Although digital breast tomosynthesis improves breast cancer detection, it is critical for radiologists to be cognizant of differences in cancer conspicuity both within the modality itself and between mammographic views; understanding differences in lesion conspicuity and learning strategies to reduce diagnostic errors can help readers improve the clinical outcomes of imaging with DBT.
Abstract
Digital breast tomosynthesis (DBT) represents a valuable addition to breast cancer screening by decreasing recall rates while increasing cancer detection rates. The increased accuracy achieved with DBT is due to the quasi–three-dimensional format of the reconstructed images and the ability to “scroll through” breast tissue in the reconstructed images, thereby reducing the effect of tissue superimposition found with conventional planar digital mammography. The margins of both benign and malignant lesions are more conspicuous at DBT, which allows improved lesion characterization, increased reader confidence, and improved screening outcomes. However, even with the improvements in accuracy achieved with DBT, there remain differences in breast cancer conspicuity by mammographic view. Early data suggest that breast cancers may be more conspicuous on craniocaudal (CC) views than on mediolateral oblique (MLO) views. While some very laterally located breast cancers may be visualized on only the MLO view, the increased conspicuity of cancers on the CC view compared with the MLO view suggests that DBT screening should be performed with two-view imaging. Even with the improved conspicuity of lesions at DBT, there may still be false-negative studies. Subtle lesions seen on only one view may be discounted, and dense and/or complex tissue patterns may make some cancers occult or extremely difficult to detect. Therefore, radiologists should be cognizant of both perceptual and cognitive errors to avoid potential pitfalls in lesion detection and characterization.
©RSNA, 2016
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Describe differences in cancer conspicuity between DBT and DM, by modality as well as by mammographic view.
■ Discuss strategies for improving detection of lesions seen better or only on one DBT view.
■ Identify sources of false-negative findings at DBT.
Introduction
Screening mammography, despite ongoing controversy regarding its risk-to-benefit ratio, remains the mainstay of early breast cancer detection, with routine screening shown to reduce breast cancer mortality by up to 30% (1). Digital breast tomosynthesis (DBT) is rapidly being implemented in breast clinics as the “better mammogram” because it addresses some of the limitations of conventional two-dimensional mammography by alleviating the effect of superimposed structures that lead to both false-positive and false-negative results at breast cancer screening (2–13).
In the largest prospective clinical screening trial that compared digital mammography (DM) alone to combined DM and DBT in 12 631 patients, Skaane et al (4) demonstrated a 15% reduction in recall rate and a 27% increase in cancer detection rate, with a 40% increase in detection of invasive cancers. Similar findings were reported by Friedewald et al (5), who demonstrated a 15% reduction in recall rate and a 29% increase in cancer detection rate when DBT was used in conjunction with DM and compared with DM screening alone. These benefits have been shown to be achievable across all breast densities but appear to be especially large in women younger than 50 years (6,7). In addition, Margolies et al (8) found that DBT significantly changed management in women with dense breasts (13% of cases) compared with women with less dense breasts (9% of cases) (P = .03). More recently, McDonald et al (14) have shown that improvements in screening outcomes with DBT were sustainable over consecutive years of screening in an entire clinic population.
Several studies have demonstrated that DBT results in increased radiologist performance, regardless of an individual’s experience with the modality. Alakhras et al (15) found that radiologist performance was significantly better with combined DBT and DM than with DM alone, regardless of the reader’s experience with the modality, and Thomassin-Naggara et al (16) reported increased sensitivity and improved negative predictive value when one-view DBT was added to DM and compared with DM alone, noting that the benefit was greatest for radiologists less experienced with the modality.
Cancer Conspicuity and Detection at DBT
Several studies have described the increased conspicuity of breast cancers at DBT compared with DM. Andersson et al (17) evaluated the conspicuity of 40 breast cancers at one-view DBT compared with one- and two-view DM and reported that cancers were significantly more conspicuous at one-view DBT (P < .01) and that one-view DBT resulted in a significant increase in Breast Imaging Reporting and Data System (BI-RADS) category upgrades compared with both one- and two-view DM (P < .01 for both). In a study of 199 screening-detected cancers in patients undergoing concurrent DBT and DM, Korhonen et al (18) reported that cancers were more conspicuous on the DBT images than in the DM portion of the study when evaluating the modalities as a whole, as well as when compared by view—the craniocaudal (CC) and mediolateral oblique (MLO) views of each modality (P < .001 for all). In addition, the authors reported that 8.27% of invasive breast cancers were seen at DBT only and not at DM, with only 0.47% of cancers seen at DM alone.
Many factors contribute to an abnormality being seen at DBT only and not at DM. In a series of 268 consecutive BI-RADS category 4 or 5 screening-detected lesions, Ray et al (19) reported that 7% (19 of 268) of lesions were seen at DBT only and were occult at DM, of which 63% occurred in women with heterogeneously dense or extremely dense breasts. Architectural distortion accounted for 74% of the DM-occult lesions, while masses accounted for 26%. Of the 19 lesions seen at DBT only, 53% (10 of 19) were found to be invasive breast cancers, while 70% were invasive lobular carcinomas and 30% were invasive ductal carcinomas (19). Similarly, Partyka et al (20) reviewed 9982 combined DM and DBT screening examinations and showed that of the 26 cases of architectural distortion, 73% (19 of 26) were seen at DBT only, with six of seven of the remaining cases of architectural distortion seen significantly better at DBT compared with DM. Of the cases of architectural distortion seen at DBT only, 21% (four of 19) were breast cancers.
As such, DBT appears to be particularly useful in detection of lesions with architectural distortion that are occult at conventional imaging (Figs 1, 2). Although architectural distortion is only the third most common mammographic manifestation of nonpalpable breast cancer, it is estimated to account for 12%–45% of cases of missed breast cancer (20,21). Burrell et al (22) have reported that architectural distortion also is the most commonly missed mammographic abnormality on false-negative screening studies.
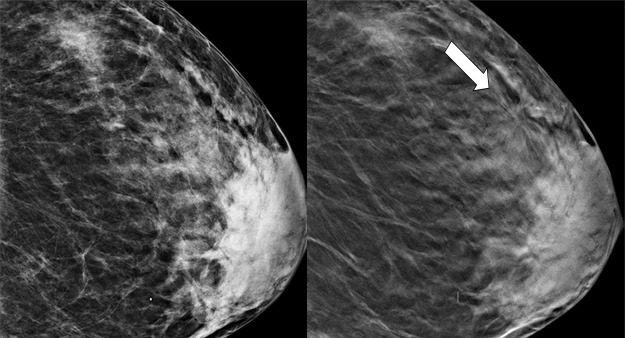
Figure 1a.
Cancer seen only at DBT at routine screening of a 48-year-old woman. CC DM (left) and CC DBT (right) images (a) and MLO DM (left) and MLO DBT (right) images (b) demonstrate that architectural distortion (arrow) is visible in the lateral breast on the DBT images only. Biopsy revealed intermediate-grade invasive ductal carcinoma.
Figure 2a.
Cancer seen only at DBT at routine screening of a 46-year-old woman. (a, b) CC DM (left) and CC DBT (right) images (a) and MLO DM (left) and MLO DBT (right) images (b) demonstrate that an area of subtle architectural distortion (arrow) is visible in the upper outer quadrant on the DBT images only. (c) Ultrasonographic (US) image shows an ill-defined hypoechoic mass. (d) Sagittal magnetic resonance (MR) subtraction image shows a region of architectural distortion with nonmasslike enhancement. Biopsy revealed a 6-cm invasive lobular carcinoma.
Figure 1b.
Cancer seen only at DBT at routine screening of a 48-year-old woman. CC DM (left) and CC DBT (right) images (a) and MLO DM (left) and MLO DBT (right) images (b) demonstrate that architectural distortion (arrow) is visible in the lateral breast on the DBT images only. Biopsy revealed intermediate-grade invasive ductal carcinoma.
Figure 2b.
Cancer seen only at DBT at routine screening of a 46-year-old woman. (a, b) CC DM (left) and CC DBT (right) images (a) and MLO DM (left) and MLO DBT (right) images (b) demonstrate that an area of subtle architectural distortion (arrow) is visible in the upper outer quadrant on the DBT images only. (c) Ultrasonographic (US) image shows an ill-defined hypoechoic mass. (d) Sagittal magnetic resonance (MR) subtraction image shows a region of architectural distortion with nonmasslike enhancement. Biopsy revealed a 6-cm invasive lobular carcinoma.
Figure 2c.

Cancer seen only at DBT at routine screening of a 46-year-old woman. (a, b) CC DM (left) and CC DBT (right) images (a) and MLO DM (left) and MLO DBT (right) images (b) demonstrate that an area of subtle architectural distortion (arrow) is visible in the upper outer quadrant on the DBT images only. (c) Ultrasonographic (US) image shows an ill-defined hypoechoic mass. (d) Sagittal magnetic resonance (MR) subtraction image shows a region of architectural distortion with nonmasslike enhancement. Biopsy revealed a 6-cm invasive lobular carcinoma.
Figure 2d.
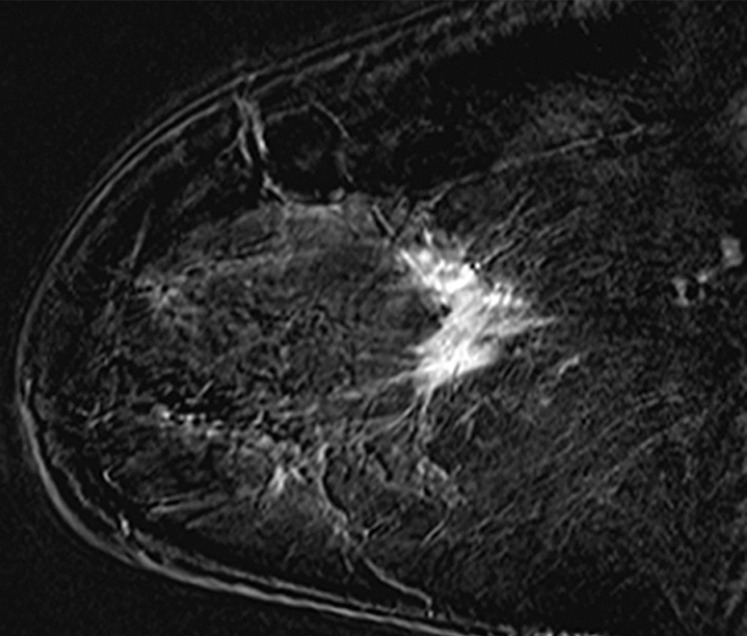
Cancer seen only at DBT at routine screening of a 46-year-old woman. (a, b) CC DM (left) and CC DBT (right) images (a) and MLO DM (left) and MLO DBT (right) images (b) demonstrate that an area of subtle architectural distortion (arrow) is visible in the upper outer quadrant on the DBT images only. (c) Ultrasonographic (US) image shows an ill-defined hypoechoic mass. (d) Sagittal magnetic resonance (MR) subtraction image shows a region of architectural distortion with nonmasslike enhancement. Biopsy revealed a 6-cm invasive lobular carcinoma.
Differences in Conspicuity by View
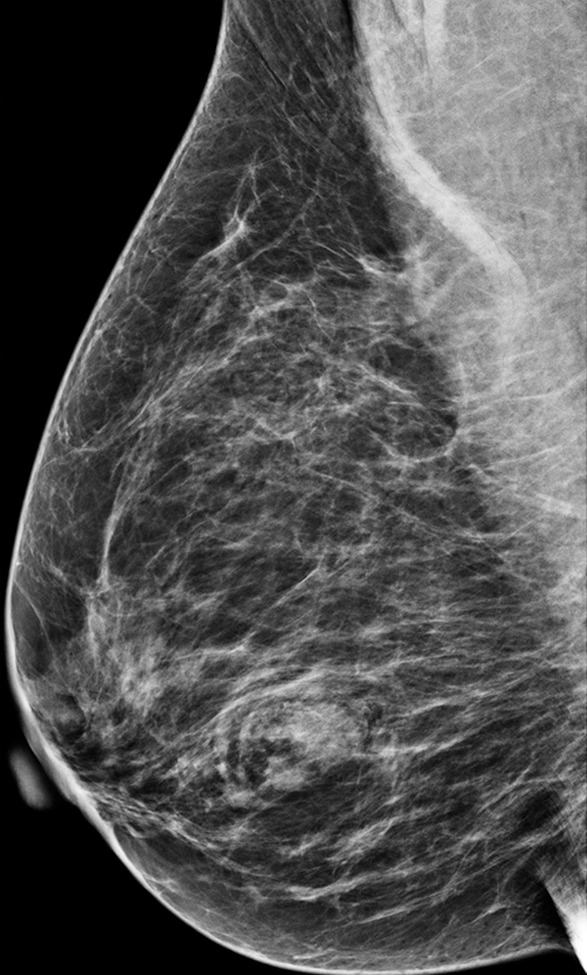
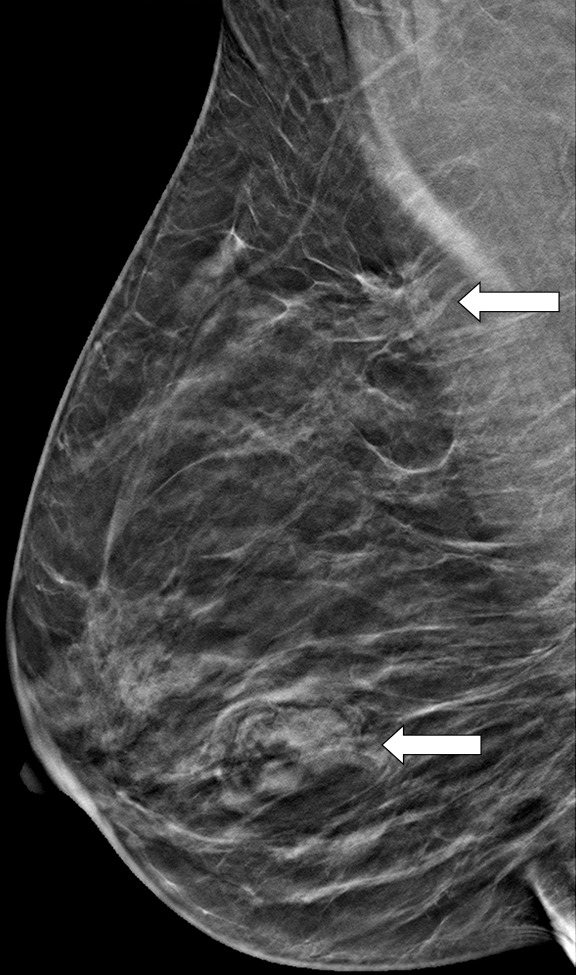
The additional cancer detection achieved with DBT screening is well recognized; however, the conspicuity of lesions on each specific view is not well defined. Beck et al (23), in a study of 115 malignant lesions, found that 35% of cancers were seen better or only on the DBT CC view, whereas only 11% of cancers were seen better or only on the DBT MLO view. Similarly, in a study by Rafferty et al (24) of 34 mixed benign and malignant lesions seen at DBT, 15% of lesions were seen better on the DBT CC view. In addition, Korhonen et al (18), in a reader study reviewing 199 breast cancers imaged with combination DM and DBT screening, reported that cancers were significantly more conspicuous on the CC view than the MLO view at both DM and DBT (P < .001) (Fig 3).
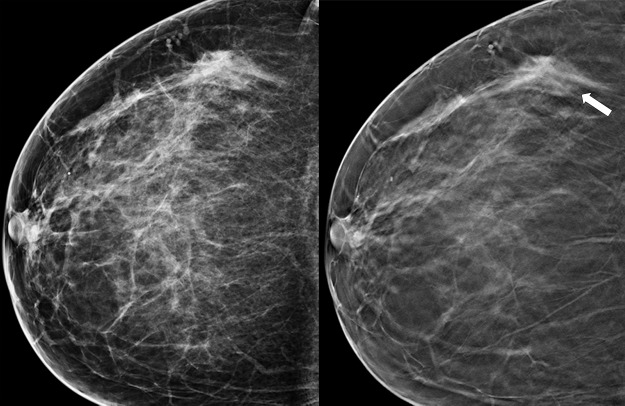
Figure 3a.

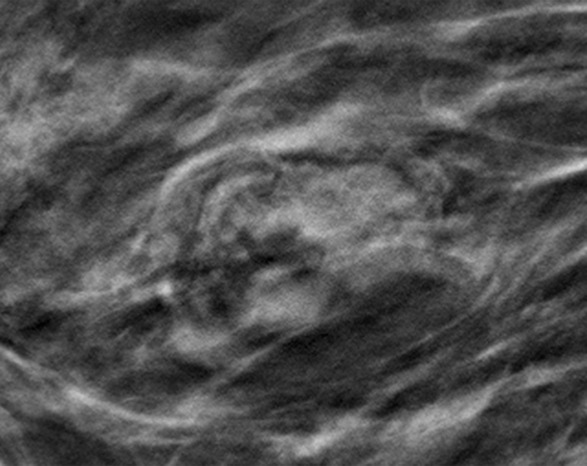
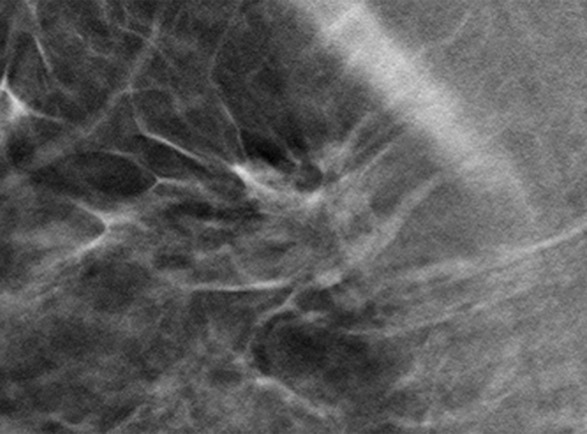
Cancer seen better on CC views than MLO views at both DM and DBT at routine screening of a 78-year-old woman. (a, b) CC DBT image (a) shows an irregular mass with spiculation (arrow in a and b) that was also seen on a CC DM view (not shown). Using the quasi–three-dimensional localization available with the CC DBT view, the lesion was triangulated to the medial and mid breast on the MLO DBT view (b), where it is subtle yet visible. The lesion was not visible on an MLO DM view (not shown) because of superimposed tissue and lack of any detectable distortion. (c) US image shows an irregular hypoechoic mass. Biopsy revealed intermediate-grade invasive ductal carcinoma.
Figure 3b.

Cancer seen better on CC views than MLO views at both DM and DBT at routine screening of a 78-year-old woman. (a, b) CC DBT image (a) shows an irregular mass with spiculation (arrow in a and b) that was also seen on a CC DM view (not shown). Using the quasi–three-dimensional localization available with the CC DBT view, the lesion was triangulated to the medial and mid breast on the MLO DBT view (b), where it is subtle yet visible. The lesion was not visible on an MLO DM view (not shown) because of superimposed tissue and lack of any detectable distortion. (c) US image shows an irregular hypoechoic mass. Biopsy revealed intermediate-grade invasive ductal carcinoma.
Figure 3c.
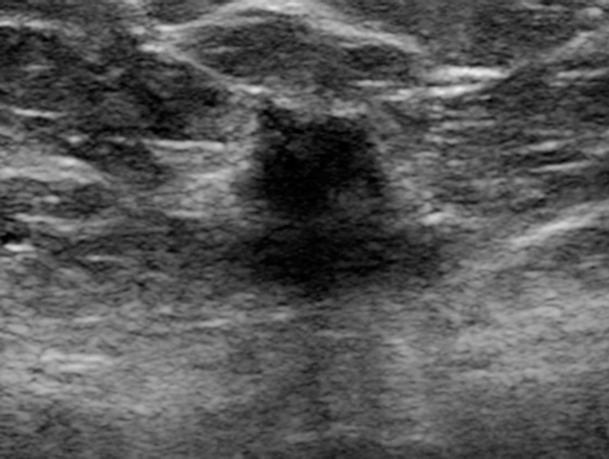
Cancer seen better on CC views than MLO views at both DM and DBT at routine screening of a 78-year-old woman. (a, b) CC DBT image (a) shows an irregular mass with spiculation (arrow in a and b) that was also seen on a CC DM view (not shown). Using the quasi–three-dimensional localization available with the CC DBT view, the lesion was triangulated to the medial and mid breast on the MLO DBT view (b), where it is subtle yet visible. The lesion was not visible on an MLO DM view (not shown) because of superimposed tissue and lack of any detectable distortion. (c) US image shows an irregular hypoechoic mass. Biopsy revealed intermediate-grade invasive ductal carcinoma.
Although there are many potential nonmalignant causes of suspicious findings seen on only one mammographic view, the possibility of malignancy should always be considered because several studies suggest that some subtle breast cancers may be detected on only one view (18,23,24). Potential causes of abnormalities seen on only one view include pseudolesions caused by summation artifacts from overlapping tissues, differences in breast compression due to positioning or nonuniformity of breast tissue, or lesions that are included in the field of view on only one view. For example, the axillary tail of the breast is more optimally viewed on the MLO view than the CC view, but the inferior medial breast is less well seen on MLO views. Portions of the breast high on the chest wall, as well as the most lateral portions of the breast, are less well seen on CC views (25). A review of 2023 one-view–only abnormalities at screening DM by Sickles (26) revealed that 82.7% of the abnormalities were due to summation artifact; however, 36 of the lesions (1.8%) were found to represent malignancies. Because the quasi–three-dimensional format of DBT reduces the confounding effect of tissue superimposition, aiding in visualization and characterization of both benign and malignant breast lesions, malignancy should always be a diagnostic consideration for abnormalities seen better or only on one view at DBT. Careful attention to the quasi–three-dimensional information provided on the DBT image set allows triangulation of subtle one-view–only lesions so that targeted diagnostic imaging is possible. Several studies estimate that 7%–9% of breast cancers are seen on only one view at DBT, while 5%–6% of breast cancers are seen on only the CC view at DBT (18,23,24). If a cancer is visible on only one view, it is more likely to be visible on the CC view than the MLO view (Fig 4).
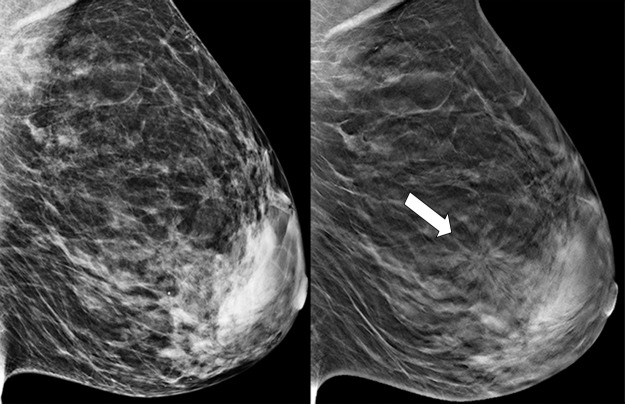
Figure 4a.
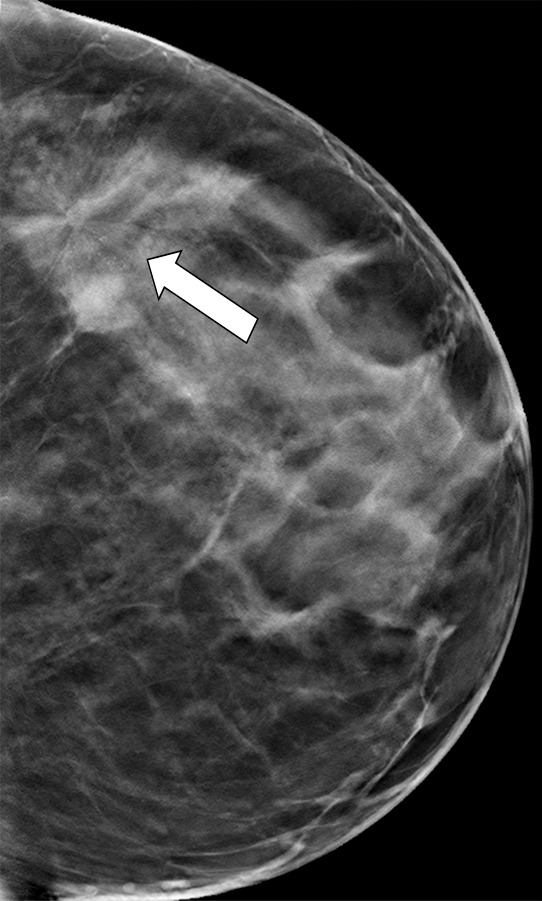
Cancer seen on only the CC DBT view in a 49-year-old woman at routine screening. (a, b) CC DBT image (a) and same view with magnification (b) show an area of architectural distortion (arrow in a) with faint calcifications that was seen only in the lateral breast on the CC DBT view only. (c) MLO DBT image fails to show a definite correlative area of distortion (?). (d) CC DM image shows no evidence of distortion. No corresponding lesions were identified at US. Biopsy revealed intermediate-grade invasive ductal carcinoma associated with ductal carcinoma in situ. Subtle findings seen on only one view at DBT, particularly architectural distortion, should be aggressively pursued because some cancers are not always evident on both CC and MLO views.
Figure 4b.
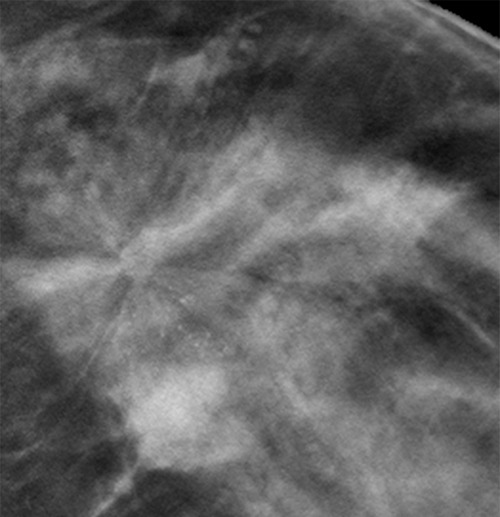
Cancer seen on only the CC DBT view in a 49-year-old woman at routine screening. (a, b) CC DBT image (a) and same view with magnification (b) show an area of architectural distortion (arrow in a) with faint calcifications that was seen only in the lateral breast on the CC DBT view only. (c) MLO DBT image fails to show a definite correlative area of distortion (?). (d) CC DM image shows no evidence of distortion. No corresponding lesions were identified at US. Biopsy revealed intermediate-grade invasive ductal carcinoma associated with ductal carcinoma in situ. Subtle findings seen on only one view at DBT, particularly architectural distortion, should be aggressively pursued because some cancers are not always evident on both CC and MLO views.
Figure 4c.
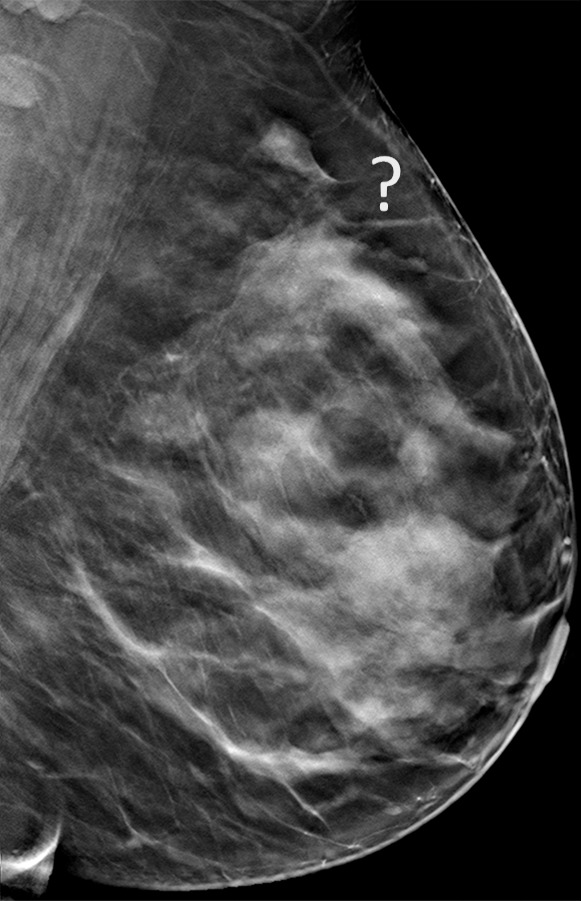
Cancer seen on only the CC DBT view in a 49-year-old woman at routine screening. (a, b) CC DBT image (a) and same view with magnification (b) show an area of architectural distortion (arrow in a) with faint calcifications that was seen only in the lateral breast on the CC DBT view only. (c) MLO DBT image fails to show a definite correlative area of distortion (?). (d) CC DM image shows no evidence of distortion. No corresponding lesions were identified at US. Biopsy revealed intermediate-grade invasive ductal carcinoma associated with ductal carcinoma in situ. Subtle findings seen on only one view at DBT, particularly architectural distortion, should be aggressively pursued because some cancers are not always evident on both CC and MLO views.
Figure 4d.
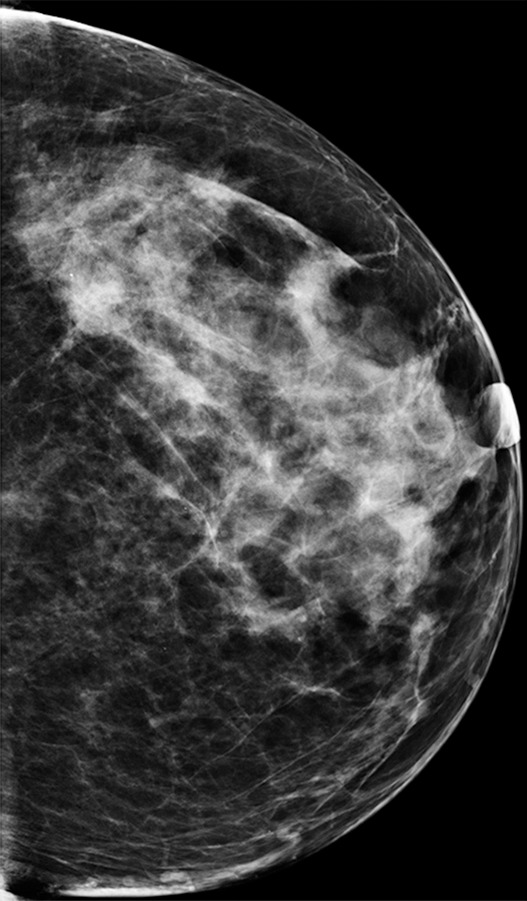
Cancer seen on only the CC DBT view in a 49-year-old woman at routine screening. (a, b) CC DBT image (a) and same view with magnification (b) show an area of architectural distortion (arrow in a) with faint calcifications that was seen only in the lateral breast on the CC DBT view only. (c) MLO DBT image fails to show a definite correlative area of distortion (?). (d) CC DM image shows no evidence of distortion. No corresponding lesions were identified at US. Biopsy revealed intermediate-grade invasive ductal carcinoma associated with ductal carcinoma in situ. Subtle findings seen on only one view at DBT, particularly architectural distortion, should be aggressively pursued because some cancers are not always evident on both CC and MLO views.
Despite evidence that cancers may be seen on only one view, recent studies have explored the use of imaging with one-view–only MLO DBT (27–29). Lång et al (27) reported that the cancer detection rate at one-view MLO DBT was significantly better than at two-view DM (8.9 per 1000 vs 6.3 per 1000) and suggested that one-view MLO DBT could be a feasible stand-alone screening modality without DM. Gennaro et al (28) found that one-view MLO DBT in combination with CC DM was superior to two-view DM for both lesion detection and characterization (overall and for benign lesions) and was noninferior to two-view DM for cancer detection and characterization of malignant lesions. Haq et al (29) argued against the use of second-view DBT in their study because of concerns regarding radiation dose. However, their study evaluated DBT as an alternative to spot-compression views because their patients all had an abnormality seen on one view at DM, with two-view DBT only used to determine if the abnormality seen at DM was seen at same-view DBT, orthogonal-view DBT, neither, or both.
Screening with DBT improves breast cancer detection. However, not all cancers are easily detected, and some lesions may be visible on only one view at two-view DBT. Therefore, suspicious lesions seen on only one view should be thoroughly evaluated.
Interval Cancers
An interval cancer is defined as a symptomatic breast cancer diagnosed in a woman within 1 year of a negative screening mammogram (30). Although false-negative studies represent an unfortunate yet expected component of any breast cancer screening program, interval cancers are associated with less-favorable tumor characteristics as well as less-favorable survival rates compared with screening-detected cancers (31). Therefore, continued review of interval cancers is crucial to any practice, as the rate of interval cancers reflects the efficacy of the screening program as well as a valuable opportunity for improving health care quality. In a study of 231 false-negative cancer screenings, Hofvind et al (31) characterized the cancers into one of three categories: (a) “missed” interval cancers (35% of cases), in which the cancer was clearly present at screening mammography and the patient should have been recalled; (b) “minimal sign” interval cancers (23% of cases), in which subtle or slowly developing signs of cancer were present at screening mammography and the requirement for recall was not obvious; or (c) “true negative” interval cancers (42% of cases), in which the cancer was not present at screening mammography. The authors reported a rate of 1.9 interval cancers for every 1000 patients screened, noting that interval cancer was defined in this study as a cancer manifesting either symptomatically (true interval cancers) or asymptomatically (false negatives) between routine 2-year screening intervals.
Although less is known regarding the interval cancer rate for DBT, there are three studies that have early follow-up data to begin investigation of the interval cancer rate (4,14,32). In Skaane et al’s (4) interval analysis of the first 12 621 subjects screened in a multiarm prospective trial with only 9 months of follow-up, there were three known interval cancers, for a rate of 0.2 per 1000 screened. In the STORM (Screening with Tomosynthesis or Standard Mammography) multiarm reader trial, with a minimum of 13 months of follow-up, the interval cancer rate was 0.82 per 1000 screenings for both the DM and DBT reading arms (each arm had readings under both modalities yet was randomized, so that a comparison of interval cancer between modalities is not possible) (32). In a recent study reporting 3 consecutive years of DBT screening, the interval cancer rate for DBT in year 1 was 0.5, versus 0.7 per 1000 screened for the comparison prior year of DM screening (14).
In the next sections, we explore sources of error in mammographic breast cancer detection to better understand potential pitfalls and identify potential pearls useful at DBT screening.
Error Categorization
Broadly speaking, errors in diagnostic radiology can be categorized as either perceptual errors or cognitive (interpretive) errors, with perceptual errors considerably more common and estimated to account for approximately 60%–80% of diagnostic radiology errors (33,34). Awareness of potential causes of perceptual and interpretive errors can decrease false-negative interpretations. Perceptual errors occur when an abnormality is determined to have been present in retrospect but was not detected prospectively. Several factors contribute to perceptual errors, including poor lesion conspicuity, subtle or atypical manifestations of cancer, the “satisfaction of search” phenomenon, radiologist fatigue, and workplace distractions or interruptions. In the case of perceptual errors, the lesion is not even detected.
Technical factors, such as those related to poor patient positioning or poor breast compression, can contribute to decreased lesion conspicuity. In addition, the location of an abnormality can contribute to decreased lesion conspicuity, particularly for lesions located at the edge of the field of view or at the edge of an image. It is well known that the potential for perceptual errors, both false positive and false negative, is increased in women with dense or complex breast patterns because subtle abnormalities may be obscured by superimposed breast tissue or may be difficult to detect in the setting of busy confounding background breast parenchyma (Fig 5).
Figure 5a.
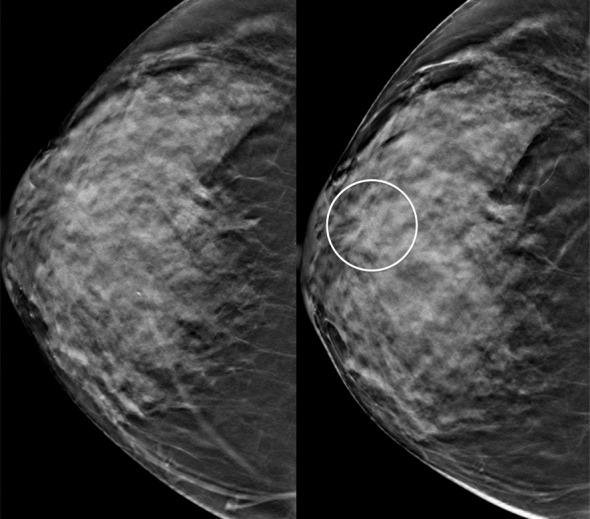
Interval cancer at DBT. (a) CC DBT image obtained at routine screening (left) was interpreted as negative. The patient returned 6 months later with a palpable mass in the subareolar location, which was then detected at DBT on the CC view only (right) as an area of subtle distortion (circle). (b) US image shows an irregular mass with calcifications. Biopsy revealed intermediate-grade invasive ductal carcinoma with associated ductal carcinoma in situ. Dense breast tissue may cause perceptual errors at both DM and DBT because cancers may be obscured by overlying complex tissue patterns. Although DBT has improved cancer detection even in dense breasts, some cancers will be subtle and difficult or impossible to detect.
Figure 5b.
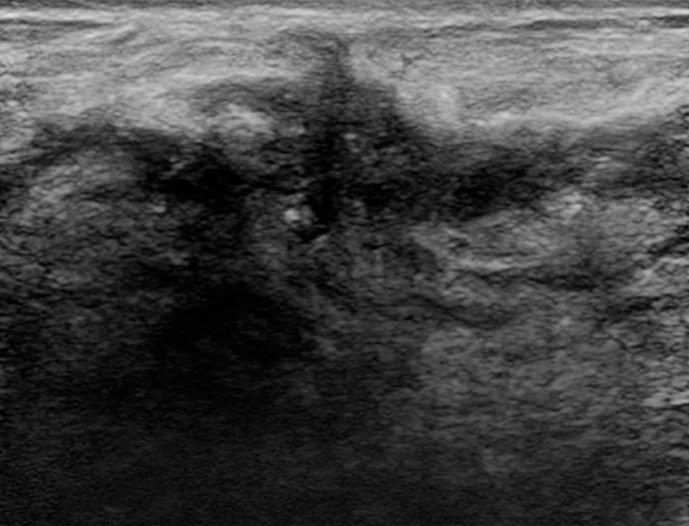
Interval cancer at DBT. (a) CC DBT image obtained at routine screening (left) was interpreted as negative. The patient returned 6 months later with a palpable mass in the subareolar location, which was then detected at DBT on the CC view only (right) as an area of subtle distortion (circle). (b) US image shows an irregular mass with calcifications. Biopsy revealed intermediate-grade invasive ductal carcinoma with associated ductal carcinoma in situ. Dense breast tissue may cause perceptual errors at both DM and DBT because cancers may be obscured by overlying complex tissue patterns. Although DBT has improved cancer detection even in dense breasts, some cancers will be subtle and difficult or impossible to detect.
The satisfaction of search phenomenon represents an additional source of perceptual errors (Fig 6). This occurs when a second abnormality is missed after the reader becomes content after finding an initial abnormality—either benign or malignant. Frequently, these errors occur when readers deviate from their normal search pattern because of the distraction from the detected abnormality. Strategies to reduce perceptual error include minimizing interruptions, working in a quiet environment, and diligently following search patterns to avoid a satisfaction of search error or missing subtle abnormalities seen at only the edge of the image (33,34).
Figure 6a.
Interval cancer missed because of a “satisfaction of search” error in a 73-year-old woman at routine screening. MLO DM (a) and MLO DBT (b) images show a hamartoma in the inferior breast (bottom arrow in b), a finding that is better seen on an electronically magnified DBT image (c). A few days after this negative screening study, the patient’s health care provider detected a lump in the patient’s upper breast. In retrospect, an area of architectural distortion (top arrow in b) is visible in the upper breast on the MLO DBT view and on a magnified view (d). The superior lesion was out of the field of view and therefore not visible on CC DM and DBT views (not shown). Biopsy revealed invasive lobular carcinoma. The satisfaction of search phenomenon represents a common cause of perceptual errors. The radiologist should not stop searching for abnormalities after the first finding because there may be multiple significant findings, including malignancies.
Figure 6b.
Interval cancer missed because of a “satisfaction of search” error in a 73-year-old woman at routine screening. MLO DM (a) and MLO DBT (b) images show a hamartoma in the inferior breast (bottom arrow in b), a finding that is better seen on an electronically magnified DBT image (c). A few days after this negative screening study, the patient’s health care provider detected a lump in the patient’s upper breast. In retrospect, an area of architectural distortion (top arrow in b) is visible in the upper breast on the MLO DBT view and on a magnified view (d). The superior lesion was out of the field of view and therefore not visible on CC DM and DBT views (not shown). Biopsy revealed invasive lobular carcinoma. The satisfaction of search phenomenon represents a common cause of perceptual errors. The radiologist should not stop searching for abnormalities after the first finding because there may be multiple significant findings, including malignancies.
Figure 6c.
Interval cancer missed because of a “satisfaction of search” error in a 73-year-old woman at routine screening. MLO DM (a) and MLO DBT (b) images show a hamartoma in the inferior breast (bottom arrow in b), a finding that is better seen on an electronically magnified DBT image (c). A few days after this negative screening study, the patient’s health care provider detected a lump in the patient’s upper breast. In retrospect, an area of architectural distortion (top arrow in b) is visible in the upper breast on the MLO DBT view and on a magnified view (d). The superior lesion was out of the field of view and therefore not visible on CC DM and DBT views (not shown). Biopsy revealed invasive lobular carcinoma. The satisfaction of search phenomenon represents a common cause of perceptual errors. The radiologist should not stop searching for abnormalities after the first finding because there may be multiple significant findings, including malignancies.
Figure 6d.
Interval cancer missed because of a “satisfaction of search” error in a 73-year-old woman at routine screening. MLO DM (a) and MLO DBT (b) images show a hamartoma in the inferior breast (bottom arrow in b), a finding that is better seen on an electronically magnified DBT image (c). A few days after this negative screening study, the patient’s health care provider detected a lump in the patient’s upper breast. In retrospect, an area of architectural distortion (top arrow in b) is visible in the upper breast on the MLO DBT view and on a magnified view (d). The superior lesion was out of the field of view and therefore not visible on CC DM and DBT views (not shown). Biopsy revealed invasive lobular carcinoma. The satisfaction of search phenomenon represents a common cause of perceptual errors. The radiologist should not stop searching for abnormalities after the first finding because there may be multiple significant findings, including malignancies.
Conversely, cognitive errors occur when an abnormality is prospectively identified at the initial image interpretation but its significance is incorrectly understood, resulting in an incorrect diagnosis (33,34). Factors contributing to cognitive errors include lack of knowledge, bias on the part of the radiologist, and incorrect or misleading clinical information (Fig 7) (33). For example, a stable or very slowly growing lesion over time could be attributed to a benign cause secondary to the lack of change when compared with prior studies (Fig 8). Similarly, a suspicious finding at the site of a prior biopsy could be incorrectly attributed to postsurgical change rather than malignancy (Fig 9). Strategies to reduce cognitive errors include careful side-to-side comparison with multiple prior studies as well as careful attention to the patient’s known clinical history. For example, the imaging appearance of postbiopsy changes after a remote breast biopsy should not change over time, and the location of the patient’s scar should always be confirmed before attributing an abnormality to secondary biopsy-related change.
Figure 7a.
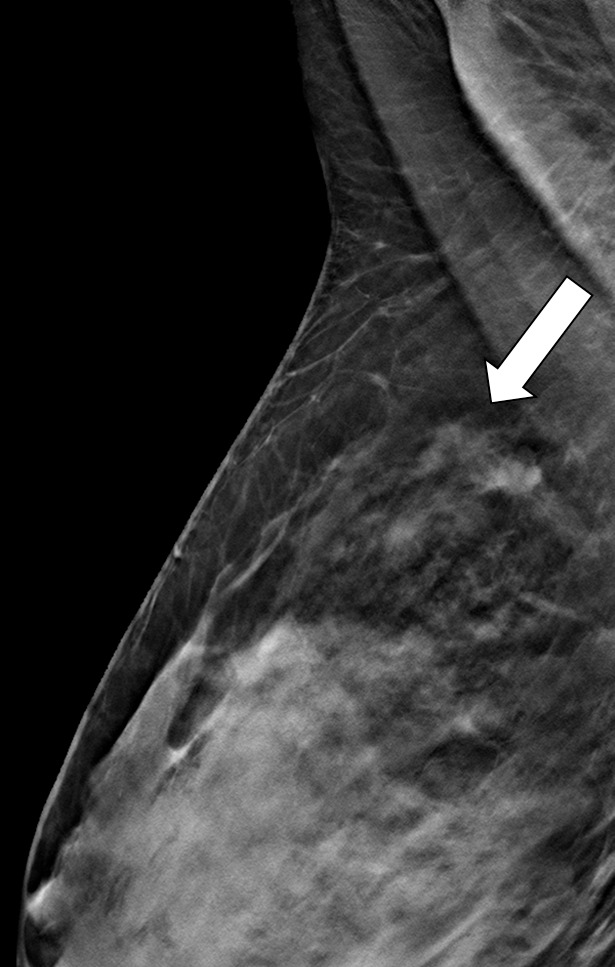
Interval cancer in a 49-year-old woman. (a) MLO DBT image obtained at routine screening shows a possible area of architectural distortion (arrow) in the superior breast that was seen on this view only. (b) MLO DM spot compression image obtained at recall was interpreted as negative. An enlarged lymph node at the edge of the field of view (arrow) was not noted. (c, d) US (c) and sagittal contrast-enhanced T1-weighted MR (d) images obtained 8 months later, when the patient presented with an axillary tail mass, show a corresponding irregular mass (arrow in d). Biopsy revealed poorly differentiated invasive ductal carcinoma. Both a cognitive error (not recognizing the subtle signs of malignancy on the recalled diagnostic study) and a perceptual error (not recognizing the enlarged lymph node at the corner of the image) contributed to a missed opportunity to detect malignancy earlier.
Figure 8a.
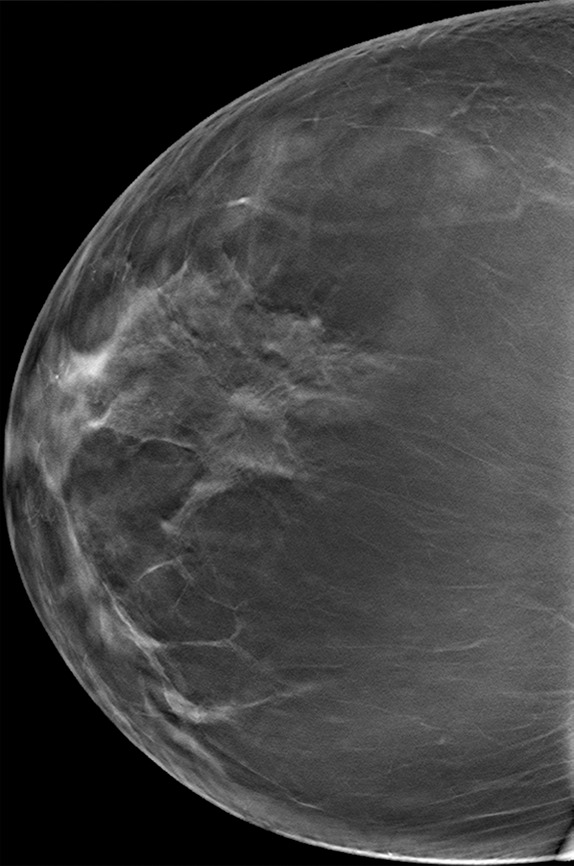
Subtle developing architectural distortion in a 54-year-old woman who presented for 2 consecutive years of screening. (a) CC DBT image obtained at the first screening was interpreted as negative, but in retrospect, a subtle distortion is present centrally. (b) CC DBT image obtained at screening 1 year later shows increasing architectural distortion (oval). Biopsy revealed invasive lobular carcinoma. Subtle distortion such as that seen in a may be difficult to detect, even at DBT, especially if it appears on only one view. At the time of the second study, a side-by-side comparison with the earlier study will aid in detection of malignancy.
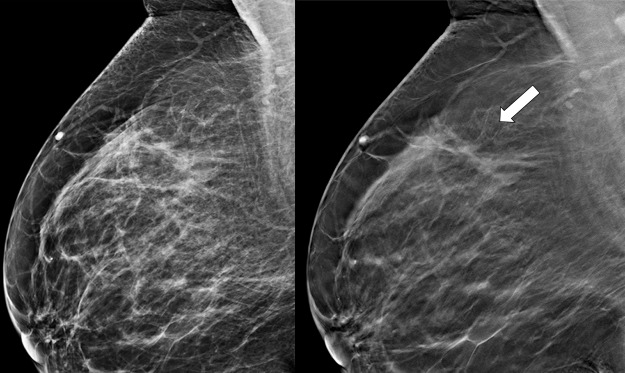
Figure 9a.
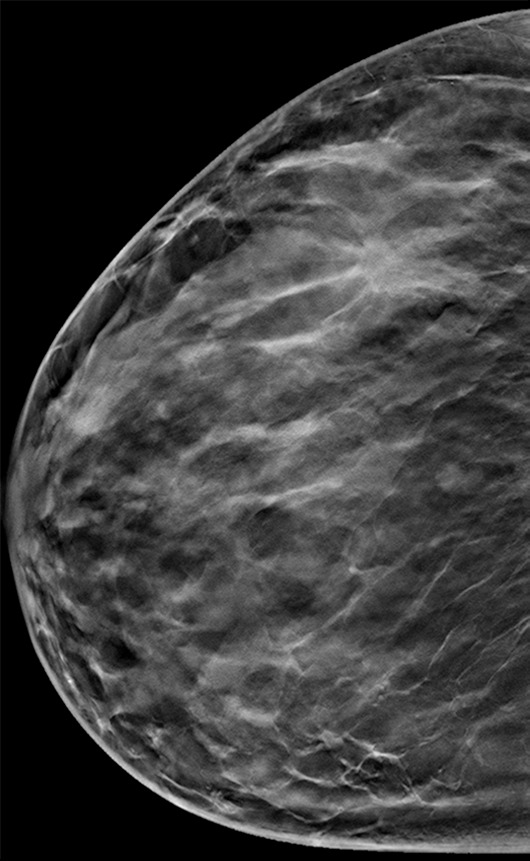
Suspicious finding attributed to postoperative change in a 51-year-old woman with a history of a benign remote surgical biopsy in the upper outer quadrant who presented for routine screening. (a) CC DBT image shows architectural distortion in the right lateral breast, a finding that was incorrectly attributed to the site of the patient’s remote biopsy (which was in the upper outer breast). (b) CC DBT image obtained at screening 1 year later again demonstrates increasing architectural distortion (arrow) in the lateral breast. The finding was correctly triangulated on the single-view CC DBT as in the lower breast (not at the site of the prior surgery in the upper outer breast). (c) With careful attention to the quasi–three-dimensional information obtained from the CC DBT view, the subtle lesion (arrow) can now be seen on an MLO DBT view. (d) US image reveals a hypoechoic spiculated mass. (e) Sagittal maximum intensity projection (MIP) MR image shows an enhancing spiculated mass (arrow) in the central breast. Biopsy revealed intermediate-grade invasive ductal carcinoma. The site of a patient’s surgical scar should always be confirmed before attributing a finding to postbiopsy changes.
Figure 7b.
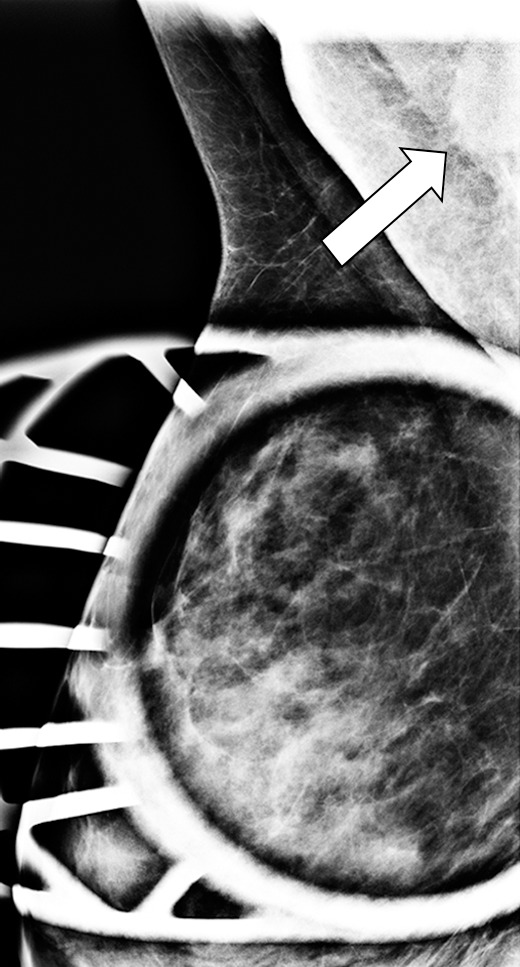
Interval cancer in a 49-year-old woman. (a) MLO DBT image obtained at routine screening shows a possible area of architectural distortion (arrow) in the superior breast that was seen on this view only. (b) MLO DM spot compression image obtained at recall was interpreted as negative. An enlarged lymph node at the edge of the field of view (arrow) was not noted. (c, d) US (c) and sagittal contrast-enhanced T1-weighted MR (d) images obtained 8 months later, when the patient presented with an axillary tail mass, show a corresponding irregular mass (arrow in d). Biopsy revealed poorly differentiated invasive ductal carcinoma. Both a cognitive error (not recognizing the subtle signs of malignancy on the recalled diagnostic study) and a perceptual error (not recognizing the enlarged lymph node at the corner of the image) contributed to a missed opportunity to detect malignancy earlier.
Figure 7c.
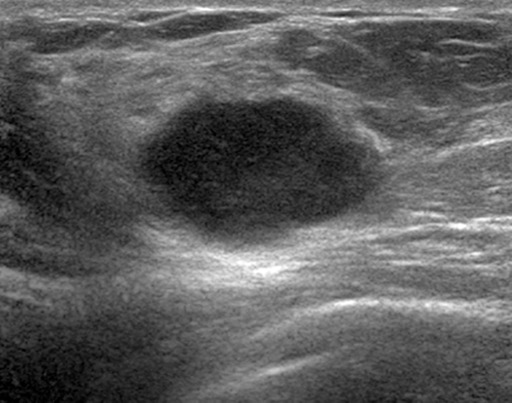
Interval cancer in a 49-year-old woman. (a) MLO DBT image obtained at routine screening shows a possible area of architectural distortion (arrow) in the superior breast that was seen on this view only. (b) MLO DM spot compression image obtained at recall was interpreted as negative. An enlarged lymph node at the edge of the field of view (arrow) was not noted. (c, d) US (c) and sagittal contrast-enhanced T1-weighted MR (d) images obtained 8 months later, when the patient presented with an axillary tail mass, show a corresponding irregular mass (arrow in d). Biopsy revealed poorly differentiated invasive ductal carcinoma. Both a cognitive error (not recognizing the subtle signs of malignancy on the recalled diagnostic study) and a perceptual error (not recognizing the enlarged lymph node at the corner of the image) contributed to a missed opportunity to detect malignancy earlier.
Figure 7d.
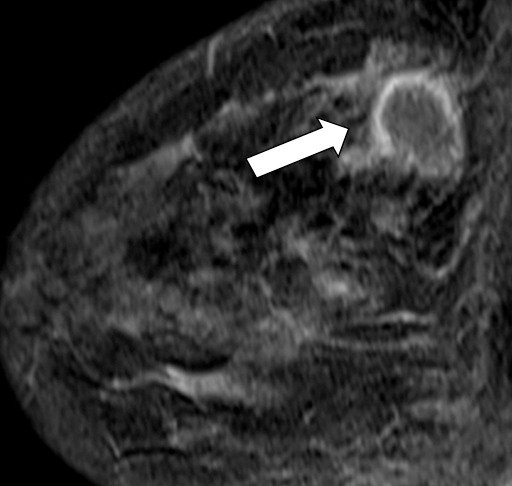
Interval cancer in a 49-year-old woman. (a) MLO DBT image obtained at routine screening shows a possible area of architectural distortion (arrow) in the superior breast that was seen on this view only. (b) MLO DM spot compression image obtained at recall was interpreted as negative. An enlarged lymph node at the edge of the field of view (arrow) was not noted. (c, d) US (c) and sagittal contrast-enhanced T1-weighted MR (d) images obtained 8 months later, when the patient presented with an axillary tail mass, show a corresponding irregular mass (arrow in d). Biopsy revealed poorly differentiated invasive ductal carcinoma. Both a cognitive error (not recognizing the subtle signs of malignancy on the recalled diagnostic study) and a perceptual error (not recognizing the enlarged lymph node at the corner of the image) contributed to a missed opportunity to detect malignancy earlier.
Figure 8b.
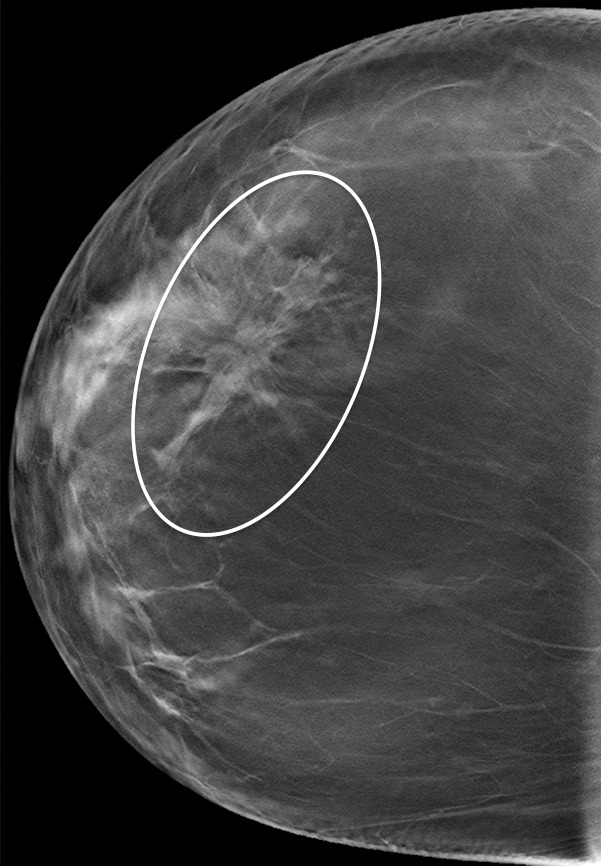
Subtle developing architectural distortion in a 54-year-old woman who presented for 2 consecutive years of screening. (a) CC DBT image obtained at the first screening was interpreted as negative, but in retrospect, a subtle distortion is present centrally. (b) CC DBT image obtained at screening 1 year later shows increasing architectural distortion (oval). Biopsy revealed invasive lobular carcinoma. Subtle distortion such as that seen in a may be difficult to detect, even at DBT, especially if it appears on only one view. At the time of the second study, a side-by-side comparison with the earlier study will aid in detection of malignancy.
Figure 9b.
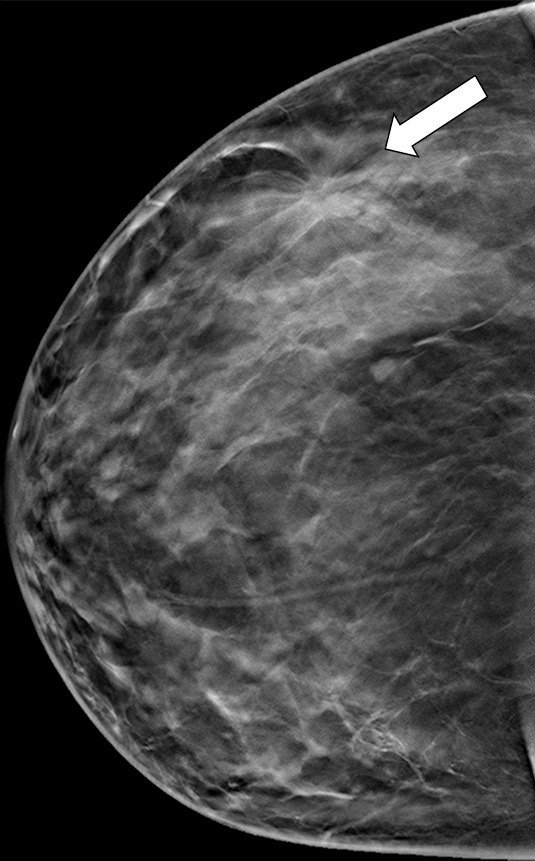
Suspicious finding attributed to postoperative change in a 51-year-old woman with a history of a benign remote surgical biopsy in the upper outer quadrant who presented for routine screening. (a) CC DBT image shows architectural distortion in the right lateral breast, a finding that was incorrectly attributed to the site of the patient’s remote biopsy (which was in the upper outer breast). (b) CC DBT image obtained at screening 1 year later again demonstrates increasing architectural distortion (arrow) in the lateral breast. The finding was correctly triangulated on the single-view CC DBT as in the lower breast (not at the site of the prior surgery in the upper outer breast). (c) With careful attention to the quasi–three-dimensional information obtained from the CC DBT view, the subtle lesion (arrow) can now be seen on an MLO DBT view. (d) US image reveals a hypoechoic spiculated mass. (e) Sagittal maximum intensity projection (MIP) MR image shows an enhancing spiculated mass (arrow) in the central breast. Biopsy revealed intermediate-grade invasive ductal carcinoma. The site of a patient’s surgical scar should always be confirmed before attributing a finding to postbiopsy changes.
Figure 9c.
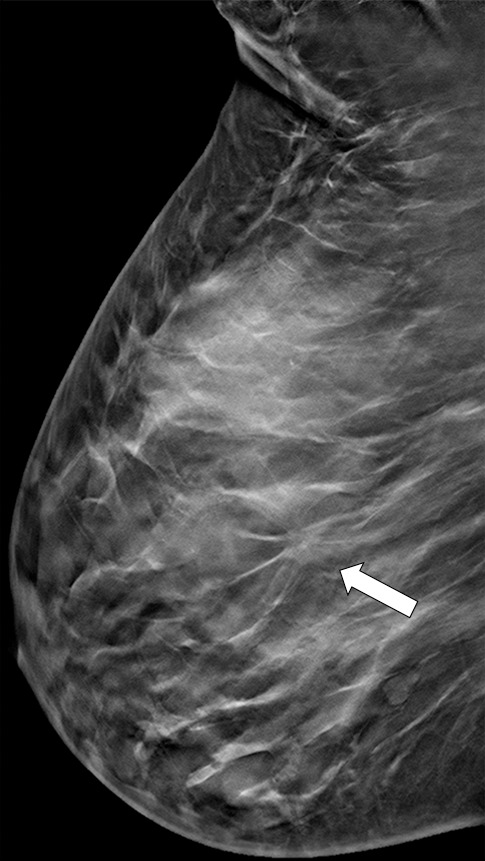
Suspicious finding attributed to postoperative change in a 51-year-old woman with a history of a benign remote surgical biopsy in the upper outer quadrant who presented for routine screening. (a) CC DBT image shows architectural distortion in the right lateral breast, a finding that was incorrectly attributed to the site of the patient’s remote biopsy (which was in the upper outer breast). (b) CC DBT image obtained at screening 1 year later again demonstrates increasing architectural distortion (arrow) in the lateral breast. The finding was correctly triangulated on the single-view CC DBT as in the lower breast (not at the site of the prior surgery in the upper outer breast). (c) With careful attention to the quasi–three-dimensional information obtained from the CC DBT view, the subtle lesion (arrow) can now be seen on an MLO DBT view. (d) US image reveals a hypoechoic spiculated mass. (e) Sagittal maximum intensity projection (MIP) MR image shows an enhancing spiculated mass (arrow) in the central breast. Biopsy revealed intermediate-grade invasive ductal carcinoma. The site of a patient’s surgical scar should always be confirmed before attributing a finding to postbiopsy changes.
Figure 9d.
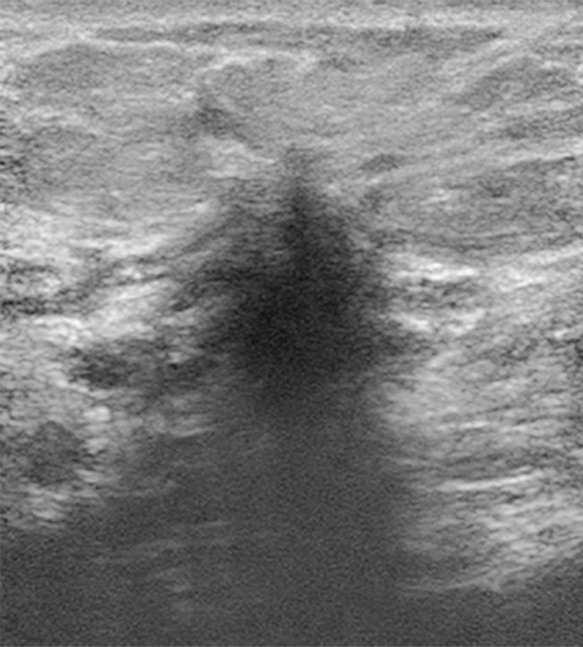
Suspicious finding attributed to postoperative change in a 51-year-old woman with a history of a benign remote surgical biopsy in the upper outer quadrant who presented for routine screening. (a) CC DBT image shows architectural distortion in the right lateral breast, a finding that was incorrectly attributed to the site of the patient’s remote biopsy (which was in the upper outer breast). (b) CC DBT image obtained at screening 1 year later again demonstrates increasing architectural distortion (arrow) in the lateral breast. The finding was correctly triangulated on the single-view CC DBT as in the lower breast (not at the site of the prior surgery in the upper outer breast). (c) With careful attention to the quasi–three-dimensional information obtained from the CC DBT view, the subtle lesion (arrow) can now be seen on an MLO DBT view. (d) US image reveals a hypoechoic spiculated mass. (e) Sagittal maximum intensity projection (MIP) MR image shows an enhancing spiculated mass (arrow) in the central breast. Biopsy revealed intermediate-grade invasive ductal carcinoma. The site of a patient’s surgical scar should always be confirmed before attributing a finding to postbiopsy changes.
Figure 9e.
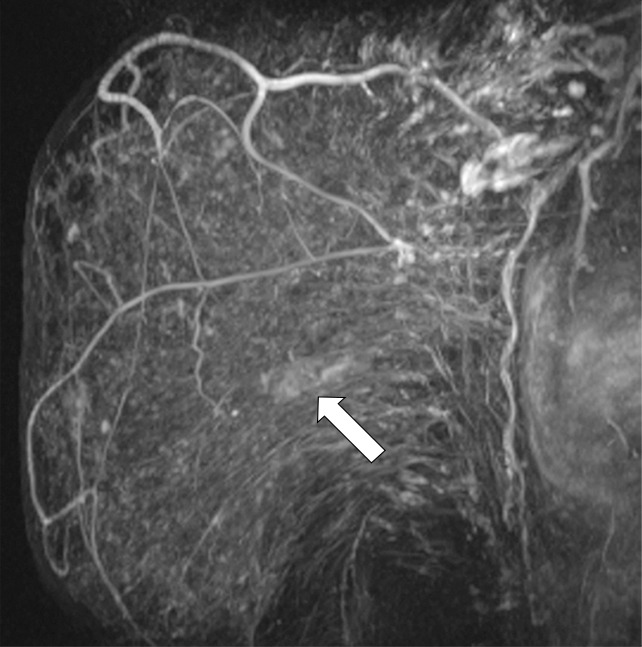
Suspicious finding attributed to postoperative change in a 51-year-old woman with a history of a benign remote surgical biopsy in the upper outer quadrant who presented for routine screening. (a) CC DBT image shows architectural distortion in the right lateral breast, a finding that was incorrectly attributed to the site of the patient’s remote biopsy (which was in the upper outer breast). (b) CC DBT image obtained at screening 1 year later again demonstrates increasing architectural distortion (arrow) in the lateral breast. The finding was correctly triangulated on the single-view CC DBT as in the lower breast (not at the site of the prior surgery in the upper outer breast). (c) With careful attention to the quasi–three-dimensional information obtained from the CC DBT view, the subtle lesion (arrow) can now be seen on an MLO DBT view. (d) US image reveals a hypoechoic spiculated mass. (e) Sagittal maximum intensity projection (MIP) MR image shows an enhancing spiculated mass (arrow) in the central breast. Biopsy revealed intermediate-grade invasive ductal carcinoma. The site of a patient’s surgical scar should always be confirmed before attributing a finding to postbiopsy changes.
Cancers Not Seen at DBT
Although the false-negative rate of DBT screening is less than that of screening with DM alone (14), some cancers are still difficult to detect or nondetectable with DBT. DBT remains an anatomic study without the physiologic information that can be provided by contrast-enhanced imaging modalities such as MR imaging. In our practice, we have encountered several cases of breast cancer that were occult at both DM and DBT imaging but visible at either US or MR imaging (Figs 10, 11). Dense or complex breast parenchymal patterns, subtle or atypical symptoms of breast cancer at presentation, and a lack of secondary signs of malignancy contribute to some breast cancers being occult or missed at DBT.
Figure 10a.

Cancer not initially seen at routine screening in a 53-year-old woman. (a, b) CC DBT (a) and CC DM (b) images were initially interpreted as negative. However, the patient requested screening breast US. (c) US image shows a 7-mm hypoechoic mass in the upper inner quadrant. In retrospect, a small focal asymmetry (circle in a) can be seen on the CC DBT view only. Biopsy revealed intermediate-grade invasive ductal carcinoma. Although the sensitivity of mammography in nondense breasts is high, subtle signs of malignancy may still be overlooked.
Figure 11a.
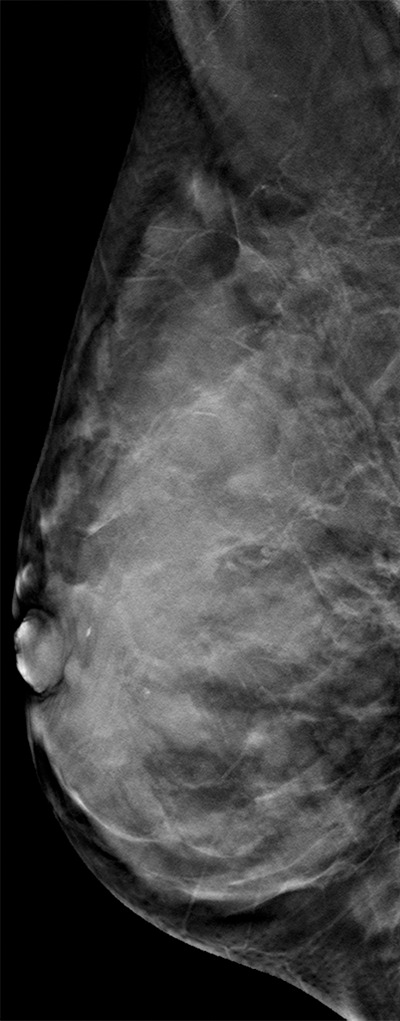
Interval cancer that was DBT occult at routine screening of a 54-year-old woman. (a) MLO DBT image obtained at screening was interpreted as normal. Five months after screening, the patient presented with diffuse pain and thickening in the right breast. (b) Diagnostic MLO DBT image (b) and targeted US images (not shown) were also interpreted as negative. Given the patient’s symptoms, MR imaging was performed. (c) Contrast-enhanced MIP MR image shows diffuse, asymmetric, nonmasslike enhancement. (d) MIP MR image of the normal contralateral breast is shown for comparison. (e) Axial postcontrast fat-suppressed T1-weighted MR image shows global asymmetric nonmasslike enhancement of the right breast relative to the left breast. Biopsy revealed diffuse micropapillary ductal carcinoma in situ with areas of microinvasion. The patient’s clinical symptoms should always guide management, and not all cancers may be seen at DBT.
Figure 10b.
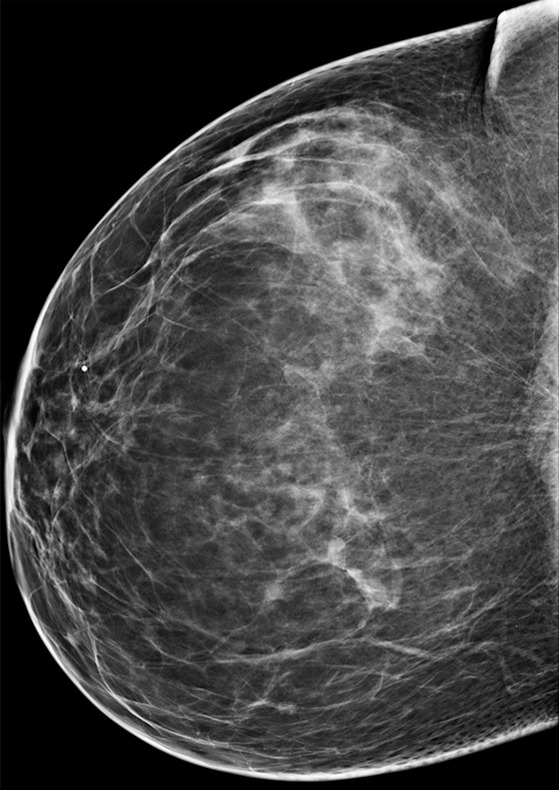
Cancer not initially seen at routine screening in a 53-year-old woman. (a, b) CC DBT (a) and CC DM (b) images were initially interpreted as negative. However, the patient requested screening breast US. (c) US image shows a 7-mm hypoechoic mass in the upper inner quadrant. In retrospect, a small focal asymmetry (circle in a) can be seen on the CC DBT view only. Biopsy revealed intermediate-grade invasive ductal carcinoma. Although the sensitivity of mammography in nondense breasts is high, subtle signs of malignancy may still be overlooked.
Figure 10c.
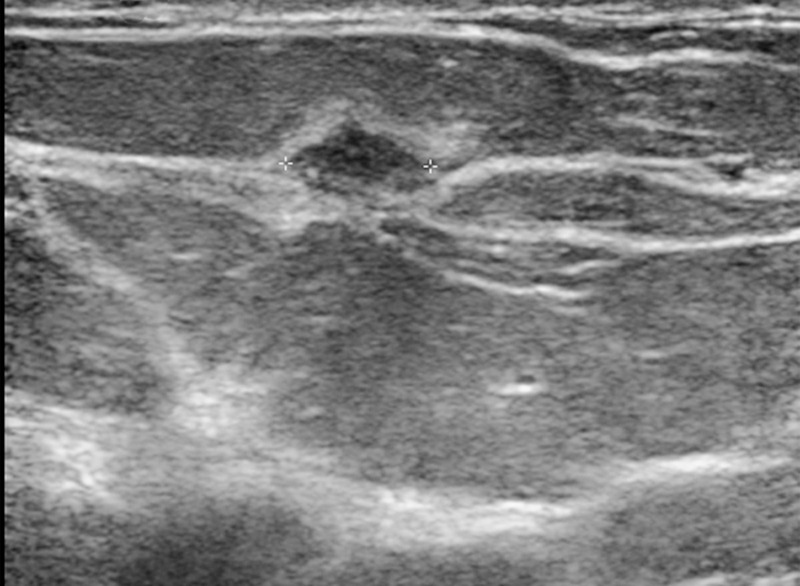
Cancer not initially seen at routine screening in a 53-year-old woman. (a, b) CC DBT (a) and CC DM (b) images were initially interpreted as negative. However, the patient requested screening breast US. (c) US image shows a 7-mm hypoechoic mass in the upper inner quadrant. In retrospect, a small focal asymmetry (circle in a) can be seen on the CC DBT view only. Biopsy revealed intermediate-grade invasive ductal carcinoma. Although the sensitivity of mammography in nondense breasts is high, subtle signs of malignancy may still be overlooked.
Figure 11b.
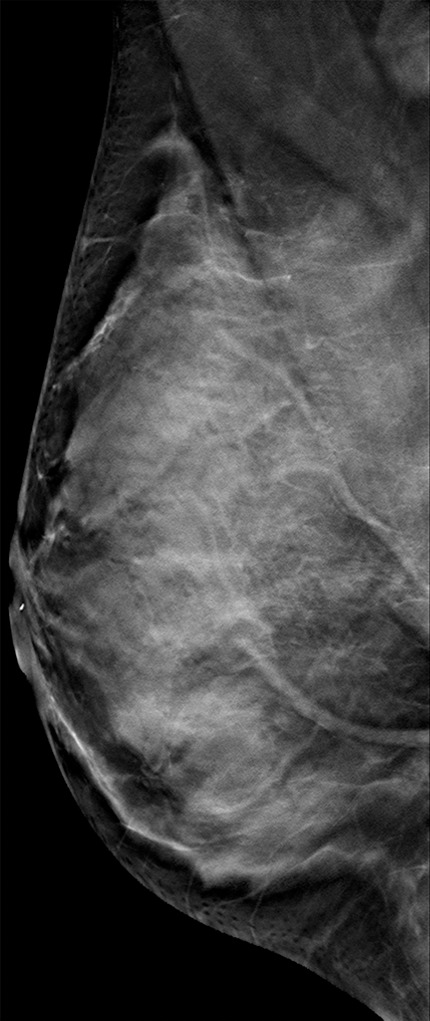
Interval cancer that was DBT occult at routine screening of a 54-year-old woman. (a) MLO DBT image obtained at screening was interpreted as normal. Five months after screening, the patient presented with diffuse pain and thickening in the right breast. (b) Diagnostic MLO DBT image (b) and targeted US images (not shown) were also interpreted as negative. Given the patient’s symptoms, MR imaging was performed. (c) Contrast-enhanced MIP MR image shows diffuse, asymmetric, nonmasslike enhancement. (d) MIP MR image of the normal contralateral breast is shown for comparison. (e) Axial postcontrast fat-suppressed T1-weighted MR image shows global asymmetric nonmasslike enhancement of the right breast relative to the left breast. Biopsy revealed diffuse micropapillary ductal carcinoma in situ with areas of microinvasion. The patient’s clinical symptoms should always guide management, and not all cancers may be seen at DBT.
Figure 11c.
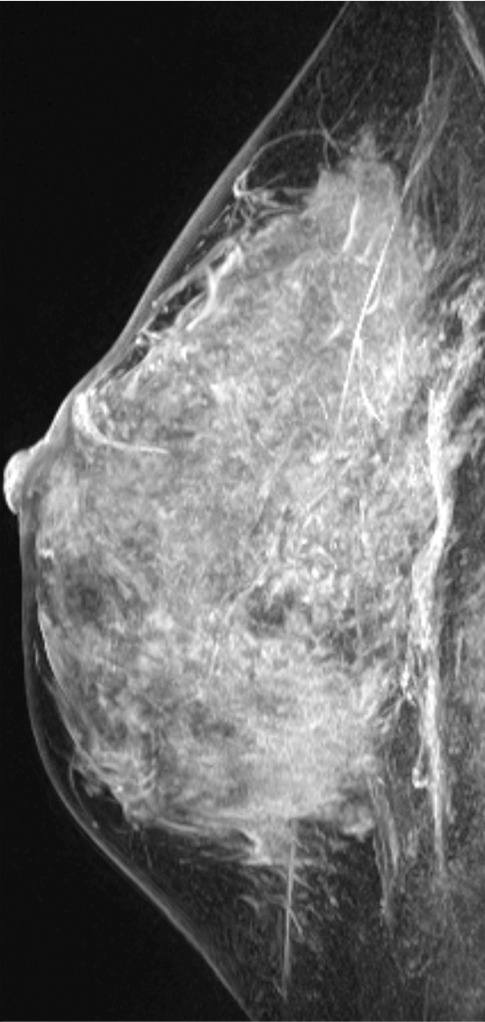
Interval cancer that was DBT occult at routine screening of a 54-year-old woman. (a) MLO DBT image obtained at screening was interpreted as normal. Five months after screening, the patient presented with diffuse pain and thickening in the right breast. (b) Diagnostic MLO DBT image (b) and targeted US images (not shown) were also interpreted as negative. Given the patient’s symptoms, MR imaging was performed. (c) Contrast-enhanced MIP MR image shows diffuse, asymmetric, nonmasslike enhancement. (d) MIP MR image of the normal contralateral breast is shown for comparison. (e) Axial postcontrast fat-suppressed T1-weighted MR image shows global asymmetric nonmasslike enhancement of the right breast relative to the left breast. Biopsy revealed diffuse micropapillary ductal carcinoma in situ with areas of microinvasion. The patient’s clinical symptoms should always guide management, and not all cancers may be seen at DBT.
Figure 11d.
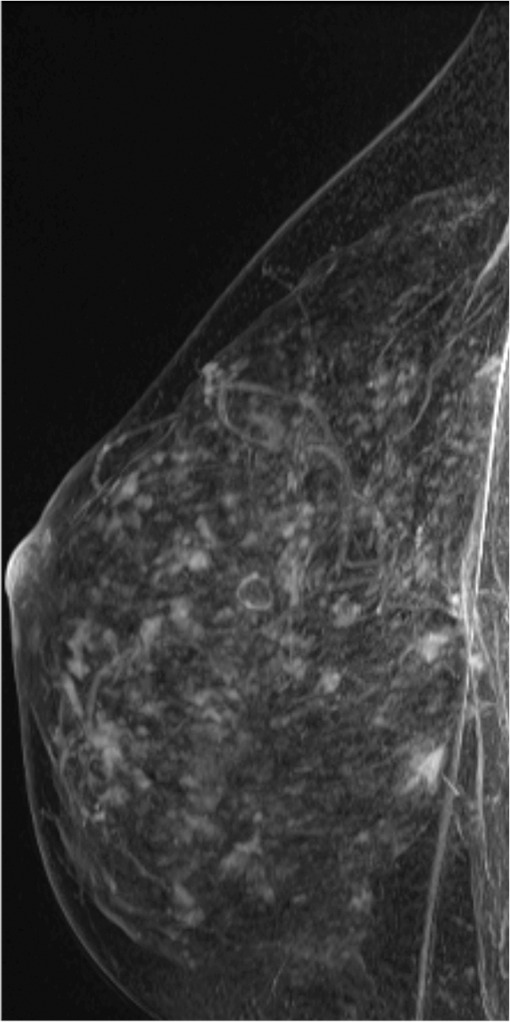
Interval cancer that was DBT occult at routine screening of a 54-year-old woman. (a) MLO DBT image obtained at screening was interpreted as normal. Five months after screening, the patient presented with diffuse pain and thickening in the right breast. (b) Diagnostic MLO DBT image (b) and targeted US images (not shown) were also interpreted as negative. Given the patient’s symptoms, MR imaging was performed. (c) Contrast-enhanced MIP MR image shows diffuse, asymmetric, nonmasslike enhancement. (d) MIP MR image of the normal contralateral breast is shown for comparison. (e) Axial postcontrast fat-suppressed T1-weighted MR image shows global asymmetric nonmasslike enhancement of the right breast relative to the left breast. Biopsy revealed diffuse micropapillary ductal carcinoma in situ with areas of microinvasion. The patient’s clinical symptoms should always guide management, and not all cancers may be seen at DBT.
Figure 11e.
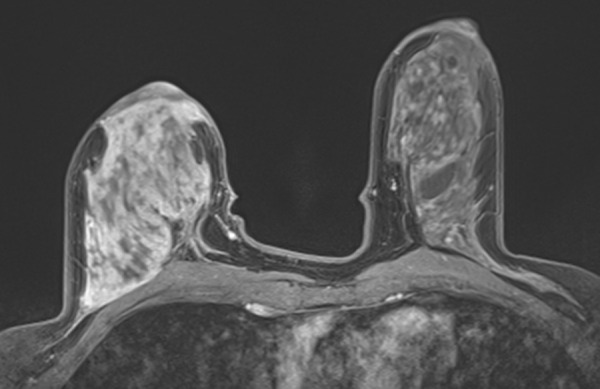
Interval cancer that was DBT occult at routine screening of a 54-year-old woman. (a) MLO DBT image obtained at screening was interpreted as normal. Five months after screening, the patient presented with diffuse pain and thickening in the right breast. (b) Diagnostic MLO DBT image (b) and targeted US images (not shown) were also interpreted as negative. Given the patient’s symptoms, MR imaging was performed. (c) Contrast-enhanced MIP MR image shows diffuse, asymmetric, nonmasslike enhancement. (d) MIP MR image of the normal contralateral breast is shown for comparison. (e) Axial postcontrast fat-suppressed T1-weighted MR image shows global asymmetric nonmasslike enhancement of the right breast relative to the left breast. Biopsy revealed diffuse micropapillary ductal carcinoma in situ with areas of microinvasion. The patient’s clinical symptoms should always guide management, and not all cancers may be seen at DBT.
In addition, although some of these cases occurred in high-risk women undergoing routine surveillance with MR imaging, other cases occurred in women with few or no risk factors who presented with symptoms such as bloody nipple discharge after negative DM and DBT screening. Because not all cancers can be detected at DBT, the patient’s personal breast cancer risk level and clinical symptoms should always guide management. For example, women who present with signs or symptoms of breast cancer should be thoroughly evaluated with multimodality breast imaging, including US and/or MR imaging. In addition, women at high risk for development of breast cancer (ie, BRCA mutation carriers, women with a lifetime risk greater than 20%) should consider supplemental screening with breast MR imaging (35).
Conclusion
DBT represents an important advancement in breast cancer screening. Although cancers are more conspicuous at DBT than at DM, there remain differences in breast cancer conspicuity between mammographic views, with cancer conspicuity improved on the CC view compared with the MLO view. In addition, although cancers may be seen better on one view, we have found several cancers in our practice that were seen on only one view, which suggests a valuable role of two-view DBT in screening.
Although DBT represents a valuable imaging tool, some cancers may be difficult to impossible to detect, and being cognizant of the most common pitfalls in image interpretation as well as of technologic limitations will improve clinical outcomes. Although diagnostic errors cannot be totally eliminated, strategies to limit errors should be incorporated into everyday clinical practice. These strategies include full utilization of the quasi–three-dimensional information included in the DBT image set, adherence to consistent search patterns, avoidance of distractions while reading images, careful comparison with multiple prior studies, and careful attention to the patient’s clinical history.
Interactive Presentation
Recipient of a Magna Cum Laude award for an education exhibit at the 2015 RSNA Annual Meeting.
For this journal-based SA-CME activity, the author E.F.C. has provided disclosures (see “Disclosures of Conflicts of Interest”); all other authors, the editor, and the reviewers have disclosed no relevant relationships.
Supported by the National Institutes of Health Penn Center for Innovation in Personalized Breast Screening (U54CA163313).
Disclosures of Conflicts of Interest.—: E.F.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: personal fees from Hologic and Siemens Healthineers. Other activities: disclosed no relevant relationships.
Abbreviations:
- BI-RADS
- Breast Imaging Reporting and Data System
- CC
- craniocaudal
- DBT
- digital breast tomosynthesis
- DM
- digital mammography
- MIP
- maximum intensity projection
- MLO
- mediolateral oblique
References
- 1.Tabár L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011;260(3):658–663. [DOI] [PubMed] [Google Scholar]
- 2.Roth RG, Maidment ADA, Weinstein SP, Roth SO, Conant EF. Digital breast tomosynthesis: lessons learned from early clinical implementation. Radiographics 2014;34(4):E89–E102. http://pubs.rsna.org/doi/pdf/10.1148/rg.344130087. Published July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology 2013;269(3):694–700. [DOI] [PubMed] [Google Scholar]
- 4.Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013;267(1):47–56. [DOI] [PubMed] [Google Scholar]
- 5.Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2015;311(24):2499–2507. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy AM, Kontos D, Synnestvedt M, et al. Screening outcomes following implementation of digital breast tomosynthesis in a general-population screening program. J Natl Cancer Inst 2014;106(11):dju316. doi:10.1093/jnci/dju316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose SL, Tidwell AL, Bujnoch LJ, Kushwaha AC, Nordmann AS, Sexton R, Jr. Implementation of breast tomosynthesis in a routine screening practice: an observational study. AJR Am J Roentgenol 2013;200(6):1401–1408. [DOI] [PubMed] [Google Scholar]
- 8.Margolies L, Cohen A, Sonnenblick E, et al. Digital breast tomosynthesis changes management in patients seen at a tertiary care breast center. ISRN Radiol 2014;2014:658929. doi:10.1155/2014/658929. Published March 3, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert FJ, Tucker L, Gillan MGC, et al. The TOMMY trial: a comparison of TOMosynthesis with digital MammographY in the UK NHS Breast Screening Programme—a multicentre retrospective reading study comparing the diagnostic performance of digital breast tomosynthesis and digital mammography with digital mammography alone. Health Technol Assess 2015;19(4):i–xxv, 1–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durand MA, Haas BM, Yao X, et al. Early clinical experience with digital breast tomosynthesis for screening mammography. Radiology 2015;274(1):85–92. [DOI] [PubMed] [Google Scholar]
- 11.Rafferty EA, Park JM, Philpotts LE, et al. Diagnostic accuracy and recall rates for digital mammography and digital mammography combined with one-view and two-view tomosynthesis: results of an enriched reader study. AJR Am J Roentgenol 2014;202(2):273–281. [DOI] [PubMed] [Google Scholar]
- 12.Rose SL, Tidwell AL, Ice MF, Nordmann AS, Sexton R, Jr, Song R. A reader study comparing prospective tomosynthesis interpretations with retrospective readings of the corresponding FFDM examinations. Acad Radiol 2014;21(9):1204–1210. [DOI] [PubMed] [Google Scholar]
- 13.Gennaro G, Toledano A, di Maggio C, et al. Digital breast tomosynthesis versus digital mammography: a clinical performance study. Eur Radiol 2010;20(7):1545–1553. [DOI] [PubMed] [Google Scholar]
- 14.McDonald ES, McCarthy AM, Akhtar A, Synnestvedt MB, Schnall M, Conant EF. Baseline screening mammography: performance of full-field digital mammography versus digital breast tomosynthesis. AJR Am J Roentgenol 2015;205(5):1143–1148. [DOI] [PubMed] [Google Scholar]
- 15.Alakhras MM, Brennan PC, Rickard M, Bourne R, Mello-Thoms C. Effect of radiologists’ experience on breast cancer detection and localization using digital breast tomosynthesis. Eur Radiol 2015;25(2):402–409. [DOI] [PubMed] [Google Scholar]
- 16.Thomassin-Naggara I, Perrot N, Dechoux S, et al. Added value of one-view breast tomosynthesis combined with digital mammography according to reader experience. Eur J Radiol 2015;84(2):235–241. [DOI] [PubMed] [Google Scholar]
- 17.Andersson I, Ikeda DM, Zackrisson S, et al. Breast tomosynthesis and digital mammography: a comparison of breast cancer visibility and BIRADS classification in a population of cancers with subtle mammographic findings. Eur Radiol 2008;18(12):2817–2825. [DOI] [PubMed] [Google Scholar]
- 18.Korhonen K, Weinstein S, McDonald ES, Zuckerman S, Conant EF. Screen-detected breast cancer conspicuity on digital breast tomosynthesis versus digital mammography. Presented at the Radiological Society of North America scientific assembly and annual meeting, Chicago, Ill, November 29–December 4, 2015. [Google Scholar]
- 19.Ray KM, Turner E, Sickles EA, Joe BN. Suspicious findings at digital breast tomosynthesis occult to conventional digital mammography: imaging features and pathology findings. Breast J 2015;21(5):538–542. [DOI] [PubMed] [Google Scholar]
- 20.Partyka L, Lourenco AP, Mainiero MB. Detection of mammographically occult architectural distortion on digital breast tomosynthesis screening: initial clinical experience. AJR Am J Roentgenol 2014;203(1):216–222. [DOI] [PubMed] [Google Scholar]
- 21.Knutzen AM, Gisvold JJ. Likelihood of malignant disease for various categories of mammographically detected, nonpalpable breast lesions. Mayo Clin Proc 1993;68(5):454–460. [DOI] [PubMed] [Google Scholar]
- 22.Burrell HC, Sibbering DM, Wilson AR, et al. Screening interval breast cancers: mammographic features and prognosis factors. Radiology 1996;199(3):811–817. [DOI] [PubMed] [Google Scholar]
- 23.Beck N, Butler R, Durand M, et al. One-view versus two-view tomosynthesis: a comparison of breast cancer visibility in the mediolateral oblique and craniocaudal views. Presented at the annual meeting of the American Roentgen Ray Society, Washington, DC, April 2013. [Google Scholar]
- 24.Rafferty EA, Niklason L, Jameson-Meehan L. Breast tomosynthesis: one view or two? [abstr]. In: Radiological Society of North America scientific assembly and annual meeting program. Oak Brook, Ill: Radiological Society of North America, 2006; 335. [Google Scholar]
- 25.Giess CS, Frost EP, Birdwell RL. Interpreting one-view mammographic findings: minimizing callbacks while maximizing cancer detection. RadioGraphics 2014;34(4):928–940. [DOI] [PubMed] [Google Scholar]
- 26.Sickles EA. Findings at mammographic screening on only one standard projection: outcomes analysis. Radiology 1998;208(2):471–475. [DOI] [PubMed] [Google Scholar]
- 27.Lång K, Andersson I, Rosso A, Tingberg A, Timberg P, Zackrisson S. Performance of one-view breast tomosynthesis as a stand-alone breast cancer screening modality: results from the Malmö Breast Tomosynthesis Screening Trial, a population-based study. Eur Radiol 2016;26(1):184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gennaro G, Hendrick RE, Toledano A, et al. Combination of one-view digital breast tomosynthesis with one-view digital mammography versus standard two-view digital mammography: per lesion analysis. Eur Radiol 2013;23(8):2087–2094. [DOI] [PubMed] [Google Scholar]
- 29.Haq R, Lim YY, Maxwell AJ, et al. Digital breast tomosynthesis at screening assessment: are two views always necessary? Br J Radiol 2015;88(1055):20150353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houssami N, Abraham LA, Kerlikowske K, et al. Risk factors for second screen-detected or interval breast cancers in women with a personal history of breast cancer participating in mammography screening. Cancer Epidemiol Biomarkers Prev 2013;22(5):946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofvind S, Geller B, Skaane P. Mammographic features and histopathological findings of interval breast cancers. Acta Radiol 2008;49(9):975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houssami N, Macaskill P, Bernardi D, et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading: evidence to guide future screening strategies. Eur J Cancer 2014;50(10):1799–1807. [DOI] [PubMed] [Google Scholar]
- 33.Bruno MA, Walker EA, Abujudeh HH. Understanding and confronting our mistakes: the epidemiology of error in radiology and strategies for error reduction. RadioGraphics 2015;35(6):1668–1676. [DOI] [PubMed] [Google Scholar]
- 34.Donald JJ, Barnard SA. Common patterns in 558 diagnostic radiology errors. J Med Imaging Radiat Oncol 2012;56(2):173–178. [DOI] [PubMed] [Google Scholar]
- 35.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57(2):75–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.