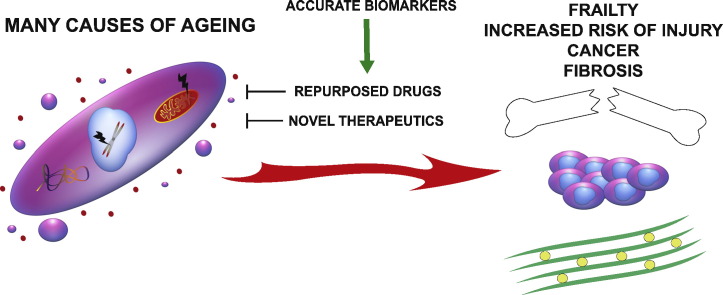
Abstract
Ageing is a leading risk factor for many debilitating diseases. While age-related diseases have been the subject of over a century of intense investigation, until recently, physiological ageing was considered unavoidable. Pharmacological and genetic studies have since shown that ageing is a malleable process and that its abrogation can prevent its associated diseases. This review summarises a sample of the most promising efforts to deliver the products of ageing research to the clinic. Current efforts include the use of clinically approved drugs that have since been repurposed, as well as the development of novel therapeutics, to target ageing. Furthermore, ongoing research has sought reliable biomarkers of ageing that will accelerate the development of such therapeutics. Development of these technologies will improve quality of late-life and help relieve the enormous stress placed on state healthcare systems by a rapidly ageing global population. Thus, for both medical and socioeconomic reasons, it is imperative that ageing is made to yield to intervention.
Keywords: Ageing, Biomarkers, Drug repurposing, Clinical trials
Graphical abstract
Highlights
-
•
The rate of ageing is malleable and its suppression has potential to suppress disease.
-
•
Clinically approved therapeutics that act on ageing pathways could be repurposed to inhibit ageing.
-
•
Development of accurate biomarkers for ageing will increase the speed at which geroscience can be translated to the clinic.
1. Introduction
The ageing global population represents one of the greatest challenges to modern society. Ageing is the biggest risk factor for some of the most debilitating and distressing diseases known, including neurodegenerative diseases such as Parkinson's and Alzheimer's, as well as cardiovascular, inflammatory and metabolic disease, as well as cancer. Collectively, the diseases of ageing represent the biggest causes of morbidity and mortality in the developed world (Kennedy and Pennypacker, 2014). While the study and treatment of these diseases in isolation has yielded a great deal of knowledge and a significant improvement in patient quality of life, it is important to acknowledge that these diseases do not exist in isolation. An aged individual may possess any number of co-morbidities, each of which can exponentially complicate any required therapeutic interventions. Given the complexity of treating age-related co-morbidities separately, the idea of targeting ageing itself as a means of reversing the pathogenesis of several diseases at once is appealing (Faragher et al., 2009).
This review will evaluate current developments in translating basic ageing research into useful therapeutics. Particular attention will be paid to ongoing attempts to repurpose already available drugs, before touching on promising drugs that, based on recent evidence in pre-clinical models, could also be repurposed. Additionally, new therapeutics being developed specifically to target ageing are discussed. Finally, since any appraisal of anti-ageing function will only be possible with robust biomarkers of ageing (Kenessary et al., 2013), we present a brief review of the search for powerful predictors of physiological age. To highlight strategies with greatest potential to manipulate human ageing, only drugs that have demonstrable effects in mammals will be considered. Other reviews have covered longevity-enhancing treatments in non-mammalian models (Vaiserman and Marotta, 2016, Vaiserman et al., 2016).
2. Ageing as a Clinical Indication
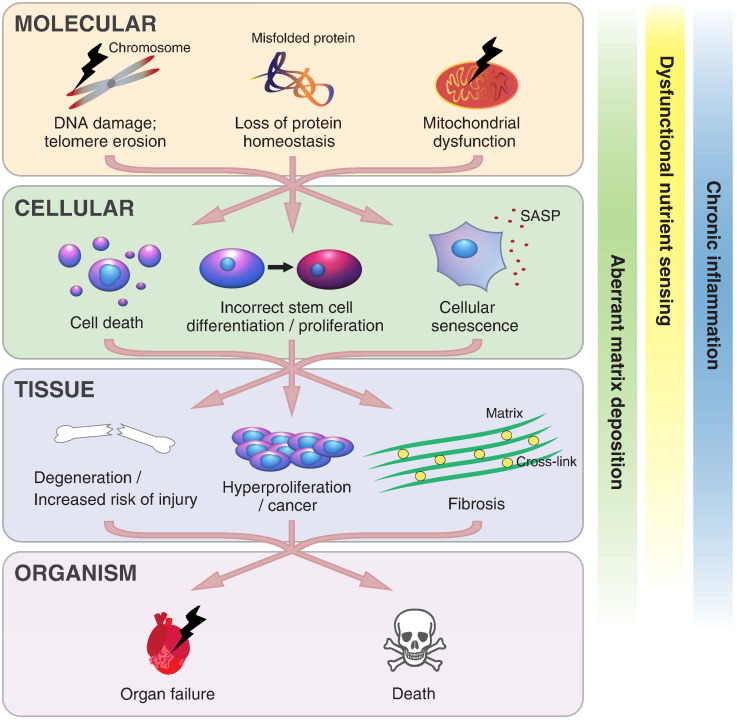
Twenty years ago, the eminent epidemiologist Richard Doll argued that ageing as a single unified phenomenon did not exist (Peto and Doll, 1997). Doll proposed that, on the balance of evidence available at the time, it was better to consider each of the apparently independent diseases of ageing as separate in nature and thus separate in possible treatments. Current advances in gerontology have since shown that many age-related diseases do indeed share common causes and thus may be reversed or prevented through common cures. Since then the shift in scientific consensus from regarding ageing as an inexorable, entropic process to one that is mediated by well-defined - and importantly, malleable - biochemical pathways has been dramatic. Initial studies in nematodes demonstrated genetic manipulation of longevity was possible (Kenyon et al., 1993). Subsequent studies are just beginning to tease out the many genetic determinants of human longevity (Zhang et al., 2016). Briefly, ageing is thought to be caused by developmental processes that evolved to ensure successful reproductive maturation but carry on long after reproductive maturity is reached (antagonistic pleiotropy; Williams, 1957), resulting in molecular and physiological defects that render organisms frail and less able to respond to stress. These molecular and physiological defects manifest in many forms over several hierarchical layers of molecular, cellular and tissue biology. Cells within old individuals may possess aggregated proteins, dysfunctional mitochondria, (epi)genetic lesions and eroded telomeres. This can lead to senescence and subsequent depletion of active stem cell populations, impairing regenerative capacity and further promoting tissue ageing. Systemically, age-related immunodegeneration, disrupted circadian rhythms, impaired nutrient sensing and improper autocrine and paracrine signalling processes combine to promote an “aged” extracellular environment, causing or exacerbating organ failure and leading to the exponential increase in morbidity and mortality that characterises an ageing population (reviewed by López-Otín et al., 2013, Kirkwood, 2005; summarised in Fig. 1).
Fig. 1.
Mechanisms of human ageing. Ageing affects several systems acting at several hierarchical levels from molecular events such as mitochondrial dysfunction and genomic lesions, through to cellular events such as senescence or over-proliferation. Dysfunctional cells lead to tissue dysfunction, culminating in organ failure and death. Interactions between levels serve to exacerbate pathology at all levels. Black lightening symbolises damage or failure; SASP refers to the senescence-associated secretory phenotype.
Given that ageing is the biggest risk factor for many debilitating diseases that render individuals frail and rob them of their independence and dignity, there are strong moral and economic imperatives to tackle it and prevent the associated pathologies. Newly armed with an idea of how humans age, numerous companies and government-funded programmes have sprung up to address human ageing as a problem in and of itself, rather than trying to address the diseases of ageing separately. High profile examples include the (formerly Google) Alphabet-funded ageing research venture, Calico (California Life Sciences Company); the interventions testing program (ITP) run by the National Institute on Aging (NIA), designed to test the longevity-enhancing potential of a variety of different drugs; and Human Longevity Inc., co-founded by J. Craig Venter, which aims to elucidate and treat the (epi)genetic causes of age-related diseases. Furthermore, the SENS (Strategies for Engineered Negligible Senescence) Research Foundation performs its own research and helps fund the research of other institutes, and focuses on utilising combinations of regenerative medicine, gene therapy and pharmacology to reverse ageing. More recently, work showing that clearance of senescent cells improves health in old mice (Baker et al., 2016; and discussed later) prompted the creation of Unity Biotechnology. Unity is attempting to develop “senolytics” – therapeutics that recapitulate this effect in humans. Meanwhile in Oxford, UK, Chronos Therapeutics is developing pharmaceuticals to combat age-related diseases. Far from being an inscrutable, purely academic problem, both young start-ups and established companies are now seeing ageing as a tractable and potentially profitable venture.
3. Repurposing Drugs to Combat Ageing
Finding new applications for existing therapeutics (drug repurposing) is a commonly used strategy to allow drugs to reach wider markets and greater numbers of patients (Dudley et al., 2011). Repurposing drugs that have already made it to the market is an attractive strategy for many reasons. Drug development can often cost billions of dollars (Adams and Brantner, 2006) and take 10–17 years to go from early phase clinical trials to market-ready product. Drug repurposing circumvents much of the exploratory work needed to determine mechanisms of action, formulation, manufacture procedure and pharmacokinetics. Importantly, market-tested drugs have already gone through clinical trials and have proven their safety and efficacy in their initially intended application. Consequently, the development time for repurposed drugs can be markedly reduced (3–12 years).
There are two major approaches of drug repurposing: drug-centred and disease-centred (reviewed in greater detail by Dudley et al., 2011). Briefly, drug-centred repurposing relies on the physicochemical properties (e.g. structural similarity to other drugs) or biological effects (e.g. similar transcriptome response between different drugs) of the drug in question. Indirect properties, such as common side-effects between drugs indicated for different diseases can also be used. More recently, computational modelling of drug-protein interactions (molecular docking simulations) can also be used to perform high-throughput screens of possible interaction partners. Disease-centred approaches rely on using similarities between different diseases to suggest potentially repurposable drugs. Generally, if two different diseases possess similar molecular aetiologies, then therapies used to treat one may also work for the other. The two approaches are not mutually exclusive and depending on the knowledge available regarding drug and/or disease of interest, one or both approaches can be utilised.
To facilitate drug-centred repurposing approaches to target ageing, such as the NIA's ITP, geroprotectors.org provides a database of > 250 compounds found to extend lifespan in various model organisms (Moskalev et al., 2015). Of the 103 compounds listed by geroprotectors.org that are approved for use in humans, there is already considerable interest in repurposing at least two of them: the immunosuppressant rapamycin and the anti-diabetic drug, metformin.
3.1. Rapamycin and mTOR Inhibition
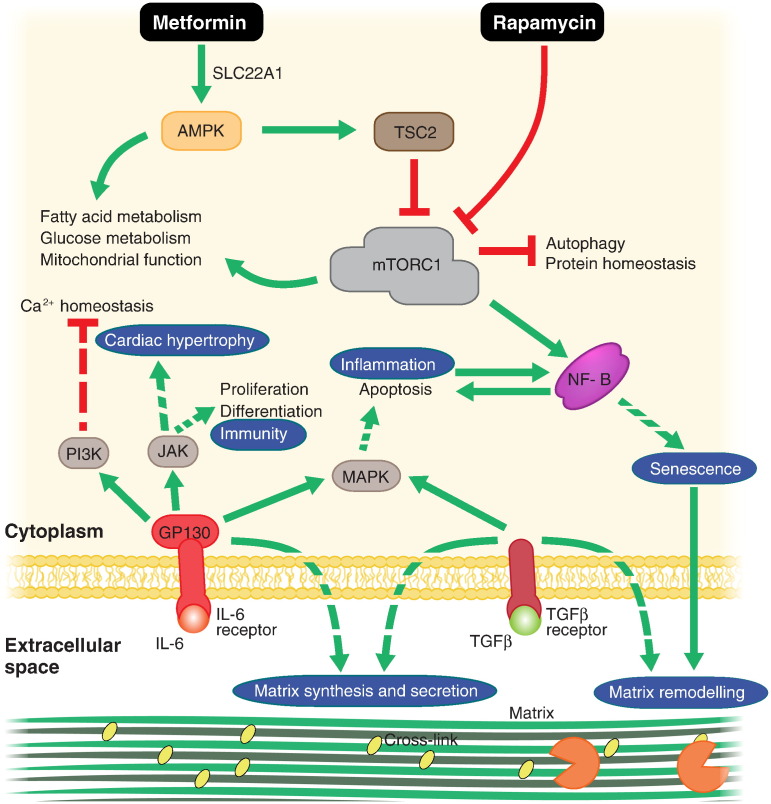
Within the gerontology literature, few compounds can claim to have received as much attention as rapamycin. Rapamycin has been shown to extend murine lifespan and healthspan (Wilkinson et al., 2012), even when administered late in life (Harrison et al., 2009). Rapamycin binds to FK506 binding protein and serves to inhibit the nutrient-sensitive kinase mTOR (mechanistic target of rapamycin) complex I (mTORC1) (Banaszynski et al., 2005). Inhibition of mTORC1 leads to pleiotropic effects that are thought to improve protein and organelle homeostasis by relieving inhibition of autophagy and proteasome function while simultaneously suppressing protein translation (Hill et al., 2010, Yu et al., 2010). mTOR also regulates mitochondrial biogenesis and lipid homeostasis through PPARγ and PGC1α (Fig. 2; reviewed by Laplante & Sabatini, 2009). Rapamycin treatment has been shown to reverse amyloid-β aggregation and improve brain vascularisation in Alzheimer's disease mouse models, as well as cognitive function in these disease models and wild-type mice (reviewed by Richardson et al., 2015). Rapamycin also prevented neuronal cell death in toxin-induced models of Parkinson's disease (Malagelada et al., 2010). Rapamycin is currently approved for clinical use as an immunosuppressant following organ transplantation. Surprisingly however, RAD001, a rapamycin analogue, was shown to improve influenza vaccination response in elderly patients (Mannick et al., 2014). Mannick et al. suggest that RAD001 may suppress chronic inflammatory phenotypes rather than general immune function, presumably by bringing mTORC1 activity back to levels observed in young individuals, although this remains to be tested. Furthermore, it is difficult to assess the relevance of any observed increase in vaccination response without comparison to young controls. Importantly, while rapamycin has been shown to extend lifespan in a variety of pre-clinical models, the precise effects in mice have been shown to be sex-specific (Garratt et al., 2016, Miller et al., 2014) and specific to particular genetic backgrounds (Lamming et al., 2013, Lamming et al., 2012). Notably, rapamycin was shown to increase mortality in diabetic mice (Sataranatarajan et al., 2016). Furthermore, a genetically heterogeneous mouse line fed high doses of rapamycin showed increased incidence of cataracts and testicular atrophy (Wilkinson et al., 2012). These studies highlight the need for greater personalisation in medicine to distinguish those that might most benefit from any give treatment. To address apparent genetic and environmental effects on rapamycin-mediated longevity extension, the Dog Aging Project (DAP; dogagingproject.com), led by researchers at the University of Washington, is currently trailing rapamycin on domesticated dogs. The logic behind DAP being that domesticated dogs possess high genetic variability due to decades of selective breeding and share the variability of environmental conditions possessed by their human owners. A significant positive effect on canine longevity and healthspan would mean that rapamycin was able to overcome any genetic or environmental variability and bodes well for potential trials on human populations.
Fig. 2.
Mechanisms of promising repurposed anti-ageing drugs. Metformin and rapamycin are thought to act synergistically to improve protein and energy homeostasis. Inhibitors of IL-6 and NF-κB could act to reverse systemic inflammation and TFGβ inhibitors have been shown to reverse aberrant signalling caused by an aged stem cell niche (of which extracellular matrix is a major component). Green arrows indicate stimulation of the indicated process. Red, flat arrows indicate inhibition. Indirect interactions are indicated by dashed arrows. Processes that have effects at the tissue or organ level are surrounded by blue circles.
3.2. TAMEing Ageing With Metformin
Metformin is an anti-diabetic drug that is thought to activate adenosine monophosphate-activated kinase (AMPK) to modulate glucose homeostasis (Shaw et al., 2005). However, other reports suggest that metformin possesses pleiotropic activity (Foretz et al., 2010) and can modulate a variety of downstream processes related to ageing (Fig. 2). Pre-clinical evidence supports metformin having a systemic anti-ageing effect when administered early in life, possibly independent of metformin's glucose-lowering effect (Anisimov et al., 2011). To capitalise on these results, metformin is now the subject of a first-of-its-kind clinical trial (funded by the American Federation of Aging Research) to see if it does indeed prevent ageing. The TAME (Targeting Ageing with Metformin) study is designed to test if metformin possesses longevity-enhancing properties independent of its anti-diabetic effects. Furthermore, the TAME study aims to establish a benchmark in clinical trial design which can be applied to test other compounds with putative anti-ageing effects (Barzilai et al., 2016). Measuring rates of ageing in human cohorts poses an interesting methodological problem. The gold standard for measuring rates of ageing in model organisms is to measure lifespans via construction of Kaplan-Meier survival curves and perform subsequent Gompertz analysis (Gompertz, 1825). However, this method cannot be applied to clinical trials involving human subjects on a reasonable time-scale. The TAME study aims to use time before appearance of next co-morbidity as a proxy for rate of ageing. Health declines exponentially with time in ageing populations, leading to the sudden drop in survival - caused by increasingly rapid appearance of co-morbidities over time - observed in survival curves assembled from ageing populations. However, this approach still requires a significant amount of time and it remains to be seen if biomarker-based approaches (discussed later) can prove quicker or more efficient.
3.3. Second Generation Repurposed Anti-ageing Compounds?
If successful, rapamycin and metformin could represent the first generation of therapeutics specifically indicated for use against ageing. Once ageing is defined as something that is treatable, other drugs that have been shown to act on some relevant ageing target could be similarly repurposed, with the knowledge gained from rapamycin and metformin informing their trial and marketing strategies. For instance, given the systemic implications of age-related immunodegeneration and chronic inflammation in ageing systems, drugs that act on the immune system show considerable promise in manipulating age-related disease pathogenesis.
Interleukin-6 (IL-6) is secreted by senescent cells and is thought to promote pleiotropic effects along with the chronic, sterile inflammation that characterises tissue ageing. Signalling through IL-6 receptor has been shown to increase with age and been associated with an increased risk of coronary heart disease (Bujak and Frangogiannis, 2007, Sarwar and Butterworth, 2012). Recently, tocilizumab, an antibody-based therapeutic that targets the IL-6 receptor to block receptor-ligand interactions passed clinical trials. Tocilizumab is currently used to treat severe rheumatoid and juvenile arthritis, but drugs like tocilizumab could show considerable promise in preventing cardiac ageing. However, it should be noted that tocilizumab is associated with several side-effects, such as increased urinary tract infections and flu-like symptoms, which may negate its use in the elderly at the doses administered for its current indications.
Nuclear factor κB (NF-κB) is a pro-inflammatory transcription factor that is upregulated with age and is also thought to promote the age-related increase in systemic inflammation (Adler et al., 2007). Inhibition of NF-κB has previously been shown to reverse cardiac hypertrophy (a common age-related complication) by modulating extracellular matrix (ECM) composition (Kumar et al., 2011). Notably, knockout of inhibitory NF-κB subunits in mice resulted in a progeroid phenotype that could be inhibited with anti-inflammatory drugs (Jurk et al., 2014). Many NF-κB inhibitors exist that act at several levels of the canonical or non-canonical NF-κB pathways with varying degrees of specificity (Gilmore and Herscovitch, 2006). Aspirin has been shown to non-specifically inhibit NF-κB (Kopp and Ghosh, 1994), as has metformin, possibly contributing to its apparent anti-ageing effects (Moiseeva et al., 2013). Several other drugs that inhibit NF-κB are currently approved for clinical use, though as with aspirin and metformin, they are thought to act indirectly (Miller et al., 2010). Drugs that directly inhibit NF-κB, such as PBS-1086 (Fabre et al., 2012) and IT-901 (Shono et al., 2016) have shown promising pre-clinical results in cancer studies but have not yet been approved for clinical use.
Aside from manipulating the immune system, recent studies using heterochronic parabiosis (surgical connection of the circulatory systems of two mice of different ages) has implicated several members of the transforming growth factor β (TGFβ) family in age-related disease (reviewed by Conboy et al., 2015). TGFβ family members are involved in regulation of a variety of developmental and regenerative processes in many tissues. TGFβ is primarily thought to regulate stem cell function through altering niche composition by promotion of ECM synthesis and secretion (Fisher et al., 2016, Trappmann et al., 2012). ECM composition has been shown to change with age in many tissues and can dictate stem cell regenerative capacity (Sun et al., 2011). Pharmacological inhibition of TGFβ signalling using 2-(3-(6-Methylpyridin- 2-yl)-1H-pyrazol-4-yl)-1,5-naphthyridine to inhibit the kinase activity of TGFβ receptor type-I (TGFBR1) reversed the deleterious effects of ageing on neurogenesis and skeletal muscle mass (Yousef et al., 2015). Several TFGβ inhibitors are currently undergoing clinical trials to test their safety and efficacy on various types of cancers (see tables 1 and 2 in Buijs et al. 2012). Among these compounds is LY2157299, a TGFBR1 inhibitor currently involved in phase II clinical trials against pancreatic, liver and central nervous system cancers. If approved, LY2157299 may also be a promising drug to repurpose against other, age-related diseases.
4. New Compounds Specifically Developed to Target Ageing
While repurposing existing therapeutics is a pragmatic, commercially sound strategy, there are numerous potential anti-ageing targets that remain to be exploited. Examples include:
4.1. NAD+ Supplementation
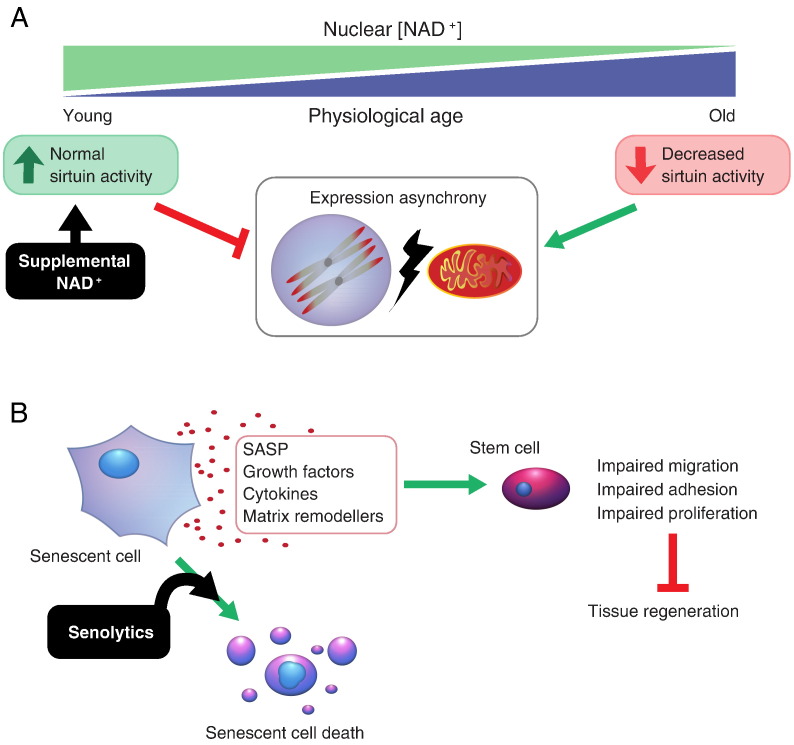
Mitochondrial dysfunction and broad metabolic perturbation are considered hallmarks of ageing (López-Otín et al., 2013). Gomes et al. (2013) showed that nicotinamide adenine dinucleotide (NAD+) levels in the nucleus decreased with age in skeletal muscle, resulting in imbalance between nuclear and mitochondrial gene expression (Fig. 3A). Furthermore, Gomes et al. went on to show that restoration of NAD+ levels could reverse many of the biochemical features of skeletal muscle ageing. While NAD+ itself is poorly absorbed, nicotinamide mononucleotide (NMN) feeding has been show to increase circulating NAD+ levels in mice eating supplemented food, leading to the prospect of NMN and another precursor, nicotinamide riboside (NR; currently available as a supplement) being marketed as anti-ageing drugs. However, while several biochemical features associated with muscle ageing were reversed by NMN feeding, it is unknown if this resulted in improved muscle function or healthspan. Furthermore, these studies have only been conducted on mice and the effects of NMN/NR/NAD+ supplementation on humans are largely untested. Additionally, NR is also found in trace amounts in milk, questioning how necessary expensive supplementation is compared to cheaper dietary adjustments.
Fig. 3.
Promising novel drugs designed to target ageing. (A) NAD+ supplementation seeks to improve nuclear-mitochondrial communication in old cells and avoid accumulation of orphaned, hydrophobic electron transport chain subunits, thus aiding protein and organelle homeostasis. (B) Senolytic drugs act to remove senescent cells from tissues and thus ablate SASP (senescence-associated secretory phenotype) mediated tissue remodelling and restore regenerative capacity by removing SASP-induced restrictions of stem cell mobility and proliferation.
4.2. Senolytics
Recently, Baker and colleagues have shown that ablation of senescent cells in old mice can extend longevity and reverse age-related decline of function in heart and kidney (Baker et al. 2016). Senescent cells accumulate in aged tissues due to DNA damage, oncogene activation, telomere erosion and mitochondrial dysfunction (Moiseeva et al., 2009, Wiley et al., 2015). Senescence of stem cell populations results in a decrease in number of stem cells able to contribute to tissue regeneration following injury or illness. Furthermore, senescent cells secrete specific growth factors, cytokines (such as IL-8 and IL-6) and matrix remodelling factors. These factors, collectively known as the senescence-associated secreted proteome (SASP) serve to remodel the surrounding environment to prevent migration and proliferation of surrounding cells. This serves two purposes: 1) to prevent cancer, and 2) to recruit macrophages to clear the senescent cells. However, due to a combination of immunodegeneration and aberrant inter-cellular signalling during ageing, senescent cells become death-resistant and can persist in their host tissues. Treatment with the small molecule, ABT-737 has been shown to selectively kill senescent cells in vivo by inhibition of the specific anti-apoptotic proteins which they depend on for survival (Yosef et al., 2016). Clearance of senescent cells may prevent further SASP-mediated remodelling (Fig. 3B) and allow matrix deposition and remodelling that increases regenerative capability (Calhoun et al., 2015). However, senescent cells are also thought to play important roles in wound healing and regeneration. For example, SASP-mediated leukocyte infiltration has been shown to mediate the potent regenerative capabilities possessed by certain salamander species (Yun et al., 2015). Furthermore, senescence of pancreatic β-cells has been shown to increase insulin secretion and improve glucose homeostasis in a mouse model of diabetes (Helman et al., 2016). A finer, potentially complementary approach may be to manipulate the SASP itself to eliminate long-term detrimental effects, such as chronic inflammation and impaired regeneration, while maintaining short-term beneficial effects (e.g. cancer prevention and wound healing; reviewed by Malaquin et al. 2016).
5. Biomarkers for Detecting Drug Efficacy Against Ageing
To accurately assess the impact of possible therapeutics on human ageing, suitable biomarkers that correlate with, and are likely causative of, increased physiological age are needed. A biomarker for ageing can be conservatively defined as “a biological parameter of an organism that either alone or in some multivariate composite will, in the absence of disease, better predict functional capacity at some late age than will chronological age” (Baker and Sprott, 1988, Martin-Ruiz and von Zglinicki, 2014). Ideal biomarkers must also be simple and uninvasive to assess (i.e. not causing lethality in model organisms or significant discomfort in humans). Molecular biomarkers of physiological age have historically been controversial; often showing little predictive power of subsequent morbidity or mortality. Notably, variation in sampling methods for the same parameter can make comparison difficult (Cooper et al., 2011). Proposed molecular biomarkers of ageing also tend to be specific to ageing in certain cell-types and not representative of the physiological age of the organism as a whole. Notably, in humans, a meta-analysis by Chen et al. (2016) has shown that age-related changes in methylation pattern in different sets of CpG dinucleotides strongly correlate with all-cause mortality across a variety of ethnic groups.
Controversially, telomere length has also been proposed as a biomarker for ageing (Der et al., 2012). Telomeres are structural caps that serve to protect the ends of chromosomes from loss of information by gradual erosion following successive replication cycles. Telomeres also prevent chromosome ends from being recognised by DNA repair machinery as double-strand breaks and “repaired”, which would lead to aneuploidy. However, recent studies have shown that telomere length is no better a predictor of age-related morbidity than chronological age (Glei et al., 2016). Telomere length is typically measured in leukocytes isolated from blood samples. Given that these cells are terminally differentiated and unlikely to divide again, their telomere length may not be indicative of regenerative capacity or ability to respond to stress. Nevertheless, telomere extending drugs are in development (Harley et al., 2011) and have received notable attention in the popular press (Kendrick, 2009). One such drug, TA-65, like NMN mentioned previously, is currently marketed as a supplement, rather than medicine, meaning that Geron - TA-65′s parent company - does not have to seek approval from the Food and Drug Administration (FDA) to sell it in the USA. It remains to be seen if this is a viable long-term strategy for a compound that purports to have a medically relevant impact on an integral aspect of cell biology.
Discerning which of the myriad of changes that occur with age are the most powerful predictors of morbidity or mortality may require processing large and complex datasets, potentially incorporating searches of genomic, transcriptomic and proteomic depositories. The continued development of new computational and machine learning methodologies will be required to countenance all the variables at play. Zhavoronkov and colleagues used neural networks trained on 60,000 patient blood samples and information obtained from routine health checks (Putin et al., 2016). They found that the ensemble of neural networks that performed best in predicting age-related outcomes were those that relied on a large combination of biomarkers to make their prediction. This result suggests that, given the complexity of ageing, it is likely that no single biomarker will have the greatest predictive power under all circumstances.
6. Conclusions
Ultimately, the most promising therapeutics for targeting ageing appear to be those that produce systemic effects at several hierarchical levels. For example, manipulation of autophagy, the unfolded protein response and mitochondrial function with rapamycin has systemic effects on cell physiology, but also has been shown to markedly affect immune function with systemic consequences for the whole organism. Furthermore, ablation of senescent cells is thought to be beneficial due to abrogation of the SASP and its detrimental downstream signalling effects that can propagate senescence through a tissue and possibly beyond. The nature of the currently most promising therapeutics highlights a fundamental aspect of human ageing – that there is likely no singular cause and that many separate causes must be addressed simultaneously to observe significant gains in maximum healthspan. Although some causes are easier to address than others, the commercial applicability of much of ageing research is still a long way off. Study design will play a major role and is a subject of intense scrutiny (discussed further by Justice et al. (2016)). Furthermore, accuracy of current ageing biomarkers is still poor and will continue to hamper therapeutic efforts until addressed. Importantly, current efforts to identify ageing biomarkers typically try to find correlations with mortality. Few attempts have been made (see Martin-Ruiz et al., 2011) to correlate them with indicators of frailty or multi-morbidity which might enhance their prognostic potential.
7. Outstanding Questions
The therapeutics mentioned in this review are a small sample of some of the most promising clinical pharmacological approaches to tackling age-related diseases. Assessing the potential systemic anti-ageing effects of therapeutics currently used to treat specific age-related diseases could provide additional repurposable drugs. However, any possible translational benefits will be contingent on greater enrolment of the elderly in clinical trials. Elderly individuals (65 or older) are typically under-represented in clinical trials. This is understandable to an extent; age represents a significant source of variability between individuals (i.e. different people age differently), even in identical twins (Brodin et al., 2015), and the presence of different co-morbidities between elderly individuals is a major confounding variable that can exponentially complicate the interpretation of trial data. The exorbitant cost of clinical trials means that companies will try to limit variability to increase the likelihood of correctly discerning statistically significant effects. Unfortunately for those involved however, this variability and resulting expense must be endured if anti-ageing therapeutics are to be trialled properly. Devising novel strategies that mitigate costs to allow greater enrolment of elderly individuals will be a considerable challenge in a nascent anti-ageing biotech industry.
In regards to research, other nascent strategies, such as stimulating IL-33 signalling to provoke clearance of amyloid-β plaques and restoration of synaptic plasticity in Alzheimer's disease (Fu et al., 2016), will continue to excite the gerontology research community and hammer home the notion that physiological ageing is not an inexorable process. Instead, like any other pathology, ageing is something tangible that can be addressed by modern medicine. Successful application of these new strategies in the clinic will significantly reduce the economic burden of our rapidly ageing population and improve both quality and quantity of life.
Author Contributions
Authors contributed equally to the preparation of the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Search Strategy and Selection Criteria
More recent studies were preferred except where specific historical reasons warranted citation of older works. Specific attention was preferably given to work that forms the basis of clinical or preclinical studies (i.e. work performed on mammalian models or humans).
Acknowledgements
JS was supported by a BBSRC David Phillips Fellowship. Funder had no role in writing the manuscript or decision to publish. The authors apologise to those colleagues whose work could not be cited due to lack of space.
Contributor Information
Venkatesh Mallikarjun, Email: venkatesh.mallikarjun@postgrad.manchester.ac.uk.
Joe Swift, Email: joe.swift@manchester.ac.uk.
References
- Adams C.P., Brantner V.V. Estimating the cost of new drug development: is it really 802 million dollars? Health Aff. 2006;25:420–428. doi: 10.1377/hlthaff.25.2.420. [DOI] [PubMed] [Google Scholar]
- Adler A.S., Sinha S., Kawahara T.L.A., Zhang J.Y., Segal E., Chang H.Y. Motif module map reveals enforcement of aging by continual NF-kappa B activity. Genes Dev. 2007;21:3244–3257. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov V.N., Berstein L.M., Popovich I.G., Zabezhinski M.A., Egormin P.A., Piskunova T.S., Semenchenko A.V., Tyndyk M.L., Yurova M.N., Kovalenko I.G., Poroshina T.E. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011;3:148–157. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D.J., Childs B.G., Durik M., Wijers M.E., Sieben C.J., Zhong J., Saltness R.A., Jeganathan K.B., Verzosa G.C., Pezeshki A., Khazaie K., Miller J.D., van Deursen J.M. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature. 2016:1–20. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker G.T., Sprott R.L. Biomarkers of aging. Exp. Gerontol. 1988;23:223–239. doi: 10.1016/0531-5565(88)90025-3. [DOI] [PubMed] [Google Scholar]
- Banaszynski L.A., Liu C.W., Wandless T.J. Characterization of the FKBP-rapamycin-FRB ternary complex. J. Am. Chem. Soc. 2005;127:4715–4721. doi: 10.1021/ja043277y. [DOI] [PubMed] [Google Scholar]
- Barzilai N., Crandall J.P., Kritchevsky S.B., Espeland M.A. Metformin as a tool to target aging. Cell Metab. 2016;23:1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin P., Jojic V., Gao T., Bhattacharya S., Angel C.J.L., Furman D., Shen-Orr S., Dekker C.L., Swan G.E., Butte A.J., Maecker H.T., Davis M.M. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160:37–47. doi: 10.1016/j.cell.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs J.T., Stayrook K.R., Guise T.A. The role of TGF-β in bone metastasis: novel therapeutic perspectives. Bonekey Rep. 2012;1:96. doi: 10.1038/bonekey.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujak M., Frangogiannis N.G. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc. Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun C., Shivshankar P., Saker M., Sloane L.B., Livi C.B., Sharp Z.D., Orihuela C.J., Adnot S., White E.S., Richardson A., Jourdan Le Saux C. Senescent cells contribute to the physiological remodeling of aged lungs. J. Gerontol. A Biol. Sci. Med. Sci. 2015;1:1–9. doi: 10.1093/gerona/glu241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.H., Marioni R.E., Colicino E., Peters M.J., Ward-Caviness C.K., Tsai P.-C., Roetker N.S., Just A.C., Demerath E.W., Guan W., Bressler J., Fornage M., Studenski S., Vandiver A.R., Moore A.Z., Tanaka T., Kiel D.P., Liang L., Vokonas P., Schwartz J., Lunetta K.L., Murabito J.M., Bandinelli S., Hernandez D.G., Melzer D., Nalls M., Pilling L.C., Price T.R., Singleton A.B., Gieger C., Holle R., Kretschmer A., Kronenberg F., Kunze S., Linseisen J., Meisinger C., Rathmann W., Waldenberger M., Visscher P.M., Shah S., Wray N.R., McRae A.F., Franco O.H., Hofman A., Uitterlinden A.G., Absher D., Assimes T., Levine M.E., Lu A.T., Tsao P.S., Hou L., Manson J.E., Carty C.L., LaCroix A.Z., Reiner A.P., Spector T.D., Feinberg A.P., Levy D., Baccarelli A., Meurs J.V., Bell J.T., Peters A., Deary I.J., Pankow J.S., Ferrucci L., Horvath S. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY) 2016;8:1–22. doi: 10.18632/aging.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy I.M., Conboy M.J., Rebo J. Systemic problems : a perspective on stem cell aging and rejuvenation. Aging (Albany NY) 2015;7:1–11. doi: 10.18632/aging.100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R., Hardy R., Aihie Sayer A., Ben-Shlomo Y., Birnie K., Cooper C., Craig L., Deary I.J., Demakakos P., Gallacher J., McNeill G., Martin R.M., Starr J.M., Steptoe A., Kuh D., HALCyon Study Team Age and gender differences in physical capability levels from mid-life onwards: the harmonisation and meta-analysis of data from eight UK cohort studies. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der G., Batty G.D., Benzeval M., Deary I.J., Green M.J., McGlynn L., McIntyre A., Robertson T., Shiels P.G. Is telomere length a biomarker for aging: cross-sectional evidence from the west of Scotland? PLoS One. 2012;7 doi: 10.1371/journal.pone.0045166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J.T., Deshpande T., Butte A.J. Exploiting drug-disease relationships for computational drug repositioning. Brief. Bioinform. 2011;12:303–311. doi: 10.1093/bib/bbr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre C., Mimura N., Bobb K., Kong S.-Y., Gorgun G., Cirstea D., Hu Y., Minami J., Ohguchi H., Zhang J., Meshulam J., Carrasco R.D., Tai Y.-T., Richardson P.G., Hideshima T., Anderson K.C. Dual inhibition of canonical and noncanonical NF-B pathways demonstrates significant antitumor activities in multiple myeloma. Clin. Cancer Res. 2012;18:4669–4681. doi: 10.1158/1078-0432.CCR-12-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faragher R.G.A., Sheerin A.N., Ostler E.L. Can we intervene in human ageing? Expert Rev. Mol. Med. 2009;11 doi: 10.1017/S1462399409001197. [DOI] [PubMed] [Google Scholar]
- Fisher G.J., Shao Y., He T., Qin Z., Perry D., Voorhees J.J., Quan T. Reduction of fibroblast size/mechanical force down-regulates TGF-β type II receptor: implications for human skin aging. Aging Cell. 2016;15:67–76. doi: 10.1111/acel.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M., Hébrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G., Sakamoto K., Andreelli F., Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu A.K.Y., Hung K.-W., Yuen M.Y.F., Zhou X., Mak D.S.Y., Chan I.C.W., Cheung T.H., Zhang B., Fu W.-Y., Liew F.Y., Ip N.Y. IL-33 ameliorates Alzheimer's disease-like pathology and cognitive decline. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E2705–E2713. doi: 10.1073/pnas.1604032113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt M., Nakagawa S., Simons M.J.P. Comparative idiosyncrasies in life extension by reduced mTOR signalling and its distinctiveness from dietary restriction. Aging Cell. 2016;15:737–743. doi: 10.1111/acel.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore T.D., Herscovitch M. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene. 2006;25:6887–6899. doi: 10.1038/sj.onc.1209982. [DOI] [PubMed] [Google Scholar]
- Glei D.A., Goldman N., Risques R.A., Rehkopf D.H., Dow W.H., Rosero-Bixby L., Weinstein M. Predicting survival from telomere length versus conventional predictors: a multinational population-based cohort study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A.P., Price N.L., Ling A.J.Y., Moslehi J.J., Montgomery M.K., Rajman L., White J.P., Teodoro J.S., Wrann C.D., Hubbard B.P., Mercken E.M., Palmeira C.M., de Cabo R., Rolo A.P., Turner N., Bell E.L., Sinclair D.A. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompertz B. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Philos. Trans. R. Soc. Lond. 1825;115:513–583. doi: 10.1098/rstb.2014.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C.B., Liu W., Blasco M., Vera E., Andrews W.H., Briggs L.A., Raffaele J.M. A natural product telomerase activator as part of a health maintenance program. Rejuvenation Res. 2011;14:45–56. doi: 10.1089/rej.2010.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., Nadon N.L., Wilkinson J.E., Frenkel K., Carter C.S., Pahor M., Javors M.A., Fernandez E., Miller R.A. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman A., Klochendler A., Azazmeh N., Gabai Y., Horwitz E., Anzi S., Swisa A., Condiotti R., Granit R.Z., Nevo Y., Fixler Y., Shreibman D., Zamir A., Tornovsky-Babeay S., Dai C., Glaser B., Powers A.C., Shapiro A.M.J., Magnuson M.A., Dor Y., Ben-Porath I. p16(Ink4a)-induced senescence of pancreatic beta cells enhances insulin secretion. Nat. Med. 2016;22:412–420. doi: 10.1038/nm.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C., Carolina N., Qian S.-B., Zhang X., Sun J., Bennink J.R., Yewdell J.W., Patterson C. mTORC1 links protein quality and quantity control by sensing chaperone availability. J. Biol. Chem. 2010;285:27385–27395. doi: 10.1074/jbc.M110.120295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurk D., Wilson C., Passos J.F., Oakley F., Correia-Melo C., Greaves L., Saretzki G., Fox C., Lawless C., Anderson R., Hewitt G., Pender S.L., Fullard N., Nelson G., Mann J., van de Sluis B., Mann D.A., von Zglinicki T. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2014;2:4172. doi: 10.1038/ncomms5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice J., Miller J.D., Newman J.C., Hashmi S.K., Halter J., Austad S.N., Barzilai N., Kirkland J.L. Frameworks for proof-of-concept clinical trials of interventions that target fundamental aging processes. J. Gerontol. A Biol. Sci. Med. Sci. 2016;00:1–9. doi: 10.1093/gerona/glw126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick M. Anti-Aging Pill Targets Telomeres at the Ends of Chromosomes [WWW Document] Sci. Am. 2009 URL scientificamerican.com/article/anti-aging-pill-targets-telomeres. (accessed 7.12.16) [Google Scholar]
- Kenessary A., Zhumadilov Z., Nurgozhin T., Kipling D., Yeoman M., Cox L., Ostler E., Faragher R. Biomarkers, interventions and healthy ageing. New Biotechnol. 2013;30:373–377. doi: 10.1016/j.nbt.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Kennedy B.K., Pennypacker J.K. Drugs that modulate aging: the promising yet difficult path ahead. Transl. Res. 2014;163:456–465. doi: 10.1016/j.trsl.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C., Chang J., Gensch E., Rudner A., Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood T.B.L. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Kopp E., Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- Kumar S., Seqqat R., Chigurupati S., Kumar R., Baker K.M., Young D., Sen S., Gupta S. Inhibition of nuclear factor kappa B regresses cardiac hypertrophy by modulating the expression of extracellular matrix and adhesion molecules. Free Radic. Biol. Med. 2011;50:206–215. doi: 10.1016/j.freeradbiomed.2010.10.711. [DOI] [PubMed] [Google Scholar]
- Lamming D.W., Ye L., Astle C.M., Baur J.A., Sabatini D.M., Harrison D.E. Young and old genetically heterogeneous HET3 mice on a rapamycin diet are glucose intolerant but insulin sensitive. Aging Cell. 2013;12:712–718. doi: 10.1111/acel.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming D.W., Ye L., Katajisto P., Goncalves M.D., Saitoh M., Stevens D.M., Davis J.G., Salmon A.B., Richardson A., Ahima R.S., Guertin D.A., Sabatini D.M., Baur J.A. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M., Sabatini D.M. mTOR signaling at a glance. J. Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. 10.1242/jcs.051011 doi:122/20/3589 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagelada C., Jin Z.H., Jackson-Lewis V., Przedborski S., Greene L.A. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson's disease. J. Neurosci. 2010;30:1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaquin N., Martinez A., Rodier F. Keeping the senescence secretome under control: molecular reins on the senescence-associated secretory phenotype. Exp. Gerontol. 2016;82:39–49. doi: 10.1016/j.exger.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Mannick J.B., Del Giudice G., Lattanzi M., Valiante N.M., Praestgaard J., Huang B., Lonetto M.A., Maecker H.T., Kovarik J., Carson S., Glass D.J., Klickstein L.B. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3009892. 10.1126/scitranslmed.3009892 268ra179–268ra179. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C., Jagger C., Kingston A., Collerton J., Catt M., Davies K., Dunn M., Hilkens C., Keavney B., Pearce S.H.S., den Elzen W.P.J., Talbot D., Wiley L., Bond J., Mathers J.C., Eccles M.P., Robinson L., James O., Kirkwood T.B.L., von Zglinicki T. Assessment of a large panel of candidate biomarkers of ageing in the Newcastle 85 + study. Mech. Ageing Dev. 2011;132:496–502. doi: 10.1016/j.mad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C., von Zglinicki T. Biomarkers of healthy ageing: expectations and validation. Proc. Nutr. Soc. 2014;73:422–429. doi: 10.1017/S0029665114000147. [DOI] [PubMed] [Google Scholar]
- Miller R.A., Harrison D.E., Astle C.M., Fernandez E., Flurkey K., Han M., Javors M.A., Li X., Nadon N.L., Nelson J.F., Pletcher S., Salmon A.B., Sharp Z.D., Van Roekel S., Winkleman L., Strong R. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.C., Huang R., Sakamuru S., Shukla S.J., Attene-Ramos M.S., Shinn P., Van Leer D., Leister W., Austin C.P., Xia M. Identification of known drugs that act as inhibitors of NF-κB signaling and their mechanism of action. Biochem. Pharmacol. 2010;79:1272–1280. doi: 10.1016/j.bcp.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva O., Bourdeau V., Roux A., Deschênes-Simard X., Ferbeyre G. Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol. Cell. Biol. 2009;29:4495–4507. doi: 10.1128/MCB.01868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva O., Deschênes-Simard X., St-Germain E., Igelmann S., Huot G., Cadar A.E., Bourdeau V., Pollak M.N., Ferbeyre G. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell. 2013;12:489–498. doi: 10.1111/acel.12075. [DOI] [PubMed] [Google Scholar]
- Moskalev A., Chernyagina E., de Magalhaes J.P., Barardo D., Thoppil H., Shaposhnikov M., Budovsky A., Fraifeld V.E., Garazha A., Tsvetkov V., Bronovitsky E., Bogomolov V., Scerbacov A., Kuryan O., Gurinovich R., Jellen L.C., Kennedy B., Mamoshina P., Dobrovolskaya E., Aliper A., Kaminsky D., Zhavoronkov A. Geroprotectors.org: a new, structured and curated database of current therapeutic interventions in aging and age-related disease. Aging (Albany NY) 2015;7:616–628. doi: 10.18632/aging.100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Doll R. There is no such thing as aging. BMJ. 1997;315:1030–1032. doi: 10.1136/bmj.315.7115.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putin E., Mamoshina P., Aliper A., Korzinkin M., Moskalev A., Kolosov A., Ostrovskiy A., Cantor C., Vijg J., Zhavoronkov A. Deep biomarkers of human aging: application of deep neural networks to biomarker development. Impact Aging. 2016;8:1–13. doi: 10.18632/aging.100968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A., Galvan V., Lin A.-L., Oddo S. How longevity research can lead to therapies for Alzheimer's disease: the rapamycin story. Exp. Gerontol. 2015;68:51–58. doi: 10.1016/j.exger.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar N., Butterworth A.S. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sataranatarajan K., Ikeno Y., Bokov A., Feliers D., Yalamanchili H., Lee H.J., Mariappan M.M., Tabatabai-Mir H., Diaz V., Prasad S., Javors M.A., Ghosh Choudhury G., Hubbard G.B., Barnes J.L., Richardson A., Kasinath B.S. Rapamycin increases mortality in db/db mice, a mouse model of type 2 diabetes. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:850–857. doi: 10.1093/gerona/glv170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R.J., Lamia K.A., Vasquez D., Koo S., Depinho R.A., Montminy M., Cantley L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. 10.1126/science.1120781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shono Y., Tuckett A.Z., Liou H.-C., Doubrovina E., Derenzini E., Ouk S., Tsai J.J., Smith O.M., Levy E.R., Kreines F.M., Ziegler C.G.K., Scallion M.I., Doubrovin M., Heller G., Younes A., O'Reilly R.J., van den Brink M.R.M., Zakrzewski J.L. Characterization of a c-Rel inhibitor that mediates anticancer properties in hematologic malignancies by blocking NF-κB-controlled oxidative stress responses. Cancer Res. 2016;76:377–389. doi: 10.1158/0008-5472.CAN-14-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Li W., Lu Z., Chen R., Ling J., Ran Q., Jilka R.L., Chen X.-D. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J. 2011;25:1474–1485. doi: 10.1096/fj.10-161497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappmann B., Gautrot J.E., Connelly J.T., Strange D.G.T., Li Y., Oyen M.L., Cohen Stuart M.A., Boehm H., Li B., Vogel V., Spatz J.P., Watt F.M., Huck W.T.S. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012;11:742. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- Vaiserman A.M., Lushchak O.V., Koliada A.K. Anti-aging pharmacology: promises and pitfalls. Ageing Res. Rev. 2016 doi: 10.1016/j.arr.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Vaiserman A.M., Marotta F. Longevity-promoting pharmaceuticals: is it a time for implementation? Trends Pharmacol. Sci. 2016;37:331–333. doi: 10.1016/j.tips.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Wiley C.D., Velarde M.C., Lecot P., Liu S., Sarnoski E.A., Freund A., Shirakawa K., Lim H.W., Davis S.S., Ramanathan A., Gerencser A.A., Verdin E., Campisi J. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 2015;23:303–314. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J.E., Burmeister L., Brooks S.V., Chan C.C., Friedline S., Harrison D.E., Hejtmancik J.F., Nadon N., Strong R., Wood L.K., Woodward M.A., Miller R.A. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G.C. Pleiotropy, Natural Selection, and the Evolution of Senescence. Evolution. 1957;11:398. 10.2307/2406060 N. Y. [Google Scholar]
- Yosef R., Pilpel N., Tokarsky-Amiel R., Biran A., Ovadya Y., Cohen S., Vadai E., Dassa L., Shahar E., Condiotti R., Ben-Porath I., Krizhanovsky V. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016;7:11190. doi: 10.1038/ncomms11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef H., Conboy M.J., Morgenthaler A., Bugaj L., Paliwal P., Greer C., Conboy I.M. Systemic attenuation of the TGF-β pathway by a single drug simultaneously rejuvenates hippocampal neurogenesis and myogenesis in the same old mammal. Oncotarget. 2015;6:11959–11978. doi: 10.18632/oncotarget.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., McPhee C.K., Zheng L., Mardones G.A., Rong Y., Peng J., Mi N., Zhao Y., Liu Z., Wan F., Hailey D.W., Oorschot V., Klumperman J., Baehrecke E.H., Lenardo M.J. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun M.H., Davaapil H., Brockes J.P. Recurrent turnover of senescent cells during regeneration of a complex structure. Elife. 2015;4:1–16. doi: 10.7554/eLife.05505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Nogales-Cadenas R., Lin J.-R., Zhang W., Cai Y., Vijg J., Zhang Z.D. Systems-level analysis of human aging genes shed new light on mechanisms of aging. Hum. Mol. Genet. 2016 doi: 10.1093/hmg/ddw145. 10.1093/hmg/ddw145 ddw145–ddw145. [DOI] [PMC free article] [PubMed] [Google Scholar]