Abstract
Background
Decisions on the intensity of analgesic therapy and judgments regarding its efficacy are difficult at the end of life, when many patients are not fully conscious and pain is a very common symptom. In healthy individuals and in postoperative settings, nociception and subsequent pain relief have been shown to induce changes in the autonomic nervous system (ANS), which can be detected by measuring heart rate variability (HRV).
Objectives
The changes in the ANS were studied by measuring HRV during opioid therapy for cancer breakthrough pain (CBTP) in palliative-care patients with cancer and compared these changes with patient-reported pain levels on a numeric rating scale (NRS).
Patients and methods
The study included ten patients with advanced cancer and baseline opioid therapy. In each patient, a 24-hour peak-to-peak HRV measurement with a sampling rate of 4,000 Hz was performed. High frequency (HF), low frequency (LF), total power, pNN50 (indicating parasympathetic activity), and log LF/HF were obtained in two intervals prior to therapy and in four intervals thereafter. Intensity of CBTP was recorded using a patient-reported NRS prior to therapy and 30 minutes afterward.
Results
CBTP occurred in seven patients (three males and four females; mean age: 62 ± 5.2 years) and was treated with opioids. A highly significant positive correlation was found between opioid-induced reduction in patient-reported pain intensity based on NRS and changes in log LF/HF (r > 0.700; p < 0.05). Log LF/HF decreased in patients who had a reduction in pain of >2 points on the NRS but remained unchanged in the other patients.
Conclusion
Our data suggest that log LF/HF may be a useful surrogate marker for alleviation of CBTP in patients with advanced cancer and might allow detection of pain without active contribution from patients.
Keywords: heart rate variability, cancer breakthrough pain, advanced cancer, palliative care
Introduction
Pain is a subjective sensation and therefore difficult to measure. The intensity of pain can be reported by patients on a scale from 0 to 10. The numeric rating scale (NRS) is a well-studied method of measuring both acute and chronic pain, has been validated by several investigators, and is widely used to measure pain in clinical practice and in clinical studies.1–3
The NRS has some practical limitations in a clinical setting. There is no clear evidence about its optimal cut points, and it requires the patient’s ability to understand the abstract concept of the NRS.4 Therefore, it can hardly be used in end-of-life care situations, when many patients are not fully conscious and pain is a very common symptom.
In this setting, decisions about the intensity of analgesic therapy and judgments regarding its efficacy are usually based on interpretation of possibly pain-related symptoms by clinical impression. This may result in inadequate treatment of a considerable proportion of these patients. Therefore, it would be desirable to have a tool to measure pain intensity and analgesia-induced reduction in pain intensity without active contribution by the patient.
The Expert Working Group on Pain of the European Association of Palliative Care discourages inclusion of these patients in interventional pain studies but recommends the development of alternative ways to diagnose pain.5
The aim of our study was to investigate the autonomic nervous system (ANS) as a predictor of response to analgesic treatment by measuring heart rate variability (HRV). Jeanne et al used HRV measurement to diagnose pain during general anesthesia in patients undergoing surgery. They found that the high-frequency (HF) power of HRV reflecting parasympathetic (vagal) activity decreased in a sensitive, reproducible way in patients under general anesthesia when light sedation was used. This dysregulation was not seen when patients were treated with adequate analgesia using opioids. Therefore, the authors speculated that HRV might be useful for monitoring the adequacy of analgesia during anesthesia.6 This has led to the development of an Analgesia/Nociception Index, which might help to anticipate analgesic response.7
Similar results associating HRV with pain have been obtained in patients with irritable bowel syndrome,8 in patients with pain who are undergoing physiotherapy9 and in postoperative pain,10 occupational pain,11 and experimentally induced pain in healthy volunteers.12 In the latter two studies, nociception was concomitant with an increase in low frequency (LF), reflecting both sympathetic and parasympathetic (vagal) and an increase in log LF/HF, indicating overall balance between sympathetic and parasympathetic (vagal) activities. These data suggest a possible role for these parameters in monitoring and measuring pain reduction during opioid treatment of patients with advanced cancer.
Patients and methods
Patients
The study admitted ten consecutive patients (aged >18 years) with terminal cancer who were admitted to the palliative-care unit for treatment of uncontrolled pain, were capable of giving informed consent, had a baseline opioid therapy for cancer-related pain, had previous episodes of cancer breakthrough pain (CBTP), and were judged by the recruiting physician to be able to complete the study diary. The study used a portable 5-point electrocardiogram with a sampling rate of 4,000 Hz to measure HRV over 1 day (20–24 hours).
The study excluded patients with atrial fibrillation, those taking beta blockers, those with a pacemaker, and those with heart or lung transplants, because physiologic HRV is no longer present in these conditions.
The power analysis was not conducted, as the aim was to obtain preliminary data that could be used for planning definite studies.
The study was approved by the ethics committee of the Medical University of Vienna, Vienna, Austria. All patients provided written informed consent.
Managing CBTP
The study analyzed the first episode of CBTP after the start of monitoring of HRV in each patient. Therapy for CBTP consisted of the following opioids: buccal fentanyl, short-acting oral hydromorphone, or short-acting morphine. The decision to use any of them was made by the prescribing physician together with the patient. In the cases of hydromorphone and morphine, the dose of breakthrough pain medication was one-sixth of the total daily dose. The dose of transmucosal fentanyl was determined by titration to provide an optimal effect.
Monitoring CBTP
The study monitored for changes in LF and log LF/HF in patients with opioid treatment for CBTP, which is an abrupt, short-lived, and intense pain that “breaks through” the sustained-released analgesia provided to control persistent pain13 and responds to treatment with opioids within minutes.14,15 Pain was assessed using an NRS with a range from 0 to 10 on which 0 was defined as “no pain” and 10 was defined as “worst pain imaginable”. Assessments of pain by NRS were performed immediately before opioid administration for CBTP and 30 minutes afterward.
Monitoring and analyzing HRV
In each patient, a 24-hour peak-to-peak HRV measurement with a sampling rate of 4,000 Hz (Medilog® AR12plus; Schiller Handelsgesellschaft GmbH, Linz, Austria) was performed. HF, LF, total power (TP), pNN50, log LF/HF, and heart rate were compared. To provide validity and reliability, the guidelines of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology were applied.16
HF is a band of power spectrum ranging from 0.15 to 0.4 Hz that reflects parasympathetic (vagal) activity. LF is a band of power spectrum ranging from 0.04 to 0.15 Hz. It reflects both sympathetic and parasympathetic (vagal) activities. TP is the subsumption of measurements between 0.003 and 0.4 Hz and serves as a benchmark of total variability.
pNN50 measures the percentage of successive inter-beat (RR) intervals that differ from one another by >50 milliseconds. Higher parasympathetic (vagal) activity results in higher pNN50 values.
Log LF/HF is the ratio between the power of LF and HF bands. It indicates overall balance between sympathetic and parasympathetic (vagal) activities.
Data for HF, LF, log LF/HF, TP, heart rate, and pNN50 were obtained for the following time intervals.
T − 2: 30 minutes before the start of opioid medication;
T − 1: 15 minutes before the start of opioid medication;
T + 1: 10–25 minutes after the start of opioid medication;
T + 2: 10–45 minutes after the start of opioid medication;
T + 3: 10–75 minutes after the start of opioid medication; and
T + 4: 10–135 minutes after the start of opioid medication.
Statistical analysis
For metric data, the mean value, standard deviation (SD), minimum (min) value, maximum (max) value, and difference between max and min (range) were reported, based on skewed distribution. The level of significance was set at p = 0.05, and p-values were corrected for multiple tests after Bonferroni–Holm test. To estimate effect sizes, Pearson’s correlation coefficient (r) was reported, based on the following ratings: 0.1 = small, 0.3 = moderate, and 0.5 = large effect size. Statistical analysis was performed using the IBM Statistical Package for the Social Sciences (SPSS) version 17.0. (SPSS Inc., Chicago, IL, USA).
Results
The study included ten patients with advanced cancer. Seven patients (three males and four females; mean age: 62 ± 5.2 years) developed CBTP and were available for HRV analysis. Short-acting opioids (hydromorphone, morphine) were used in four patients. Opioids were administered in the following doses: hydromorphone 1.3, 2.6, and 5.2 mg and morphine 20 mg. Three patients were administered rapid-acting opioids (transbuccal fentanyl in two and transnasal fentanyl in one). Transbuccal fentanyl was administered in a dose of 200 µg and transnasal fentanyl in a dose of 100 µg.
Level of pain before and after treatment with opioids
Pain measured by NRS was 7.4 ± 1.3 (mean ± SD; range 6.0–9.0) before opioid administration and 5.0 ± 1.5 (mean ± SD; range 3.0–7.0) 30 minutes thereafter (p < 0.05). There was a mean reduction in pain of 2.6 points on the patient-reported NRS. Four patients had a reduction of 2 points, two patients had a reduction of 3 points, and one patient had a reduction of 4 points.
Log LF/HF before and after treatment of breakthrough pain
The mean log LF/HF showed a nonsignificant reduction after treatment (recording time intervals T + 2, T + 3, and T + 4; Table 1). Other HRV-derived parameters, including HF, LF, TP, pNN50, and heart rate, remained unchanged (data not shown).
Table 1.
Log LF/HF before and after administration of opioids (n = 7)
| Period of log LF/HF recording | Min | Max | MV | SD |
|---|---|---|---|---|
| T − 2: 30 minutes until the start of opioid treatment | −0.38 | 0.58 | 0.19 | 0.32 |
| T − 1: 15 minutes until the start of opioid treatment | −0.68 | 0.57 | 0.14 | 0.38 |
| T + 1: 10–30 minutes after the start of opioid therapy | −0.43 | 0.59 | 0.17 | 0.33 |
| T + 2: 10–45 minutes after the start of opioid therapy | −0.39 | 0.55 | 0.10 | 0.35 |
| T + 3: 10–75 minutes after the start of opioid therapy | −0.62 | 0.60 | 0.07 | 0.37 |
| T + 4: 10–135 minutes after the start of opioid therapy | −0.24 | 0.65 | 0.12 | 0.29 |
Abbreviations: LF, low frequency; HF, high frequency; Min, minimum; Max, maximum; MV, mean value; SD, standard deviation.
Log LF/HF before and 30 minutes after treatment of pain
A highly significant (p < 0.05) positive correlation with a correlation coefficient (r) of >0.700 was found between changes in NRS from T + 1 to T + 2 and changes in log LF/HF. Other tested HRV-derived parameters, including HF, LF, TP, pNN50, and heart rate, showed no significant correlation with the patient-reported changes in NRS. Log LF/HF decreased in all patients who had a reduction in pain of >2 points on the NRS (Figure 1) but remained unchanged in patients who had reductions of up to 2 points (Figure 2 and Table 2).
Figure 1.
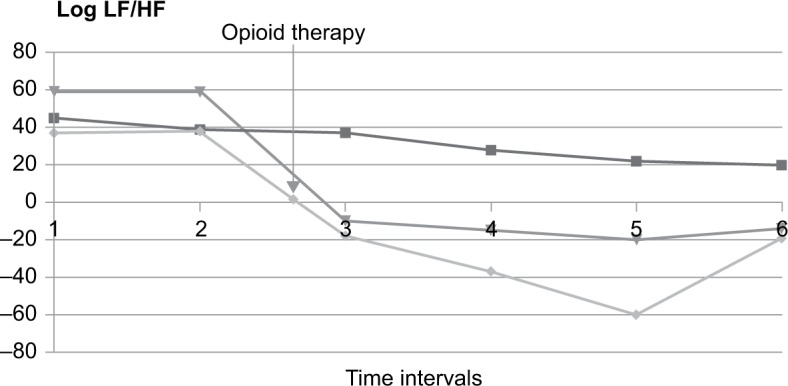
Log LF/HF in patients with decreased pain intensity >2 points on the NRS before (time intervals T − 2 and T − 1) and after (time intervals T + 1, T + 2, T + 3, and T + 4) the start of therapy.
Notes: 1 = T − 2, 2 = T − l, 3 = T + l, 4 = T + 2, 5 = T + 3, and 6 = T + 4. T − 2: 30 minutes before the start of opioid medication; T − 1: 15 minutes before the start of opioid medication; T + 1: 10–25 minutes after the start of opioid medication; T + 2: 10–45 minutes after the start of opioid medication; T + 3: 10–75 minutes after the start of opioid medication; T + 4: 10–135 minutes after the start of opioid medication.
Abbreviations: LF, low frequency; HF, high frequency; NRS, numeric rating scale.
Figure 2.
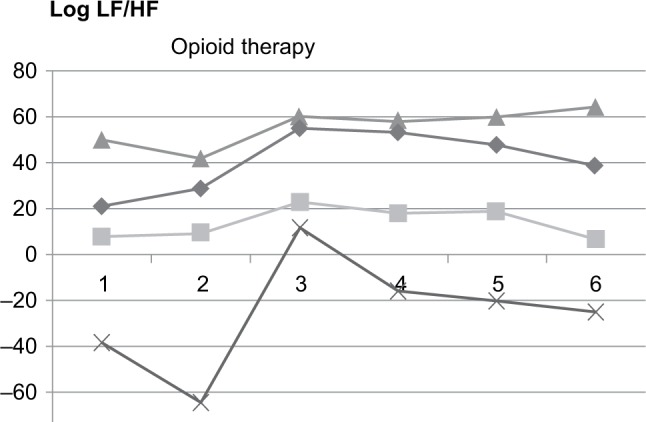
Log LF/HF in patients with decreased pain intensity >2 points on the NRS before (time intervals 1 and 2) and after (time intervals 3, 4, 5, and 6) the start of therapy.
Notes: 1 = T − 2, 2 = T − l, 3 = T + l, 4 = T + 2, 5 = T + 3, and 6 = T + 4. T − 2: 30 minutes before the start of opioid medication; T − 1: 15 minutes before the start of opioid medication; T + 1: 10–25 minutes after the start of opioid medication; T + 2: 10–45 minutes after the start of opioid medication; T + 3: 10–75 minutes after the start of opioid medication; and T + 4: 10–135 minutes after the start of opioid medication.
Abbreviations: LF, low frequency; HF, high frequency; NRS, numeric rating scale.
Table 2.
Delta of log LF/HF between posttreatment recording periods T + 1 through T + 4 and period T − 1 (pretreatment) and its correlation with delta of levels of intensity of pain prior to pain treatment (PT0) and 30 minutes after initiation of pain treatment (PT30)
| Period of log LF/HF recording and correlation coefficient | PT0 – PT30 | |
|---|---|---|
| Log LF/HF, T − 1 minus T + 1 | r | 0.653 |
| p | 0.079 | |
| Log LF/HF, T − 1 minus T + 2 | r | 0.730* |
| p | 0.040 | |
| Log LF/HF, T − 1 minus T + 3 | r | 0.866* |
| p | 0.005 | |
| Log LF/HF, T − 1 minus T + 4 | r | 0.898* |
| p | 0.002 | |
Notes: T + 1: 10–25 minutes after the start of opioid medication; T + 4: 10–135 minutes after the start of opioid medication; T − 1: 15 minutes before the start of opioid medication; T + 2: 10–45 minutes after the start of opioid medication; T + 3: 10–75 minutes after the start of opioid medication.
Abbreviations: LF, low frequency; HF, high frequency.
Discussion
The aim of the present study was to investigate the ANS as a predictor of response to analgesic treatment by measuring HRV.
The results suggested that pain-associated changes in the ANS can be detected in palliative-care patients suffering from advanced cancer despite the fact that dysfunctions in this system in these patients have been reported, while current evidence indicates longer survival in cancer patients who have higher vagal nerve activity.17–19
The results of the present study showed a decline in log LF/HF, reflecting the overall balance between sympathetic and parasympathetic activities after CBTP treatment in the group of palliative-care patients. These data are in line with the results of previous studies, which showed decreased log LF/HF after easing experimentally induced or postoperative pain.10,12
Log LF/HF is the ratio between the power of LF and HF bands. It can be used to quantify the overall balance between the sympathetic and parasympathetic systems. Higher log LF/HF values reflect stress-induced domination of the sympathetic system, and lower levels reflect domination of the parasympathetic system during relaxation. In the present study, reduced log LF/HF after CBTP treatment with opioids suggests relaxation of patients due to relief from pain-induced stress.
This hypothesis is supported by the fact that opioid treatment of CBTP resulted in a median reduction in pain of 2.6 points on the patient-reported NRS. A similar amount of pain reduction following treatment was observed in several intervention studies on CBTP.14,15
Limitations
The present study had several limitations. 1) It did not define a standardized protocol, and physicians were able to choose short- or rapid-acting opioids based on clinical impression. We believe that this limitation is acceptable because our intention was to objectify treatment response to various formulations of opioids. 2) The sample size was small. 3) This was a hypothesis-generating study, and a larger, confirmatory study is needed. The strengths of the present study are that it tested our research hypothesis in a small sample size to limit resource expenditure and that measuring HRV is a feasible, noninvasive tool.
The findings suggest a causal relationship between patient-reported pain and log LF/HF after CBTP treatment. Therefore, if our results can be substantiated in a larger trial, log LF/HF might be an ideal HRV-derived parameter to monitor opioid-induced pain relief, even in unconscious patients.
In a previous pilot study with healthy volunteers, measuring HRV did not differentiate nociception of painful, nonpainful, or sham stimuli. Otherwise, in the 62 patients undergoing general anesthesia, nervous system-stimulating effects differed while the influence of various sedatives on HRV was being studied.20,21 Therefore, this topic warrants further investigation. We suggest comparing HRV outcomes and pain intensity at defined time intervals. It is interesting to note that patients with a reduction of >2 points on the NRS had a clear decline in their log LF/HF, while the log LF/HF remained more or less unchanged in patients who had a decline in NRS of only 2 points. This might reflect a cut point of ANS before it reacts to pain relief, but this needs to be investigated in further studies. Concerning dyspnea, a 1-point reduction in NRS has been deemed a minimally clinically important difference and has been used to define a response to palliative treatment in randomized controlled trials.22–24
Conclusion
This pilot study indicated that measuring HRV might provide data about analgesic response, even in unconscious patients. It is a noninvasive tool that can be ethically applied in patients suffering from advanced illnesses. Data from this preliminary study showed that the ANS of patients with advanced cancer reacted to opioid-induced relief of CBTP in a way that might allow pain detection without active contribution from patients.
Acknowledgments
This research was funded in part by the Hilde Umdasch Privatstiftung, which had no involvement in designing the study; in collecting, analyzing, or interpreting the data; in writing the article; or in the decision to submit the article for publication.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Paice JA, Cohen FL. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs. 1997;20(2):88–93. doi: 10.1097/00002820-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175–184. [PubMed] [Google Scholar]
- 3.Breivik EK, Bjornsson GA, Skovlund E. A comparison of pain rating scales by sampling from clinical trial data. Clin J Pain. 2000;16(1):22–28. doi: 10.1097/00002508-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Oldenmenger WH, de Raaf PJ, de Klerk C, van der Rijt CC. Cut points on 0–10 numeric rating scales for symptoms included in the Edmonton symptom assessment scale in cancer patients: a systematic review. J Pain Symptom Manage. 2013;45(6):1083–1093. doi: 10.1016/j.jpainsymman.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Caraceni A, Cherny N, Fainsinger R, et al. Pain measurement tools and methods in clinical research in palliative care: recommendations of an Expert Working Group of the European Association of Palliative Care. J Pain Symptom Manage. 2002;23(3):239–255. doi: 10.1016/s0885-3924(01)00409-2. [DOI] [PubMed] [Google Scholar]
- 6.Jeanne M, Logier R, De Jonckheere J, Tavernier B. Heart rate variability during total intravenous anesthesia: effects of nociception and analgesia. Auton Neurosci. 2009;147(1–2):91–96. doi: 10.1016/j.autneu.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Jeanne M, Logier R, De Jonckheere J, Tavernier B. Validation of a graphic measurement of heart rate variability to assess analgesia/nociception balance during general anesthesia. Conf Proc IEEE Eng Med Biol Soc. 2009;3(10):5332598. doi: 10.1109/IEMBS.2009.5332598. [DOI] [PubMed] [Google Scholar]
- 8.Cain KC, Jarrett ME, Burr RL, Hertig VL, Heitkemper MM. Heart rate variability is related to pain severity and predominant bowel pattern in women with irritable bowel syndrome. Neurogastroenterol Motil. 2007;19(2):110–118. doi: 10.1111/j.1365-2982.2006.00877.x. [DOI] [PubMed] [Google Scholar]
- 9.Roy RA, Boucher JP, Comtois AS. Heart rate variability modulation after manipulation in pain-free patients vs patients in pain. J Manipulative Physiol Ther. 2009;32(4):277–286. doi: 10.1016/j.jmpt.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Chang LH, Ma TC, Tsay SL, Jong GP. Relationships between pain intensity and heart rate variability in patients after abdominal surgery: a pilot study. Chin Med J. 2012;125(11):1964–1969. [PubMed] [Google Scholar]
- 11.Koenig J, Jarczok MN, Fischer JE, Thayer JF. The association of (effective and ineffective) analgesic intake, pain interference and heart rate variability in a cross-sectional occupational sample. Pain Med. 2015;16(12):2261–2270. doi: 10.1111/pme.12825. [DOI] [PubMed] [Google Scholar]
- 12.Koenig J, Jarczok MN, Ellis RJ, Hillecke TK, Thayer JF. Heart rate variability and experimentally induced pain in healthy adults: a systematic review. Eur J Pain. 2013;18(3):301–314. doi: 10.1002/j.1532-2149.2013.00379.x. [DOI] [PubMed] [Google Scholar]
- 13.Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1–2):129–134. doi: 10.1016/s0304-3959(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 14.Thronaes M, Popper L, Eeg M, Jaatun E, Kvitberg M, Kaasa S. Efficacy and tolerability of intranasal fentanyl spray in cancer patients with breakthrough pain. Clin Ther. 2015;37(3):585–596. doi: 10.1016/j.clinthera.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Velázquez Rivera I, Muñoz Garrido JC, Garcia Velasco P, España Ximenez de Enciso I, Velázquez Clavarana L. Efficacy of sublingual fentanyl vs. oral morphine for cancer-related breakthrough pain. Adv Ther. 2014;31(1):107–117. doi: 10.1007/s12325-013-0086-4. [DOI] [PubMed] [Google Scholar]
- 16.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 17.Guo Y, Palmer JL, Strasser F, Yusuf SW, Bruera E. Heart rate variability as a measure of autonomic dysfunction in men with advanced cancer. Eur J Cancer Care. 2013;22(5):612–616. doi: 10.1111/ecc.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masel EK, Huber P, Schur S, Kierner KA, Nemecek R, Watzke HH. Predicting discharge of palliative care inpatients by measuring their heart rate variability. Ann Palliat Med. 2014;3(4):244–249. doi: 10.3978/j.issn.2224-5820.2014.08.01. [DOI] [PubMed] [Google Scholar]
- 19.Zhou X, Ma Z, Zhang L, et al. Heart rate variability in the prediction of survival in patients with cancer: a systematic review and meta-analysis. J Psychosom Res. 2016;89:20–25. doi: 10.1016/j.jpsychores.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Shin S, Lee JW, Kim SH, Jung YS, Oh YJ. Heart rate variability dynamics during controlled hypotension with nicardipine, remifentanil and dexmedetomidine. Acta Anaesthesiol Scand. 2014;58(2):168–176. doi: 10.1111/aas.12233. [DOI] [PubMed] [Google Scholar]
- 21.Jess G, Pogatzki-Zahn EM, Zahn PK, Meyer-Friessem CH. Monitoring heart rate variability to assess experimentally induced pain using the analgesia nociception index: a randomised volunteer study. Eur J Anaesthesiol. 2016;33(2):118–125. doi: 10.1097/EJA.0000000000000304. [DOI] [PubMed] [Google Scholar]
- 22.Abernethy AP, McDonald CF, Frith PA, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet. 2010;376(9743):784–793. doi: 10.1016/S0140-6736(10)61115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui D, Kilgore K, Frisbee-Hume S, et al. Dexamethasone for dyspnea in cancer patients: a pilot double-blind, randomized, controlled trial. J Pain Symptom Manage. 2016;52(1):8–16. doi: 10.1016/j.jpainsymman.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]


