Abstract
Objective
To evaluate the effect of the fetal head station at attempted operative vaginal delivery (aOVD), and specifically midpelvic or low aOVD, on urinary incontinence (UI), anal incontinence (AI), and perineal pain at 6 months.
Design
Prospective cohort study.
Setting
1941 women with singleton term fetuses in vertex presentation with midpelvic or low aOVD between 2008 and 2013 in a tertiary care university hospital.
Methods
Symptoms of urinary incontinence (UI) using the Bristol Female Lower Urinary Tract Symptoms questionnaire, and symptoms of anal incontinence (AI) severity using Fecal Incontinence Severity Index (FISI) were assessed 6 months after aOVD. We measured the association between midpelvic or low aOVD and symptoms of UI, AI, and perineal pain at 6 months using multiple regression and adjusting for demographics, and risk factors of UI and AI, with adjusted odds ratios (aORs) and 95% confidence intervals (95% CI).
Results
The study included 907 women (46.7%) who responded to the questionnaire; 18.4% (167/907) had midpelvic aOVD, and 81.6% (740/907) low; and none of women with symptoms of UI (26.6%, and 22.4%, respectively; p = 0.31), AI (15.9%, and 21.8%; p = 0.09), the FISI score, and perineal pain (17.2%, and 12.7%; p = 0.14) differed significantly between groups. The same was true for stress, urge, and mixed-type UI, severe UI and difficulty voiding. Compared with low pelvic aOVD, the aORs for symptoms of UI in midpelvic aOVD were 0.70 (0.46–1.05) and AI 1.42 (0.85–2.39). Third- and fourth-degree tears were a major risk factor of symptoms of UI (aOR 3.08, 95% CI 1.35–7.00) and AI (aOR 3.47, 95% CI 1.43–8.39).
Conclusion
Neither symptoms of urinary nor anal incontinence differed at 6 months among women who had midpelvic and low pelvic aOVD. These findings are reassuring and need further studies at long-term to confirm these short-term data.
Introduction
Although most women want a spontaneous vaginal delivery, a non-negligible proportion of them have a labor that fails to progress during the second stage of labor and can thus leave the fetus at midpelvic station. Obstetricians are then faced with a choice between a potentially difficult operative vaginal delivery (OVD) and cesarean delivery at full dilatation with a fetus in midpelvis, each with its own immediate and long-term maternal and neonatal risks. This situation seems to present the perfect setting for a randomised trial, which would probably fail to achieve due to a neither feasible nor ethical recruitment in the antenatal period. Furthermore, when the fetus is at low pelvic station, OVD is not discussed for maternal or fetal indication, and must be preferred to caesarean delivery [1]. Our previous report that midpelvic attempted operative vaginal delivery (aOVD) was not associated with a higher rate of severe short-term maternal and neonatal morbidity than attempted low pelvic delivery suggests that midpelvic aOVD is an appropriate option in selected women [2].
However, if its mid- and long-term maternal or neonatal outcome is worse than that for low pelvic OVD, cesarean delivery might be preferred over an OVD when the fetus is at midpelvis. Many studies have assessed pelvic floor symptoms, urinary incontinence (UI), anal incontinence (AI) and perineal pain during the postpartum period according to the mode of delivery (spontaneous vaginal delivery, OVD, caesarean), or complications of pregnancy or delivery [3–13]. Findings vary widely. These discrepancies may be explained by methodological differences (retrospective, prospective or ambispective designs, sample size, or time during the postpartum period chosen for evaluation). To our knowledge, no study has specifically evaluated postpartum pelvic floor symptoms after an aOVD according to the fetal head station. In a prospective based cohort study, we aimed to compare pelvic floor symptoms after aOVD for midpelvic and for low aOVDs at 6 months, and to analyse the risk factors of symptoms of UI and AI on this population.
Material and Methods
Study sample
The study protocol was approved by the Institutional Review Board at the Angers University Hospital, France, on November 2008 (Study ID: 2008) [2]. This study was conducted in accordance with French law. All subjects were told about the study and were given oral information. Verbal consent was provided to the medical team in charge of the study. Our study was done using data from a prospective based cohort study including 1,941 women with live singleton term fetuses in vertex presentation who underwent an aOVD at midpelvic or low from December 2008 through October 2013 in a tertiary care university hospital with more than 4,000 annual deliveries [2]. Pre-specified study design was to analyse short-term maternal and neonatal morbidity according to the fetal head station (midpelvic or low aOVD) [2], and to prospectively analyse long-term maternal complication (pelvic floors disorders, sexual dysfunction, maternal postpartum depressive symptoms at 6 months and 5 years), and children development at 5 years, according to the fetal head station, and specifically in midpelvic or low pelvic aOVD. As described in detail previously [2], this study included all women with an aOVD, defined by the placement of at least one blade for forceps or spatula or a vacuum, regardless of its success (i.e., whether delivery was finally vaginal or caesarean), and a live singleton pregnancy in vertex presentation at term (equal to or later than 37 weeks of gestation) [2]. Exclusion criteria were multiple gestations, fetal growth restriction (FGR), defined as <10th centile for gestational age on Hadlock curves [14,15], a known congenital anomaly, vaginal breech delivery, and the absence of fetal station information according to the American College of Obstetricians and Gynaecologists (ACOG) classification [16]. Specifically, station was defined by the level of the leading bony point of the fetal head in centimetres at or below the level of maternal ischial spines (0 and +1 = midpelvic; +2 and +3 = low). Two cohorts of women were assessed separately: those with a midpelvic aOVD (N = 391; 20.6%), and a lowpelvic aOVD (N = 1,550; 79.4%).
Measures
Information about pelvic floor disorders was obtained from a questionnaire sent 6 months after delivery. A second mailing was sent to the women from whom we received no response. A questionnaire asked about postpartum pelvic floor exercises and pelvic floor symptoms during the preceding 4 weeks. Those women who answered yes to the entry question “Do you have involuntary loss of urine?” were considered to have symptoms of UI and were then asked further questions from the French version of a validated questionnaire Bristol Female Lower Urinary Tract Symptoms (BFLUTS) [8,17,18], about the frequency, amount, and circumstances of leakage and if incontinence was a problem for them. Stress urinary incontinence (SUI) was assessed by responses to the question "Does urine leak when you are physically active, cough or sneeze?" Possible responses were as follows: never, occasionally, sometimes, often or all the time. Occasionally was defined as less than one-third of the time; sometimes as between one and two-thirds of the time; and often as more than two-thirds of the time. Women who answered “often” or “all the time” were considered to have severe SUI [8,17,18]. Urge incontinence was assessed by any positive response to "Does urine leak before you go to the toilet?", and mixed incontinence by a positive response to both of previous questions. Voiding difficulty was assessed by response to the question "Do you have difficulties in emptying your bladder?" [8,17].
As previously reported [8], perineal pain was evaluated through the question: “Do you have chronic perineal pain (perineum designates the skin and muscle around the vaginal and anal outlets)?”. This question was dichotomous with two possible answers: “yes” and “no” [8]. Episiotomy complications were evaluated through the question: "Do you have any complications concerning your episiotomy (hematoma, abscess, scar disunion, surgery)?" They were defined by the existence of at least one of the following criteria: hematoma, abscess, scar disunion, or required surgery for episiotomy.
Anal incontinence was defined by yes (versus no) response to “Do you have involuntary loss of flatus or stool?” [8]. The severity of symptoms of AI was assessed with the French version of the American Society of Colon and Rectal Surgeon’s Fecal Incontinence Severity Index (FISI) [19,20]. The FISI is based on [(type of incontinence) x (frequency matrix)]. The matrix includes four types of leakage commonly found in the faecal-incontinent population (gas, mucus, and liquid and solid stool) and five frequencies classified as involuntary loss 2 or more times a day, once a day, 2 or more times a week, once a week, 1–3 times a month, or never. To create the FISI, responses for each of the four items were summed, with a higher FISI indicating greater perceived symptoms. The questionnaire used for the study is available (S1 Questionnaire).
Statistical analysis
Continuous data were described by their means ± standard deviations and compared by t-tests (or Mann-Whitney tests when appropriate); categorical data were described by percentages and compared by chi-square tests (or Fisher exact tests when appropriate). Univariate and multivariate logistic regression were used to study the association between UI or AI at 6 months (considered as a dichotomous variable) and aOVD classification. Univariate and multivariable linear regression analysis were conducted to study the association between AI severity (FISI index) at 6 months (considered as a continuous variable) and aOVD classification. Multivariate models were built with a stepwise procedure based on the Akaike criterion [21,22], and the covariate "fetal head station" was systematically forced into the models. Confounders associated in univariate analysis at a 0.2 level were included in this stepwise procedure. To check the fit of the multivariate models, we studied the studentized residuals. Wald tests were performed for testing the significance of the covariates included in the models. STATA 13.1 software (StataCorp, College Station, TX, USA) was used for all analyses. Statistical significance was defined as a P value < 0.05.
Results
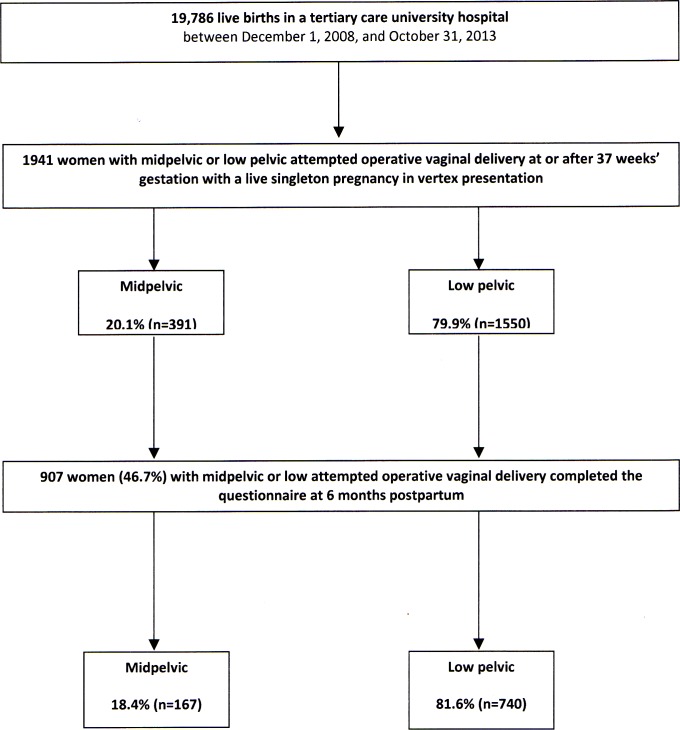
Six months after delivery, 907 women (46.7%) of the 1941 deliveries with an aOVD completed the questionnaire: 18.4% (N = 167) had been midpelvic, and 81.6% (N = 740) low attempted deliveries (Fig 1). The main differences between respondents and non-respondents were maternal age at delivery (29.0 ± 4.8 years compared to 27.6 ± 5.2 years; p<0.001), marital status (96.1% married or living with a partner compared to 91.9%; p<0.001), severe neonatal morbidity (9.6% compared to 12.8%; p = 0.03), rates of NICU transfer (5.0% compared to 8.4%; p = 0.003), and prolonged hospitalisation (>24 hours) in NICU (4.2% compared to 7.4%; p = 0.003). The fetal head station did not differ significantly between respondents and non-respondents (S1 Table).
Fig 1. Cohort flowchart.
Table 1 summarizes the characteristics of the women and their labour, and the maternal and neonatal outcomes among respondents. Among women with aOVD, the prevalence of symptoms of UI at 6 months was 21.6% (N = 196), the prevalence of symptoms of AI 19.0% (n = 172), and 113 women (12.5%) reported perineal pain. No significant differences were observed for symptoms of UI (26.6% for midpelvic, and 22.4% for low deliveries; p = 0.31), symptoms of AI (15.9%, and 21.8%, respectively; p = 0.09), and perineal pain (17.2%, and 12.7%; p = 0.14) between these groups. The mean FISI scores in women with AI were low and did not differ between the groups (11.9±6.3 vs. 11.8±6.8, respectively; p = 0.93), nor did the prevalence of stress, urge, mixed-type incontinence, severe urinary incontinence or difficulty voiding (Table 1). Women with symptoms of UI were compared to women without symptoms of UI, and women with symptoms of AI compared to women without symptoms of AI (Table 2). These groups differed according to maternal age, third/fourth degree perineal tears, perineal pain, and breast-feeding, but not according to head station at aOVD (Table 2). Finally, episiotomy was not also significantly associated with any of the pelvic floor disorders considered at 6 months (Table 2).
Table 1. Characteristics of mothers and their labor and maternal and neonatal outcomes in respondents, according to the ACOG classification.
| Mid (N = 167) | Low (N = 740) | P value | |
|---|---|---|---|
| Maternal and labor characteristics | |||
| Maternal age, (years)1 | 29.2 ± 5.3 | 29.0 ± 4.7 | 0.59* |
| Geographic origin | 0.34** | ||
| Europe, n (%) | 156 (93.4) | 699 (94.5) | |
| Sub-Saharan Africa, n (%) | 5 (3.0) | 8 (1.1) | |
| North Africa, n (%) | 1 (0.6) | 6 (0.8) | |
| Asia, n (%) | 2 (1.2) | 17 (2.3) | |
| Other, n (%) | 3 (1.8) | 10 (1.3) | |
| Married or living with a partner, n (%) | 158 (95.8) | 709 (96.2) | 0.79** |
| Nulliparity, n (%) | 118 (70.7) | 564 (76.2) | 0.13** |
| Previous cesarean delivery, n (%) | 20 (42.6) | 66 (37.5) | 0.52** |
| Previous 3rd or 4th-degree perineal lacerations, n (%) | 0 | 1 (0.6) | 0.60*** |
| Previous depression, n (%) | 5 (3.0) | 38 (5.1) | 0.25** |
| BMI before pregnancy (kg/m2)1 | 22.8 ± 4.1 | 22.7 ± 3.9 | 0.72* |
| Gestational weight gain (kg) 1 | 13.8 ± 4.5 | 13.3 ± 4.4 | 0.23* |
| Antenatal suspicion of macrosomia 2, n (%) | 18 (10.8) | 48 (6.5) | 0.05** |
| Gestational age at delivery (weeks) 1 | 39.5 ± 1.5 | 39.4 ± 1.4 | 0.63* |
| Induced labor, n (%) | 30 (18.0) | 125 (16.9) | 0.74** |
| Length of labor (min) 1 | 395.7 ± 179.1 | 388.4 ± 165.0 | 0.61* |
| Length of 2nd stage (min) 1 | 103.3 ± 73.8 | 108.8 ± 67.3 | 0.35* |
| Active phase of 2nd stage > 30 min, n (%) | 51 (30.5) | 267 (36.1) | 0.28** |
| Dose of oxytocin (mUI) 1 | 1976.3 ± 2228.8 | 1620.9 ± 2084.8 | 0.05* |
| Epidural analgesia, n (%) | 164 (98.2) | 698 (94.5) | 0.04** |
| Manual rotation, n (%) | 30 (18.0) | 81 (11.0) | 0.01** |
| Persistent occiput | 0.05** | ||
| Anterior, n (%) | 139 (83.2) | 662 (89.8) | |
| Posterior, n (%) | 21 (12.6) | 56 (7.6) | |
| Transverse, n (%) | 7 (4.2) | 19 (2.6) | |
| Indications for OVD | 0.02** | ||
| Non reassuring FHR only, n (%) | 86 (51.5) | 301 (40.7) | |
| Arrested progress only, n (%) | 51 (30.5) | 313 (42.3) | |
| Non reassuring FHR and arrested progress, n (%) | 30 (18.0) | 129 (17.4) | |
| OVD in operating room, n (%) | 12 (7.2) | 4 (0.5) | <0.001*** |
| Provider attending delivery | <0.001** | ||
| Senior obstetrician, n (%) | 88 (54.7) | 159 (21.7) | |
| Resident, n (%) | 73 (45.3) | 573 (78.3) | |
| Instrument type | |||
| Vacuum, n (%) | 13 (7.8) | 237 (32.0) | <0.001** |
| Forceps, n (%) | 20 (12.0) | 38 (5.1) | <0.001*** |
| Spatula, n (%) | 140 (84.3) | 485 (65.5) | <0.001** |
| Sequential use of instrument, n (%) | 6 (3.6) | 21 (2.8) | 0.59*** |
| Maternal outcome | |||
| Cesarean section after failed OVD, n (%) | 12 (7.2) | 4 (0.5) | <0.001*** |
| Episiotomy, n (%) | 144 (87.3) | 652 (88.1) | 0.77** |
| 3rd or 4th-degree perineal lacerations, n (%) | 3 (1.8) | 25 (3.4) | 0.30*** |
| Perineal hematomas, n (%) | 0 | 1 (0.1) | 0.63*** |
| Abscesses/hematoma required surgery, n (%) | 1 (0.7) | 3 (0.4) | 0.69*** |
| Postpartum hemorrhage (PPH), n (%) | 35 (20.9) | 128 (17.3) | 0.27** |
| Severe PPH (blood loss>1500mL), n (%) | 5 (3.0) | 16 (2.2) | 0.52*** |
| Second-line therapies3, n (%) | 1 (1.0) | 1 (0.2) | 0.25*** |
| Blood transfusion, n (%) | 7 (4.2) | 12 (1.6) | 0.04*** |
| Infections4, n (%) | 1 (0.7) | 1 (0.1) | 0.25*** |
| Thromboembolic events, n (%) | 0 | 2 (0.3) | 0.50*** |
| Maternal hospitalization in intensive care unit, n (%) | 0 | 0 | - |
| Severe maternal morbidity5, n (%) | 13 (7.8) | 64 (8.7) | 0.71** |
| Neonatal outcome | |||
| Birth weight≥4000 g, n (%) | 12 (7.2) | 38 (5.1) | 0.30** |
| 5-min Apgar score<7, n (%) | 1 (0.6) | 5 (0.7) | 0.91*** |
| pH<7.00, n (%) | 4 (2.5) | 10 (1.4) | 0.32*** |
| Transfer to NICU, n (%) | 9 (5.4) | 36 (4.9) | 0.78** |
| NICU hospitalisation>24 h, n (%) | 9 (5.4) | 29 (3.9) | 0.39** |
| Respiratory distress syndrome, n (%) | 8 (4.8) | 30 (4.1) | 0.66** |
| Neonatal trauma6, n (%) | 3 (1.8) | 3 (0.4) | 0.05*** |
| Shoulder dystocia, n (%) | 7 (4.4) | 15 (2.0) | 0.08*** |
| Need for resuscitation or intubation, n (%) | 0 | 8 (1.1) | 0.18*** |
| Severe neonatal morbidity7, n (%) | 25 (15.0) | 62 (8.4) | 0.01** |
| Registered variables at 6 months postpartum | |||
| Urinary incontinence, n (%) | 41 (26.6) | 155 (22.4) | 0.31** |
| Stress urinary incontinence, n (%) | 5 (12.2) | 16 (10.5) | 0.51*** |
| Urge urinary incontinence, n (%) | 13 (31.7) | 52 (33.3) | 0.72** |
| Mixed urinary incontinence, n (%) | 25 (15.0) | 91 (12.3) | 0.35** |
| Difficulty voiding, n (%) | 13 (29.6) | 50 (31.8) | 0.63** |
| Severe urinary incontinence, n (%) | 0 | 2 (1.3) | 0.67*** |
| Anal incontinence, n (%) | 24 (15.9) | 148 (21.8) | 0.09** |
| FISI score1 | 11.9 ± 6.3 | 11.8 ± 6.8 | 0.93* |
| Perineal pain, n (%) | 26 (17.2) | 87 (12.7) | 0.14** |
| Breastfeeding, n (%) | 95 (93.1) | 470 (93.3) | 0.63** |
| Episiotomy complications8, n (%) | 54 (38.9) | 245 (37.7) | 0.80** |
| Pelvic floor muscle training, n (%) | 119 (77.8) | 544 (79.1) | 0.72** |
1 Values are given as mean ± standard deviation.
2 Antenatal suspicion of macrosomia: fundal height measurement at delivery > 37cm and/or ultrasonographic fetal abdominal circumference > 90th p. for gestational age and sex on Hadlock curves [14].
3 Second-line therapies were uterine compression sutures, uterine artery embolization, and peripartum hysterectomy for management of massive primary postpartum hemorrhage after failure of uterine massage and uterotonic agents to stop bleeding [2].
4 Infections were defined by the existence of at least one of the following criteria: endometritis, episiotomy infection and wound infection needed surgery [2].
5 Severe maternal morbidity was defined by the existence of at least one of the following criteria: third or fourth-degree perineal lacerations, perineal hematomas, cervical laceration, extension of uterine incision at cesarean section, PPH>1500 mL, surgical haemostatic procedure, uterine artery embolization, blood transfusion, infections (endometritis, episiotomy infection, wound infection needed surgery), thromboembolic events (deep vein thrombophlebitis and pulmonary embolism), hospitalization in intensive care unit, and maternal death [2].
6 Neonatal trauma was defined by the existence of at least one of the following criteria: fracture of the clavicle or a long bone, brachial plexus injury, and cephalhematoma [2].
7 Severe neonatal morbidity was defined by at least one of the following criteria: 5-minute Apgar score<7, umbilical artery pH < 7.00, need for resuscitation or intubation, neonatal trauma, intraventricular hemorrhage > grade 2, admission to the NICU (neonatal intensive care unit) for>24 hours, convulsions, sepsis, and neonatal death [2].
8 Episiotomy complications were defined by the existence of at least one of the following criteria: hematoma, abscess, scar disunion, or required surgery for episiotomy.
FISI: American Society of Colon and Rectal Surgeon’s faecal incontinence severity index [19].
* Student t test
** χ2 test
*** Fisher exact test.
Statistical significance was defined as a P value < 0.05.
Table 2. Univariate analysis of urinary and anal incontinence at 6 months after midpelvic and low attempted operative vaginal delivery.
| Urinary incontinence | Anal incontinence | |||||
|---|---|---|---|---|---|---|
| Yes (N = 196) | No (N = 711) | P value | Yes (N = 172) | No (N = 735) | P value | |
| Maternal and labor characteristics | ||||||
| Maternal age (years) 1 | 30.0 ± 5.0 | 28.7 ± 4.7 | 0.001* | 30.5 ± 4.6 | 28.7 ± 4.7 | <0.001* |
| Multiparity, n (%) | 50 (25.5) | 159 (24.5) | 0.76** | 55 (32.0) | 149 (22.6) | 0.01** |
| Previous delivery with birth weight > 4000g, n (%) | 1 (2.0) | 7 (4.5) | 0.43*** | 6 (11.1) | 2 (1.3) | 0.002*** |
| Weight before pregnancy (kg)1 | 62.9 ± 12.1 | 61.1 ± 11.1 | 0.05* | 62.3 ± 11.1 | 61.2 ± 11.2 | 0.24* |
| BMI ≥ 30 kg/m2 before pregnancy, n (%) | 14 (7.1) | 36 (5.6) | 0.41** | 14 (8.2) | 32 (4.9) | 0.09** |
| Gestational weight gain >20 kg, n (%) | 19 (10.0) | 49 (7.8) | 0.35** | 14 (8.2) | 53 (8.3) | 0.92** |
| Antenatal suspicion of macrosomia 2, n (%) | 9 (4.6) | 51 (7.9) | 0.12** | 19 (11.0) | 37 (5.6) | 0.01** |
| Gestational age at delivery (weeks) 1 | 39.5 ± 1.3 | 39.4 ± 1.4 | 0.75* | 39.5 ± 1.3 | 39.4 ± 1.4 | 0.30* |
| Induced labor, n (%) | 35 (17.9) | 113 (17.4) | 0.88** | 42 (24.4) | 104 (15.8) | 0.008** |
| Length of labor (min)1 | 388.5 ± 164.4 | 391.9 ± 168.4 | 0.81* | 390.7 ± 171.2 | 392.8 ± 166.9 | 0.88* |
| 2nd stage>3 hours, n (%) | 33 (16.8) | 111 (17.1) | 0.93** | 34 (19.8) | 109 (16.6) | 0.32** |
| Active phase of 2nd stage > 30 min | 76 (38.8) | 223 (34.3) | 0.25** | 67 (39.0) | 227 (34.5) | 0.27** |
| Epidural analgesia, n (%) | 182 (92.9) | 624 (96.1) | 0.05** | 167 (97.1) | 626 (95.1) | 0.27** |
| Persistent occiput position | 0.22** | 0.24** | ||||
| Anterior, n (%) | 175 (90.2) | 574 (88.4) | 157 (91.3) | 580 (88.4) | ||
| Posterior, n (%) | 17 (8.8) | 53 (8.2) | 9 (5.2) | 59 (9.0) | ||
| Transverse, n (%) | 2 (1.0) | 22 (3.4) | 6 (3.5) | 17 (2.6) | ||
| ACOG classification | 0.26** | 0.11** | ||||
| Mid, n (%) | 41 (20.9) | 113 (17.4) | 24 (14.0) | 127 (19.3) | ||
| Low, n (%) | 155 (79.1) | 537 (82.6) | 148 (86.0) | 532 (80.7) | ||
| Obstetrician performing delivery | 0.33** | 0.62** | ||||
| Senior obstetrician, n (%) | 49 (25.0) | 182 (28.6) | 50 (29.2) | 117 (27.4) | ||
| Obstetric registrar, n (%) | 147 (75.0) | 455 (71.4) | 121 (70.8) | 470 (72.6) | ||
| Instrument type | ||||||
| Vacuum, n (%) | 50 (25.5) | 177 (27.3) | 0.63** | 39 (22.7) | 182 (27.7) | 0.19** |
| Forceps, n (%) | 16 (8.2) | 36 (5.6) | 0.18** | 13 (7.6) | 38 (5.8) | 0.39** |
| Spatula, n (%) | 138 (70.4) | 450 (69.3) | 0.78** | 125 (72.7) | 454 (69.0) | 0.35** |
| Sequential use of two instruments, n (%) | 8 (4.1) | 14 (2.2) | 0.14*** | 6 (3.5) | 15 (2.3) | 0.37*** |
| Indications for aOVD | 0.89** | 0.25** | ||||
| Non-reassuring FHR only, n (%) | 81 (41.3) | 280 (43.1) | 65 (37.8) | 289 (43.9) | ||
| Arrested progress only, n (%) | 80 (40.8) | 264 (40.6) | 72 (41.9) | 267 (40.5) | ||
| Non-reassuring FHR and arrested progress, n (%) | 35 (17.9) | 109 (16.8) | 35 (20.4) | 106 (16.1) | ||
| Maternal outcome | ||||||
| Cesarean delivery after failed operative vaginal delivery, n (%) | 2 (1.0) | 14 (2.1) | 0.31*** | 3 (1.7) | 13 (2.0) | 0.85*** |
| Episiotomy, n (%) | 170 (86.7) | 574 (88.6) | 0.48** | 155 (90.1) | 577 (87.8) | 0.40** |
| 3rd or 4th-degree perineal lacerations, n (%) | 11 (5.6) | 15 (2.3) | 0.02*** | 10 (5.8) | 15 (2.3) | 0.02*** |
| PPH (blood loss>500mL), n (%) | 35 (17.8) | 117 (18.0) | 0.96** | 39 (22.7) | 109 (16.5) | 0.06** |
| Severe PPH (blood loss>1500 mL), n (%) | 4 (2.0) | 17 (2.6) | 0.65*** | 6 (3.5) | 15 (2.3) | 0.37*** |
| Severe maternal morbidity 3, n (%) | 20 (10.2) | 54 (8.3) | 0.41** | 20 (11.6) | 52 (7.9) | 0.12** |
| Neonatal outcome | ||||||
| Birth weight (g) 1 | 3314 ± 396 | 3322 ± 435 | 0.81* | 3405 ± 420 | 3300 ± 426 | 0.004* |
| Birth weight > 4000 g, n (%) | 8 (4.1) | 38 (5.9) | 0.34** | 12 (7.0) | 34 (5.2) | 0.36** |
| Cephalic perimeter1 | 34.5 ± 1.3 | 34.4 ± 1.5 | 0.54* | 34.8 ± 1.5 | 34.5 ± 1.5 | 0.003* |
| Severe neonatal morbidity 4, n (%) | 14 (7.1) | 66 (10.1) | 0.21** | 14 (8.1) | 66 (10.0) | 0.46** |
| Mid-term registered variables | ||||||
| Breastfeeding, n (%) | 149 (74.5) | 419 (64.6) | 0.01** | 131 (76.2) | 424 (64.4) | 0.004* |
| Episiotomy complications 5, n (%) | 60 (32.8) | 239 (39.5) | 0.10** | 50 (30.5) | 245 (40.0) | 0.03* |
| Pelvic floor muscle training, n (%) | 162 (83.1) | 500 (77.5) | 0.10** | 148 (86.6) | 504 (77.1) | 0.007** |
| Perineal pain, n (%) | 34 (17.7) | 79 (12.3) | 0.06** | 29 (17.1) | 83 (12.7) | 0.14** |
| Urinary incontinence, n (%) | 196 (100) | 0 | - | 66 (38.4) | 130 (19.7) | < 0.001** |
| Stress urinary incontinence, n (%) | 20 (10.5) | - | 3 (4.8) | 17 (14.4) | 0.05*** | |
| Urge urinary incontinence, n (%) | 62 (32.1) | - | 13 (20.3) | 49 (38.6) | 0.01** | |
| Mixed urinary incontinence, n (%) | 115 (58.7) | - | 49 (28.5) | 66 (10.0) | <0.001** | |
| Difficulty voiding, n (%) | 61 (31.9) | - | 30 (46.2) | 31 (24.6) | 0.002** | |
| Severe urinary incontinence, n (%) | 2 (1.0) | - | 1 (1.6) | 1 (0.8) | 0.62*** | |
| Anal incontinence, n (%) | 63 (33.2) | 109 (17.0) | <0.001** | 172 (100) | 0 | - |
| FISI score1 | 12.6 ± 7.2 | 6.4 ± 5.9 | 0.02* | 12.0 ± 6.7 | 7.5 ± 4.9 | 0.04* |
Values are crude and adjusted linear regression coefficients (R) with their 95% confidence intervals (CI).
1 Values are given as mean ± standard deviation.
2 Antenatal suspicion of macrosomia: fundal height measurement at delivery > 37cm and/or ultrasonographic fetal abdominal circumference > 90th p. for gestational age and sex on Hadlock curves [14].
3 Severe maternal morbidity was defined by the existence of at least one of the following criteria: third or fourth-degree perineal lacerations, perineal hematomas, cervical laceration, extension of uterine incision at cesarean section, PPH>1500 mL, surgical haemostatic procedure, uterine artery embolization, blood transfusion, infections (endometritis, episiotomy infection, wound infection needed surgery), thromboembolic events (deep vein thrombophlebitis and pulmonary embolism), hospitalization in intensive care unit, and maternal death [2].
4 Severe neonatal morbidity was defined by at least one of the following criteria: 5-minute Apgar score<7, umbilical artery pH < 7.00, need for resuscitation or intubation, neonatal trauma, intraventricular hemorrhage > grade 2, admission to the NICU (neonatal intensive care unit) for>24 hours, convulsions, sepsis, and neonatal death [2].
5 Episiotomy complications were defined by the existence of at least one of the following criteria: hematoma, abscess, scar disunion, or required surgery for episiotomy.
* Student t test
** χ2 test
*** Fisher exact test.
Statistical significance was defined as a P value < 0.05.
In the multiple logistic regression analysis, attempted midpelvic (compared with low pelvic) aOVD was not significantly associated with either symptoms of UI (adjusted odds ratio (aOR) 0.70, 95% CI 0.46–1.05), controlling for maternal age, weight before pregnancy, epidural use, ACOG classification, 3rd or 4th-degree perineal lacerations, anal incontinence, and breast-feeding; or symptoms of AI (aOR 1.42, 95% CI 0.85–2.39), controlling for maternal age, parity, body mass index before pregnancy, antenatal suspicion of macrosomia, ACOG classification, 3rd or 4th-degree perineal lacerations, cephalic perimeter, breast-feeding, pelvic floor muscle training, episiotomy complications and urinary incontinence (Tables 3 and 4). Third/fourth degree perineal tears were a major risk factor for symptoms of UI (aOR 3.08, 95% CI 1.35–7.00) and symptoms of AI (aOR 3.47, 95% CI 1.43–8.39) 6 months after aOVD. Maternal age over 30 years and breastfeeding were also significant risk factors for symptoms of UI (aOR 1.66, 95% CI 1.19–2.31 and aOR 1.64, 95% CI 1.13–2.38, respectively) and symptoms of AI (aOR 1.81, 95% CI 1.25–2.62 and aOR 1.65, 95% CI 1.09–2.50, respectively) at 6 months postpartum (Tables 3 and 4).
Table 3. Multivariate analysis for urinary incontinence at 6 months after midpelvic and low attempted operative vaginal delivery.
| Variables | Urinary incontinence (N = 196) | |
|---|---|---|
| Adjusted OR (95% CI) | P value | |
| Maternal age > 30 years | 1.66 (1.19–2.31) | 0.003 |
| Weight before pregnancy > 55 kg | 1.73 (1.12–2.66) | 0.01 |
| Epidural analgesia | 0.52 (0.25–1.06) | 0.07 |
| ACOG classification | ||
| Mid | 0.70 (0.46–1.05) | 0.08 |
| Low | Reference | - |
| 3rd or 4th-degree perineal lacerations | 3.08 (1.35–7.00) | 0.007 |
| Breastfeeding | 1.64 (1.13–2.38) | 0.009 |
Values are adjusted odds ratios (OR) with 95% confidence intervals (CI).
Wald tests were performed for testing the significance of the covariates included in the models.
Table 4. Multivariate analysis for anal incontinence at 6 months after midpelvic and low attempted operative vaginal delivery.
| Variables | Anal incontinence (N = 172) | |
|---|---|---|
| Adjusted OR (95% CI) | P value | |
| Maternal age > 30 years | 1.81 (1.25–2.62) | 0.002 |
| BMI before pregnancy ≥ 30 kg/m2 | 2.29 (1.12–4.71) | 0.02 |
| ACOG classification | ||
| Mid | 1.42 (0.85–2.39) | 0.19 |
| Low | Reference | - |
| 3rd or 4th-degree perineal lacerations | 3.47 (1.43–8.39) | 0.006 |
| Cephalic perimeter > 36 cm | 1.74 (1.13–2.65) | 0.01 |
| Breastfeeding | 1.65 (1.09–2.50) | 0.02 |
Values are adjusted odds ratios (OR) with 95% confidence intervals (CI).
Wald tests were performed for testing the significance of the covariates included in the models.
Discussion
Main findings
We investigated the association between the fetal head station, and specifically midpelvic or low pelvic, and pelvic floors disorders, specifically symptoms of UI and AI, 6 months after aOVD, using a prospective based cohort analysis. We found that midpelvic aOVD was not associated with a higher rate of symptoms of UI and AI at 6 months postpartum compared to attempted low pelvic delivery. After multivariate analysis, third/fourth degree perineal tears and maternal age older than 30 years were significant risk factors for symptoms of UI and AI at 6 months postpartum.
Interpretation
It is difficult to compare our results with the literature because, to our knowledge, previous studies of pelvic floor disorders in the postpartum period after aOVD have never detailed their results according to fetal head station at instrument application [3–5,7,8,11].
Nevertheless, our results are consistent with other well-established findings in the literature about health problems after delivery: frequency of UI around 20% after OVD [23–26], frequency of AI around 20% after OVD [27–32], risk factor for AI (obstetric anal sphincter injury) after OVD [6,30,31,33], perineal tears of third/fourth degree [11,31,33,34], advanced maternal age and pre-pregnancy BMI as major risks factors for UI and AI after delivery [35–39]. We also demonstrated that breastfeeding is a significant risk factor for symptoms of UI and AI at 6 months postpartum. This has previously been demonstrated in a longitudinal cohort study that postpartum incontinence at 24 months was significantly associated with persistent breastfeeding [39], probably explained by hormonal changes [40].
Strengths and limitations
The principal strength of this study is the use of validated instruments for symptoms of UI, AI and pelvic floor disorders at 6 months postpartum in a large, prospective based cohort study with carefully characterized obstetric patients. This allowed a complete characterization of symptoms in this population. In particular, this is, to our knowledge, the first prospective based cohort study, that directly compares midpelvic and low aOVDs for pelvic floor disorders at 6 months postpartum. Tähtinen et al. [13] noted in a recent systematic review and meta-analysis concerning the long-term impact of mode of delivery on stress urinary incontinence and urgency urinary incontinence that the limitations of their review were largely the weaknesses of the eligible studies with only one randomized trial concerning breech presentation at term [41], and only one prospective cohort [12].
Our study has several limitations. First, as described in detail previously [2], determination of the station of the fetal head and thus classification of the OVDs is relatively subjective and is influenced by fetal head position, molding, and time of assessment (before or after regional analgesia) [1]. Nevertheless, the prevalence of midpelvic aOVD was similar to that in other study [42], and the rates of induced labor, persistent occiput posterior or transverse positions, manual rotation, forceps and spatula, aOVD performed by senior obstetricians and aOVD in an operating room were significantly higher in the midpelvic compared to the low-pelvic aOVD group, as previously shown [2]. This finding suggests that the risk of contamination between the two groups was low [2]. Second, we used the French versions of validated questionnaires which were previously used in published works [8,43], but the French versions of these questionnaires were not validated in French. Third, we reported a relative low rate of respondents (46.7%), but this rate was consistent with others large postpartum evaluations using mailed questionnaire [35,36]. It is plausible that participants declining to respond to a questionnaire at 6 months were at higher risk of anal and/or urinary incontinence than participants included in the study. Nevertheless, the non-respondents differed mainly from the respondents in their rate of neonatal morbidity, a factor that was not related to either symptoms of UI or AI in our study. Four, we reported pelvic floor disorders after midpelvic and low pelvic aOVD at 6 months postpartum without any objective testing regarding urinary leakage or anal incontinence. This time frame in the context of the development of pelvic floor disorders after delivery is considered very short term. Urogynecologic evaluation between 2 and 5 years out from aOVD should be done to be meaningful to patients and physicians [4,35,37].
Conclusion
We found that neither urinary nor anal incontinence differed at 6 months among women who had midpelvic and low pelvic aOVD. These findings may suggest that midpelvic aOVD should be an alternative valid option to cesarean delivery when the fetus is at midpelvis, because short-term maternal and neonatal morbidity and pelvic morbidity at 6 months are not significantly different between midpelvic and low pelvic aOVD. The data at 6 months postpartum are reassuring and need further studies at long-term to confirm these short-term data.
Supporting Information
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The Angers University Hospital provided the funding for the study. The funding source had no role in the design or conduct of the study, collection, analysis, or interpretation of the date, or writing the article or decision to submit for publication.
References
- 1.Committee on Practice Bulletins-Obstetrics (2015) ACOG Practice Bulletin No. 154: Operative Vaginal Delivery. Obstet Gynecol 126: e56–e65. 10.1097/AOG.0000000000001147 [DOI] [PubMed] [Google Scholar]
- 2.Ducarme G, Hamel JF, Bouet PE, Legendre G, Vandenbroucke L, Sentilhes L (2015) Maternal and neonatal morbidity after attempted operative vaginal delivery according to fetal head station. Obstet Gynecol 126: 521–529. 10.1097/AOG.0000000000001000 [DOI] [PubMed] [Google Scholar]
- 3.Lal MH, Mann C, Callender R, Radley S (2003) Does cesarean delivery prevent anal incontinence? Obstet Gynecol 101: 305–312. [DOI] [PubMed] [Google Scholar]
- 4.Bahl R, Strachan B, Murphy DJ (2005) Pelvic floor morbidity at 3 years after instrumental delivery and cesarean delivery in the second stage of labor and the impact of a subsequent delivery. Am J Obstet Gynecol 192: 789–794. 10.1016/j.ajog.2004.10.601 [DOI] [PubMed] [Google Scholar]
- 5.Solans-Domènech M, Sanchez E, Espuna-Pons M, Pelvic Floor Research Group (Grup de Recerca del Sòl Pelvià; GRESP) (2010) Urinary and anal incontinence during pregnancy and postpartum: incidence, severity, and risk factors. Obstet Gynecol 115: 618–628. 10.1097/AOG.0b013e3181d04dff [DOI] [PubMed] [Google Scholar]
- 6.Pretlove SJ, Thompson PJ, Toozs-Hobson PM, Radley S, Khan KS (2008) Does the mode of delivery predispose women to anal incontinence in the first year postpartum? A comparative systematic review. BJOG 115: 421–434. 10.1111/j.1471-0528.2007.01553.x [DOI] [PubMed] [Google Scholar]
- 7.Herbruck LF (2008) The impact of childbirth on the pelvic floor. Urol Nurs 28: 173–184; quiz 185. [PubMed] [Google Scholar]
- 8.Fritel X, Schaal JP, Fauconnier A, Bertrand V, Levet C, Pigné A (2008) Pelvic floor disorders 4 years after first delivery: a comparative study of restrictive versus systematic episiotomy. BJOG 115: 247–252. 10.1111/j.1471-0528.2007.01540.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S (2003) Vaginal delivery parameters and urinary incontinence: the Norwegian EPINCONT study. Am J Obstet Gynecol 189: 1268–1274. [DOI] [PubMed] [Google Scholar]
- 10.Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S (2003) Urinary incontinence after vaginal delivery or cesarean section. NEJM 348: 900–907. 10.1056/NEJMoa021788 [DOI] [PubMed] [Google Scholar]
- 11.Handa VL, Blomquist JL, McDermott KC, Friedman S, Munoz A (2012) Pelvic floor disorders after childbirth: effect of episiotomy, perineal laceration, and operative birth. Obstet Gynecol 119: 233–239. 10.1097/AOG.0b013e318240df4f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handa VL, Pierce CB, Muñoz A, Blomquist JL (2015) Longitudinal changes in overactive bladder and stress incontinence among parous women. Neurourol Urodyn 34: 356–361. 10.1002/nau.22583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tähtinen RM, Cartwright R, Tsui JF, Aaltonen RL, Aoki Y, Cardenas JL, et al. (2016) Long-term Impact of Mode of Delivery on Stress Urinary Incontinence and Urgency Urinary Incontinence: A Systematic Review and Meta-analysis. Eur Urol 70:148–158. 10.1016/j.eururo.2016.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK (1985) Estimation of fetal weight with the use of head, body, and femur measurements–a prospective study. Am J Obstet Gynecol 151: 333–337. [DOI] [PubMed] [Google Scholar]
- 15.French College of Gynecologists and Obstetricians (2013) [Intra-uterine growth retardation: guidelines for clinical practice–short text]. J Gynecol Obstet Biol Reprod 42: 1018–1025. [DOI] [PubMed] [Google Scholar]
- 16.Obstetrics forceps (1988) ACOG committee opinion, no. 59. Washington, DC: American College of Obstetricians and Gynecologists.
- 17.Jackson S, Donovan J, Brookes S, Eckford S, Swithinbank L, Abrams P (1996) The Bristol Female Lower Urinary Tract Symptoms questionnaire: development and psychometric testing. Br J Urol 77: 805–812. [DOI] [PubMed] [Google Scholar]
- 18.Brookes S, Donovan J, Wright M, Jackson S, Abrams P (2004) A scored form of the Bristol Female Lower Urinary Tract Symptoms questionnaire: data from a randomized controlled trial of surgery for women with stress incontinence. Am J Obstet Gynecol 191: 73–82. 10.1016/j.ajog.2003.12.027 [DOI] [PubMed] [Google Scholar]
- 19.Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. (1999) Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum 42: 1525–1532. [DOI] [PubMed] [Google Scholar]
- 20.Rockwood TH (2004) Incontinence severity and QOL scales for fecal incontinence. Gastroenterology 126: S106–S113. [DOI] [PubMed] [Google Scholar]
- 21.Bozdogan H (1987) Model Selection and Akaike's Information Criterion (AIC): The General Theory and Its Analytical Extensions. Psychometrika 52: 345–370. [Google Scholar]
- 22.Burnham KP, Anderson DR (2002) Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (2nd ed.), Springer-Verlag. [Google Scholar]
- 23.Brown S, Lumley J (1998) Maternal health after childbirth: results of an Australian population based survey. Br J Obstet Gynaecol 105: 156–161. [DOI] [PubMed] [Google Scholar]
- 24.Johanson RB, Heycock E, Carter J, Sultan AH, Walklate K, Jones PW (1999) Maternal and child health after assisted vaginal delivery: five-year follow up of a randomised controlled study comparing forceps and ventouse. Br J Obstet Gynaecol 106: 544–549. [DOI] [PubMed] [Google Scholar]
- 25.Farrell SA, Allen VM, Baskett TF (2001) Parturition and urinary incontinence in primiparas. Obstet Gynecol 97: 350–356. [DOI] [PubMed] [Google Scholar]
- 26.Liebling RE, Swingler R, Patel RR, Verity L, Soothill PW, Murphy DJ (2004) Pelvic floor morbidity up to one year after difficult instrumental delivery and cesarean section in the second stage of labor: a cohort study. Am J Obstet Gynecol 191: 4–10. 10.1016/j.ajog.2004.01.013 [DOI] [PubMed] [Google Scholar]
- 27.Sultan AH, Kamm MA, Hudson CN, Bartram CI (1994) Third degree obstetric anal sphincter tears: risk factors and outcome of primary repair. BMJ 308: 887–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zetterström JP, Lopez A, Anzen B, Dolk A, Norman M, Mellgren A (1999) Anal incontinence after vaginal delivery: a prospective study in primiparous women. Br J Obstet Gynaecol 106: 324–330. [DOI] [PubMed] [Google Scholar]
- 29.MacArthur C, Glazener CM, Wilson PD, Herbison GP, Gee H, Lang GD, et al. (2001) Obstetric practice and faecal incontinence three months after delivery. BJOG 108:678–683. [DOI] [PubMed] [Google Scholar]
- 30.Eason E, Labrecque M, Marcoux S, Mondor M (2002) Anal incontinence after childbirth. CMAJ 166: 326–330. [PMC free article] [PubMed] [Google Scholar]
- 31.Johannessen HH, Wibe A, Stordahl A, Sandvik L, Backe B, Mørkved S (2014) Prevalence and predictors of anal incontinence during pregnancy and 1 year after delivery: a prospective cohort study. BJOG 121: 269–279. 10.1111/1471-0528.12438 [DOI] [PubMed] [Google Scholar]
- 32.Johannessen HH, Wibe A, Stordahl A, Sandvik L, Mørkved S (2015) Anal incontinence among first time mothers—What happens in pregnancy and the first year after delivery? Acta Obstet Gynecol Scand 94: 1005–1013. 10.1111/aogs.12689 [DOI] [PubMed] [Google Scholar]
- 33.Huebner M, Gramlich NK, Rothmund R, Nappi L, Abele H, Becker S (2013) Fecal incontinence after obstetric anal sphincter injuries. Int J Gynaecol Obstet 121: 74–77. 10.1016/j.ijgo.2012.10.023 [DOI] [PubMed] [Google Scholar]
- 34.Borello-France D, Burgio KL, Richter HE, Zyczynski H, Fitzgerald MP, Whitehead W, et al. (2006) Fecal and urinary incontinence in primiparous women. Obstet Gynecol 108: 863–872. 10.1097/01.AOG.0000232504.32589.3b [DOI] [PubMed] [Google Scholar]
- 35.Macarthur C, Glazener C, Lancashire R, Herbison P, Wilson D, Grant A (2005) Faecal incontinence and mode of first and subsequent delivery: a six-year longitudinal study. BJOG 112: 1075–1082. 10.1111/j.1471-0528.2005.00721.x [DOI] [PubMed] [Google Scholar]
- 36.Buhling KJ, Schmidt S, Robinson JN, Klapp C, Siebert G, Dudenhausen JW (2006) Rate of dyspareunia after delivery in primiparae according to mode of delivery. Eur J Obstet Gynecol Reprod Biol 124: 42–46. 10.1016/j.ejogrb.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 37.Fritel X, Fauconnier A, Levet C, Bénifla JL (2004) Stress urinary incontinence 4 years after the first delivery: a retrospective cohort survey. Acta Obstet Gynecol Scand 83: 941–945. 10.1111/j.0001-6349.2004.00457.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hijaz A, Sadeghi Z, Byrne L, Hou JC, Daneshgari F (2012) Advanced maternal age as a risk factor for stress urinary incontinence: a review of the literature. Int Urogynecol J 23: 395–401. 10.1007/s00192-011-1562-5 [DOI] [PubMed] [Google Scholar]
- 39.Quiboeuf E, Saurel-Cubizolles MJ, Fritel X, EDEN Mother-Child Cohort Study Group (2015) Trends in urinary incontinence in women between 4 and 24 months postpartum in the EDEN cohort. BJOG 123: 1222–1228. 10.1111/1471-0528.13545 [DOI] [PubMed] [Google Scholar]
- 40.Burgio KL, Zyczynski H, Locher JL, Richter HE, Redden DT, Wright KC (2003) Urinary incontinence in the 12-month postpartum period. Obstet Gynecol 102: 1291–1298. [DOI] [PubMed] [Google Scholar]
- 41.Hannah ME, Whyte H, Hannah WJ, Hewson S, Amankwah K, Cheng M, et al. (2004) Maternal outcomes at 2 years after planned cesarean section versus planned vaginal birth for breech presentation at term: the international randomized Term Breech Trial. Am J Obstet Gynecol 191: 917–927. 10.1016/j.ajog.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 42.Murphy DJ, Liebling RE, Verity L, Swingler R, Patel R (2001) Early maternal and neonatal morbidity associated with operative delivery in second stage of labour: a cohort study. Lancet 358: 1203–1207. 10.1016/S0140-6736(01)06341-3 [DOI] [PubMed] [Google Scholar]
- 43.Fritel X, Ringa V, Varnoux N, Fauconnier A, Piault S, Bréart G (2005) Mode of delivery and severe stress incontinence. a cross-sectional study among 2,625 perimenopausal women. BJOG 112: 1646–1651. 10.1111/j.1471-0528.2005.00763.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.