Abstract
Introduction
The aim of this study was to determine whether the orthopedic forces of rapid maxillary expansion cause significant quantitative changes in the cranial and the circummaxillary sutures.
Methods
Twenty patients (mean age, 12.3 ± 1.9 years) who required rapid maxillary expansion as a part of their comprehensive orthodontic treatment had preexpansion and postexpansion computed tomography scans. Ten cranial and circummaxillary sutures were located and measured on one of the axial, coronal, or sagittal sections of each patient’s preexpansion and postexpansion computed tomography scans. Quantitative variables between the 2 measurements were compared by using the Wilcoxon signed rank test. A P value less than 0.05 was considered statistically significant.
Results
Rapid maxillary expansion produced significant width increases in the intermaxillary, internasal, maxillonasal, frontomaxillary, and frontonasal sutures, whereas the frontozygomatic, zygomaticomaxillary, zygomaticotemporal, and pterygomaxillary sutures showed nonsignificant changes. The greatest increase in width was recorded for the intermaxillary suture (1.7 ± 0.9 mm), followed by the internasal suture (0.6 ± 0.3 mm), and the maxillonasal suture (0.4 ± 0.2 mm). The midpalatal suture showed the greatest increase in width at the central incisor level (1.6 ± 0.8 mm) followed by the increases in width at the canine level (1.5 ± 0.8 mm) and the first molar level (1.2 ± 0.6 mm).
Conclusions
Forces elicited by rapid maxillary expansion affect primarily the anterior sutures (intermaxillary and maxillary frontal nasal interfaces) compared with the posterior (zygomatic interface) craniofacial structures.
Rapid maxillary expansion (RME) is indicated for the treatment of maxillary transverse deficiency. The expansion occurs when the force applied to the teeth and the maxilla exceeds the limits needed for tooth movement. The applied force causes widening and gradual opening of the midpalatal suture, compression of the periodontal ligament, bending of the alveolar processes, and dental tipping.1–3
Although the main purpose of RME is to correct maxillary arch discrepancies, its effect is not limited to the maxillary alveolus and midpalatal suture but is expected to affect several other adjacent structures in the face and the cranium. The maxilla articulates with other skull bones through the cranial and circummaxillary sutures. Sutures in the skull have several functions. They unite bones, absorb forces, act as joints that permit relative movement between bones, and play a role as growth sites.4–7
Several histologic investigations have studied sutural responses to orthopedic forces in monkeys,8–12 cats,13 rats,14,15 and biopsies from children.16 Histologically, the sutures of the maxilla are heavily affected by the orthopedic forces used in orthodontic treatment.17 Experimental separation of sutures in monkeys resulted in skeletal remodeling in all the circummaxillary sutures; the amount of remodeling appeared to be proportional to the sutures distance from and orientation to the applied force.8 However, differences in sutural morphology, together with differences in sequence and degree of closure between humans and different animal species, bring doubt on the clinical application of the animal experiments.
Mechanical stresses induced by RME result in the transmission of forces that produce tensile and compressive strains on the facial bones and the adjacent structures. Craniomaxillary sutures, which then absorb and transmit these forces, are likely to be modified by these mechanical forces.18 Many investigators have pointed out that RME results in dramatic changes in the craniofacial structures. Gardner and Kronman12 in their RME study on rhesus monkeys found that the lambdoid, parietal, and midsagittal sutures of the vault of the cranium showed evidence of distortion, and in 1 animal a split of 1.5 mm was reported. Kudlick19 concluded that all craniofacial bones directly articulating with the maxilla were displaced, except the sphenoid bone. Wertz and Dreskin20 showed that the maxilla moved downward and usually forward during suture opening, whereas Timms21 showed that the maxilla and the palatine bones moved apart, along with the pterygoid processes of the sphenoid bone. Jafari et al22 in their 3-dimensional study on a finite element model of a human skull indicted that the transverse orthopedic forces of RME produced not only an expansive force at the intermaxillary suture but also high forces on various structures on the craniofacial complex, particularly the sphenoid and zygomatic bones.
Although a number of clinical and radiographic studies have evaluated the effects of RME on the craniomaxillary complex, only limited information is available on the response of the cranial sutures. Computed tomography (CT), as an imaging modality, offers many advantages over conventional radiographs, since it permits clear visualization of sections of the human body and the skull without overlap of structures. Therefore, the aim of this study, by using low-dose multiplanar CT scans, was to examine the effects of orthopedic forces on the cranial and circummaxillary sutures in adolescents treated with RME.
MATERIAL AND METHODS
This prospective study included 20 patients with transverse maxillary deficiency. The patients’ ages ranged from 8 to 15 years (mean, 12.3 ± 1.9 years). They were treated by RME with the hyrax appliance as part of their comprehensive orthodontic treatment. The appliance design required banding 4 teeth (2 maxillary first premolars or deciduous first molars and 2 maxillary first molars). The appliance screw was activated 2 quarter turns (0.2 mm/turn) twice a day until the required amount of expansion was achieved and the palatal cusp of the maxillary first molar contacted the buccal cusp of the mandibular first molar. The appliance was left in situ as a passive retainer for 3 months, after which it was removed. The study was approved by the ethical committee of the dental faculty, Al-Azhar University of Cairo, Egypt, and each parent signed an informed consent form.
The spiral CT machine (model X, vision; GE Medical Systems, Milwaukee, Wis) was used to obtain pre-RME (T1) CT images before orthodontic treatment and post-RME (T2) images immediately after the hyrax appliance was removed. The CT scans were made at 120 kV and 20 mA, with a scanning time of 2 seconds per section. Scan parameters included an A2-90 scanning filter, a 25-cm field of view, and a matrix of 512 ± 512 pixels. The window width was 2400 Hounsfield units with a center of 1300 Hounsfield units. The machine’s perpendicular light beam was used to standardize the head position in all 3 planes and thus allow comparison of the images before and after expansion. The scans were taken of each patient in the supine position, with the palatal plane perpendicular to the ground. The subject was positioned so that the longitudinal light beam passed through the center of the glabella and the philtrum, and the transverse light beam passed through the lateral eye canthi. One-millimeter thick axial sections were made parallel to the palatal plane, incorporating the dentoalveolar and the basal areas of the maxilla up to the cranial base.
The digital imaging and communications in medicine (DICOM) images for each patient were assessed by using the InVivo Dental imaging software program (Anatomage, San Jose, Calif). Ten cranial and circummaxillary sutures (Fig 1) were precisely located and measured in width on 1 axial, coronal, or sagittal section of the CT scans showing the greatest width and according to the orientation of the suture (Figs 2–6). The location of each suture was determined in reference to its location on the 3-dimensional volumetric model by adjusting the cursor over the same reference point for T1 and T2; subsequently, the corresponding location on the different sections (axial, coronal, and sagittal) was updated.
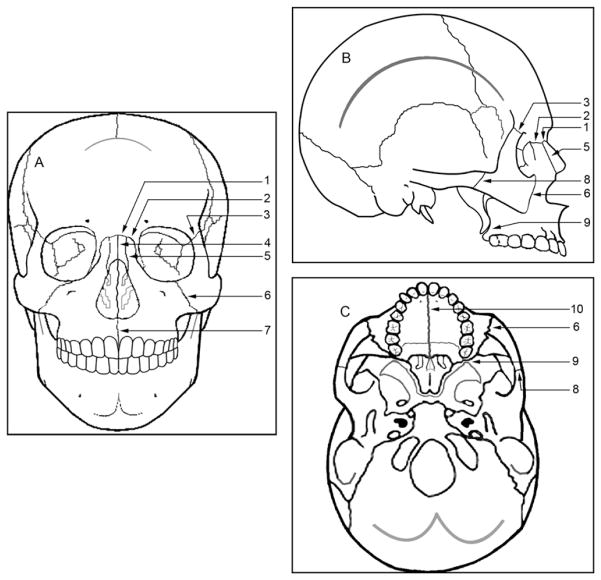
Fig 1.
Schematic representation of the sutures used in the study: A, frontal; B, lateral; C, axial views. 1, Frontonasal; 2, frontomaxillary; 3, frontozygomatic; 4, internasal; 5, nasomaxillary; 6, zygomaticomaxillary; 7, intermaxillary; 8, temporozygomatic; 9, pterygomaxillary; 10, midpalatal suture.
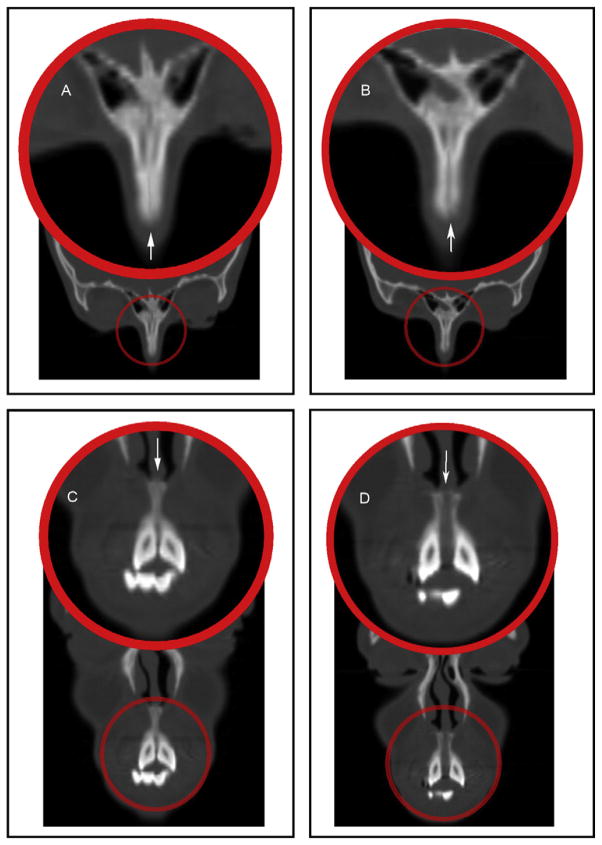
Fig 2.
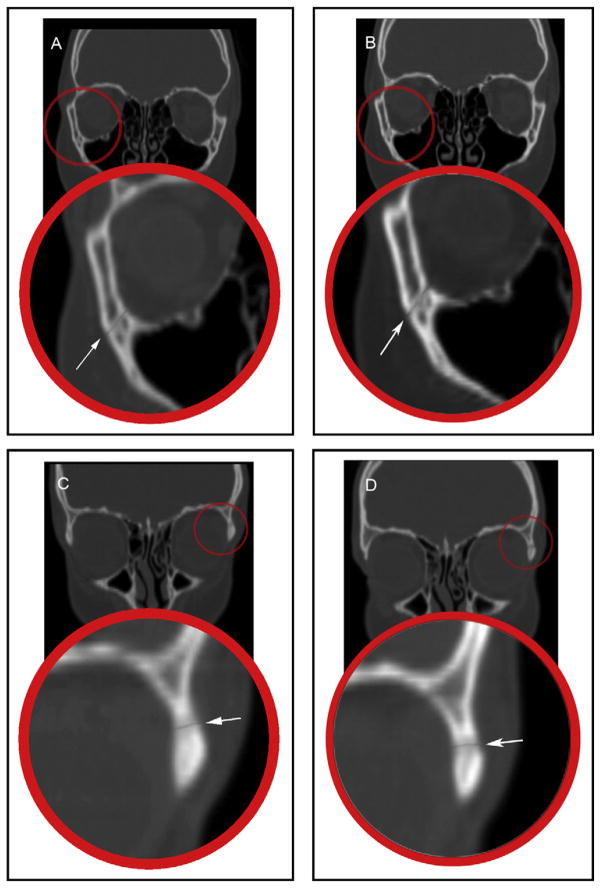
Coronal CT sections showing: A, the frontomaxillary suture before RME; B, the frontomaxillary suture after RME; C, the frontonasal suture before RME; D, the frontonasal suture after RME.
Fig 6.
Axial CT sections showing: A, the zygomaticotemporal suture before RME; B, the zygomaticotemporal suture after RME; C, the midpalatal suture before RME; D, the midpalatal suture after RME.
The frontomaxillary, frontonasal, frontozygomatic, zygomaticomaxillary, internasal, and intermaxillary sutures were located and measured on the coronal sections (Figs 2–4). The internasal suture was measured on a 45° clockwise rotated coronal section to represent the full length of the suture. The pterygomaxillary and the nasomaxillary sutures were located and measured on the sagittal sections (Fig 5). T1 and T2 measurements were determined for each suture by measuring the width of the radiolucent area located between the 2 bony edges at the middle of the suture. The difference between T2 and T1 indicates the increase or decrease in the suture width.
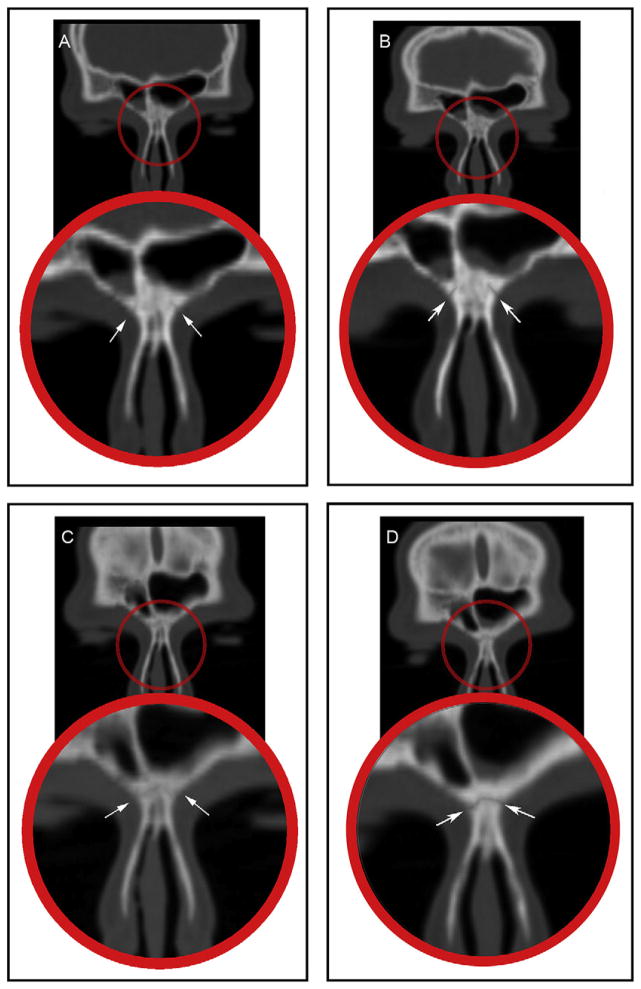
Fig 4.
Coronal CT section rotated in a 45° clockwise direction showing: A, the internasal suture before RME; B, the internasal suture after RME; and coronal CT sections showing: C, the intermaxillary suture before RME; D, the intermaxillary suture after RME.
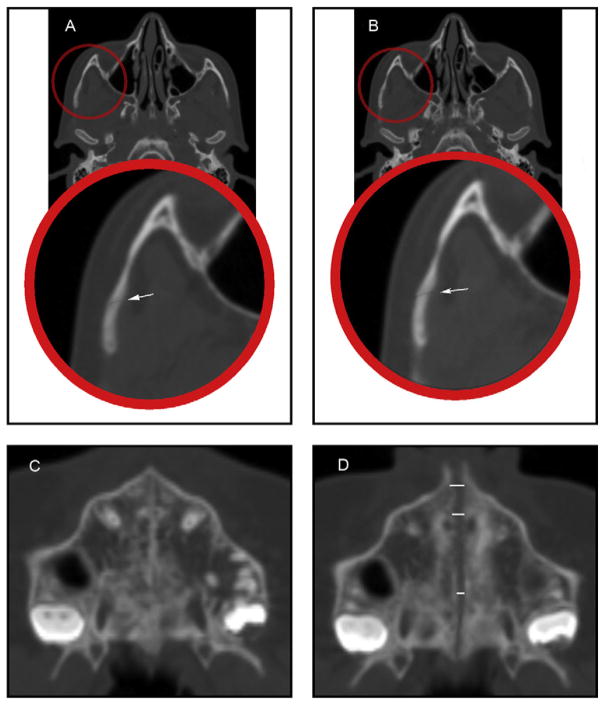
Fig 5.
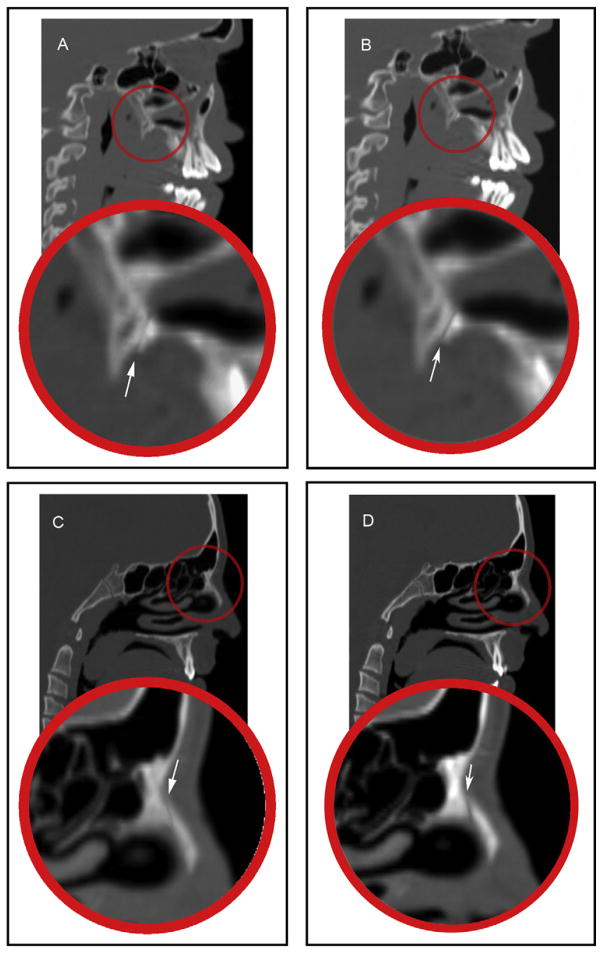
Sagittal CT sections showing: A, the pterygomaxillary suture before RME; B, the pterygomaxillary suture after RME; C, the nasomaxillary suture before RME; D, the nasomaxillary suture after RME.
The midpalatal and zygomaticotemporal sutures were evaluated on the axial section (Fig 6). The midpalatal suture was measured at the level of the maxillary permanent central incisors, canines, and first molars. The exact location of the maxillary central incisors, canines, and first molars was determined with the marker (cursor) feature in the Anatomage software, which can show the exact corresponding position of the teeth on the axial section from a reference level on the coronal section (Fig 7). The difference between T2 and T1 indicates the change and the amount of suture opening.
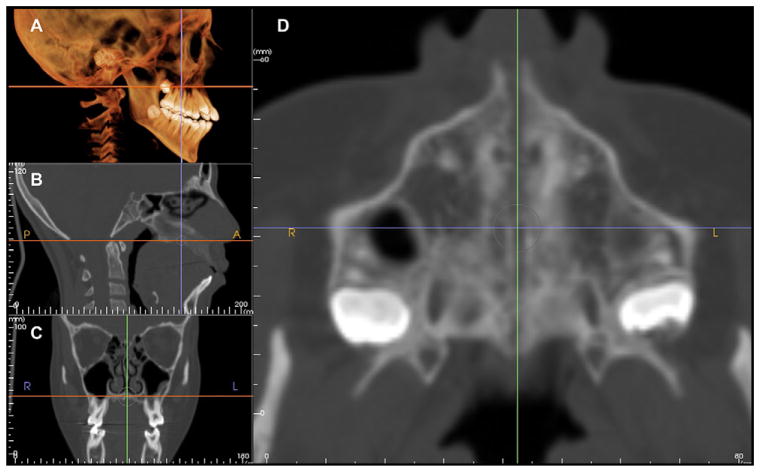
Fig 7.
The rendering window of the software allows viewing of: A, 3-dimensional volume; B, sagittal, C, coronal, and D, axial sections. By adjusting the cursor over the point of interest, the corresponding images are updated.
All measurements were performed twice at a 2-week interval by the same examiner (A.G.). Differences between the 2 readings for intraexaminer variation were tested by using paired t tests. The results were based on the averages of the 2 readings. Descriptive statistics (means and standard deviations) were obtained for each variable at T1 and T2, and for the changes from T2 to T1. Within-group comparisons of quantitative variables between T1 and T2 were made by using the Wilcoxon signed rank test. A probability value less than 0.05 was considered statistically significant. All statistical calculations were done by using the computer programs Excel 2007 (Microsoft, Redmond, Wash) and the Statistical Package for the Social Science (version 15; SPSS, Chicago, Ill) for Windows.
RESULTS
The intraexaminer reliability test showed no statistically significant difference between the 2 readings. Descriptive statistics for the measurements at T1 and T2 and for the changes from T2 to T1 are presented in Tables I and II. The comparison of the changes represents the overall treatment effect.
Table I.
Comparison of the measurements at T1, T2, and the changes in the cranial and circummaxillary sutures (n = 20)
| T1
|
T2
|
Change
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Max | Min | Mean | SD | Max | Min | Mean | SD | Max | Min | P value | |
| Frontozygomatic width Rt (mm) | 0.6 | 0.3 | 1.2 | 0.1 | 0.6 | 0.3 | 1.2 | 0.2 | 0.0 | 0.0 | 0.2 | 0.1 | 0.065 |
|
| |||||||||||||
| Frontozygomatic width Lt (mm) | 0.6 | 0.3 | 1.3 | 0.2 | 0.7 | 0.3 | 1.3 | 0.2 | 0.1 | 0.1 | 0.3 | 0.1 | 0.063 |
|
| |||||||||||||
| Frontomaxillary width Rt (mm) | 0.6 | 0.2 | 0.9 | 0.4 | 0.7 | 0.2 | 1.3 | 0.4 | 0.1 | 0.1 | 0.8 | 0.1 | 0.006* |
|
| |||||||||||||
| Frontomaxillary width Lt (mm) | 0.6 | 0.2 | 1.0 | 0.4 | 0.7 | 0.3 | 1.6 | 0.4 | 0.1 | 0.2 | 1.5 | 0.1 | 0.003* |
|
| |||||||||||||
| Zygomaticomaxillary width Rt (mm) | 0.5 | 0.1 | 0.8 | 0.3 | 0.6 | 0.1 | 0.8 | 0.5 | 0.1 | 0.1 | 0.3 | 0.1 | 0.055 |
|
| |||||||||||||
| Zygomaticomaxillary width Lt (mm) | 0.5 | 0.1 | 0.8 | 0.3 | 0.6 | 0.1 | 0.8 | 0.4 | 0.1 | 0.1 | 0.2 | 0.1 | 0.055 |
|
| |||||||||||||
| Zygomaticotemporal width Rt (mm) | 0.6 | 0.3 | 1.4 | 0.5 | 0.7 | 0.2 | 1.5 | 0.6 | 0.0 | 0.1 | 0.2 | 0.1 | 0.065 |
|
| |||||||||||||
| Zygomaticotemporal width Lt (mm) | 0.6 | 0.3 | 1.3 | 0.4 | 0.7 | 0.2 | 1.3 | 0.5 | 0.0 | 0.1 | 0.3 | 0.1 | 0.065 |
|
| |||||||||||||
| Pterygomaxillary width Rt (mm) | 0.5 | 0.1 | 0.8 | 0.3 | 0.6 | 0.2 | 0.9 | 0.4 | 0.1 | 0.2 | 0.6 | 0.2 | 0.065 |
|
| |||||||||||||
| Pterygomaxillary width Lt (mm) | 0.5 | 0.2 | 0.9 | 0.3 | 0.6 | 0.2 | 1.0 | 0.4 | 0.1 | 0.2 | 0.7 | 0.1 | 0.065 |
|
| |||||||||||||
| Frontonasal width Rt (mm) | 0.5 | 0.2 | 0.9 | 0.2 | 0.7 | 0.2 | 1.1 | 0.4 | 0.2 | 0.1 | 0.6 | 0.1 | <0.001* |
|
| |||||||||||||
| Frontonasal width Lt (mm) | 0.5 | 0.2 | 0.9 | 0.3 | 0.7 | 0.2 | 1.1 | 0.5 | 0.2 | 0.1 | 0.6 | 0.1 | <0.001* |
|
| |||||||||||||
| Nasomaxillary width Rt (mm) | 0.5 | 0.1 | 0.8 | 0.4 | 0.9 | 0.2 | 1.5 | 0.6 | 0.4 | 0.2 | 1.0 | 0.1 | <0.001* |
|
| |||||||||||||
| Nasomaxillary width Lt (mm) | 0.6 | 0.1 | 0.8 | 0.4 | 1.0 | 0.2 | 1.5 | 0.7 | 0.4 | 0.2 | 1.1 | 0.1 | <0.001* |
|
| |||||||||||||
| Internasal width (mm) | 0.5 | 0.1 | 0.7 | 0.3 | 1.1 | 0.3 | 1.9 | 0.7 | 0.6 | 0.3 | 2.7 | 0.5 | <0.001* |
|
| |||||||||||||
| Intermaxillary width (mm) | 0.5 | 0.1 | 0.6 | 0.2 | 2.2 | 0.9 | 4.3 | 1.1 | 1.7 | 0.9 | 3.6 | 0.6 | <0.001* |
T1, Pretreatment measurements; T2, posttreatment measurements; Rt, right; Lt, left; Max, maximum; Min, minimum.
Statistically significant at P <0.05.
Table II.
Comparison of the measurements at T1, T2, and the changes in the midpalatal suture (n = 20)
| T1
|
T2
|
Change
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Max | Min | Mean | SD | Max | Min | Mean | SD | Max | Min | P value | |
| Midpalatal suture width at the central incisor level (mm) | 0.5 | 0.1 | 0.6 | 0.0 | 2.1 | 0.8 | 5.2 | 1.0 | 1.6 | 0.8 | 4.8 | 0.5 | <0.001* |
|
| |||||||||||||
| Midpalatal suture width at the canine level (mm) | 0.5 | 0.1 | 0.6 | 0.0 | 2.0 | 0.8 | 4.9 | 1.0 | 1.5 | 0.8 | 4.5 | 0.5 | <0.001* |
|
| |||||||||||||
| Midpalatal suture width at the first molar level (mm) | 0.6 | 0.1 | 0.7 | 0.2 | 1.7 | 0.6 | 2.7 | 0.8 | 1.2 | 0.6 | 2.1 | 0.2 | <0.001* |
T1, Pretreatment measurements; T2, posttreatment measurements; Max, maximum; Min, minimum.
Statistically significant at P <0.05.
Between T2 and T1 (Table I), all cranial and circummaxillary sutures showed significant increases in width except for the frontozygomatic, zygomaticomaxillary, zygomaticotemporal, and pterygomaxillary sutures. The greatest increases in width were recorded in the intermaxillary suture (1.7 ± 0.9 mm) and the internasal suture (0.6 ± 0.3 mm), followed by the right and left nasomaxillary sutures (0.4 ± 0.2 mm for each). For the right and left frontonasal sutures, a significant increase in width (0.2 ± 0.1 mm for each) was recorded, whereas the increases in the width of the frontomaxillary suture were 0.1 ± 0.1 mm and 0.1 ± 0.2 mm for the right and the left sutures, respectively.
In the comparison of the significant changes in mid-palatal suture width (Table II), the greatest increase was recorded at the central incisor level (1.6 ± 0.8 mm) followed by the increases in width at the canine level (1.5 ± 0.8 mm) and the first molar level (1.2 ± 0.6 mm), indicating that the opening of the suture was not symmetric, since it was wider at the central incisors and less at the first molar level. Although it was only a small absolute difference, the amount of expansion at the first molar level was 75% of the expansion at the central incisor level.
DISCUSSION
All cranial and circummaxillary sutures measured in this study showed significant increases in width except for the frontozygomatic, zygomaticomaxillary, zygomaticotemporal, and pterygomaxillary sutures. The results of significant increases in the width of the intermaxillary, internasal, nasomaxillary, frontomaxillary, and frontonasal sutures agree with previous studies showing that these areas are affected by the generated forces.12,23–25 A study using the technique of photoelasticity reported that the stresses generated by the expansion appliance activation during RME produced a demonstrable increase in the stresses at the frontonasal suture.26 Similarly, a finite element study evaluating stress distribution along the craniofacial sutures with RME demonstrated that the maximum stresses were experienced by the medial aspect of the frontomaxillary suture, the superior portion of the nasomaxillary suture, and the lateral aspect of the frontonasal suture.2 These stresses were also consistent with observations on monkeys and humans.27–31
The results of 0.6-mm internasal, 0.4-mm nasomaxillary, and 0.2-mm frontonasal width changes suggest a slightly increased nasal cavity width that could be considered an anatomic confirmation of the results from previous studies, demonstrating that increased nasal cavity width and subsequent reduction in the nasal airflow resistance is an orthopedic influence of RME.27,30,32–34
The nonsignificant difference in the width of the pterygomaxillary suture indicates its rigid interdigitation and high resistance to expansion, consistent with the results from studies on dry skulls and human autopsy material. For example, it was demonstrated that disarticulation of the palatal bone from the pterygoid process is possible only on infantile and juvenile (early mixed dentition) skulls. Attempted disarticulation in the late juvenile (late mixed dentition) and adolescent periods was always accompanied by fracture of the heavily interdigitated osseous surfaces.35 On dry human skulls, the pterygomaxillary sutural area at 6.5 years of age was found to be linear with evidence of a gap between the pterygoid process of the sphenoid bone and the maxillary tuberosity. In contrast, the anatomy of this region is changed dramatically by 12 years of age, when the 2 bony surfaces were more interdigitated. This supports the conclusion that by this age it becomes increasingly difficult to protract the maxilla from the pterygoid processes without creating microfractures that can be an obstacle for further growth.36
The lack of significant differences in the widths of the frontozygomatic, zygomaticomaxillary, and zygomaticotemporal sutures in this study might be explained also by their increased interdigitation and rigidity. Both Lines37 and Bell and Epker38 stated that the reason for failure of nonsurgically assisted RME in their studies was the increased rigidity of the facial skeleton. They cited fusion of various combinations of zygomaticotemporal, zygomaticofrontal, and zygomaticomaxillary sutures as the primary anatomic sites of resistance to RME. Although sutural widths in our study might decrease between the end of expansion and the removal of the appliance (end of retention), the differences in width between the sutures at the end of retention suggest that some sutures are affected differentially compared with others and thus affect orthopedic movement.
In our study, the midpalatal suture was opened successfully after RME in all subjects, with a greater magnitude anteriorly than posteriorly. On the axial images, it appeared as a triangular radiolucent area with the base turned anteriorly. Quantitative changes at the midpalatal suture confirmed that the greatest significant increase in width was at the central incisor level followed by the increases in width at the canine and first molar levels, indicating the unparallel pattern of suture opening. The amount of expansion at the first molar level was 75% of the expansion at the central incisor level. These values of suture expansion were similar to those found by some authors and different from others.27,39,40 The differences could be mainly explained by different methods of investigation, times of examination after removing the appliances, and the age groups of the patients in each study.
CONCLUSIONS
Cranial sutures respond differently to the external orthopedic forces according to their anatomic location and the degree of interdigitation. These findings showed that many cranial and circummaxillary sutures are significantly affected by the mechanical forces generated by RME in adolescent subjects. However, the lack of significant opening of other craniofacial sutures supports clinical findings of reduced effectiveness of maxillary expansion and protraction facemasks in adolescents.
Fig 3.
Coronal CT sections showing: A, the zygomaticomaxillary suture before RME; B, the zygomaticomaxillary suture after RME; C, the frontozygomatic suture before RME; D, the frontozygomatic suture after RME.
Footnotes
The authors report no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.da Silva Filho OG, Montes LA, Torelly LF. Rapid maxillary expansion in the deciduous and mixed dentition evaluated through posteroanterior cephalometric analysis. Am J Orthod Dentofacial Orthop. 1995;107:268–75. doi: 10.1016/s0889-5406(95)70142-7. [DOI] [PubMed] [Google Scholar]
- 2.Gautam P, Valiathan A, Adhikari R. Stress and displacement patterns in the craniofacial skeleton with rapid maxillary expansion: a finite element method study. Am J Orthod Dentofacial Orthop. 2007;132:5, e1–511. doi: 10.1016/j.ajodo.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 3.Al-Battikki R. Rapid maxillary expansion: review of literature. Saudi Dent J. 2001;13:161–7. [Google Scholar]
- 4.Davis WM, Kronman JH. Anatomical changes induced by splitting of the midpalatal suture. Angle Orthod. 1969;39:126–32. doi: 10.1043/0003-3219(1969)039<0126:ACIBSO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Pritchard JJ, Scott JH, Girgis FG. The structure and development of cranial and facial sutures. J Anat. 1956;90:73–86. [PMC free article] [PubMed] [Google Scholar]
- 6.Wagemans PA, van de Velde JP, Kuijpers-Jagtman AM. Sutures and forces: a review. Am J Orthod Dentofacial Orthop. 1988;94:129–41. doi: 10.1016/0889-5406(88)90361-7. [DOI] [PubMed] [Google Scholar]
- 7.Persson M. The role of sutures in normal and abnormal craniofacial growth. Acta Odontol Scand. 1995;53:152–61. doi: 10.3109/00016359509005965. [DOI] [PubMed] [Google Scholar]
- 8.Jackson GW, Kokich VG, Shapiro PA. Experimental and postexperimental response to anteriorly directed extraoral force in young Macaca nemestrina. Am J Orthod. 1979;75:318–33. doi: 10.1016/0002-9416(79)90278-1. [DOI] [PubMed] [Google Scholar]
- 9.Kambara T. Dentofacial changes produced by extraoral forward force in the Macaca irus. Am J Orthod. 1977;71:249–77. doi: 10.1016/0002-9416(77)90187-7. [DOI] [PubMed] [Google Scholar]
- 10.Nanda R. Protraction of maxilla in rhesus monkeys by controlled extraoral forces. Am J Orthod. 1978;74:121–41. doi: 10.1016/0002-9416(78)90080-5. [DOI] [PubMed] [Google Scholar]
- 11.Brandt HC, Shapiro PA, Kokich VG. Experimental and postexperimental effects of posteriorly directed extraoral traction in adult Macaca fascicularis. Am J Orthod. 1979;75:301–17. doi: 10.1016/0002-9416(79)90277-x. [DOI] [PubMed] [Google Scholar]
- 12.Gardner GE, Kronman JH. Cranioskeletal displacements caused by rapid palatal expansion in the rhesus monkey. Am J Orthod. 1971;59:146–55. doi: 10.1016/0002-9416(71)90046-7. [DOI] [PubMed] [Google Scholar]
- 13.Debbane EF. A cephalometric and histologic study of the effect of orthodontic expansion of the midpalatal suture of the cat. Am J Orthod. 1958;44:187–219. [Google Scholar]
- 14.Ten Cate AR, Freeman E, Dickinson JB. Sutural development: structure and its response to rapid expansion. Am J Orthod. 1977;71:622–36. doi: 10.1016/0002-9416(77)90279-2. [DOI] [PubMed] [Google Scholar]
- 15.Katsaros C, Kiliaridis S, Berg R. Functional influence on sutural growth. A morphometric study in the anterior facial skeleton of the growing rat. Eur J Orthod. 1994;16:353–60. doi: 10.1093/ejo/16.5.353. [DOI] [PubMed] [Google Scholar]
- 16.Melsen B. A histological study of the influence of sutural morphology and skeletal maturation on rapid palatal expansion in children. Trans Eur Orthod Soc. 1972:499–507. [PubMed] [Google Scholar]
- 17.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129:469, e1–32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Mao JJ. Mechanobiology of craniofacial sutures. J Dent Res. 2002;81:810–6. doi: 10.1177/154405910208101203. [DOI] [PubMed] [Google Scholar]
- 19.Kudlick EM. A study utilizing direct human skulls as models to determine how bones of the craniofacial complex are displaced under the influence of mid-palatal expansion [thesis] Rutherford, NJ: Fairleigh Dickinson University; 1973. [Google Scholar]
- 20.Wertz R, Dreskin M. Midpalatal suture opening: a normative study. Am J Orthod. 1977;71:367–81. doi: 10.1016/0002-9416(77)90241-x. [DOI] [PubMed] [Google Scholar]
- 21.Timms DJ. A study of basal movement with rapid maxillary expansion. Am J Orthod. 1980;77:500–7. doi: 10.1016/0002-9416(80)90129-3. [DOI] [PubMed] [Google Scholar]
- 22.Jafari A, Shetty KS, Kumar M. Study of stress distribution and displacement of various craniofacial structures following application of transverse orthopedic forces–a three-dimensional FEM study. Angle Orthod. 2003;73:12–20. doi: 10.1043/0003-3219(2003)073<0012:SOSDAD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Chaconas SJ, Caputo AA. Observation of orthopedic force distribution produced by maxillary orthodontic appliances. Am J Orthod. 1982;82:492–501. doi: 10.1016/0002-9416(82)90318-9. [DOI] [PubMed] [Google Scholar]
- 24.Shetty V, Caridad JM, Caputo AA, Chaconas SJ. Biomechanical rationale for surgical-orthodontic expansion of the adult maxilla. J Oral Maxillofac Surg. 1994;52:742–9. doi: 10.1016/0278-2391(94)90492-8. [DOI] [PubMed] [Google Scholar]
- 25.Starnbach H, Bayne D, Cleall J, Subtelny JD. Facioskeletal and dental changes resulting from rapid maxillary expansion. Angle Orthod. 1966;36:152–64. doi: 10.1043/0003-3219(1966)036<0152:FADCRF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.Kusakabe T, Caputo AA, Shetty V, Iida J. Biomechanical rationale for surgically facilitated expansion of the maxilla in the cleft palate patient. World J Orthod. 2007;8:167–73. [PubMed] [Google Scholar]
- 27.Haas AJ. Rapid expansion of the maxillary dental arch and nasal cavity by opening the midpalatal suture. Angle Orthod. 1961;31:73–90. [Google Scholar]
- 28.Garib DG, Henriques JF, Janson G, Freitas MR, Coelho RA. Rapid maxillary expansion-tooth tissue-borne versus tooth-borne expanders: a computed tomography evaluation of dentoskeletal effects. Angle Orthod. 2005;75:548–57. doi: 10.1043/0003-3219(2005)75[548:RMETVT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Chung CH, Font B. Skeletal and dental changes in the sagittal, vertical, and transverse dimensions after rapid palatal expansion. Am J Orthod Dentofacial Orthop. 2004;126:569–75. doi: 10.1016/j.ajodo.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 30.Babacan H, Sokucu O, Doruk C, Ay S. Rapid maxillary expansion and surgically assisted rapid maxillary expansion effects on nasal volume. Angle Orthod. 2006;76:66–71. doi: 10.1043/0003-3219(2006)076[0066:RMEASA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Iseri H, Tekkaya AE, Oztan O, Bilgic S. Biomechanical effects of rapid maxillary expansion on the craniofacial skeleton, studied by the finite element method. Eur J Orthod. 1998;20:347–56. doi: 10.1093/ejo/20.4.347. [DOI] [PubMed] [Google Scholar]
- 32.Cross DL, McDonald JP. Effect of rapid maxillary expansion on skeletal, dental, and nasal structures: a postero-anterior cephalometric study. Eur J Orthod. 2000;22:519–28. doi: 10.1093/ejo/22.5.519. [DOI] [PubMed] [Google Scholar]
- 33.Doruk C, Sokucu O, Bicakci AA, Yilmaz U, Tas F. Comparison of nasal volume changes during rapid maxillary expansion using acoustic rhinometry and computed tomography. Eur J Orthod. 2007;29:251–5. doi: 10.1093/ejo/cjl069. [DOI] [PubMed] [Google Scholar]
- 34.Ramires T, Maia RA, Barone JR. Nasal cavity changes and the respiratory standard after maxillary expansion. Braz J Otorhinolaryngol. 2008;74:763–9. doi: 10.1016/S1808-8694(15)31388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melsen B, Melsen F. The postnatal development of the palatomaxillary region studied on human autopsy material. Am J Orthod. 1982;82:329–42. doi: 10.1016/0002-9416(82)90467-5. [DOI] [PubMed] [Google Scholar]
- 36.Baccetti T, Franchi L. The fourth dimension in dentofacial orthopedic: treatment timing for Class II and Class III malocclusion. World J Orthod. 2001;2:159–67. [Google Scholar]
- 37.Lines PA. Adult rapid maxillary expansion with corticotomy. Am J Orthod. 1975;67:44–56. doi: 10.1016/0002-9416(75)90128-1. [DOI] [PubMed] [Google Scholar]
- 38.Bell WH, Epker BN. Surgical-orthodontic expansion of the maxilla. Am J Orthod. 1976;70:517–28. doi: 10.1016/0002-9416(76)90276-1. [DOI] [PubMed] [Google Scholar]
- 39.da Silva Filho OG, Lara TS, de Almeida AM, da Silva HC. Evaluation of the midpalatal suture during rapid palatal expansion in children: a CT study. J Clin Pediatr Dent. 2005;29:231–8. doi: 10.17796/jcpd.29.3.kvu17822u2056508. [DOI] [PubMed] [Google Scholar]
- 40.Podesser B, Williams S, Crismani AG, Bantleon HP. Evaluation of the effects of rapid maxillary expansion in growing children using computer tomography scanning: a pilot study. Eur J Orthod. 2007;29:37–44. doi: 10.1093/ejo/cjl068. [DOI] [PubMed] [Google Scholar]