Abstract
Introduction
Patients with severe eosinophilic asthma have an unmet need for novel and efficacious treatments. Reslizumab is one of three monoclonal antibodies targeting the interleukin-5 (IL5) pathway and has been found in phase 3b clinical trials to reduce asthma exacerbations, control asthma-related symptoms and improve pulmonary function in patients with eosinophilic asthma.
Areas covered
In this article we will discuss the results of asthma clinical trials using reslizumab, beginning with a discussion of the relationship between eosinophils, IL5 and asthma. We conducted PubMed searches using the terms ‘reslizumab’, ‘anti-IL5’, ‘eosinophilic asthma’, ‘IL5 asthma’. We also searched ClinicalTrials.gov for ‘reslizumab’, ‘reslizumab asthma’, ‘SCH 55700’, ‘SCH 55700 asthma’, ‘Cinquil’, and ‘Cinquil asthma’.
Expert opinion
Reslizumab and other anti-IL5 therapies have seen success in recent trials through more stringent study participant selection targeting eosinophilic inflammation. This selection can now be based on simple blood counts. These drugs have shown a very good safety profile, but long-term safety data is not yet available. Approval for these drugs is eagerly awaited by clinicians and patients alike.
Keywords: Reslizumab, Anti-IL5 therapy, Asthma, Interleukin 5, Eosinophil, Monoclonal antibody, Eosinophilic inflammation
1. Introduction: an unmet need for novel asthma therapies
Asthma is a common chronic respiratory illness posing a substantial burden to society in terms of morbidity, mortality and cost1, 2. This burden is disproportionately born by the subset of patients with ‘severe’ asthma, who by definition depend on daily medications otherwise found to be efficacious in most asthmatics3,4. Considerable efforts have been directed at distinguishing this treatment-resistant patient subset from others, which has resulted in the categorization of asthma according to inflammatory signatures 5, 6, clusters7, and pathophysiologic mechanisms8. The development of novel asthma therapies is grounded on these categories, and many small molecule and monoclonal antibodies targeting these biomolecular specificities are being investigated9–11. One major asthma subtype, constituting roughly half of all patients with asthma6, is phenotypically characterized by eosinophilic inflammation12.
Interleukin-5 (IL5) is the chief cytokine responsible for eosinophil production, survival and maturation13. Since eosinophils are thought to play a pathophysiologic role in asthma, energies have been invested in generating therapies that target IL5 and its receptor. The aim of such therapies is to reduce eosinophilic inflammation in the hopes of controlling asthma. Anti-IL5 therapies in eosinophilic diseases have been reviewed elsewhere14–19. In this article, we will discuss the clinical performance of reslizumab, including data from recently completed phase 3b clinical trials using this antibody. We will begin with a review the biology of eosinophils and IL5 and their role in asthma, explore the biochemical differences between reslizumab and other anti-IL5 therapies, and consider the potential hurdles limiting the clinical efficacy of targeting eosinophils as a therapeutic goal in asthma.
2. The role of eosinophils in asthma
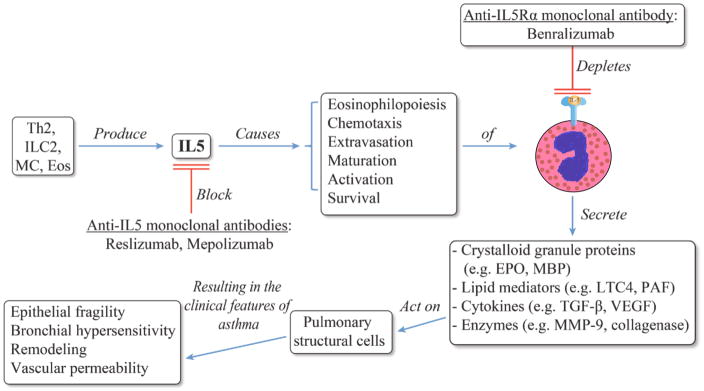
The connection between eosinophils and asthma was made over a century ago20, and their role as culprit vs. bystander in the pathobiology of this disease has been debated ever since. Although no definitive proof for causality has been provided, multiple lines of evidence suggest that airway eosinophils are capable of causing the clinical and pathologic traits seen in asthma. Following their discovery, the abnormal presence of eosinophils in the airway and peripheral blood in cases of fatal asthma were frequently (but not universally) reported21. Peripheral blood eosinophilia was later found to correlate with clinical and physiological features of asthma such as symptoms22 and bronchial hyperreactivity23. Repeated allergen challenges were found to result in both airway eosinophilia and airway hyperreactivity in primates24. Simultaneously, eosinophil-derived chemical mediators were determined to recreate the pathologic features of asthma (Figure 1). BAL-derived eosinophils and their cationic protein contents were found to correlate with severity in patients with asthma. Importantly, degranulated intraepithelial eosinophils were found to be a pathologic feature suggestive of severe asthma25. Co-incubation of human BAL-harvested eosinophils was shown to result in epithelial cell cytotoxicity26, and to trigger histamine release from both mast cells and basophils in vitro27. More recently, eosinophils have been shown to be important contributors to airway remodeling, partly due to their production of TGF-beta28. Major basic protein, the most abundant component of crystalloid granules in eosinophils29, was found to be elevated in sputum of patients with asthma30. It was also shown to cause airway smooth muscle contraction ex vivo31, and airway hyperresponsiveness in vivo using intratracheal instillation in primates32.
Figure 1.
Schematic representation of the biology of IL-5 [36], eosinophils and their mediators [29,73], and their effect on asthma. The point in this cascade where therapies targeting IL-5 or its receptor act is shown. Depletion of cells expressing IL-5Ra (eosinophils and basophils) results from antibody-directed cell-dependent cytotoxicity.
The HE-stained eosinophil was adapted with permission from wordpress.com [74].
Eos: Eosinophils; EPO: Eosinophil peroxidase; ILC2: Group 2 innate lymphoid cells; LTC4: Cysteinyl leukotriene C4; MBP: Major basic protein; MC: Mast cells; MMP-9: Matrix metalloproteinase 9; PAF: Platelet-activating factor.
Considering these findings, trials were conducted testing asthma treatment strategies targeting eosinophil numbers as a therapeutic aim. One such strategy adjusted the daily inhaled corticosteroid (ICS) dose to decrease sputum eosinophil numbers. This was found to reduce asthma exacerbations compared to guideline-driven decisions, suggesting a major role for eosinophils in asthma33, 34.
3. IL5, eosinophils and asthma
IL5 is the principal eosinophilopoietic cytokine in humans35. It mediates eosinophil chemotaxis, activation, maturation, and survival. Additionally, IL5 enhances eosinophil cytotoxicity and the secretion of chemical mediators36 (Figure 1). Classically, it is conceived as being predominantly produced by Th2 cells, but IL5 has also been found to be produced by group 2 innate lymphoid cells (ILC2)37, and in smaller quantities by mast cells and eosinophils38. The connection between IL5, eosinophils, and asthma was bolstered by in vivo experiments using IL5 knockout mice. These mice were sensitized and challenged with ovalbumin, and did not develop eosinophilia, pulmonary cytotoxicity, or airway hyperreactivity compared with wild-type mice. The allergic phenotype in the knockout was restored with reconstitution of IL539. In humans, the colocalization of IL5 mRNA, T cells and eosinophils was shown to occur in bronchial biopsies of patients with asthma and not in those of normal controls40. T cells were identified as a source of IL5 mRNA in BALF only of patients with asthma41. Clinically, bronchial mucosal IL5 levels were shown to correlate with asthma symptoms, pulmonary function, and degree of airway hyperreactivity42. Further, serum IL5 concentrations were reported to increase during asthma exacerbations43. IL5 was directly demonstrated to cause airway eosinophilia in asthma through instillation of purified IL5 into patients with asthma undergoing bronchoscopies44.
Design and biology of therapies targeting IL5 and its signaling pathway
Anti-IL5 therapies target elements of the IL5 signaling pathway. These monoclonal antibodies are aimed at IL5 (ligand) or the IL5 receptor. Three such monoclonal antibodies are being investigated in asthma clinical trials. Two of these target IL5 (ligand) itself, the other targets the IL5 receptor alpha subunit. The mechanism of action of antibodies targeting the IL5 ligand is thought to be disruption of the IL5 properties (described in the prior section); additional consequences of this blockade include reduction of adhesion molecule expression in eosinophils45. In this review we will focus on reslizumab. Reslizumab (Sch 55700) is a humanized monoclonal antibody against human IL5, in which the antigen recognition sites from a rat IgG2a antibody were incorporated into a human IgG4/kappa constant regions using complementarity determining region grafting technology46. Mapping of the IL5 epitope recognized by reslizumab and further characterization through site-directed mutagenesis showed that amino acids 89–93 (out of the 115 amino acids that compose IL5) are critical for recognition and for signaling through the IL5R47. In vitro studies show that reslizumab binds IL5 with high affinity (Kd =20pM) and has a long duration of action, with control of pulmonary eosinophilia in a model of eosinophilic allergic pulmonary inflammation in monkey up to 6 months after administration of reslizumab46. Similarly, mepolizumab (SB-240563) is humanized monoclonal antibody against human IL5, but from murine origin, and grafted onto a human IgG1 heavy chain48. The IgG1 subclass has a greater binding affinity to Fcγ receptors, and thus IgG2 or IgG4 are chosen in the design of monoclonal antibody therapeutics when a reduction in antibody-directed cell-mediated cytotoxicity is desired49. But since the target of both reslizumab and mepolizumab is a soluble protein, the significance of this difference is unclear.
Benralizumab (MEDI-563) is a humanized murine monoclonal directed against the alpha chain of the IL5R. It potentially causes eosinophil depletion through two mechanisms -- disruption of IL5-mediated signaling and potentially through antibody-directed cell-mediated cytotoxicity50. It also causes depletion of basophils, since these granulocytes also express the IL5Ra51.
4. Clinical studies on reslizumab and its effect on asthma, in comparison with other anti-IL-5 therapies
The first clinical trial using reslizumab for asthma was a phase 2, dose-ranging pilot study conducted by Kips et al. looking at safety, biologic activity and pharmacokinetics. Patients were selected on the basis of asthma severity and use of oral or high-dose ICS as controller therapy52. This study verified the safety of this drug, and determined that doses ≥0.3mg/kg significantly lowered peripheral blood eosinophil levels from baseline compared to placebo (a mean 53% reduction at 48 hours). This effect was significant for up to 30 days after injection, although less dramatic (mean 19% reduction). This of course implied that reslizumab did not lead to complete eosinophil depletion, at least at the dosages tested. Further, and like the early phase mepolizumab clinical trials53, 54, this study strikingly did not report clinical benefit. Reslizumab was found to be no different than placebo in terms of symptom control, peak flow recordings, or physician evaluation. FEV1 was significantly improved transiently (only 24 hours after administration), and only with a dose below the proposed threshold for biological activity (with 0.3mg/kg rather than with 1mg/kg). Furthermore, not all participants experienced a reduction in sputum eosinophilia even at the 1mg/kg dose. The lack of clinical benefit was partly attributed to the study not being powered to evaluate clinical efficacy. But importantly, participants in these studies were recruited without consideration of their inflammatory cell profile.
The initial studies on eosinophil-directed therapy in asthma were disappointing. However, as it became clear that asthma pathobiologic subtypes existed (vide supra) studies were developed targeting patients with evidence of eosinophilic inflammation. The first such studies targeted patients with persistent sputum eosinophils despite high dose inhaled corticosteroid therapy. In studies with mepolizumab, Haldar et al showed a mean 43% fewer asthma exacerbations relative to placebo over the course of 50 weeks (95% CI 8–68%; P=0.02); Nair et al reported 1 asthma exacerbation in 1 of 9 participants given mepolizumab, while 12 exacerbations occurred on 10 participants given placebo, 55, 56. Interestingly, the Nair trial which included only patients with current sputum eosinophilia (of ≥3% sputum eosinophils) reported improvement in symptoms but the Haldar trial, which enrolled subjects with a documented episode of sputum eosinophilia in the preceding two years, did not report such improvements.
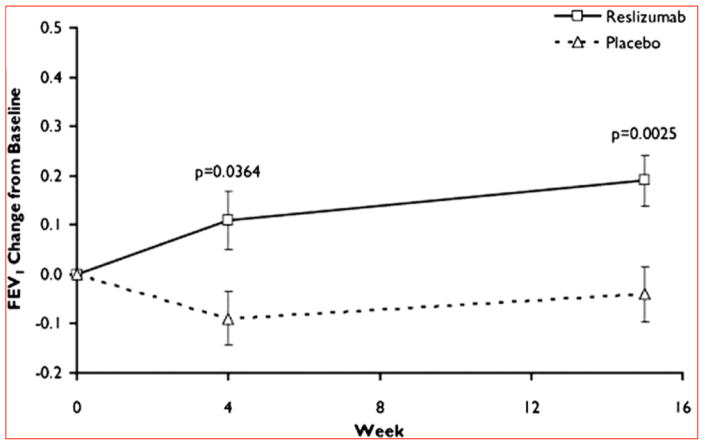
The success of those two mepolizumab trials rekindled interest in reslizumab and other drugs targeting the IL5 pathway. The first phase 2 trial to study the clinical efficacy of reslizumab was reported by Castro et al. on patients with uncontrolled asthma refractory to high-dose ICS and at least one other controller therapy57. Current eosinophilia was required and defined as ≥3% sputum eosinophils at the time of randomization. Participants were also stratified at randomization by degree of asthma control, and the primary outcome was the difference in asthma control between the treatment and control group. Although a statistical significance was not reached in their primary outcome, a non-significant trend towards improved control was observed. Importantly, on pre-specified secondary outcomes reslizumab was found to significantly decrease sputum eosinophils by 95% from baseline (95% CI −100–316%; P=0.01), to decrease blood eosinophils by 400/uL (95% CI 0–1,500; P<0.001; the median at baseline was 500/uL), and to improve lung function with an increase in FEV1 of 180mL (SD 372mL; P<0.01) (Figure 2). Pivotal lessons from this study were that the greatest improvement in asthma control was experienced by i. those most uncontrolled at baseline, ii. those with higher eosinophils levels, and iii. those with nasal polyposis. Prior evidence for the efficacy of reslizumab in treating nasal polyposis had been documented58, noting the frequent connection between nasal polyposis and eosinophilia (particularly when in the setting of asthma). These findings also lent support for trials using the anti-IL5Ra drug benralizumab. In a recently completed phase 2b trial, benralizumab given in 20mg/dose injections was found to decrease asthma exacerbations by 57% (p=0.015, 80% CI 33–72) for patients with asthma and a peripheral blood count of ≥300 eosinophils/uL as compared to those receiving placebo59. These results further support to targeting the IL5 pathway in eosinophilic asthma, and to using peripheral blood eosinophil counts to select patients for these trials.
Figure 2.
Change in lung function in a Phase II clinical trial of reslizumab on eosinophilic asthma presented by Castro et al. The addition of reslizumab to daily high-dose inhaled corticosteroid controller therapy significantly increased forced expiratory volume in 1 s (FEV1) by 4 weeks in comparison to placebo. This improvement remained at the end of the study, at week 15. FEV1 is expressed in liters.
Reprinted with permission of the American Thoracic Society. Copyright _ 2015 American Thoracic Society. Castro M, Mathur S, Hargreave F, et al. 2011.
Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. American journal of respiratory and critical care medicine.
184:1125–32. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society [57].
In turn, two larger phase 3 reslizumab studies have been completed60, 61. Preliminary data from Corren et al and Bjermer et al were presented in abstract form at the 2014 annual meeting of the European Respiratory Society. These trials used ‘uncontrolled asthma’ as an inclusion criterion—consistent with the above phase 2 trial’s observation of greatest benefit for reslizumab when used in participants with uncontrolled asthma at enrollment. As a departure from prior recruitment schemes, these studies defined persistent eosinophilia as a peripheral blood eosinophil count of >400/uL at the time of enrollment and did not require sputum eosinophil counts. In addition, controller therapy requirements were relaxed, allowing participants to be on medium dose ICS. Both investigations reported that reslizumab at 3mg/kg significantly improves lung function and symptoms compared to placebo62, 63, as follows. The increases in FEV1 in these studies were similar in magnitude to those of the above phase 2 trial. Corren et al reported a 270mL increase in FEV1 and a 0.49 drop in ACQ score after 16 weeks of reslizumab (P=0.04 for both measures). Bjermer et al reported a 160mL increase in FEV1 (P≤0.024) and a 0.359 drop in ACQ score (P≤0.033) after 16 weeks of reslizumab. These studies were first to show a significant effect for reslizumab on asthma control. They also demonstrate that identifying current eosinophilia in peripheral blood, as opposed to sputum, is sufficient to identify appropriate candidates for this therapy.
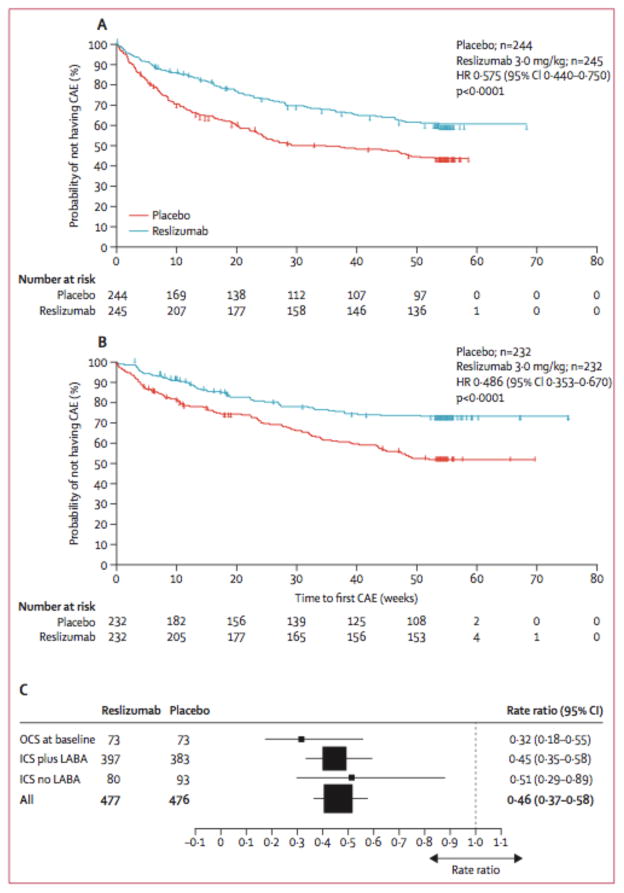
These results were recently confirmed in a report of two large phase 3 clinical trials designed to evaluate reslizumab for its effect on asthma exacerbations. The data from the two trials were pooled together for analysis and presented in a single manuscript64. Enrollment targeted patients with asthma inadequately controlled with high dose ICS and ≥400/uL eosinophils at screening. Similar to mepolizumab65–67, this year-long study found that reslizumab decreased rates of asthma exacerbations by 54% (95% CI 42–63%; P<0.001) and prolonged the time to first exacerbation compared with placebo (Figure 3). Critically, the strongest signal for these outcomes was detected in the 20% of patients who were on oral corticosteroids at entry. For those participants maintained on oral steroids at baseline, the rate of asthma exacerbations was reduced by 68% compared with placebo (95% CI 45–82%; reslizumab, N=73; placebo N=73). For those on ICS plus long-acting beta-2 adrenergic receptor agonists (LABA), the rates of asthma exacerbations were reduced by 55% (95% CI 42–65%; reslizumab, N=397; placebo N=383). For those on ICS alone, the rates of asthma exacerbations were reduced by 49% (95% CI 11–71%; reslizumab, N=80; placebo N=93) (Figure 3). The reductions in rates of asthma exacerbation in this trial are similar in magnitude to comparable mepolizumab trials (47% (95% CI, 29–61%)65; 42% (95% CI, 1–53%)66; 52% (95% CI 36–64%)67). Other efficacy outcomes such as pulmonary function and asthma control were also significantly improved compared with placebo after 52 weeks of therapy. These findings further validate the anti-IL5 strategy in general for treatment of eosinophilic asthma.
Figure 3.
Time to first exacerbation in two Phase IIIb clinical trials of reslizumab on eosinophilic asthma reported by Castro et al. The addition of reslizumab to usual asthma controller therapy significantly reduced the time to first CAE in both trials (A and B). Subgroup analysis showed that the greatest effect occurred in participants using OCS at baseline (C).
Adapted with permission from Lancet Respiratory Medicine*. Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, P [64].
CAE: Clinical asthma exacerbation; ICS: Inhaled corticosteroids; HR: Hazard ratio; LABA: Long-acting b-2 adrenergic receptor antagonists; OCS: Oral corticosteroids.
4.1. Safety
Post-injection anaphylaxis after injection was reported in two patients in the most recent reslizumab trial (out of 477 participants administered reslizumab, 0.42%; anaphylaxis was not reported on participants receiving placebo), and guidelines on how to safely administer it need to be specified. ‘Rebound eosinophilia’ after stopping treatment with anti-IL5 therapy has concerned many due to in vitro observations of up-regulation of the IL5R in eosinophils68, and due to rebound eosinophilia in patients with the hypereosinophilic syndrome after treatment with mepolizumab69. Haldar reported worsening of symptoms with discontinuation of mepolizumab70, but commented did not find evidence of ‘rebound’ airway eosinophilia to attribute this increase in asthma symptoms to. His follow-up study was conducted with 56 participants (27 who has been assigned to mepolizumab) out of the original 61 who participated in the trial. No other similar observations have been reported, and no other systematic follow-up studies have been conducted.
The long-term consequences of eosinopenia are not known. Recent animal models identified IL-10-producing eosinophils that did not contribute to the development of airway hyperresponsiveness but did promote the resolution of airway inflammation71. So far, reports on adverse effects from reslizumab has been no different than those from placebo, except for 2 cases of anaphylaxis (none in the placebo group), as mentioned above. Whether elimination of eosinophils would adversely affect ability to combat parasites is unclear. Evaluation for parasites may be advisable in those with history of exposure or travel.
5. Gaps in knowledge
Current data supports the use of reslizumab and other anti-IL5 biologics in patients with uncontrolled, persistently eosinophilic asthma. While we know that patients with eosinophils and asthma respond best to these therapies, we still do not know the cutoffs for these responses and how therapy modifies our ability to characterize responders. The optimal duration and dose of therapy are unknown. Eosinophilia was found to recur in one follow-up study for mepolizumab70, and is expected to do so after most of these therapies. Since all these therapies will be significantly more expensive than current therapies, cost-benefit analyses in relation to existing, less expensive, therapies will need to be performed72. Head-to-head trials between reslizumab and the other anti-IL5 agents have not been performed.
6. Conclusion
A substantial portion of the asthmatic population remains symptomatic despite the use of currently available controller therapies. For those in whom eosinophilic inflammation is prominent, anti-IL5 therapies promise to be a welcome addition to the asthma armamentarium. After a slow start in their drug development process, anti-IL5 therapies have seen success in newer trials through more informed study participant selection targeting eosinophilic inflammation.
7. Expert opinion
The studies with reslizumab have examined thresholds of sputum eosinophils ≥3%, or blood eosinophils ≥400/uL. However, studies with other agents suggest that in patients with two or more exacerbations in the prior year there may be effectiveness using a threshold as low as 150 eosinophils/uL65. This effect may not to extend to patients with fewer exacerbations. While these drugs appear to have a very good safety profile, long-term safety is yet to accumulate especially in areas with greater prevalence of parasites. However, these drugs appear to represent the first apparent low-morbidity therapy for patients who remain symptomatic despite our best currently available therapy and who have evidence that eosinophils play a role in their disease. That evidence can now be obtained from simple blood counts. These drugs clearly impact recurrent exacerbations in these patients and future studies will define those who achieve improvements in symptoms and lung-function. The approval of reslizumab and other drugs targeting eosinophils is eagerly awaited by the target patients and the clinicians who treat them.
Footnotes
Financial and competing interests disclosure
E Israel as received consultancy fees from AstraZeneca, travel grant support from TEVA speciality pharmaceuticals and research grants from Genentech. JC Cardet was supported by a diversity research supplement from NIAID. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Contributor Information
Juan Carlos Cardet, Brigham and Women’s Hospital, Asthma Research Center, 75, Francis St, Surgery Bldg 1, Suite 155. Boston, MA 02115, Instructor of Medicine, Harvard Medical School, Assistant Director of the Asthma Research Center at Brigham and Women’s Hospital, Divisions of Rheumatology, Immunology & Allergy, and Pulmonary and Critical Care Medicine, Telephone number: 617-732-8201, Fax number: 617-730-2858.
Elliot Israel, Brigham and Women’s Hospital, 75 Francis St, Boston, MA 02115, Professor of Medicine, Harvard Medical School, Director of Clinical Research, Division of Pulmonary and Critical Care Medicine at Brigham and Women’s Hospital, Telephone number: 617-732-8110, Fax number: 617-732-7421.
References
- 1.Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. The Journal of allergy and clinical immunology. 2011;127(1):145–52. doi: 10.1016/j.jaci.2010.10.020. Epub 2011/01/08. [DOI] [PubMed] [Google Scholar]
- 2.Moorman JE, Zahran H, Truman BI, Molla MT. Current asthma prevalence - United States, 2006–2008. MMWR Surveill Summ. 2011;60(Suppl):84–6. Epub 2011/03/25. [PubMed] [Google Scholar]
- 3.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. The European respiratory journal. 2014;43(2):343–73. doi: 10.1183/09031936.00202013. Epub 2013/12/18. [DOI] [PubMed] [Google Scholar]
- 4.Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. American journal of respiratory and critical care medicine. 2004;170(8):836–44. doi: 10.1164/rccm.200401-033OC. Epub 2004/07/17. [DOI] [PubMed] [Google Scholar]
- 5.Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. American journal of respiratory and critical care medicine. 2009;180(5):388–95. doi: 10.1164/rccm.200903-0392OC. Epub 2009/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGrath KW, Icitovic N, Boushey HA, et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. American journal of respiratory and critical care medicine. 2012;185(6):612–9. doi: 10.1164/rccm.201109-1640OC. Epub 2012/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. American journal of respiratory and critical care medicine. 2010;181(4):315–23. doi: 10.1164/rccm.200906-0896OC. Epub 2009/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. The Journal of allergy and clinical immunology. 2011;127(2):355–60. doi: 10.1016/j.jaci.2010.11.037. Epub 2011/02/02. [DOI] [PubMed] [Google Scholar]
- 9.Olin JT, Wechsler ME. Asthma: pathogenesis and novel drugs for treatment. BMJ. 2014;349:g5517. doi: 10.1136/bmj.g5517. Epub 2014/11/26. [DOI] [PubMed] [Google Scholar]
- 10.Hambly N, Nair P. Monoclonal antibodies for the treatment of refractory asthma. Current opinion in pulmonary medicine. 2014;20(1):87–94. doi: 10.1097/MCP.0000000000000007. Epub 2013/11/28. [DOI] [PubMed] [Google Scholar]
- 11.Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: The next steps toward personalized care. The Journal of allergy and clinical immunology. 2015;135(2):299–310. doi: 10.1016/j.jaci.2014.12.1871. Epub 2015/02/11. [DOI] [PubMed] [Google Scholar]
- 12.Nair P. What is an “eosinophilic phenotype” of asthma? The Journal of allergy and clinical immunology. 2013;132(1):81–3. doi: 10.1016/j.jaci.2013.05.007. Epub 2013/06/04. [DOI] [PubMed] [Google Scholar]
- 13.Kita HaB, Bruce S. Biology of Eosinophils. In: Adkinson NFea., editor. Midleton’s Allergy: Principles and Practice. 8. Saunders, Elsevier inc; 2014. pp. 265–79. [Google Scholar]
- 14.Mukherjee M, Sehmi R, Nair P. Anti-IL5 therapy for asthma and beyond. The World Allergy Organization journal. 2014;7(1):32. doi: 10.1186/1939-4551-7-32. Epub 2015/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legrand F, Klion AD. Biologic therapies targeting eosinophils: current status and future prospects. The journal of allergy and clinical immunology In practice. 2015;3(2):167–74. doi: 10.1016/j.jaip.2015.01.013. Epub 2015/03/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia G, Taille C, Laveneziana P, Bourdin A, Chanez P, Humbert M. Anti-interleukin-5 therapy in severe asthma. European respiratory review : an official journal of the European Respiratory Society. 2013;22(129):251–7. doi: 10.1183/09059180.00004013. Epub 2013/09/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCallister JW. Reslizumab and eosinophilic asthma: one step closer to phenotype-directed therapy? American journal of respiratory and critical care medicine. 2011;184(10):1096–7. doi: 10.1164/rccm.201108-1565ED. Epub 2011/11/17. [DOI] [PubMed] [Google Scholar]
- 18.Lim HF, Nair P. Efficacy and safety of reslizumab in patients with moderate to severe eosinophilic asthma. Expert review of respiratory medicine. 2015;9(2):135–42. doi: 10.1586/17476348.2015.1000867. Epub 2015/01/13. [DOI] [PubMed] [Google Scholar]
- 19.Walsh GM. Profile of reslizumab in eosinophilic disease and its potential in the treatment of poorly controlled eosinophilic asthma. Biologics : targets & therapy. 2013;7:7–11. doi: 10.2147/BTT.S30133. Epub 2013/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellis AG. The pathological anatomy of bronchial asthma. Am J Med Sci. 1908;136:407–29. [Google Scholar]
- 21.Houston JC, De Navasquez S, Trounce JR. A clinical and pathological study of fatal cases of status asthmaticus. Thorax. 1953;8(3):207–13. doi: 10.1136/thx.8.3.207. Epub 1953/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horn BR, Robin ED, Theodore J, Van Kessel A. Total eosinophil counts in the management of bronchial asthma. The New England journal of medicine. 1975;292(22):1152–5. doi: 10.1056/NEJM197505292922204. Epub 1975/05/29. [DOI] [PubMed] [Google Scholar]
- 23.Taylor KJ, Luksza AR. Peripheral blood eosinophil counts and bronchial responsiveness. Thorax. 1987;42(6):452–6. doi: 10.1136/thx.42.6.452. Epub 1987/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gundel RH, Gerritsen ME, Gleich GJ, Wegner CD. Repeated antigen inhalation results in a prolonged airway eosinophilia and airway hyperresponsiveness in primates. J Appl Physiol (1985) 1990;68(2):779–86. doi: 10.1152/jappl.1990.68.2.779. Epub 1990/02/01. [DOI] [PubMed] [Google Scholar]
- 25.Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. The New England journal of medicine. 1990;323(15):1033–9. doi: 10.1056/NEJM199010113231505. Epub 1990/10/11. [DOI] [PubMed] [Google Scholar]
- 26.Davis WB, Fells GA, Sun XH, Gadek JE, Venet A, Crystal RG. Eosinophil-mediated injury to lung parenchymal cells and interstitial matrix. A possible role for eosinophils in chronic inflammatory disorders of the lower respiratory tract. The Journal of clinical investigation. 1984;74(1):269–78. doi: 10.1172/JCI111411. Epub 1984/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gleich GJ, Loegering DA, Adolphson CR. Eosinophils and bronchial inflammation. Chest. 1985;87(1 Suppl):10S–3S. doi: 10.1378/chest.87.1_supplement.10s. Epub 1985/01/01. [DOI] [PubMed] [Google Scholar]
- 28.Yasukawa A, Hosoki K, Toda M, et al. Eosinophils promote epithelial to mesenchymal transition of bronchial epithelial cells. PloS one. 2013;8(5):e64281. doi: 10.1371/journal.pone.0064281. Epub 2013/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gleich GJ. Mechanisms of eosinophil-associated inflammation. The Journal of allergy and clinical immunology. 2000;105(4):651–63. doi: 10.1067/mai.2000.105712. Epub 2000/04/11. [DOI] [PubMed] [Google Scholar]
- 30.Frigas E, Loegering DA, Solley GO, Farrow GM, Gleich GJ. Elevated levels of the eosinophil granule major basic protein in the sputum of patients with bronchial asthma. Mayo Clinic proceedings. 1981;56(6):345–53. Epub 1981/06/01. [PubMed] [Google Scholar]
- 31.Flavahan NA, Slifman NR, Gleich GJ, Vanhoutte PM. Human eosinophil major basic protein causes hyperreactivity of respiratory smooth muscle. Role of the epithelium. The American review of respiratory disease. 1988;138(3):685–8. doi: 10.1164/ajrccm/138.3.685. Epub 1988/09/01. [DOI] [PubMed] [Google Scholar]
- 32.Gundel RH, Letts LG, Gleich GJ. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. The Journal of clinical investigation. 1991;87(4):1470–3. doi: 10.1172/JCI115155. Epub 1991/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715–21. doi: 10.1016/S0140-6736(02)11679-5. Epub 2002/12/14.: This clinical trial compared ICS dose adjustments in response to sputum eosinophils vs guideline-driven decisions, and found that the prior strategy reduced asthma exacerbations, supporting a major role for eosinophils in asthma. [DOI] [PubMed] [Google Scholar]
- 34.Jayaram L, Pizzichini MM, Cook RJ, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. The European respiratory journal. 2006;27(3):483–94. doi: 10.1183/09031936.06.00137704. Epub 2006/03/02. [DOI] [PubMed] [Google Scholar]
- 35.Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood. 1989;73(6):1504–12. Epub 1989/05/01. [PubMed] [Google Scholar]
- 36.Steinke JW, Rosenwasser LJ, Borish L. Cytokines in Allergic Inflammation. In: Adkinson NFea., editor. Middleton’s Allergy: Principles and Practice. 8. Elsevier; 2014. pp. 65–82. [Google Scholar]
- 37.Brusselle GG, Maes T, Bracke KR. Eosinophils in the spotlight: Eosinophilic airway inflammation in nonallergic asthma. Nature medicine. 2013;19(8):977–9. doi: 10.1038/nm.3300. Epub 2013/08/08. [DOI] [PubMed] [Google Scholar]
- 38.Lalani T, Simmons RK, Ahmed AR. Biology of IL-5 in health and disease. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 1999;82(4):317–32. doi: 10.1016/S1081-1206(10)63281-4. quiz 32–3. Epub 1999/05/05. [DOI] [PubMed] [Google Scholar]
- 39.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. The Journal of experimental medicine. 1996;183(1):195–201. doi: 10.1084/jem.183.1.195. Epub 1996/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamid Q, Azzawi M, Ying S, et al. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. The Journal of clinical investigation. 1991;87(5):1541–6. doi: 10.1172/JCI115166. Epub 1991/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. The New England journal of medicine. 1992;326(5):298–304. doi: 10.1056/NEJM199201303260504. Epub 1992/01/30. [DOI] [PubMed] [Google Scholar]
- 42.Humbert M, Corrigan CJ, Kimmitt P, Till SJ, Kay AB, Durham SR. Relationship between IL-4 and IL-5 mRNA expression and disease severity in atopic asthma. American journal of respiratory and critical care medicine. 1997;156(3 Pt 1):704–8. doi: 10.1164/ajrccm.156.3.9610033. Epub 1997/10/06. [DOI] [PubMed] [Google Scholar]
- 43.Corrigan CJ, Haczku A, Gemou-Engesaeth V, et al. CD4 T-lymphocyte activation in asthma is accompanied by increased serum concentrations of interleukin-5. Effect of glucocorticoid therapy. The American review of respiratory disease. 1993;147(3):540–7. doi: 10.1164/ajrccm/147.3.540. Epub 1993/03/01. [DOI] [PubMed] [Google Scholar]
- 44.Shi H, Qin S, Huang G, et al. Infiltration of eosinophils into the asthmatic airways caused by interleukin 5. American journal of respiratory cell and molecular biology. 1997;16(3):220–4. doi: 10.1165/ajrcmb.16.3.9070605. Epub 1997/03/01. [DOI] [PubMed] [Google Scholar]
- 45.Johansson MW, Mosher DF. Integrin activation States and eosinophil recruitment in asthma. Frontiers in pharmacology. 2013;4:33. doi: 10.3389/fphar.2013.00033. Epub 2013/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Egan RW, Athwal D, Bodmer MW, et al. Effect of Sch 55700, a humanized monoclonal antibody to human interleukin-5, on eosinophilic responses and bronchial hyperreactivity. Arzneimittel-Forschung. 1999;49(9):779–90. doi: 10.1055/s-0031-1300502. Epub 1999/10/09. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Kuvelkar R, Murgolo NJ, et al. Mapping and characterization of the epitope(s) of Sch 55700, a humanized mAb, that inhibits human IL-5. International immunology. 1999;11(12):1935–44. doi: 10.1093/intimm/11.12.1935. Epub 1999/12/11. [DOI] [PubMed] [Google Scholar]
- 48.Hart TK, Cook RM, Zia-Amirhosseini P, et al. Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys. The Journal of allergy and clinical immunology. 2001;108(2):250–7. doi: 10.1067/mai.2001.116576. Epub 2001/08/10. [DOI] [PubMed] [Google Scholar]
- 49.Carter PJ. Potent antibody therapeutics by design. Nature reviews Immunology. 2006;6(5):343–57. doi: 10.1038/nri1837. Epub 2006/04/20. [DOI] [PubMed] [Google Scholar]
- 50.Ghazi A, Trikha A, Calhoun WJ. Benralizumab--a humanized mAb to IL-5Ralpha with enhanced antibody-dependent cell-mediated cytotoxicity--a novel approach for the treatment of asthma. Expert opinion on biological therapy. 2012;12(1):113–8. doi: 10.1517/14712598.2012.642359. Epub 2011/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denburg JA, Silver JE, Abrams JS. Interleukin-5 is a human basophilopoietin: induction of histamine content and basophilic differentiation of HL-60 cells and of peripheral blood basophil-eosinophil progenitors. Blood. 1991;77(7):1462–8. Epub 1991/04/01. [PubMed] [Google Scholar]
- 52.Kips JC, O’Connor BJ, Langley SJ, et al. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. American journal of respiratory and critical care medicine. 2003;167(12):1655–9. doi: 10.1164/rccm.200206-525OC. Epub 2003/03/22. [DOI] [PubMed] [Google Scholar]
- 53.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. American journal of respiratory and critical care medicine. 2003;167(2):199–204. doi: 10.1164/rccm.200208-789OC. Epub 2002/10/31. [DOI] [PubMed] [Google Scholar]
- 54.Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356(9248):2144–8. doi: 10.1016/s0140-6736(00)03496-6. Epub 2001/02/24. [DOI] [PubMed] [Google Scholar]
- 55*.Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. The New England journal of medicine. 2009;360(10):985–93. doi: 10.1056/NEJMoa0805435. Epub 2009/03/07.: one of the first trials with anti-IL5 therapies to show clinical efficacy (see reference below) [DOI] [PubMed] [Google Scholar]
- 56*.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. The New England journal of medicine. 2009;360(10):973–84. doi: 10.1056/NEJMoa0808991. Epub 2009/03/07.: one of the first trials with anti-IL5 therapies to show clinical efficacy (see reference above) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57*.Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. American journal of respiratory and critical care medicine. 2011;184(10):1125–32. doi: 10.1164/rccm.201103-0396OC. Epub 2011/08/20.: first clinically successful asthma trial with reslizumab. [DOI] [PubMed] [Google Scholar]
- 58.Gevaert P, Lang-Loidolt D, Lackner A, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. The Journal of allergy and clinical immunology. 2006;118(5):1133–41. doi: 10.1016/j.jaci.2006.05.031. Epub 2006/11/08. [DOI] [PubMed] [Google Scholar]
- 59.Castro M, Wenzel SE, Bleecker ER, et al. Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. The Lancet Respiratory medicine. 2014;2(11):879–90. doi: 10.1016/S2213-2600(14)70201-2. Epub 2014/10/13. [DOI] [PubMed] [Google Scholar]
- 60.Clinicaltrials.gov identifier NCT01508936. 2015 Available from: https://clinicaltrials.gov/show/NCT01508936.
- 61.Clinicaltrials.gov identifier NCT01270464. 2015 [04/06/2015]; Available from: http://ClinicalTrials.gov/show/NCT01270464.
- 62*.Jea Corren. A randomized phase 3 study of reslizumab efficacy in relation to blood eosinophil levels in patients with moderate to severe asthma. European Respiratory Journal. 2014;44(supplement 58) one of the first reslizumab trials to identify eosinophilic asthma based on peripheral blood eosinophil levels (see reference below) [Google Scholar]
- 63*.Bjermer L. A randomized phase 3 study of the efficacy and safety of reslizumab in subjects with asthma with elevated eosinophils. The European respiratory journal. 2014;44(supplement 58) one of the first reslizumab trials to identify eosinophilic asthma based on peripheral blood eosinophil levels (see reference above). [Google Scholar]
- 64**.Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. The Lancet Respiratory medicine. 2015 doi: 10.1016/S2213-2600(15)00042-9. Epub 2015/03/05. first major trial showing efficacy in reducing asthma exacerbations with reslizumab. [DOI] [PubMed] [Google Scholar]
- 65*.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. The New England journal of medicine. 2014;371(13):1198–207. doi: 10.1056/NEJMoa1403290. Epub 2014/09/10. clinically successful asthma trial with mepolizumab showing reductions in exacerbations. [DOI] [PubMed] [Google Scholar]
- 66*.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. The New England journal of medicine. 2014;371(13):1189–97. doi: 10.1056/NEJMoa1403291. Epub 2014/09/10. clinically successful asthma trial with mepolizumab showing a steroid sparing effect for patients with prednisone-dependent severe asthma. [DOI] [PubMed] [Google Scholar]
- 67*.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–9. doi: 10.1016/S0140-6736(12)60988-X. Epub 2012/08/21. clinically successful asthma trial with mepolizumab. [DOI] [PubMed] [Google Scholar]
- 68.Stein ML, Villanueva JM, Buckmeier BK, et al. Anti-IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. The Journal of allergy and clinical immunology. 2008;121(6):1473–83. 83 e1–4. doi: 10.1016/j.jaci.2008.02.033. Epub 2008/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim YJ, Prussin C, Martin B, et al. Rebound eosinophilia after treatment of hypereosinophilic syndrome and eosinophilic gastroenteritis with monoclonal anti-IL-5 antibody SCH55700. The Journal of allergy and clinical immunology. 2004;114(6):1449–55. doi: 10.1016/j.jaci.2004.08.027. Epub 2004/12/04. [DOI] [PubMed] [Google Scholar]
- 70.Haldar P, Brightling CE, Singapuri A, et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. The Journal of allergy and clinical immunology. 2014;133(3):921–3. doi: 10.1016/j.jaci.2013.11.026. Epub 2014/01/15. [DOI] [PubMed] [Google Scholar]
- 71.Takeda K, Shiraishi Y, Ashino S, et al. Eosinophils contribute to the resolution of lung-allergic responses following repeated allergen challenge. The Journal of allergy and clinical immunology. 2015;135(2):451–60. doi: 10.1016/j.jaci.2014.08.014. Epub 2014/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nair P. Anti-interleukin-5 monoclonal antibody to treat severe eosinophilic asthma. The New England journal of medicine. 2014;371(13):1249–51. doi: 10.1056/NEJMe1408614. Epub 2014/09/10. [DOI] [PubMed] [Google Scholar]