Preface text
Natural killer (NK) and NKT cells are subsets of lymphocytes that share some phenotypic and functional similarities. Both cell types can rapidly respond to the presence of tumour cells and participate in antitumour immune responses. This has prompted interest in the development of innovative anticancer therapies that are based on the manipulation of NK and NKT cells. Recent studies have highlighted how the immune reactivity of NK and NKT cells is shaped by the environment in which they develop. The rationale use of these cells for cancer immunotherapies awaits a better understanding of their effector functions, migratory patterns and survival properties in humans.
Introduction
The immune system is classically divided into innate and adaptive branches. Adaptive immunity can be defined by the presence of cells (T cells and B cells in higher vertebrates) that can respond to many diverse environmental antigens. This is achieved by the clonal expression of a colossal repertoire of receptors with antigen specificities (T cell receptors (TCRs) and B cell receptors (BCRs)), the diversity of which results from somatic DNA rearrangements. In contrast, the recognition of various assaults by cells of the innate immune system has been described to depend so far only upon germline-encoded receptors. A recent paradigm shift in our understanding of immunity in mammals has resulted from the discovery of these recognition receptors used by the innate immune system, such as the Toll-like receptors (TLRs), and their specificity 1–3.
Besides conventional T and B cells, a series of innate lymphoid cells (ILCs) were recently identified 4. ILCs include various cells of the innate immune system, such as lymphoid tissue-inducer (LTi) cells, but also cells that produce interleukin-5 (IL-5), IL-13, IL-17 and/or IL-22 helping to initiate immune responses to pathogens. Natural killer (NK) cells are now recognized as a subset of cytotoxic ILCs that express the transcription factor E4BP4/Nfil3. NK cells also secrete cytokines, such as interferon-γ (IFN-γ), that participate in the shaping of the adaptive immune response 5. An important feature of NK cells is their capacity to distinguish stressed cells (such as tumour cells, infected cells and cells which have undergone physical or chemical injuries) from normal cells. NK cells were initially identified through their ability to kill tumour cells (hence their name) 6–8. Since then, the anti-tumour effect of NK cells has been documented in many models and instances. In vitro, mouse and human NK cells can kill a broad array of tumour cells of hematopoietic and non-hematopoietic origin. In vivo, mouse NK cells can eliminate many transplantable and spontaneous tumours 9, 10. Selective NK cell deficiencies are extremely rare11, thus preventing the monitoring of a high incidence of cancers in these patients, but also possibly testifying of the physiological importance of NK cells. Nevertheless, an epidemiological study has linked low peripheral blood NK cell activity with increased cancer risk 12. In addition, NK cell infiltration in tumour tissue is associated with better disease prognosis in non-small cell lung carcinomas 13, 14, clear cell renal cell carcinoma 15 and colorectal cancer 16. Observations in patients with advanced gastrointestinal stromal tumour treated with imatinib mesylate support the hypothesis that NK cells exert anti-tumour effects not only through direct cytolytic activity, but also indirectly through their ability to produce cytokines such as IFN-γ 17.
Natural Killer T (NKT) cells have been classified in four different groups 18. Only type I and type II NKT cells are CD1d restricted as they respond to CD1d expressing cells and are absent in CD1d deficient mice 18 (Box 1). The most studied group, type I NKT cells or invariant NKT cells (iNKT cells), is well conserved in mammals 18. iNKT cells develop within the thymus, arising from the same common lymphoid precursor pool from which conventional T cells develop 19, 20. Once the αβ T cell lineage commitment is made and double positive thymocytes are generated, the iNKT cell and conventional T cell selection pathways diverge 21, 22. iNKT cell precursors are selected following α-chain rearrangement and expression of the semi-invariant T cell receptor (Vα14-Jα18 in mice, Vα24-Jα18 in humans) 21, 23–26. In contrast to conventional T cells that are selected by antagonist/partial ligands, it is believed that iNKT cells are selected by agonist glycolipids 27–29. iNKT express respond rapidly to a variety of glycolipids presented by CD1d 30, 31 (Box 1). Strong iNKT cell agonists, such as α-galactosylceramide (α-GalCer), induce an immediate and powerful cytokine release 32–35. Similarly to NK cells, a role for iNKT cells in tumour immuno-surveillance has been documented 36–39 and their potential in anti-tumour therapy is beginning to be uncovered.
Box 1. CD1d-restricted T cells and activation of iNKT cells.
CD1d restricted cells are well conserved in mammals and have been initially classified as type I NKT cells and type II NKT cells. Type I NKT cells (iNKT cells) express a semi-invariant TCR while type II NKT cells express a more diverse TCR. iNKT cells have been characterized using the CD1d tetramer loaded with α-GalCer or other agonist in both mouse and human as well as using the 6B11 monoclonal antibody in human. Type II NKT cell functions are unclear and have been indirectly examined by comparing the phenotype and immune response of CD1d and Jα18 deficient animals. iNKT cell frequencies is lower in humans than mice while type II NKT cell frequency is mostly unknown due to lack of tools to phenotype them. Although all iNKT cells can be identified by the CD1d tetramer, recent data indicate that there are several subsets of iNKT cells with different functions and phenotype. Notably a α-GalCer-reactive NKT cell subset that expresses a canonical Vα10-Jα50 TCR was recently identified 213. iNKT cells are unique as they have the ability to respond as innate cells with minimal TCR involvement or as memory like cells through engagement of their semi invariant TCR. The TCR dependent activation has been extensively studied using the strong agonist α-GalCer. In this case, the iNKT cell response is CD1d dependent and inflammatory cytokines are dispensable. In fact α-GalCer loaded CD1d coated on a plate is sufficient to activate iNKT cells. Interestingly pathogens, which are known to express iNKT cell antigens and require iNKT cells for effective protection, predominantly depend on inflammatory cytokines such as IL-12. The indirect pathway has recently been characterized during viral infection and is active in the presence of large amounts of inflammatory cytokines such as IL-12, IL-18 and IFN-α. In this case, iNKT cells behave functionally as classical NK cells and produce mostly Th1 cytokines. iNKT cells can also be stimulated by a combination of inflammatory cytokines and CD1d/TCR dependent stimulus. This has been reported with bacteria such as Salmonella typhimurium, Staphylococcus aureus and Mycobacterium tuberculosis that lack agonist glycolipids, and activate iNKT cells through recognition of presumably endogenous glycosphingolipids such as β-D-glucopyranosylceramide 214, presented by pathogen-activated dendritic cells 215–218.
Although their developmental programmes are controlled by different transcription factors (PLZF and E4BP4/Nfil3 respectively) 40–43, iNKT and NK cells have phenotypic and functional similarities. For instance, NK cells use Ly49H, a receptor that can be seen as an invariant TCR-like receptor, to recognize the mouse cytomegalovirus (MCMV) protein m157, and functionally behave as innate T cells 44. Conversely, during certain viral infections, iNKT cells can be activated by inflammatory cytokines with minimal TCR involvement and functionally behave as NK like cells 45–47. In addition, both NK and iNKT cells are poised to secrete cytokines 48 and they depend on IL-15 and T-bet for maturation and homeostasis 49–51. However, unlike NK cells, which rely mostly on perforin/granzyme-mediated mechanisms, iNKT cell cytotoxicity is mostly restricted to the CD95/CD178 pathway 52.
In this article, we discuss recent research on the role of NK and NKT cells in the control of tumours with a special emphasis on how the molecular dissection of their mode of recognition of tumour cells is leading to the development of innovative cancer therapies. The heterogeneity of NK and NKT cells, their roles in infections or auto-immunity, as well as their regulatory function in the shaping of adaptive immunity will not be discussed here because these topics have been extensively covered elsewhere.
How do NK cells recognize tumour cells?
NK cells express an array of receptors, notably expressed at the cell surface, that enable them to detect their cellular targets while sparing normal cells. These molecules include inhibitory, activating, adhesion and cytokine receptors. NK cells integrate these various signals that might be transduced simultaneously during the contact with the interacting cells, dictating whether NK cells will be activated or not (Figure 1). Whether and how this integration of signals leads to a commensurate or all-or-none NK cell response (such as for target cell lysis) remains to be dissected.
Figure 1.
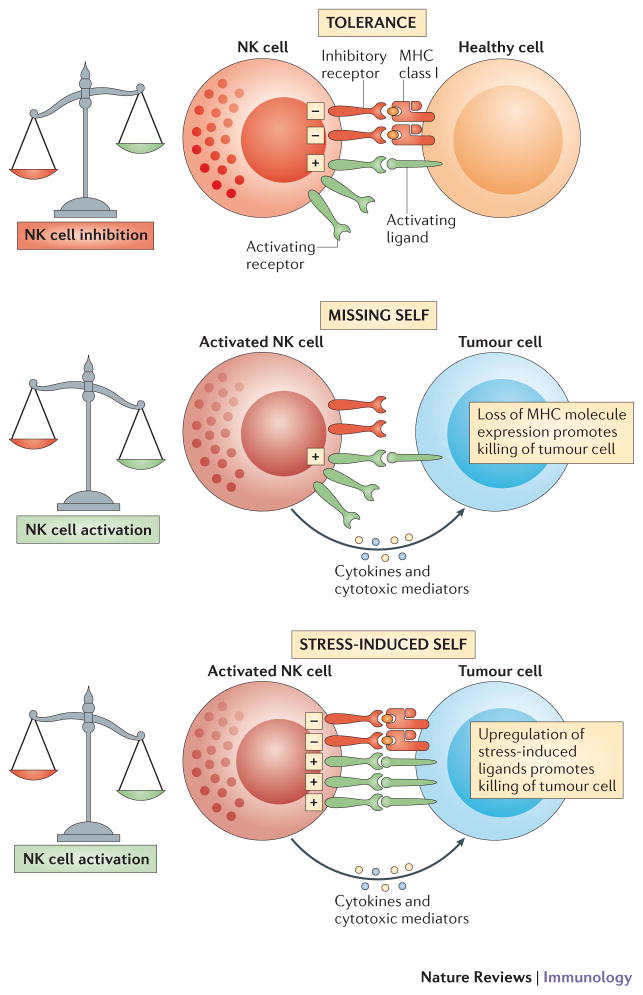
NK cell recognition of tumour cells. a | NK cells are tolerant to normal self cells as the ‘strength’ of activating signals is dampened by engagement of inhibitory receptors. b | NK cells are selectively activated by stressed cells as they express a density of cell surface ligands for activating receptors which overcomes signalling via inhibitory receptors. c | this NK cell activation leads to tumour elimination directly (cytotoxicity) or indirectly (production of cytokines such as IFN-γ).
Missing-self and NK cell education
NK cells express MHC class I-specific inhibitory receptors (Box 2). These receptors and their ligands (H-2 in mice and HLA in humans) are highly polymorphic molecules encoded by multigenic, multiallelic families of genes that are inherited independently 53, 54. NK cells have thus to discriminate self in a context where self molecules differ from individual to individual. Similarly to T cells, NK cells are educated to self versus altered-self discrimination, but the molecular strategies involved in this education are different. T cell education involves the stimulatory T cell receptor whereas NK cell education is mediated through the engagement of the MHC class I-specific inhibitory receptors. This education, also termed ‘licensing’ or ‘arming’ leads to the maturation of a NK cell functional repertoire (that is, the ensemble of signals towards which NK cells are reactive), which is adapted to self-MHC class I environment5, 55–60. In MHC class I-deficient individuals, NK cells are hyporesponsive to stimulatory receptor stimulation and thereby tolerant to self 61, 62. Hyporesponsiveness of NK cells grown in a MHC class I-deficient environment can nevertheless be overcome in inflammatory conditions 63. Thus, 2 mechanisms of self-tolerance for NK cells coexist in steady-state conditions: functionally competent NK cells, whose effector responses are inhibited by the recognition of self MHC class I molecules, and hyporesponsive NK cells that cannot detect self-MHC class I. Of course, this is a simplistic view, and NK cell education does not result in a bipolar situation, but rather in a continuum allowing fine tuning of NK cell responsiveness. For example, NK cells that express higher levels of inhibitory receptors specific for self MHC class I molecules show greater immunoreactivity that NK cells that express lower levels of inhibitory receptors specific for self MHC class I molecules 60, 64, 65. The molecular mechanisms underlying this MHC-dependent NK cell education are only partially understood. Nevertheless, a functional immunoreceptor tyrosine-based inhibitory motif (ITIM) is necessary in the intracytoplasmic tail of the mouse Ly49 inhibitory receptors 55. In addition, NK cell education via MHC class I-specific inhibitory receptors does not substantially alter the NK cell transcriptional program. Rather, it is associated with the membrane confinement of activating receptors in nanodomains, whereas these receptors are preferentially located in actin-rich membrane meshwork in uneducated NK cells 66. Thus, competent and hyporesponsive NK cells might switch from one state to another via this mechanism dependent on membrane confinement of activating receptors, conferring NK cells more plasticity than originally thought. Along this line, NK cells expressing inhibitory receptors revert from a hyporesponsive to a competent status upon exposure to cognate MHC class I molecules 67, 68. Therefore, upon encounter with target cells expressing low MHC class I surface density, one could predict that the reactivity of NK cells will decrease upon adaptation to this altered MHC class I environment. Despite the recent confirmation of the importance of ‘missing self’ recognition for NK cell responsiveness to tumours in vivo in a mouse model of chronic myeloid leukaemia 69, the kinetics of NK cells’ adaptation to their surrounding milieu which are still unclear, represent a key issue to understand the reactivity of NK cells.
Box 2. NK cells and missing-self.
NK cells use inhibitory receptors to detect the presence of constitutively expressed self-molecules on susceptible target cells. In particular, NK cells express MHC class I-specific receptors and are relieved from inhibitory signals when encountering MHC class I–deficient cells 219–224. As a consequence, NK cells can recognize ‘missing self’ on interacting cells 219. The MHC class I–specific inhibitory receptors include the killer cell immunoglobulin-like receptors (KIRs) in humans, the lectin-like Ly49 dimers in the mouse and the lectin-like CD94-NKG2A heterodimers in both species 54, 222–227. These inhibitory receptors possess intracytoplasmic inhibitory signalling domains called immunoreceptor tyrosine-based inhibition motifs (ITIMs) that mediate their inhibitory function 228–230. Other inhibitory receptors (for example, mouse NKR-P1B, human NKR-P1A and mouse 2B4) that recognize non-MHC self-molecules (for example, Clr-b, LLT-1 and CD48, respectively) also regulate NK cell activation 231.
Stress-induced self recognition
Besides using inhibitory receptors that recognize self, NK cells are also equipped with cell surface activating receptors 70, 71. In addition to the recognition of microbial molecules by a variety of innate immune receptors, the so-called ‘infectious non-self recognition’, it has been shown that several receptors of innate immune cells can detect internal changes that occur in damaged host tissues, leading to the concept of ‘stress-induced’ self recognition”72–74. This mode of detection relies on the recognition of self molecules that are barely detectable in steady-state conditions, but whose expression increases in various forms of stress. A prototypical example is the activation of NK cells via engagement of the activating NKG2D receptor. NKG2D interacts with self-molecules that are selectively up-regulated on stressed cells, such as tumour cells 75–78. In vivo, NKG2D was shown to be crucial for immunosurveillance of epithelial and lymphoid malignancies in two transgenic models of de novo tumour genesis 79. In the transgenic Eμ-Myc mouse model of spontaneous B cell lymphoma, the tumour expression of NKG2D ligands represents an early step of tumour genesis that is associated with still unknown genetic lesions of cancer cells 80. NKG2D ligands are the stress-inducible MICA/B and ULBP/RAE proteins in humans 72, 77 and Rae1, H60, and Mult-1 proteins in mice 75, 76, 78. A link between tumour genesis, DNA damage response (DDR) and the immune response has been proposed. DNA-damaging agents or DNA lesions associated with tumour genesis activate the DDR, which results in up-regulation of NKG2D ligands leading NK cells to attack the diseased cells 81. The up-regulation of NKG2D ligands depends on the PI-3 kinase-related ATM (ataxia telangiectasia, mutated) or ATR (ATM- and Rad3-related) protein kinases, which initiate the DDR pathway after exposure to DNA damage 73. Treatment with proteasome inhibitors also induces NKG2D ligand expression in multiple myeloma cells via the ATM/ATR pathway 82.
Besides NKG2D, NK cells express an array of cell surface molecules, such as the natural cytotoxicity receptors (NCR), which were shown to be involved in the activation of NK cells by tumour cells more than a decade ago. The NCR family includes NKp46 (NCR1, CD335) 83, NKp44 (NCR2, CD336) 84 and NKp30 (NCR3, CD337) 85. NCR association with immunoreceptor tyrosine-based activation motif (ITAM)-bearing transducing polypeptides is reminiscent of the architecture of other pivotal immune receptor complexes, such as the TCR-, BCR- and Fc-receptors, and makes them very potent activating receptors 71. The tumour cell surface ligands for the NCR family have remained elusive, hindering a complete understanding of their role in tumour surveillance. An exception resides in the identification of a novel member of the B7 family of immunoreceptors, B7-H6, as a cellular ligand for NKp30 86, 87. The expression of B7-H6 on tumour cells induces NKp30-dependent NK cell activation and cytotoxicity. Importantly, B7-H6 is absent from all normal cells tested in steady-state conditions, but is expressed by tumour cells. Considering that NK cells do not compromise the integrity of normal healthy cells and tissues, a reasonable hypothesis is that, as for NKG2D ligands, self-ligands for all activating NK cells receptors are tightly down-regulated in healthy cells and are up-regulated in stressed cells, such as tumour cells. There are many other activating receptors and adhesion molecules that are present on NK cells and may participate in the recognition of tumour cells. These receptors include DNAM-1 via its ligands poliovirus receptor (PVR) and Nectin-2 88, and the signalling lymphocytic activation molecule (SLAM)-receptors (2B4, NTB-A) as reviewed elsewhere 88. Importantly, both human and murine NK cells also express FcγRIIIA (CD16), which recognizes antibody-coated target cells through their Fc portion and mediates antibody-dependent cellular cytotoxicity (ADCC). Growing evidence indicates that ADCC contributes to the beneficial clinical response of human cancers to several monoclonal antibody-based therapies, including treatment with rituximab 89, 90, implying that NK cells are involved in the antitumour response in these settings.
Manipulation of NK cells in antitumour therapy
The remarkable conservation of their antitumour activity against many types of murine and human tumours suggests that NK cells detect common modifications in cellular metabolism and/or gene expression that are shared or induced by many oncogenic processes. This ability of NK cells to target a common mechanism present in cancers while respecting the integrity of healthy cells, has led to them being considered as promising therapeutic tools for cancer immunotherapy 91.
Allogeneic hematopoietic stem cell transplantation for cancer patients
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an efficient form of adoptive immunotherapy in the treatment of patients with haematological malignancies 92, although the therapeutic effect was originally thought to derive mostly from the escalated doses of cytotoxic agents and radiation therapy used in the so-called “conditioning regimen”. The reality of a graft-versus-leukaemia (GVL) effect developed by the allogeneic graft was nevertheless established more than 3 decades ago 93. Since this time, the possibility of disease control in other haematological malignancies 94 but also in solid tumours 95, 96 has been confirmed. Despite these proof-of-concepts, allo-HSCT has long been carried out in a limited subset of cases as compared to the authentic extent of potential population. This is in part due to the high rate of fatal toxicities attached to the procedure, which eventually impairs patient survival despite effective disease control. These complications are mainly related to the inner mechanism of this treatment which relies on immunological effect 97, at least when used to treat patients with various forms of advanced or poor-prognosis cancers. Indeed alongside the searched GVL effect, allogeneic immune activation is the source of a Graft-Versus-Host disease (GVHD) recognition targeting minor histocompatibility antigens on non tumour tissues. Even in the context of fully HLA identical sibling donor, this latter effect is highly effective: when fully developed, it conducts to unwanted and potentially serious injuries of normal tissue as gut, skin and liver epithelia. This has and still represents an important limitation for allo-HSCT development along with the lack of donors. Indeed, for years, allogeneic HSCT protocols have failed to manipulate the immune system to boost graft-versus-tumour (GVT) effects without inducing GVHD. In order to solve this issue, most of HSCT protocols have relied on the manipulation of T cell responses, an attempt that proved to be extremely hazardous due to the underestimated and unpredictable cross-reactivity of the TCRs. In contrast, it is now established in pre-clinical and clinical settings that NK cells have a unique capacity to exert potent GVT effects without inducing GVHD, a feature which most likely results from the differential distribution of ligands for activating NK cell receptors on hematopoietic and non-hematopoietic tissues, such as epithelial cells 98.
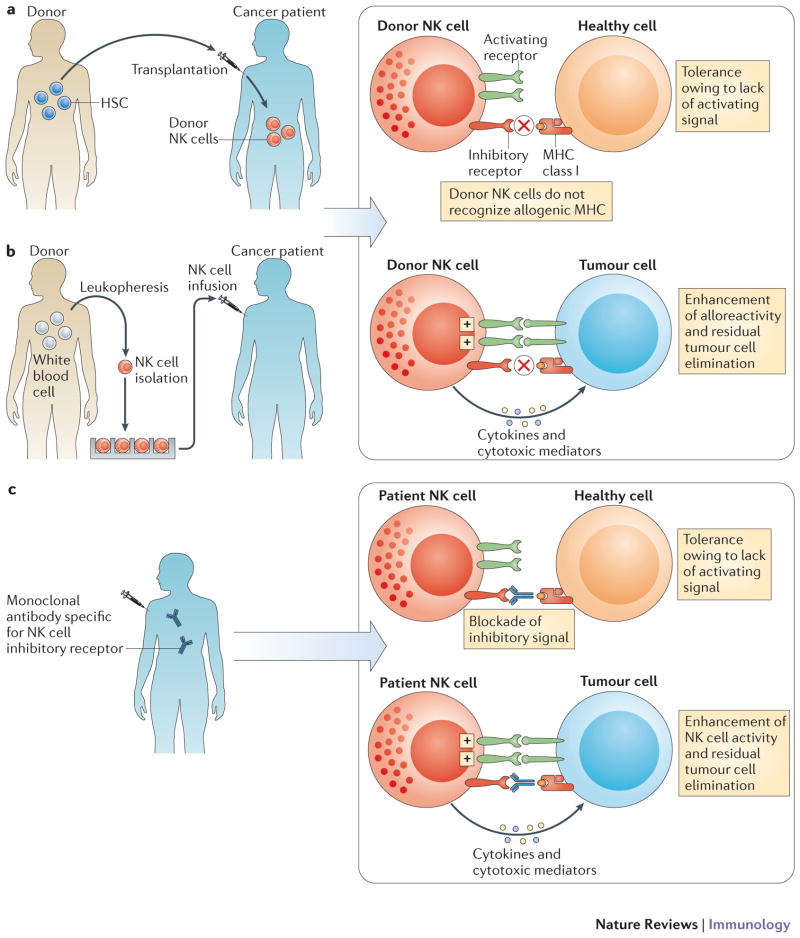
Allo-HSCT has been an evolving field in the last two decades, as a consequence of the diversification of stem cell and donor sources and changes in conditioning regimen. One type of allo-HSCT is the so-called ‘haplo-identical’ transplantation, which was developed to overcome the problem posed by a lack of an HLA-matched donor and has been recently put in light again the potential of NK cells in cancer control. In haplo-identical transplantation the related donor and recipient share only one haplotype encoding HLA molecules. In some but not all cases, the recipient expresses MHC alleles (that is, KIR ligands) that are not expressed by the donor. In this ‘KIR ligand-mismatched’ situation, subsets of donor-derived NK cells that are not restrained by host MHC class I molecules develop in the recipient and have GVL potential. In classical haplo-identical HSCT protocols, the allograft is depleted of T-cells before reinfusion to reduce the incidence of severe and potentially lethal GVHD, and the patient receive highly cytotoxic and immune-suppressive conditioning regimen to prevent graft rejection 99. In these settings, recipients with acute myeloid leukaemia (AML) who received a haplo-identical transplant from a KIR ligand-mismatched donor had a marked reduction in relapse compared with an otherwise similar group of AML patients transplanted from a KIR ligand-matched related donor100. In haplo-identical HSCT, the positive influence of KIR-incompatibility was detected in other studies with patients with AML 101, 102 or multiple myeloma 103, but not in others 104, indicating that other parameters in the treatment protocol - such as the dose of stem cells and the extent of T cell depletion - contribute to clinical outcome. Hence, the spectrum of alloreactivity displayed by donor-derived NK cells remains to be fully elucidated (Figure 2a). Clinical efficacy against residual patient tumour cells requires that fully competent NK cells of donor origin develop in the recipient following transplantation. Considering the role of MHC class I in NK cell education and the plasticity of NK cells described above, the development of alloreactive NK cells of donor origin is likely to depend upon the recipient’s conditioning regimen and the dose of donor hematopoietic progenitors (that is, the likelihood and the duration of interaction with donor or recipient MHC class I molecules). The complexity and changing nature of such interactions is a likely explanation for the apparently inconsistent observations of NK cell alloreactivity in different protocols and studies. The challenge will be to harness the therapeutic potential of NK cells in this context. NK cells could also be used as part of the conditioning regimen, as it has been shown in mouse models that donor NK cells can kill host dendritic cells impairing donor T cell-mediated GVHD 100. Irrespective of this possibility, NK cells are especially attractive therapeutic tools because their association with a reduction in relapse rates was not associated with increased GVHD incidence. Though limited, these observations thus suggest that NK cell manipulation can dissociate GVL and GVHD effects, an objective that has never been attained through the manipulation of T cell compartments. In addition, cyclosporin A that is administered to inhibit T cells and prevent GVHD can also induce NK cell expansion and boost NK cell functions105, 106. Finally, recent work has also focused on the role of activating KIR (KIR-S) in transplantation for patients with leukaemia. KIR-S are molecular homologs of the inhibitory KIRs, with shorter cytoplasmic tails that are devoid of ITIMs and with a transmembrane domain that associates with the ITAM-bearing transducing polypeptide DAP12 107–109. KIR-S are potent NK cell activators and can also be expressed by certain rare T cell subsets. The genes encoding KIR-S are absent in approximately 25% of Caucasians who are homozygous for the so-called KIR gene group A. Investigations into KIR-S genetics, showed that the presence of activating KIRs was associated with less leukaemia relapse and cytomegalovirus reactivation and improved survival in some patients 110–115. Thus, several years after the clinical proof of concept was brought by a pivotal study 100, the question remains of how NK cell alloreactivity can be fully exploited to produce clinical benefits in haplo-identical HSCT. In addition, these protocols of haplo-identical HSCT remain used only for a minor subset of patients with poor prognosis malignancies, because the protocols are associated with a high transplant-related mortality due to profound and durable immunosuppression.
Figure 2.
NK cellular therapy. a | In haplo-identical or MHC-matched HSCT, NK cells of healthy donor origin will develop into the cancer patient. b | Alternatively, NK cells can be isolated from healthy donors, activated and/or expanded in vitro prior to infusion into the cancer patient. In both cases (allo-HSCT and NK cell infusions), the aim is to promote the anti-tumour function of donor NK cells in the cancer patient; indeed a fraction of donor NK cells will be not be inhibited by the MHC class I molecules of the cancer patient as the KIR expressed by these subsets of NK cells will not interact with by the MHC class I molecules of the cancer patient (ELIMINATION). Most normal cells of the patients will not activate donor NK cells as, in contrast to cancer cells of the patient, they lack the cell surface density of ligands required to activate donor NK cells (IGNORANCE). c | Anti-KIR monoclonal antibody therapy. Fully human anti-KIR mAbs can be injected in cancer patient. Anti-KIR mAbs are designed to boost the antitumour activity of NK cells (ELIMINATION) without inducing auto-immunity (IGNORANCE).
HLA-identical HSCT is still the most frequently used and continuously expanding approach in patients with cancer and has highly benefited from the reduced toxicity associated with the new approaches for conditioning regimen. Following the initial description of the first clinical success 92, allo-HSCT procedures were carried out with basically unmodified protocols for 20 to 25 years. It begins with a preparative phase, so called conditioning. This phase is followed by allogeneic graft cell infusion and a post infusion drug immunosuppression in attempt to limit unwanted GVH reactions. The goals of conditioning are to treat residual tumour disease and to allow for donor engraftment through a profound immunosuppression of the host. Both goals have been carried out rather successfully by the association of high dose total body irradiation with chemotherapy or by the association of highly myeloablative anti-neoplasic drugs. However, the cytotoxicity of the conditioning generates pro-inflammatory cytokines which represent the starting point of the previously described negative effects 116. Later, this pro-inflammatory effect was integrated in a more complex cytokine-storm hypothesis. Indeed, these cytokines when reaching blood stream get together with the circulating immune cells of the infused graft, turning on their immune capacities and eventually generating GVH effects. This now classical scenario suggested that reducing cytokine generation could afford less toxicity. Some years later, this hypothesis was convincingly verified by the combination of distinct potent immunosuppressive agents, including notably a purine analogue compound, to carry out allogeneic engraftment, leaving the anti-tumour action of allo-HSCT mainly to the development of GVL or GVT effect 117–119. Since this time initial findings have been widely confirmed and the so-called reduced intensity conditionings (RIC) are now broadly used. Indeed the dramatic diminution of procedure related mortality120 has conducted to propose this treatment to a wider population, including patients with other diagnoses than leukaemia - lymphoid malignancies and solid tumours - and patients above the classical age of 50 years, generally not considered for transplantation because of the increased risk of fatal complication. This has conducted to a nearly doubling of the number of transplants worldwide in less than 10 years 121. Altogether these achievements justify the present attempts to further improve disease control after HLA-identical HSCT and in particular, approaches that manipulate NK cells. Indeed donor NK cells are among the first cells to arise in the recipient following allo-HSCT 122, with a rapid NK cell reconstitution evident as early as 1.5 months post-RIC HSCT 123, 124. Various studies have shown an association between higher numbers of NK cells in the graft and lower rates of relapse 115. High NK cell counts on day 30 post allo-HSCT have also been associated with improved clinical outcomes125, 126. More recently, an association between high NK cell counts at day 60 and reduced relapse after RIC HSCT was reported 127. This effect was not documented after standard conditioning regimens. Altogether, these data demonstrate that NK cells are readily present at early times following HSCT in RIC and can exert their functions even during immunosuppressive treatment.
Donor lymphocyte infusions
Nowadays, allo-HSCT anti-tumour activity can be reinforced through single or sequential reinfusions of donor-derived immune competent cells once hematopoietic chimerism is established. Initially, in the mid 90’s, donor lymphocyte infusions (DLIs) were shown to result in a high incidence of durable cytogenetic and molecular remissions when used as a treatment for chronic myeloid leukaemia (CML) that relapsed after conventional allo-HSCT 128, 129. However, despite these positive effects, significant side-effects occurred, including GVHD and secondary aplasia, as a consequence of the high numbers of cytotoxic T cells in the infusions 130. Attempts to deplete CD8+ T cells from DLIs produced encouraging, although incomplete results 131, 132. The interest of lymphocyte-driven immunotherapy led to the infusion of allogeneic lymphocytes being explored as a therapy for cancer outside the context of allogeneic HSCT 133. Short-term lymphocyte survival was noted and this prevented long-term cancer control although clinical responses were documented in some patients. In line with these initial data, a recent study infusing HLA-mismatched peripheral blood stem cells established the outcome improvement of chemotherapy for acute myeloid leukaemia in elderly patients 134. Whether the use of appropriately selected and activated donor-derived NK cells instead of regular DLIs will better support an antitumour effect remains to be shown, but this warrants further study. Several protocols of clinical grade NK cell purification and in vitro expansion are now validated, and studies have shown that the infusion of allogeneic NK cells is safe in humans 135–149 (Figure 2b). Injections of mature HLA mismatched NK cells are also well-tolerated 150. Therefore there are encouraging signs that NK cell infusion could be a useful antitumour strategy
KIR-specific monoclonal antibodies
As already mentioned, the use of allo-HSCT remains restricted to minor subsets of patients affected with poor prognosis malignancies, mainly subsets of patients presenting with acute leukaemia associated with poor-prognosis criteria (mostly cytogenetic), relapse, patients with myelodysplastic syndromes, as well as patients with lymphoid malignancies who failed or relapsed after initial therapy. Restrictions to its use include the difficulty in identifying a suitable donor as well as the physical condition of the recipient and his/her predicted ability to sustain the morbidity associated with the transplantation; as a consequence allo-HSCT can rarely be offered to elderly people, populations in which the incidence of haematological malignancies is increasing. In vivo, activation of NK cells is another avenue for medical progress, with potential applications in larger populations of patients. Fully humanized KIR-specific monoclonal antibodies have been generated to achieve such progress 151, 152 (Figure 2c). By blocking the interaction between all inhibitory KIR molecules that recognize HLA-C alleles, KIR-specific monoclonal antibodies are expected to boost the reactivity of NK cells against tumour cells expressing ligands for activating receptors, without inducing autoimmunity against normal cells, which do not express sufficient density of activating ligands 153 (Figure 2c). However, the recognition of MHC class I molecules by KIRs is crucial for NK cell education. The blocking of KIRs by monoclonal antibodies may have more complex consequences than simply triggering tumour elimination. To fully evaluate this issue in vivo, a ‘humanized’ preclinical mouse model has been developed in which all NK cells are educated by a transgenic inhibitory receptor, human KIR2DL3, through the engagement with its HLA-Cw3 ligand. This approach revealed that NK cells could be reprogrammed to kill HLA-Cw3+ target cells without compromising self-tolerance and without abolishing NK cell education 154. Following pre-clinical evaluation, 100 patients with AML or MM were treated in phase I or II clinical studies (N. Vey et al., in preparation). These studies showed that the infusion of KIR-specific monoclonal antibodies was safe, even in mostly elderly patients who had been heavily pre-treated by chemotherapy. Although the results of efficacy are awaited, the availability of KIR-specific monoclonal antibodies thus paves the way for the design of innovative NK cell-based antitumour therapies. For instance, new protocol might include the combination of KIR-specific monoclonal antibodies with HLA-identical HSCT, with the infusion of HLA-identical NK cells, with other monoclonal antibodies (for example, CD20-specific or HER2/Neu-specific monoclonal antibodies), or with drugs that induce the expression of ligands for activating NK cell receptors, such as lenalinomide 155.
In conclusion, the efficacy of NK cell-based antitumour therapies still remains to be firmly established. Question remain concerning the sensitivity of tumour cells to NK cell attack, on the migratory properties of endogenous 156 and infused NK cells 157, as well as on the survival and homeostatic proliferative capacity of donor NK cells in cancer patients in conditions of chemotherapy and/or HSCT 158. Many clinical trials have been initiated which hopefully will answer some of these key issues (Box 2).
Roles of NKT cells in cancer
In earlier studies, NK and NKT cell functions in antitumour immune responses have been sometimes confounded. Recent progress in the characterization of iNKT cells allowed for a better understanding of their functions in response to tumours. We review below how iNKT cells are believed to recognize tumour cells and how these findings have led to current strategies to target these cells for tumour therapy.
How do NKT cells recognize tumour cells?
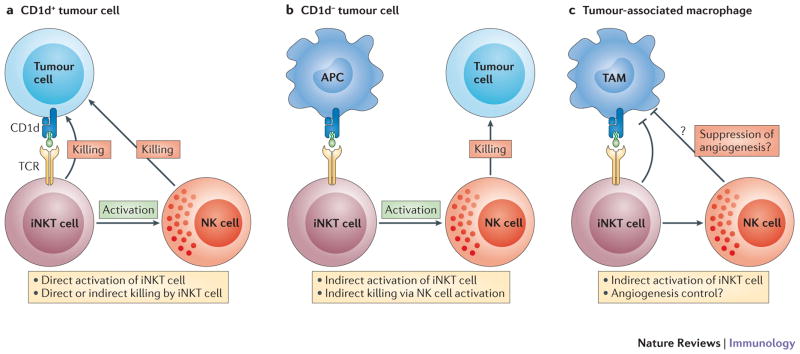
Activation of iNKT cells with strong agonists such as α-GalCer leads to strong antitumour responses in mice (Figure 3). However, in this case, the iNKT cell contribution to the antitumour response is indirect and mediated in part by downstream effectors such as NK cells (Figure 3) and by the cytokine IFN-γ, rather than directly targeting cancer cells by cytotoxic mechanisms. iNKT cells can directly respond to IL-12 in the B16 melanoma model and produce IFN-γ, yet iNKT cells are dispensable in the B16 melanoma model 159. However, their contribution in the absence of NK cells using NK cell deficient mice has not been addressed in this model. A direct recognition of CD1d-expressing tumour cells by iNKT cells has been demonstrated in vitro (Figure 3a). CD1d is expressed on some myelomonocytic leukaemia cells and it has been shown that these cells are sensitive to lysis by human NKT cells 160. Similarly, using a CD1d-transfected mouse B-cell lymphoma, it was shown that iNKT cells protect against tumour progression in a CD1d-dependent manner 161. However, it is unclear how iNKT cells distinguish CD1d expression on malignant cells from CD1d expression on normal cells. It is possible that a different set of self ligands is presented by CD1d in transformed cells, yet the evidence for this are lacking. Although CD1d expression has been demonstrated on human malignant hematopoietic cells, the majority of solid tumours and cell line models do not express CD1d or poorly express CD1d suggesting an indirect mechanism or cross-presentation to iNKT cells (Figure 3b) 162.
Figure 3.
iNKT cell recognition of tumours. a | normal and direct recognition of tumour cells by iNKT cells. b | Indirect activation and indirect killing mediated by iNKT cells. c | Control of angiogenesis by iNKT cells
Role of iNKT cells in anti-tumour immuno-surveillance
There are strong evidences for a role of iNKT cells in tumour immunosurveillance at least in mice. A protective role for iNKT cells in tumour immunosurveillance has been demonstrated in various tumour models, including MCA-induced fibrosarcomas, P53 loss and in the TRAMP prostate cancer model 36–39. These studies were performed in the absence of exogenous stimuli and by comparing Jα18-deficient and CD1d-deficient mice to wild-type mice suggesting that iNKT cells were critical while type II NKT cells were dispensable. Adoptive transfer of CD4/CD8 negative iNKT cells from the liver suggest a crucial role for this subset of iNKT cells in promoting antitumour immune responses. iNKT cell IFN-γ production has been shown to be critical at least against MCA-induced sarcomas 163 and most likely promotes NK cell activation. Overall, these studies in mice suggest that iNKT cells have an active role in tumour immunosurveillance and their absence predisposes to cancer development. However, it is not entirely clear how iNKT cells become activated in these models and which CD1d-expressing cells activate them. In addition, the contribution of endogenous lipids and inflammatory cytokines is unknown. In fact, it has been proposed that iNKT cells do not target directly the tumour but instead control CD1d-expressing tumour-associated macrophages, preventing these cells from promoting angiogenesis 164 (Figure 3c). In humans, observational studies have focused mostly on iNKT cells. Overall, iNKT frequency is decreased in solid tumours including in melanoma, colon, lung, breast and head and neck squamous cell carcinoma while an increased iNKT cell number is associated with a better prognosis 165–167. Therefore these observational studies in human are consistent with the mouse studies and suggest that iNKT cells may play a role in tumour immunosurveillance. Interestingly, type II NKT cells have been shown to suppress tumour immunosurveillance provided by iNKT cells potentially explaining the paradox in the role of CD1d restricted T cells in the regulation of tumour immunity 168.
Manipulating iNKT cells for tumour therapy
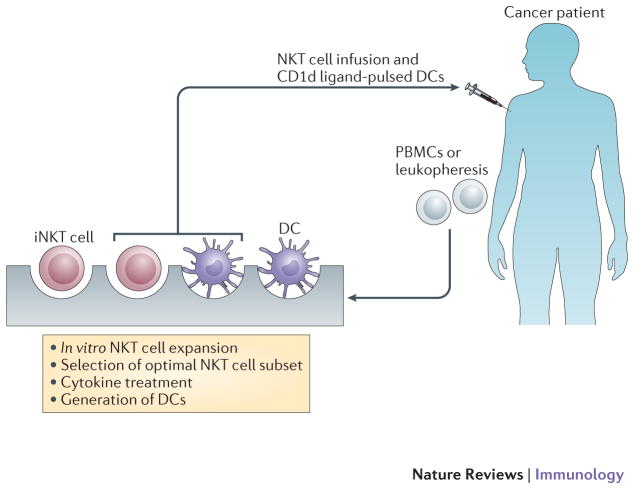
Initial studies clearly show the antitumour effects of soluble α-GalCer in mice 169, 170. In mice, α-GalCer induces a rapid iNKT cell activation that leads to downstream activation of NK cells. The cascade of activation initiated by α-GalCer-activated iNKT cells extends to cells of the adaptive immune system, although this is delayed in comparison to the rapidity at which iNKT cells promote NK cell activation. However, α-GalCer administration can also induce IL-4 production and in some cases anergy, as they become unresponsive to subsequent activation 171, 172. To circumvent these issues, other delivery methods have been examined including adoptive transfer of α-GalCer pulsed monocyte-derived dendritic cells, which induce a more potent anti-tumour effect than treatment with soluble α-GalCer alone (Figure 4) due to the dendritic cell superior ability to present antigens and expression of co-stimulatory molecules at their cell surface. A significant amount of work has been undertaken to design glycolipids that will induce a stronger Th1-type response and target a specific subset of iNKT cells known to be biased toward Th1 responses at least in human 173. Although polarization of the iNKT cell response has been observed using α-GalCer analogues, the mechanism leading to this polarization is still under intense investigation 174–176. Regardless of the mechanism(s), it was shown that although α-GalCer analogues that are associated with either a Th1-type or a Th2-type immune response can induce the predicted systemic cytokine bias in mice (production of IFN-γ or IL-4, respectively), the immediate iNKT cell response is not polarized 177. Two groups targeted murine CD1d itself and showed that injection of a CD1d-specific monoclonal antibody has the capacity to directly mature antigen-presenting cells, leading to inflammatory cytokine secretion and tumour growth prevention 178, 179. However, the CD1d mediated intracellular signalling pathway has not been defined and it is not known whether human CD1d can perform similar functions to mouse CD1d. Notably, a recent study showed that blocking antibodies specific for CD1d had opposite effects and increased tumour metastasis 180. Targeting specific CD1d-expressing cell subsets has been attempted using antigen–α-GalCer conjugate particles preferentially adapted for B cells or CD169 positive macrophages 181, 182. Another strategy was to expand autologous iNKT cells in vitro in order to compensate for the decreased iNKT cell frequency observed in cancer patients 183,184. Clinical trials using α-GalCer or α-GalCer-pulsed antigen presenting cells showed that these treatments were relatively safe and well tolerated. Soluble administration of α-GalCer did not result in significant clinical benefits 185, 186, although injection of α-GalCer-loaded immature dendritic cells led to modest iNKT cell activation in vivo 187, 188. However, when mature monocyte-derived dendritic cells were used, iNKT cell population expansion was observed and this led to an increase in serum levels of IL-12 and IFN-γ 189. Interestingly, the combination of iNKT cell transfer and α-GalCer-pulsed Dendritic cells has been reported to induce significant antitumour immunity in patients with head and neck squamous cell carcinomas 184, 190 (Figure 4). Overall, there are strong evidences for iNKT cell roles in tumour immunosurveillance and anti-tumour potential of ligand activated iNKT cells. Additional studies to optimize glycolipid delivery and to target specific iNKT cell subsets warrant future investigations.
Figure 4.
iNKT cellular therapy. Patient Specific iNKT cell subset expanded in vitro prior to infusion together with CD1d ligand pulsed Dendritic cells.
Perspectives on NK and NKT cells anti-tumour properties
Recent studies indicate that NK cell activation could lead to the generation of ‘memory’ NK cells, a feature only ascribed to B and T cells so far, and recognized as the hallmark of the adaptive immune system. In the model of MCMV infection, memory-like NK cells present increased reactivity not only to molecules that activate their MCVM receptor Ly49H but also to those that activate NK1.1 44. Similarly cytokine (IL-12/IL-18)-pre-activated NK cells respond better to NK1.1 or Ly49H stimulation 191. In addition, hapten- and virus-specific memory NK cells have been described 192, 193. As a consequence of the expression of activating receptors involved in tumour elimination on most if not all NK cells (e.g. NKp46, NKp30, NKG2D), NK cells that have experienced a non-tumour driven activation (e.g. infection, adjuvants) might display broad cross-reactivity and thus exert better anti-tumour function as shown many years ago 194. The capacity to produce primed/memory NK cells to fight against cancer, as well as the possibility to drive the expansion of tumour-specific memory NK cells thus represents an attractive avenue to explore.
α-GalCer has been tested as a potential adjuvant due to its ability to induce the activation of a variety of immune cells, including NK cells, in both mice and humans 195–202. These and other findings led to the development of α-GalCer analogs and strategies to better exploit iNKT properties in the design of adjuvants or vaccines 203–206. Therefore, anti tumour therapy taking advantage of the adjuvant potential of iNKT cell ligands and effector functions of both NK and iNKT cells warrant future investigations.
Finally, detrimental roles of NK cells have recently emerged in conditions of inflammation, where NK cells can aggravate sepsis conditions 207, in conditions of auto-immunity as they might contribute to the onset of pathology and to tissue damage 208, as well as in conditions of microbial infections where they can dampen subsequent T cell responses 209, 210. As inflammation is now recognized as a key element in tumour development 211 and anti-tumour T cell functions have been shown to increase patient survival 212, these issues should be considered for the establishment of robust immunotherapy protocols. It is also mandatory to improve blood and tissue immune-monitoring to explore in large cohorts of patient the role of NK and NKT cells during cancer.
The knowledge of antitumour immune control has recently rapidly progressed, and a new vision of immunotherapy has emerged from new concepts, medical strategies, medications and medical devices. It is likely that in the coming years the reciprocal movement from bench to bed and from bed to bench will continue to accelerate feeding both scientific knowledge and innovative treatments meeting medical needs. In this perspective, NK/NKT cells undoubtedly represent very exciting players of antitumour immunotherapy.
Box 3. NK cell clinical trials.
A survey on clinicaltrials.gov database searching for ‘clinical trials and NK cells’ indicates that more than 200 clinical trials have been registered since 2003. Of these, ≈150 are observational studies and ≈50 are interventional clinical interventions with NK cells (phase I: 20; Phase I/II: 11 Phase II: 17; Phase III: 3). Various selection and expansion (if any) procedures and clinical situations are represented, demonstrating the current lack of consensus in the field. Most protocols are designed for patients with haematological malignancies; and only 5 (10% of interventional clinical interventions with NK cells) recruit patients with solid tumours. Identically only 7 (14%) are proposed to childhood. Interestingly 28 trials are designed and conducted in the context of allo-HSCT setting (18 after partially compatible transplant); the 22 other protocols evaluate the use of allogeneic (mainly from a mismatched related donor) NK cells outside of the context of regular HSCT. Altogether, if completed they anticipate to recruit a large number of 1863 patients. To date, seven clinical trials have been completed for an initial estimated enrolment of 103 patients and only two of those (NCT00274846 and NCT00354172) have reported results.
Acknowledgments
E. V. and S. U. are supported by funds from the THINK European Research Council advanced grant, Agence Nationale de la Recherche, Ligue Nationale contre le Cancer, and institutional grants from INSERM, CNRS and Aix-Marseille Université to the CIML. E. V. is a scholar of the Institut Universitaire de France, a co-founder of and shareholder in Innate-Pharma. D. B. and C. C. are supported by Institut Paoli-Calmettes, PHRC # 2010 and Association de la Recherche contre le Cancer grants (Programme ARC, 2011). L. B. is supported by NIH research grants AI46709 and AI058181.
Glossary terms
- Toll-like receptors (TLRs)
A family of evolutionarily conserved pattern-recognition receptors. These molecules are located intracellularly and at the cell surface of macrophages, dendritic cells, B cells and intestinal epithelial cells. Their natural ligands are molecules that are found in bacteria, viruses and fungi.
- Innate lymphoid cells (ILCs)
A group of cells of lymphoid origin that includes NK cells, LTis and other non-T, non-B cells that produce distinct cytokines such as IL-5, IL-13 or IL-17.
- Natural killer cells (NK cells)
Non-T, non-B lymphocytes and can mediate natural killing against prototypical NK-cell-sensitive targets — K562 (human) and YAC-1 (mouse) and/or produce IFN-γ. In humans, NK cells are typically NKp46+CD56+CD3−, and they are NK1.1+CD3− in the C57BL/6 mouse strain and generally NKp46+CD3− in all mouse strains.
- imatinib mesylate (Gleevec®)
First of its class Tyrosine Kinase Inhibitor (TKI), with clinical activity against Chronic Myeloid Leukaemia (CML) associated with the t(9;22) reciprocal translocation. Introduction of imatinib mesylate in clinical practice at the end of the 20th century, induced a rapid shift in medical practices, leading to abandon alloHSCT for what previously was a standard indication.
- CD1d
A MHC-like molecule that associates with β2-microglobulin and present lipids.
- T-bet
A member of the T-box family of transcription factors. It is a master switch in the development of T helper 1 (TH1)-cell responses, through its ability to regulate expression of the interleukin-12 receptor, inhibit signals that promote TH2-cell development and promote the production of interferon-γ.
- perforin/granzyme-mediated mechanisms
Granzymes are serine proteinases that are found primarily in the cytoplasmic granules of cytotoxic T lymphocytes and natural killer cells. They enter target cells through perforin pores, then cleave and activate intracellular caspases and lead to target-cell apoptosis.
- CD95/CD178 pathway
CD178 (also known as FAS ligand) binds to CD95 (also known as FAS). This results in the formation of the death-inducing signalling complex and subsequent activation of caspases, which leads to apoptosis in the CD95-expressing target cell.
- Immunoreceptor tyrosine-based inhibitory motif (ITIM)
This motif is present in the cytoplasmic domain of several inhibitory receptors. After ligand binding, ITIMs are tyrosine phosphorylated and recruit lipid- or tyrosine-phosphatases.
- Immunoreceptor tyrosine-based activation motif (ITAM)
Activating receptors often have ITAMs consisting of a consensus amino-acid sequence with paired tyrosines and leucines (YxxI/Lx(6–12)YxxI/L). These are normally located in the cytoplasmic domains of ligand-binding transmembrane receptors (such as FcεRI and TCR) and they mediate interaction between the transmembrane receptor complex and protein tyrosine kinase activity, which is required to initiate early and late signalling events.
- DNA damage response (DDR)
A cell response triggered by DNA damage, such as single or double strand breaks. DDR stops cell cycle progression to enable repair before the damage is transmitted to progeny cells. Checkpoints in the mammalian DDR are the phosphatidylinositol 3-kinases ATM and ATR.
- Antibody-dependent cellular cytotoxicity (ADCC)
The capacity of NK cells to lyse antibody-coated target cells via their Fc receptor, CD16.
- Rituximab
A chimeric monoclonal antibody that is specific for the CD20 molecule, which is primarily expressed by B cells. Rituximab is the most frequently used antibody therapy for patients with cancer
- Graft-versus-leukaemia (GVL) effect
The anti-tumour activity of donor T cells against residual leukaemic cells of the graft recipient following (allogeneic) bone marrow transplantation.
- Graft-versus-host disease (GVHD)
Tissue damage in a recipient of allogeneic transplanted tissue (usually a bone-marrow transplant) that results from the activity of donor CTLs that recognize the recipient’s tissue as foreign. GVHD varies markedly in severity, but can be life threatening in severe cases. Typically, damage to the skin and gut mucosa leads to clinical manifestations.
- Reduced-intensity conditioning (RIC) regimen
A regimen that uses less chemotherapy and radiation than that normally used for myeloablation.
- cyclosporin A
A commonly used immunosuppressive drug that blocks calcineurin A and thereby inhibits T cell activation. It is used to prevent the rejection of transplanted organs and to treat some inflammatory diseases.
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–59. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 4.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–7. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 5.Vivier E, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oldham RK, Herberman RB. Evaluation of cell-mediated cytotoxic reactivity against tumor associated antigens with 125I-iododeoxyuridine labeled target cells. J Immunol. 1973;111:862–71. [PubMed] [Google Scholar]
- 7.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–29. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 8.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 9.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–61. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 10.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 11.Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol. 2006;118:1–10. doi: 10.1016/j.clim.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–9. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 13.Carrega P, et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer. 2008;112:863–75. doi: 10.1002/cncr.23239. [DOI] [PubMed] [Google Scholar]
- 14.Platonova S, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–22. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 15.Eckl J, et al. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J Mol Med (Berl) 2011 doi: 10.1007/s00109-011-0806-7. [DOI] [PubMed] [Google Scholar]
- 16.Halama N, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res. 2011;17:678–89. doi: 10.1158/1078-0432.CCR-10-2173. [DOI] [PubMed] [Google Scholar]
- 17.Menard C, et al. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 2009;69:3563–9. doi: 10.1158/0008-5472.CAN-08-3807. [DOI] [PubMed] [Google Scholar]
- 18.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 19.Tilloy F, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189:1907–21. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–5. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 21.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2:971–8. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 22.Egawa T, et al. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–16. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180:1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schumann J, Voyle RB, Wei BY, MacDonald HR. Cutting edge: influence of the TCR V beta domain on the avidity of CD1d:alpha-galactosylceramide binding by invariant V alpha 14 NKT cells. J Immunol. 2003;170:5815–9. doi: 10.4049/jimmunol.170.12.5815. [DOI] [PubMed] [Google Scholar]
- 26.Wei DG, Curran SA, Savage PB, Teyton L, Bendelac A. Mechanisms imposing the Vbeta bias of Valpha14 natural killer T cells and consequences for microbial glycolipid recognition. J Exp Med. 2006;203:1197–207. doi: 10.1084/jem.20060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sidobre S, et al. The V alpha 14 NKT cell TCR exhibits high-affinity binding to a glycolipid/CD1d complex. J Immunol. 2002;169:1340–8. doi: 10.4049/jimmunol.169.3.1340. [DOI] [PubMed] [Google Scholar]
- 28.Pellicci DG, et al. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1(-)CD4(+) CD1d-dependent precursor stage. J Exp Med. 2002;195:835–44. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantu C, 3rd, Benlagha K, Savage PB, Bendelac A, Teyton L. The paradox of immune molecular recognition of alpha-galactosylceramide: low affinity, low specificity for CD1d, high affinity for alpha beta TCRs. J Immunol. 2003;170:4673–82. doi: 10.4049/jimmunol.170.9.4673. [DOI] [PubMed] [Google Scholar]
- 30.Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 31.Burdin N, et al. Selective ability of mouse CD1 to present glycolipids: a-galactosylceramide specifically stimulates Va14+ NK T lymphocytes. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- 32.Brigl M, Brenner MB. CD1: Antigen Presentation and T Cell Function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 33.Kronenberg M. Toward an Understanding of NKT Cell Biology: Progress and Paradoxes. Annu Rev Immunol. 2004;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 34.Godfrey DI, Pellicci DG, Smyth MJ. Immunology. The elusive NKT cell antigen--is the search over? Science. 2004;306:1687–9. doi: 10.1126/science.1106932. [DOI] [PubMed] [Google Scholar]
- 35.Bendelac A, Savage PB, Teyton L. The Biology of NKT Cells. Annu Rev Immunol. 2006;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 36.Smyth MJ, et al. Differential Tumor Surveillance by Natural Killer (NK) and NKT Cells. Journal Of Experimental Medicine. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swann JB, et al. Type I natural killer T cells suppress tumors caused by p53 loss in mice. Blood. 2009;113:6382–5. doi: 10.1182/blood-2009-01-198564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowak M, et al. Defective NKT cell activation by CD1d+ TRAMP prostate tumor cells is corrected by interleukin-12 with alpha-galactosylceramide. PLoS One. 2010;5:e11311. doi: 10.1371/journal.pone.0011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellone M, et al. iNKT cells control mouse spontaneous carcinoma independently of tumor-specific cytotoxic T cells. PLoS One. 2010;5:e8646. doi: 10.1371/journal.pone.0008646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savage AK, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovalovsky D, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008 doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gascoyne DM, et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–24. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 43.Kamizono S, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. The Journal of Experimental Medicine. 2009;206:2977–2986. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wesley JD, Tessmer MS, Chaukos D, Brossay L. NK cell-like behavior of Valpha14i NK T cells during MCMV infection. PLoS Pathog. 2008;4:e1000106. doi: 10.1371/journal.ppat.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tyznik AJ, et al. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008;181:4452–6. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brigl M, et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011;208:1163–77. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stetson DB, et al. Constitutive Cytokine mRNAs Mark Natural Killer (NK) and NK T Cells Poised for Rapid Effector Function. J Exp Med. 2003;198:1069–76. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuda JL, et al. Homeostasis of V alpha 14i NKT cells. Nat Immunol. 2002;3:966–74. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 50.Ranson T, et al. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci U S A. 2003;100:2663–8. doi: 10.1073/pnas.0535482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Townsend MJ, et al. T-bet Regulates the Terminal Maturation and Homeostasis of NK and Valpha14i NKT Cells. Immunity. 2004;20:477–94. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 52.Wingender G, Krebs P, Beutler B, Kronenberg M. Antigen-Specific Cytotoxicity by Invariant NKT Cells In Vivo Is CD95/CD178-Dependent and Is Correlated with Antigenic Potency. The Journal of Immunology. 2010;185:2721–2729. doi: 10.4049/jimmunol.1001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carlyle JR, et al. Evolution of the Ly49 and Nkrp1 recognition systems. Semin Immunol. 2008;20:321–30. doi: 10.1016/j.smim.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–14. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 55.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–13. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 56.Fernandez NC, et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–23. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yokoyama WM, Kim S. How Do Natural Killer Cells Find Self to Achieve Tolerance? Immunity. 2006;24:249–257. doi: 10.1016/j.immuni.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–31. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 59.Brodin P, Karre K, Hoglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30:143–149. doi: 10.1016/j.it.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. 2010;142:847–56. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoglund P, et al. Recognition of beta 2-microglobulin-negative (beta 2m-) T-cell blasts by natural killer cells from normal but not from beta 2m-mice: nonresponsiveness controlled by beta 2m-bone marrow in chimeric mice. Proc Natl Acad Sci U S A. 1991;88:10332–6. doi: 10.1073/pnas.88.22.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liao NS, Bix M, Zilstra M, Jaenish R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253:199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 63.Orr MT, Murphy WJ, Lanier LL. ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nat Immunol. 2010;11:321–7. doi: 10.1038/ni.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182:4572–80. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113:2434–41. doi: 10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]
- 66.Guia S, et al. Confinement of activating receptors at the plasma membrane controls natural killer cell tolerance. Sci Signal. 2011;4:ra21. doi: 10.1126/scisignal.2001608. [DOI] [PubMed] [Google Scholar]
- 67.Joncker NT, Shifrin N, Delebecque F, Raulet DH. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 2010;207:2065–2072. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elliott JM, Wahle JA, Yokoyama WM. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J Exp Med. 2010;207:2073–2079. doi: 10.1084/jem.20100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kijima M, Gardiol N, Held W. Natural Killer Cell Mediated Missing-Self Recognition Can Protect Mice from Primary Chronic Myeloid Leukemia In Vivo. PLoS ONE. 2011;6:e27639. doi: 10.1371/journal.pone.0027639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bottino C, Moretta L, Moretta A. NK cell activating receptors and tumor recognition in humans. Curr Top Microbiol Immunol. 2006;298:175–82. doi: 10.1007/3-540-27743-9_9. [DOI] [PubMed] [Google Scholar]
- 71.Moretta A, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Ann Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 72.Bauer S, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 73.Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev Immunol. 2009;9:568–80. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–90. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 75.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1:119–26. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 76.Cerwenka A, et al. Retinoic Acid early Inducible genes define a Ligand family for the Activating NKG2D receptor in Mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 77.Cosman D, et al. ULBPs, Novel MHC Class I-Related Molecules, Bind to CMV Glycoprotein UL16 and Stimulate NK Cytotoxicity through the NKG2D Receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 78.Carayannopoulos LN, et al. Ligands for murine NKG2D display heterogenous binding behavior. Eur J Immunol. 2002;32:597–605. doi: 10.1002/1521-4141(200203)32:3<597::aid-immu597>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 79.Guerra N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Unni AM, Bondar T, Medzhitov R. Intrinsic sensor of oncogenic transformation induces a signal for innate immunosurveillance. Proc Natl Acad Sci U S A. 2008;105:1686–91. doi: 10.1073/pnas.0701675105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soriani A, et al. ATM-ATR dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK cell susceptibility and is associated with a senescent phenotype. Blood. 2008 doi: 10.1182/blood-2008-08-173914. [DOI] [PubMed] [Google Scholar]
- 83.Sivori S, et al. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med. 1997;186:1129–1136. doi: 10.1084/jem.186.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vitale M, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J ExpMed. 1998;187:2065–2072. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pende D, et al. Identification and Molecular Characterization of NKp30, a Novel Triggering Receptor Involved in Natural Cytotoxicity Mediated by Human Natural Killer Cells. J Exp Med. 1999;190:1505–1516. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brandt CS, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Y, Wang Q, Mariuzza RA. Structure of the human activating natural cytotoxicity receptor NKp30 bound to its tumor cell ligand B7-H6. J Exp Med. 2011 doi: 10.1084/jem.20102548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bottino C, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198:557–67. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cartron G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 90.Veeramani S, et al. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood. 2011;118:3347–9. doi: 10.1182/blood-2011-05-351411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–94. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 92.Thomas E, et al. Bone Marrow Transplantation. The New England Journal of Medicine. 1975;292:832–843. 895–902. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- 93.Weiden PLF, Thomas NED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. New England Journal of Medicine. 1979;300:1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 94.Storb R. Allogeneic hematopoietic stem cell transplantation--yesterday, today, and tomorrow. Exp Hematol. 2003;31:1–10. doi: 10.1016/s0301-472x(02)01020-2. [DOI] [PubMed] [Google Scholar]
- 95.Childs R, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343:750–8. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 96.Blaise D, et al. Reduced-intensity preparative regimen and allogeneic stem cell transplantation for advanced solid tumors. Blood. 2004;103:435–41. doi: 10.1182/blood-2003-07-2236. [DOI] [PubMed] [Google Scholar]
- 97.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dong Z, et al. Essential function for SAP family adaptors in the surveillance of hematopoietic cells by natural killer cells. Nat Immunol. 2009;10:973–80. doi: 10.1038/ni.1763. [DOI] [PubMed] [Google Scholar]
- 99.Aversa F, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–93. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 100.Ruggeri L, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 101.Giebel S, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–819. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 102.Ruggeri L, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–40. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kroger N, et al. Comparison between antithymocyte globulin and alemtuzumab and the possible impact of KIR-ligand mismatch after dose-reduced conditioning and unrelated stem cell transplantation in patients with multiple myeloma. Br J Haematol. 2005;129:631–43. doi: 10.1111/j.1365-2141.2005.05513.x. [DOI] [PubMed] [Google Scholar]
- 104.Bignon JD, Gagne K. KIR matching in hematopoietic stem cell transplantation. Curr Opin Immunol. 2005;17:553–9. doi: 10.1016/j.coi.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 105.Poggi A, Zocchi MR. Cyclosporin A regulates human NK cell apoptosis induced by soluble HLA-I or by target cells. Autoimmun Rev. 2005;4:532–6. doi: 10.1016/j.autrev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 106.Wang H, et al. The unexpected effect of cyclosporin A on CD56+CD16 and CD56+CD16+ natural killer cell subpopulations. Blood. 2007;110:1530–1539. doi: 10.1182/blood-2006-10-048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moretta A, et al. Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells. J Exp Med. 1995;182:875–884. doi: 10.1084/jem.182.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Olcese L, et al. Killer-cell activatory receptors for MHC Class I molecules are included in a multimeric complex expressed by human killer cells. J Immunol. 1997;158:5083–5086. [PubMed] [Google Scholar]
- 109.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 110.Verheyden S, Schots R, Duquet W, Demanet C. A defined donor activating natural killer cell receptor genotype protects against leukemic relapse after related HLA-identical hematopoietic stem cell transplantation. Leukemia. 2005;19:1446–51. doi: 10.1038/sj.leu.2403839. [DOI] [PubMed] [Google Scholar]
- 111.De Santis D, et al. Natural killer cell HLA-C epitopes and killer cell immunoglobulin-like receptors both influence outcome of mismatched unrelated donor bone marrow transplants. Tissue Antigens. 2005;65:519–28. doi: 10.1111/j.1399-0039.2005.00396.x. [DOI] [PubMed] [Google Scholar]
- 112.Cook M, et al. Donor KIR genotype has a major influence on the rate of cytomegalovirus reactivation following T-cell replete stem cell transplantation. Blood. 2006;107:1230–2. doi: 10.1182/blood-2005-03-1039. [DOI] [PubMed] [Google Scholar]
- 113.Chen C, et al. Activating KIR genes are associated with CMV reactivation and survival after non-T-cell depleted HLA-identical sibling bone marrow transplantation for malignant disorders. Bone Marrow Transplant. 2006;38:437–44. doi: 10.1038/sj.bmt.1705468. [DOI] [PubMed] [Google Scholar]
- 114.McQueen KL, et al. Donor-recipient combinations of group A and B KIR haplotypes and HLA class I ligand affect the outcome of HLA-matched, sibling donor hematopoietic cell transplantation. Hum Immunol. 2007;68:309–23. doi: 10.1016/j.humimm.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Clausen J, et al. Impact of natural killer cell dose and donor killer-cell immunoglobulin-like receptor (KIR) genotype on outcome following human leucocyte antigen-identical haematopoietic stem cell transplantation. Clin Exp Immunol. 2007;148:520–8. doi: 10.1111/j.1365-2249.2007.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holler E, et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990;75:1011–6. [PubMed] [Google Scholar]
- 117.Storb R, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–54. [PubMed] [Google Scholar]
- 118.Giralt S, et al. Engrafment of allogeneic hematopoietic progenitor cells ith purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89:4531–4536. [PubMed] [Google Scholar]
- 119.Slavin S, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 120.Blaise D, Vey N, Faucher C, Mohty M. Current status of reduced-intensity-conditioning allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2007;92:533–41. doi: 10.3324/haematol.10867. [DOI] [PubMed] [Google Scholar]
- 121.Gratwohl A, et al. The EBMT activity survey 2007 with focus on allogeneic HSCT for AML and novel cellular therapies. Bone Marrow Transplant. 2009;43:275–91. doi: 10.1038/bmt.2009.7. [DOI] [PubMed] [Google Scholar]
- 122.Pende D, et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009;113:3119–29. doi: 10.1182/blood-2008-06-164103. [DOI] [PubMed] [Google Scholar]
- 123.Mohty M, et al. Recovery of lymphocyte and dendritic cell subsets following reduced intensity allogeneic bone marrow transplantation. Hematology. 2002;7:157–64. doi: 10.1080/10245330210000013898. [DOI] [PubMed] [Google Scholar]
- 124.Larosa F, et al. Peripheral T-cell expansion and low infection rate after reduced-intensity conditioning and allogeneic blood stem cell transplantation. Bone Marrow Transplant. 2005;35:859–68. doi: 10.1038/sj.bmt.1704889. [DOI] [PubMed] [Google Scholar]
- 125.Kim DH, et al. Non-CD34+ cells, especially CD8+ cytotoxic T cells and CD56+ natural killer cells, rather than CD34 cells, predict early engraftment and better transplantation outcomes in patients with hematologic malignancies after allogeneic peripheral stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:719–28. doi: 10.1016/j.bbmt.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 126.Savani BN, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21:2145–52. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]
- 127.Dunbar EM, et al. The relationship between circulating natural killer cells after reduced intensity conditioning hematopoietic stem cell transplantation and relapse-free survival and graft-versus-host disease. Haematologica. 2008;93:1852–8. doi: 10.3324/haematol.13033. [DOI] [PubMed] [Google Scholar]
- 128.Kolb HJ, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia. Blood. 1995;86:2041–50. [PubMed] [Google Scholar]
- 129.Dazzi F, et al. Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood. 2000;96:2712–6. [PubMed] [Google Scholar]
- 130.Marks DI, et al. The toxicity and efficacy of donor lymphocyte infusions given after reduced-intensity conditioning allogeneic stem cell transplantation. Blood. 2002;100:3108–3114. doi: 10.1182/blood-2002-02-0506. [DOI] [PubMed] [Google Scholar]
- 131.Shimoni A, et al. Long-Term follow-up of recipients of CD8-depleted donor lymphocyte infusions for the treatment of chronic myelogenous leukemia relapsing after allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant. 2001;7:568–75. doi: 10.1016/s1083-8791(01)70017-1. [DOI] [PubMed] [Google Scholar]
- 132.Alyea EP, et al. CD8+ cell depletion of donor lymphocyte infusions using cd8 monoclonal antibody-coated high-density microparticles (CD8-HDM) after allogeneic hematopoietic stem cell transplantation: a pilot study. Bone Marrow Transplant. 2004;34:123–8. doi: 10.1038/sj.bmt.1704536. [DOI] [PubMed] [Google Scholar]
- 133.Porter DL, et al. Graft-versus-tumor induction with donor leukocyte infusions as primary therapy for patients with malignancies. J Clin Oncol. 1999;17:1234. doi: 10.1200/JCO.1999.17.4.1234. [DOI] [PubMed] [Google Scholar]
- 134.Guo M, et al. Infusion of HLA-mismatched peripheral blood stem cells improves the outcome of chemotherapy for acute myeloid leukemia in elderly patients. Blood. 2011;117:936–41. doi: 10.1182/blood-2010-06-288506. [DOI] [PubMed] [Google Scholar]
- 135.Miller JS, et al. Large scale ex vivo expansion and activation of human natural killer cells for autologous therapy. Bone Marrow Transplant. 1994;14:555–62. [PubMed] [Google Scholar]
- 136.Passweg JR, et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18:1835–8. doi: 10.1038/sj.leu.2403524. [DOI] [PubMed] [Google Scholar]
- 137.McKenna DH, Jr, et al. Good manufacturing practices production of natural killer cells for immunotherapy: a six-year single-institution experience. Transfusion. 2007;47:520–8. doi: 10.1111/j.1537-2995.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 138.Lundqvist A, McCoy JP, Samsel L, Childs R. Reduction of GVHD and enhanced antitumor effects after adoptive infusion of alloreactive Ly49-mismatched NK cells from MHC-matched donors. Blood. 2007;109:3603–6. doi: 10.1182/blood-2006-05-024315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Castriconi R, et al. Human NK cell infusions prolong survival of metastatic human neuroblastoma-bearing NOD/scid mice. Cancer Immunol Immunother. 2007;56:1733–42. doi: 10.1007/s00262-007-0317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shi J, et al. Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br J Haematol. 2008;143:641–53. doi: 10.1111/j.1365-2141.2008.07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Arai S, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–32. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- 142.Cho D, Campana D. Expansion and activation of natural killer cells for cancer immunotherapy. Korean J Lab Med. 2009;29:89–96. doi: 10.3343/kjlm.2009.29.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barkholt L, et al. Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: a phase I clinical study. Immunotherapy. 2009;1:753–64. doi: 10.2217/imt.09.47. [DOI] [PubMed] [Google Scholar]
- 144.Bachanova V, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010;59:1739–44. doi: 10.1007/s00262-010-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nguyen S, et al. Infusion of allogeneic natural killer cells in a patient with acute myeloid leukemia in relapse after haploidentical hematopoietic stem cell transplantation. Transfusion. 2011;51:1769–78. doi: 10.1111/j.1537-2995.2010.03058.x. [DOI] [PubMed] [Google Scholar]
- 146.Geller MA, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.De Somer L, et al. Recipient lymphocyte infusion in MHC-matched bone marrow chimeras induces a limited lymphohematopoietic host-versus-graft reactivity but a significant antileukemic effect mediated by CD8+ T cells and natural killer cells. Haematologica. 2011;96:424–31. doi: 10.3324/haematol.2010.035329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Curti A, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118:3273–9. doi: 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- 149.Brehm C, et al. IL-2 Stimulated but Not Unstimulated NK Cells Induce Selective Disappearance of Peripheral Blood Cells: Concomitant Results to a Phase I/II Study. PLoS One. 2011;6:e27351. doi: 10.1371/journal.pone.0027351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Miller JS, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 151.Alici E. IPH-2101, a fully human anti-NK-cell inhibitory receptor mAb for the potential treatment of hematological cancers. Curr Opin Mol Ther. 2010;12:724–33. [PubMed] [Google Scholar]
- 152.Romagne F, et al. Pre-clinical characterization of 1-7F9, a novel human anti-KIR therapeutic antibody that augments NK-mediated killing of tumor cells. Blood. 2009;114:2667–2677. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Romagne F, Vivier E. Natural killer cell-based therapies. F1000 Med Rep. 2011;3:9. doi: 10.3410/M3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sola C, et al. Genetic and antibody-mediated reprogramming of natural killer cell missing-self recognition in vivo. Proc Natl Acad Sci U S A. 2009;106:12879–84. doi: 10.1073/pnas.0901653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Benson DM, Jr, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood. 2011;118:6387–91. doi: 10.1182/blood-2011-06-360255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Walzer T, Vivier E. G-protein-coupled receptors in control of natural killer cell migration. Trends Immunol. 2011;32:486–92. doi: 10.1016/j.it.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 157.Brand JM, et al. Kinetics and organ distribution of allogeneic natural killer lymphocytes transfused into patients suffering from renal cell carcinoma. Stem Cells Dev. 2004;13:307–14. doi: 10.1089/154732804323099235. [DOI] [PubMed] [Google Scholar]
- 158.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8 T cells. Nat Rev Immunol. 2011;11:645–57. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Park SH, Kyin T, Bendelac A, Carnaud C. The contribution of NKT cells, NK cells, and other gamma-chain-dependent non-T non-B cells to IL-12-mediated rejection of tumors. J Immunol. 2003;170:1197–201. doi: 10.4049/jimmunol.170.3.1197. [DOI] [PubMed] [Google Scholar]
- 160.Metelitsa LS, Weinberg KI, Emanuel PD, Seeger RC. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003;17:1068–77. doi: 10.1038/sj.leu.2402943. [DOI] [PubMed] [Google Scholar]
- 161.Renukaradhya GJ, et al. Type I NKT cells protect (and type II NKT cells suppress) the host’s innate antitumor immune response to a B-cell lymphoma. Blood. 2008;111:5637–45. doi: 10.1182/blood-2007-05-092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198:173–81. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Crowe NY, Smyth MJ, Godfrey DI. A Critical Role for Natural Killer T Cells in Immunosurveillance of Methylcholanthrene-induced Sarcomas. J Exp Med. 2002;196:119–27. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Song L, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119:1524–36. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tahir SM, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–50. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 166.Yanagisawa K, et al. Impaired Proliferative Response of Vα24 NKT Cells from Cancer Patients Against α-Galactosylceramide. The Journal of Immunology. 2002;168:6494–6499. doi: 10.4049/jimmunol.168.12.6494. [DOI] [PubMed] [Google Scholar]
- 167.Tachibana T, et al. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11:7322–7. doi: 10.1158/1078-0432.CCR-05-0877. [DOI] [PubMed] [Google Scholar]
- 168.Terabe M, et al. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–33. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kawano T, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated valpha14 NKT cells [In Process Citation] ProcNatl AcadSciUS A. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Swann JB, Coquet JM, Smyth MJ, Godfrey DI. CD1-restricted T cells and tumor immunity. Curr Top Microbiol Immunol. 2007;314:293–323. doi: 10.1007/978-3-540-69511-0_12. [DOI] [PubMed] [Google Scholar]
- 171.Parekh VV, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–83. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim S, et al. Impact of bacteria on the phenotype, functions, and therapeutic activities of invariant NKT cells in mice. J Clin Invest. 2008;118:2301–15. doi: 10.1172/JCI33071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cerundolo V, Barral P, Batista FD. Synthetic iNKT cell-agonists as vaccine adjuvants--finding the balance. Curr Opin Immunol. 2010;22:417–24. doi: 10.1016/j.coi.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 174.Stanic AK, et al. Another view of T cell antigen recognition: cooperative engagement of glycolipid antigens by Va14Ja18 natural T(iNKT) cell receptor [corrected] J Immunol. 2003;171:4539–51. doi: 10.4049/jimmunol.171.9.4539. [DOI] [PubMed] [Google Scholar]
- 175.Oki S, Tomi C, Yamamura T, Miyake S. Preferential T(h)2 polarization by OCH is supported by incompetent NKT cell induction of CD40L and following production of inflammatory cytokines by bystander cells in vivo. Int Immunol. 2005;17:1619–29. doi: 10.1093/intimm/dxh342. [DOI] [PubMed] [Google Scholar]
- 176.Im JS, et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30:888–98. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sullivan BA, et al. Mechanisms for glycolipid antigen-driven cytokine polarization by Valpha14i NKT cells. J Immunol. 2010;184:141–53. doi: 10.4049/jimmunol.0902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yue SC, et al. Direct CD1d-mediated stimulation of APC IL-12 production and protective immune response to virus infection in vivo. J Immunol. 2010;184:268–76. doi: 10.4049/jimmunol.0800924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Teng MW, Yue S, Sharkey J, Exley MA, Smyth MJ. CD1d activation and blockade: a new antitumor strategy. J Immunol. 2009;182:3366–71. doi: 10.4049/jimmunol.0802964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hix LM, et al. CD1d-expressing breast cancer cells modulate NKT cell-mediated antitumor immunity in a murine model of breast cancer metastasis. PLoS One. 2011;6:e20702. doi: 10.1371/journal.pone.0020702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Barral P, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci U S A. 2008;105:8345–50. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Barral P, et al. CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nat Immunol. 2010;11:303–12. doi: 10.1038/ni.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Motohashi S, et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12:6079–86. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- 184.Yamasaki K, et al. Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol. 2011;138:255–65. doi: 10.1016/j.clim.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 185.Giaccone G, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–9. [PubMed] [Google Scholar]
- 186.Schneiders FL, et al. Clinical experience with alpha-galactosylceramide (KRN7000) in patients with advanced cancer and chronic hepatitis B/C infection. Clin Immunol. 2011;140:130–41. doi: 10.1016/j.clim.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 187.Nieda M, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–9. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 188.Ishikawa A, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910–7. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 189.Chang DH, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–17. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kunii N, et al. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009;100:1092–8. doi: 10.1111/j.1349-7006.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cooper MA, et al. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106:1915–9. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–35. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 194.Bukowski JF, Biron CA, Welsh RM. Elevated natural killer cell-mediated cytotoxicity, plasma interferon, and tumor cell rejection in mice persistently infected with lymphocytic choriomeningitis virus. J Immunol. 1983;131:991–6. [PubMed] [Google Scholar]
- 195.Carnaud C, et al. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 196.Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000;30:985–992. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 197.Hermans IF, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–7. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 198.Wesley JD, et al. Cutting edge: IFN-gamma signaling to macrophages is required for optimal Valpha14i NK T/NK cell cross-talk. J Immunol. 2005;174:3864–8. doi: 10.4049/jimmunol.174.7.3864. [DOI] [PubMed] [Google Scholar]
- 199.Gonzalez-Aseguinolaza G, et al. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195:617–24. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang Y, et al. Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, alpha-galactosylceramide. Vaccine. 2008;26:1807–16. doi: 10.1016/j.vaccine.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 201.Galli G, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A. 2007;104:3984–9. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kamijuku Mechanism of NKT cell activation by intranasal coadministration of alpha-galactosylceramide, which can induce cross-protection against influenza viruses. Mucosal Immunology. 2008 doi: 10.1038/mi.2008.2. [DOI] [PubMed] [Google Scholar]
- 203.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 204.Oki S, Chiba A, Yamamura T, Miyake S. The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells. J Clin Invest. 2004;113:1631–40. doi: 10.1172/JCI20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yu KO, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. Proc Natl Acad Sci U S A. 2005;102:3383–8. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med. 2003;198:1631–41. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chiche L, et al. The role of natural killer cells in sepsis. J Biomed Biotechnol. 2011;2011:986491. doi: 10.1155/2011/986491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Schleinitz N, Vely F, Harle JR, Vivier E. Natural killer cells in human autoimmune diseases. Immunology. 2010;131:451–8. doi: 10.1111/j.1365-2567.2010.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Andrews DM, et al. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J Exp Med. 2010;207:1333–43. doi: 10.1084/jem.20091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2011 doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 212.Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer immunology-analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8:711–9. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pellicci DG, et al. Recognition of beta-linked self glycolipids mediated by natural killer T cell antigen receptors. Nat Immunol. 2011;12:827–33. doi: 10.1038/ni.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Brennan PJ, et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12:1202–11. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;26:26. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 216.Mattner J, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 217.Salio M, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci U S A. 2007;104:20490–5. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Paget C, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 219.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defense strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 220.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–44. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 221.Moretta L, et al. Allorecognition by NK cells nonself or no self? Immunol. 1992;13:300. doi: 10.1016/0167-5699(92)90042-6. [DOI] [PubMed] [Google Scholar]
- 222.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 223.Moretta A, et al. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol. 1996;14:619–48. doi: 10.1146/annurev.immunol.14.1.619. [DOI] [PubMed] [Google Scholar]
- 224.Kärre K. How to recognize a foreign submarine. ImmunolRev. 1997;155:5–9. doi: 10.1111/j.1600-065x.1997.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 225.Braud VM, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 226.Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A Is a Natural Killer Cell Receptor for the Nonclassical Major Histocompatibility Complex (MHC) Class I Molecule Qa-1(b) J Exp Med. 1998;188:1841–1848. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol. 2002;20:217–51. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 228.Olcese L, et al. Human and mouse killer-cell inhibitory receptors recruit PTP1C and PTP1D protein tyrosine phosphatases. J Immunol. 1996;156:4531–4534. [PubMed] [Google Scholar]
- 229.Burshtyn DN, et al. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitory receptor. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–9. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 231.Kumar V, McNerney ME. A new self: MHC-class-I-independent Natural-killer-cell self-tolerance. Nat Rev Immunol. 2005;5:363–74. doi: 10.1038/nri1603. [DOI] [PubMed] [Google Scholar]