Summary
Drug development for psychiatric disorders has virtually ground to a halt. Some recent drugs are better tolerated and others safer than earlier ones, but they are no more effective. Years of failure in preventing or delaying onset of illness, in ameliorating symptoms, in lowering suicide rates, in improving quality of life, have put at risk the commercial investments that have, in the past, funded the various stages of drug development. To promote the development of drugs with greater efficacy, psychiatry needs to improve the way it brings potentially helpful drugs to market. Psychiatry needs to learn from other fields that human beings differ in their response to drugs. Psychiatric drug research needs to be grounded in a better understanding of molecular brain mechanisms, neural circuits, and their relationships to clinical diseases. Using this understanding, drugs need to be more precisely directed at specific brain targets. In psychiatric drug development, government, industry, regulators and academia must realign to meet medicine’s responsibilities and use science in the best interests of patients.
Keywords: Psychotropic Drugs, Clinical Drug Trials, Alzheimer Drugs, Pharmaceutical Industry, Alzheimer’s disease, Depression, Schizophrenia, Anxiety
Why are psychiatric drugs developed 70 years ago are just as effective as recently developed drugs in the battle against mental illness? How is it that just as many people today as 70 years ago get depressed, take their lives, fail to improve? How come we can recognize mental illness earlier than we ever did, but cannot prevent its progression? Are our theories and models inadequate? Are our research methods flawed? Are we off base in our classification of psychiatric disease? Is this the fault of basic scientists, of clinical trialists, of the pharmaceutical industry? Is drug discovery not a scientific process after all, but solely a matter of luck? In this paper we provide critical analyses of the current state of psychiatric drug developments and, using the results from these analyses, indicate the range of moves forward that are required for improvements to take place (Table 1).
Table 1.
Current state of neuropsychiatric drug developments
| Issues in Drug Development | Indicated Methodological or Other Changes | Potential Advantages for Drug Development |
|---|---|---|
| Plethora of ME-TOO new drugs fail to advance efficacy. | Clinical trials evaluating all new drugs against a current standard of care. | Improved focus on efficacy advancement and identification of more clinically consequential targets for drugs. |
| Pharmacogenetically non-homogeneous subject groups in clinical trials due to mixed mechanisms associated with a clinical disorder. | For research purposes, ground diagnoses in the mechanistic sources of clinical disorders. | Specific clinical guidance for selection of patients to treat. |
| Pharmacogenetically non-homogeneous subject groups in clinical trials due to subject differences in drug pharmacokinetics. | Screen proposed clinical trial research subjects to insure that optimal drug concentrations will be reached at targets for each subject. | Specific clinical guidance for selection of patients to treat. |
| Lack of adequate knowledge of disease mechanisms to advance efficacy using mechanistic-predictive approaches to drug development | Incorporate the testing of the mechanism of drug efficacy in each clinical trial. | Both mechanisms relevant to and unrelated to clinically desired benefits can be identified and inform subsequent development. |
| Failures of preclinical studies, especially animal models, to advance efficacy. | Reposition preclinical models in more specific relations to their homologous mechanisms in humans | Refinement of preclinical studies as models predictive of drug effects in humans. |
| Academic bias from conflicts of interest in commercialization of new drugs. | Specific increased funding for academic clinical pharmacology. Prohibitions against academics, medical school departments, or medical schools receiving other than unrestricted gifts from commercial entities. | Academia can be positioned as a critic of commercial methods of drug development and as an innovator of new methods and compound candidate drugs. |
| Failures to advance neuroscience knowledge by using clinical trials as human experiments able to test mechanisms of disease and therapy | Commitment of clinical neuroscience to develop a multi-science based Standard Model of the Brain and to test it in all research. | A self sustaining and self correcting scientific model for new drug development |
Search strategy and selection criteria
The data for this paper were obtained from publications cited in the references, and on references within these publications, and on updates of these data, from the initial publication to the present, using searches on Medline and Google Scholar for each of the main topics of this paper: history of psychiatric drug development; development of drugs for Alzheimer’s disease; neuropathological mechanisms of psychiatric disorders and of Alzheimer’s disease; sources of medical errors and errors in neuropsychiatric drug developments; pharmaceutical industry policies towards psychiatric drug development; and variations in clinical pharmacology science and practices across the field of medicine. Because of the breadth of this critical review, only representative articles were sought and each search was concluded when further references contributed no further knowledge.
The advent of modern neuropsychiatric drug developments
Modern psychiatric neuropharmacology began with mid-20th century serendipitous observations. The efficacy of lithium for mania was discovered when guinea pigs became tranquil. Chlorpromazine, an adjunct to anesthesia, improved manic and psychotic symptoms in patients. Iproniazid, an anti-TB drug, improved mood. Meprobamate, a preservative for penicillin, showed tranquilizing properties.1
Rather than relying on chance and serendipity, modern drug development, as illustrated by Alzheimer’s disease (AD), has pursued an understanding of the molecular basis of the targeted disease. Following the discovery in 1987 of cholinergic cell loss in the Nucleus Basalis of Meynart (NBM) in the brains of AD patients, the deficiency of the neurotransmitter acetylcholine (ACh) suggested a range of provided drug targets: enhanced ACh synthesis and decreased ACh metabolism.2 Acetylchoinesterase inhibitors (AChEIs) were then shown to improve cognition in AD patients and commercialized. This approach, based on scientific understanding, was easier in AD than it is in most psychiatric conditions because no specific brain pathology associates with schizophrenic, anxiety, manic or depressive symptoms in the same way that NBM lesions associate with cognitive impairments in AD. The approach requires a theoretical understanding of the underlying problem explored in preclinical tests of candidate drugs and then clinical trials for safety and efficacy. This method, successful for AD, hasn’t worked for psychiatry.
Following the commercialization of ACHEIs and of the partial antagonist of glutamate at NMDA receptors, memantine, therapeutic attempts to stop or slow the progress of Mild Cognitive Impairment (MCI) to full blown AD failed.3,4 The initial hope had been that these drugs would both prevent the progression from MCI into AD, and also modify the course of clinically diagnosed AD. Similar to drug outcomes for other psychiatric conditions, safety, tolerability, and symptomatic relief were present, but not efficacy against the progression of disease.
Without efficacy against disease progression, AD research turned to characterizing the amyloid formations and neurofibrillary tangles described by Alois Alzheimer in 1906.5 This led to the current theoretical formulation that the toxic peptide Aβ42 begins to aggregate within brain as amyloid plaques up to 30 years before AD becomes clinically apparent.6,7 The accumulation of amyloid is followed 10 to 15 years later by the appearance of hyperphosphorylated tau (p-tau).8–10 Whether p-tau is induced by Aβ42 or from an independent mechanism is not known. This neuropathological cascade is followed in some but not all patients by clinical AD.11,12 The association of the ACh deficit with cognitive impairment was clear. The roles of Aβ, neuroinflammation, and other factors in clinical AD and other brain diseases are nebulous. This may explain why AD drugs aimed at these targets have failed in more than 200 clinical trials. First investigators can offer no confident predictions of success since current knowledge of AD pathogenesis provides no critical target that can be addressed by a drug. Second, clinical trials cannot advance the science of AD because failures due to drugs and targets cannot be distinguished.13 Clinical trials have remained exercises in trial and error dependent on chance and serendipity.14 Without firm understandings of underlying pathology, investigators take “a shot on goal” without knowledge of the precise goal!15 Dependent upon trial and error, psychiatry hopes that serendipity will provide a way forward.
Psychiatry’s chronic diseases pose problems of timing for drug interventions. The current conception of AD assumes that pathology evolves over time and can be stopped at various stages during its progression.16 In AD, drugs capable of reducing Aβ42 brain accumulations may not have been administered early enough in the disease course.13,17 Currently research into pathophysiology of AD does not indicate how early or when in the progressive cascade of pathology an intervention is needed.18 The present strategy, to target asymptomatic patients with risk factors and monitor for disease progression, in schizophrenia has not sat well with ethics committees. 16,19,20 Preventive intervention clinical trials, difficult to justify in asymptomatic middle age and elderly with AD risk factors, present even greater problems in younger, asymptomatic, at risk persons. Furthermore, brain targets for most psychiatric disorders have not been as robustly associated with their diseases, as have amyloid and p-tau neurofibrillary tangles with AD. Some psychiatric disorders have been hypothesized to start very early, including in utero.21
The presence of early pathology presents ‘Catch-22’ problems for investigators.18. First investigators lack knowledge of disease mechanisms that would lend importance to drug-induced alterations in pathophysiology potentially able to control disease progression. Important drugs may be unappreciated even though they counter important pathological changes. Second, regulatory policies require demonstrations of clinical benefits, which will not be available prior to the emergence of symptoms. How can clinical benefits be demonstrated if the patient-to-be is not yet sick? For AD and psychiatry, a successful shot on a well-characterized goal may not score in a way that is acceptable under current regulatory policies. Third, drugs effective against pathologies in clinically ill patients may exhibit adverse effects that would make difficult or even exclude their use in early preventive interventions in at risk patients. Psychiatry’s drug development faces new challenges in the 21st century.
In psychiatry, the efficacy shown by lithium, chlorpromazine, iproniazid and meprobamate led to these drugs being intensively studied to uncover relevant brain targets. Many were found, but this did not, in the longer run, lead to better products.22–24 In AD, the identification of possibly relevant brain targets responsible for disease progression has yet to lead to a drug that prevents progression. Animal models with relatively good predictive properties were developed for the various psychiatric diseases and for AD pathologies, but animals provide imperfect models for human diseases.25 Using these models and other resources, after initial successes in a disease, the only new neuropsychiatric drugs that have emerged over the last 70 years have been Me-Too drugs (Figure 1).26–27 In the absence of alternatives, preclinical drug development strategies have tried to identify new drug candidates based on chemical structural similarity to currently used drugs. This strategy has not worked very well. To reduce psychiatry’s dependence on serendipity and iterations of Me-Too drugs, it has been proposed that psychiatry needs detailed knowledge of the development and progression of pathogenesis of disorders and integration of discovery and translational phases of neuroscience. 28,29 To further psychiatry’s theoretical understanding, as human experiments clinical trials will usefully be re-designed and used to inform basic research.
Figure 1.
Current psychiatric drug development appears to be ‘stuck in a rut’ with ‘me too’ drugs – leading to a famine of new innovative agents reaching patients.
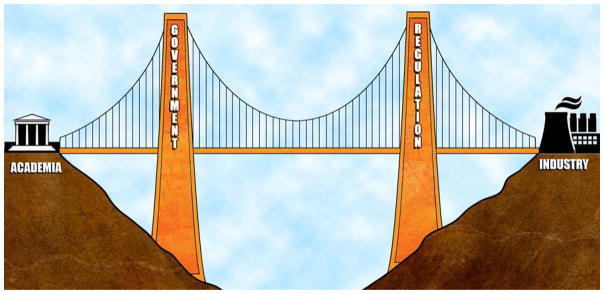
Industry has, to a large extent, withdrawn support for psychiatric drug discovery.30 To avoid a crisis created by loss of industry investment, international change is needed in the environment of drug development. It would be in the best interests of all for regulators, academics, funding agencies, and industry to collaborate to ensure the probity, scientific rigour, affordability, profitability, and patient benefits from neuropsychiatric drug developments. The European Union Innovative Medicines Initiative (EU-IMI) in neuropsychiatry offers a possible model to reposition current resources (Figure 2).31
Figure 2.
A new model separates academia and industry to function as two independent but collaborative sectors to control conflicts of interest. Governmental funding agencies create an environment that bridges these resources
How can we currently advance the efficacy of psychiatric drugs?
Psychiatry faces daunting problems. No single intervention appears capable of improving the efficacy of neuropsychiatric drugs. Evidence implicates errors in development, pharmaceutical industry resistance to methodological innovations, the inability to understand the reasons for drug efficacy and to build on them, compromises to evidence-based medicine, and so forth. Each easily undermines any systematic and stepwise attempts to place clinical drug developments in AD and psychiatry on sound scientific foundations. In combinations their cumulative effects may account for some of psychiatry’s current problems.
Errors
Mistakes can derail the development of potentially groundbreaking drugs. An extensive literature has raised concerns over the last several decades about human errors that continue to plague both basic research and clinical phases of drug developments.15,17,32–34 While no drug failures have been specifically ascribed to errors in design or methodology, numerous failures to replicate earlier studies have been related to errors that were disregarded by sponsors when deciding whether or not to advance drugs into later stages.32,34 Since drugs are regularly abandoned for commercial development after a failure, we cannot know whether some drugs have failed due only to human errors. One step towards more effective drug development in psychiatry and AD would be to recognize the inevitability of human error and adopt new technologies and practices to identify errors early and prevent them from influencing subsequent development.32
Pharmaceutical industry resistance to methodological innovations
One major problem in neuropsychiatric drug development is the imbalanced domination of the pharmaceutical industry in all clinical phases of development. Industry lobbying has historically delayed the introduction of methodological rigor, such as safety requirements and routine comparisons against drugs of proven efficacy.35,36 Pharma dominates decisions as to which drug avenues are pursued, the methods used, and how much time and money will be invested in each drug.
Academic and industry investigators who seek to rationalize drug discovery face important limits in our understanding of genomic analyses and the roles of mutations in disease. In medicine generally, as in psychiatric genetics, complex arrays of inherited and mutated genes associate with diseases. Medicine is struggling to target genetic sources for chronic diseases and arrest disease progression. Psychiatry’s experiences with targeting gamma-secretase, where presenilin 1 genetic abnormalities associate with AD, may have failed by interfering with normal functions carried out by the enzyme.37 Daunting challenges like this face investigators sorting through the over 100 gene loci identified in schizophrenia: the role of mutations, the functional importance of these loci and mutations in the general population compared with those affected by disease, and the timing of gene expressions and mutations in relation to clinical disease.38 Each of these factors affects priorities for drug development. Funding more balanced between basic research into the pathogenesis of diseases and attempts at drug development may be more effective.
In our view, the National Institutes of Health, EU-IMI, and other national regulatory bodies can possibly be repositioned to function as mediators among academia, patient care, and for-profit sectors (Figure 2). There is inherent conflict between medicine’s commitment to the best interests of patients and industry’s responsibilities to its investors. For psychiatric drug development to go forward a more functional and mutually beneficial balance of these competing interests may be needed. As an example, industry and psychiatry could together address the astonishingly high placebo response in clinical trials of psychotropic drugs.39 In pursing collaborations such as this, industry may face issues difficult to resolve. For example, because of the need for large numbers of subjects, in some research areas contract research organizations recruit trial participants. One can be concerned that over time, less severely ill subjects have come to dominate in subject pools. For this or other reasons the placebo rate may be sustained making true drug responses difficult to distinguish.40 Solving psychiatry’s problems may first involve understanding psychiatry’s problems in conjunction with all stakeholders.
Funding may be more effectively used after revisions in current practices. It is our impression that, were academia, industry and practitioners to address the errors that have led to failures of psychiatric research, drugs and patient care, the funds wasted could become available to basic research studies designed to be replicated to more soundly ground successful stepwise clinical drug developments. We are concerned with wasted funding when more than 75% of published basic research cannot be replicated in other laboratories,41,42 clinical trials are destined to fail by reusing methods already shown as flawed,13,32 and patients suffer morbidity and even die from medical errors.43 Clinical trials fail by proceeding from seriously flawed earlier phases into later phases,18 by proceeding into a clinical trial when the preliminary research used to originally justify the trial has already been shown to not be replicated in other than the original laboratory,44 and so forth.
When predicting from neuroscience and riffs on chemical structure fail
In psychiatry knowledge of neuropathology and predicting improved drugs from the pharmacological properties of earlier partially successful drugs have both failed. The story of clozapine is instructive.45–48 Synthesized in 1959 as a take off on the structure of imipramine, clozapine was expected to be antidepressant. When it demonstrated sedative properties in animals, it was compared to placebo in the treatment of psychosis and found effective. Since all antipsychotics led to extrapyramidal side-effects and clozapine did not, so it was considered weak. In a 1974 clinical trial in Finland there were 16 cases of agranulocytosis, eight of which proved fatal. The company shut down further trials and provided drug for compassionate use in trial patients who were doing well. In fact, they were doing so well, that investigators in the U.S. demanded a fresh trial of the drug. To provide a rigorous test of efficacy it was tested in patients who had not responded to other antipsychotic drugs. Clozapine passed the test with flying colours. Since then, the clozapine molecule has been intensely studied to see what makes it work in non-responders to other drugs. There are many clues, but no real answers. 49 The ‘atypical’ antipsychotics, built to emulate the structure of clozapine, do not appear to be any more effective than first generation antipsychotics.50
Hype replaces evidence
The lack of superior efficacy of the ‘atypical’ antipsychotics and their serious metabolic and cardiovascular side effects did not stop the fanfare that accompanied the emergence of each successive drug.51,52 The same occurs with each new antidepressant. For example, the recent release of vortioxetine was lauded for its superior action at 5-HT receptors, a claim the FDA did not allow.53,54 Here academics suggest clinically relevant advances when only biochemical, not clinically relevant, differences, have been evidenced. Both pharmaceutical companies and academics ‘spin’ the results of clinical trials. For example, after acknowledging that, in Phase 2 trials, vortioxetine showed no efficacy advantage over active comparators, an academic commentator concluded that it still could be a valuable antidepressant since “patients enrolled in double-blind, placebo-controlled trials are not representative of patients seen and treated in clinical practice.”54
Bias
Bias can lead to human error. Psychiatry may be particularly vulnerable to external biases due to the almost unique status of psychiatry’s dependence on clinical trial funding from pharmaceutical companies to support faculty and staff. There are good reasons to believe that psychiatrists’ human judgments have had to overcome biases introduced by the dependence on pharmaceutical funding as the financial support from NIH and foundations decreased.55 It is common to see long lists of potential conflicts of interest following papers and oral presentations.55 Neuropsychologists have reported that such recently used memories bias judgments.56 Conflicts of interest in assessments of head injury outcomes in football, in the effects of smoking on health, in the efforts to control antibiotic uses to arrest emerged resistance, in drug developments, and elsewhere in medicine illustrate that “it is difficult to get a man to understand something, when his salary depends on his not understanding it.”57
Rules and regulations can interfere with drug development
The Kefauver-Harris Drug Amendments to the Federal Food, Drug and Cosmetic Act resulted in FDA requirements that efficacy be demonstrated in clinical trials.36 These trials, however, almost always compare drug candidates against placebo and not against already effective drugs. When the CATIE58 (for antipsychotics) and STAR*D59 (for antidepressants) adopted new methodologies and compared newer drugs to older ones, they found no efficacy advantage for the newer drugs. This lack of advance has been widely acknowledged in psychiatry despite attempts by some commentators to rescue the negative STAR*D and CATIE reports.60
Until recently, there have been no obligations to study mechanisms of action when designing a drug trial. Only now has the National Institute of Mental Health (NIMH) begun requiring grant applicants to test the hypothesized mechanism of action of a drug used in a clinical trial.61,62 Most clinical trials of psychiatric and AD drugs are sponsored by industry and not by NIMH. Drug development for AD has no similar requirement. If psychiatry is to derive drugs from knowledge of human targets, an important next step for the science behind drug development will be for the National Institute of Aging (NIA) and industry to adopt the NIMH clinical trial designs that include the study of mechanisms of drug action. In this way clinical trials will contribute to knowledge of disease mechanisms.
Another important step for advancing efficacy will be obligatory comparisons of new drugs against active compounds. In other words, potential new drugs should be tested for comparative efficacy and, at the same time, be used as pharmacological probes to evaluate disease mechanisms. These requirements of improved efficacy for registration and information for theory would shift industry and academia away from the pursuit of Me-Too drugs.
Even these steps will not protect clinical trials of well-targeted psychiatric drugs from being misinterpreted, as they have been in the past. For example, to witness disease-modifying effects current trials of anti-Aβ drugs in AD depend on modifications of cognitive and behavioral functions in patients. Since trials normally compare drug to placebo and the cognitive-functional course of AD varies considerably among subjects, improvements may be interpreted as evidence for a disease modifying effect from engagement of the drug target when the same difference would have occurred with existing ACHE drug treatments. To avoid the temptations to over-promote new drugs, as occurred in the 20th century, psychiatry will need rigorous neuropathological and clinical criteria for recognizing an advance in efficacy as evidence for disease course modification.
The development of new psychotropic drugs carries financial risks. In response, the pharmaceutical industry has decreased its investment in psychiatric disorders, even for those disorders for which no effective treatments currently exist.30,63 The industry necessarily focuses on genetic-biochemical brain states which can discount psychological-experiential causes of symptoms and patient wellbeing. This may have undermined efficacy evaluations in clinical trials, led to distortions in assessments of drug efficacy and conditions of clinical use, and distracted attention from the understanding and efficacy of alternative therapeutic approaches to mental disorders. For example, in AD, there are many symptoms such as sleep disturbances, agitation, or delusions that can, in some patients, respond best to non-pharmacologic interventions.64,65 This is equally true for many of the distressing symptoms of psychiatric disorders. American psychiatry, as applied neuroscience, may too readily study the mind through understanding the brain without equivalent regard for the dependence of the state of the brain on personal experiences, attitudes, values, beliefs, and so forth. Social and cultural resources of human beings determine the way their brains respond to circumstance and to drugs, yet are easily ignored.66,67 Drug development can be misled by failing to understand that the response to a drug may depend on many factors, among them: the background and experience and individuality of the patient, previous exposure to drugs and other substances, the nature of the relationship between patient and prescriber, and the timing of the intervention in relation to the stage of the person’s disease. Psychiatry needs an inclusive consideration of all the biological and social sciences to create a context for drug development adequately diverse to accommodate the range of mechanism relevant for diseases and human wellbeing.13
Looking for guidance from outside of psychiatry
Both the AD and psychiatry fields can benefit from advances in oncological drug development. 21st century oncological drug developments emphasize the pharmacogenetics that govern drug action. In cancer chemotherapy trials, even when a new drug is a failure in the sense that the majority of trial subjects do not show benefit, the few whose tumours do seem to shrink are intensively studied.68 There is much to be learned from the genetic and epigenetic makeup of such outliers.69,70 In psychiatry, outliers have yet to be studied. Outliers may benefit our knowledge of pharmacogenetics and individual experiential factors that can affect drug development and provide insight into mechanisms and drug targets.
Psychiatrists are accustomed to a diversity of responses to drugs. Disease categories are indistinct and symptoms multi-determined. DSM groupings are not good predictors of how patients respond to any intervention. Response of psychiatric symptoms to drugs is probably governed to a great degree, as it is in oncology, by pharmacogenetics. Currently, the widespread custom of polypharmacy for almost every psychiatric disorder clouds the issue with respect to any one drug. When a person fails to respond adequately to a drug regimen, another drug is added.71 This is a practice that needs to be replaced by an understanding of the reasons for failures and successes so this knowledge can be applied more widely in patient care. Identifying drug response or lack of response could help build a better nosology and therapeutics for psychiatry. Studying the effect of drugs can lead backward to the causes of disease.
Discussion
A plethora of problems: a dearth of solutions!
Others have already discussed areas in theory, research methods, nosology, academia-industry relations and potential regulatory requirements that require attention in AD and psychiatric drug developments. Drug discoveries in these fields have been more a matter of luck than a consequence of scientific discovery. Next steps are to balance the dependence on serendipity by strengthening our science. This strengthening potentially will include targeting those not yet ill but demonstrably at risk, choosing inclusion criteria for clinical trials more wisely, staying aware of the pharmacogenetic diversity in populations, freeing researchers from ties with pharmaceutical companies, comparing new drugs against drugs in current use rather than against placebos, forgoing financial incentives, studying drug response outliers, paying attention to non-drug effects, figuring out molecular mechanisms of symptom formation and disease progression, designing new drugs accordingly, and not losing advances to the minefields of errors.
The last half-century of failures indicates that psychiatric drug development requires more focused investments of energies and resources. One important source for new drugs can be the repurposing of drugs used in unrelated disorders, a major source of the initial drugs identified in the mid 20th century.72 Currently, drugs are approved for clinical use in specific disorders. In psychiatry, this is an obstacle because disorders overlap. It may make more sense in psychiatry to approve drugs according to how well they target a specific brain receptor and how well they provide specific clinical benefits associated with that target across disorders. The dopamine receptor, for instance, is a potential target in addictions, in attention deficit hyperactivity disorder, in depression, and in schizophrenia. Drug agonists or antagonists at one of the dopamine receptors may benefit patients across a range of clinical disorders.35
We are beginning to understand that very few, if any, psychiatric drugs will have efficacy across an entire population even when everyone suffers from the same disorder. This is because individual genomic differences govern drug response. Psychiatric drugs are not like antibiotics that attack a strain of bacteria, each bacterial population being identical in its drug response to others of the type (at least until it develops resistance). Psychiatric drugs target a protein receptor target in the brain and proteins differ among individuals. Moreover, drug metabolism varies among individuals so that, at any given dose, the amount of drug reaching the target protein differs substantially. This is now well known, even though pharmaceutical companies and we clinicians would prefer a drug that affects the whole population in the same way. We are understandably reluctant to accept the fact that people are different because such an admission both potentially limits profits for industry and complicates patient care. We currently are also reluctant to examine outliers in psychiatric clinical trials and to determine the cause of what appear to be drug failures. Pharmaceutical companies tend in those cases to cut their losses and abandon further research, moving on to the next agent in the pipeline. They are mistaken because understanding failures can generate new insights into mechanisms of illness and trial methodology, outliers can open new understanding of diseases and therapies.
What is psychiatry to do? Rethinking 5 decades of experience
There are no immediate and at-hand solutions. We interpret this review as indicating a need to pursue important priorities, such as putting psychiatric practice on the soundest possible scientific grounding. We have found five major problems raised by others: theory, research methods, nosology, academia-industry relations, and regulatory requirements. To this list we have added the effects of human errors in development,73 pharmaceutical industry resistance to methodological innovations, inadequate understanding of the reasons for drug efficacy, commercially-promoted compromises to evidence-based medicine, unforeseen effects from legislative actions and inactions, and inadequate mechanisms in place to address errors. Psychiatry faces a full agenda of challenges (Table 2).
Table 2.
Solutions to failures in neuropsychiatric drug development
| Aims: | Response |
|---|---|
| Establish tight industry-academic partnerships that focus drug development towards mechanisms of disease | Investigate all drugs in relationship to biological targets and mechanisms of disease development. |
| Improve rate of drug development by regulatory, financial, legal incentives | Advocate in the right quarters for steps that will encourage successful competition in the neuropsychiatric drug market. |
| Prevent errors in drug developments | Accept the inevitability of man made error and initiate systematic intervention for prevention and correction. |
| Revive the prestige of scientific psychiatric clinical pharmacology | Encourage academic interest in the science of clinical pharmacology based on basic neuropsychiatric principles. Encourage academics to take the initiative in industry-academia collaborations. |
| Improve efficacy | Promote public health campaigns against the introduction of new drugs that do not improve efficacy. Require new drugs to be tested against older ones whose efficacy has been established. Do not permit academic involvement in clinical trials of drugs unless they have shown a clear advantage over established drugs. |
Conclusions
In comparison to psychiatric disorders, we know a lot about the nature of AD and how it differs from other brain diseases. We have established molecular targets that should allow for effective novel drug development, and yet this has not happened. With psychiatric diseases, we are at a greater disadvantage – we don’t know what separates one illness from another except by symptoms, which themselves change over time and overlap. There is no specific brain pathology that defines and separates with clear boundaries the various psychiatric disorders. We know, more or less, how antipsychotics, antidepressants and anxiolytic drugs work in the brain and how they affect patients in the clinic, and we have tried for 70 years to improve on the original serendipitously found agents - but without much success. Some recent drugs are safer and better tolerated, but are no more effective. Years of failure in preventing or delaying onset of illness, in ameliorating symptoms, in lowering suicide rates, in improving quality of life, have put at risk the commercial investments that have, in the past, funded the various clinical stages of drug development. To avoid a flight of commercial interests to other fields in medicine, we need to improve the way we bring potentially helpful drugs to market in the field of psychiatry.18 To avoid a loss of the Hippocratic tradition that commits medicine to the most effective uses of science in patient care, we need to create a more win-win (for patients) collaboration between academia and industry.74 One proposal would be for government to take on this role as they have for aviation safety.73,75 Given the error losses of research studies and error associated morbidity and mortality, government may be needed to address the error problems psychiatry shares with the rest of medicine. Government could also mediate between the best interests of patients and of the industries that serve them.
We would give priority to three specific issues. First, to overcome the invalidation of research by errors. Second, to address the possibility that symptomatic drugs have reached the limits of their effectiveness. It may be time to turn our attention to modifications of disease pathologies and to abandon investment in symptomatic interventions. Third, to concentrate on preclinical studies and efficient, small, human proof-of-concept studies designed to inform basic research and to exclude latent errors before they end in phase III mishaps and wasted funding.73 One aim of this focus would be to decease the costs of drug developments and subsequent marketed products.
Our hope is that these activities would support the understanding of molecular brain mechanisms that result in neuropathology and symptoms. Psychiatric drug research would depend on a refined soundly evidenced brain-behavior theory that is able to predict clinical success from interventions that address specific mechanisms. Our response to the data in this review is that we need to think more clearly about stages of illness. We need to think more frequently about variations in individual drug response. We need to better target drugs to specific faulty proteins to advance our science. We need to rely on expanded basic research and feed what we learn from clinical trials back into the laboratory and from the laboratory back into the clinic. We need to learn how to prevent and how to correct errors. We need to empower psychiatry to rely on brain sciences and become less dependent on serendipity, trial and error, and untested impressions about psychiatric diseases.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health (NHG), and by grants from the National Institutes of Health (NIA-R01 and -R21) and Alzheimer’s Association (IIRG) (DKL). The authors thank Jimmy Burril, Visual Media Services, Intramural Research Program, National Institute on Aging, in relation to Figures 1 and 2.
Footnotes
Conflicts of Interest
REB, NHG and DKL declare no conflicts of interest. MVS Consults to Clera Inc., a start-up pharmaceutical company and, otherwise, declares no conflicts of interest.
References
- 1.Maxwell RA, Eckhardt SB. Drug discovery. Humana Press; NYC, NY: 1990. pp. 143–54. [Google Scholar]
- 2.Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–6. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 3.Summers WK, Majovski LV, Marsh GM, Tachiki K, Kling A. Oral tetrahydroaminoacridin in long-term treatment of senile dementia, Alzheimer’s type. N Engl J Med. 1986;324:352. doi: 10.1056/NEJM198611133152001. [DOI] [PubMed] [Google Scholar]
- 4.Schneider LS, Mangialasche F, Andreasen N, et al. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med. 2014;275:251–83. doi: 10.1111/joim.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vishal S, Sourabh A, Harkirat S. Alois Alzheimer (1864–1915) and the Alzheimer syndrome. J Med Biogr. 2011;19:32–3. doi: 10.1258/jmb.2010.010037. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–7. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigue KM, Kennedy KM, Devous MD, Sr, et al. β-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78:387–95. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fein JA, Sokolow S, Miller CA, et al. Co-localization of amyloid beta and tau pathology in Alzheimer’s disease synaptosomes. Am J Pathol. 2008;172:1683–92. doi: 10.2353/ajpath.2008.070829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crimins JL, Pooler A, Polydoro M, Luebke JL, Spires-Jones TL. The intersection of amyloid beta and tau in glutamatergic synaptic dysfunction and collapse in Alzheimer’s disease. Ageing Res Rev. 2013;12:757–63. doi: 10.1016/j.arr.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckerström C, Olsson E, Klasson N, et al. Multimodal Prediction of Dementia with up to 10 Years Follow Up: The Gothenburg MCI Study. J Alzheimers Dis. 2014;44(1):205–14. doi: 10.3233/JAD-141053. [DOI] [PubMed] [Google Scholar]
- 12.Landau SM, Frosch MP. Tracking the earliest pathologic changes in Alzheimer disease. Neurology. 2014;82:1576–7. doi: 10.1212/WNL.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 13.Becker RE, Greig NH, Giacobini E, Schneider LS, Ferrucci L. A new roadmap for drug development for Alzheimer’s disease. Nat Rev Drug Discov. 2013;13:156. doi: 10.1038/nrd3842-c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott T. Scientists can’t get their minds around Alzheimer’s. Los Angeles Times. 2007 Dec 27; < http://articles.latimes.com/2007/dec/27/nation/la-na-alzheimers27dec27> (last viewed 04/14/2015)
- 15.Vellas B. Tarenflurbil for Alzheimer’s disease: a “shot on goal” that missed. Lancet Neurol. 2010;9:235–7. doi: 10.1016/S1474-4422(10)70030-2. [DOI] [PubMed] [Google Scholar]
- 16.McGorry P, Keshavan M, Goldstone S, et al. Biomarkers and clinical staging in psychiatry. World Psychiatry. 2014;13:211–23. doi: 10.1002/wps.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aisen PS, Vellas B, Hampel H. Moving towards early clinical trials for amyloid-targeted therapy in Alzheimer’s disease. Nat Rev Drug Discov. 2013;12:324. doi: 10.1038/nrd3842-c1. [DOI] [PubMed] [Google Scholar]
- 18.Becker RE, Greig NH. A new regulatory road-map for Alzheimer’s disease drug development. Curr Alzheimer Res. 2014;11:215–20. doi: 10.2174/156720501103140329210642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farlane WR. Prevention of the first episode of psychosis. Psychiatr Clin North Am. 2011;34:95–107. doi: 10.1016/j.psc.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornblatt BA, Lencz T, Kane JM. Treatment of the schizophrenia prodrome: is it presently ethical? Schizophr Res. 2001;51:31–38. doi: 10.1016/s0920-9964(01)00236-5. [DOI] [PubMed] [Google Scholar]
- 21.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–38. doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ban T. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3:495–500. [PMC free article] [PubMed] [Google Scholar]
- 23.Carlsson A, Lindqvist M. Effect of chlorpromazine and haloperidol on formation of 3-methoxytyramine and normetanephrine on mouse brain. Acta Pharmacol Toxicol (Copenh) 1963;20:140–4. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 24.López-Muñoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15:1563–86. doi: 10.2174/138161209788168001. [DOI] [PubMed] [Google Scholar]
- 25.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–69. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuchs E, Flugge G. Experimental animal models for the simulation of depression and anxiety. Dialogues Clin Neurosci. 2006;8:323–333. doi: 10.31887/DCNS.2006.8.3/efuchs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shekhar A, McCann UD, Meaney MJ, et al. Summary of a National Institute of Mental Health workshop: developing animal models of anxiety disorders. Psychopharmacol (Berl) 2001;157:327–39. doi: 10.1007/s002130100859. [DOI] [PubMed] [Google Scholar]
- 28.Hyman SE. Psychiatric drug development: diagnosing a crisis. Cerebrum. 2013;2013:5. [PMC free article] [PubMed] [Google Scholar]
- 29.Lane RM. Antidepressant drug development: focus on triple reuptake inhibition. J Psychopharmacol. doi: 10.1177/0269881114553252. Published online 14 October 2014. [DOI] [PubMed] [Google Scholar]
- 30.Klein DF, Glick ID. Industry withdrawal from psychiatric medication development. Rev Bras Psiquiatr. 2014;36:259–61. doi: 10.1590/1516-4446-2014-3603. [DOI] [PubMed] [Google Scholar]
- 31.IMI. Introducing IMI. 2014 < http://www.imi.europa.eu/content/mission>last viewed 04/14/2015.
- 32.Becker RE, Greig NH. Neuropsychiatric Clinical Trials: Lost in Translation. Sci Transl Med. 2010;2(61):61rv6. doi: 10.1126/scitranslmed.3000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilcock GK, Black SE, Hendrix SB, Zavitz KH, Swabb EA, Laughlin MA. Tarenflurbil Phase II Study investigators, Efficacy and safety of tarenflurbil in mild to moderate Alzheimer’s disease: A randomised phase II trial. Lancet Neurol. 2008;7:483–93. doi: 10.1016/S1474-4422(08)70090-5. [DOI] [PubMed] [Google Scholar]
- 34.Selkoe DJ. Resolving controversies on the path to Alzheimer’s therapeutics. Nat Med. 2011;12:1060–5. doi: 10.1038/nm.2460. [DOI] [PubMed] [Google Scholar]
- 35.Borchers AT, Hagie F, Keen CL, Gershwin ME. The history and contemporary challenges of the US Food and Drug Administration. Clin Ther. 2007;29:1–16. doi: 10.1016/j.clinthera.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Janssen WF. The story of the laws behind the labels. US Food and Drug Administration; < http://www.fda.gov/AboutFDA/WhatWeDo/History/Overviews/ucm056044.htm>(last viewed 04/14/2015) [Google Scholar]
- 37.Krishnaswamy S, Verdile G, Groth D, Kanyenda L, Martins RN. The structure and function of Alzheimer’s gamma secretase enzyme complex. Crit Rev Clin Lab Sci. 2009;46(5–6):282–301. doi: 10.3109/10408360903335821. [DOI] [PubMed] [Google Scholar]
- 38.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Potter WZ, Mallinckrodt CH, Detke MJ. Controlling placebo response in drug development: lessons learned from psychopharmacology, making it difficult to detect. Pharmaceutical Med. 2014;28(2):53–65. [Google Scholar]
- 40.Rutherford BR, Pott E, Tandler JM, Wall MM, Roose SP, Lieberman JA. Placebo response in antipsychotic clinical trials: a meta-analysis. JAMA Psychiatry. 2014;71:1409–21. doi: 10.1001/jamapsychiatry.2014.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halford A. Global survey of academic translational researchers: reproducibility, current practices, barriers to progress, and future directions. 2014 < http://go.sigmaaldrich.com/Translational-Survey>. (last viewed 03/23/2015)
- 42.Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov. 2011;10:712. doi: 10.1038/nrd3439-c1. [DOI] [PubMed] [Google Scholar]
- 43.James JT. A new, evidence-based estimate of patient harms. J Patient Saf. 2013;9:122–8. doi: 10.1097/PTS.0b013e3182948a69. [DOI] [PubMed] [Google Scholar]
- 44.Cummings J. Phase 2A Trial of bexarotene in patients with Alzheimer’s disease. The AlzForum. 2013 Jun 21; http://www.alzforum.org/pap/annotation.asp?powID=147562 (last viewed 04/14/2015)
- 45.Hippius H. The history of clozapine. Psychopharmacology (Berl) 1989;99:S3–S5. doi: 10.1007/BF00442551. [DOI] [PubMed] [Google Scholar]
- 46.Crilly J. The history of clozapine and its emergence in the US market: a review and analysis. Hist Psychiatry. 2007;18:39–60. doi: 10.1177/0957154X07070335. [DOI] [PubMed] [Google Scholar]
- 47.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 48.Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329:162–7. doi: 10.1056/NEJM199307153290303. [DOI] [PubMed] [Google Scholar]
- 49.Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:27–38. [PubMed] [Google Scholar]
- 50.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(Suppl 1):1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 51.Heres S, Davis J, Maino K, Jetzinger E, Kissling W, Leucht S. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: an exploratory analysis of head-to-head comparison studies of second-generation antipsychotics. Am J Psychiatry. 2006;163:185–94. doi: 10.1176/appi.ajp.163.2.185. [DOI] [PubMed] [Google Scholar]
- 52.Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166:152–63. doi: 10.1176/appi.ajp.2008.08030368. [DOI] [PubMed] [Google Scholar]
- 53.Cutler AJ, Schwartz TL. The Pharmacology of MDD Treatment: Building a Foundation With a Focus on 5-HT. Medscape Education Psychiatry & Mental Health CME Released: 11/22/2013. < http://www.medscape.org/viewarticle/814593> (last viewed 04/14/2015)
- 54.Correll CU. Vortioxetine: A New Antidepressant Choice in the United States. Medscape. 2013 Oct 02; < http://www.medscape.com/viewarticle/811959_3> (last viewed 03/23/2015)
- 55.Appelbaum PS, Gold A. Psychiatrists’ relationships with industry: the principal-agent problem. Harv Rev Psychiatry. 2010;18(5):255–65. doi: 10.3109/10673229.2010.507038. [DOI] [PubMed] [Google Scholar]
- 56.Wimber M, Alink A, Charest I, Kriegeskorte N, Anderson MC. Retrieval induces adaptive forgetting of competing memories via cortical pattern suppression. Nat Neurosci. 2015 Mar 16; doi: 10.1038/nn.3973. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewis S. Candidate for Governor: And How I Got Licked (1935) University of California Press; Berkeley, CA: 1994. p. 109. [Google Scholar]
- 58.Swartz MS, Stroup TS, McEvoy JP, et al. What CATIE found: results from the schizophrenia trial. Psychiatr Serv. 2008;59:500–6. doi: 10.1176/ps.2008.59.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 60.Wisniewski SR, Rush AJ, Nierenberg AA, et al. Can phase III trial results of antidepressant medications be generalized to clinical practice? A STAR*D report. Am J Psychiatry. 2009;166:599–607. doi: 10.1176/appi.ajp.2008.08071027. [DOI] [PubMed] [Google Scholar]
- 61.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–7. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 62.Insel T. Director’s Blog: A new approach to clinical trials. 2014 Feb 27; < http://www.nimh.nih.gov/about/director/2014/a-new-approach-to-clinical-trials.shtml> (last viewed 04/14/2015)
- 63.Miller G. Is pharma running out of brainy ideas? Science. 2010;329:502–4. doi: 10.1126/science.329.5991.502. [DOI] [PubMed] [Google Scholar]
- 64.Lyketsos CG1, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7:532–539. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Becker RE, Vicari S. Management of Alzheimer’s disease: community based care for patients and families. Home Health Care Consultant. 1998;5(5):11–17. [Google Scholar]
- 66.Kleinman A. Rebalancing academic psychiatry: why it needs to happen – and soon. Br J Psychiatry. 2012;201:421–2. doi: 10.1192/bjp.bp.112.118695. [DOI] [PubMed] [Google Scholar]
- 67.Bracken P, Thomas P, Timimi S, et al. Psychiatry beyond the current paradigm. Br J Psychiatry. 2012;201:430–4. doi: 10.1192/bjp.bp.112.109447. [DOI] [PubMed] [Google Scholar]
- 68.Chang DK, Grimmond SM, Evans TRJ, Blankin AV. Mining the genomes of exceptional responders. Nat Cancer Rev. 2014;14:291–2. doi: 10.1038/nrc3723. [DOI] [PubMed] [Google Scholar]
- 69.De Palma M, Hanahan D. The biology of personalized cancer medicine: Facing individual complexities underlying hallmark capabilities. Mol Oncol. 2012;6:2111–27. doi: 10.1016/j.molonc.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zawia NH, Lahiri DK. (2012) Epigenetics: A paradigm shift in understanding Alzheimer’s Disease. Curr Alzheimer Res. 2012;9:525–6. doi: 10.2174/156720512800617973. [DOI] [PubMed] [Google Scholar]
- 71.Buckley PF. Antipsychotic polypharmacy in schizophrenia: ‘secret sauce or wild abandon?’. In: Ritsner MS, editor. Polypharmacy in Psychiatry Practice. II. Springer; Netherlands: 2013. pp. 3–10. [Google Scholar]
- 72.National Center for Advancing Translational Sciences. Repurposing drugs. < http://www.ncats.nih.gov/research/reengineering/rescue-repurpose/rescue-repurpose.html> (last viewed 04/14/2015)
- 73.Reason J. Human Error. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 74.Becker RE. Remembering Sir William Osler 100 years following his death: what we can learn from his legacy. The Lancet. 2014;384:2260–63. doi: 10.1016/S0140-6736(14)61887-0. [DOI] [PubMed] [Google Scholar]
- 75.Becker RE, Greig NH. Why so few drugs for Alzheimer’s disease? Are methods failing drugs? Curr Alzheimer Res. 2010;7:642–51. doi: 10.2174/156720510793499075. [DOI] [PMC free article] [PubMed] [Google Scholar]