser residues to Ala (3T2S; Chi et al. 2001). Both Gcn4-K50,58R and Gcn4-3T2S (Chi et al. 2001; Rosonina et al. 2012) strains grew as well as the Gcn4-WT strain on a range of media with increasingly depleted levels of Val and Ile (i.e., increasing SM levels), indicating that neither Gcn4 sumoylation nor phosphorylation are necessary for its role in activating transcription of amino acid biosynthesis genes (Figure 2A; Chi et al. 2001; Rosonina et al. 2012). In contrast, the Gcn4-ΔCT mutant that cannot bind DNA was unable to grow on the SM-containing media. These results imply that Gcn4 SUMO and phosphorylation modifications might both be involved in regulating the protein after it has functioned in gene activation on target gene promoters.
Figure 2.
Gcn4 sumoylation does not depend on its prior phosphorylation. (A) Yeast spot assay comparing growth of strains expressing indicated forms of Gcn4, including a form that cannot be phosphorylated by Pho85 or Cdk8 (3T2S; Chi et al. 2001). Minimal medium was used lacking Ura, Val, and Ile, and supplemented with either no SM (top), 0.5 μg/ml SM, or 10× or 20× this concentration, as indicated. Plates were photographed after indicated number of days of growth. Strains analyzed are ERYM663, ERYM664F, YAA003, and ERYM709. (B) HA and SUMO immunoblot analyses of HA IPs from strains expressing indicated HA-tagged forms of Gcn4, as in Figure 1A. Open circles indicate the positions of the two major sumoylated forms of Gcn4. Strains analyzed are ERYM663, ERYM664F, and YAA003. (C) Level of Gcn4 sumoylation in Gcn4-WT and -3T2S strains. Densitometry was performed on HA IP-immunoblots (as in Figure S1C) by measuring the intensity of the two major sumoylated forms of Gcn4, and representing it as a percent fraction of the total Gcn4 signal on the immunoblot. Strains analyzed are ERYM663 and YAA003. (D) HA immunoblot analysis of the Gcn4-HA isoforms in the indicated strains was performed, as in Figure 1A, after mock treatment (−) or addition of Lambda protein phosphatase (+). Open circles indicate the position of the major sumoylated form of Gcn4 (the additional sumoylated form detected in IPs is only barely visible near the top of the blot). Closed circles indicate the positions of the differentially phosphorylated unsumoylated forms of Gcn4. Strains analyzed are ERYM663, ERYM664F, ERYM665, and ERYM666. (E) Gcn4-HA immunoblot analysis in cdk8Δ and cdk8Δ pho85Δ strains. Strains analyzed are ERYM663, ERYM667, and ERYM671. (F) HA immunoblot analysis of Gcn4-WT in a cdk8Δ strain and Gcn4-3T2S in a CDK8 strain from lysates treated (+), or mock treated (−), with Lambda phosphatase. Strains analyzed are ERYM663, ERYM667, and YAA003. (G) Flag immunoblot analysis of strains expressing plasmid-derived Flag-tagged Gcn4-WT or Gcn4-K50,58R in a time-course after addition of SM to growth medium. Strains analyzed are ERYM665 and ERYM666.
We next examined whether Gcn4 sumoylation depends on its prior phosphorylation by either Pho85 or Cdk8. Gcn4 was immunoprecipitated from Gcn4-WT, Gcn4-K50,58R, and Gcn4-3T2S strains, and examined by HA and SUMO immunoblot (Figure 2B). Multiple forms of Gcn4-3T2S migrate further in SDS-PAGE gels compared to corresponding forms of Gcn4-WT, confirming that, in this strain, Gcn4 is indeed deficient in phosphorylation (Figure S1, B and C). However, preventing Gcn4 phosphorylation through mutation of the five phosphorylation sites, or by deleting CDK8 and PHO85, had no effect on its sumoylation pattern (Figure 2, B and E). Indeed, we determined the level of Gcn4 sumoylation in Gcn4-WT and Gcn4-3T2S strains by quantifying the abundance of the two major SUMO isoforms in an HA immunoblot relative to all forms of Gcn4, and found that, in both strains, ∼60% of Gcn4 is sumoylated (Figure 2C and Figure S1C). Thus, Gcn4 sumoylation can occur independently of its phosphorylation by either Pho85 or Cdk8.
Blocking Gcn4 sumoylation affects its phosphorylation pattern
We further examined the pattern of Gcn4 modifications in the Gcn4-WT and Gcn4-K50,58R strains to determine whether prior sumoylation affects Gcn4 phosphorylation. Three major forms of Gcn4 are detected by HA immunoblot analysis of Gcn4-WT, which we attribute to monosumoylated Gcn4, and two forms of unsumoylated Gcn4 that appear as a doublet of bands (open and closed circles, respectively, in Figure 2D). The additional, higher molecular weight sumoylated form of Gcn4, which is readily detected after IP, is only weakly detectable by HA immunoblot analysis (cf. Input and IP in Figure S1B). Both monosumoylated and unsumoylated forms of Gcn4-WT showed increased migration when lysates were treated with Lambda protein phosphatase, indicating that all major forms of Gcn4 are normally phosphorylated, and that the doublet represents two forms of Gcn4 that differ in levels of phosphorylation (Figure 2D). Because a similar pattern was also observed in strains lacking Pho85 (Figure 2D, lanes 5–8), Cdk8 is likely the kinase responsible for generating the phosphorylated forms of Gcn4 detected. Indeed, immunoblot analysis of Gcn4 in cdk8Δ and cdk8Δ pho85Δ strains showed a pattern that was virtually identical to phosphatase-treated Gcn4 (cf. Figure 2, D and E), and phosphatase treatment of lysate from the cdk8Δ strain resulted in only a modest further reduction of phosphorylated Gcn4 forms (Figure 2F), strongly implicating Cdk8, and not Pho85, as the kinase responsible for the majority of phosphorylated forms of Gcn4 detected under the conditions used for our analyses. The phosphatase-dependent shift in migration indicates that virtually all monosumoylated Gcn4 (open circles in Figure 2D) is normally also phosphorylated, pointing to a tight relationship between Cdk8-mediated phosphorylation and sumoylation of Gcn4.
Supporting the notion that Gcn4 sumoylation influences its phosphorylation, Gcn4-WT and Gcn4-K50,58R display different phosphorylation patterns when examined by HA immunoblot. This is reflected in the relative intensities of bands in the fast-migrating doublet, with Gcn4-WT showing approximately equal levels of each band in the doublet, and Gcn4-K50,58R showing higher levels of the lower, least-phosphorylated form (cf. lanes 1 and 5 with 3 and 7, respectively in Figure 2D). This was also observed in strains expressing Flag-tagged Gcn4-WT and K50,58R, in which a time-course of SM-induction was performed (Figure 2G). The time-course shows that the upper band of the doublet appears after about 15 min of induction, but barely appears in the SUMO-deficient mutant by this time. This suggests that, although sumoylation is not absolutely necessary for subsequent Gcn4 phosphorylation, SUMO modification greatly stimulates the production of this specific phosphoisoform of Gcn4. Together, these results demonstrate that impairing Gcn4 sumoylation through the K50,58R mutation also results in reduced phosphorylation of the TF by Cdk8.
Most Gcn4 ubiquitination occurs independently of its sumoylation
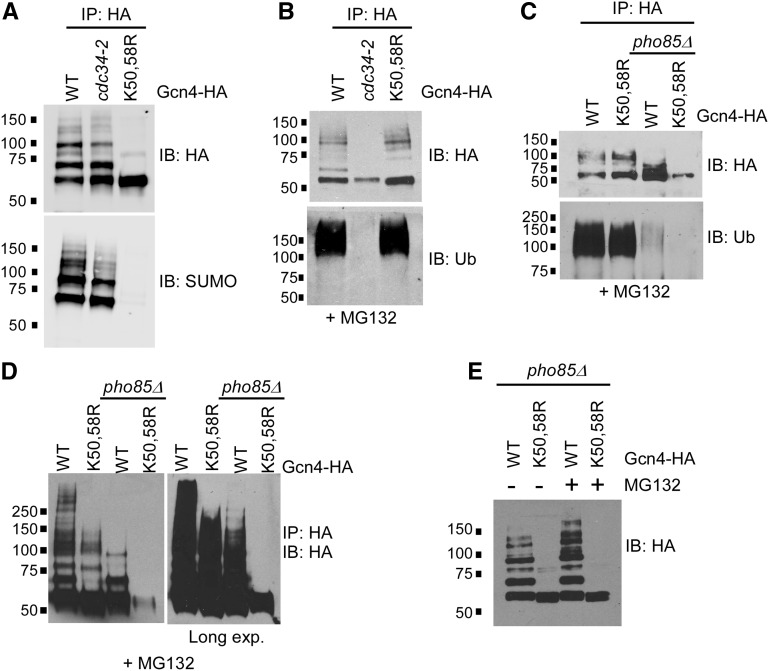
Sumoylation and ubiquitination can be mutually exclusive modifications, occurring on the same Lys residue (Desterro et al. 1998; Yao et al. 2011), whereas, in other cases, sumoylation can trigger subsequent ubiquitination through recognition by SUMO-targeted ubiquitin ligases (STUbLs; Sriramachandran and Dohmen 2014). SUMO-modified residues K50 and K58 lie outside of ubiquitin-targeted Gcn4 degradation domains, indicating that it is not likely that sumoylation and ubiquitination occur on the same Lys residues (Meimoun et al. 2000). However, to determine whether Gcn4 ubiquitination and sumoylation show any codependence, we used a strain expressing a defective form of the Gcn4 ubiquitin ligase, Cdc34 (cdc34-2; Meimoun et al. 2000). Since ubiquitinated Gcn4 is subject to degradation through the 26S proteasome (Kornitzer et al. 1994), to observe its ubiquitination, Gcn4, Gcn4-WT, Gcn4-K50,58R, and cdc34-2 strains were treated with the 26S proteasome inhibitor MG132 prior to lysis and IP. As expected, immunoblot analysis with a ubiquitin antibody (Ub) showed a heavy smear of ubiquitinated forms of Gcn4 derived from the Gcn4-WT strain, but no ubiquitinated species were detected in the cdc34-2 strain (Figure S1D). Analysis of the IPs with a SUMO antibody showed that, although some high-molecular weight forms of sumoylated Gcn4 are reduced, the vast majority of sumoylated Gcn4 species are retained in the cdc34-2 strain, even in the absence of its ubiquitination (Figure 3A). Furthermore, analysis of Gcn4 ubiquitination in the Gcn4-K50,58R strain indicates that the bulk of Gcn4 ubiquitination has no dependence on its ability to be sumoylated (Figure 3B). At first examination therefore, Gcn4 sumoylation and ubiquitination appear to occur independently of each other.
Figure 3.
Gcn4 sumoylation promotes further modifications that are stabilized by blocking the 26S proteasome. (A) HA and SUMO immunoblot analysis of HA IPs of Gcn4-WT from CDC34 (WT) or cdc34-2 strains, or from a Gcn4-K50,58R-expressing strain, as in Figure 1A. Strains analyzed are ERYM663, YAA002, and ERYM664F. (B) HA and ubiquitin (Ub) immunoblot analysis of HA IPs from strains expressing WT, cdc34-2, or K50,58R forms of Gcn4. Cultures were treated with MG132 prior to induction with SM as in Figure 1A. Strains analyzed are ERYM663, YAA002, and ERYM664F. (C, D) HA and Ub immunoblot analysis of HA IPs of Gcn4-WT or Gcn4-K50,58R from PHO85 or pho85Δ strains. Cultures were treated with MG132 prior to induction with SM as in Figure 1A. Two exposures of a higher resolution HA immunoblot of immunoprecipitated samples derived from the same strains appears in (D). Strains analyzed are ERYM663, ERYM664F, ERYM665, and ERYM666. (E) HA immunoblot analysis of HA IPs of Gcn4-WT or Gcn4-K50,58R in pho85Δ strains either mock treated, or treated with MG132 prior to induction with SM as in Figure 1A. Because pho85Δ strains grow slowly, cultures analyzed in (C–E) were not necessarily matched for cell density, which can result in variable of Gcn4 expression signals. Strains analyzed are ERYM665 and ERYM666.
Pho85-independent modifications of Gcn4 are impaired by SUMO-site mutations
In our previous study, we demonstrated that sumoylation of Gcn4 reduces its levels on DNA near target gene promoters in a manner dependent on Cdk8 (Rosonina et al. 2012). This prompted us to speculate that sumoylation of DNA-bound Gcn4 triggers its degradation through Cdk8 phosphorylation-mediated ubiquitination and proteolysis. Pho85 phosphorylation of Gcn4, which mediates degradation of Gcn4 independently of its sumoylation, occurs at a significantly higher level than phosphorylation by Cdk8 (Chi et al. 2001; Shemer et al. 2002; Rosonina et al. 2012). However, as mentioned above, the specific phosphorylated forms of Gcn4 detected in the analyses in Figure 2 are largely due to Cdk8 phosphorylation, suggesting that Gcn4 that is phosphorylated by Pho85 is rapidly degraded, and therefore not readily detected. In support of this, treatment of WT, pho85Δ, cdk8Δ, and pho85Δ cdk8Δ cells with MG132 demonstrates that the vast majority of MG132-stabilized forms of ubiquitinated Gcn4 depend on Pho85 (Figure S1E). These observations suggest that Gcn4 that is phosphorylated by Pho85 is far more rapidly ubiquitinated and targeted for degradation than Cdk8-mediated phosphorylated forms of Gcn4.
In order to examine the relationship between sumoylation and Cdk8-mediated ubiquitination of Gcn4 specifically, we used a strain lacking Pho85. Although pho85Δ strains grow slower than Pho85-expressing counterparts (e.g., Rosonina et al. 2012), they are capable of inducing Gcn4 expression, albeit at somewhat variable levels (see Figure 3, C–E). Ub immunoblot analysis of Gcn4-WT or Gcn4-K50,58R immunoprecipitated from pho85Δ cells showed low levels of ubiquitination that were only slightly detectable above background, making it difficult to determine whether blocking Gcn4 sumoylation affects Pho85-independent ubiquitination in this strain (Figure 3C). Instead, we examined whether MG132-stabilized forms of Gcn4, which represent proteasome-targeted ubiquitinated Gcn4, could be detected in HA immunoblots. As expected, compared to Pho85-containing cells, considerably fewer immunoprecipitated forms of Gcn4 were detected in pho85Δ cells in the presence of MG132 (Figure 3D). Nonetheless, a long exposure of the HA immunoblot showed that whereas a significant amount of modified Gcn4-WT was detectable in pho85Δ cells, essentially no modified forms of Gcn4-K50,58R could be detected in the absence of Pho85 (Figure 3D, right). Furthermore, the HA immunoblot analysis shown in Figure 3E confirms that, in pho85Δ cells, MG132 stabilizes modified forms of Gcn4-WT, specifically, but not of Gcn4-K50,58R. Taken together, our results point to a role for sumoylation in stimulating Pho85-independent ubiquitination of Gcn4, which is consistent with a model in which sumoylation triggers Cdk8 phosphorylation-mediated degradation of Gcn4 near target promoters.
Gcn4 sumoylation is transcription-independent
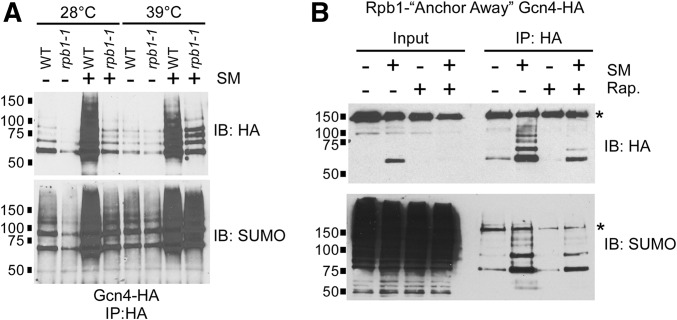
Once bound to DNA, Gcn4 activates transcription by interacting with coactivators, such as Gal11, leading to the recruitment of RNAP II to target promoters (Herbig et al. 2010). To determine whether activation itself provides an additional signal for Gcn4 sumoylation, we generated a number of Gcn4 mutant strains with substitutions at residues previously shown to function in Gcn4 activation (Drysdale et al. 1995; Brzovic et al. 2011; Warfield et al. 2014), or lacking Gal11, and examined Gcn4 sumoylation levels in these strains. However, none of the mutant strains showed defective induction of Gcn4 target genes, and all were able to grow on amino acid starvation medium (Figure S2), suggesting that Gcn4 uses multiple independent mechanisms to activate transcription (Drysdale et al. 1995), and negating the utility of these mutant strains. Instead, to obtain a more detailed picture of how Gcn4 is regulated on target genes, we examined whether Gcn4 sumoylation is dependent on transcription by RNAP II. That is, does Gcn4 sumoylation take place only after the activator successfully recruits RNAP II to target promoters? Our first approach involved the use of the rpb1-1 strain, in which the largest subunit of RNAP II, Rpb1, is inactivated when grown at elevated temperatures (Nonet et al. 1987). WT and rpb1-1 strains were grown at normal (28°) or restrictive (39°) temperatures, then treated with SM to induce expression of Gcn4-6HA. As expected, impairment of RNAP II resulted in reduced induction of Gcn4 target genes ARG1 and CPA2, as determined by RT-PCR (Figure S1F). Although induction of Gcn4 expression is largely at the level of translation (Hinnebusch 2005), its expression in rpb1-1 was significantly reduced compared to WT, at both the permissive and restrictive temperatures (Figure 4A, top). Despite this, Gcn4 expressed in rpb1-1 cells at the restrictive temperature was heavily sumoylated (Figure 4A, bottom). This indicates that Gcn4 sumoylation takes place even when transcription is impaired.
Figure 4.
Gcn4 sumoylation does not depend on active transcription or RNAP II recruitment. (A) HA and SUMO immunoblot analysis of HA IPs of Gcn4-WT from WT or rpb1-1 strains. Strains were grown either at 28°, then left at that temperature, or switched to 39° for 15 min, which is the nonpermissive temperature for the rpb1-1 strain (Nonet et al. 1987). Strains were then either mock treated (−) or induced with SM (+) for an additional 15 min at the same temperatures prior to lysis and IP. Strains analyzed are YAA010 and YAA011. (B) HA and SUMO immunoblot analysis of HA IPs of Gcn4-WT expressed in the Rpb1-FRB (Anchor Away) strain (YAA032). Cultures of the strain were either mock treated (−), or treated with 1 μg/ml rapamycin (+ Rap.) for 20 min prior to a further 20 min treatment with either DMSO (mock; −) or SM, to induce expression of Gcn4, prior to lysis and IP. Asterisks indicate unrelated cross-reacting protein detected in immunoblot analyses in this strain.
To address the possibility that elevated temperature itself triggered Gcn4 sumoylation in the experiments described above, we employed an alternate method of impairing transcription to examine its effect on Gcn4 sumoylation. The “anchor-away” approach was recently applied to Rpb1 as a method of blocking transcription without the need for elevated temperature (Haruki et al. 2008; Moqtaderi et al. 2014). Briefly, Rpb1 is expressed as a fusion with the FRB domain of human mTOR in a yeast strain that also expresses the ribosomal protein RPL13A fused to FKBP12, which binds to FRB in the presence of rapamycin. Exposing this strain to rapamycin causes Rpb1-FRB to bind nuclear RPL13A-FKB12, which rapidly translocates to the cytoplasm, thereby halting transcription. As shown in Figure 4B, Gcn4 expression in the Rpb1-FRB strain is induced by SM, but prior treatment with rapamycin results in reduced Gcn4 levels (top). Nonetheless, SUMO immunoblot analysis shows that Gcn4 is sumoylated in the absence of nuclear Rpb1 (Figure 4B, bottom). Based on the results of these analyses, we conclude that SUMO marks DNA-bound Gcn4 independently of transcription or the recruitment of RNAP II to its target genes.
Hyper-sumoylation of Gcn4 reduces its occupancy on target promoters
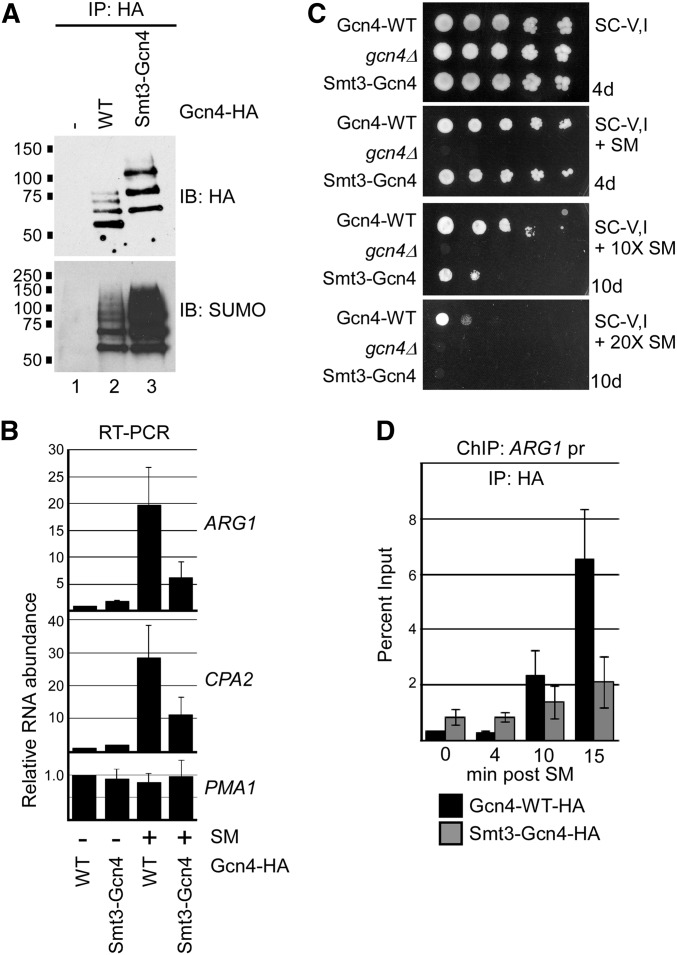
To further explore the consequences of sumoylation on Gcn4 function, we generated a yeast strain that expresses a SUMO-fused form of the protein. The strain produces a fusion of the yeast SUMO peptide, Smt3 (lacking the C-terminal diglycine motif that is targeted by SUMO proteases), at the N-terminus of Gcn4, expressed from the natural GCN4 locus. Considering that the SUMO-modified residues on Gcn4 are at positions 50 and 58, we reasoned that the N-terminal fusion might effectively mimic sumoylated Gcn4 in the cell. Immunoprecipitated Gcn4 was examined from cells expressing wild type (WT) or Smt3-fusion forms of the protein (Figure 5A). Smt3-Gcn4 generated three prominent bands on an HA immunoblot, which comigrate with bands likely attributed to mono-, di-, and tri-sumoylated Gcn4-WT (cf. lanes 2 and 3), indicating that some of the Smt3-Gcn4 fusion becomes further SUMO modified. Notably, the Smt3-Gcn4 fusion protein displays a significantly higher level of overall sumoylation than Gcn4-WT (Figure 5A, bottom).
Figure 5.
Hyper-sumoylation of Gcn4 reduces its occupancy on target DNA. (A) HA and SUMO immunoblot analysis of HA IPs of Gcn4-WT, or of the fusion Smt3-Gcn4-WT, both of which contain the usual C-terminal 6× HA tag. A strain with no HA tag on Gcn4 (−) was analyzed in parallel as a control. Strains analyzed are ERYM615, ERYM613, and YAA030H. (B) qRT-PCR analysis of RNA isolated from Gcn4-WT or Smt3-Gcn4 strains mock-treated (−) or induced for Gcn4 expression with SM (+). Genes analyzed include Gcn4-targets ARG1 and CPA2, and the constitutively expressed PMA1 gene. The average of three independent experiments is shown, with SD shown as error bars. Strains analyzed are ERYM613 and YAA030H. (C) Yeast spot assay comparing growth of strains expressing Gcn4-WT, no Gcn4 (gcn4Δ), or the fusion Smt3-Gcn4 on minimal medium lacking Val and Ile, and supplemented with either no SM (top), 0.5 μg/ml SM, or 10× or 20× this concentration, as indicated. Plates were photographed after indicated number of days of growth. Strains analyzed are ERYM613, ERYM625, and YAA030H. (D) Comparison of Gcn4 and Smt3-Gcn4 occupancy on target DNA. HA ChIP analysis of the promoter-proximal region of the ARG1 gene was performed in the Gcn4-WT or Smt3-Gcn4 strains at indicated times post induction with SM. The average of three independent experiments is shown with SD shown as error bars. Strains analyzed are ERYM613 and YAA030H.
We next examined the effects of higher levels of Gcn4 sumoylation on the expression of target genes. As seen in Figure 5B, quantitative RT-PCR (qRT-PCR) analysis showed that cells expressing the Smt3-Gcn4 fusion produced significantly fewer ARG1 and CPA2 transcripts during amino acid starvation than cells expressing Gcn4-WT, whereas there was no difference for PMA1, a constitutively expressed gene. This was not due to reduced abundance or stability of the Smt3-Gcn4 fusion relative to Gcn4-WT, as immunoblot analysis showed higher levels of the Smt3-fusion form of the protein during amino acid starvation, and both forms showed similar rates of degradation after the addition of concentrated Val and Ile to the growth medium, which was previously shown to trigger rapid Gcn4 degradation (Figure S3A; Rosonina et al. 2012). As expression of amino acid biosynthesis genes is essential for viability during starvation, we examined whether reduced expression of Gcn4 target genes reflected defects in growth in SM-containing medium. As shown in Figure 5C, yeast cells expressing Smt3-Gcn4 showed a significant growth defect compared with cells expressing Gcn4-WT on medium containing elevated levels of SM, when amino acid biosynthesis is likely critical for survival (Figure 5C). These results correlate higher levels of Gcn4 sumoylation with less target gene activation, and are consistent with a model in which SUMO stimulates removal of Gcn4 from promoters.
To test this specifically, we compared the levels of Gcn4-WT and Smt3-Gcn4 on DNA near the promoter of the Gcn4-targeted gene ARG1 over a time-course of amino acid starvation, by ChIP. At 10 and 15 min postinduction, significantly lower levels of Smt3-Gcn4 were detected near the ARG1 promoter, compared to Gcn4-WT (Figure 5D). We do not believe this is due to a defect in recruitment of the fusion protein to target DNA, because, prior to induction of ARG1, and 4 min thereafter, we detected significantly higher levels of Smt3-Gcn4 than Gcn4-WT. Although it is not known why Smt3-Gcn4 occupies DNA near the ARG1 promoter prior to its induction, this observation indicates that the fusion protein is not defective in binding target DNA. Instead, the results of this analysis are consistent with increased removal of hyper-sumoylated Gcn4 from promoter-proximal DNA, compared to normal Gcn4, with a consequential reduction in activation of target genes.
Cdk8 is required for removal of hyper-sumoylated Gcn4 from DNA
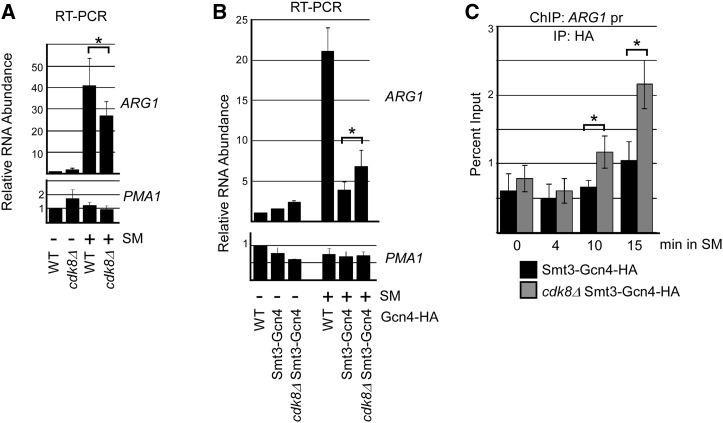
To provide further evidence that Gcn4 sumoylation promotes its Cdk8-mediated phosphorylation in order to clear it from target promoters, we examined whether deletion of CDK8 can reverse the effects of hyper-sumoylation of Gcn4 on expression of target genes. Cdk8 plays both positive and negative roles in regulating RNAP II (Galbraith et al. 2010). For ARG1, qRT-PCR analysis indicates that deletion of CDK8 results in reduced induction of activated ARG1, probably reflecting its positive roles in regulating RNAP II transcription (Figure 6A). However, in cells expressing the Smt3-Gcn4 fusion, no decrease in expression of ARG1 was detected in the absence of Cdk8 (Figure 6B). Instead, expression was significantly elevated, indicating that the drop in expression due to hyper-sumoylation of Gcn4 was reversed by eliminating Cdk8. Importantly, this correlated with an increased occupancy of Smt3-Gcn4 in cdk8Δ cells near the ARG1 promoter as determined by time-course ChIP (Figure 6C). Taken together, these data indicate that Cdk8 is at least partly required for the reduced occupancy of hyper-sumoylated Gcn4 on the ARG1 gene and its reduced expression, and strongly supports the notion that sumoylated, DNA-bound Gcn4 is targeted for removal through a Cdk8-mediated pathway.
Figure 6.
Deletion of CDK8 partially restores occupancy of Smt3-Gcn4 on target DNA. (A) Comparison of ARG1 and PMA1 RNA levels in WT and cdk8Δ strains. qRT-PCR analysis was performed on RNA isolated from WT or cdk8Δ strains in mock-treated (−) or SM-induced cells. The average of three independent experiments is shown with SD shown as error bars. Asterisk indicates statistically different values. Strains analyzed are ERYM613 and YAA034B. (B) Analysis of ARG1 and PMA1 RNA levels by qRT-PCR on RNA isolated from indicated strains that were mock-treated (−) or treated with SM. The average of three independent experiments is shown with SD shown as error bars. Asterisk indicates statistically different values. Strains analyzed are ERYM613, YAA030H, and YAA034B. (C) Comparison of DNA occupancy of Smt3-Gcn4 in CDK8 and cdk8Δ strains on ARG1 promoter-proximal DNA. HA ChIP analysis was performed at time-points indicated after induction with SM. The average of three independent experiments is shown with SD shown as error bars. Asterisks indicate statistically different values. Strains analyzed are YAA030H and YAA034B.
Discussion
In response to amino acid starvation in yeast, Gcn4 levels increase rapidly, primarily through derepression of GCN4 mRNA translation, and by blocking the Pho85-mediated pathway of Gcn4 degradation (Irniger and Braus 2003; Hinnebusch 2005). Accumulation of Gcn4 then allows it to bind and activate target amino acid biosynthesis genes (Hinnebusch and Natarajan 2002). We previously demonstrated that a significant fraction of Gcn4 becomes sumoylated during this process (Rosonina et al. 2012). Here, we have examined the determinants for Gcn4 sumoylation, and found that neither prior phosphorylation or ubiquitination are required. Instead, we have shown that DNA binding is both necessary and sufficient for the modification to occur in vivo, in a manner independent of amino acid starvation conditions. Gcn4 forms dimers when bound to DNA (Guarnaccia et al. 2004), and it is therefore possible that only the dimeric form of Gcn4 is recognized by the sumoylation machinery, thereby explaining the requirement for DNA binding. Furthermore, our finding that recruitment of RNAP II is not necessary for Gcn4 sumoylation strongly points to DNA binding as the principal, if not sole, criteria for Gcn4 sumoylation. As such, the SUMO mark can serve to distinguish DNA-bound from unbound Gcn4, and restrict downstream regulatory processes, such as Cdk8-mediated degradation, to Gcn4 molecules that have already associated with target genes.
Our results provide strong evidence that the major function for sumoylation of Gcn4 is to control its occupancy on target DNA sequences. Our previous work demonstrated that SUMO-blocking Gcn4 mutations (in the K50,58R mutant) led to increased DNA occupancy (Rosonina et al. 2012), and we have now shown that hyper-sumoylation of Gcn4 results in a dramatic decrease in Gcn4 occupancy on the Gcn4-targeted ARG1 gene. Both of these observations showed dependence on Cdk8, which, as a component of the Mediator complex, is recruited to promoters by Gcn4. At least two major forms of Mediator have been described, one which interacts with a Cdk8-containing module but not with RNAP II (Ebmeier and Taatjes 2010), and another that associates with RNAP II (Näär et al. 2002), suggesting that binding of Cdk8 or RNAP II to the Mediator is mutually exclusive. This has led some to propose that the Cdk8 module interacts with the promoter-bound Mediator only after RNAP II is released and engaged in elongation (Allen and Taatjes 2015). By this model, Cdk8 can access and phosphorylate promoter-bound Gcn4 only after successful activation of target genes. Our results now show that Cdk8-mediated phosphorylation of Gcn4 is reduced when Gcn4 cannot be sumoylated, suggesting that sumoylation of the DNA-bound Gcn4 dimer stimulates optimal phosphorylation by Cdk8. Cdk8-mediated phosphorylation of Gcn4, and consequent ubiquitination and degradation, is therefore directed at Gcn4 molecules that are bound by DNA (sumoylated) and that have already functioned in gene activation. This high level of regulation can serve to prevent mistargeting of unbound or unused Gnc4 molecules for phosphorylation-triggered ubiquitination-mediated proteasomal degradation.
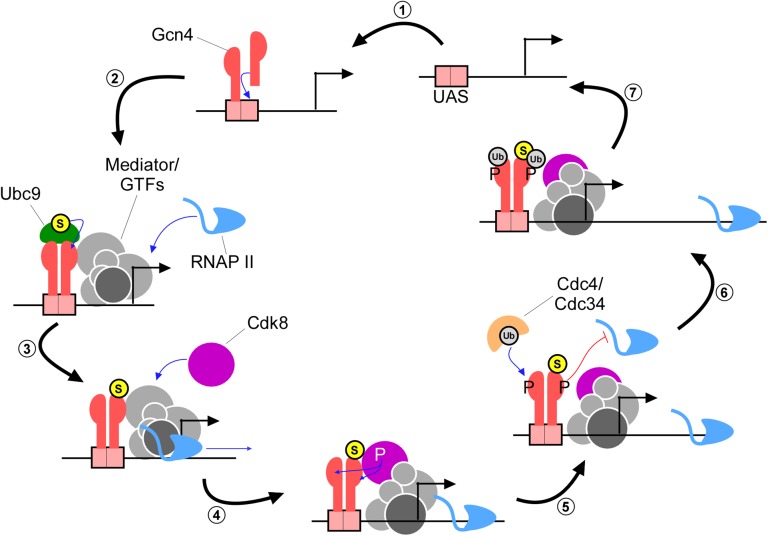
Our analysis provides key contributions to an emerging, highly detailed picture of how an important transcription factor is regulated once it binds DNA, which we summarize here as a model (Figure 7). As Gcn4 levels rise during amino acid starvation, individual Gcn4 molecules assemble as dimers on cognate DNA sites across the genome (Figure 7, step “1”) (Ellenberger et al. 1992; Natarajan et al. 2001; Guarnaccia et al. 2004). We propose that, although the DNA-bound Gcn4 dimer is then recognized by Ubc9, only one of the two Gcn4 subunits becomes sumoylated (“2”). This is supported by two observations. First, fractionation analysis shows that both unsumoylated and sumoylated Gcn4 are associated with chromatin (Rosonina et al. 2012). Second, both unsumoylated and sumoylated forms of Gcn4 were purified in an IP performed with a SUMO antibody, even under conditions where only tightly interacting proteins (such as monomers of a Gcn4 dimer) are expected to remain associated (>0.5 M NaCl; Figure S3B). This data points to the presence of Gcn4 dimers in which only one subunit is SUMO modified. Recruitment of Ubc9 might occur concurrently with gene activation (i.e., recruitment of coactivators), since, as we have shown, Gcn4 sumoylation and target gene activation are not dependent on each other (Rosonina et al. 2012; and present study). Through direct interaction between Gcn4 and Gal11 or other factors, the Mediator complex is recruited to target promoters, which facilitates assembly of the PIC (“3”; Swanson et al. 2003; Brzovic et al. 2011). As RNAP II is released from the PIC and engages in transcriptional elongation, as mentioned above, the Cdk8 module can then assemble with the remainder of the Mediator complex, where it can access Gcn4 (“4”; Allen and Taatjes 2015). Sumoylation significantly enhances phosphorylation of Gcn4 by Cdk8, and both subunits of the Gcn4 dimer are likely targeted by Cdk8, which is supported by our detection of phosphorylated SUMO-modified and unsumoylated forms of Gcn4 (“5”). Phosphorylated Gcn4, which has reduced ability to activate transcription (Lipford et al. 2005), is then cleared from promoters by subsequent ubiquitination and 26S proteasome-mediated degradation (“6” and “7”; Kornitzer et al. 1994; Meimoun et al. 2000; Chi et al. 2001). Once cleared of Gcn4, target genes can undergo further rounds of activation driven by additional Gcn4 molecules, or they can be shut off if Gcn4 levels are depleted, as by Pho85-mediated degradation when amino levels are restored.
Figure 7.
Model depicting how coordinated modifications regulate Gcn4 after it binds DNA. Refer to text for a detailed description. Encircled S represents SUMO modification, encircled Ub is ubiquitin, and P represents phosphorylation. The 26 S proteasome, which targets ubiquitinated Gcn4 for degradation, is not shown, but acts in step 7.
Gene-specific TFs represent one of the largest groups of SUMO targets in both yeast and mammals (Gill 2005; Makhnevych et al. 2009; Cubenas-Potts and Matunis 2013; Chymkowitch et al. 2015b). In many cases, mutations that impair sumoylation of these TFs result in increased activation of target genes, which has led to a general association of the SUMO mark with transcriptional repression (e.g., Gill 2005; Cheng et al. 2014; Ng et al. 2015; Sarkar et al. 2015). Different mechanisms have been proposed for explaining how the SUMO mark inhibits transcription, including sumoylation-mediated recruitment of histone deacetylases, and retention of sumoylated TFs in the cytoplasm (Yang et al. 2003; Morita et al. 2005). However, we propose that SUMO might have a more general, evolutionarily conserved, role in marking DNA-bound forms of TFs to limit their occupancy on chromatin, as we have seen with Gcn4. A consequence of blocking sumoylation of TFs, therefore, would be unrestricted association with chromatin, and increased expression of target genes, which might be interpreted as SUMO having a repressive effect on transcription of target genes. In support of the idea that SUMO functions to mark DNA-bound TFs, ChIP experiments have shown that sumoylated proteins are significantly enriched near promoters of transcriptionally active genes in both yeast and mammals (Rosonina et al. 2010; Liu et al. 2012; Neyret-Kahn et al. 2013; Chymkowitch et al. 2015a). Furthermore, supporting a role for SUMO in restricting TF association with DNA, recent studies examined effects of sumoylation on the human TFs MITF and c-Fos, and found that SUMO-impairing mutations resulted in significantly increased occupancy of both TFs on target genes, as well as elevated expression of these genes (Bertolotto et al. 2011; Tempe et al. 2014). It remains to be determined, however, whether SUMO imparts such an effect on its other numerous TF conjugates, and whether Cdk8, which shows genome-wide distribution, and phosphorylates many known gene-specific TFs (Andrau et al. 2006; Zhu et al. 2006; Poss et al. 2013), has a general role in preferentially targeting SUMO-conjugated TFs after they have functioned in gene activation. Nonetheless, our analysis has demonstrated a major novel role for SUMO in marking the DNA-bound form of a TF as a means to restrict its association with chromatin, and ensure that target gene expression levels are well controlled.
Acknowledgments
We thank David Bentley (University of Colorado), Kevin Struhl (Harvard Medical School) and Mike Tyers [Institute for Research in Immunology and Cancer (IRIC), Université de Montréal] for kindly sharing yeast strains. This work was supported by a Discovery Grant to E.R. from the Natural Sciences and Engineering Council of Canada (NSERC; grant number RGPIN-04208-2014).
Footnotes
Communicating editor: M. Hampsey
Supplemental material is available online at http://www.genetics.org/cgi/content/full/genetics.116.194134/DC1.
Literature Cited
- Allen B. L., Taatjes D. J., 2015. The Mediator complex: a central integrator of transcription. Nat. Rev. Mol. Cell Biol. 16: 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg D. C., D. J. Burke, and J. N. Strathern, 2006 Yeast RNA isolation: small-scale. CSH Protoc. 2006: pii: pdb.prot4155. [DOI] [PubMed]
- Andrau J.-C., van de Pasch L., Lijnzaad P., Bijma T., Koerkamp M. G., et al. , 2006. Genome-wide location of the coactivator mediator: binding without activation and transient Cdk8 interaction on DNA. Mol. Cell 22: 179–192. [DOI] [PubMed] [Google Scholar]
- Bertolotto C., Lesueur F., Giuliano S., Strub T., de Lichy M., et al. , 2011. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature 480: 94–98. [DOI] [PubMed] [Google Scholar]
- Brzovic P. S., Heikaus C. C., Kisselev L., Vernon R., Herbig E., et al. , 2011. The acidic transcription activator Gcn4 binds the mediator subunit Gal11/Med15 using a simple protein interface forming a fuzzy complex. Mol. Cell 44: 942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoensawan V., Martinho C., Wigge P. A., 2015. “Hit-and-run”: transcription factors get caught in the act. BioEssays 37: 748–754. [DOI] [PubMed] [Google Scholar]
- Cheng J., Huang M., Zhu Y., Xin Y.-J., Zhao Y.-K., et al. , 2014. SUMOylation of MeCP2 is essential for transcriptional repression and hippocampal synapse development. J. Neurochem. 128: 798–806. [DOI] [PubMed] [Google Scholar]
- Chi Y., Huddleston M. J., Zhang X., Young R. A., Annan R. S., et al. , 2001. Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev. 15: 1078–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chymkowitch P., Le May N., Charneau P., Compe E., Egly J.-M., 2011. The phosphorylation of the androgen receptor by TFIIH directs the ubiquitin/proteasome process. EMBO J. 30: 468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chymkowitch P., Nguéa A. P., Aanes H., Koehler C. J., Thiede B., et al. , 2015a Sumoylation of Rap1 mediates the recruitment of TFIID to promote transcription of ribosomal protein genes. Genome Res. 25: 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chymkowitch P., Nguéa P. A., Enserink J. M., 2015b SUMO-regulated transcription: challenging the dogma. BioEssays 37: 1095–1105. [DOI] [PubMed] [Google Scholar]
- Cubenas-Potts C., Matunis M. J., 2013. SUMO: a multifaceted modifier of chromatin structure and function. Dev. Cell 24: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis R. W., Maluf N. K., Yang Q., Lambert J. R., Bain D. L., 2015. Glucocorticoid receptor-DNA dissociation kinetics measured in vitro reveal exchange on the second time scale. Biochemistry 54: 5306–5314. [DOI] [PubMed] [Google Scholar]
- Desterro J. M., Rodriguez M. S., Hay R. T., 1998. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol. Cell 2: 233–239. [DOI] [PubMed] [Google Scholar]
- Drysdale C. M., Duenas E., Jackson B. M., Reusser U., Braus G. H., et al. , 1995. The transcriptional activator GCN4 contains multiple activation domains that are critically dependent on hydrophobic amino acids. Mol. Cell. Biol. 15: 1220–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebmeier C. C., Taatjes D. J., 2010. Activator-Mediator binding regulates Mediator-cofactor interactions. Proc. Natl. Acad. Sci. USA 107: 11283–11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger T. E., Brandl C. J., Struhl K., Harrison S. C., 1992. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted α helices: crystal structure of the protein-DNA complex. Cell 71: 1223–1237. [DOI] [PubMed] [Google Scholar]
- Falco S. C., Dumas K. S., 1985. Genetic analysis of mutants of Saccharomyces cerevisiae resistant to the herbicide sulfometuron methyl. Genetics 109: 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filtz T. M., Vogel W. K., Leid M., 2014. Regulation of transcription factor activity by interconnected post-translational modifications. Trends Pharmacol. Sci. 35: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith M. D., Donner A. J., Espinosa J. M., 2010. CDK8: a positive regulator of transcription. Transcription 1: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng F., Wenzel S., Tansey W. P., 2012. Ubiquitin and proteasomes in transcription. Annu. Rev. Biochem. 81: 177–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G., 2005. Something about SUMO inhibits transcription. Curr. Opin. Genet. Dev. 15: 536–541. [DOI] [PubMed] [Google Scholar]
- Grimaldi Y., Ferrari P., Strubin M., 2014. Independent RNA polymerase II preinitiation complex dynamics and nucleosome turnover at promoter sites in vivo. Genome Res. 24: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnaccia C., Raman B., Zahariev S., Simoncsits A., Pongor S., 2004. DNA-mediated assembly of weakly interacting DNA-binding protein subunits: in vitro recruitment of phage 434 repressor and yeast GCN4 DNA-binding domains. Nucleic Acids Res. 32: 4992–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S., Young E. T., 2011. Transcriptional regulation in Saccharomyces cerevisiae: transcription factor regulation and function, mechanisms of initiation, and roles of activators and coactivators. Genetics 189: 705–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruki H., Nishikawa J., Laemmli U. K., 2008. The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol. Cell 31: 925–932. [DOI] [PubMed] [Google Scholar]
- Herbig E., Warfield L., Fish L., Fishburn J., Knutson B. A., et al. , 2010. Mechanism of Mediator recruitment by tandem Gcn4 activation domains and three Gal11 activator-binding domains. Mol. Cell. Biol. 30: 2376–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietakangas V., Anckar J., Blomster H. A., Fujimoto M., Palvimo J. J., et al. , 2006. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc. Natl. Acad. Sci. USA 103: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G., 2005. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 59: 407–450. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G., Natarajan K., 2002. Gcn4p, a master regulator of gene expression, is controlled at multiple levels by diverse signals of starvation and stress. Eukaryot. Cell 1: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes B. C., LeBlanc J. F., Hawley D. K., 1992. Kinetic analysis of yeast TFIID-TATA box complex formation suggests a multi-step pathway. J. Biol. Chem. 267: 11539–11547. [PubMed] [Google Scholar]
- Hope I. A., Struhl K., 1985. GCN4 protein, synthesized in vitro, binds HIS3 regulatory sequences: implications for general control of amino acid biosynthetic genes in yeast. Cell 43: 177–188. [DOI] [PubMed] [Google Scholar]
- Irniger S., Braus G. H., 2003. Controlling transcription by destruction: the regulation of yeast Gcn4p stability. Curr. Genet. 44: 8–18. [DOI] [PubMed] [Google Scholar]
- Keaveney M., Struhl K., 1998. Activator-Mediated Recruitment of the RNA polymerase II machinery is the predominant mechanism for transcriptional activation in yeast. Mol. Cell 1: 917–924. [DOI] [PubMed] [Google Scholar]
- Kitazono A. A., Tobe B. T. D., Kalton H., Diamant N., Kron S. J., 2002. Marker-fusion PCR for one-step mutagenesis of essential genes in yeast. Yeast 19: 141–149. [DOI] [PubMed] [Google Scholar]
- Knop M., Siegers K., Pereira G., Zachariae W., Winsor B., et al. , 1999. Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast 15: 963–972. [DOI] [PubMed] [Google Scholar]
- Kornitzer D., Raboy B., Kulka R. G., Fink G. R., 1994. Regulated degradation of the transcription factor Gcn4. EMBO J. 13: 6021–6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickwar C. R., Mueller F., Hanlon S. E., McNally J. G., Lieb J. D., 2012. Genome-wide protein-DNA binding dynamics suggest a molecular clutch for transcription factor function. Nature 484: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipford J. R., Deshaies R. J., 2003. Diverse roles for ubiquitin-dependent proteolysis in transcriptional activation. Nat. Cell Biol. 5: 845–850. [DOI] [PubMed] [Google Scholar]
- Lipford J. R., Smith G. T., Chi Y., Deshaies R. J., 2005. A putative stimulatory role for activator turnover in gene expression. Nature 438: 113–116. [DOI] [PubMed] [Google Scholar]
- Liu C., Apodaca J., Davis L. E., Rao H., 2007. Proteasome inhibition in wild-type yeast Saccharomyces cerevisiae cells. Biotechniques 42: 158, 160, 162. [DOI] [PubMed] [Google Scholar]
- Liu H. W., Zhang J., Heine G. F., Arora M., Gulcin Ozer H., et al. , 2012. Chromatin modification by SUMO-1 stimulates the promoters of translation machinery genes. Nucleic Acids Res. 40: 10172–10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhnevych T., Sydorskyy Y., Xin X., Srikumar T., Vizeacoumar F. J., et al. , 2009. Global map of SUMO function revealed by protein-protein interaction and genetic networks. Mol. Cell 33: 124–135. [DOI] [PubMed] [Google Scholar]
- McNally J. G., Müller W. G., Walker D., Wolford R., Hager G. L., 2000. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science 287: 1262–1265. [DOI] [PubMed] [Google Scholar]
- Meimoun A., Holtzman T., Weissman Z., McBride H. J., Stillman D. J., et al. , 2000. Degradation of the transcription factor Gcn4 requires the kinase Pho85 and the SCF(CDC4) ubiquitin-ligase complex. Mol. Biol. Cell 11: 915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqtaderi Z., Geisberg J. V., Struhl K., 2014. Secondary structures involving the poly(A) tail and other 3′ sequences are major determinants of mRNA isoform stability in yeast. Microb. Cell 1: 137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y., Kanei-Ishii C., Nomura T., Ishii S., 2005. TRAF7 sequesters c-Myb to the cytoplasm by stimulating its sumoylation. Mol. Biol. Cell 16: 5433–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratani M., Kung C., Shokat K. M., Tansey W. P., 2005. The F box protein Dsg1/Mdm30 is a transcriptional coactivator that stimulates Gal4 turnover and cotranscriptional mRNA processing. Cell 120: 887–899. [DOI] [PubMed] [Google Scholar]
- Näär A. M., Taatjes D. J., Zhai W., Nogales E., Tjian R., 2002. Human CRSP interacts with RNA polymerase II CTD and adopts a specific CTD-bound conformation. Genes Dev. 16: 1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K., Meyer M. R., Jackson B. M., Slade D., Roberts C., et al. , 2001. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol. Cell. Biol. 21: 4347–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyret-Kahn H., Benhamed M., Ye T., Le Gras S., Cossec J. C., et al. , 2013. Sumoylation at chromatin governs coordinated repression of a transcriptional program essential for cell growth and proliferation. Genome Res. 23: 1563–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C. H., Akhter A., Yurko N., Burgener J. M., Rosonina E., et al. , 2015. Sumoylated controls the timing of Tup1-mediated transcriptional deactivation. Nat. Commun. 6: 6610.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Bruce C., Hart C., Leigh-Bell J., Gelperin D., et al. , 2009. Dynamic and complex transcription factor binding during an inducible response in yeast. Genes Dev. 23: 1351–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M., Scafe C., Sexton J., Young R., 1987. Eucaryotic RNA polymerase conditional mutant that rapidly ceases mRNA synthesis. Mol. Cell. Biol. 7: 1602–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson C., Cyr D. M., 2005. Ubiquitin-Proteasome Protocols. Humana Press, Totowa, NJ. [Google Scholar]
- Poss Z. C., Ebmeier C. C., Taatjes D. J., 2013. The Mediator complex and transcription regulation. Crit. Rev. Biochem. Mol. Biol. 48: 575–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries R., Bomeke K., Irniger S., Grundmann O., Braus G. H., 2002. Amino acid-dependent Gcn4p stability regulation occurs exclusively in the yeast nucleus. Eukaryot. Cell 1: 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal Y., Qiu H., Hinnebusch A. G., 2014. Accumulation of a threonine biosynthetic intermediate attenuates general amino acid control by accelerating degradation of Gcn4 via Pho85 and Cdk8. PLoS Genet. 10: e1004534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G., Hubner M. R., Metivier R., Brand H., Denger S., et al. , 2003. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol. Cell 11: 695–707. [DOI] [PubMed] [Google Scholar]
- Rosonina E., Duncan S. M., Manley J. L., 2010. SUMO functions in constitutive transcription and during activation of inducible genes in yeast. Genes Dev. 24: 1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosonina E., Duncan S. M., Manley J. L., 2012. Sumoylation of transcription factor Gcn4 facilitates its Srb10-mediated clearance from promoters in yeast. Genes Dev. 26: 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar K., Sadhukhan S., Han S.-S., Vyas Y. M., 2015. SUMOylation-disrupting WAS mutation converts WASp from a transcriptional activator to a repressor of NF-κB response genes in T cells. Blood 126: 1670–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer R., Meimoun A., Holtzman T., Kornitzer D., 2002. Regulation of the transcription factor Gcn4 by Pho85 cyclin PCL5. Mol. Cell. Biol. 22: 5395–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyueva D., Stampfel G., Stark A., 2014. Transcriptional enhancers: from properties to genome-wide predictions. Nat. Rev. Genet. 15: 272–286. [DOI] [PubMed] [Google Scholar]
- Sriramachandran A. M., Dohmen R. J., 2014. SUMO-targeted ubiquitin ligases. Biochim. Biophys. Acta 1843: 75–85. [DOI] [PubMed] [Google Scholar]
- Sundqvist A., Ericsson J., 2003. Transcription-dependent degradation controls the stability of the SREBP family of transcription factors. Proc. Natl. Acad. Sci. USA 100: 13833–13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. J., Qiu H., Sumibcay L., Krueger A., Kim S., et al. , 2003. A multiplicity of coactivators is required by Gcn4p at individual promoters in vivo. Mol. Cell. Biol. 23: 2800–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey W. P., 2001. Transcriptional activation: risky business. Genes Dev. 15: 1045–1050. [DOI] [PubMed] [Google Scholar]
- Tempe D., Vives E., Brockly F., Brooks H., De Rossi S., et al. , 2014. SUMOylation of the inducible (c-Fos:c-Jun)/AP-1 transcription complex occurs on target promoters to limit transcriptional activation. Oncogene 33: 921–927. [DOI] [PubMed] [Google Scholar]
- Thomas M. C., Chiang C.-M. M., 2006. The general transcription machinery and general cofactors. Crit. Rev. Biochem. Mol. Biol. 41: 105–178. [DOI] [PubMed] [Google Scholar]
- van Werven F. J., van Teeffelen H. A., Holstege F. C., Timmers H. T., 2009. Distinct promoter dynamics of the basal transcription factor TBP across the yeast genome. Nat. Struct. Mol. Biol. 16: 1043–1048. [DOI] [PubMed] [Google Scholar]
- Vernimmen D., Bickmore W. A., 2015. The hierarchy of transcriptional activation: from enhancer to promoter. Trends Genet. 31: 696–708. [DOI] [PubMed] [Google Scholar]
- Wang X., Muratani M., Tansey W. P., Ptashne M., 2010. Proteolytic instability and the action of nonclassical transcriptional activators. Curr. Biol. 20: 868–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield L., Tuttle L. M., Pacheco D., Klevit R. E., Hahn S., 2014. A sequence-specific transcription activator motif and powerful synthetic variants that bind Mediator using a fuzzy protein interface. Proc. Natl. Acad. Sci. USA 111: E3506–E3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. H., Jaffray E., Hay R. T., Sharrocks A. D., 2003. Dynamic interplay of the SUMO and ERK pathways in regulating Elk-1 transcriptional activity. Mol. Cell 12: 63–74. [DOI] [PubMed] [Google Scholar]
- Yao Q., Li H., Liu B. Q., Huang X. Y., Guo L., 2011. SUMOylation-regulated protein phosphorylation, evidence from quantitative phosphoproteomics analyses. J. Biol. Chem. 286: 27342–27349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Wirén M., Sinha I., Rasmussen N. N., Linder T., et al. , 2006. Genome-wide occupancy profile of mediator and the Srb8–11 module reveals interactions with coding regions. Mol. Cell 22: 169–178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. All yeast strains and plasmids are available upon request.