Abstract
Autophagy is a catabolic process that facilitates nutrient recycling via degradation of damaged organelles and proteins through lysosomal mediated degradation. Alterations in this complex, and tightly regulated process, lead to disease. Autophagy is widely accepted as cytoprotective against neurodegenerative diseases and a variety of clinical interventions are moving forward to increase autophagy as a therapeutic intervention. Autophagy has both positive and negative roles in cancer and this has led to controversy over whether or how autophagy manipulation should be attempted in cancer therapy. Nevertheless, cancer is the disease where most current activity in trying to manipulate autophagy for therapy is taking place and dozens of clinical trials are using autophagy inhibition with Chloroquine or Hydroxychloroquine in combination with other drugs for the treatment of multiple neoplasms. Here, we review recent literature implicating autophagy in neurodegenerative diseases and cancer and highlight some of the opportunities, controversies and potential pitfalls of therapeutically targeting autophagy.
Keywords: Autophagy, Neurodegenerative disease, Cancer, Clinical trials
Highlights
-
•
Autophagy is a complex process that can be pharmacologically targeted at multiple steps.
-
•
Autophagy protects against neurodegeneration and enhanced autophagy decreases pathogenesis of neurodegenerative diseases.
-
•
Autophagy is both pro and anti-tumorigenic depending on the stage of tumorigenesis and mutational and oncogenic background.
-
•
Clinical trials are ongoing to increase autophagy in neurodegenerative diseases and inhibit autophagy to treat cancer.
1. Introduction
The award of the 2016 Nobel Prize for Medicine or Physiology to Professor Yoshinori Ohsumi for his work on elucidating mechanisms of autophagy recognizes the importance of autophagy for human disease. Autophagy allows cells to maintain intracellular homeostasis and respond to stress by degrading proteins, organelles and other cellular components via the lysosome. This process is evolutionally conserved and acts as a critical cellular response to nutrient and oxygen deprivation, resulting in recycled amino acids, nutrients, and lipids. Alterations in autophagy and inherited mutations in autophagy related genes, known as ATG genes, that control autophagy have been linked to human disease, including neurological diseases, autoimmune disease, metabolic disorders, infectious disease and cancer. These connections imply that therapeutic interventions to boost or inhibit autophagy may be useful to treat or prevent disease (Rubinsztein et al., 2012). Here we review the roles of autophagy in two such diseases– neurodegenerative disease and cancer– where direct therapeutic targeting intended to stimulate and inhibit autophagy is moving forward in the clinic.
2. Opportunities to Target Autophagy for Therapy
Three types of autophagy have been characterized– microautophagy, chaperone mediated autophagy (CMA), and macroautophagy. Microautophagy involves direct engulfument and lysosomal degradation of cytosolic material, (Li et al., 2012) while CMA is facilitated by chaperones that target proteins containing a specific amino acid motif to the lysosome. Although CMA is implicated in disease (Cuervo and Wong, 2014), this review will focus on macroautophagy.
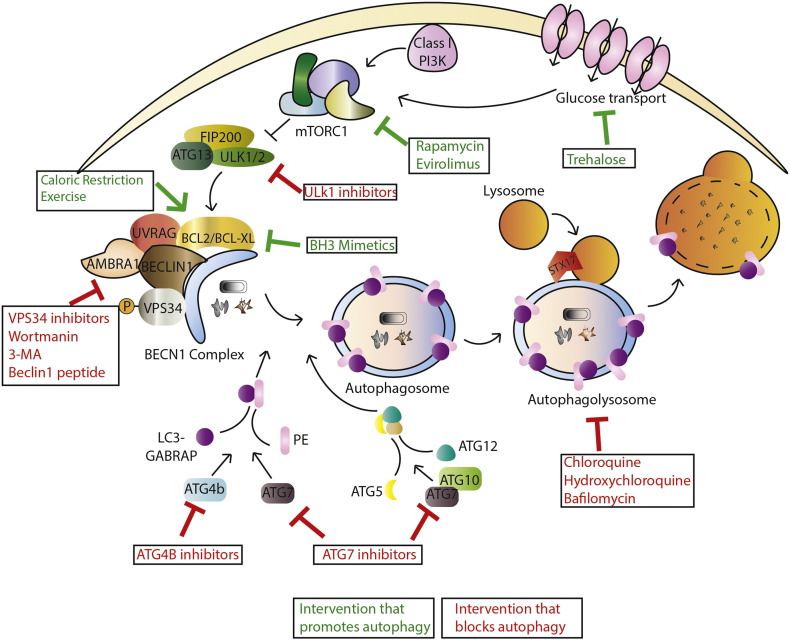
Macroautophagy (from here on termed autophagy) is characterized by the formation of double membrane vesicles called autophagosomes that engulf cytoplasmic material then fuse with the lysosome to degrade their contents. The process involves various steps: initiation, nucleation, elongation, and closure of the membranes that form the autophagosome, fusion with the lysosome and recycling of macromolecular precursors. Each step is regulated by particular autophagy-related proteins (ATGs) (Mizushima et al., 2011). Autophagy is controlled transcriptionally by MITF and FOXO families of transcription factors (Füllgrabe et al., 2014), as well as CREB and ATF (Amaravadi, 2015) and is subject to post-translational regulation allowing its pharmacological manipulation both positively and negatively (Fig. 1). For example, the mammalian target of rapamycin (mTOR) complex, mTORC1 inhibits autophagy. Thus, mTOR inhibitors are often used to stimulate autophagy. mTORC2 has also been linked to autophagy, although this may be specific to CMA (Arias et al., 2015). Autophagy can also be induced independently of mTOR. For example, the naturally occurring disaccharide, trehalose, which works independently of mTOR (Sarkar et al., 2007a), induces autophagy to protect against liver disease by affecting glucose transporters (DeBosch et al., 2016).
Fig. 1.
Interventions that target autophagy. Macroautophagy is controlled by nutrient availability via regulation by mTORC1, which under conditions of nutrient availability can inhibit the formation of Ulk1/2 complexes. Ulk complexes facilitate Beclin-1 complex formation and phagaphore initiation. Phagaphore elongation is then mediated by LC3-PE conjugation and ATG5-12 conjugation. Finally, double membrane autophagasomes fuse with lysosomes to form autophagolysosomes resulting in degradation of autophagosome contents by lysosomal hydrolases. A number of pharmacological and naturally occurring agents have been designed/discovered that target this pathway allowing interventions that upregulate and down regulate autophagy.
Other interventions inhibit autophagy, including inhibitors of the protein kinases, ULK1 and ULK2 (Egan et al., 2015, Petherick et al., 2015), and the class III phosphoinositide-3-kinase, (VPS34) (Bago et al., 2014, Dowdle et al., 2014, Ronan et al., 2014). VPS34 is a component of the BCL-2 interacting moesin-like coiled-coil protein 1, (Beclin-1) signaling complex. Beclin-1 contains a BH3 domain, allowing it to interact with other BH3 containing proteins, including B cell CLL/lymphoma 2 (BCL-2), and the downstream functions of the Beclin-1 complex can differ with respect to autophagy, dependent on the molecules found in complex with it (Sinha and Levine, 2008). Other components of the complex include ultraviolet irradiation resistant-associated gene (UVRAG), SH3GLB2, (also known as BIF-1), and activating molecule in Beclin-1 regulated autophagy 1 (AMBRA1). Some of these interactions can be pharmacologically targeted. BH3 mimetics like Venetoclax, which was designed to induce apoptosis by blocking BCL-2 interactions at the mitochondria, also disrupt interactions between Beclin-1 and BCL-2 to induce autophagy (Maiuri et al., 2007). However, this mechanism has been challenged and it has been proposed that BH3 mimetics may only affect autophagy via indirect mechanisms that occur sometime after they have hit their direct target (Reljic et al., 2016). A cell-permeable peptide, Tat-Beclin1, disrupts another interaction in the Beclin-1 complex to induce autophagy (Shoji-Kawata et al., 2013). In addition to the many drugs that can modulate autophagy (Levine et al., 2015), non-pharmacological interventions including caloric restriction and exercise also induce autophagy. For example, exercise targets the Beclin-1 signaling complex to induce autophagy that can protect mice against diabetes (He et al., 2012). Two ubiquitin-like conjugation systems elongate the autophagosome membrane. The ubiquitin-like protein ATG12 is conjugated to ATG5 in a process requiring the E1-like enzyme, ATG7. A similar lipid conjugation system (also using ATG7) attaches phosphatidylethanolamine (PE) to the Microtubule Associated Protein 1 light chain 3 (MAP1LC3) and GABA type A receptor-associated protein (GABARAP) families of proteins. Pharmacological inhibitors of ATG7 that block these processes have been discussed at meetings. LC3-PE conjugation occurs after cleavage and processing of LC3 by the protease, ATG4B, which can also be inhibited pharmacologically (Akin et al., 2014). LC3-PE is incorporated into the autophagosome membrane and is the most common marker of autophagy (Klionsky et al., 2016). The final step is to fuse the autophagosome with the lysosome by the SNARE protein, STX17 (Itakura et al., 2012). This step can be blocked with lysosomal inhibitors such as chloroquine (CQ) and hydroxychloroquine (HCQ) or Bafilomycin A1. Once fusion is complete, the lysosomal hydrolases degrade the contents of the autophagosomes producing amino acids, nutrients, and lipids that are available to fuel protein and other macromolecular synthesis and metabolism.
3. Targeting Autophagy in Neurological Diseases
Autophagy has been implicated in neurodegenerative disease for two reasons. First, defects in autophagy cause neurodegeneration. For example, although systemic knockout of Atg7 leads to neonatal death in mice (Komatsu et al., 2005), neuronal deletion allows the mice to survive to adulthood, however the animals succumb to neurodegenerative disease (Komatsu et al., 2006). Moreover, when Atg7 is acutely deleted in all cells in adult animals, the most common cause of death is neurodegenerative disease with accumulation of ubiquitinated protein aggregates (Karsli-Uzunbas et al., 2014). A mutation in the human ATG5 gene that inhibits autophagy also causes congenital ataxia and developmental delay (Kim et al., 2016). The second link to neurodegenerative diseases including Alzheimers Disease (AD), Parkinson's Disease (PD), and Huntington's Disease (HD) is the involvement of toxic protein aggregates that can be degraded via autophagy in the pathology of all of these diseases (Nah et al., 2015).
Alzheimer's disease is the most common progressive neurodegenerative dementia and is characterized by the accumulation of plaques and intra-neuronal fibrillary tangles, where the former consists of beta-amyloid peptides (Aβ) that are highly insoluble and toxic (Querfurth and LaFerla, 2010), and the latter is composed of hyperphosphorylated microtubule-associated protein TAU aggregates (Wang et al., 2013). Defective lysosomal proteolysis and compromised autophagosome transport occurs in AD resulting in an accumulation of autophagosomes as well as Aβ peptides and Tau protein aggregates (Zhang et al., 2016).
Huntington's disease is an autosomal dominant disease caused by CAG repeat expansion in the portion of the gene containing several glutamines, known as the polyglutamine (Poly Q) tract of the HTT gene (Novak and Tabrizi, 2010) and patients present with cognitive dysfunction and a loss of motor control thought to be due to toxic aggregates from the polyQ containing protein. Cytoplasmic aggregates formed by mutant forms of HTT (mHTT) can be removed by autophagy, and stimulation of autophagy with mTOR inhibitors, as well as mTOR independent autophagy inducers, can reduce neuronal toxicity in Huntington's disease models (Sarkar et al., 2007b) (Table 1). Autophagosomes in HD disease may also be defective and are often devoid of contents resulting in abnormal amounts of mHTT protein aggregates that are not degraded by the lysosome (Martinez-Vicente et al., 2010). Additionally, mHTT itself affects autophagy resulting in accumulation of autophagosomes that are unable to fuse with lysosomes (Wong and Holzbaur, 2014).
Table 1.
Compounds that directly/indirectly upregulate autophagy in neurodegenerative diseases.
| Disease | Compound | Mechanism | Model tested/clinical trial phase | Reference |
|---|---|---|---|---|
| Alzheimer's | AVN-211 | Antagonist of 5-HT6R (mTOR activator) | Rodent models | (Ivachtchenko et al., 2016) |
| Lu AE58054 (AKA idalopirdine) | Antagonist of 5-HT6R (mTOR activator) | Phase III | (Wilkinson et al., 2014) | |
| SB-742457 | Antagonist of 5-HT6R (mTOR activator) | Phase II | (Maher-Edwards et al., 2010) | |
| AUTEN-67 | MTMR-14 (autophagy inhibitor) | Drosophilia, Zebrafish and preclinical rodent model | (Papp et al., 2016) | |
| rAAV/Aβ vaccine | AKT/mTOR | Rodent models | (Wang et al., 2015) | |
| ACAT1 | Unknown | Neuroblastoma cells and rodent models | (Shibuya et al., 2015) | |
| Rapamycin | Antagonist to mTOR | Rodent models | (Caccamo et al., 2010, Siman et al., 2015) | |
| Nicotinamide | Lysosomal acidification | Phase I | (Liu et al., 2013) | |
| Resveratrol | Antagonist to TORC1 | Phase III | (Vingtdeux et al., 2010) | |
| Lithium | AMPK | Phase II | (Forlenza et al., 2012) | |
| Latrepirdine | Antagonist to mTOR | Phase III | (Steele and Gandy, 2013) | |
| Metformin | Antagonist to mTOR/PP2A | Phase II | (Kickstein et al., 2010) | |
| Parkinson's | DMF | Activation of NRF2 | Rodent models | (Lastres-Becker et al., 2016) |
| Curcumin | TFEB | Rodent models | (Ji and Shen, 2014, Song et al., 2016) | |
| BECN1 gene | Beclin-1 complex | Rodent model | (Spencer et al., 2009) | |
| TFEB gene | TFEB regulation | Rodent model | (Decressac et al., 2013) | |
| Huntington's | AUTEN-67 | MTMR-14 | Drosophila | (Billes et al., 2016) |
| Rapamycin/CCI-779 | Antagonist to mTOR | Drosophila and Rodent models | (Ravikumar et al., 2004) | |
| Berberine | Unknown | Rodent models | (Jiang et al., 2015) | |
| Rheb gene | Activator of mTOR | Rodent models | (Lee et al., 2015) | |
| Calpastatin | Calpain inhibitor | Rodent models | (Menzies et al., 2015) | |
| Rilmenidine | Unknown | Rodent models | (Rose et al., 2010) | |
| Trehalose | Unknown | Rodent models | (Sarkar and Rubinsztein, 2008) |
Accumulation of α-synuclein (SNCA) defines the molecular pathology of Parkinson's disease, a progressive disorder that results from the death of dopaminergic neurons in the substantia nigra. Patients accumulate Lewy bodies, intracytoplasmic inclusions containing SNCA, which act as a neurotoxin. Macroautophagy, CMA, as well as mitophagy (i.e. selective autophagy of mitochondria) have all been implicated in PD (Nah et al., 2015, Narendra et al., 2008). Notably, Pink1 and Parkin, two proteins whose loss of function is directly linked to Parkinson's disease, also help facilitate the removal of damaged mitochondria by the process of mitophagy, and loss of this process resulting in an accumulation of dysfunctional mitochondria has been shown to be causative for the disease (Rub et al., 2016). Additionally, loss of autophagy results in increased oxidative stress, which exacerbates PD pathology (Surendran and Rajasankar, 2010).
In light of these and other studies suggesting that autophagy plays a cytoprotective role against neurodegenerative diseases (Rubinsztein et al., 2015), a number of interventions to enhance autophagic flux, that is the fusion of lysosomes and autophagosomes and degradation of autophagosome cargo, have been tested (Table 1). One study shows that in an animal model of AD, the Vitamin B precursor, Nicotinimide could reduce Aβ and hyperphosphorylated Tau as well as improve cognitive performance, all dependent on restoration of autophagy (Liu et al., 2013). Resveratrol inhibits rotenone-induced dopaminergic neuron cell death in a model of PD (Lin et al., 2014). Because the effects were abrogated by an autophagy inhibitor this is thought to be due to autophagy. mTOR inhibitors such as rapamycin and CCI-779 can induce autophagy to improve motor neuron functioning in fly and mouse models of HD (Ravikumar et al., 2004). Consequently, several compounds including many that feed into mTOR signaling are moving forward towards clinical trials for different diseases (Table 1). One such class of drugs antagonize the G-protein coupled receptor, 5-HT6R, known to activate mTOR signaling in the brain (Meffre et al., 2012), and include the compounds AVN-211 (Ivachtchenko et al., 2016) (Morozova et al., 2014) (Phase IIa clinical trial for Schizophrenia, highlighting safety), Lu AE58054 (AKA idalopirdine)(Wilkinson et al., 2014)(Phase III clinical trial for AD - NCT02006641, NCT02006654, NCT02079246), and SB-742457(Maher-Edwards et al., 2010) (Phase II clinical trial for AD- NCT00224497, NCT0034819, NCT00708552, NCT00710684).
There are caveats with the above mentioned studies. First, there is no good method for accurately quantifying autophagy in vivo, and especially in human patients. This makes it difficult to be sure that drugs that increase autophagic flux in cultured cells are also doing so in vivo. Second, agents that activate autophagy also have other effects, for example mTOR inhibitors also affect protein, nucleotide, and lipid synthesis as well as glutamine metabolism and glycolysis all via autophagy independent mechanisms (Li et al., 2014). This makes it difficult to know if the beneficial effects are due to increased autophagy or other effects. An advantage in preclinical models is the ability to design controlled experiments where an intervention intended to increase autophagy is also tested in animals with a separate genetic interference of autophagy, which should abrogate the protective effects seen by the intervention. However, this kind of experiment is also subject to criticism; it may show that autophagy is necessary for protection against disease but doesn't necessarily mean that the increase in autophagy is responsible. A major hurdle for the field is to develop better ways to stimulate autophagy in a more specific manner. In summary, while more work is needed to fully elucidate the mechanisms of these compounds and their therapeutic potential, upregulation of autophagy remains a promising therapeutic approach for neurodegenerative diseases such as Alzheimer's, Parkinson's and Huntington's disease.
4. Targeting Autophagy in Cancer
Most clinical studies that are attempting to therapeutically target autophagy are in cancer. A recent search of ClinicalTrials.Gov using the search term “autophagy” brought up over 60 clinical trials, of which > 50 are cancer trials attempting to inhibit autophagy. This is somewhat surprising because, unlike with neurodegenerative diseases where the consensus view is that autophagy protects against disease, autophagy can both promote and inhibit tumor growth and autophagy's roles vary in different context (White, 2015).
Autophagy was first suggested to be a tumor suppressor when a study from Beth Levine's group (Liang et al., 1999) found that Beclin-1 interacts with an oncogenic protein (BCL-2) and some breast, prostate, and ovarian cancer tumors had a monoallelic loss in BECN1 (encoding Beclin-1). In human tumors, these results are complicated by close proximity of the BECN1 gene and the potent tumor suppressor BRCA1 (Laddha et al., 2014, Tang et al., 2015). This makes it difficult to rule out the possibility that in human tumors, it is loss of BRCA1 rather than BECN1 that is important. Indeed, TCGA data shows there is no significant loss of BECN1 independent of BRCA1 loss (White, 2015). In mice, monoallelic deletion of the Becn1 gene alone is sufficient to cause mammary hyperplasia, (Qu et al., 2007, Yue et al., 2003) with possible context-specific effects with different oncogenic drivers (Cicchini et al., 2014). However, the Becn1+/− genotype still maintains functional autophagy, while other autophagy knockouts that are much more systemically deficient in autophagy (Yoshii et al., 2016) do not develop cancer suggesting that the Becn1+/− tumor phenotype may be autophagy independent. Mutation of other autophagy regulators (e.g. the UVRAG (Liang et al., 2006), BIF1 (Coppola et al., 2008), and AMBRA proteins (Cianfanelli et al., 2015)) that are all in the Beclin-1 complex have also been implicated in cancer. However, all of these molecules and Beclin1 itself also have autophagy independent roles. These complications mean that it is possible that some tumor suppressor effects of Beclin1 and other components of the complex are actually due to autophagy-independent functions.
In KRAS-driven mouse models, knockout of Atg5 or Atg7 often promotes pre-malignant lesions (Rao et al., 2014, Rosenfeldt et al., 2013, Yang et al., 2014,). KO of Atg5 delays the onset of lung cancer in KRAS-driven model of lung cancer, although once tumors do progress, Atg5 loss enhances survival (Rao et al., 2014). Likewise, in a KRAS-driven pancreatic cancer model, loss of Atg5 or Atg7 accelerates tumor initiation but blocks malignant progression (Rosenfeldt et al., 2013). Although not fully understood, both these studies also demonstrated a role for TP53 in switching between pro and anti-tumorigenic roles of autophagy. A recent study found that mutations in a splicing factor increased cell transformation by incorrectly processing the ATG7 mRNA resulting in decreased ATG7 protein and defective autophagy (Park et al., 2016), again suggesting that autophagy protects against early steps in tumor development. Taken together, these various studies suggest that autophagy may play a role in suppressing tumor initiation but can promote tumor progression. Consistent with this idea, genetic enhancement of autophagy (by overexpression of active TFEB, a master transcriptional regulator of autophagy) can increase growth of established tumors (Wei et al., 2014).
In vitro studies using shRNAs to target > 100 autophagy regulators identified a number of breast cancer cell lines that are dependent upon functional autophagy for growth even in the absence of stress (Maycotte et al., 2014). Importantly however, some tumor cell lines were barely affected by autophagy gene knockdown indicating that tumor cells differ greatly in their dependence on autophagy. This work followed studies suggesting that cancer cell lines with mutant RAS genes are “autophagy addicted” (Guo et al., 2011) and some tumor types, e.g. pancreas cancer, which transcriptionally induces high levels of autophagy (Perera et al., 2015), are highly dependent on autophagy (Yang et al., 2011). However, RAS mutation alone does not determine sensitivity to autophagy inhibition in vitro (Morgan et al., 2014) highlighting the need for more rigorous studies to identify which tumor cells are, or are not, autophagy-dependent.
Similarly, in vivo studies have indicated the necessity of Atg5 or Atg7 in KRAS- or BRAF-driven tumor models (Karsli-Uzunbas et al., 2014, Rao et al., 2014, Rosenfeldt et al., 2013, Yang et al., 2014, Guo et al., 2013, Strohecker et al., 2013, Xie et al., 2015). A requirement for autophagy to maintain tumors in vivo has also been demonstrated for cancers driven by PTEN loss (Santanam et al., 2016). Moreover, additional autophagy regulators such as FIP200, have been shown to maintain tumor growth due to autophagy and not other functions of the protein (Chen et al., 2016). One caveat for many of the mouse studies is that deletion of the Atg gene occurs simultaneously with activation of the oncogenic driver and occurs only in the tumor cells. These experiments do not adequately model what autophagy inhibition would be like in the clinic. A patient would likely only be treated after they had a full-blown cancer with an autophagy inhibitor that cannot discriminate between autophagy in the cancer cells and autophagy in the rest of the body. This has been modeled in KRAS-driven lung cancer, where tumors were allowed to develop before systemic loss of the Atg7 gene was achieved (Karsli-Uzunbas et al., 2014). Complete, irreversible inhibition of autophagy by Atg7 deletion in adult mice caused death by bacterial infection or, after several weeks, neurodegeneration and the animals displayed defects in glucose homeostasis and extreme sensitivity to starvation. However, these results also indicate a therapeutic window where the tumor can be eradicated without wholesale toxicity when autophagy is inhibited. The existence of such a therapeutic window– a given dose and/or length of treatment time that can be effective in inhibiting tumor growth without overt toxicity, provides an opportunity for therapeutic inhibition. In the clinic we would likely never have a drug that is as good at inhibiting autophagy as complete knockout of an ATG gene. Moreover, autophagy inhibition with a drug could be reversed by removing the drug, thus this work tells us that even a “perfect” autophagy inhibitor might not be so toxic that it couldn't be used in cancer therapy.
As with its tumor suppressing roles, the mechanisms by which autophagy supports tumor growth are poorly understood. Autophagy has been implicated in the pro-tumorigenic processes of glycolysis, oxidative metabolism, cell proliferation and anchorage-independent growth (Lock et al., 2011, Vander Heiden et al., 2009). In addition autophagy is involved in metastasis (Kenific et al., 2010). For example, it is important in tumor cell invasion and migration, and metastasis in vivo due to autophagy induced focal adhesion disassembly (Kenific et al., 2016, Sharifi et al., 2016) and autophagy-regulated cytokine secretion (Lock et al., 2014). Autophagy deficiency in cancer cells results in multiple metabolic problems and the accumulation of defective mitochondria (Guo et al., 2016). Additionally autophagy in stromal cells can promote tumor cell metabolism by feeding amino acids (specifically alanine) to the tumor cells (Sousa et al., 2016). It is likely that a combination of metabolic effects and other cellular functions such as cytokine secretion contribute to autophagy's ability to promote tumor growth and progression and thus explain how, for some tumors, autophagy inhibition alone can have profound anti-cancer effects.
Autophagy also plays a role in response to treatment. An extensive literature suggests that autophagy can protect cancer cells against commonly used cancer therapies including a wide array of different drugs and radiation (Thorburn et al., 2014, Rebecca and Amaravadi, 2015). Such studies are the basis for most of the current efforts to target autophagy in patients (Table 2). Perhaps even more important than chemo-sensitization, autophagy inhibition may overcome acquired resistance to other anti-cancer agents. The best evidence for this is in tumors with activating mutations in BRAF that have become resistant to BRAF inhibitors like vemurafenib. Vemurafenib resistance in melanoma is associated with increased autophagy and autophagy inhibition can reverse resistance associated with endoplasmic reticulum stress (Ma et al., 2014). There is also evidence that autophagy inhibition to overcome drug resistance can be effective in patients. A brain cancer patient with a BRAF mutant tumor that had become resistant to vemurafenib was successfully treated with a combination of CQ and vemurafenib experiencing long term tumor regression on the combination treatment (Levy et al., 2014). Importantly, this patient had periods of time when the autophagy inhibitor CQ was maintained but the BRAF inhibitor was discontinued for periods of time. In every instance, this led to increased tumor growth that was reversed when the combination treatment was re-established. This case study is the first to suggest that autophagy inhibition with CQ can overcome acquired resistance to the kinase inhibitor but that only combination treatment with both the BRAF inhibitor and the autophagy inhibitor is effective. Continued follow up of this patient has demonstrated sustained tumor regression for over two and a half years and more recent studies submitted for publication from Mulcahy-Levy et al. have extended these findings to two other patients both of whom acquired resistance to vemurafenib following successful therapy and then experienced clinical improvement on the combination of CQ and vemurafenib.
Table 2.
Active and recently completed clinical trials (last 2 years) with autophagy inhibition for the treatment of cancer.
| Tumor type | Clinical trial phase | Drug combination | Reference |
|---|---|---|---|
| Breast | Phase II | CQ + Taxols (microtubule inhibitors) | NCT01446016 |
| Breast | Phase II | CQ | NCT02333890 |
| Breast (DCIS*) | Phase I/II | CQ | NCT01023477 |
| Pancreatic | Phase I | CQ + gemcitabine | NCT01777477 |
| Pancreatic | Phase I/II | HCQ + gemcitabine | NCT01506973 |
| Pancreatic | Phase II | HCQ + gemcitabine (DNA damaging) + abraxane (microtubule inhibitor) | NCT01978184 |
| Pancreatic | Phase I/II | HCQ + gemcitabine (DNA damaging) | NCT01128296 |
| Melanoma | Phase I | CQ + radiation + DT01 (DNA repair inhibitor) | NCT01469455 |
| Melanoma | Phase I/II | HCQ + Trametinib (MEK inhibitor) | NCT02257424 |
| Small cell lung | Phase I | CQ | NCT00969306 |
| Small cell lung | Phase I | CQ + radiotherapy | NCT01575782 |
| Non-small cell lung | Phase II | HCQ + Paclitaxol (mycrotubule inhibitor) + carboplatin (DNA damaging) + Bevacizumab (angiogenesis inhibitor) | NCT01649947 |
| Colorectal | Phase I/II | HCQ + Oxaliplatin (alylating) + 5-FU (DNA damaging) + Bevacizumab (angiogenesis inhibitor) | NCT01206530 |
| Colorectal | Phase II | HCQ + Bevacizumab (angiogenesis inhibitor) + XELOX (Alkylating + antimetabolite) | NCT01006369 |
| Colorectal | Phase II | HCQ + Vorinostat (HDAC inhibitor) | NCT02316340 |
| Prostate | Phase II | HCQ + ABT-263 (Bcl inhibitor) + Abiraterone (antiandrogen) | NCT01828476 |
| Prostate | Phase II | HCQ | NCT00726596 |
| Renal cell carcinoma | Phase I/II | HCQ + RAD001 (mTOR inhibitor) | NCT01510119 |
| Glioblastoma, astrocytoma | Phase II | CQ + chemoradiation with temezolomide (alkylating agent) | NCT02432417 |
| Glioma, chondrosarcoma, intrahepatic cholangiocarcinoma | Phase I/II | CQ + Metformin | NCT02496741 |
| Glioblastoma | Phase I | CQ + chemoradiation with temezolomide (alkylating agent) | NCT02378532 |
| Brain metastasis | Not provided | CQ + radiotherapy | NCT01727531 |
| Adult solid neoplasm | Phase I | HCQ + Sunitinib Malate (RTKi) | NCT00813423 |
| Multiple myeloma | Phase II | CQ + Velcade (protease inhibitor) + Cyclophosphamide (alkylating agent) | NCT01438177 |
| Relapsed solid tumors | Phase I | HCQ + Sorafenib (RTKi) | NCT01634893 |
| Advanced solid tumors | Phase I | HCQ + MK2206 (Akt inhibitor) | NCT01480154 |
| Solid tumors | Phase I | CQ + carboplatin/gemcitabine (DNA damaging) | NCT02071537 |
| Advanced cancers | Phase I | HCQ + vorinostat (HDAC inhibitor) HCQ + Sirolimus (mTOR inhibitor) |
NCT01266057 |
| Solid tumor | Phase I | HCQ + vorinostat (HDAC inhibitor) | NCT01023737 |
| Adult solid tumor | Phase I | HCQ + temsirolimus | NCT00909831 |
Although most current clinical trials (Table 2) involve autophagy inhibition, there are arguments against this idea often revolving around effects of autophagy inhibition on the immune response to cancer (Zhong et al., 2016). It has been reported that “immunogenic” tumor cell killing– i.e. killing cancer cells with chemotherapy in a way that will lead to effective engagement of an anti-tumor immune response – requires that the dying cancer cells have functional autophagy (Michaud et al., 2011) leading to suggestions that we should try to enhance autophagy to improve cancer immunotherapy (Zhong et al., 2016). Consistent with this idea, a recent study concluded that caloric restriction could enhance tumor immunosurveillance only for autophagy-proficient tumors (Pietrocola et al., 2016). However, autophagy inhibition can enhance immune cell-mediated, anti-tumor effects under at least some circumstances (Liang et al., 2012, Baginska et al., 2013). More work is needed to better understand the interplay between autophagy's role(s) in the anti-tumor immune response.
5. Clinical Trials of Targeted Autophagy Inhibition
Despite the caveats discussed above, there are already many studies attempting to inhibit autophagy in cancer therapy. All the current studies use CQ or HCQ. These drugs inhibit the lysosome and block autophagy while causing accumulation of autophagosomes and LC3, which have been used as pharmacodynamic markers of the inhibitor's activity. CQ and HCQ are inexpensive, approved drugs that have been used for decades to treat malaria and arthritis but have some caveats as autophagy inhibitors. First, they can have anti-tumor effects through other mechanisms such as reducing nutrient scavenging and can sensitize to other chemotherapies by autophagy-independent mechanisms (Eng et al., 2016, Maycotte et al., 2012). Second, alterations in tumor pH may affect drug bioavailability (Pellegrini et al., 2014).
Clinical trials targeting multiple neoplasms have been launched to assess the effects of autophagy inhibition in combination with radiation, conventional cytotoxic chemotherapies and targeted agents including DNA damaging agents, HDAC inhibitors, proteasome inhibitors, mitotic inhibitors, antiandrogens, and several kinase inhibitors (ClinicalTrials.gov, Table 2). Additional studies in melanoma, glioblastoma, pancreatic, breast, lung, and prostate cancer are testing CQ/HCQ as a single agent (ClinicalTrials.gov). A major caveat with the current clinical trials is that we have no way to identify patients who are most likely to benefit. At least initially, one strategy to do this may be to focus on tumors with mutations that confer sensitivity to autophagy inhibitors like the BRAF mutant brain tumor studies mentioned above (Levy et al., 2014).
The first Phase I and I/II trials have been reported testing autophagy inhibition alone or in combination with a proteosome inhibitor (bortezomib), radiation therapy, DNA damaging agents, i.e. temazolomide and doxorubicin, as well as the mTOR inhibitor, temsirolimus, and the HDAC inhibitor vorinostat (Rosenfeld et al., 2014, Rangwala et al., 2014b, Rangwala et al., 2014a, Barnard et al., 2014, Mahalingam et al., 2014, Vogl et al., 2014, Wolpin et al., 2014, Rojas-Puentes et al., 2013, Patel et al., 2016). Doses effective for autophagy inhibition can be reached with minimal side effects, although one study in glioblastoma patients did report grade 3 and 4 neutropenia and thrombopenia (Rosenfeld et al., 2014). Thus far, no studies have reported adverse side effects expected of autophagy inhibition based on pre-clinical animal studies, such as increased incidence of neuropathy, secondary cancers, infections, or metabolic disturbances. Encouragingly, some studies suggest favorable clinical responses. Rangwala et al. reported a partial response and stable disease in 14% and 27%, respectively, of metastatic melanoma patients treated with HCQ in combination with temozolomide, with a complete response and prolonged stable disease seen in 2/6 patients with WT BRAF refractory melanoma (Rangwala et al., 2014b). Moreover, a phase II study of CQ and radiation therapy on brain metastasis in patients with advanced solid tumors report progress free survival after one year in 84% of patients as compared to 55% of patients in the control arm (radiation therapy alone) (Rojas-Puentes et al., 2013).
6. Outstanding Questions
In this article we have focused on just two types of disease (neurodegenerative disease and cancer) where different approaches to therapeutically manipulate autophagy have been adopted and where clinical studies are underway. However there are still many outstanding questions even for just these two areas. For example, although there is considerable evidence that autophagy inhibition can make other anti-cancer drugs more effective, this may only apply in tumor cells that start off being dependent on autophagy. Indeed, in cancer cells that are not autophagy-dependent the same drug combination may be antagonistic rather than synergistic (Levy et al., 2014, Maycotte et al., 2014); we are currently unable to predict if cancer cells will or won't be autophagy-dependent. Some drugs are also thought to require autophagy in order to kill cancer cells (Thorburn et al., 2014). In neurodegenerative diseases it is unclear whether or not different ways of activating autophagy will be more or less effective and we are greatly hampered by the fact that even for laboratory studies we lack good tools to specifically activate autophagy without affecting other cellular processes. In cancer therapy we have poor understanding of when autophagy will increase tumor cell killing and when it will decrease it and we need to better understand the interplay between autophagy and the immune response to cancer. For any therapy involving autophagy manipulation, a major problem is our inability to accurately measure autophagic flux in vivo to assess if the intervention actually worked.
Given the competing roles described above, an important question is whether treatment of cancer by autophagy inhibition will lead to neurodegenerative side effects and likewise, that treatment of neurodegenerative diseases with autophagy promoting agents might promote pre-malignant tumors. There are currently no studies that specifically induce autophagy, making it difficult to address the risk of autophagy inducing agents. However, because activities like exercise and caloric restriction that induce autophagy systemically (He et al., 2012, Pietrocola et al., 2016) are known for their health advantages, we speculate that specific autophagy induction may be safe. We must also consider the role of autophagy manipulation intended to treat one disease on other systems and organs, such as the liver (Madrigal-Matute and Cuervo, 2016).
7. Concluding Remarks
A danger is that if clinical studies of therapeutic targeting of autophagy are ineffective, it may be because of our lack of knowledge about who to treat and how best to manipulate autophagy and not because autophagy manipulation is an ineffective therapy. It would be a pity if autophagy-targeted therapies were abandoned when the problem was that we didn't fully understand their effects rather than that autophagy manipulation was never going to be effective. As with many areas of biology where competing effects are seen– e.g. the immune system can both promote and inhibit tumorigenesis while affecting a multitude of other diseases, the key to successful implementation of therapies designed to target a specific process lies in understanding the biology and trying to specifically target the processes you want to enhance or inhibit. Continued progress on understanding how autophagy affects diseases like cancer and neurodegeneration and the development of better ways to manipulate and measure these processes should hopefully allow us to maximize the potential for autophagy manipulation to treat disease.
Footnotes
Search strategy and selection criteria: Data for this review were obtained from PubMed searches using the criteria: “autophagy and cancer”, “Huntington's disease”, “Parkinson's disease”, and “Alzheimer's disease”. Clinical trial information was obtained from Clinicaltrials.gov using the search criteria: “autophagy”, “chloroquine”, and “hydroxychloroquine”. Only articles published in English from 1993 to 2016 were included with particular emphasis on studies from 2014 to 2016.
References
- Akin D., Wang S.K., Habibzadegah-Tari P., Law B., Ostrov D., Li M., Yin X.-M., Kim J.-S., Horenstein N., Dunn W.A. A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors. Autophagy. 2014;10:2021–2035. doi: 10.4161/auto.32229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaravadi R.K. Transcriptional regulation of autophagy in RAS-driven cancers. J. Clin. Invest. 2015;125:1393–1395. doi: 10.1172/JCI81504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias E., Koga H., Diaz A., Mocholi E., Patel B., Cuervo A.M. Lysosomal mTORC2/PHLPP1/Akt regulate chaperone-mediated autophagy. Mol. Cell. 2015;59:270–284. doi: 10.1016/j.molcel.2015.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baginska J., Viry E., Berchem G., Poli A., Noman M.Z., Van Moer K., Medves S., Zimmer J., Oudin A., Niclou S.P., Bleackley R.C., Goping I.S., Chouaib S., Janji B. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proc. Natl. Acad. Sci. U. S. A. 2013;110:17450–17455. doi: 10.1073/pnas.1304790110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bago R., Malik N., Munson M.J., Prescott A.R., Davies P., Sommer E., Shpiro N., Ward R., Cross D., Ganley I.G., Alessi D.R. Characterization of VPS34-IN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase. Biochem. J. 2014;463:413–427. doi: 10.1042/BJ20140889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard R.A., Wittenburg L.A., Amaravadi R.K., Gustafson D.L., Thorburn A., Thamm D.H. Phase I clinical trial and pharmacodynamic evaluation of combination hydroxychloroquine and doxorubicin treatment in pet dogs treated for spontaneously occurring lymphoma. Autophagy. 2014;10:1415–1425. doi: 10.4161/auto.29165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billes V., Kovacs T., Hotzi B., Manzeger A., Tagscherer K., Komlos M., Tarnoci A., Padar Z., Erdos A., Bjelik A., Legradi A., Gulya K., Gulyas B., Vellai T. AUTEN-67 (autophagy enhancer-67) hampers the progression of neurodegenerative symptoms in a Drosophila model of Huntington's disease. J. Huntingtons Dis. 2016 doi: 10.3233/JHD-150180. [DOI] [PubMed] [Google Scholar]
- Caccamo A., Majumder S., Richardson A., Strong R., Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J. Biol. Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Wang C., Yeo S., Liang C.-C., Okamoto T., Sun S., Wen J., Guan J.-L. Distinct roles of autophagy-dependent and -independent functions of FIP200 revealed by generation and analysis of a mutant knock-in mouse model. Genes Dev. 2016;30:856–869. doi: 10.1101/gad.276428.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfanelli V., Fuoco C., Lorente M., Salazar M., Quondamatteo F., Gherardini P.F., de Zio D., Nazio F., Antonioli M., D'orazio M., Skobo T., Bordi M., Rohde M., Dalla Valle L., Helmer-Citterich M., Gretzmeier C., Dengjel J., Fimia G.M., Piacentini M., Di Bartolomeo S., Velasco G., Cecconi F. AMBRA1 links autophagy to cell proliferation and tumorigenesis by promoting c-Myc dephosphorylation and degradation. Nat. Cell Biol. 2015;17:706. doi: 10.1038/ncb3171. [DOI] [PubMed] [Google Scholar]
- Cicchini M., Chakrabarti R., Kongara S., Price S., Nahar R., Lozy F., Zhong H., Vazquez A., Kang Y., Karantza V. Autophagy regulator BECN1 suppresses mammary tumorigenesis driven by WNT1 activation and following parity. Autophagy. 2014;10:2036–2052. doi: 10.4161/auto.34398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola D., Khalil F., Eschrich S.A., Boulware D., Yeatman T., Wang H.G. Down-regulation of Bax-interacting factor-1 in colorectal adenocarcinoma. Cancer. 2008;113:2665–2670. doi: 10.1002/cncr.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo A.M., Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2014;24:92–104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debosch B.J., Heitmeier M.R., Mayer A.L., Higgins C.B., Crowley J.R., Kraft T.E., Chi M., Newberry E.P., Chen Z., Finck B.N., Davidson N.O., Yarasheski K.E., Hruz P.W., Moley K.H. Trehalose inhibits solute carrier 2A (SLC2A) proteins to induce autophagy and prevent hepatic steatosis. Sci. Signal. 2016;9:ra21. doi: 10.1126/scisignal.aac5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decressac M., Mattsson B., Weikop P., Lundblad M., Jakobsson J., Bjorklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-ynuclein toxicity. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E1817–E1826. doi: 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle W.E., Nyfeler B., Nagel J., Elling R.A., Liu S., Triantafellow E., Menon S., Wang Z., Honda A., Pardee G., Cantwell J., Luu C., Cornella-Taracido I., Harrington E., Fekkes P., Lei H., Fang Q., Digan M.E., Burdick D., Powers A.F., Helliwell S.B., D'aquin S., Bastien J., Wang H., Wiederschain D., Kuerth J., Bergman P., Schwalb D., Thomas J., Ugwonali S., Harbinski F., Tallarico J., Wilson C.J., Myer V.E., Porter J.A., Bussiere D.E., Finan P.M., Labow M.A., Mao X., Hamann L.G., Manning B.D., Valdez R.A., Nicholson T., Schirle M., Knapp M.S., Keaney E.P., Murphy L.O. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 2014 doi: 10.1038/ncb3053. [DOI] [PubMed] [Google Scholar]
- Egan D.F., Chun M.G.H., Vamos M., Zou H., Rong J., Miller C.J., Lou H.J., Raveendra-Panickar D., Yang C.-C., Sheffler D.J., Teriete P., Asara J.M., Turk B.E., Cosford N.D.P., Shaw R.J. Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol. Cell. 2015;59:285–297. doi: 10.1016/j.molcel.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng C.H., Wang Z., Tkach D., Toral-Barza L., Ugwonali S., Liu S., Fitzgerald S.L., George E., Frias E., Cochran N., de Jesus R., Mcallister G., Hoffman G.R., Bray K., Lemon L., Lucas J., Fantin V.R., Abraham R.T., Murphy L.O., Nyfeler B. Macroautophagy is dispensable for growth of KRAS mutant tumors and chloroquine efficacy. Proc. Natl. Acad. Sci. U. S. A. 2016;113:182–187. doi: 10.1073/pnas.1515617113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlenza O.V., de Paula V.J., Machado-Vieira R., Diniz B.S., Gattaz W.F. Does lithium prevent Alzheimer's disease? Drugs Aging. 2012;29:335–342. doi: 10.2165/11599180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Füllgrabe J., Klionsky D.J., Joseph B. The return of the nucleus: transcriptional and epigenetic control of autophagy. Nat. Rev. Mol. Cell Biol. 2014;15:65–74. doi: 10.1038/nrm3716. [DOI] [PubMed] [Google Scholar]
- Guo J.Y., Chen H.Y., mathew r., Fan J., Strohecker A.M., Karsli-Uzunbas G., Kamphorst J.J., Chen G., Lemons J.M., Karantza V., Coller H.A., Dipaola R.S., Gelinas C., Rabinowitz J.D., White E. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.Y., Karsli-Uzunbas G., Mathew R., Aisner S.C., Kamphorst J.J., Strohecker A.M., Chen G., Price S., Lu W., Teng X., Snyder E., Santanam U., Dipaola R.S., Jacks T., Rabinowitz J.D., White E. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev. 2013;27:1447–1461. doi: 10.1101/gad.219642.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.Y., Teng X., Laddha S.V., Ma S., Van Nostrand S.C., Yang Y., Khor S., Chan C.S., Rabinowitz J.D., White E. Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes Dev. 2016;30:1704–1717. doi: 10.1101/gad.283416.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Bassik M.C., Moresi V., Sun K., Wei Y., Zou Z., An Z., Loh J., Fisher J., Sun Q., Korsmeyer S., Packer M., May H.I., Hill J.A., Virgin H.W., Gilpin C., Xiao G., Bassel-Duby R., Scherer P.E., Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura E., Kishi-Itakura C., Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151:1256–1269. doi: 10.1016/j.cell.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Ivachtchenko A.V., Lavrovsky Y., Ivanenkov Y.A. AVN-211, novel and highly selective 5-HT6 receptor small molecule antagonist, for the treatment of Alzheimer's disease. Mol. Pharm. 2016;13:945–963. doi: 10.1021/acs.molpharmaceut.5b00830. [DOI] [PubMed] [Google Scholar]
- Ji H.F., Shen L. The multiple pharmaceutical potential of curcumin in Parkinson's disease. CNS Neurol. Disord. Drug Targets. 2014;13:369–373. doi: 10.2174/18715273113129990077. [DOI] [PubMed] [Google Scholar]
- Jiang W., Wei W., Gaertig M.A., Li S., Li X.J. Therapeutic effect of berberine on Huntington's disease transgenic mouse model. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsli-Uzunbas G., Guo J.Y., Price S., Teng X., Laddha S.V., Khor S., Kalaany N.Y., Jacks T., Chan C.S., Rabinowitz J.D., White E. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov. 2014;4:914–927. doi: 10.1158/2159-8290.CD-14-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenific C.M., Thorburn A., Debnath J. Autophagy and metastasis: another double-edged sword. Curr. Opin. Cell Biol. 2010;22:241–245. doi: 10.1016/j.ceb.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenific C.M., Stehbens S.J., Goldsmith J., Leidal A.M., Faure N., Ye J., Wittmann T., Debnath J. NBR1 enables autophagy-dependent focal adhesion turnover. J. Cell Biol. 2016;212:577–590. doi: 10.1083/jcb.201503075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kickstein E., Krauss S., Thornhill P., Rutschow D., Zeller R., Sharkey J., Williamson R., Fuchs M., Kohler A., Glossmann H., Schneider R., Sutherland C., Schweiger S. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21830–21835. doi: 10.1073/pnas.0912793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Sandford E., Gatica D., Qiu Y., Liu X., Zheng Y., Schulman B.A., Xu J., Semple I., Ro S.-H., Kim B., Mavioglu R.N., Tolun A., Jipa A., Takats S., Karpati M., Li J.Z., Yapici Z., Juhasz G., Lee J.H., Klionsky D.J., Burmeister M. Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay. eLife. 2016;5 doi: 10.7554/eLife.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A., Adachi H., Adams C.M., Adams P.D., Adeli K., Adhihetty P.J., Adler S.G., Agam G., Agarwal R., Aghi M.K., Agnello M., Agostinis P., Aguilar P.V., Aguirre-Ghiso J., Airoldi E.M., Ait-Si-Ali S., Akematsu T., Akporiaye E.T., AL-Rubeai M., Albaiceta G.M., Albanese C., Albani D., Albert M.L., Aldudo J., Algül H., Alirezaei M., Alloza I., Almasan A., Almonte-Beceril M., Alnemri E.S., Alonso C., Altan-Bonnet N., Altieri D.C., Alvarez S., Alvarez-Erviti L., Alves S., Amadoro G., Amano A., Amantini C., Ambrosio S., Amelio I., Amer A.O., Amessou M., Amon A., An Z., Anania F.A., Andersen S.U., Andley U.P., Andreadi C.K., Andrieu-Abadie N., Anel A., Ann D.K., Anoopkumar-Dukie S., Antonioli M., Aoki H., Apostolova N., Aquila S., Aquilano K., Araki K., Arama E., Aranda A., Araya J., Arcaro A., Arias E., Arimoto H., Ariosa A.R., Armstrong J.L., Arnould T., Arsov I., Asanuma K., Askanas V., Asselin E., Atarashi R., Atherton S.S., Atkin J.D., Attardi L.D., Auberger P., Auburger G., Aurelian L., Autelli R., Avagliano L., Avantaggiati M.L., Avrahami L., Awale S., Azad N., Bachetti T., Backer J.M., Bae D.-H., Bae J.-S., Bae O.-N., Bae S.H., Baehrecke E.H., Baek S.-H., Baghdiguian S., Bagniewska-Zadworna A. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M., Waguri S., Ueno T., Iwata J., Murata S., Tanida I., Ezaki J., Mizushima N., Ohsumi Y., Uchiyama Y., Kominami E., Tanaka K., Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M., Waguri S., Chiba T., Murata S., Iwata J., Tanida I., Ueno T., Koike M., Uchiyama Y., Kominami E., Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Laddha S.V., Ganesan S., Chan C.S., White E. Mutational landscape of the essential autophagy Gene BECN1 in human cancers. Mol. Cancer Res. 2014;12:485–490. doi: 10.1158/1541-7786.MCR-13-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastres-Becker I., Garcia-Yague A.J., Scannevin R.H., Casarejos M.J., Kugler S., Rabano A., Cuadrado A. Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in Parkinson's disease. Antioxid. Redox Signal. 2016 doi: 10.1089/ars.2015.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Tecedor L., Chen Y.H., Monteys A.M., Sowada M.J., Thompson L.M., Davidson B.L. Reinstating aberrant mTORC1 activity in Huntington's disease mice improves disease phenotypes. Neuron. 2015;85:303–315. doi: 10.1016/j.neuron.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- levine B., Packer M., Codogno P. Development of autophagy inducers in clinical medicine. J. Clin. Invest. 2015;125:14–24. doi: 10.1172/JCI73938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J.M.M., Thompson J.C., Griesinger A.M., Amani V., Donson A.M., Birks D.K., Morgan M.J., Mirsky D.M., Handler M.H., Foreman N.K., Thorburn A. Autophagy inhibition improves chemosensitivity in BRAFV600E brain tumors. Cancer Discov. 2014;4:773–780. doi: 10.1158/2159-8290.CD-14-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.W., Li J., Bao J.K. Microautophagy: lesser-known self-eating. Cell. Mol. Life Sci. 2012;69:1125–1136. doi: 10.1007/s00018-011-0865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Kim S.G., Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19:373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X.H., Jackson S., Seaman M., Brown K., Kempkes B., Hibshoosh H., Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Liang C., Feng P., Ku B., Dotan I., Canaani D., Oh B.H., Jung J.U. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat. Cell Biol. 2006;8:688–699. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- Liang X., de Vera M.E., Buchser W.J., Romo de Vivar Chavez A., Loughran P., Beer Stolz D., Basse P., Wang T., van Houten B., Zeh H.J., Lotze M.T. Inhibiting systemic autophagy during interleukin 2 immunotherapy promotes long-term tumor regression. Cancer Res. 2012;72:2791–2801. doi: 10.1158/0008-5472.CAN-12-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.K., Chen S.D., Chuang Y.C., Lin H.Y., Huang C.R., Chuang J.H., Wang P.W., Huang S.T., Tiao M.M., Chen J.B., Liou C.W. Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int. J. Mol. Sci. 2014;15:1625–1646. doi: 10.3390/ijms15011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Pitta M., Jiang H., Lee J.H., Zhang G., Chen X., Kawamoto E.M., Mattson M.P. Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol. Aging. 2013;34:1564–1580. doi: 10.1016/j.neurobiolaging.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock R., Roy S., Kenific C.M., Su J.S., Salas E., Ronen S.M., Debnath J. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol. Biol. Cell. 2011;22:165–178. doi: 10.1091/mbc.E10-06-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock R., Kenific C.M., Leidal A.M., Salas E., Debnath J. Autophagy-dependent production of secreted factors facilitates oncogenic RAS-driven invasion. Cancer Discov. 2014;4:466–479. doi: 10.1158/2159-8290.CD-13-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X.-H., Piao S.-F., Dey S., Mcafee Q., Karakousis G., Villanueva J., Hart L.S., Levi S., Hu J., Zhang G., Lazova R., Klump V., Pawelek J.M., Xu X., Xu W., Schuchter L.M., Davies M.A., Herlyn M., Winkler J., Koumenis C., Amaravadi R.K. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J. Clin. Invest. 2014;124:1406–1417. doi: 10.1172/JCI70454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal-Matute J., Cuervo A.M. Regulation of liver metabolism by autophagy. Gastroenterology. 2016;150:328–339. doi: 10.1053/j.gastro.2015.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam D., Mita M., Sarantopoulos J., Wood L., Amaravadi R.K., Davis L.E., Mita A.C., Curiel T.J., Espitia C.M., Nawrocki S.T., Giles F.J., Carew J.S. Combined autophagy and HDAC inhibition: a phase I safety, tolerability, pharmacokinetic, and pharmacodynamic analysis of hydroxychloroquine in combination with the HDAC inhibitor vorinostat in patients with advanced solid tumors. Autophagy. 2014;10:1403–1414. doi: 10.4161/auto.29231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher-Edwards G., Zvartau-Hind M., Hunter A.J., Gold M., Hopton G., Jacobs G., Davy M., Williams P. Double-blind, controlled phase II study of a 5-HT6 receptor antagonist, SB-742457, in Alzheimer's disease. Curr. Alzheimer Res. 2010;7:374–385. doi: 10.2174/156720510791383831. [DOI] [PubMed] [Google Scholar]
- Maiuri M.C., Le Toumelin G., Criollo A., Rain J.C., Gautier F., Juin P., Tasdemir E., Pierron G., Troulinaki K., Tavernarakis N., Hickman J.A., Geneste O., Kroemer G. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M., Talloczy Z., Wong E., Tang G., Koga H., Kaushik S., de Vries R., Arias E., Harris S., Sulzer D., Cuervo A.M. Cargo recognition failure is responsible for inefficient autophagy in Huntington's disease. Nat. Neurosci. 2010;13:567–576. doi: 10.1038/nn.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycotte P., Aryal S., Cummings C.T., Thorburn J., Morgan M.J., Thorburn A. Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy. 2012;8:200–212. doi: 10.4161/auto.8.2.18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycotte P., Gearheart C.M., Barnard R., Aryal S., Mulcahy Levy J.M., Fosmire S.P., Hansen R.J., Morgan M.J., Porter C.C., Gustafson D.L., Thorburn A. STAT3-mediated autophagy dependence identifies subtypes of breast cancer where autophagy inhibition can be efficacious. Cancer Res. 2014;74:2579–2590. doi: 10.1158/0008-5472.CAN-13-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffre J., Chaumont-Dubel S., Mannoury La Cour C., Loiseau F., Watson D.J., Dekeyne A., Seveno M., Rivet J.M., Gaven F., Deleris P., Herve D., Fone K.C., Bockaert J., Millan M.J., Marin P. 5-HT(6) receptor recruitment of mTOR as a mechanism for perturbed cognition in schizophrenia. EMBO Mol. Med. 2012;4:1043–1056. doi: 10.1002/emmm.201201410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies F.M., Garcia-Arencibia M., Imarisio S., O'sullivan N.C., Ricketts T., Kent B.A., Rao M.V., Lam W., Green-Thompson Z.W., Nixon R.A., Saksida L.M., Bussey T.J., O'kane C.J., Rubinsztein D.C. Calpain inhibition mediates autophagy-dependent protection against polyglutamine toxicity. Cell Death Differ. 2015;22:433–444. doi: 10.1038/cdd.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M., Martins I., Sukkurwala A.Q., Adjemian S., Ma Y., Pellegatti P., Shen S., Kepp O., Scoazec M., Mignot G., Rello-Varona S., Tailler M., Menger L., Vacchelli E., Galluzzi L., Ghiringhelli F., Di Virgilio F., Zitvogel L., Kroemer G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- Mizushima N., Yoshimori T., Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- Morgan M.J., Gamez G., Menke C., Hernandez A., Thorburn J., Gidan F., Staskiewicz L., Morgan S., Cummings C., Maycotte P., Thorburn A. Regulation of autophagy and chloroquine sensitivity by oncogenic RAS in vitro is context-dependent. Autophagy. 2014;10:1814–1826. doi: 10.4161/auto.32135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova M.A., Lepilkina T.A., Rupchev G.E., Beniashvily A.G., Burminskiy D.S., Potanin S.S., Bondarenko E.V., Kazey V.I., Lavrovsky Y., Ivachtchenko A.V. Add-on clinical effects of selective antagonist of 5HT6 receptors AVN-211 (CD-008-0173) in patients with schizophrenia stabilized on antipsychotic treatment: pilot study. CNS Spectr. 2014;19:316–323. doi: 10.1017/S1092852913000394. [DOI] [PubMed] [Google Scholar]
- Nah J., Yuan J., Jung Y.K. Autophagy in neurodegenerative diseases: from mechanism to therapeutic approach. Mol. Cell. 2015;38:381–389. doi: 10.14348/molcells.2015.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D., Tanaka A., Suen D.F., Youle R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak M.J., Tabrizi S.J. Huntington's disease. BMJ. 2010;340:c3109. doi: 10.1136/bmj.c3109. [DOI] [PubMed] [Google Scholar]
- Papp D., Kovacs T., Billes V., Varga M., Tarnoci A., Hackler L., Jr., Puskas L.G., Liliom H., Tarnok K., Schlett K., Borsy A., Padar Z., Kovacs A.L., Hegedus K., Juhasz G., Komlos M., Erdos A., Gulyas B., Vellai T. AUTEN-67, an autophagy-enhancing drug candidate with potent antiaging and neuroprotective effects. Autophagy. 2016;12:273–286. doi: 10.1080/15548627.2015.1082023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.M., Ou J., Chamberlain L., Simone T.M., Yang H., Virbasius C.M., Ali A.M., Zhu L.J., Mukherjee S., Raza A., Green M.R. U2AF35(S34F) promotes transformation by directing aberrant ATG7 pre-mRNA 3′ end formation. Mol. Cell. 2016;62:479–490. doi: 10.1016/j.molcel.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Hurez V., Nawrocki S.T., Goros M., Michalek J., Sarantopoulos J., Curiel T., Mahalingam D. Vorinostat and hydroxychloroquine improve immunity and inhibit autophagy in metastatic colorectal cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini P., Strambi A., Zipoli C., Hägg-Olofsson M., Buoncervello M., Linder S., de Milito A. Acidic extracellular pH neutralizes the autophagy-inhibiting activity of chloroquine: implications for cancer therapies. Autophagy. 2014;10:562–571. doi: 10.4161/auto.27901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera R.M., Stoykova S., Nicolay B.N., Ross K.N., Fitamant J., Boukhali M., Lengrand J., Deshpande V., Selig M.K., Ferrone C.R., Settleman J., Stephanopoulos G., Dyson N.J., Zoncu R., Ramaswamy S., Haas W., Bardeesy N. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature. 2015;524:361–365. doi: 10.1038/nature14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petherick K.J., Conway O.J.L., Mpamhanga C., Osborne S.A., Kamal A., Saxty B., Ganley I.G. Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycin (mTOR)-dependent autophagy. J. Biol. Chem. 2015;290:11376–11383. doi: 10.1074/jbc.C114.627778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrocola F., Pol J., Vacchelli E., Rao S., Enot D.P., Baracco E.E., Levesque S., Castoldi F., Jacquelot N., Yamazaki T., Senovilla L., Marino G., Aranda F., Durand S., Sica V., Chery A., Lachkar S., Sigl V., Bloy N., Buque A., Falzoni S., Ryffel B., Apetoh L., Di Virgilio F., Madeo F., Maiuri M.C., Zitvogel L., Levine B., Penninger J.M., Kroemer G. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell. 2016;30:147–160. doi: 10.1016/j.ccell.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X., Zou Z., Sun Q., Luby-Phelps K., Cheng P., Hogan R.N., Gilpin C., Levine B. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–946. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- Querfurth H.W., Laferla F.M. Alzheimer's disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Rangwala R., Chang Y.C., Hu J., Algazy K.M., Evans T.L., Fecher L.A., Schuchter L.M., Torigian D.A., Panosian J.T., Troxel A.B., Tan K.S., Heitjan D.F., Demichele A.M., Vaughn D.J., Redlinger M., Alavi A., Kaiser J., Pontiggia L., Davis L.E., O'Dwyer P.J., Amaravadi R.K. Combined MTOR and autophagy inhibition: phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy. 2014;10:1391–1402. doi: 10.4161/auto.29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala R., Leone R., Chang Y.C., Fecher L.A., Schuchter L.M., Kramer A., Tan K.S., Heitjan D.F., Rodgers G., Gallagher M., Piao S., Troxel A.B., Evans T.L., Demichele A.M., Nathanson K.L., O'Dwyer P.J., Kaiser J., Pontiggia L., Davis L.E., Amaravadi R.K. Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma. Autophagy. 2014;10:1369–1379. doi: 10.4161/auto.29118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Tortola L., Perlot T., Wirnsberger G., Novatchkova M., Nitsch R., Sykacek P., Frank L., Schramek D., Komnenovic V., Sigl V., Aumayr K., Schmauss G., Fellner N., Handschuh S., Glosmann M., Pasierbek P., Schlederer M., Resch G.P., Ma Y., Yang H., Popper H., Kenner L., Kroemer G., Penninger J.M. A dual role for autophagy in a murine model of lung cancer. Nat. Commun. 2014;5:3056. doi: 10.1038/ncomms4056. [DOI] [PubMed] [Google Scholar]
- Ravikumar B., Vacher C., Berger Z., Davies J.E., Luo S., Oroz L.G., Scaravilli F., Easton D.F., Duden R., O'kane C.J., Rubinsztein D.C. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- Rebecca V.W., Amaravadi R.K. Emerging strategies to effectively target autophagy in cancer. Oncogene. 2015 doi: 10.1038/onc.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reljic B., Conos S., Lee E.F., Garnier J.-M., Dong L., Lessene G., Fairlie W.D., Vaux D.L., Lindqvist L.M. BAX-BAK1-independent LC3B lipidation by BH3 mimetics is unrelated to BH3 mimetic activity and has only minimal effects on autophagic flux. Autophagy. 2016;12:1083–1093. doi: 10.1080/15548627.2016.1179406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Puentes L.L., Gonzalez-Pinedo M., Crismatt A., Ortega-Gomez A., Gamboa-Vignolle C., Nunez-Gomez R., Dorantes-Gallareta Y., Arce-Salinas C., Arrieta O. Phase II randomized, double-blind, placebo-controlled study of whole-brain irradiation with concomitant chloroquine for brain metastases. Radiat. Oncol. 2013;8:209. doi: 10.1186/1748-717X-8-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronan B., Flamand O., Vescovi L., Dureuil C., Durand L., Fassy F., Bachelot M.-F., Lamberton A., Mathieu M., Bertrand T., marquette J.-P., El-ahmad Y., Filoche-Romme B., Schio L., Garcia-Echeverria C., Goulaouic H., Pasquier B. A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat. Chem. Biol. 2014;10:1013–1019. doi: 10.1038/nchembio.1681. [DOI] [PubMed] [Google Scholar]
- Rose C., Menzies F.M., Renna M., Acevedo-Arozena A., Corrochano S., Sadiq O., Brown S.D., Rubinsztein D.C. Rilmenidine attenuates toxicity of polyglutamine expansions in a mouse model of Huntington's disease. Hum. Mol. Genet. 2010;19:2144–2153. doi: 10.1093/hmg/ddq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M.R., Ye X., Supko J.G., Desideri S., Grossman S.A., Brem S., Mikkelson T., Wang D., Chang Y.C., Hu J., Mcafee Q., Fisher J., Troxel A.B., Piao S., Heitjan D.F., Tan K.S., Pontiggia L., O'Dwyer P.J., Davis L.E., Amaravadi R.K. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy. 2014;10:1359–1368. doi: 10.4161/auto.28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeldt M.T., O'Prey J., Morton J.P., Nixon C., Mackay G., Mrowinska A., Au A., Rai T.S., Zheng L., Ridgway R., Adams P.D., Anderson K.I., Gottlieb E., Sansom O.J., Ryan K.M. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- Rub C., Wilkening A., Voos W. Mitochondrial quality control by the Pink1/Parkin system. Cell Tissue Res. 2016 doi: 10.1007/s00441-016-2485-8. [DOI] [PubMed] [Google Scholar]
- Rubinsztein D.C., Codogno P., Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 2012;11:709–730. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein D.C., Bento C.F., Deretic V. Therapeutic targeting of autophagy in neurodegenerative and infectious diseases. J. Exp. Med. 2015;212:979–990. doi: 10.1084/jem.20150956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanam U., Banach-Petrosky W., Abate-Shen C., Shen M.M., White E., Dipaola R.S. Atg7 cooperates with Pten loss to drive prostate cancer tumor growth. Genes Dev. 2016;30:399–407. doi: 10.1101/gad.274134.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Rubinsztein D.C. Huntington's disease: degradation of mutant huntingtin by autophagy. FEBS J. 2008;275:4263–4270. doi: 10.1111/j.1742-4658.2008.06562.x. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Davies J.E., Huang Z., Tunnacliffe A., Rubinsztein D.C. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J. Biol. Chem. 2007;282:5641–5652. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Perlstein E.O., Imarisio S., Pineau S., Cordenier A., Maglathlin R.L., Webster J.A., Lewis T.A., O'Kane C.J., Schreiber S.L., Rubinsztein D.C. Small molecules enhance autophagy and reduce toxicity in Huntington's disease models. Nat. Chem. Biol. 2007;3:331–338. doi: 10.1038/nchembio883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi M.N., Mowers E.E., Drake L.E., Collier C., Chen H., Zamora M., Mui S., Macleod K.F. Autophagy promotes focal adhesion disassembly and cell motility of metastatic tumor cells through the direct interaction of paxillin with LC3. Cell Rep. 2016;15:1660–1672. doi: 10.1016/j.celrep.2016.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya Y., Niu Z., Bryleva E.Y., Harris B.T., Murphy S.R., Kheirollah A., Bowen Z.D., Chang C.C., Chang T.Y. Acyl-coenzyme A:cholesterol acyltransferase 1 blockage enhances autophagy in the neurons of triple transgenic Alzheimer's disease mouse and reduces human P301L-tau content at the presymptomatic stage. Neurobiol. Aging. 2015;36:2248–2259. doi: 10.1016/j.neurobiolaging.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji-Kawata S., Sumpter R., Leveno M., Campbell G.R., Zou Z., Kinch L., Wilkins A.D., Sun Q., Pallauf K., Macduff D., Huerta C., Virgin H.W., Helms J.B., Eerland R., Tooze S.A., Xavier R., Lenschow D.J., Yamamoto A., King D., Lichtarge O., Grishin N.V., Spector S.A., Kaloyanova D.V., Levine B. Identification of a candidate therapeutic autophagy-inducing peptide. Nature. 2013;494:201–206. doi: 10.1038/nature11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R., Cocca R., Dong Y. The mTOR inhibitor rapamycin mitigates perforant pathway neurodegeneration and synapse loss in a mouse model of early-stage Alzheimer-type tauopathy. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene. 2008;27(Suppl 1):S137–S148. doi: 10.1038/onc.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.X., Sun Y.R., Peluso I., Zeng Y., Yu X., Lu J.H., Xu Z., Wang M.Z., Liu L.F., Huang Y.Y., Chen L.L., Durairajan S.S., Zhang H.J., Zhou B., Zhang H.Q., Lu A., Ballabio A., Medina D.L., Guo Z., Li M. A novel curcumin analog binds to and activates TFEB in vitro and in vivo independent of MTOR inhibition. Autophagy. 2016:1–18. doi: 10.1080/15548627.2016.1179404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa C.M., Biancur D.E., Wang X., Halbrook C.J., Sherman M.H., Zhang L., Kremer D., Hwang R.F., Witkiewicz A.K., Ying H., Asara J.M., Evans R.M., Cantley L.C., Lyssiotis C.A., Kimmelman A.C. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;536:479–483. doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B., Potkar R., Trejo M., Rockenstein E., Patrick C., Gindi R., Adame A., Wyss-Coray T., Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J. Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele J.W., Gandy S. Latrepirdine (Dimebon(R)), a potential Alzheimer therapeutic, regulates autophagy and neuropathology in an Alzheimer mouse model. Autophagy. 2013;9:617–618. doi: 10.4161/auto.23487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohecker A.M., Guo J.Y., Karsli-Uzunbas G., Price S.M., Chen G.J., Mathew R., Mcmahon M., White E. Autophagy sustains mitochondrial glutamine metabolism and growth of BRAFV600E-driven lung tumors. Cancer Discov. 2013;3:1272–1285. doi: 10.1158/2159-8290.CD-13-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran S., Rajasankar S. Parkinson's disease: oxidative stress and therapeutic approaches. Neurol. Sci. 2010;31:531–540. doi: 10.1007/s10072-010-0245-1. [DOI] [PubMed] [Google Scholar]
- Tang H., Sebti S., Titone R., Zhou Y., Isidoro C., Ross T.S., Hibshoosh H., Xiao G., Packer M., Xie Y., Levine B. Decreased BECN1 mRNA expression in human breast cancer is associated with estrogen receptor-negative subtypes and poor prognosis. EBioMedicine. 2015;2:255–263. doi: 10.1016/j.ebiom.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn A., Thamm D.H., Gustafson D.L. Autophagy and cancer therapy. Mol. Pharmacol. 2014;85:830–838. doi: 10.1124/mol.114.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V., Giliberto L., Zhao H., Chandakkar P., Wu Q., Simon J.E., Janle E.M., Lobo J., Ferruzzi M.G., Davies P., Marambaud P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl D.T., Stadtmauer E.A., Tan K.S., Heitjan D.F., Davis L.E., Pontiggia L., Rangwala R., Piao S., Chang Y.C., Scott E.C., Paul T.M., Nichols C.W., Porter D.L., Kaplan J., Mallon G., Bradner J.E., Amaravadi R.K. Combined autophagy and proteasome inhibition: a phase 1 trial of hydroxychloroquine and bortezomib in patients with relapsed/refractory myeloma. Autophagy. 2014;10:1380–1390. doi: 10.4161/auto.29264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.Z., Xia Y.Y., Grundke-Iqbal I., Iqbal K. Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J. Alzheimers Dis. 2013;33(Suppl 1):S123–S139. doi: 10.3233/JAD-2012-129031. [DOI] [PubMed] [Google Scholar]
- Wang H.C., Zhang T., Kuerban B., Jin Y.L., Le W., Hara H., Fan D.S., Wang Y.J., Tabira T., Chui D.H. Autophagy is involved in oral rAAV/Abeta vaccine-induced Abeta clearance in APP/PS1 transgenic mice. Neurosci. Bull. 2015;31:491–504. doi: 10.1007/s12264-015-1546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Wang C., Croce C.M., Guan J.-L. p62/SQSTM1 synergizes with autophagy for tumor growth in vivo. Genes Dev. 2014;28:1204–1216. doi: 10.1101/gad.237354.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E. The role for autophagy in cancer. J. Clin. Invest. 2015;125:42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D., Windfeld K., Colding-Jorgensen E. Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer's disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2014;13:1092–1099. doi: 10.1016/S1474-4422(14)70198-X. [DOI] [PubMed] [Google Scholar]
- Wolpin B.M., Rubinson D.A., Wang X., Chan J.A., Cleary J.M., Enzinger P.C., Fuchs C.S., Mccleary N.J., Meyerhardt J.A., Ng K., Schrag D., Sikora A.L., Spicer B.A., Killion L., Mamon H., Kimmelman A.C. Phase II and pharmacodynamic study of autophagy inhibition using hydroxychloroquine in patients with metastatic pancreatic adenocarcinoma. Oncologist. 2014;19:637–638. doi: 10.1634/theoncologist.2014-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y.C., Holzbaur E.L. The regulation of autophagosome dynamics by huntingtin and HAP1 is disrupted by expression of mutant huntingtin, leading to defective cargo degradation. J. Neurosci. 2014;34:1293–1305. doi: 10.1523/JNEUROSCI.1870-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Koh J.Y., Price S., White E., Mehnert J.M. Atg7 overcomes senescence and promotes growth of BrafV600E-driven melanoma. Cancer Discov. 2015;5:410–423. doi: 10.1158/2159-8290.CD-14-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Wang X., Contino G., liesa M., Sahin E., Ying H., Bause A., Li Y., Stommel J.M., Dell'Antonio G., Mautner J., Tonon G., Haigis M., Shirihai O.S., Doglioni C., Bardeesy N., Kimmelman A.C. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A., Rajeshkumar N.V., wang X., Yabuuchi S., Alexander B.M., Chu G.C., von Hoff D.D., MAITRA A., Kimmelman A.C. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 2014;4:905–913. doi: 10.1158/2159-8290.CD-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii S.R., Kuma A., Akashi T., Hara T., Yamamoto A., Kurikawa Y., Itakura E., Tsukamoto S., Shitara H., Eishi Y., Mizushima N. Systemic analysis of Atg5-null mice rescued from neonatal lethality by transgenic ATG5 expression in neurons. Dev. Cell. 2016;39:116–130. doi: 10.1016/j.devcel.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Yue Z., Jin S., Yang C., Levine A.J., Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Miah M., Culbreth M., Aschner M. Autophagy in neurodegenerative diseases and metal neurotoxicity. Neurochem. Res. 2016;41:409–422. doi: 10.1007/s11064-016-1844-x. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Sanchez-Lopez E., Karin M. Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell. 2016;166:288–298. doi: 10.1016/j.cell.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]