Abstract
Background
We present the results of a Phase 2a randomized controlled trial investigating the safety, and secondary endpoints of subretinal rAAV.sFLT-1 gene therapy in patients with active wet age-related macular degeneration (wAMD).
Methods
All patients (n = 32), (ClinicalTrials.gov; NCT01494805), received ranibizumab injections at baseline and week 4, and thereafter according to prespecified criteria. Patients in the gene therapy group (n = 21) received rAAV.sFLT-1 (1 × 1011 vg). All patients were assessed every 4 weeks to the week 52 primary endpoint.
Findings
Ocular adverse events (AEs) in the rAAV.sFLT-1 group were mainly procedure related and self-resolved. All 11 phakic patients in the rAAV.sFLT-1 group showed progression of cataract following vitrectomy. No systemic safety signals were observed and none of the serious AEs were associated with rAAV.sFLT-1. AAV2 capsid was not detected and rAAV.sFLT-1 DNA was detected transiently in the tears of 13 patients. ELISPOT analysis did not identify any notable changes in T-cell response. In the rAAV.sFLT-1 group 12 patients had neutralizing antibodies (nAb) to AAV2. There was no change in sFLT-1 levels in bodily fluids. In the rAAV.sFLT-1 group, Best Corrected Visual Acuity (BCVA) improved by a median of 1.0 (IQR: − 3.0 to 9.0) Early Treatment Diabetic Retinopathy Study (ETDRS) letters from baseline compared to a median of − 5.0 (IQR: − 17.5 to 1.0) ETDRS letters change in the control group. Twelve (57%) patients in the rAAV.sFLT-1 group maintained or improved vision compared to 4 (36%) in the control group. The median number of ranibizumab retreatments was 2.0 (IQR: 1.0 to 6.0) for the gene therapy group compared to 4.0 (IQR: 3.5 to 4.0) for the control group.
Interpretation rAAV.sFLT-1 combined with the option for co-treatment appears to be a safe and promising approach to the treatment of wAMD.
Funding
National Health and Medical Research Council of Australia (AP1010405), Lions Eye Institute, Perth Australia, Avalanche Biotechnologies, Menlo Pk, CA, USA.
Keywords: Wet age related macular degeneration, AAV.sFLT-1, Gene therapy, Clinical trial
Highlights
-
•
Subretinal injection of rAAV.sFLT-1 was found to be safe in 21 patients with wet age-related macular degeneration.
-
•
Visual acuity was maintained in gene therapy treated patients over the 52 week period.
-
•
The gene therapy treated group of patients had a trend towards fewer ranibizumab retreatments than the control group.
Wet age-related macular degeneration (wAMD) is a common cause of vision loss in the elderly. We propose to use gene therapy to treat this disease. This study reinforces the findings from our Phase 1 study examining the safety of the delivery of rAAV.sFLT-1 by subretinal injection as a form of treatment of wAMD. The findings from this trial demonstrate that the surgical subretinal administration of rAAV.sFLT-1 has a favorable safety profile and that it can potentially be used in conjunction with current therapies to provide an improved outcome in the treatment of wAMD.
1. Introduction
Age-related macular degeneration (AMD) is a common cause of vision loss in the elderly (Congdon et al., 2004). The role of vascular endothelial growth factor (VEGF) in the pathogenesis of the neovascular form of AMD, wet AMD (wAMD), is well established (Kliffen et al., 1997), and the efficacy of anti-VEGF molecules such as ranibizumab, bevacizumab and aflibercept in treating wAMD has been extensively studied (Kaiser et al., 2007, Rosenfeld et al., 2006, Schmidt-Erfurth et al., 2014). More recently, down-regulation of naturally occurring anti-angiogenic factors such as pigment epithelium-derived factor (PEDF) and soluble fms-like tyrosine kinase-1 (sFLT-1) in wAMD has been found to exacerbate the degenerative process (Bouck, 2002, Luo et al., 2013, Ohno-Matsui et al., 2001).
The advent of recombinant adeno-associated virus (rAAV) vector gene-therapy has enabled exploration of treatments with the potential for longer-term control or reversal of pathogenic processes (Naldini, 2015). rAAV vectors have been studied in individuals with monogenic conditions, such as choroideremia (Vasireddy et al., 2013), Leber's congenital amaurosis (LCA) (Maguire et al., 2008), and X-linked retinoschisis (XLRS). Further, the potential to deliver long-term, stable protein expression via gene therapy intraocularly to treat a complex disease like wAMD creates the possibility of a paradigm shift in the delivery of ophthalmic healthcare.
sFLT-1 is a naturally occurring VEGF inhibitor that is a soluble variant of the full length membrane bound VEGFR-1 protein (Kendall and Thomas, 1993). It has been reported that serum sFLT-1 is lower in wAMD patients (Uehara et al., 2015) and that sFLT-1 alone is sufficient to confer protection against choroidal neovascularization (CNV) in rodent and primate models (Lai et al., 2001, Lai et al., 2012, Lai et al., 2009, Lai et al., 2005, Lai et al., 2002, Rakoczy et al., 2015). Recombinant vector mediated gene delivery using subretinal injection of rAAV.sFLT-1 has been one approach used to demonstrate this (Lai et al., 2001, Lai et al., 2012, Lai et al., 2009, Lai et al., 2005, Lai et al., 2003, Lai et al., 2002, Rakoczy et al., 2015). This allows for the vector to be placed directly adjacent to the retinal pigment epithelial (RPE) cells and photoreceptors, enabling uptake and transduction of the viral vector. The expression of sFLT-1 is then accomplished through the normal protein-producing machinery of the host cells.
We previously published our findings on the safety of rAAV.sFLT-1 administered subretinally to 6 patients with advanced wAMD in a Phase 1 trial (Rakoczy et al., 2015) (www.youtube.com/watch?v=CUiSFAi2_EY). Here, we present the results of our Phase 2a randomized clinical trial, investigating the safety, immunologic and other secondary endpoints of rAAV.sFLT-1 gene therapy delivered subretinally to 21 patients with active wAMD compared to 11 control patients.
2. Methods
The design and methods of the study were previously described in detail (Rakoczy et al., 2015) and they are summarized as follows:
2.1. Study Design
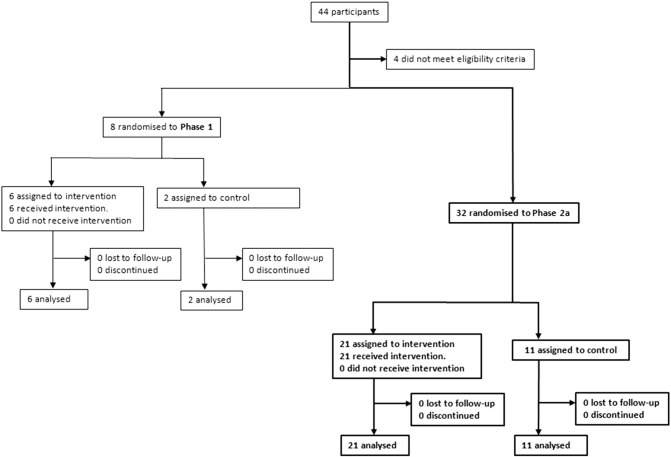
The primary objective of the study was to assess the safety and tolerability of rAAV.sFLT-1 in subjects with wAMD. This Phase 2a study was a single center, investigator-sponsored trial registered at ClinicalTrials.gov; NCT01494805 (Rakoczy et al., 2015). Patients were recruited from the Lions Eye Institute (LEI), Nedlands, WA, Australia and Sir Charles Gairdner Hospital (SCGH) Nedlands, WA, Australia. This study involved 32 patients with active wAMD who received either rAAV.sFLT-1, 1 × 1011 vg subretinally (N = 21), and/or intravitreal ranibizumab pro re nata (PRN) (Fig. 1). Patients were assessed every 4 weeks to the week 52 primary endpoint, with long-term follow-up to continue through to 36 months. Any ocular or systemic adverse events (AEs) were reported to the principal investigator (IJC) who determined whether they were related to gene therapy, ranibizumab therapy, study procedures, or unrelated. Gene therapy patients had all AEs over the 52-week duration recorded in the case report form (CRF), whereas in control patients AEs were recorded in the CRF for up to 30 days after which only AEs deemed related to study procedures, including ranibizumab injections, were recorded. Serious AEs (SAEs) in both groups were reported for the duration of the study and followed up until resolution.
Fig. 1.
Trial profile for Phase 1 (Rakoczy et al., 2015) and Phase 2a clinical trials.
The protocol was approved by the Australian Therapeutic Goods Administration. The trial was performed at the LEI. Approval was obtained from The University of Western Australia Institutional Biosafety Committee and the SCGH Human Ethics Committee. The tenets of the Declaration of Helsinki were observed, and all patients provided written informed consent.
2.2. Participants
Eligibility criteria included patients 55 years or older, with a best corrected visual acuity (BCVA) worse than 20/40 secondary to wAMD due to active subfoveal choroidal CNV demonstrated by leakage on fluorescein angiography (FA), or the presence of fluid on spectral domain optical coherence tomography (SD-OCT). The vast majority of patients had previously received anti-VEGF therapy, and a washout period for the anti-VEGF was not required prior to the baseline visit.
2.3. Randomization and Masking
Since this was a Phase 2a study, no power calculation was done to determine sample size. The patients were randomized in a 2:1 ratio (gene therapy: control) according to a computer-generated permuted block randomization list prepared at the start of the study and held off-site. The procedure staff and patients were not masked to the treatment received. Staff members performing the assessments at study visits were masked to the study group.
2.4. Procedures
The subretinal gene-therapy delivery procedure was described previously in the Phase 1 study (Rakoczy et al., 2015). All 32 patients received intravitreal 0·5 mg ranibizumab in the study eye at baseline and at the week 4 visit. The patients in the gene therapy group received subretinal injection of 100 μL rAAV.sFLT-1 (1 × 1011 vg) following a core vitrectomy at day 7. Primary and secondary end points were preselected. The primary end point was measured using physical and ophthalmic examinations, vital signs and clinical laboratory testing. Study assessments included ophthalmic examination, BCVA, and SD-OCT every 4 weeks. FA was performed quarterly. Ranibizumab retreatment was indicated for worsening disease: > 10 Early Treatment Diabetic Retinopathy Study (ETDRS) letter loss (from patient's previous visit) attributable to CNV or > 5 EDTRS letter loss if apparent to the patient, any increased, new or persistent subsensory, sub-RPE, or intraretinal fluid on SD-OCT > 50 μm from previous visit, or signs of increased CNV leakage on FA. Laboratory tests included routine hematology, renal and hepatic function tests, urine protein, serum IgM, IgG, IgA levels and protein electrophoresis, and enumeration of peripheral blood lymphocyte subsets (B cells - CD19 +; T cells - CD3 +, CD4 +, CD8 +; and natural killer cells - CD3 −, CD16 +, CD56 +). Biodistribution of rAAV.sFLT-1 was assessed by qPCR for rAAV.sFLT-1 DNA and AAV2 capsid detection by ELISA in tears from treated and fellow eye, serum, urine, and saliva. AAV2-specific immune responses assessed included neutralizing antibodies (nAb), total antibodies to AAV2 capsid proteins detected by ELISA and AAV2-specific T-cells detected by interferon-gamma (IFN-γ) ELISpot assay.
2.5. Outcomes
This study was primarily designed to assess the safety and tolerability of rAAV.sFLT-1 in patients with wAMD. Secondary objectives were to assess the effect of rAAV.sFLT-1 using analysis of BCVA, number of ranibizumab retreatments received, and retinal thickness on SD-OCT. There were no specific data exclusion criteria used. Biodistribution of rAAV.sFLT-1 and the immune response to AAV2 were also assessed. A further post hoc analysis of the fundus photographs, fluorescein angiograms, and SD-OCT images for each patient was completed by the Doheny Image Reading Center (DIRC), Los Angeles, USA.
2.6. Statistical Analysis
The primary analysis was conducted when week 52 data were available and locked for all patients. LEI transferred the data to CPR Pharma Services PTY LTF (Therbarton, Australia). Safety analyzes were conducted according to the randomized treatment group. There were no specific treatments for outliers. Comparisons between groups were performed using t-tests, Wilcoxon rank sum tests, and Fisher's exact tests as appropriate for each endpoint. Analyzes were conducted using SAS software, version 9·4 (SAS Institute, Inc.) or “R”-statistics.
3. Results
3.1. Disposition
Between August 2012 and March 2014, 32 patients were enrolled in this Phase 2a study and all completed 52 weeks of follow-up.
3.2. Demographics
The patient cohort was 97% (31) white and 63% (20) female, with a median age of 80.0 years (IQR: 74.0 to 83.0). The Phase 2a patients were similar in age to the Phase 1 patients (Rakoczy et al., 2015), but had less advanced although still active wAMD in the study eye (Table S1). Twenty-nine Phase 2a patients were previously treated for wAMD and 3 (1 gene therapy, 2 controls) were treatment-naïve patients. Within the Phase 2a study, the active and control arms were otherwise comparable in terms of age, BCVA and other ocular parameters (Table 1).
Table 1.
Baseline characteristics of Phase 2a patients.
| Active | Control | All patients | |
|---|---|---|---|
| N = 21 | N = 11 | N = 32 | |
| Age (years), median (Q1, Q3) | 80 (73, 81) | 79 (77, 83) | 80 (74, 83) |
| Baseline BCVA (ETDRS letters), median (Q1, Q3) | 63 (50, 71) | 63 (53, 69) | 63 (50, 70) |
| Baseline center point thickness (μm), median (Q1, Q3) | 328 (285, 388) | 349 (307, 515) | 333 (296,460) |
| Number treatment naïve | 1 (5%) | 2 (18%) | 3 (9%) |
| Previous anti-VEGF injections, median (Q1, Q3) | 10 (5,13) | 8 (3,24) | 9 (5,14) |
| Fibrosis at baseline | 17 (81%) | 10 (91%) | 27 (84%) |
| PED at baseline | 7 (33%) | 4 (36%) | 11 (34%) |
3.3. Primary Endpoint: Safety
3.3.1. Ocular Safety
No serious ocular AE was reported in the gene therapy group and 1 endophthalmitis event was reported in the control group (Table S2). There were 51 ocular AEs in the rAAV.sFLT-1 group which included subconjunctival (10), minor subretinal (8) or vitreous hemorrhage (4) and anterior chamber inflammation/uveitis (3). Twenty-six (51·0%) of these AEs were related to the study procedure and occurred within 30 days post administration (Table S3). The majority of cases were deemed to be mild in nature, visually insignificant with no permanent sequelae. Two ocular AEs were considered possibly related to rAAV.sFLT-1 were eye inflammation, and anterior chamber inflammation, which were mild in nature and resolved without sequelae. As expected in this age group, visually significant nuclear cataract developed in all phakic patients (n = 11) following vitrectomy. One case of significant vision loss (≥ 15 ETDRS letters) at week 52 was observed in the gene therapy group and was attributed to progression of wAMD.
The control group had 3 cases of significant vision loss (≥ 15 ETDRS letters) at week 52. One case was attributed to endophthalmitis 3 days after an intravitreal ranibizumab injection, the second to a large submacular hemorrhage, and the third due to the progression of wAMD. The control group also reported one case each of posterior vitreous detachment, corneal opacities, and cataract (Table S3). Of the 11 controls, 7 were phakic. One had cataract removed at 9 months following endophthalmitis treated with vitrectomy.
3.3.2. Systemic Safety
No systemic safety signals were observed relative to treatment. None of the 7 SAEs in the gene therapy group were attributed to rAAV.sFLT-1 gene therapy (Table S2) and no clinically significant abnormalities in laboratory tests values were reported. Two transient laboratory AEs normalized to within reference range by the following visit were deemed by the investigator (IJC) to be possibly but not likely related to gene therapy. Over the 52 weeks follow up, all other non-ocular AEs were classified as mild or moderate and were deemed unrelated to the study procedure (Table S3). The most commonly reported non-ocular AEs involved the respiratory system. In the gene therapy group there were 4 episodes of upper respiratory tract infection (URTI, 2 undefined, 1 sinusitis and 1 nasopharyngitis) and 2 episodes of lower respiratory tract infection (LRTI). These respiratory tract infections were mild and self-limiting and occurred between 75 and 358 days post entering the trial, without any trend. One URTI and one LRTI were reported prior to receiving rAAV.sFLT-1 at baseline and at day 19 after gene therapy, respectively. In the control group there were 4 AEs with an infectious nature, including 2 URTI (1 undefined and 1 sinusitis). Other non-ocular adverse events in both groups were deemed unrelated to the study procedure (Table S3).
3.4. Biodistribution
AAV2 capsid was not detected in samples collected at any time point. In the gene therapy group, rAAV.sFLT-1 DNA was detected transiently in the tears from the treated eye in a subset of patients (13/21), in the tears of the fellow eye in one patient, in the saliva in one patient, and in the urine in one patient. All these occurrences resolved prior to the week 4 visit and none of the patients had detectable vector DNA in blood (Table S4). The measured systemic levels of sFLT-1 and VEGF proteins in serum, saliva, and urine did not show any statistically significant difference at baseline between the control and gene therapy group. There was also no statically significant difference between the sFLT-1 and VEGF levels at baseline and week 52 of the gene therapy group. The limited number of vitreous samples did not show any change in sFLT-1 protein levels between the baseline and week 4 (Table S5).
3.5. Immune Response to AAV2
At baseline 9 of 21 rAAV.sFLT-1-injected patients had no detectable nAbs to AAV2, 5 patients had low nAb titre (1:20–1:100) and 7 patients had nAb titres (> 1:100). Three of the 9 nAb-negative patients seroconverted after rAAV.sFLT-1 therapy. Serum levels of antibodies to AAV2 capsid proteins were variable and uninformative. Enumeration of AAV2-specific T-cells in peripheral blood mononuclear cells, as determined by IFN-γ ELISpot assay, demonstrated that there were no notable changes in T-cell responses to AAV2 during the study period in all, but 1 patient who exhibited elevated responses at random multiple time points post-treatment.
3.6. Secondary Endpoints and Post Hoc Analysis
3.6.1. BCVA
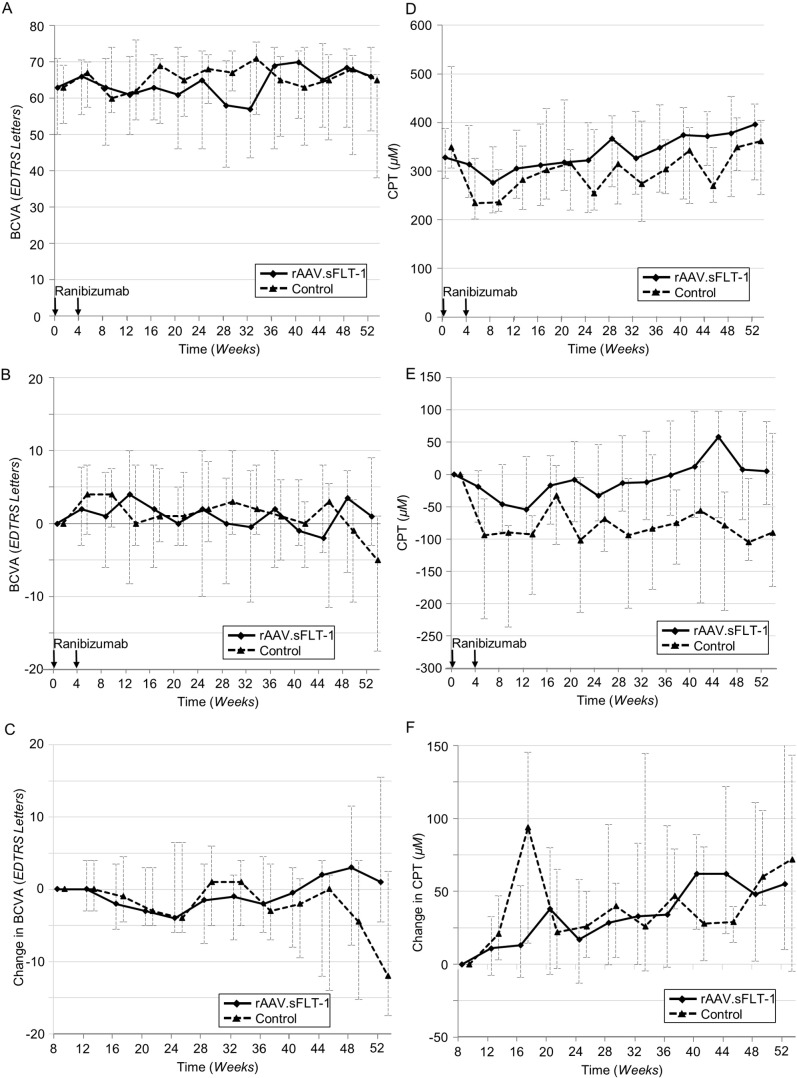
In the gene therapy group (n = 21), the median change in BCVA was 1.0 (IQR: − 3.0 to 9.0) ETDRS letters from baseline (median: 63.0, IQR: 50.0 to 71.0) to week 52 (median: 66.0, IQR: 51.0 to 74.0). In the control group (n = 11), the median change in BCVA from baseline (median: 63.0, IQR: 53.0 to 69.0) to week 52 (median: 65.0, IQR: 38.0 to 66.5) was − 5.0 (IQR: − 17.5 to 1.0) ETDRS letters. The difference in median changes between groups was 6.0 ETDRS letters (Fig. 2a and b). In a post hoc analysis, when the change in BCVA was measured from week 8 (the time point at which gene therapy is believed to potentially take effect) there was also a difference in median changes between the gene therapy (median: 1.0, IQR: − 4.0 to 10.0 ETDRS letters) and control groups (median: − 12.0, IQR: − 17.5 to 2.5 ETDRS letters) at week 52 (Fig. 2c).
Fig. 2.
Graphs of median BCVA and CPT over time A: Median BCVA over 52 weeks. B: Median change in BCVA from baseline to week 52. C: Median change in BCVA from week 8 (activation of gene therapy) to week 52. D: Median CPT over 52 weeks. E: Median change in CPT from baseline to week 52. F: Median change in CPT from week 8 (activation of gene therapy) to week 52. BCVA = Best Corrected Visual Acuity; CPT = Center Point Thickness.
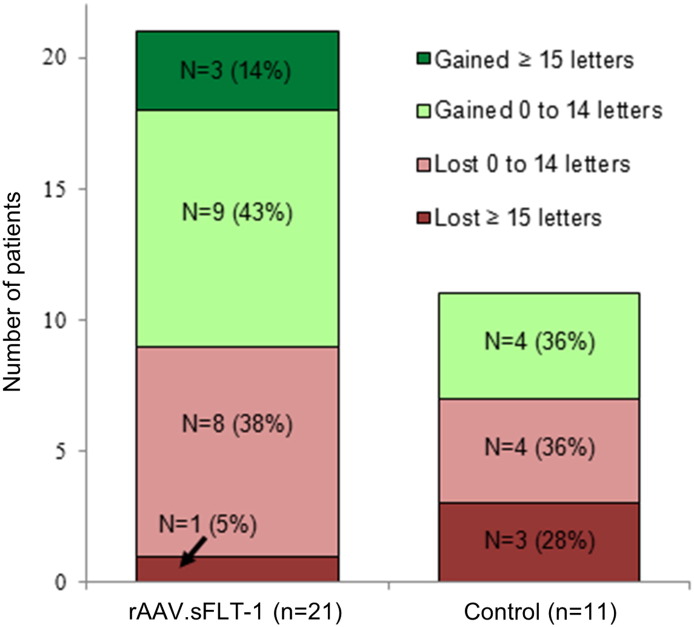
Twelve of 21 (57%) gene therapy patients experienced maintenance or improvement of vision versus 4 of 11 (36%) control patients. The percentage of patients gaining ≥ 15 ETDRS letters from baseline to week 52 was 14% (3) for the gene therapy group and 0% for the control group (Fig. 3). In the gene therapy group 1 patient lost > 15 ETDRS letters versus 3 in the control group. When the 3 control subjects losing 3 lines of vision (outliers) were removed there was no statistically significant difference in BCVA improvement between the gene therapy and control groups (Fig. S1). Post hoc analysis of initial lens status showed no change in average BCVA in the pseudophakic subset at week 52 suggesting that surgery in the phakic subset only restored vision to pre-cataract levels (Figs. S2 and S3). There was a significant drop in BCVA in the pseudophakic control group which was due to significant loss of vision in a patient with endophthalmitis and a patient with submacular hemorrhage (Fig. S2).
Fig. 3.
BCVA change category by treatment group. Patients categorized by change in BCVA at week 52. BCVA = Best Corrected Visual Acuity.
3.6.2. Spectral Domain Optical Coherence Tomography
The SD-OCT images are presented in Fig. S4. In the gene therapy group (n = 21), a median change in center point thickness (CPT) on SD-OCT of 5.0 (IQR: − 47.0 to 82.0) μm from baseline to week 52 was observed. In the control group (n = 11), the median change in CPT was − 90.0 (IQR: − 173.5 to 64.0) μm (Fig. 2d and e). The difference in median change in CPT between the gene therapy and control groups was 95.0 μm. Analyzes of central subfield thickness (CST) showed similar results to those for CPT (Fig. S5). There was no clinically important difference between the median CPT at baseline for the gene therapy group 328 μm and the control group at 349 μm (Fig. 2c). When the median change in CPT over time was measured from a new baseline at week 8, no difference was found between the gene therapy and control groups at week 52 (Fig. 2d). Similar results were observed for CST (Fig. S6). Analysis of the SD-OCTs at DIRC demonstrated that results from manual segmentation and automated segmentation were comparable (Figs. S7 and S8).
3.6.3. Number of Ranibizumab Retreatments
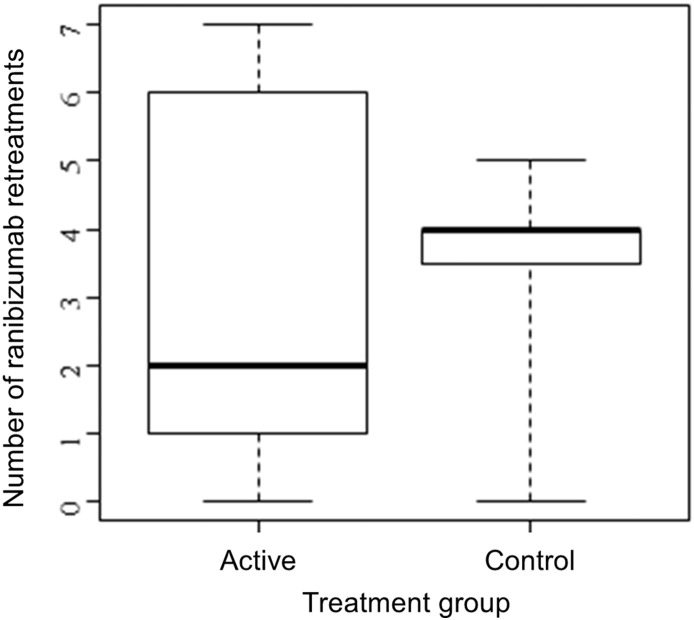
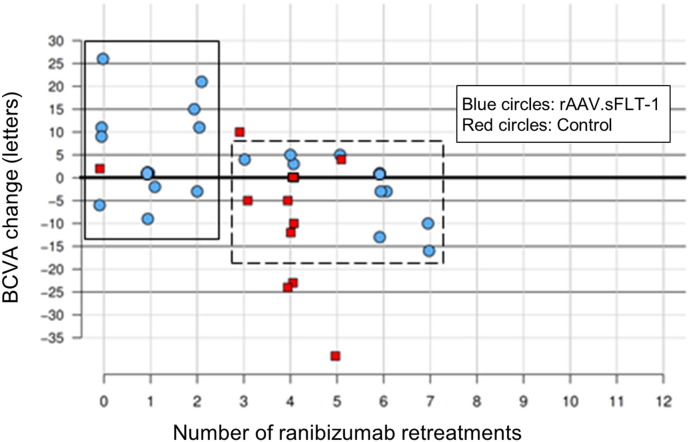
As a decreased need for ranibizumab retreatment injections would signal rAAV.sFLT-1 response and disease control, the number of ranibizumab retreatments performed by week 52 under pre-specified criteria was assessed. Excluding the initial 2 mandatory injections in both groups as per protocol at baseline and week 4, the median number of ranibizumab injections was 2.0 (IQR: 1.0 to 6.0) for the gene therapy group compared to 4.0 (IQR: 3.5 to 4.0) for the control group (Fig. 4). Further analysis suggested a negative association between the number of ranibizumab retreatments and BCVA change in the gene therapy group (Fig. 5). Eleven (52·4%) rAAV.sFLT-1-treated patients received 2 or fewer ranibizumab retreatments with 3 (14·3%) achieving notable BCVA gains of ≥ 15 ETDRS letters. It was also noted that 10 of these 11 (90·9%) patients had nAb to AAV2 present at baseline. The other 10 patients (47·6%) in the rAAV.sFLT-1-treated group received more than 2 ranibizumab retreatments, with no clinically significant vision gain. Only 2 of these 10 (20%) patients had nAb to AAV2 at baseline. In the control group, 10 of 11 patients (90·9%) received more than 2 ranibizumab retreatments and none achieved a gain of ≥ 15 ETDRS letters. Two of the control patients had nAb to AAV2 at baseline.
Fig. 4.
Ranibizumab retreatments by treatment group.
Distribution of the number of ranibizumab retreatments in each treatment group, showing the median (thickest line), the 25th and 75th percentiles (box boundaries), and the range (whiskers).
Fig. 5.
BCVA vs number of ranibizumab retreatments at week 52.
Linear regression of BCVA change on the number of ranibizumab retreatments showed a significant negative association (P = 0.01) for Phase 2a gene therapy patients. A subset of rAAV.sFLT-1 treated patients received 2 or fewer ranibizumab retreatments (solid box). A second subset of rAAV.sFLT-1 treated patients received > 2 ranibizumab retreatments (dashed box). BCVA = Best Corrected Visual Acuity.
4. Discussion
This Phase 2a study of 32 patients is the largest ocular AAV clinical trial to date and serves to confirm the results of the Phase 1 study (Rakoczy et al., 2015), which showed that subretinal injection of rAAV.sFLT-1 is safe. The study also investigates the potential association between the safety and patient-focused efficacy of rAAV.sFLT-1 gene therapy delivered subretinally.
Results of the study showed no serious gene therapy-related ocular or systemic side effects. All AEs related to gene therapy or study procedure were mild or moderate and resolved without sequelae. These findings were consistent with the Phase 1 study (Rakoczy et al., 2015), as well as other AAV ocular studies (Vasireddy et al., 2013, Maguire et al., 2008, Pierce and Bennett, 2015, Simonelli et al., 2010, Testa et al., 2013). The majority of mild and moderate ocular AEs were associated with the surgery involving vitrectomy and subretinal injection (Maguire et al., 2008, Rakoczy et al., 2015). As these procedures were a necessary component of the AAV therapy, they are an important consideration for safety. A variety of ocular hemorrhages associated with the surgery were mild in nature without any visual significance and with no permanent sequelae. Cataracts are a known side effect of vitrectomy (Blankenship and Machemer, 1985) and progression to visually significant nuclear cataract in all phakic gene therapy patients and in 1 control patient was noted. It is not yet clear if subretinal injection can be done safely without vitrectomy. A higher number of systemic AEs were reported in the gene therapy group compared to the control group, which we interpret as due to the reporting procedures described in the Methods section. Respiratory system-related AEs were reported more frequently in patients treated with rAAV.sFLT-1 (28%) compared to controls (18%). However, the infections were heterogeneous in character, and differences in frequency between the gene therapy group and control group were not statistically significant. Overall the incidence of respiratory system-related AEs in the gene therapy group was less frequent than rates published for existing anti-VEGF agents in late-stage wAMD trials, so an association with rAAV.sFLT-1 gene therapy seems unlikely.
Results of rAAV.sFLT-1 qPCR, AAV2 capsid ELISA, and sFLT-1 protein quantitation indicate that the biodistribution of rAAV.sFLT-1 outside the target tissue (retina) after subretinal injection is limited and transient. No rAAV.sFLT-1 DNA or AAV2 capsid were detected in any of the assessed samples at or after the week 4 visit. The measured systemic levels of sFLT-1 and VEGF in serum, urine, and saliva were highly variable, fluctuating and dependent on the individual and we could not identify any trend that would have suggested systemic effect. The only immune response to rAAV.sFLT-1 therapy detected was sero conversion of nAbs observed in 3 patients which is consistent with previously published reports showing that subretinal administration of AAV does not frequently induce humoral immune responses to the AAV capsid (Li et al., 2008). Recent reports show that subretinal delivery of rAAV does not affect the efficacy of rAAV.sFLT-1 therapy when subsequently administered to the fellow eye (Li et al., 2008, Bennett et al., 2012).
Amongst the patients who received the rAAV.sFLT-1 gene therapy, 12 (57·1%) had pre-existing nAbs to AAV2 and it seems that pre-existing nAbs to AAV2 do not necessarily decrease the efficacy of the gene therapy administered by subretinal injection. This study does not raise any new concerns for ocular gene therapy with respect to the variables of patient safety and immune response and confirms the published work of others (Bennett et al., 2012).
Unlike many of the previously reported anti-VEGF trials (CATT Research Group et al., 2011, IVAN Study Investigators et al., 2012, Heier et al., 2012) for wAMD, 29 of 32 (90·6%) recruited patients in this study were not treatment-naïve, having received a median of 9.0 (IQR: 5.0 to 14.0) previous anti-VEGF injections. The chronic nature of baseline disease in the patients participating in this safety trial may have diminished the potential for large vision gains or fluid loss on SD-OCT. In addition the study was designed to assess the safety of rAAV.sFLT-1 delivered by subretinal injection and sought to provide PRN ranibizumab therapy in both arms. Therefore, no significant difference in BCVA and CPT between the gene therapy and control groups was expected. Nevertheless, the median change in BCVA for the gene therapy group was + 1 · 0 ETDRS letters at week 52, and for the control group was − 5 · 0 ETDRS letters confirming that rAAVsFLT-1 did not have a deleterious effect. In the control group, this loss of ETDRS letters potentially could suggest under-treatment of the wAMD with ranibizumab, greater intrinsic disease activity or a higher complication rate for intravitreal injections. However, closer examination reveals that this loss was due to 3 of 11 (27·3%) patients who lost in excess of 20 ETDRS letters caused by infrequent but well known complications of the natural history of wAMD and PRN intravitreal therapy. Once these 3 patients were removed from the analysis there was no statistically significant difference in BCVA improvement between the gene therapy and the control groups. The incidence of moderate vision loss was similar in the treatment and control groups suggesting that there might be a subgroup of patients who do not have the capacity to respond to anti-VEGF treatments. As all the phakic patients in the gene therapy group required cataract surgery presumed secondary to the initial vitrectomy, lens status and cataract removal were investigated as possible explanations for the median final BCVA of this group being higher than the control group. Although the numbers are small, post hoc analysis of initial lens status and need for cataract surgery was not found to drive the BCVA in the rAAV.sFLT-1-treated group and the control group at the week 52 time point.
This study found that the median CPT over time showed a greater decrease in the control group than the gene therapy group. While both groups were given ranibizumab injections at baseline and week 4, the gene therapy group patients received a core vitrectomy, associated with subretinal gene therapy, at day 7. This may have removed any remaining active ranibizumab from the first intravitreal injection, thus leading to a smaller initial response in the gene therapy group. In most studies (CATT Research Group et al., 2011, Lalwani et al., 2009), the greatest decreases in CPT occurred in the first 2 months of the study.
The gene therapy group had a trend towards fewer ranibizumab retreatments than the control group, and in a post hoc analysis 11 (53%) gene therapy patients received ≤ 2 ranibizumab retreatments, with the majority having improved vision. A cohort of the gene therapy group received >2 ranibizumab retreatments, but lost or gained < 5 letters, suggestive of either under treatment or a bimodal response alluding to either lack of gene therapy effect, less susceptibility to anti-VEGF therapy, or greater intrinsic disease activity. We were not able to identify any reliable indicators to suggest a cause for this potential divergent response such as pre-existing immunity to AAV, anti-VEGF resistance, under treatment, variability of successful delivery or the location of the delivery. Potential reasons for variability between this Phase 2a study and the previous Phase 1 (Rakoczy et al., 2015) in BCVA and CPT outcomes were examined (Table S1). Product quality was evaluated and found to meet drug specifications in the lots used in both trials and showed no difference in biologic activity in vitro. Other possible causes include differing disease stage and anatomic appearance such as degree of subretinal fluid, geographic atrophy, subretinal fibrosis, pigment epithelial detachment, and CNV location. These variations in anatomic appearance and disease stage may also have impacted certain technical factors of therapy including diffusion in the retina and subretinal space. In the Phase 1 study, the baseline median BCVA in ETDRS letters was 40.0 (IQR: 33.0 to 54.0) versus 63.0 (IQR: 50.0 to 71.0) in the Phase 2a patients, while the median CPT was 549.0 (IQR: 268.0 657.0) compared to 332.5 (IQR: 295.5 to 459.8) μm, respectively. The difference in thickness could have had an impact on the mobility of sFLT-1 to the area of the subfoveal neovascularization via changing pharmacokinetic properties (Holash et al., 2002). Other possibilities are difficult to quantify but may include intrinsic VEGF load or the degree of anti-VEGF responsiveness in this cohort of patients. Additional preclinical laboratory studies evaluating potential sources of variation in the apparent clinical effect between the patients in this study and our initial report (Rakoczy et al., 2015) using this approach are underway. These include a determination of the optimal vector copy number to be injected, the impact of site of injection and the efficacy of alternative modified AAV vectors and cDNA constructs. There were several limitations to this study. These include the fact that this was an open label study as the gene therapy required a surgery (subretinal injection and vitrectomy) that could not be mimicked by a sham treatment due to the constraints of a clinical trial. Although the technicians measuring BCVA were masked, the open label study design allowed for potential patient or investigator bias. Also, the optimal method for subretinal administration has not been standardized relative to both safety and potential efficacy in this patient population with an anatomically abnormal macula due to intrinsic disease relative to the normal eye. The retreatment criteria differed slightly from other published studies (CATT Research Group et al., 2011, Lalwani et al., 2009), but were designed to allow for assessment of gene therapy activity as evidenced by reduced need for ranibizumab retreatments. There exist a number of different and evolving community standards for anti-VEGF retreatment including programmed monthly injections, treat and extend regimens, and PRN regimens. Furthermore, this Phase 2a study only assessed 1 dose of gene therapy against control, which prevented an understanding of a possible dose-response effect. Finally, 29 of 32 patients had previously been treated to varying degrees for wAMD, in contrast to most wAMD studies that have enrolled only treatment-naïve patients and that decision may have also affected the results of the trial.
Based upon the results of this study, rAAV.sFLT-1 is safe when injected under the retina, but further clinical trials are needed to prove gene therapy is a viable option for wAMD. Although we performed several post hoc analyzes, the number of patients involved in the study was too small to make useful assessments of clinical outcomes.
Conflict of Interest
CMP, MADE, MF, ILM have no conflict of interest. IJC, CML, PER have a patent pending (PCT14/281,783), CML and ALM receive financial support, SB and SBB employed by Adverum (Formerly Avalanche) Biotechnologies and SDS and MSB have financial interest.
Role of Funding Sources
Funding Sources: National Health and Medical Research Council of Australia (AP1010405), Lions Eye Institute, Perth Australia, Avalanche Biotechnologies, Menlo Pk, CA, USA. Avalanche Biotechnologies had no role in the design but participated in the data management and data analysis of the manuscript. The other two funding agencies contribution was only financial.
Authors Contribution
IJC and EPR take responsibility for the decision to publish the results. IJC, CMP, CML, ALM, SB, MSB and EPR had full access to the data. EPR did the conceptual design. IJC, C-ML, MAD-E, SDS, MSB and EPR participated in the study design. IJC, C-ML, CMP, MAD-E and EPR contributed to regulatory approval. IJC, C-ML, ALM, CMP, MAF and EPR contributed to data collection. IJC and ILM performed the surgeries. IJC, C-ML, ALM, MAD-E, MAF, SBB, SB and EPR participated in data analysis. IJC, C-ML, ALM, MAD-E, SBB, SDS, MSB and EPR participated in writing the paper.
Acknowledgements
The authors sincerely thank Dr. Vignesh Raja and Dr. Sendhil K Somasundaram, SCGH, Perth, for participating in some of the follow up studies and Dr. Alia Rashid from Avalanche Biotechnologies Inc., for her contribution to the medical writing.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.11.016.
Appendix A. Supplementary data
Supplementary information on methods
Supplementary tables and figures
References
- Bennett J., Ashtari M., Wellman J., Marshall K.A., Cyckowski L.L., Chung D.C., Mccague S., Pierce E.A., Chen Y., Bennicelli J.L., Zhu X., Ying G.S., Sun J., Wright J.F., Auricchio A., Simonelli F., Shindler K.S., Mingozzi F., High K.A., Maguire A.M. AAV2 gene therapy readministration in three adults with congenital blindness. Sci. Transl. Med. 2012;4:120ra15. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship G.W., Machemer R. Long-term diabetic vitrectomy results. Report of 10 year follow-up. Ophthalmology. 1985;92:503–506. doi: 10.1016/s0161-6420(85)34015-0. [DOI] [PubMed] [Google Scholar]
- Bouck N. PEDF: anti-angiogenic guardian of ocular function. Trends Mol. Med. 2002;8:330–334. doi: 10.1016/s1471-4914(02)02362-6. [DOI] [PubMed] [Google Scholar]
- Congdon N., O'Colmain B., Klaver C.C., Klein R., Munoz B., Friedman D.S., Kempen J., Taylor H.R., Mitchell P., Eye Diseases Prevalence Research G. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- CATT Research Group, Martin D.F., Maguire M.G., Ying G.S., Grunwald J.E., Fine S.L., Jaffe G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier J.S., Brown D.M., Chong V., Korobelnik J.F., Kaiser P.K., Nguyen Q.D., Kirchhof B., Ho A., Ogura Y., Yancopoulos G.D., Stahl N., Vitti R., Berliner A.J., Soo Y., Anderesi M., Groetzbach G., Sommerauer B., Sandbrink R., Simader C., Schmidt-Erfurth U., View & Groups, V. S Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Holash J., Davis S., Papadopoulos N., Croll S.D., Ho L., Russell M., Boland P., Leidich R., Hylton D., Burova E., Ioffe E., Huang T., Radziejewski C., Bailey K., Fandl J.P., Daly T., Wiegand S.J., Yancopoulos G.D., Rudge J.S. VEGF-trap: a VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVAN Study Investigators, Chakravarthy U., Harding S.P., Rogers C.A., Downes S.M., Lotery A.J., Wordsworth S., Reeves B.C. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Kaiser P.K., Brown D.M., Zhang K., Hudson H.L., Holz F.G., Shapiro H., Schneider S., Acharya N.R. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J. Ophthalmol. 2007;144:850–857. doi: 10.1016/j.ajo.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Kendall R.L., Thomas K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. U. S. A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliffen M., Sharma H.S., Mooy C.M., Kerkvliet S., De Jong P.T. Increased expression of angiogenic growth factors in age-related maculopathy. Br. J. Ophthalmol. 1997;81:154–162. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.M., Brankov M., Zaknich T., Lai Y.K., Shen W.Y., Constable I.J., Kovesdi I., Rakoczy P.E. Inhibition of angiogenesis by adenovirus-mediated sFlt-1 expression in a rat model of corneal neovascularization. Hum. Gene Ther. 2001;12:1299–1310. doi: 10.1089/104303401750270959. [DOI] [PubMed] [Google Scholar]
- Lai C.M., Estcourt M.J., Himbeck R.P., Lee S.Y., Yew-San Yeo I., Luu C., Loh B.K., Lee M.W., Barathi A., Villano J., Ang C.L., Van Der Most R.G., Constable I.J., Dismuke D., Samulski R.J., Degli-Esposti M.A., Rakoczy E.P. Preclinical safety evaluation of subretinal AAV2.sFlt-1 in non-human primates. Gene Ther. 2012;19:999–1009. doi: 10.1038/gt.2011.169. [DOI] [PubMed] [Google Scholar]
- Lai C.M., Estcourt M.J., Wikstrom M., Himbeck R.P., Barnett N.L., Brankov M., Tee L.B., Dunlop S.A., Degli-Esposti M.A., Rakoczy E.P. rAAV.sFlt-1 gene therapy achieves lasting reversal of retinal neovascularization in the absence of a strong immune response to the viral vector. Invest. Ophthalmol. Vis. Sci. 2009;50:4279–4287. doi: 10.1167/iovs.08-3253. [DOI] [PubMed] [Google Scholar]
- Lai C.M., Shen W.Y., Brankov M., Lai Y.K., Barnett N.L., Lee S.Y., Yeo I.Y., Mathur R., Ho J.E., Pineda P., Barathi A., Ang C.L., Constable I.J., Rakoczy E.P. Long-term evaluation of AAV-mediated sFlt-1 gene therapy for ocular neovascularization in mice and monkeys. Mol. Ther. 2005;12:659–668. doi: 10.1016/j.ymthe.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Lai Y.K., Sharma S., Lai C.M., Brankov M., Constable I.J., Rakoczy P.E. Virus-mediated secretion gene therapy – a potential treatment for ocular neovascularization. Adv. Exp. Med. Biol. 2003;533:447–453. doi: 10.1007/978-1-4615-0067-4_57. [DOI] [PubMed] [Google Scholar]
- Lai Y.K., Shen W.Y., Brankov M., Lai C.M., Constable I.J., Rakoczy P.E. Potential long-term inhibition of ocular neovascularisation by recombinant adeno-associated virus-mediated secretion gene therapy. Gene Ther. 2002;9:804–813. doi: 10.1038/sj.gt.3301695. [DOI] [PubMed] [Google Scholar]
- Lalwani G.A., Rosenfeld P.J., Fung A.E., Dubovy S.R., Michels S., Feuer W., Davis J.L., Flynn H.W., Jr., Esquiabro M. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO study. Am J. Ophthalmol. 2009;148:43–58. doi: 10.1016/j.ajo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Li Q., Miller R., Han P.Y., Pang J., Dinculescu A., Chiodo V., Hauswirth W.W. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol. Vis. 2008;14:1760–1769. [PMC free article] [PubMed] [Google Scholar]
- Luo L., Uehara H., Zhang X., Das S.K., Olsen T., Holt D., Simonis J.M., Jackman K., Singh N., Miya T.R., Huang W., Ahmed F., Bastos-Carvalho A., Le Y.Z., Mamalis C., Chiodo V.A., Hauswirth W.W., Baffi J., Lacal P.M., Orecchia A., Ferrara N., Gao G., Young-Hee K., Fu Y., Owen L., Albuquerque R., Baehr W., Thomas K., Li D.Y., Chalam K.V., Shibuya M., Grisanti S., Wilson D.J., Ambati J., Ambati B.K. Photoreceptor avascular privilege is shielded by soluble VEGF receptor-1. Elife. 2013;2 doi: 10.7554/eLife.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N., Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K.A., Testa F., Surace E.M., Rossi S., Lyubarsky A., Arruda V.R., Konkle B., Stone E., Sun J., Jacobs J., Dell'osso L., Hertle R., Ma J.X., Redmond T.M., Zhu X., Hauck B., Zelenaia O., Shindler K.S., Maguire M.G., Wright J.F., Volpe N.J., Mcdonnell J.W., Auricchio A., High K.A., Bennett J. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–360. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- Ohno-Matsui K., Morita I., Tombran-Tink J., Mrazek D., Onodera M., Uetama T., Hayano M., Murota S.I., Mochizuki M. Novel mechanism for age-related macular degeneration: an equilibrium shift between the angiogenesis factors VEGF and PEDF. J. Cell. Physiol. 2001;189:323–333. doi: 10.1002/jcp.10026. [DOI] [PubMed] [Google Scholar]
- Pierce E.A., Bennett J. The status of RPE65 Gene therapy trials: safety and efficacy. Cold Spring Harb. Perspect. Med. 2015;5:a017285. doi: 10.1101/cshperspect.a017285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoczy E.P., Lai C.M., Magno A.L., Wikstrom M.E., French M.A., Pierce C.M., Schwartz S.D., Blumenkranz M.S., Chalberg T.W., Degli-Esposti M.A., Constable I.J. Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomised clinical trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)00345-1. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P.J., Brown D.M., Heier J.S., Boyer D.S., Kaiser P.K., Chung C.Y., Kim R.Y., Group M.S. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U., Kaiser P.K., Korobelnik J.F., Brown D.M., Chong V., Nguyen Q.D., Ho A.C., Ogura Y., Simader C., Jaffe G.J., Slakter J.S., Yancopoulos G.D., Stahl N., Vitti R., Berliner A.J., Soo Y., Anderesi M., Sowade O., Zeitz O., Norenberg C., Sandbrink R., Heier J.S. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201. doi: 10.1016/j.ophtha.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Simonelli F., Maguire A.M., Testa F., Pierce E.A., Mingozzi F., Bennicelli J.L., Rossi S., Marshall K., Banfi S., Surace E.M., Sun J., Redmond T.M., Zhu X., Shindler K.S., Ying G.S., Ziviello C., Acerra C., Wright J.F., Mcdonnell J.W., High K.A., Bennett J., Auricchio A. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol. Ther. 2010;18:643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa F., Maguire A.M., Rossi S., Pierce E.A., Melillo P., Marshall K., Banfi S., Surace E.M., Sun J., Acerra C., Wright J.F., Wellman J., High K.A., Auricchio A., Bennett J., Simonelli F. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital amaurosis type 2. Ophthalmology. 2013;120:1283–1291. doi: 10.1016/j.ophtha.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara H., Mamalis C., Mcfadden M., Taggart M., Stagg B., Passi S., Earle P., Chakravarthy U., Hogg R.E., Ambati B.K. The reduction of serum soluble Flt-1 in patients with neovascular age-related macular degeneration. Am J. Ophthalmol. 2015;159(92–100):e1–e2. doi: 10.1016/j.ajo.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasireddy V., Mills J.A., Gaddameedi R., Basner-Tschakarjan E., Kohnke M., Black A.D., Alexandrov K., Zhou S., Maguire A.M., Chung D.C., Mac H., Sullivan L., Gadue P., Bennicelli J.L., French D.L., Bennett J. AAV-mediated gene therapy for choroideremia: preclinical studies in personalized models. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information on methods
Supplementary tables and figures