Abstract
B-type cyclins promote mitotic entry and inhibit mitotic exit. In Saccharomyces cerevisiae, four B-type cyclins, Clb1–4, carry out essential mitotic roles, with substantial but incomplete overlap of function among them. Previous work in many organisms has indicated that B-type cyclin-dependent inhibition of mitotic exit imposes a requirement for mitotic destruction of B-type cyclins. For instance, precise genomic removal of the Clb2 destruction box (D box) prevents mitotic proteolysis of Clb2, and blocks mitotic exit. Here, we show that, despite significant functional overlap between Clb2 and Clb3, D-box-dependent Clb3 proteolysis is completely dispensable for mitotic exit. Removal of the Clb3 D box results in abundant Clb3 protein and associated kinase throughout the cell cycle, but mitotic exit occurs with close to normal timing. Clb3 degradation is required for pre-Start G1 control in the succeeding cell cycle. Deleting the CLB3 D box essentially eliminates all time delay before cell cycle Start following division, even in very small newborn cells. CLB3∆db cells show no cell cycle arrest response to mating pheromone, and CLB3∆db completely bypasses the requirement for CLN G1 cyclins, even in the absence of the early expressed B-type cyclins CLB5,6. Thus, regulated mitotic proteolysis of Clb3 is specifically required to make passage of Start in the succeeding cell cycle “memoryless”—dependent on conditions within that cycle, and independent of events such as B-type cyclin accumulation that occurred in the preceding cycle.
Keywords: Clb3, destruction box, mitotic exit, G1 cyclin control, Start
THE eukaryotic cell cycle is regulated by cyclin-dependent kinases (CDKs) bound to cyclins (Bloom and Cross 2007). In Saccharomyces cerevisiae, the Cdc28 CDK binds to nine cyclins: G1 cyclins Cln1-3; S phase B-type cyclins Clb5,6; and mitotic cyclins Clb1–4. These cyclins have sharply different biological functions: cln1,2,3 null strains have an absolute defect in cell cycle initiation (“Start”) but no defect in any post-Start events (DNA replication, spindle assembly, nuclear division, and cytokinesis) (Richardson et al. 1989; Cross 1990). In contrast, clb1,2,3,4 null strains execute Start and DNA replication, but fail to execute cell division (Fitch et al. 1992; Richardson et al. 1992).
Specificity of cyclin function can derive from differential regulation at many levels: cyclin abundance or subcellular localization, response to inhibitors, degree of activation of Cdc28 kinase activity, and cyclin-specific substrate targeting by docking motifs (Loog and Morgan 2005; Bloom and Cross 2007; Kõivomägi et al. 2011). These diverse controls may be coordinated to regulate the overall temporal pattern of specific CDK activity. On the other hand, deletion of many cyclin genes leads to, at most, minor defects. Thus, cyclin specificity is a strong, but not absolute, determinant of function (Roberts 1999; Bloom and Cross 2007).
B-type cyclins are essential for entry into mitosis; subsequent mitotic exit (cytokinesis, telophase, and resetting the system to G1 in newborn cells) requires mitotic cyclin degradation (Murray and Kirschner 1989; Murray et al. 1989; King et al. 1996). Degradation requires cyclin ubiquitination by the anaphase-promoting complex (APC), targeted by the cyclin destruction box (D box) or KEN box motifs (Glotzer et al. 1991; Pfleger and Kirschner 2000). Consistent with the requirement for mitotic cyclin degradation for mitotic exit, precise genomic removal of the D box and KEN boxes from the budding yeast mitotic cyclin Clb2 caused a first-cycle block to mitotic exit (Wäsch and Cross 2002).
The ability of mitotic B-type cyclins to both induce mitotic entry and block mitotic exit may tightly couple many aspects of cell cycle progression to once-per-CDK-cycle (Nasmyth 1996). As B-type cyclin-CDK activity rises, mitotic entry is induced, but exit is suppressed; upon B-type cyclin degradation, no further mitotic entry events occur, but mitotic exit is allowed (Nasmyth 1996). Systematic variation in “locked” levels of the Clb2 mitotic cyclin led to the need to revise this “ratchet” model to include a key role for the regulated Cdc14 phosphatase (Drapkin et al. 2009). Cdc14 activation, in turn, is under partially autonomous oscillatory control, requiring a mechanism for oscillator coordination (Lu and Cross 2010).
The CLB1/2 and CLB3/4 gene pairs are highly similar, but the CLB3/4 vs. CLB1/2 divergence is ancient (Archambault et al. 2005). Of CLB1-4, clb2 deletion led to the most extreme phenotypes; CLB3 has mitotic functions partially overlapping with CLB2 (Fitch et al. 1992; Richardson et al. 1992). Clb3 and Clb2 are similarly abundant through the cell cycle (Cross et al. 2002), but differ in activity toward diverse substrates (Kõivomägi et al. 2011).
Clb3 is degraded upon mitotic exit in parallel with Clb2 (Cross et al. 2002). Removal of the Clb2 D box results in failure of mitotic exit and consequent lethality (Wäsch and Cross 2002). Here, we characterize the requirement for the Clb3 D box for proteolytic regulation and for cell cycle control.
Materials and Methods
Strains and plasmids
Standard methods were used for transformation, mating, and tetrad analysis. All strains were derivatives of W303. All strains with CLB3∆db were generated using HO-induced exact gene replacement of the CLB3 allele (Cross and Pecani 2011).
Construction of clb1clb2CLB3∆db required some more complex procedures. We crossed a clb1clb2::GALL-CLB2-URA3clb6::kanMX strain with a MATainc GAL-HOCLB3∆db-URA3-CLB3clb4::HIS3 strain on a YEPD plate to keep GAL-HO inactive, then dissected tetrads on galactose medium to simultaneously maintain viability of segregants bearing clb1clb2::GALL-CLB2, and induce cleavage of the HO cut site to obtain CLB3∆db recombinants. (clb6:kanMX was used in the experiment for technical convenience because of its tight linkage to the unmarked clb1 deletion; previous findings (Epstein and Cross 1992; Fitch et al. 1992; Richardson et al. 1992; Schwob and Nasmyth 1993; Cross et al. 1999, 2002) make it unlikely that clb6 deletion has a significant effect on these results.) We identified strains that were MATainc GAL-HOCLB3∆db clb1clb2::GALL-CLB2clb6::kanMX (clb6 was maintained due to linkage with clb1).
Elutriation
Elutriation was carried out in a Beckman J-6M centrifuge at 3000 rpm and 4°. YEPD cultures (1 liter, OD660 ≈ 0.4) were collected by filtration, resuspended in 100 ml water, and sonicated in three 1-min intervals at power setting “4” in a Misonix XL2020 sonicator. Cell suspension fractions were collected after 10% increases in pump speed. Half of each cell fraction was fixed with 70% EtOH for subsequent flow cytometric analysis; the other half was used for protein preparation for Western blotting.
Western blotting and kinase activity assays
Western blotting was carried out using standard methods. Anti-Pgk1 (Invitrogen) and anti-Clb2 (Covance) were used at 1:10,000 dilution. Peroxidase anti-peroxidase complex (Sigma P1291) for detection of Protein A-tagged proteins was used at 1:5000 dilution. Anti-mouse and anti-rabbit secondary antibodies (GE) were used at 1:5000 dilution.
For kinase activity assays, cells were washed once in LSHN (50 mM NaCl, 10 mM HEPES pH 7.5, 10% Glycerol). Glass beads (400 μl) and 400 μl LSHNN (LSHN + 0.1% NP-40) with protease and phosphatase inhibitors were added, and cells were broken with a FastPrep FP120 Cell Disruptor (Thermo Electron Corp.) in two 20 sec intervals at setting 5. Cell lysates were centrifuged for 2 min, and the supernatant was incubated with 30 μl Rabbit IgG-Agarose beads (Sigma A2909) for 1 hr at 4°. Bound beads were washed four times with LSHNN, once with HNN (250 mM NaCl, 10 mM HEPES pH 7.5, 10% Glycerol, 0.1% NP-40), once with kinase buffer (10 mM HEPES pH 7.5, 10 mM MgCl2, 1 mM DTT), and resuspended in 60 μl kinase buffer. The kinase activity assay was carried out essentially as described by Levine et al. (1996). Histone H1 radioactivity was detected using a Typhoon 9400 variable imager (Amersham Biosciences). Both Western blot and kinase activity images were quantitated using ImageJ software (Schneider et al. 2012; Schindelin et al. 2015).
Time-lapse and fixed cell microscopy
Time-lapse and fixed cell microscopy were carried out essentially as previously described (Di Talia et al. 2007; Oikonomou and Cross 2011; Rahi et al. 2016). Fixed cell images were acquired with Micro-Manager software (Edelstein et al. 2010, 2014). The flow cell experiments were performed using the ONIX Microfluidic Perfusion System (CellASIC) with a Leica DMI6000B inverted fluorescence microscope. Cell segmentation and quantification were carried out with custom Matlab software as in Rahi et al. (2016).
Flow cytometry and cell size measurements
For flow cytometry measurements, cells were fixed in 70% ethanol, stained with propidium iodide (PI), and analyzed as described (Epstein and Cross 1992) using a BD FACSCalibur, or a BD Accuri C6 instrument (Becton Dickinson). Cell size was measured using a Z2 Coulter Cell and Particle Counter (Beckman Coulter), and analyzed with Z2 AccuComp software (Beckman Coulter).
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Strains and data used in this study are available upon request.
Results
The Clb3 D box is not required for cell cycle progression
All mitotic cyclins in S. cerevisiae contain a nine amino acid D box, which serves as a target for ubiquitination and subsequent proteolysis toward the end of mitosis (Glotzer et al. 1991; Fitch et al. 1992; Richardson et al. 1992). Removal of the D box from CLB2 effectively prevents Clb2 degradation (Wäsch and Cross 2002). CLB2∆db cells are not viable without overexpression of the Clb-CDK inhibitor Sic1. Turning off SIC1 expression in CLB2∆db GAL-SIC1 strains leads to arrest at mitotic exit: long mitotic spindles with separated chromosomes, without cytokinesis or rereplication of DNA (Wäsch and Cross 2002).
Clb3 and Clb2 overlap functionally (see Introduction); also, Clb3 and Clb2 oscillate nearly in phase in wild-type cells, and have similar abundance (Cross et al. 2002). Thus, removal of the D box from CLB3 could have similar results as with CLB2. We employed GAL-HO mediated exact gene replacement in a novel method to construct an exact endogenous replacement of the wild-type CLB3 allele with an allele lacking the D box sequence RVALSRVTN (Cross and Pecani 2011). This method allows recovery of CLB3∆db or CLB3 in individual cells (depending on crossover point) without selection, and efficiently detects lethal recombinants. Contrary to expectation, fully viable recombinants bearing the CLB3∆db allele were readily recovered. Here, we characterize the phenotype of these cells.
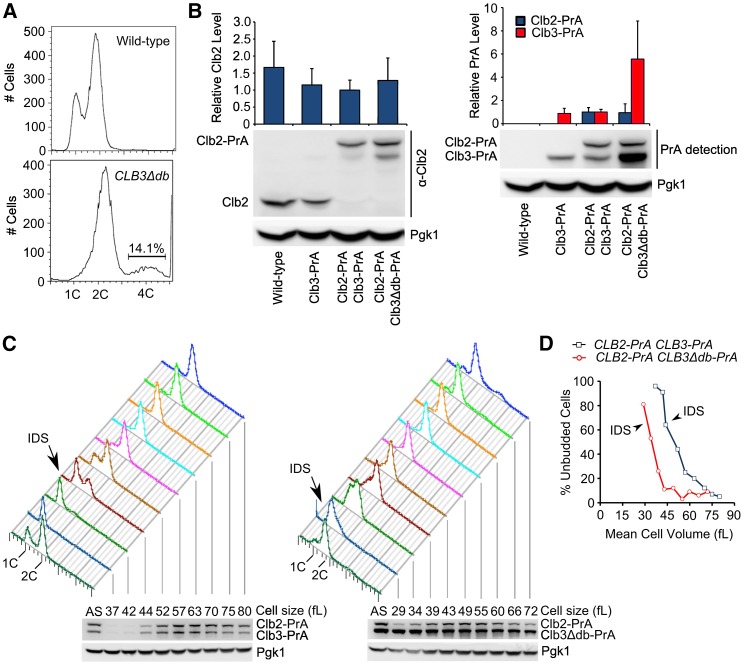
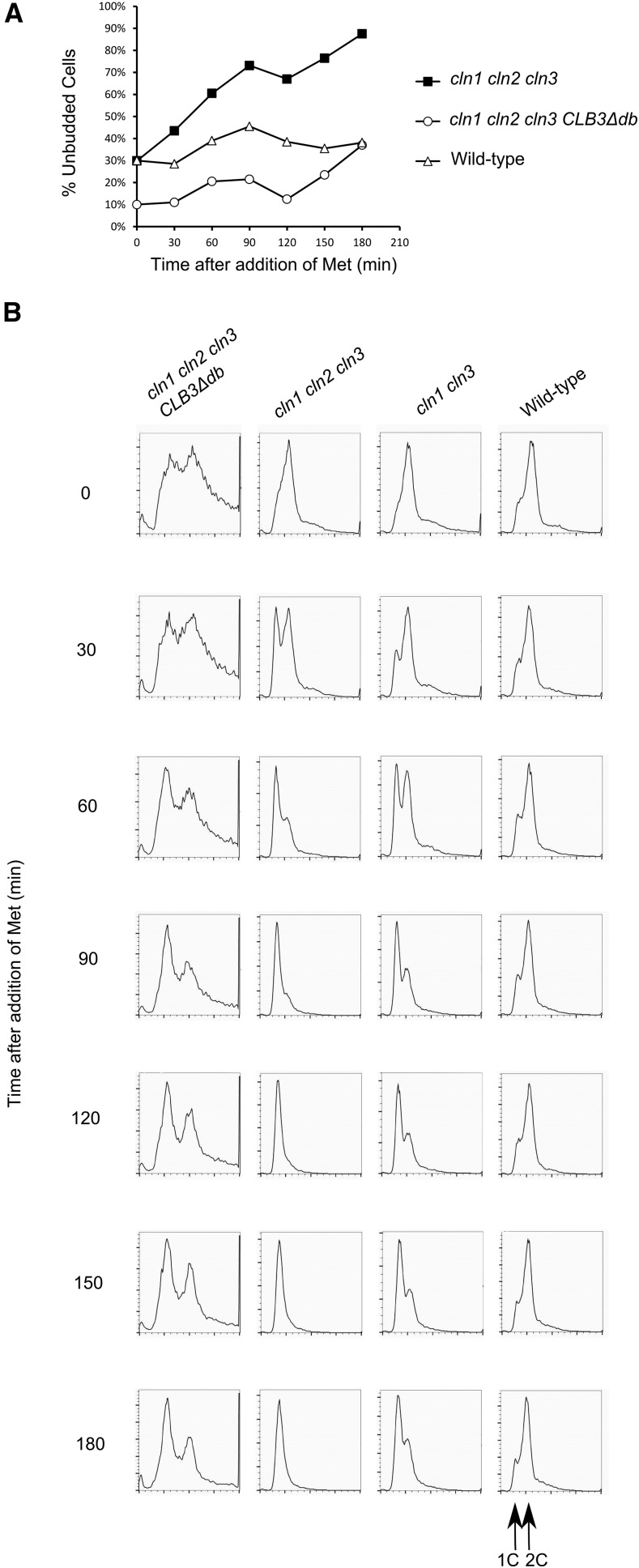
DNA flow cytometry profiles from asynchronous CLB3∆db cultures are depleted of 1C DNA and enriched for 4C (Figure 1A). However, microscopy showed that the average number of nuclei per cell body (mother or bud), was about 0.67 for both wild type and CLB3∆db, indicating normal coordination of budding and nuclear division. In the CLB3∆db population, aberrant cells with >2 cell bodies were observed with frequency similar to the 4C FACS signal (13% vs. 14%). Therefore, CLB3∆db likely causes a delay in cytokinesis or cell separation. If both daughter nuclei in a conjoined pair replicated DNA, 1C cells would be rare, and replication would result in a 4C FACS signal. Since most cells are 2C, any such delay must be transient.
Figure 1.
DNA content and protein abundance in asynchronous and elutriated cultures of CLB3∆db cells. (A) Representative FACS profiles of wild-type and CLB3∆db strains in log-phase asynchronous culture. (B) Clb2 and Protein A immunoblots from asynchronous cultures of Clb3-PrA Clb2-PrA and Clb3∆db-PrA Clb2-PrA strains (standardized to Pgk1, then to PrA signal in Clb3-PrA Clb2-PrA strain). Error bars are SD from two parallel Western blots with the same strains. (C) DNA content and Protein A level in elutriation fractions of Clb3-PrA Clb2-PrA and Clb3∆db-PrA Clb2-PrA strains. DNA content was determined by flow cytometric analysis. Protein levels in elutriation fractions, as shown in the immunoblot, were normalized to Pgk1. The first sample in FACS profiles and immunoblot (denoted by “AS”) is from asynchronous culture taken immediately before fractionation by elutriator. (D) Percent unbudded cells vs. mean cell volume in each elutriation fraction. Percentage of unbudded cells was determined by visual microscopic examination of a subset of each fraction (∼200 cells). Mean cell volume was measured by Coulter cell size analyzer. IDS, Initiation of DNA synthesis [as determined by flow cytometric analysis of DNA content; shown in (C)].
To test for cytokinesis delay, we measured the time of Myo1 disappearance relative to Whi5 nuclear entry. Myo1 forms a ring at the bud neck, which disappears at the completion of cytokinesis (Bi et al. 1998); the Whi5 transcriptional repressor enters the nucleus 6 min before disappearance of the Myo1 ring (Figure 2; Di Talia et al. 2007). We confirmed this measurement, and found it to be identical in CLB3 and in CLB3∆db cells (Table 1, T0). Therefore, the 4C peak in CLB3∆db FACS is likely due to a transient delay in cell separation after cytokinesis. This delay will reduce the 1C population, and increase 4C, if replication completes before cell separation.
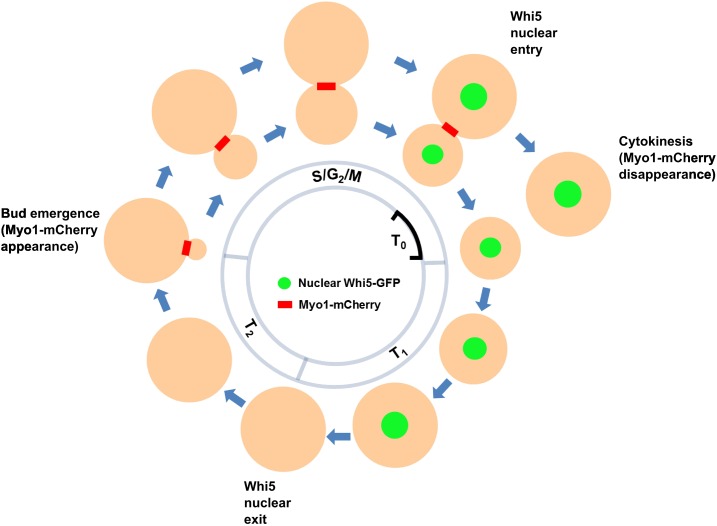
Figure 2.
Illustration of cell cycle periods T0, T1, T2, G1, and S/G2/M. Periods T1 and T2 were defined by Di Talia et al. (2007). T0 is defined in this study as the time between Whi5 nuclear entry and Myo1-mCherry disappearance from the bud neck (as shown).
Table 1. Cell cycle timing in daughter cells.
| Strain | T0 (min) | T1 (min) | T2 (min) | S/G2/M (min) |
|---|---|---|---|---|
| Wild type | 6 ± 2 (50) | 19 ± 10 (50) | 17 ± 9 (50) | 53 ± 8 (50) |
| CLB3∆db | 6 ± 2 (50) | 0 ± 1 (50) | 18 ± 10 (50) | 71 ± 16 (50) |
Times are mean ± SD. Number of cells scored in parentheses. We define T0 as the time from Whi5 entry to cytokinesis (Myo1 ring disappearance) (Figure 2); T1, time from Myo1 ring disappearance to Whi5 exit; T2, time from Whi5 exit to bud emergence (Di Talia et al. 2007); S/G2/M, time from Myo1 ring appearance at incipient bud neck to disappearance at cytokinesis. The frame resolution was 3 min.
The Clb3 D box regulates Clb3 protein abundance
To determine if the Clb3 D box was required for cell-cycle-dependent Clb3 proteolysis, we employed the same gene replacement method in a strain containing a C-terminal Protein A epitope tag on the endogenous CLB3 [PrA-tagged Clb3 is fully functional (Cross et al. 2002)]. We then compared protein abundance of endogenously expressed Clb3-PrA to Clb3∆db-PrA. We detected Protein A with IgG, and Clb2 with specific antibodies, in extracts from asynchronous cultures of CLB3∆db-PrA CLB2-PrA, CLB3-PrA CLB2-PrA, CLB3-PrA, and CLB2CLB3 strains (Figure 1B). Clb3-PrA and Clb2-PrA migrate to different gel positions and can be detected simultaneously by PrA detection, allowing direct comparison of Clb2 and Clb3 levels in doubly tagged cells (Cross et al. 2002). Clb3-PrA and Clb2-PrA levels were comparable (Figure 1B, this study; Cross et al. 2002). Clb3∆db-PrA abundance, however, was almost sixfold higher than Clb3-PrA or Clb2-PrA (Figure 1B). To follow Clb3∆db levels through the cell cycle, we fractionated cells according to cell size by elutriating log-phase CLB3∆db-PrA CLB2-PrA and CLB3-PrA CLB2-PrA cultures (Figure 1C). Small newborn G1 cells contain almost no Clb2 or Clb3, which accumulate in parallel with respect to time of DNA replication and budding. In contrast, Clb3∆db-PrA is present throughout the cell cycle at a level ∼3- to 10-fold higher than the peak level of Clb2-PrA. This defect in regulation of Clb3-PrA levels was not due to global failure of cyclin proteolysis, since Clb2-PrA in the same cells was degraded approximately normally.
In both CLB3-PrA and CLB3∆db-PrA elutriated cultures, the smallest cells had 1C DNA content, with increasing proportions of 2C observed in progressively larger cell fractions. CLB3∆db-PrA cultures showed 2C cells in fractions collected at significantly smaller cell size than CLB3-PrA, indicating initiation of DNA replication at smaller cell size in CLB3∆db-PrA cells. Essentially identical results were obtained in a repeat experiment with the same strains, and in an experiment with strains lacking the Protein A tag (data not shown). CLB3∆db cells (with or without the Protein A tag) also showed budding at significantly smaller cell size than CLB3 controls (Figure 1D).
The implications of apparent loss of size control over DNA replication and budding in CLB3∆db strains are pursued in more detail below.
Clb3-associated protein kinase activity persists through mitotic exit in the absence of the Clb3 D box
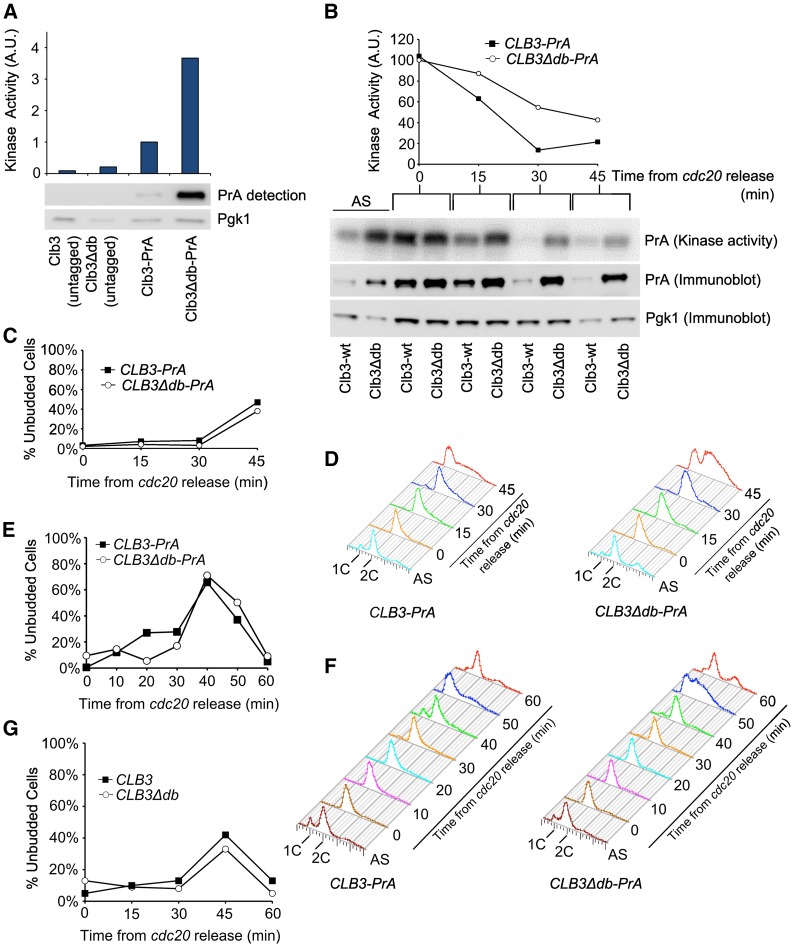
It was surprising to observe high levels of Clb3∆db apparently persisting throughout the cell cycle, given the ability of undegradable Clb2 to block mitotic exit (Wäsch and Cross 2002). Both Clb2 and Clb3 function by activating the Cdc28 protein kinase (Bloom and Cross 2007), so we considered the possibility that Clb3∆db might be specifically defective in Cdc28 activation. CLB3∆db-PrA, and CLB3-PrA strains were grown to log-phase, and protein extracts were incubated with IgG beads to purify Clb3-PrA complexes. Kinase activity was then assayed by phosphorylation of histone H1 (Figure 3A). Kinase activity in the CLB3∆db-PrA strain was significantly higher than in the CLB3-PrA strain (Figure 3A), although this difference was less pronounced than the difference in Clb3 protein levels (Figure 1B). Clb3∆db could have an intrinsic partial defect in activating Cdc28; alternatively, high levels of Clb3∆db might result in limitation for free Cdc28 or other components. Cdc28 is present in excess of cyclins (Cross et al. 2002), but not by a large factor, so Cdc28 may be limiting in CLB3∆db cells.
Figure 3.
Clb3∆db-associated kinase activity, DNA content, and budding in asynchronous cultures, and after release from MET-CDC20 block. (A) Kinase activity in IgG pull-downs from asynchronous cultures. (B) Kinase activity in IgG pull-downs from MET-CDC20 block/release time-course with CLB3-PrA and CLB3db∆PrA strains. Immunoblot is from total cell lysate before IgG pull-down. (C) Percent unbudded cells following cdc20 release [same strains as in (B)]. Percentage of unbudded cells was determined by visual microscopic examination of a subset (∼200 cells) of samples taken at each timepoint. (D) DNA content in same samples as in (B), as measured by flow cytometric analysis. (E) Timecourse in (B), (C), (D) was repeated with 10 min timepoints to more accurately determine division time. (F) DNA content, as measured by flow cytometric analysis, is also shown for the repeat timecourse in (E). (G) Percent unbudded cells following cdc20 release in strains without the PrA tag.
If Clb3∆db or its associated kinase activity was low specifically during mitotic exit, this could result in viability of CLB3∆db cells despite these cells containing high levels of Clb3∆db-associated kinase activity through most of the cell cycle. To examine Clb3∆db levels, and associated kinase activity, specifically during exit from mitosis, we introduced CLB3∆db-PrA and CLB3-PrA into a MET-CDC20 background. MET-CDC20 places the essential APC activator Cdc20 under methionine control, allowing a rapidly reversible block to mitotic exit (Yeong et al. 2000). Methionine was added to log-phase cultures to turn off MET-CDC20 expression and block cells in metaphase, then methionine was removed to release the block (Figure 3, B–G). Clb3 protein and associated kinase activity were comparably high at the block in CLB3-PrA and CLB3∆db-PrA strains (Figure 3B). After release, both declined sharply in the CLB3-PrA strain. Although kinase activity in the CLB3∆db-PrA strains decreased by 50% 45 min after the release from MET-CDC20 block, it was significantly higher than in the CLB3-PrA strain throughout the time-course, and about half the level in CLB3-PrA at the cdc20 block [which is very likely elevated significantly over the normal peak level due to continued synthesis without degradation (Drapkin et al. 2009)]. Despite persistence of substantial Clb3∆db-associated kinase activity after release of the cdc20 block, cell division (indicated by accumulation of unbudded cells; Figure 3C) was nearly identical in CLB3∆db-PrA and CLB3-PrA strains. This result was confirmed in strains without the PrA tag (Figure 3G).
In the CLB3-PrA strain, accumulation of unbudded cells was associated with transition from a uniform 2C FACS peak to accumulation of mostly cells with 1C DNA content; subsequently this 1C peak transited to 2C, consistent with DNA replication in the succeeding cell cycle (Figure 3, C and D). This picture was less clear in the CLB3∆db-PrA strain, where a significant 2C population was present at all time points (also apparent in the longer time-course; Figure 3F). This could have indicated some mitotic delay; however, the same cell populations showed almost exactly comparable cell division and rebudding as wild type, as indicated by the proportion of unbudded cells (Figure 3C). The lack of strong accumulation of a 1C peak is likely due to a very short G1 period, consistent with the analysis of elutriated asynchronous cultures above (Figure 1, C and D), and with the analysis of Whi5 nuclear residence times below (Figure 7 and Figure 8).
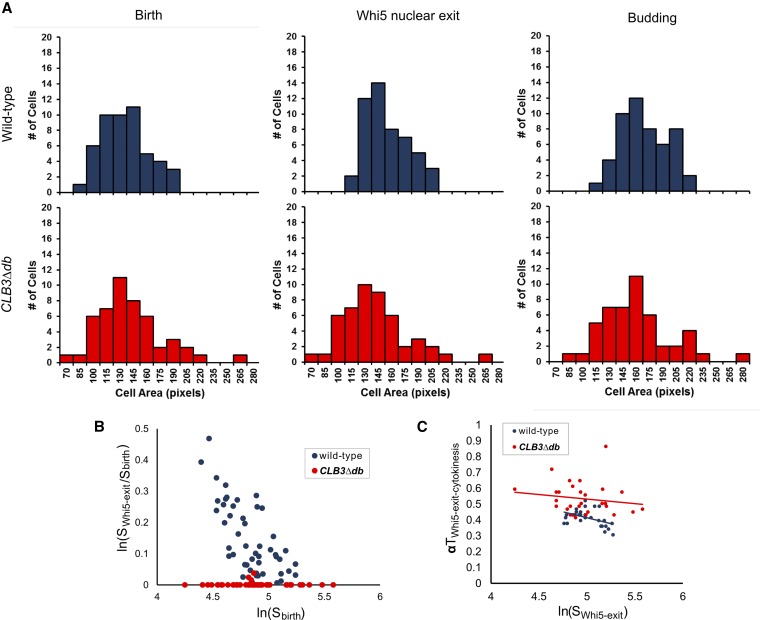
Figure 7.
Testing for cell size control in wild-type and CLB3∆db cells. (A) Distribution of cell areas in wild-type and CLB3∆db cells at birth, time of Whi5 nuclear exit, and time of budding, as measured from time-lapse microscopy. (B) Log relative growth [ln(SWhi5-exit/Sbirth)] vs. log of cell size at birth [ln(Sbirth)] in wild-type and CLB3∆db cells. (C) αT (time from Whi5 nuclear exit to cytokinesis multiplied by growth rate) vs. log of cell area at time of Whi5 nuclear exit.
Figure 8.
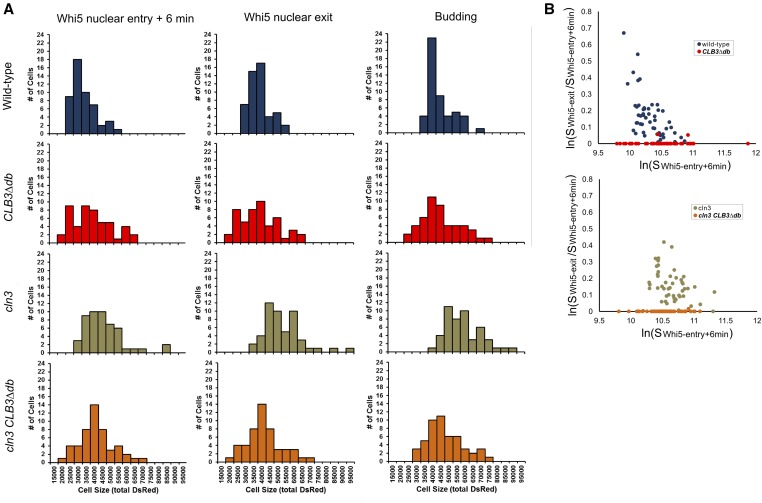
Analysis of cell size control in wild-type, CLB3∆db, cln3, and cln3 CLB3∆db cells. (A) Distribution of cell sizes (total DsRed) in wild-type, CLB3∆db, cln3, and cln3 CLB3∆db cells at birth (estimated as time of Whi5 nuclear entry + 6 min), time of Whi5 nuclear exit, and time of budding. (B) Log relative growth [ln(SWhi5-exit/SWhi5-entry+6min)] vs. log of cell size at birth [ln(SWhi5-entry+6min)] in wild-type, CLB3∆db, cln3, and cln3 CLB3∆db cells.
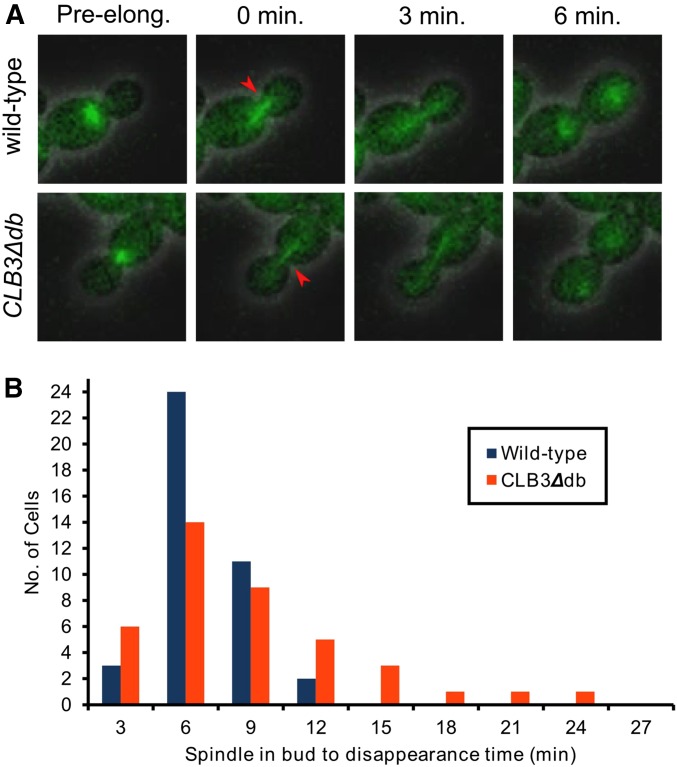
To detect possible mitotic delay at the single-cell level, specifically during anaphase and telophase, we used time-lapse microscopy of log-phase CLB3∆db and CLB3 strains with GFP-tagged tubulin (TUB1-GFP). We recorded the time from spindle entry into the bud (AE) to its disappearance (AD) as an estimate for the length of anaphase. Average AE to AD time was ∼7 min in wild-type and ∼9 min in CLB3∆db cells (Figure 4). There was, however, much more variability in AE to AD times in CLB3∆db cells compared to wild type, with CLB3∆db cells having more short (3 min) and long (>12 min) anaphase times (Figure 4).
Figure 4.
Measurement of anaphase length in wild-type and CLB3∆db cells using time-lapse microscopy. (A) Representative TUB1-GFP images. Length of anaphase was scored as entry of spindle into the bud (AE) to spindle disappearance (AD). Red arrows indicate newly formed spindle. (B) Distribution of anaphase times (AE to AD) in wild-type and CLB3∆db cells.
In summary, while the Clb3 D box is needed to provide regulation of Clb3 protein and associated kinase activity, absence of this regulation does not impede mitotic exit by more than a short interval.
Fully functional Cdc14 is required for viability in the absence of the Clb3 D box
Mitotic exit is likely dependent on the balance of Clb-Cdc28 kinase activity and the antagonistic Cdc14 phosphatase activity on mitotic targets (Drapkin et al. 2009). Mitotic exit can be blocked when relevant targets are kept above a phosphorylation threshold by high Clb-Cdc28 activity, and allowed when Cdc14 dephosphorylates these targets below the critical threshold (Drapkin et al. 2009). If Clb3∆db and Cdc14 share at least one common target, the dephosphorylation of which is required for mitotic exit, then, according to this model, mitotic exit will only be allowed if the increased kinase activity from Clb3∆db is still not sufficiently high to overcome dephosphorylation of the relevant target(s) by Cdc14. If the activity of Cdc14 is reduced, however, the phosphatase/kinase ratio might be decreased enough to bring the phosphorylation of the relevant target(s) above the threshold required for mitotic exit, so mitotic exit is blocked.
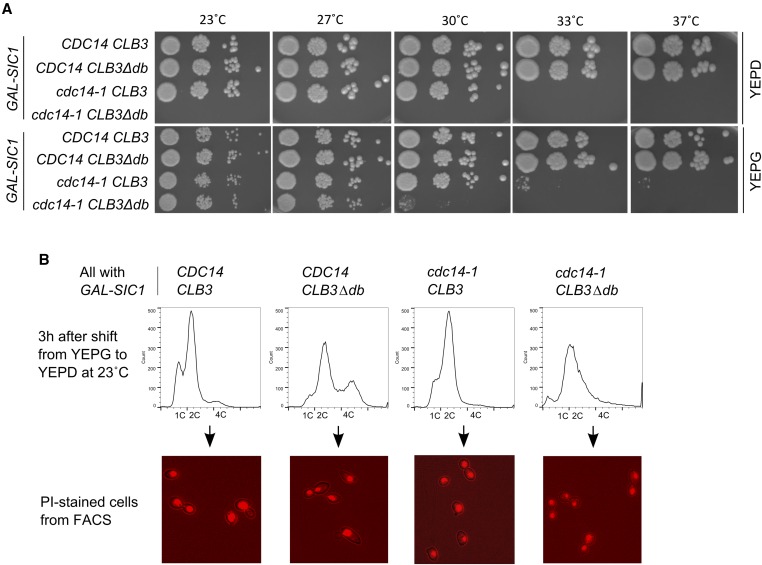
To test for viability of CLB3∆db cells with lowered Cdc14 activity, we used the temperature-sensitive allele cdc14-1 (Hartwell et al. 1974), which can be partially rescued at nonpermissive temperature by overexpressed SIC1 (Jaspersen et al. 1998; Yuste-Rojas and Cross 2000). We made 10× serial dilutions of CDC14CLB3, CDC14CLB3∆db, cdc14-1 CLB3, and cdc14-1 CLB3∆db cells (all bearing GAL-SIC1) on YEP-Glucose (“YEPD”; GAL off) and YEP-Galactose (“YEPG”; GAL on) plates, and placed them at various temperatures (Figure 5A). Because the product of cdc14-1 is temperature-sensitive, its activity should decrease with increasing temperature, and it may not be fully functional even at permissive temperature.
Figure 5.
Characterization of cdc14-1 CLB3∆db cells. (A) 10× serial dilutions of CDC14 CLB3, CDC14 CLB3∆db, cdc14-1 CLB3, and cdc14-1 CLB3∆db cells (all with GAL-SIC1) on YEP-Glucose (“YEPD”; GAL off) and YEP-Galactose (“YEPG”; GAL on) plates at various temperatures. (B) Top: DNA content measured 3 hr after shift from YEPG to YEPD medium at 23° (GAL-SIC1 off). Bottom: microscopic images of cells used for flow cytometry. Nuclei stained with PI.
Viability of GAL-SIC1CDC14CLB3 and GAL-SIC1CDC14CLB3∆db cells was unaffected by temperature or growth medium (Figure 5A). GAL-SIC1cdc14-1 CLB3 cells were viable up to 30° with GAL-SIC1 off (YEPD), and were rescued weakly at higher temperatures with GAL-SIC1 on (YEPG), consistent with previous observations (Jaspersen et al. 1998; Yuste-Rojas and Cross 2000). GAL-SIC1cdc14-1 CLB3∆db cells were inviable at all tested temperatures with GAL-SIC1 off, and were rescued with GAL-SIC1 on up to 27° (with partial rescue at 30°).
To determine whether the inviability of cdc14-1 CLB3∆db cells is due to arrest at mitotic exit, we cultured the strains above in liquid YEPG medium at 23°, shifted to YEPD to turn off GAL-SIC1, and measured DNA content after 3 hr (Figure 5B). cdc14-1 CLB3∆db cells mostly had 2C DNA, and the 4C peak characteristic of CLB3∆db cells was not present, suggesting that these cells replicated DNA once, arrested at mitotic exit, and did not rereplicate DNA. Microscopic examination of the cells used for flow cytometry revealed that nearly all cdc14-1 CLB3∆db cells were large-budded, with two separated nuclei and no rebudding, consistent with blocked mitotic exit, whereas the CDC14CLB3, CDC14CLB3∆db, and cdc14-1 CLB3 populations were a mixture of cells with single or double nuclei, consistent with continued cycling.
Therefore, it is likely that, with fully functional Cdc14, Clb3∆db kinase activity is not high enough to keep relevant targets above the phosphorylation threshold needed to block mitotic exit. With lowered Cdc14 activity in the cdc14-1 mutant, the phosphatase/kinase ratio could be reduced enough to bring the phosphorylation of those targets above threshold, blocking mitotic exit.
It is notable that cdc14-1 CLB3∆db cells are completely inviable even at 23°, while cdc14-1 CLB3 cells are fully viable up to 30°. Thus, CLB3∆db does not block mitotic exit in a CDC14-wt background, but it makes the mitotic exit control system much less robust to normally inconsequential reductions in Cdc14 activity. SIC1 overexpression probably lowers Clb3∆db-associated kinase activity, rebalancing the system, and allowing mitotic exit. SIC1 overexpression has very little effect at any temperature on cdc14-1 CLB3 cell viability.
Swe1, Sic1, and Swi5 are not essential for viability in the absence of the Clb3 D box
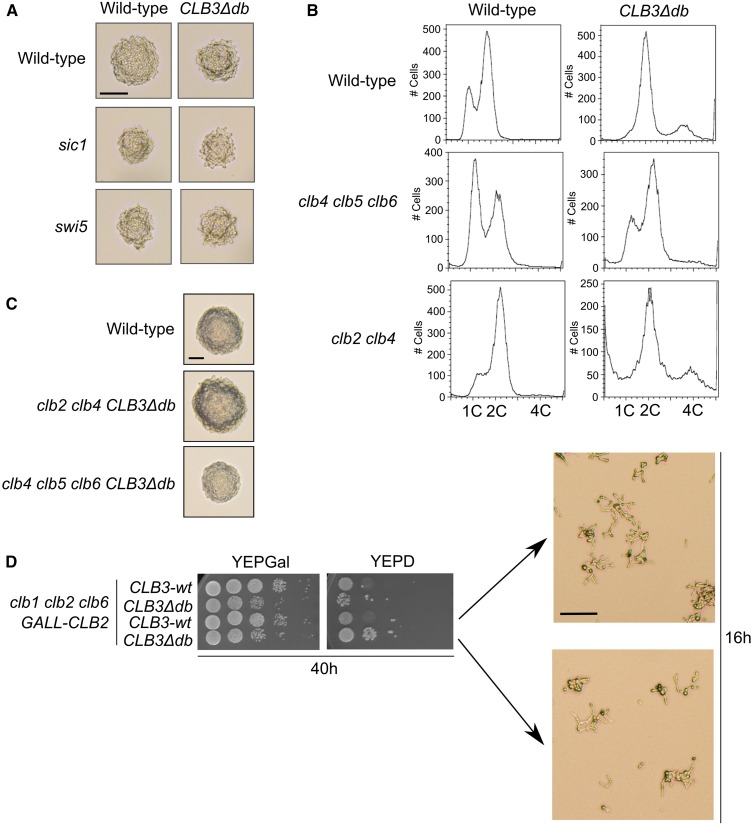
The experiments above might not detect a brief window of inhibition of Clb3∆db-associated kinase activity. If such a drop were essential for mitotic exit, then a normally nonessential Clb3 inhibitor might be essential in a CLB3∆db background. We tested the known inhibitors of Clb-Cdc28: Sic1 (a stoichiometric inhibitor), Swi5 (the major SIC1 transcription factor), and Swe1 (a protein kinase that phosphorylates and inhibits Clb-Cdc28 complexes) (Booher et al. 1993; Mendenhall 1993; Schwob et al. 1994; Knapp et al. 1996; Toyn et al. 1997). We made CLB3∆db and CLB3 alleles using GAL-HO-mediated exact gene replacement in swe1, sic1, or swi5 backgrounds. Microscopic observation of CLB3∆db recombinants in swe1, sic1, or swi5 backgrounds showed no obvious defects compared to isogenic CLB3 strains (Figure 6A).
Figure 6.
Analysis of Clb3∆db function in mitosis, and testing for essential Clb-CDK inhibitors in a CLB3∆db background. (A) CLB3∆db/wt strains with and without sic1 or swi5 deletions. Images were taken 24 hr after induction of HO site cleavage. Bar, 50 μm. (B) FACS profiles of CLB3∆db/wt strains in either clb2 clb4 or clb4 clb5 clb6 backgrounds. (C) CLB3∆db/wt strains in either clb2 clb4 or clb4 clb5 clb6 backgrounds 24 hr after induction of HO site cleavage. Bar, 50 μm. (D) Serial dilutions of clb1 clb2::GALL-CLB2 CLB3∆db cells after 40 hr on YEPD to shut off GALL-CLB2. Bar, 50 μm.
Therefore, Swe1, Sic1, and Swi5 have no essential role in maintaining viability of CLB3∆db cells. Combined with direct measurements of protein abundance and associated kinase activity, these observations suggest that even superphysiological levels of undegradable, active Clb3 are essentially fully permissive for mitotic exit.
The Clb3 D box is not required for normal Clb3 functions
We tested genetic function of Clb3∆db, by introducing CLB3∆db into backgrounds (clb2clb4 or clb4clb5clb6) where Clb3 is essential (Epstein and Cross 1992; Fitch et al. 1992; Richardson et al. 1992; Schwob and Nasmyth 1993). Both the clb2clb4CLB3∆db and clb4clb5clb6CLB3∆db strains were fully viable (Figure 6C), indicating that Clb3∆db can carry out normal Clb3 functions.
clb5clb6 strains bud on schedule, but exhibit a pronounced delay in S phase initiation; this is because normal rapid initiation of DNA replication after Start is dependent on the early expression of Clb5 and Clb6 (Epstein and Cross 1992; Schwob and Nasmyth 1993). In their absence, DNA replication initiation is delayed until later expression of Clb1–4 (Epstein and Cross 1992; Schwob and Nasmyth 1993; Donaldson et al. 1998). Confirming this finding, CLB3clb4,5,6 mutants exhibit a large accumulation of G1 cells in asynchronous FACS profiles (Figure 6B); this increase in the G1 population is almost entirely eliminated in a CLB3∆db clb4,5,6 mutant (Figure 6B), indicating that the stabilized Clb3∆db is able to promote replication initiation early in the cell cycle. This is consistent with other findings on early replication initiation due to CLB3∆db (Figure 1 and Figure 3; see above).
clb1clb2 strains are inviable (Fitch et al. 1992; Richardson et al. 1992) despite possessing CLB3. We wondered whether the high level of Clb3∆db might allow rescue of a clb1clb2 background. We constructed clb1,2,6 GALL-CLB2 strains that were CLB3 or CLB3∆db (see Materials and Methods), maintaining strains on galactose, then examined the effect of shutting off GALL-CLB2 on glucose medium. Both clb1,2,6 CLB3 and clb1,2,6 CLB3∆db cells exhibited rapid arrest as long-budded cells, indicating no rescue of clb1,2 lethality by CLB3∆db (Figure 6D). The clb1clb2CLB3∆db population did contain a small fraction of cells with no morphological abnormalities, which formed viable colonies; the nature of this leakage was not investigated. It is clear that in the great majority of cells, CLB3∆db had no ability to rescue clb1,2 arrest.
Clb3 proteolysis may be required for cell size control at Start
Analysis of elutriated fractions from asynchronous cultures suggested that CLB3∆db strains bud and initiate DNA replication at a smaller cell size than wild type (Figure 1, C and D), implying that Clb3∆db promotes both budding and initiation of DNA replication in cells that are below the wild type critical size for these events.
Budding and DNA replication are dependent on the Start transition, which is controlled by the G1 cyclins Cln1, Cln2, and Cln3 (Lew and Reed 1993; Cross 1995; Dirick et al. 1995). The Cln regulatory hierarchy starts with Cln3 inactivating the Whi5 transcriptional repressor of the G1/S regulon, which includes Cln1 and Cln2 (Costanzo et al. 2004; De Bruin et al. 2004; Di Talia et al. 2007; Skotheim et al. 2008). Initial, partial Whi5 inactivation is followed by rapid completion of Whi5 inactivation by Cln1 and Cln2, in a transcriptional positive feedback loop (Skotheim et al. 2008). Cell size control is coupled to the Cln3-Whi5 part of this regulatory hierarchy (Di Talia et al. 2007): small cells, born with a high Whi5 concentration, must increase cellular volume to decrease Whi5 concentration to a critical level that is sensitive to inhibition by Cln3 (Schmoller et al. 2015).
The unbudded interval can be divided into two discrete, independent periods: an initial interval (T1) during which cells are subject to size control, and a subsequent “timing” interval (T2) (Di Talia et al. 2007). Whi5 exits the nucleus at the end of T1 (Figure 2). The Start transition coincides with Whi5 nuclear exit, when the Cln1,2 positive feedback loop fires (Di Talia et al. 2007; Skotheim et al. 2008; Doncic et al. 2011). T1 is longer in small daughter cells since these cells must reach a critical size before progression through Start (Di Talia et al. 2007; Schmoller et al. 2015).
We used time-lapse microscopy of log-phase CLB3∆db and CLB3 strains with MYO1-mCherry and WHI5-GFP to determine the effect of CLB3∆db on cell cycle timing. We restricted our analysis to daughter cells only since there is almost no size control period in mother cells (Di Talia et al. 2007). T1 was eliminated in CLB3∆db daughters, while T0 (Whi5 entry to cytokinesis) and T2 (Whi5 exit to budding) were unaffected (Table 1). Elimination of T1 in CLB3∆db cells was compensated by an increase in the interval between bud emergence and cytokinesis, so that the overall division time was unaffected (Table 1).
It is possible that Whi5-GFP enters the nucleus briefly during T0 because of inhibition of Clb3∆db by Sic1. To test this, we measured Whi5-GFP nuclear residence times in wild-type, CLB3∆db, sic1∆, and CLB3∆db sic1∆ cells (Table 2). We did not detect Whi5-GFP nuclear entry in >90% of CLB3∆db sic1∆ daughter cells with a 3 min frame resolution, while the Whi5-GFP nuclear residence time in CLB3sic1∆ cells was nearly comparable to that of CLB3SIC1, suggesting that the short interval of Whi5-GFP nuclear entry is indeed due to Sic1 inhibition of Clb3∆db.
Table 2. Whi5-GFP nuclear residence times in daughter cells.
| Strain | Wild Type | CLB3∆db | sic1∆ | CLB3∆db sic1∆ |
|---|---|---|---|---|
| Whi5-GFP in nucleus (min) | 25 ± 11 (50) | 6 ± 3 (50) | 23 ± 14 (50) | 0 ± 2 (50) |
Times are mean ± SD. Number of cells scored in parentheses.
T1 is the interval subject to size control (Di Talia et al. 2007), so a simple explanation of ablation of T1 in CLB3∆db cells would be if these cells are born larger than wild-type cells. To test this, we measured distributions of cell sizes at birth (time of Myo1 ring disappearance) in these movies. Birth sizes were largely overlapping between CLB3∆db and wild-type cells (Figure 7A); however, the distribution of size of CLB3∆db cells was wider, with more extremely small and large cells. Wild-type cell size increased between cell birth and Whi5 nuclear exit (Figure 7A), reflecting the requirement for cell growth to a critical size for Whi5 exit. In contrast, since Whi5 exit was almost immediate upon birth in CLB3∆db cells, there was no change in cell size, even in small newborn cells.
A metric for the degree of size control is derived from a plot of the log of relative growth between birth and Whi5 exit, vs. log of size at birth (Di Talia et al. 2007). Assuming perfect size control, then cell size at Whi5 exit (SWhi5-exit) is a constant. In this case, a plot of ln(SWhi5-exit/Sbirth) against ln(Sbirth) has a slope of −1, with an x-intercept at ln(SWhi5-exit). Previous measurements resulted in a slope of ∼ −0.4, indicating strong, but imperfect, size control (Di Talia et al. 2007). We reproduced this result for wild type (Figure 7B). In contrast, the slope for CLB3∆db cells was essentially 0, indicating absence of detectable size control, even in daughter cells with sizes associated with strong delays in wild type (Figure 7B).
To see if size control is instead shifted to a later phase of the cell cycle in CLB3∆db cells, we plotted αT, the time from Whi5 exit to Myo1 ring disappearance (cytokinesis) multiplied by the growth rate, vs. the log of cell size at the time of Whi5 exit (Figure 7C). If small CLB3∆db cells are delayed in Myo1 ring disappearance, then αT will be larger for these cells, since their growth rate is comparable. αT showed only moderate dependence on cell size at the time of Whi5 exit from the nucleus in either wild-type or CLB3∆db cells (Figure 7C); this moderate dependence may provide some level of size control to CLB3∆db cells. We noted above that there is considerable variability in the sizes of CLB3∆db cells, consistent with a global deficit in cell size control.
Deletion of the CLB3 D box bypasses Start control by Cln3 G1 cyclin
Cln3, which promotes the nuclear exit of Whi5, can control the duration of T1: deletion or overexpression of CLN3 prolongs or shortens T1, respectively (Di Talia et al. 2007). Thus, Clb3∆db could eliminate T1 through hyperactivating Cln3, or alternatively by bypassing Cln3.
To test the dependence of CLB3∆db on CLN3 for elimination of T1, we carried out a similar analysis to that in Figure 7, by time-lapse microscopy of log-phase CLB3, CLB3∆db, cln3, and cln3CLB3∆db strains with WHI5-GFP and ACT1pr-DsRed. In this experiment we used total DsRed signal, since this provides a more accurate estimate of cell size than cell area (Di Talia et al. 2007). T1 was elongated in cln3CLB3 cells, as reported (Di Talia et al. 2007) but there was no detectable T1 period in CLB3db cells, with or without CLN3 (Table 3). T2 remained nearly unchanged between wild type and CLB3∆db, and between cln3 and cln3CLB3∆db. Therefore, elimination of T1 by Clb3∆db is not dependent on Cln3.
Table 3. Daughter cell T1 and T2 times with and without CLN3.
| Genotype | T1 (min) | T2 (min) |
|---|---|---|
| Wild type | 18 ± 11 (50) | 18 ± 7 (50) |
| CLB3∆db | 0 ± 1 (50) | 18 ± 11 (50) |
| cln3 | 26 ± 10 (50) | 16 ± 7 (50) |
| cln3 CLB3∆db | 0 ± 1 (50) | 18 ± 9 (50) |
Times are mean ± SD. Number of cells scored in parentheses. We consider T1 as time from cytokinesis (estimated as Whi5 nuclear entry + 6 min) to Whi5 exit; T2: time from Whi5 exit to bud emergence (Di Talia et al. 2007).
Deletion of CLN3 resulted in larger cells, at birth, Whi5 exit, and budding, but this effect was nearly eliminated by CLB3∆db (Figure 8A). The “size control” plot ln(SWhi5-exit/Sbirth) against ln(Sbirth) showed readily detectable size control (negative slope) in CLB3CLN3. Size control was also clearly detected in CLB3cln3, but shifted to larger cell size, as reported (Di Talia et al. 2007). There was no detectable size control in CLB3∆db cells with or without CLN3, even at sizes where CLB3 cells exhibit clear size control (Figure 8B). These results confirm the findings in Figure 7 obtained using microscopic cell “area,” using instead the ACT1-DsRed cell size reporter.
Thus, removal of the Clb3 D box eliminates pre-Start cell size control independently of CLN3, even in very small daughter cells.
In the absence of the CLB3 D box G1/S cyclins Cln1,2,3 and Clb5,6 are no longer required for viability
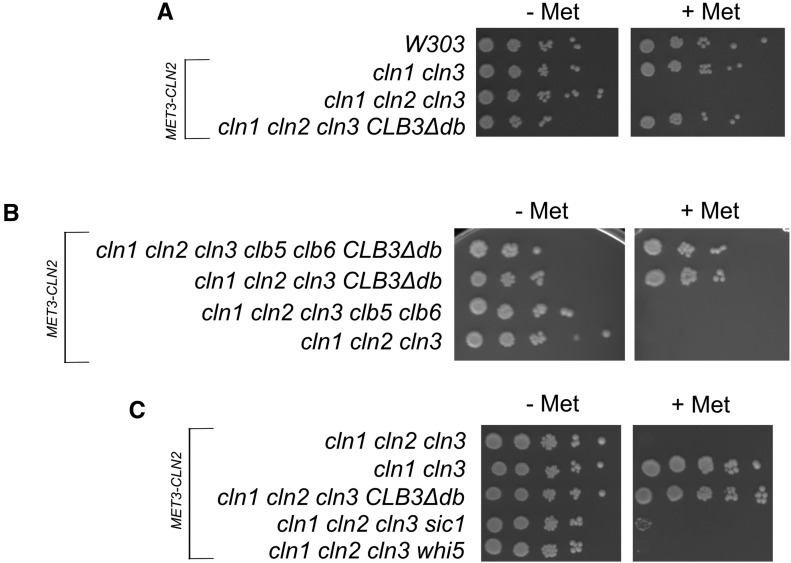
As shown above, Clb3∆db bypasses the requirement for Cln3. The main function of Cln3 is likely to trigger the CLN1,2 positive feedback loop (Skotheim et al. 2008). We were interested to know whether Clb3∆db could also bypass the need for Cln1,2. We constructed cln1cln2cln3MET3-CLN2 strains that were CLB3∆db or CLB3, and grew log-phase cultures on medium lacking methionine to keep MET3-CLN2. Methionine was then added to the cultures, and samples of cells were taken every 30 min up to 3 hr to measure DNA content and budding index. Immediately before the addition of methionine, some cells were also placed in 10-fold serial dilutions on solid agar medium with or without methionine (Figure 9A). As expected, the cln1,2,3 CLB3 strain arrested in the first cell cycle after shutoff of MET3-CLN2, accumulating as unbudded cells with unreplicated DNA (Figure 10). In contrast, the cln1cln2cln3CLB3∆db strain exhibited only a partial accumulation of unbudded cells (up to ∼40%), and no accumulation of cells with unreplicated DNA. This result suggests efficient bypass of the CLN requirement. Consistently, serial dilutions on methionine-containing medium show that cln1cln2cln3CLB3∆db cells can form colonies with high efficiency, and an approximately similar growth rate as CLN+ wild-type controls (Figure 9A).
Figure 9.
Testing bypass of CLNs and CLB5/6 in CLB3∆db strains. (A) Serial dilutions of CLB3∆db/wt strains with a cln1 cln2 cln3 MET3-CLN2 background. Cells were grown to log-phase in medium lacking methionine, then plated in 10-fold serial dilutions on solid medium containing methionine to shut off MET3-CLN2. (B) CLB3∆db/wt strains with a clb5 clb6 cln1 cln2 cln3 MET3-CLN2 background were plated in 10-fold serial dilutions as in (A). (C) cln- cells with either wild-type, CLN2, CLB3∆db, sic1, or whi5 backgrounds were plated in 10-fold serial dilutions as in (A).
Figure 10.
DNA content and budding in cln1 cln2 cln3 CLB3∆db cells after shutoff of MET3-CLN2. (A) Percent unbudded cells after block of MET3-CLN2 by addition of methionine. First sample (t = 0) was taken before addition of methionine. (B) DNA content after block of MET3-CLN2.
Despite their rapid growth rate, microscopic examination of cln1,2,3 CLB3∆db MET3-CLN2 colonies grown on +Met revealed significant cell size heterogeneity. To further characterize this, we inoculated CLB3∆db cln1cln2cln3MET3-CLN2 cells (pregrown in medium lacking methionine) onto solid medium containing methionine, then separated five individual unbudded or very small budded cells with a microneedle to a fresh area, and examined them microscopically at intervals. All five cells had divided multiple times after 6 hr, forming microcolonies, with some variation in cell size notable. After 24 hr, all five microcolonies had continued to proliferate into a mixture of small and large cells. With the microneedle, we moved the large cells (which were unbudded) to a fresh area of the plate. After another 24 hr, most of these unbudded large cells eventually formed microcolonies again consisting of a mixture of small and large cells. We conclude, therefore, that CLB3∆db effectively bypasses cln1cln2cln3 inviability, but some aspects of the rescue are irregular compared to wild type.
Clb5 and Clb6 are early expressed B-type cyclins that promote S phase; moderate overexpression of CLB5 can bypass the Cln requirement (Epstein and Cross 1992). However, the bypass of CLN deficiency by Clb3∆db is not dependent on CLB5/6 (Figure 9B).
We showed above that CLB3∆db cells efficiently inactivated Whi5. To test whether this could be sufficient to account for cln1,2,3 bypass by CLB3∆db, we constructed a cln1,2,3 whi5 MET-CLN2 strain. This strain was completely inviable on +Met, showing that CLB3∆db has broader Start-promoting activities than just inactivating Whi5 (Figure 9C). cln1,2,3 bypass by CLB3∆db is not simply a result of CLB3∆db cells being born with no nuclear Whi5.
Comparison of cln1,2,3 bypass by CLB3∆db and sic1∆
Removing the Clb3 D box prevents negative control of Clb3 by the APC (see Introduction). Another negative control on B-type cyclins is due to the inhibitor Sic1, and deletion of SIC1 was reported to bypass the cln1,2,3 requirement (Tyers 1996). To compare the efficiency of CLN bypass by CLB3∆db to that of a sic1 deletion, we made cln1, cln2, cln3MET3-CLN2 strains in wild-type, CLB3∆db, and sic1 backgrounds (with a cln1CLN2+ cln3 strain as a positive control) and grew log-phase cultures on medium lacking methionine. The cultures were then plated on YEPD plates (with methionine) to shut off MET3-CLN2 (Figure 9C). Consistent with the experiment above, cln1cln2cln3 cells quickly arrested as large unbudded cells, but cln1cln2cln3CLB3∆db cells formed microcolonies with similar efficiency and timing as the cln1CLN2+ cln3 control. By comparison, the bypass of cln- by sic1 was poor: only ∼1% of cells had formed microcolonies after 3 days on the YEPD plate. [cln1,2,3 bypass seems less efficient in the W303 background than in the experiments of Tyers (1996) in the BF305-15d background; but even in that background, sic1cln1cln2cln3 cells were large and slow-growing compared to controls (Epstein and Cross 1994; Tyers 1996)].
The Clb3 D box is required for mating pheromone-induced block to Start
The mating pheromone alpha-factor arrests cells before Start via a signal transduction pathway that inhibits CLNs (Peter and Herskowitz 1994; Cross 1995). Given that Clb3∆db bypasses the cln1,2,3 requirement, we tested whether CLB3∆db cells were sensitive to mating pheromone. To test for alpha-factor-induced arrest of CLB3∆db cells, we placed log-phase bar1 CLB3∆db and bar1CLB3 cells (MATa-mating type bar1) on a YEPD plate with 10−7 M alpha-factor, and checked for arrest after 24 hr. (The bar1 deletion prevents alpha-factor degradation [Chan and Otte 1982]). All bar1CLB3 cells were arrested after 24 hr on alpha-factor, but only ∼10% of bar1CLB3∆db cells were arrested. Therefore, Clb3 proteolysis is required for effective mating pheromone regulation of Start.
Discussion
Mitotic degradation of Clb3 is not required for mitotic exit
Destruction box-dependent degradation of mitotic cyclins at the end of mitosis has been proposed to be essential for mitotic exit (Murray and Kirschner 1989; Murray et al. 1989; Ghiara et al. 1991). Here, we show that Clb3∆db, though functional, stable, and active through the cell cycle, is fully permissive for mitotic exit; the only mitotic defect we noted was a moderate delay in cell separation after cytokinesis. This is unexpected given the significant functional overlap of CLB3 and CLB2 (Fitch et al. 1992; Richardson et al. 1992), and the complete block to mitotic exit imposed by CLB2∆db at the endogenous locus (Wäsch and Cross 2002).
Clb2-Cdc28 may be the most active of the Clb-Cdc28 complexes, although Clb2 lacks docking sites possessed by Clb5 and Clb3 (Kõivomägi et al. 2011). If block of mitotic exit by Clb2 involves low-affinity substrates not recognized by docking sites (Loog and Morgan 2005; Kõivomägi et al. 2011), then perhaps Clb3, even at high levels, cannot effectively phosphorylate these targets. This is supported by our observation that deletion of the Clb3 D box can result in mitotic exit block if Cdc14 activity is reduced; therefore, Clb3 likely shares at least one target with Cdc14, but could be unable to phosphorylate such a target to levels sufficiently high to block mitotic exit. Components of the mitotic exit network Cdc15, Mob1, and Dbf2 have been previously identified as targets of Clb2-Cdc28 (Jaspersen and Morgan 2000; Ubersax et al. 2003; König et al. 2010) and could possibly be targets of Clb3 as well. Indeed, Mob1 and Dbf2 have been shown to interact with Clb3 (Archambault et al. 2004).
Absence of mitotic cyclin activity after mitosis is likely required in order to reassemble prereplicative complexes for DNA replication (Dahmann et al. 1995; Nasmyth 1996; Detweiler and Li 1998), and to permit budding at the G1/S phase transition (Lew and Reed 1993; Amon et al. 1994). Since both DNA replication and budding occur in CLB3∆db cells, even when present at high levels, Clb3 may only inefficiently phosphorylate the relevant substrates for these controls as well.
Mitotic degradation of Clb3 is required to eliminate “memory” of the preceding cell cycle and allow Start control
In contrast to the absence of any significant requirement for Clb3 proteolysis for mitotic exit, mitotic Clb3 proteolysis is required for control of Start. Start is conditional on cells attaining a sufficient cell size; it depends on Cln3 as an initial upstream signal, and on the Cln1,2-dependent positive feedback loop (Cross and Tinkelenberg 1991; Dirick and Nasmyth 1991; Cross 1995; Skotheim et al. 2008). Start is also specifically blocked by mating pheromones (Cross 1995). All of these controls are abrogated by removal of the Clb3 D box: CLB3∆db cells pass Start even when very small, as indicated by the absence of nuclear Whi5, and by accelerated budding and DNA replication, in a Cln3-independent fashion; CLB3∆db results in mating factor insensitivity, and eliminates the requirement for any of CLN1,2,3 CLB5,6. Rescue of CLN deficiency by cyclins has been previously reported, but has involved expression of the rescuing cyclins from a strong promoter such as ADH1 (Koff et al. 1991; Léopold and O’Farrell 1991; Lew et al. 1991), resulting in gross overexpression of the foreign cyclin. In this study, we have removed only a small regulatory segment of a B-type cyclin expressed under its endogenous promoter. Very efficient CLN bypass was observed with mutants in the CDC28 cyclin-dependent kinase evolved by directed evolution to partial cyclin independence (Levine et al. 1999). The mutant Cdc28 proteins had cyclin-independent kinase activity, which would presumably be present in small newborn cells even after complete mitotic cyclin proteolysis.
The CLN gene family has been maintained in almost all members of the large fungal lineage (N. Buchler, personal communication), and the functionally cognate G1 cyclins D and E are widespread in animal lineages, although they are also largely dispensable for the core cell cycle (Sicinski et al. 1995; Geng et al. 2003). Continued selection for presence of G1 cyclins is likely due to their role in providing a “starter switch” that allows connection of the cell cycle to regulatory signals, such as cell size in the case of budding yeast. There is an absolute requirement for CLN gene expression after cell birth, even in very large newborn cells (Richardson et al. 1989; Cross 1990). This means that every cell cycle is effectively memoryless: the firing of the CLN switch in the preceding cell cycle is irrelevant. As a consequence, each cell cycle is directly coupled to regulatory systems that target G1 cyclins. This is presumably also the reason for the high instability of Cln proteins—stabilization of Cln3 by removing its degradation signal eliminates Start control by mating pheromone and cell size, and previously synthesized stable Cln3 can promote multiple cell cycles in a cln1,2,3 background with no further CLN3 expression (Cross 1990).
Without mitotic destruction, Clb3 synthesized in the preceding cell cycle may directly activate Start, bypassing the CLN regulatory loop. We do not know what phosphorylation targets are responsible for this activity. Whi5 and Sic1 are both proposed Cdk targets that restrain Start, but genetic results above show that neither of these targets is sufficient to account for CLB3∆db cln-bypass. Other Clbs could have a similar activity if not degraded, although this role is obscured by inhibition of mitotic exit (since exit is a prerequisite for Start). Indeed, Drapkin et al. (2009) showed that cells containing a level of Clb2∆db low enough to allow escape of the block to mitotic exit had perturbed regulation of the succeeding Start, since they did not block budding in response to the mating pheromone alpha-factor. The absence of inhibition of mitotic exit by Clb3∆db allows a much clearer view of this new role of mitotic cyclin degradation: blocking memory of the previous cell cycle in newborn cells.
Acknowledgments
We thank all members of the Cross laboratory for insightful discussions. Funding support was from Public Health Service grant 5RO1-GM078153.
Footnotes
Communicating editor: S. Biggins
Literature Cited
- Amon A., Irniger S., Nasmyth K., 1994. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell 77: 1037–1050. [DOI] [PubMed] [Google Scholar]
- Archambault V., Chang E. J., Drapkin B. J., Cross F. R., Chait B. T., et al. , 2004. Targeted proteomic study of the cyclin-Cdk module. Mol. Cell 14: 699–711. [DOI] [PubMed] [Google Scholar]
- Archambault V., Buchler N. E., Wilmes G. M., Jacobson M. D., Cross F. R., 2005. Two-faced cyclins with eyes on the targets. Cell Cycle 4: 125–130. [DOI] [PubMed] [Google Scholar]
- Bi E., Maddox P., Lew D. J., Salmon E. D., McMillan J. N., et al. , 1998. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J. Cell Biol. 142: 1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom J., Cross F. R., 2007. Multiple levels of cyclin specificity in cell-cycle control. Nat. Rev. Mol. Cell Biol. 8: 149–160. [DOI] [PubMed] [Google Scholar]
- Booher R. N., Deshaies R. J., Kirschner M. W., 1993. Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 12: 3417–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin R. A. M., McDonald W. H., Kalashnikova T. I., Yates J., Wittenberg C., 2004. Cln3 activates G1-specific transcription via phosphorylation of the SBF bound repressor Whi5. Cell 117: 887–898. [DOI] [PubMed] [Google Scholar]
- Chan R. K., Otte C. A., 1982. Physiological characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol. Cell. Biol. 2: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M., Nishikawa J. L., Tang X., Millman J. S., Schub O., et al. , 2004. CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell 117: 899–913. [DOI] [PubMed] [Google Scholar]
- Cross F., 1990. Cell cycle arrest caused by CLN gene deficiency in Saccharomyces cerevisiae resembles START-I arrest and is independent of the mating-pheromone signalling pathway. Mol. Cell. Biol. 10: 6482–6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., 1995. Starting the cell cycle: what’s the point? Curr. Opin. Cell Biol. 7: 790–797. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Pecani K., 2011. Efficient and rapid exact gene replacement without selection. Yeast 28: 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Tinkelenberg A. H., 1991. A potential positive feedback loop controlling CLN1 and CLN2 gene expression at the start of the yeast cell cycle. Cell 65: 875–883. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Yuste-Rojas M., Gray S., Jacobson M. D., 1999. Specialization and targeting of B-type cyclins. Mol. Cell 4: 11–19. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Archambault V., Miller M., Klovstad M., 2002. Testing a mathematical model of the yeast cell cycle. Mol. Biol. Cell 13: 52–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmann C., Diffley J. F., Nasmyth K. A., 1995. S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transition of replication origins to a pre-replicative state. Curr. Biol. 5: 1257–1269. [DOI] [PubMed] [Google Scholar]
- Detweiler C. S., Li J. J., 1998. Ectopic induction of Clb2 in early G1 phase is sufficient to block prereplicative complex formation in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 95: 2384–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirick L., Nasmyth K., 1991. Positive feedback in the activation of G1 cyclins in yeast. Nature 351: 754–757. [DOI] [PubMed] [Google Scholar]
- Dirick L., Böhm T., Nasmyth K., 1995. Roles and regulation of Cln-Cdc28 kinases at the start of the cell cycle of Saccharomyces cerevisiae. EMBO J. 14: 4803–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Talia S., Skotheim J. M., Bean J. M., Siggia E. D., Cross F. R., 2007. The effects of molecular noise and size control on variability in the budding yeast cell cycle. Nature 448: 947–951. [DOI] [PubMed] [Google Scholar]
- Donaldson A. D., Raghuraman M. K., Friedman K. L., Cross F. R., Brewer B. J., et al. , 1998. CLB5-dependent activation of late replication origins in S. cerevisiae. Mol. Cell 2: 173–182. [DOI] [PubMed] [Google Scholar]
- Doncic A., Falleur-Fettig M., Skotheim J. M., 2011. Distinct interactions select and maintain a specific cell fate. Mol. Cell 43: 528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapkin B. J., Lu Y., Procko A. L., Timney B. L., Cross F. R., 2009. Analysis of the mitotic exit control system using locked levels of stable mitotic cyclin. Mol. Syst. Biol. 5: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein A., Amodaj N., Hoover K., Vale R., Stuurman N., 2010. Computer control of microscopes using manager. Curr. Protoc. Mol. Biol. Chapter 14: Unit14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein A. D., Tsuchida M. A., Amodaj N., Pinkard H., Vale R. D., et al. , 2014. Advanced methods of microscope control using μManager software. J. Biol. Methods 1: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein C. B., Cross F. R., 1992. CLB5: a novel B cyclin from budding yeast with a role in S phase. Genes Dev. 6: 1695–1706. [DOI] [PubMed] [Google Scholar]
- Epstein C. B., Cross F. R., 1994. Genes that can bypass the CLN requirement for Saccharomyces cerevisiae cell cycle START. Mol. Cell. Biol. 14: 2041–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch I., Dahmann C., Surana U., Amon A., Nasmyth K., et al. , 1992. Characterization of four B-type cyclin genes of the budding yeast Saccharomyces cerevisiae. Mol. Biol. Cell 3: 805–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Yu Q., Sicinska E., Das M., Schneider J. E., et al. , 2003. Cyclin E ablation in the mouse. Cell 114: 431–443. [DOI] [PubMed] [Google Scholar]
- Ghiara J. B., Richardson H. E., Sugimoto K., Henze M., Lew D. J., et al. , 1991. A cyclin B homolog in S. cerevisiae: chronic activation of the Cdc28 protein kinase by cyclin prevents exit from mitosis. Cell 65: 163–174. [DOI] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W., 1991. Cyclin is degraded by the ubiquitin pathway. Nature 349: 132–138. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Pringle J. R., Reid B. J., 1974. Genetic control of the cell division cycle in yeast. Science 183: 46–51. [DOI] [PubMed] [Google Scholar]
- Jaspersen S. L., Morgan D. O., 2000. Cdc14 activates Cdc15 to promote mitotic exit in budding yeast. Curr. Biol. 10: 615–618. [DOI] [PubMed] [Google Scholar]
- Jaspersen S. L., Charles J. F., Tinker-Kulberg R. L., Morgan D. O., 1998. A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Mol. Biol. Cell 9: 2803–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Deshaies R. J., Peters J. M., Kirschner M. W., 1996. How proteolysis drives the cell cycle. Science 274: 1652–1659. [DOI] [PubMed] [Google Scholar]
- Knapp D., Bhoite L., Stillman D. J., Nasmyth K., 1996. The transcription factor Swi5 regulates expression of the cyclin kinase inhibitor p40SIC1. Mol. Cell. Biol. 16: 5701–5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff A., Cross F., Fisher A., Schumacher J., Leguellec K., et al. , 1991. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell 66: 1217–1228. [DOI] [PubMed] [Google Scholar]
- Kõivomägi M., Valk E., Venta R., Iofik A., Lepiku M., et al. , 2011. Dynamics of Cdk1 substrate specificity during the cell cycle. Mol. Cell 42: 610–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König C., Maekawa H., Schiebel E., 2010. Mutual regulation of cyclin-dependent kinase and the mitotic exit network. J. Cell Biol. 188: 351–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léopold P., O’Farrell P. H., 1991. An evolutionarily conserved cyclin homolog from Drosophila rescues yeast deficient in G1 cyclins. Cell 66: 1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine K., Huang K., Cross F. R., 1996. Saccharomyces cerevisiae G1 cyclins differ in their intrinsic functional specificities. Mol. Cell. Biol. 16: 6794–6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine K., Kiang L., Jacobson M. D., Fisher R. P., Cross F. R., 1999. Directed evolution to bypass cyclin requirements for the Cdc28p cyclin-dependent kinase. Mol. Cell 4: 353–363. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Reed S. I., 1993. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J. Cell Biol. 120: 1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. J., Dulić V., Reed S. I., 1991. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell 66: 1197–1206. [DOI] [PubMed] [Google Scholar]
- Loog M., Morgan D. O., 2005. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature 434: 104–108. [DOI] [PubMed] [Google Scholar]
- Lu Y., Cross F. R., 2010. Periodic cyclin-cdk activity entrains an autonomous cdc14 release oscillator. Cell 141: 268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall M. D., 1993. An inhibitor of p34CDC28 protein kinase activity from Saccharomyces cerevisiae. Science 259: 216–219. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W., 1989. Dominoes and clocks: the union of two views of the cell cycle. Science 246: 614–621. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W., 1989. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature 339: 280–286. [DOI] [PubMed] [Google Scholar]
- Nasmyth K., 1996. At the heart of the budding yeast cell cycle. Trends Genet. 12: 405–412. [DOI] [PubMed] [Google Scholar]
- Oikonomou C., Cross F. R., 2011. Rising cyclin-CDK levels order cell cycle events. PLoS One 6: e20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M., Herskowitz I., 1994. Direct inhibition of the yeast cyclin-dependent kinase Cdc28-Cln by Far1. Science 265: 1228–1231. [DOI] [PubMed] [Google Scholar]
- Pfleger C. M., Kirschner M. W., 2000. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14: 655–665. [PMC free article] [PubMed] [Google Scholar]
- Rahi S. J., Pecani K., Ondracka A., Oikonomou C., Cross F. R., 2016. The CDK-APC/C oscillator predominantly entrains periodic cell-cycle transcription. Cell 165: 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H. E., Wittenberg C., Cross F., Reed S. I., 1989. An essential G1 function for cyclin-like proteins in yeast. Cell 59: 1127–1133. [DOI] [PubMed] [Google Scholar]
- Richardson H., Lew D. J., Henze M., Sugimoto K., Reed S. I., 1992. Cyclin-B homologs in Saccharomyces cerevisiae function in S phase and in G2. Genes Dev. 6: 2021–2034. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., 1999. Evolving ideas about cyclins. Cell 98: 129–132. [DOI] [PubMed] [Google Scholar]
- Schindelin J., Rueden C. T., Hiner M. C., Eliceiri K. W., 2015. The ImageJ ecosystem: an open platform for biomedical image analysis. Mol. Reprod. Dev. 82: 518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoller K. M., Turner J. J., Kõivomägi M., Skotheim J. M., 2015. Dilution of the cell cycle inhibitor Whi5 controls budding-yeast cell size. Nature 526: 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S., Eliceiri K. W., 2012. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob E., Nasmyth K., 1993. CLB5 and CLB6, a new pair of B cyclins involved in DNA replication in Saccharomyces cerevisiae. Genes Dev. 7: 1160–1175. [DOI] [PubMed] [Google Scholar]
- Schwob E., Bohm T., Mendenhall M. D., Nasmyth K., 1994. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae. (erratum: Cell 84(1): following 174] Cell 79: 233–244. [DOI] [PubMed] [Google Scholar]
- Sicinski P., Donaher J. L., Parker S. B., Li T., Fazeli A., et al. , 1995. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82: 621–630. [DOI] [PubMed] [Google Scholar]
- Skotheim J. M., Di Talia S., Siggia E. D., Cross F. R., 2008. Positive feedback of G1 cyclins ensures coherent cell cycle entry. Nature 454: 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyn J. H., Johnson A. L., Donovan J. D., Toone W. M., Johnston L. H., 1997. The Swi5 transcription factor of Saccharomyces cerevisiae has a role in exit from mitosis through induction of the cdk-inhibitor Sic1 in telophase. Genetics 145: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyers M., 1996. The cyclin-dependent kinase inhibitor p40SIC1 imposes the requirement for Cln G1 cyclin function at Start. Proc. Natl. Acad. Sci. USA 93: 7772–7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubersax J. A., Woodbury E. L., Quang P. N., Paraz M., Blethrow J. D., et al. , 2003. Targets of the cyclin-dependent kinase Cdk1. Nature 425: 859–864. [DOI] [PubMed] [Google Scholar]
- Wäsch R., Cross F. R., 2002. APC-dependent proteolysis of the mitotic cyclin Clb2 is essential for mitotic exit. Nature 418: 556–562. [DOI] [PubMed] [Google Scholar]
- Yeong F. M., Lim H. H., Padmashree C. G., Surana U., 2000. Exit from mitosis in budding yeast: biphasic inactivation of the Cdc28-Clb2 mitotic kinase and the role of Cdc20. Mol. Cell 5: 501–511. [DOI] [PubMed] [Google Scholar]
- Yuste-Rojas M., Cross F. R., 2000. Mutations in CDC14 result in high sensitivity to cyclin gene dosage in Saccharomyces cerevisiae. Mol. Gen. Genet. 263: 60–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Strains and data used in this study are available upon request.