FIGURE 3.
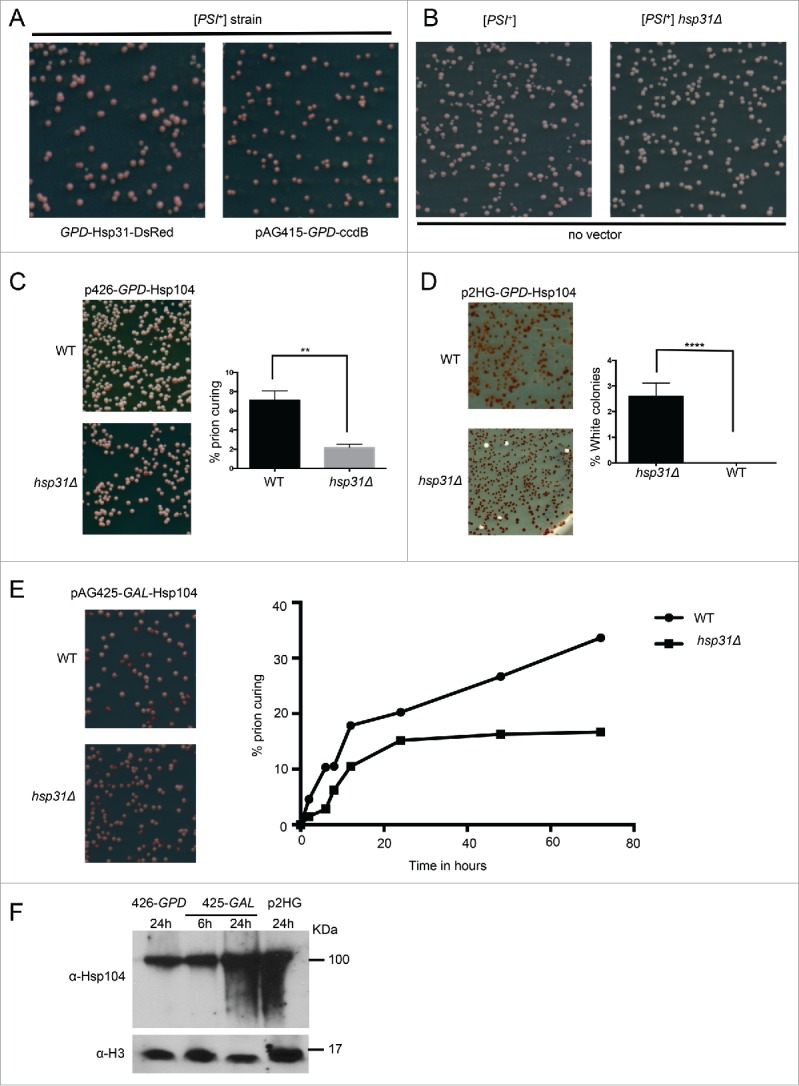
Hsp31 is required for optimal Hsp104-induced curing of the [PSI+] phenotype. (A) To determine the effect of Hsp31 on [PSI+] prion curing, the WT and hsp31Δ [PSI+] strains harboring the GPD-Hsp31 expression vector (pAG415-GPD-Hsp31-DsRed) or the vector (pAG415-GPD-ccdB-DsRed) were grown for 12 h at 30°C before plating on ¼ YPD plates. (B) The WT and hsp31Δ [PSI+] strains with no vector were also grown and treated in the same way. Plates were grown for 2–3 d at 30°C and transferred at 4°C for increased color development. No difference in colony color was observed in these strains. (C) Low-level overexpression of Hsp104 was used to induce prion curing in [PSI+] hsp31Δ and WT strains. Cells were grown in liquid media for 12 h at 30°C before plating on ¼ YPD plates. Significantly less prion curing was observed in the [PSI+] hsp31Δ strain (**unpaired Student's t-test; p ≤ 0.001, n = 3). (D) High-level overexpression of Hsp104 was used to induce prion curing in [PSI+] hsp31Δ and WT strains. A 100% curing level was observed in WT strain. In the [PSI+] hsp31Δ strain, 100% curing was never achieved. White color colonies were plotted for the WT and [PSI+] hsp31Δ strain (****unpaired Student's t-test; p ≤ 0.0001, n = 3 biological replicates). (E) Hsp104 expression under the GAL promoter for 2 to 72 h in WT and [PSI+] hsp31Δ strain. At each indicated time point, cells were plated on ¼ YPD plates. Percentage of prion curing was calculated at each point for both WT and [PSI+] hsp31Δ strain. The plotted graph is one representation of 3 independent biological repeats. (unpaired Student's t-test; p ≤ 0.001 at 24, 48 and 72 h; n = 3). (F) Western blot demonstrating the relative expression levels of Hsp104. Time points for the pGAL plasmid are represent time after switching the strain to inducing galactose media. Equal amount of cells lysates were loaded in each lane and anti-histone H3 antibody was used as a loading control.
