Abstract
Monocyte/macrophages of patients with mild cognitive impairment (MCI) and Alzheimer disease (AD) are defective in phagocytosis and degradation amyloid β1–42 (Aβ1–42), but are improved by ω-3 fatty acids (ω-3s). The hypothesis of this study was that active Aβ1–42 phagocytosis by macrophages prevents brain amyloidosis and thus maintains cognition. We studied the effects of self-supplementation with a drink with ω-3s, antioxidants, and resveratrol on Mini-Mental State Examination (MMSE) scores, macrophage M1M2 phenotype [the ratio of inflammatory cluster of differentiation (CD)54+CD80 and proresolution markers CD163+CD206], and Aβ1–42 phagocytosis in patients initially diagnosed as having MCI or subjective cognitive impairment (SCI). At baseline, the median MMSE score in patients in both the apolipoprotein E (ApoE) ε3/ε3 and ApoE ε3/ε4 groups was 26.0 and macrophage Aβ1–42 phagocytosis was defective. The MMSE rate of change increased in the ApoE ε3/ε3 group a median 2.2 points per year (P = 0.015 compared to 0) but did not change in the ApoE ε3/ε4 group (P = 0.014 between groups). In the ApoE ε3/ε3 group, all patients remained cognitively stable or improved; in the ApoE ε3/ε4 group, 1 recovered from dementia, but 3 lapsed into dementia. The macrophage phenotype polarized in patients bearing ApoE ε3/ε3 to an intermediate (green zone) M1-M2 type at the rate of 0.226 U/yr, whereas in patients bearing ApoE ε3/ε4, polarization was negative (P = 0.08 between groups). The baseline M1M2 type in the extreme M1 (red zone) or M2 (white zone) was unfavorable for cognitive outcome. Aβ1–42 phagocytosis increased in both ApoE groups (P = 0.03 in each groups). In vitro, the lipidic mediator resolvin D1 (RvD1) down regulated the M1 type in patients with ApoE ε3/ε3 but in some patients with ε3/ε4, paradoxically up-regulated the M1 type. Antioxidant/ω-3/resveratrol supplementation was associated with favorable immune and cognitive responses in ApoE ε3/ε3 and individual patients bearing ApoE ε3/ε4, and brings into personalized clinical practice the immune benefits expected from ω-3 mediators called resolvins. The validity of this study is limited by its small size and uncontrolled design.—Famenini, S., Rigali, E. A., Olivera-Perez, H. M., Dang, J., Chang, M T., Halder, R., Rao, R. V., Pellegrini, M., Porter, V., Bredesen, D., Fiala, M. Increased intermediate M1-M2 macrophage polarization and improved cognition in mild cognitive impairment patients on ω-3 supplementation.
Keywords: ω-3 fatty acids, resolvin D1, amyloid-β phagocytosis, inflammation, ApoE genotype
Despite more than 200 clinical trials in Alzheimer disease (AD), there is no disease-modifying therapy for AD or for mild cognitive impairment (MCI). Actively or passively administered antibodies against amyloid β1–42 (Aβ1–42) have taken the lead in AD immunotherapy, but these approaches have so far encountered insurmountable difficulties caused by autoimmune manifestations and imaging abnormalities and have largely failed in clinical trials (1). We discovered the underlying immune defect of AD in phagocytosis of Aβ1–42 by macrophages in 2007 (2) and since then, have developed an immune approach with lipid mediators that regains functional Aβ1–42 phagocytosis (3). Innate immunity plays a bifunctional role in AD immunopathology: a negative role through IL-1-orchestrated inflammation (4) with activation of the membrane attack complex of complement C5b-9 (5, 6) and macrophage infiltration of the brain with disruption of the blood–brain barrier (7), and a positive role in uptake and clearance of Aβ1–42 by macrophages of healthy subjects (2). Aβ1–42 is the principal pathogenic molecule in the AD brain causing inflammation, neuronal apoptosis, disruption of neuronal connections, and congophilic angiopathy (8). Thus, appropriately regulating inflammation and increasing macrophage Aβ1–42 phagocytosis by ω-3 fatty acids (ω-3s) has a high therapeutic potential against MCI or subjective cognitive impairment (SCI).
The immunopathological mechanisms of failed Aβ1–42 clearance in patients with AD have not been reproduced in mouse models. In the mouse brain, Aβ1–42 clearance is attributable primarily to microglia (9). In the human brain, the macrophages have arguably a critical role in Aβ1–42 clearance, surmised from the defective macrophage phenotype of patients with AD (2) and disruption of the blood–brain barrier by apoptotic macrophages releasing Aβ1–42 into the vessel wall (10). In addition, the transcription of inflammatory genes in peripheral blood mononuclear cells (PBMCs) of patients with AD is deregulated either up or down in comparison to those in control subjects. Therefore, the increase of Aβ1–42 phagocytosis and modulation of inflammation by ω-3 docosahexaenoic acid (DHA), resolvin D1 (RvD1), and 1,25-dihydroxyvitamin D3 (1,25D3) (11) have therapeutic potential. The polyphenol resveratrol activates neuroprotective sirtuin-1 (Sirt1) and the genes for mitochondrial oxidative phosphorylation (12). Functioning macrophages could clear the AD brain, but patients have genetic heterogeneity in the apolipoprotein E (ApoE) gene and differences in macrophage responses. ApoE is a major lipid carrier in the brain. The ApoE ε4 allele is associated with late-onset AD through multiple mechanisms (13), including decreased Aβ1–42 clearance from the brain as observed in a mouse model (14), and functions as a transcription factor through binding to ∼1700 gene promoter regions associated with neuronal health (15). ApoE4 induces a proinflammatory state that is mediated, at least in part, by NF-κB, including a significant elevation of the proinflammatory cytokines IL6 and -8, which are associated with the pathologic changes found in AD (16). In addition, ApoE4 associates with soluble amyloid-precursor protein α (APPα) and reduces Sirt1 mRNA and protein expression (16, 15).
Brain-intrinsic mechanisms of DHA interacting with ApoE have been proposed in mouse models, including a change in the blood–brain barrier (17). However, these mechanisms do not address the immune defects of patients with MCI with specific changes in inflammatory activation, Aβ1–42 phagocytosis, and macrophage phenotype (see below). Health benefits of ω-3s have been extensively investigated and their effectiveness documented in rheumatoid arthritis (18). Epidemiologic studies of subjects at risk of dementia, with or without ω-3 intervention, have examined ω-3 blood levels and demonstrated that lower plasma concentrations of DHA are associated with cognitive decline (17). Seafood consumption correlates with lower AD neuropathology (19). In retrospective and some prospective intervention studies, ω-3s (20, 21) and vitamin D3 (22) had a positive effect on prevention of cognitive decline. The benefits of fish oil were stronger in non-ApoE ε4 subjects (23). In a randomized, placebo-controlled study, ω-3 supplementation reduced the rate of decline of the Mini–Mental State Examination (MMSE) score in comparison with a placebo, but the final MMSE score was below baseline (24). Thus, ω-3s, by themselves or as precursors of specialized proresolving mediators (25), appear appropriate for immunotherapy through brain-intrinsic and -extrinsic effects (26).
In this study, ω-3s were used in a combination with other active ingredients: antioxidants, vitamin D3, and resveratrol. This combination approach is in line with successful combination therapies for AIDS and tuberculosis, as well as with the metabolic enhancement of MCI therapy (27, 28). In a previous study, the Smartfish drink (Smartfish AS, Oslo, Norway) improved Aβ1–42 phagocytosis, normalized inflammation, and increased the anti-inflammatory mediator RvD1 (3). RvD1 is produced endogenously in macrophages from ω-3s (29) (30) and stimulates phagocytosis of Aβ1–42 (11). RvD1 signals through the GPCRs ALX and GPR32 (31), and Aβ1–42 phagocytosis enhancement by RvD1 is inhibited by a blockade of GPR32 (11). In addition, RvD1 promotes the proresolution phenotype of microglia via STAT-6 and PPAR-γ signaling (32).
We examined the effects of ω-3 supplementation on macrophage phenotype, phagocytosis, and MMSE in patients with subjective and objective defects in memory. Macrophage phenotype is involved in the outcome of many diseases, tissue homeostasis, and resolution or nonresolution of inflammation (33). The M1 status is associated with inflammatory conditions and is mitigated by ω-3s (34). Alternative activation of macrophages to M2 status has a bifunctional role in diseases, such as asthma (35, 36) and pancreatic fibrosis (37). We speculate that the outcome of immune therapy for patients with MCI depends in part on modulating inflammatory M1 (red zone) or anti-inflammatory M2 (white zone) macrophages at baseline to a proresolution and prophagocytic intermediate M1-M2 (green zone) phenotype.
Given the failures of previous monotherapeutic regimens and the advantages of combination therapies (27), we provided all but 1 patient with a drink containing ω-3s in combination with antioxidants, vitamin D3, and resveratrol. This drink has been used with success in controlled trials of behavioral problems (38) and obesity management. Nonetheless, in comparison to other disorders, the investigation of patients with MCI has been complicated by their baseline heterogeneity (11, 39), in part related to ApoE genotype. The patients selected for the study qualified as having MCI or SCI on admission, according to cognitive testing and shared the universal defect in Aβ1–42 phagocytosis. Imaging later showed specific diagnosis in 2 patients as Lewy body disease and vascular dementia. Our study has relevance to independently living patients with memory problems, irrespective of their pathology or brain imaging results.
MATERIALS AND METHODS
Study design and population
We performed an observational study by immune and cognitive tests of 18 independently living patients taking voluntary supplementation with the Smartfish ω-3 drink. Fifteen patients had the initial diagnosis of MCI by the criteria of Petersen et al. (40) and 3 patients the diagnosis of SCI, according to subjective complaints and subtle cognitive defects (41). Eleven patients (5 ApoE ε3/ε3 and 6 ApoE ε3/ε4) were followed by M1M2 testing in 90 visits. Cognition was tested by MMSE administered by the investigator. All subjects signed an informed consent approved by the University of California, Los Angeles Institutional Review Board.
ω-3 nutritional supplementation
The patients took voluntary daily supplementation with the ω-3 drink (Smartfish AS) [a 200-ml emulsion containing 1000 mg DHA and 1000 mg eicosapentaenoic acid (EPA), both Pure arctic 360; Denomega, Oslo, Norway] protected against oxidation through nano-sized, stabilized emulsion droplets; 5 g whey protein (Lacprodan DI-7017; Arla Foods; Aarhus, Denmark), 0.2 g lactose; 18 g carbohydrate from pomegranate and chokeberry juice; 10 μg vitamin D3 stabilized with tocopherol (DSM Nutritional Products, Basel, Switzerland); and 150 mg resveratrol (resVida, DSM). One patient had been taking a softgel ω-3 capsule (300 mg ω-3) per day (Nature’s Bounty, Bohemia, NY, USA).
Isolation of PBMCs and macrophage cultures
PBMCs were isolated from diluted heparin-anticoagulated blood by the Ficoll-Hypaque (Sigma-Aldrich, St. Louis, MO, USA) gradient method at 3000 rpm centrifugation for 20 min at room temperature. The mononuclear fraction was collected and washed 2 times with PBS, and the cells were resuspended with Iscove’s modified Dulbecco’s medium (IMDM). Macrophages were differentiated from 50,000 mononuclear cells by culture in IMDM with 10% autologous serum for 8–12 d in 8-well culture plates (Discovery Labware; BD Biosciences, Bedford, MA, USA).
Macrophage phenotyping
Macrophages in 8-well chambers were washed with PBS, fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and stained with antibodies against cluster of differentiation (CD)54 (intercellular adhesion molecule-1), CD80 (B7-1), CD163 (scavenger receptor), and CD206 (mannose receptor) (all from BioLegend, San Diego, CA, USA; 2.5 μg/ml in PBS containing 1% bovine serum albumin), followed by appropriate secondary Alexa 565 antibodies and phalloidin-FITC (Sigma-Aldrich). Other markers included arginase-1 for M2 macrophages, and NOS2, TNF-α, IL-1β, IL-6, and IL-12 for M1 macrophages. The preparations were examined with a BX60 microscope with a ×20 objective (Olympus, Melville, NY, USA). Three images were obtained in the order top, middle, and bottom of the dense macrophage growth in each well. The images were scanned with Image-Pro software (MediaCybernetics, Rockville, MD, USA). The M1-M2 type was calculated as the ratio of (CD54+CD80)/(CD163+CD206) in mean fluorescence intensity (MFI) units.
Modulation of macrophage type in vitro
Macrophages were incubated for 18 h, with or without RvD1 or -3 (27 ng/ml; Cayman Chemicals, Ann Arbor, MI, USA) and were phenotyped with CD54, CD80, CD163, and CD206 before and after the treatment.
Flow cytometric fluorescent Aβ phagocytosis assay by monocytes
PBMCs (0.5 × 106) were suspended in IMDM with 10% autologous serum and were incubated with or without 2 µg/ml of fluorescent Aβ1–42, HiLyte Fluor 488 (Anaspec, Fremont, CA, USA) overnight at 37°C in a 5% CO2 incubator. The cells were washed 2 times with a fluorescence-activated cell sorting (FACS) buffer and then labeled for 30 min at 4°C with anti-CD14 PE (BD Biosciences). After incubation, the cells were washed 2 times with FACS buffer and fixed with 1% paraformaldehyde. Flow cytometry was performed on FACSCalibur (BD Biosciences). The data were analyzed with FlowJo software (Tree Star, Ashland, OR, USA) with a monocyte gating, based on forward and side scatter, and the results are expressed in MFI units.
Restriction isotyping of human ApoE
ApoE genotyping to identify the ApoE alleles was performed as described elsewhere (16). The method involved using primer pairs to amplify the region set in the common sequence parts of ApoE isoforms. The amplified products were later digested with HhaI and subjected to electrophoresis on polyacrylamide gels. Each of the isoforms was distinguished by a unique combination of HhaI fragment sizes that enabled explicit typing of all homozygotic and heterozygotic combinations.
Statistical methods
For a given subject and outcome, the MMSE and MFI rates of change were computed as rate of change per year = 12[(last value − first value)/n mo follow-up] from first to last measurement. The M1/M2 ratio was coded 1 if the value was between 1 and 4 (in the green zone) and was coded 0 if below 1 or greater than 4 (outside of the green zone). The rates of change of these 0, 1 values were then computed for each subject as above. Rates of change were summarized with their medians. The P values for comparing continuous variables between groups including rates were computed with the nonparametric Kruskal-Wallis test, because the data did not follow normal distribution. The nonparametric Wilcoxon signed rank test was used to compute P values for comparing median rates to 0. Computations were performed with SAS 9.4 and JMP 12.0.1 (SAS, Inc., Cary, NC, USA).
RESULTS
Design of the study and overall results
The study was an observation of patients with MCI on voluntary self-supplementation by ω-3s in the Smartfish drink. The patients were examined at each visit in 1–3 mo intervals by history and by testing macrophage type, level of Aβ1–42 phagocytosis, and MMSE score. The phenotype was scored according to the ratio of inflammatory over proresolution markers: (CD54+CD90)/(CD163+CD206). The M1M2 phenotype was considered to be noninflammatory (white zone) when the ratio was <1.0; inflammatory M1 (red zone) when the ratio was >4.0; and a proresolution M1-M2 (green zone associated with optimal Aβ1–42 phagocytosis) when the ratio was 1.0–4.0. The M1M2 results in the green zone were scored as 1 and in the white or red zone as 0. The results were analyzed according to the ApoE genotype (Fig. 1 and Table 1).
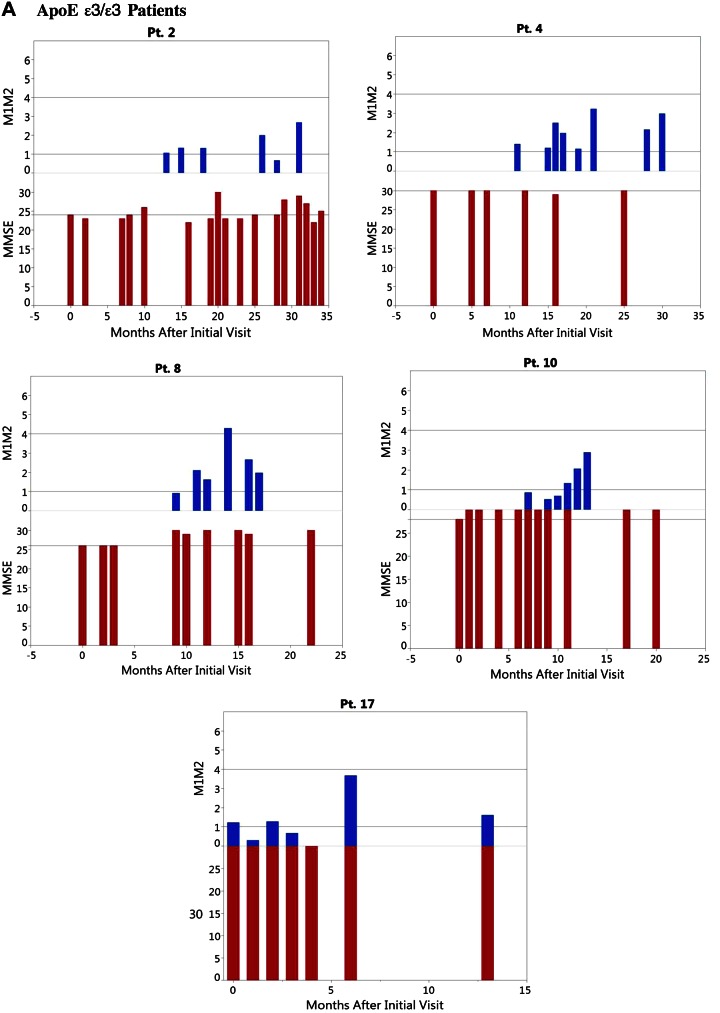
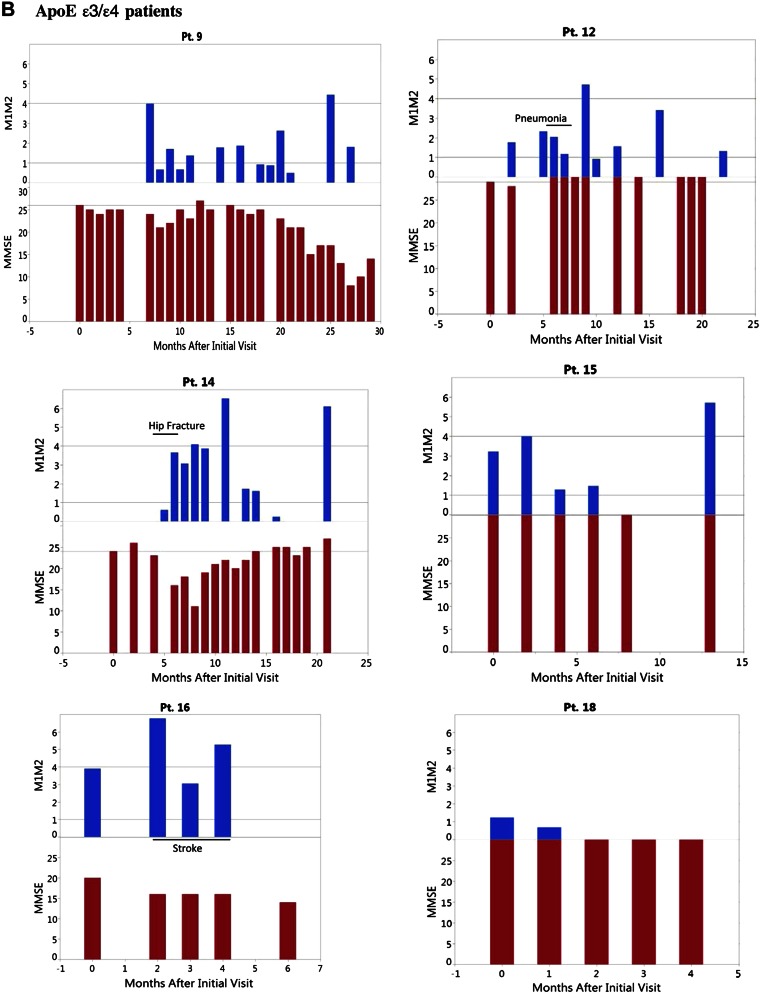
Figure 1.
Immune and cognitive results in 5 patients with APO ε3/ε3 (A) and 6 with APO ε3/ε4 (B), according to the time after onset of ω-3 supplementation.
TABLE 1.
Immune (M1-M2) and cognitive (MMSE) results in patients bearing ApoE ε3/ε3 and ApoE ε3/ε4 MCI and taking the ω-3-supplemented drink
|
P |
|||||||
|---|---|---|---|---|---|---|---|
| Variable | Group | n | Median | Min | Max | Between groups | Within group vs. 0 |
| M1/M2 | |||||||
| Follow-up (mo) | ε3/ε3 | 5 | 6.0 | 5 | 15 | 0.5065 | |
| ε3/ε4 | 5 | 5.0 | 0 | 18 | |||
| Green zone | |||||||
| Proportion | |||||||
| 60% | ε3/ε3 | 5 | 1.05 | 0.86 | 1.4 | 0.1877 | |
| 70% | ε3/ε4 | 5 | 1.97 | 0.26 | 4.0 | ||
| Rate of change (yr−1) | ε3/ε3 | 5 | 0.23 | −0.27 | 2.6 | 0.3750 | |
| ε3/ε4 | 5 | −0.06 | −2.06 | 0.0 | 0.0820 | 0.1250 | |
| MFI, M1-M2 | |||||||
| Follow-up (mo) | ε3/ε3 | 9 | 3 | 1 | 22 | 0.5915 | |
| ε3/ε4 | 6 | 5 | 3 | 16 | |||
| Initial value | ε3/ε3 | 9 | 437 | 54.1 | 1,011 | 0.2875 | |
| ε3/ε4 | 6 | 414 | 399 | 433 | |||
| Rate of change (yr−1) | ε3/ε3 | 9 | 1041 | −2,064 | 10,308 | 0.4795 | 0.0391 |
| ε3/ε4 | 6 | 2213 | 648 | 4,026 | 0.0313 | ||
| MMSE | |||||||
| Follow-up (mo) | ε3/ε3 | 9 | 11 | 1 | 29 | 0.7217 | |
| ε3/ε4 | 9 | 6 | 1 | 25 | |||
| Initial value | ε3/ε3 | 9 | 26.0 | 24 | 30 | 0.4416 | |
| ε3/ε4 | 9 | 26.0 | 20 | 30 | |||
| Rate of change (yr−1) | ε3/ε3 | 9 | 2.2 | 0.0 | 48.0 | 0.0146 | 0.0156 |
| ε3/ε4 | 9 | 0.0 | −20.0 | 12.0 | 0.4063 | ||
Significant differences within and between groups are in italics. Rate of change (yr−1) into the green zone (M1/M2 ratio = 1–4) based on the coding as 1 (M1/M2 ratio = 1–4) ϴ (M1/M2 ratio <1 or >4).
The purpose of the study was to demonstrate the effect of ω-3s on 3 main objectives: 1) MMSE rate of change (data available in 9 patients with ApoE ε3/ε3 and 9 with ApoE ε3/ε4); 2) green zone M1-M2 phenotype change (data available in 5 patients with ApoE ε3/ε3 and 6 with ApoE ε3/ε4); and 3) correlation between MMSE and M1-M2 phenotype (Table 1). The overall results were as presented below.
Objective 1
MMSE rate in the ApoE ε3/ε3 group (9 patients): median change by 0 points per year (P = 0.015 compared to 0); in the ApoE ε3/ε4 group (9 patients): median change by 0 points (P = 0.40 compared to 0); significant difference between groups (P = 0.014) (Table 1). In the ApoE ε3/ε3 group, 4 patients had cognitive improvement of 2 or more points, but in the ApoE ε3/ε4 group 4 patients showed severe cognitive loss at the last follow-up (Table 2).
TABLE 2.
Individual immune and cognitive results in patients taking the ω-3-supplmented drink
| M1/M2 |
MFI |
MMSE |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Age/sex | Diagnosis | Time in study (mo) | Initial visit | Final visit | Initial visit | Final visit | Initial visit | Final visit |
| ApoE ε3/ε3 | |||||||||
| 2 | 81/M | MCI | 27 | 1.06 | 0.70 | 54 | 1186 | 24 | 29 |
| 4 | 78/M | Pre-MCI | 21 | 1.40 | 3.23 | 377 | 1768 | 30 | 30 |
| 8 | 66/F | MCI | 16 | 0.91 | 1.97 | 437 | 1825 | 26 | 29 |
| 10 | 54/F | MCI | 19 | 0.86 | 2.88 | 679 | 1968 | 28 | 30 |
| 17 | 53/F | MCI | 8 | 1.05 | 3.67 | 1011 | 1101 | 28 | 30 |
| 20 | 80/M | MCI | 9 | 298 | 1157 | 26 | 30 | ||
| 21 | 70/M | MCI | 4 | 437 | 1339 | 26 | 26 | ||
| 22 | 82/F | MCI | 3 | 22 | 736 | 28 | 29 | ||
| Mean | 70 | 13.38 | 1.06 | 2.49 | 414.3 | 1385.0 | 27.0 | 29.1 | |
| sd | 0.21 | 1.18 | 322.0 | 426.1 | 1.85 | 1.36 | |||
| ApoE ε3/ε4 | |||||||||
| 3 | 40/M | MCI | 10 | 0.26 | 1.06 | 399 | 1466 | 24 | 21 |
| 9 | 79/M | MCI | 19 | 3.99 | 4.44 | 414 | 1880 | 26 | 17 |
| 12 | 76/M | MCI | 17 | 1.76 | 3.41 | 1280.0 | 3267 | 29 | 30 |
| 14 | 90/F | MCI | 17 | 0.61 | 0.25 | 400.0 | 1726 | 25 | 23 |
| 15 | 60/M | Pre-MCI | 9 | 3.20 | 1.46 | 419.0 | 743 | 30 | 30 |
| 16 | 81/M | MCI-AD | 10 | 4.03 | 5.28 | 433.0 | 1775 | 20 | 14 |
| 18 | 81/F | Pre-MCI | 4 | 1.22 | 0.67 | 30 | 30 | ||
| 19 | 63/F | MCI | 11 | 2.17 | 679 | 1616 | 26 | 30 | |
| 23 | 88/F | MCI | 8 | 414.0 | 1315.0 | 25 | 21 | ||
| Mean | 74 | 11.9 | 2.15 | 2.37 | 554.8 | 1723.5 | 26.1 | 24.0 | |
| sd | 1.46 | 1.99 | 307.4 | 718.0 | 3.22 | 6.24 | |||
M1-M2 phenotype was tested by immunofluorescence microscopy, MFI by flow cytometry, and cognition by MMSE testing.
Objective 2
Median M1-M2 green zone rate change in the ApoE ε3/ε3 group was 0.226 per year (P = 0.375 compared to 0) and in the ApoE ε3/ε4 group median green zone change was −0.063 (P = 0.125 compared to 0); significant difference between groups, P = 0.082 (Table 1).
Objective 3
Correlation between MMSE and macrophage M1M2 phenotype: Spearman correlation in the ApoE ε3/ε3 group = 0.200 (P = 0.747) and in the ApoE ε3/ε4 group, Spearman correlation = 0.771 (P = 0.072); Spearman correlation of all patients = 0.483 (P = 0.132).
On ω-3 supplementation ApoE ε3/ε3 macrophages polarize to the intermediate M1-M2 phenotype, whereas ApoE ε3/ε4 macrophages show irregular polarization
At baseline, patients bearing ApoE ε3/ε3 had low M1/M2 ratio (median M1/M2 ratio = 1.06), whereas patients with ApoE ε3/ε4 had higher M1/M2 ratio (median M1/M2 ratio = 1.97) (Tables 1 and 2). Macrophages of patients bearing ApoE ε3/ε3 increased at the median M1-M2 rate of 0.226 U/yr (P = 0.375 compared to 0), and macrophages of patients with ApoE ε3/ε4 had negative M1-M2 polarization of −0.063 (P = 0.125 compared to 0). The significance of the M1-M2 rate change difference between the ApoE groups was (P = 0.082) (Table 1). The irregularities of patients with ApoE ε3/ε4 were related in part to intercurrent health problems and lack of ω-3 supplementation (Fig. 1). In patients with ApoE ε3/ε4, the baseline M1M2 type was in the red zone in 3 patients and increased further (4.4, 5.7, 5.28) and in 1 patient was in the white zone type and decreased further (from 0.61 to 0.25). All of these 4 patients had poor cognitive outcome (Table 2).
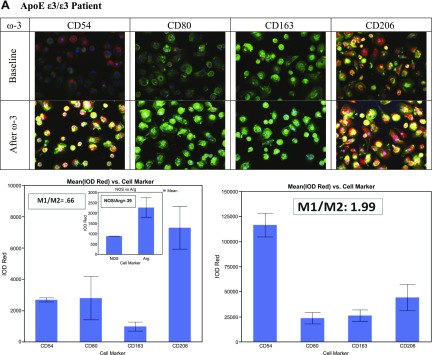
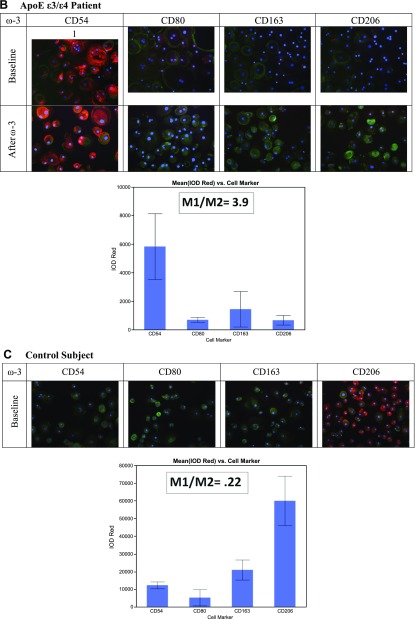
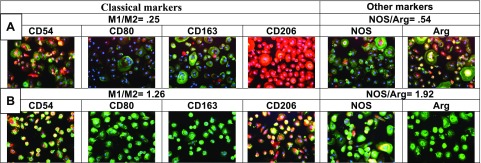
An example of M1M2 polarization in the ApoE ε3/ε3 group (Fig. 2A) shows the M1/M2 ratio increasing from baseline 0.66 to 1.99 (this patient had a baseline NOS/arginase ratio of 0.39). An example is shown where the ratio increased from 3.9 to 5.27 (Fig. 2B). A control age-matched subject had M2 type (ratio 0.22) macrophages (Fig. 2C) with excellent Aβ1–42 phagocytosis. Macrophage phenotype was confirmed by additional nonclassic markers, with the inflammatory marker NOS and the proresolution marker arginase (Fig. 3).
Figure 2.
Macrophage type at baseline and after ω-3 supplementation. A) Polarization in patients with ApoE ε3/ε3 from baseline M2 to M1-M2 after ω-3 supplementation. B) Polarization in patients with ApoE ε3/ε4 from baseline M1 to greater M1 type after ω-3. C) Control subject M2 type at baseline (no ω-3 supplementation).
Figure 3.
Classic and other markers of M2 macrophages (A) and M1-M2 macrophages (B).
Increased Aβ1–42 phagocytosis on ω-3 supplementation
On ω-3 supplementation, Aβ1–42 phagocytosis increased in both groups (Table 1). In the ApoE ε3/ε3 group, MFI increased with a median rate of change of 1041 MFI U/yr (P = 0.03 within group); in the ApoE ε3/ε4 group, with a median rate of change of 2213 MFI units per year (P = 0.03 within group; 0.47 between groups) (Table 1).
Case histories of patients with ApoE ε3/ε3
Patient 2
A 79-yr-old man with diabetes mellitus under good control at baseline in 2014 with a history of memory problems in 2013. His initial MMSE was 23 and his diagnosis was MCI. He was in the study for 30 mo except during short periods of travel. He had prostatitis in July 2014. His MMSE continued to fluctuate, with a maximum MMSE score of 29. He was an avid bicyclist throughout the study until 2016 when he had a hip fracture after falling off his bicycle. Three months after his fall and hospitalization without the ω-3 drink, his MMSE dropped from 29 to 22.
Patient 4
A 77-yr-old active film producer had recurring diverticulitis and underwent a colectomy in March 2014. At baseline he complained of mental fog with poor memory for names and words, and forgetfulness of simple tasks. His MMSE was 30, and his diagnosis was SCI. He had been taking the drink for 27 mo, except during extensive periods of foreign travel. All his MMSE results were 30. His general condition improved after colectomy: his word memory improved, and he was active in his profession.
Patient 8
A 66-yr-old woman born in Afghanistan who received a PhD in international relations and was active as an advisor on women’s issues in Afghanistan. She developed problems with adding numbers in 2014. Her initial MMSE was 26, and her diagnosis was MCI. She took the drink for 22 mo with intermittent noncompliance because of her travels. Her MMSE improved from 26 to 30.
Patient 10
A 63-yr-old woman developed problems with remote memory and word- and concept-finding. Her initial MMSE was 28, and her diagnosis was SCI. She took the drink for 21 mo and increased complex carbohydrates and fish in her diet. Her remote memory improved and she developed a successful landscaping business.
Patient 17
A 54-yr-old woman with a strong family history of dementia developed problems with finding appropriate words and addition of numbers in March 2015. Her initial MMSE was 28, and her diagnosis was MCI. She had history of attention deficit hyperactivity disorder and Graves’ hyperthyroidism. She took a commercial fish oil preparation (not the Smartfish drink) for 8 mo. Her SPECTscan (single photon emission computed tomography) showed low activity overall. Her final MMSE was 30, but she continued to have word-finding difficulty.
Case histories of patients with ApoE ε3/ε4
Patient 9
An 80-yr-old man, a retired civil engineer, took the drink for 22 mo. Before starting the study, he had a 2 yr history of memory and orientation problems. His first MMSE score was 26, and his diagnosis was MCI. His lowest MMSE was 6 in December 2015 when he was severely depressed and without supplementation. His MMSE improved to 23 in January 2016, but then his MMSE declined to 8, despite supervised supplementation. He had an increased homocysteine level of 15.6 μM (normal, 10.4–11.4 μM), normal C-reactive protein, a low DHEA level of 97 ng/ml (normal, 180–1250 ng/ml) and low testosterone of 0.7 pg/mo (normal, 30–135 pg/ml). Recently, his sensorium fluctuated, visual hallucinations were noted by his caregiver, and the fluorodeoxyglucose (FDG)–positron emission tomography/computed tomography (PET/CT) scan was consistent with dementia with Lewy bodies. He stopped taking the drink.
Patient 12
A 74-yr-old man, a retired attorney, had a 5 yr history of anxiety, poor sleep, poor memory, and word-finding difficulty. His MRI showed ventricular enlargement and temporal lobe atrophy; his FDG-PET showed reduced glucose utilization in the temporal lobes. His initial MMSE was 29, and his diagnosis was MCI. His lowest MMSE was 28 in August 2015 after having pneumonia in July 2015—final MMSE, 30. He took supplements for 20 mo. His homocysteine level was 16.4 μM (increased above normal) and DHEA was 1.56 ng/ml (below normal).
Patient 14
An 88-yr-old woman misplaced objects and had poor short-term memory, with an initial MMSE of 25; her diagnosis was MCI. She had taken the supplementation for 6 mo and then broke her hip in February 2015 and had a prolonged hospitalization without ω-3 supplementation until August 2015. Four months after the hip surgery, her MMSE declined to 11. She was restarted on the drink under supervision, and 4 mo later her MMSE increased to 25 and has remained between 24 and 27. She had low DHEA, but pregnenolone, progesterone, thyroid panel, and cortisol were normal. Her FDG-PET/CT showed hypometabolism of the posterior cingulate and parietotemporal cortex consistent with mild dementia. She did not take any AD drugs.
Patient 15
A 66-yr-old physician with MMSE 30 and depression at the start of study and took the supplemented drink. His initial MMSE was 30, and his diagnosis was SCI. His mood and office work improved while he took supplementation for 7 mo. He then failed to continue in the study for 6 mo, but returned complaining of failing recent memory, poor judgement, and depression. His MMSE remained at 30.
Patient 16
An 81-yr-old man was in the study for 11 mo. His initial diagnosis was MCI. He was taking supplements for 5 mo but had minor strokes that were diagnosed as multi-infarct dementia. His MMSE became unmeasurable, and he developed behavioral problems and was removed from the study by the caregiver.
Polarization of macrophages by RvD1 in vitro
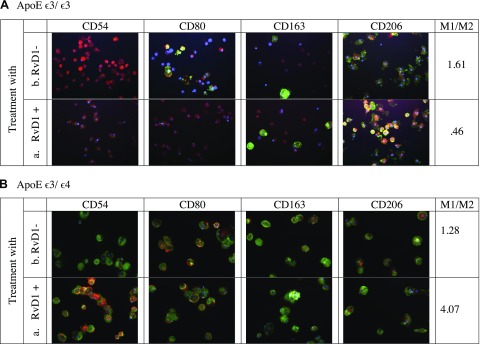
In patients bearing ApoE ε3/ε3, in vitro treatment of macrophages by RvD1 reduced the M1/M2 ratio from 2.3 to 1.61, whereas in patients with ApoE ε3/ε4, this treatment increased the M1/M2 ratio from 1.89 to 2.35 (Table 3). An example of in vitro polarization by RvD1 in a patient with ApoE ε3/ε3 from 1.61 to 0.46 is shown (Fig. 4A). An example of idiosyncratic M1 polarization by RvD1 is shown where the ratio increased from 1.28 to 4.07 in a patient with ApoE ε3/ε4 (Fig. 4B).
TABLE 3.
In vitro modulation of macrophage type by RvD1
| Patient | Date | No stimulation | RvD1 stimulation |
|---|---|---|---|
| ApoE ε3/ε3 | |||
| 2 | 5/31/2015 | 4.83 | 4.84 |
| 2 | 12/30/2015 | 1.99 | 0.89 |
| 2 | 2/21/2016 | 0.66 | 0.25 |
| 8 | 9/16/2015 | 1.97 | 1.27 |
| 10 | 5/31/2015 | 2.06 | 0.82 |
| Mean | 2.30 | 1.61 | |
| se | 0.68 | 0.82 | |
| ApoE ε3/ε4 | |||
| 12 | 9/30/2015 | 1.55 | 0.89 |
| 12 | 7/19/2016 | 1.31 | 2.58 |
| 14 | 5/31/2015 | 4.09 | 8.57 |
| 14 | 9/16/2015 | 1.76 | 1.66 |
| 14 | 12/31/2015 | 1.61 | 0.46 |
| 14 | 2/22/2016 | 0.25 | 0.67 |
| 15 | 9/17/2015 | 1.28 | 3.35 |
| 15 | 6/30/2016 | 5.71 | 3.45 |
| 16 | 9/17/2015 | 1.28 | 3.35 |
| 18 | 4/13/2016 | 0.67 | 0.30 |
| 19 | 4/13/2016 | 2.17 | 1.79 |
| 19 | 4/13/2016 | 1.07 | 1.19 |
| Mean | 1.90 | 2.36 | |
| se | 0.44 | 0.66 | |
Data are the M1/M2 ratio, with and without RvD1 stimulation. Macrophage phenotype was determined by immunofluorescence staining of macrophage with antibodies to CD54, CD80, CD163, and CD206.
Figure 4.
In vitro modulation of macrophage type by RvD1 stimulation. A) Patient with ApoE ε3/ε3, with (a) and without (b) RvD1. B) Patient with ApoE ε3/ε4, with (a) and without (b) RvD1.
DISCUSSION
Our observational study of patients with MCI examined the effects of ω-3, antioxidant, and resveratrol drink supplementation on innate immunity and cognitive status in relationship to the ApoE genotype. Clinically and statistically significant findings (but limited by the study design and low number of subjects) were: 1) in patients with ApoE ε3/ε3, a small improvement of cognitive level: MMSE improved by 2.2 points, whereas patients with ApoE ε3/ε4 did not change; the change within the ApoE ε3/ε3 group and between the groups was significant (P = 0.01); 2) M1M2 polarization of macrophages in patients with ApoE ε3/ε3 to an M1-M2 type of 0.226 compared to the ApoE ε3/ε4 group rate of −0.063 was nearly significant (P = 0.08); 3) correlation between the M1M2 rate and MMSE neared significance in the ApoE ε3/ε4 group (Spearman coefficient = 0.771, P = 0.072). The baseline M1M2 type in the red or white zone was a poor prognostic sign for cognition. The outstanding clinical “pearls” include patient 14, who recovered from dementia (MMSE 11) to her previous functioning (MMSE between 23 and 27) and patient 2, with improved MMSE score after 27 mo (initial 24, final 29). These results are limited by the small sample size; lack of randomization design to ω-3s vs. a placebo; intermittent compliance with ω-3 supplementation due to travel by patients 2, 4, and 12; diagnostic heterogeneity at baseline: 3 patients with SCI in the ApoE ε3/ε3 group vs. 2 with SCI and 1 with MCI-AD in the ApoE ε3/ε4 group; and the definition of the green zone M1/M2 ratio as 1–4 based on the observed relation of optimal M1-M2 to optimal MFI.
Overall results suggest that ω-3, antioxidant, and resveratrol supplementation were associated with stabilization or improvement for as long as 3 yr in the cognition of patients bearing ApoE ε3/ε3. The progress of those with ApoE ε3/ε4 was interrupted by intercurrent conditions, lack of supplementation, or a new diagnosis (Lewy body disease and multi-infarct dementia). An example of intercurrent conditions (hip fracture and hospitalization off supplementation), which were repaired by renewal of supplementation, is patient 14, who had a positive dementia FDG scan and lapsed into the dementia stage after surgery, but recovered to her previous cognitive status after discharge from the hospital and renewal of ω-3 supplementation.
Most patients with ApoE ε3/ε3 had M2 type at baseline, whereas 4 with ApoE ε3/ε4 had either extreme M1 red zone or M2 white zone macrophages. In the patients with ApoE ε3/ε3, ω-3 supplementation was associated with an increased rate of macrophage polarization to the M1-M2 type. In patients with ApoE ε3/ε4, polarization was irregular and associated with more extreme M1 macrophage type and poor outcome. Supplementation with ω-3 was associated with a significant improvement of MMSE status in patients with ApoE ε3/ε3 whose rate was significantly greater than 0 and superior in comparison to patients with ApoE ε3/ε4 (P = 0.01). The inferior results in patients with ApoE ε3/ε4 may be related to the known negative effects of ApoE ε4, such as a gain of toxic function through Aβ1–42 aggregation and decreased clearance (14), inflammatory activation of macrophages (42), decreased lipid and glucose metabolism, and interference with neuronal signaling and mitochondrial function (15). As expected, in vitro stimulation by RvD1 decreased the M1/M2 ratio in patients with ApoE ε3/ε3, but this stimulation paradoxically increased the M1/M2 ratio in some patients with ApoE ε3/ε4. The results of ω-3s are limited to patients with MCI and SCI, as there were no cognitive benefits of ω-3 supplementation in patients with AD (3).
CONCLUSIONS
Supplementation with ω-3s/antioxidant/resveratrol provided in a drink with ω-3s and resveratrol is significantly (P = 0.01) associated with improved MMSE status and nonsignificantly (P = 0.08) associated with polarization of macrophages to an intermediate prophagocytic M1-M2 type in patients with ApoE ε3/ε3 in comparison to those with ApoE ε3/ε4. Supplementation with ω-3 brings into personalized clinical practice the immune benefits expected from ω-3 mediators called resolvins, and the results suggest a relationship between the improvements of innate immunity and cognition. Although the validity of this study is limited due to its small size and uncontrolled design, the results suggest a new immunologic approach to the widespread public health problem of dementia. The benefits of ω-3 supplementation should be clarified in larger controlled studies.
ACKNOWLEDGMENTS
The authors thank Jeffrey Gornbein for statistical analysis of the data and Sarah Weitzman and Leonard Haller (all from the University of California, Los Angeles) for data collection and assistance with statistical analysis. The studies were supported by U.S. National Institutes of Health Institute of General Medical Sciences Grant 5P01GM099134-03 (to M.P.), a grant from the Alzheimer’s Association, and a donation from Smartfish AS (to M.F.). Smartfish AS supplied the drink to the patients who requested it. Smartfish was not involved in the design of the study, analysis or interpretation of the data, or the decision to submit the manuscript for publication. M.F. received paid travel to meetings and honoraria from Smartfish. The remaining authors declare no conflicts of interest.
Glossary
- 1,25D3
1,25-dihydroxyvitamin D3
- ω-3
ω-3 fatty acid
- Aβ1–42
amyloid β1–42
- AD
Alzheimer’s disease
- ApoE
apolipoprotein E
- APP
amyloid-precursor protein
- CD
cluster of differentiation
- Cur
curcumin
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FACS
fluorescence-activated cell sorting
- FDG
fluorodeoxyglucose
- IMDM
Iscove's modified Dulbecco's medium
- MCI
mild cognitive impairment
- MFI
mean fluorescence intensity
- MMSE
Mini–Mental State Examination
- PET
positron emission tomography
- PBMC
peripheral blood mononuclear cell
- PPAR
peroxisome proliferator-activated receptor
- Res
resveratrol
- RvD
resolvin D
- SCI
subjective cognitive impairment
- SIRT
sirtuin
- SPM
specialized proresolving mediator
AUTHOR CONTRIBUTIONS
S. Famenini performed research and analyzed data; E. Rigali analyzed data; H. Olivera-Perez, J. Dang, M. Chang, and R. Halder performed research; R. Rao tested the ApoE genotype; M. Pellegrini, V. Porter, and D. Bredesen reviewed the results and contributed to the paper; and M. Fiala designed research, analyzed data, and wrote the paper.
REFERENCES
- 1.McGeer P. L., McGeer E. G. (2015) Targeting microglia for the treatment of Alzheimer’s disease. Expert Opin. Ther. Targets 19, 497–506 [DOI] [PubMed] [Google Scholar]
- 2.Fiala M., Liu P. T., Espinosa-Jeffrey A., Rosenthal M. J., Bernard G., Ringman J. M., Sayre J., Zhang L., Zaghi J., Dejbakhsh S., Chiang B., Hui J., Mahanian M., Baghaee A., Hong P., Cashman J. (2007) Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer’s disease patients are improved by bisdemethoxycurcumin. Proc. Natl. Acad. Sci. USA 104, 12849–12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiala M., Halder R. C., Sagong B., Ross O., Sayre J., Porter V., Bredesen D. E. (2015) ω-3 Supplementation increases amyloid-β phagocytosis and resolvin D1 in patients with minor cognitive impairment. FASEB J. 29, 2681–2689 [DOI] [PubMed] [Google Scholar]
- 4.Mrak R. E., Griffin W. S. (2001) Interleukin-1, neuroinflammation, and Alzheimer’s disease. Neurobiol. Aging 22, 903–908 [DOI] [PubMed] [Google Scholar]
- 5.McGeer P. L., Akiyama H., Itagaki S., McGeer E. G. (1989) Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neurosci. Lett. 107, 341–346 [DOI] [PubMed] [Google Scholar]
- 6.Rogers J., Schultz J., Brachova L., Lue L. F., Webster S., Bradt B., Cooper N. R., Moss D. E. (1992) Complement activation and beta-amyloid-mediated neurotoxicity in Alzheimer’s disease. Res. Immunol. 143, 624–630 [DOI] [PubMed] [Google Scholar]
- 7.Fiala M., Liu Q. N., Sayre J., Pop V., Brahmandam V., Graves M. C., Vinters H. V. (2002) Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood-brain barrier. Eur. J. Clin. Invest. 32, 360–371 [DOI] [PubMed] [Google Scholar]
- 8.Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356 [DOI] [PubMed] [Google Scholar]
- 9.ElAli A., Rivest S. (2016) Microglia in Alzheimer’s disease: a multifaceted relationship. Brain Behav. Immun. 55, 138–150 [DOI] [PubMed] [Google Scholar]
- 10.Zaghi J., Goldenson B., Inayathullah M., Lossinsky A. S., Masoumi A., Avagyan H., Mahanian M., Bernas M., Weinand M., Rosenthal M. J., Espinosa-Jeffrey A., de Vellis J., Teplow D. B., Fiala M. (2009) Alzheimer disease macrophages shuttle amyloid-beta from neurons to vessels, contributing to amyloid angiopathy. Acta Neuropathol. 117, 111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizwicki M. T., Liu G., Fiala M., Magpantay L., Sayre J., Siani A., Mahanian M., Weitzman R., Hayden E. Y., Rosenthal M. J., Nemere I., Ringman J., Teplow D. B. (2013) 1α,25-Dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-β phagocytosis and inflammation in Alzheimer’s disease patients. J. Alzheimers Dis. 34, 155–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127, 1109–1122 [DOI] [PubMed] [Google Scholar]
- 13.Liu C. C., Kanekiyo T., Xu H., Bu G. (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 9, 106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellano J. M., Kim J., Stewart F. R., Jiang H., DeMattos R. B., Patterson B. W., Fagan A. M., Morris J. C., Mawuenyega K. G., Cruchaga C., Goate A. M., Bales K. R., Paul S. M., Bateman R. J., Holtzman D. M. (2011) Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 3, 89ra57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theendakara V., Peters-Libeu C. A., Spilman P., Poksay K. S., Bredesen D. E., Rao R. V. (2016) Direct transcriptional effects of apolipoprotein E. J. Neurosci. 36, 685–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theendakara V., Patent A., Peters Libeu C. A., Philpot B., Flores S., Descamps O., Poksay K. S., Zhang Q., Cailing G., Hart M., John V., Rao R. V., Bredesen D. E. (2013) Neuroprotective Sirtuin ratio reversed by ApoE4. Proc. Natl. Acad. Sci. USA 110, 18303–18308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salem N. Jr., Vandal M., Calon F. (2015) The benefit of docosahexaenoic acid for the adult brain in aging and dementia. Prostaglandins Leukot. Essent. Fatty Acids 92, 15–22 [DOI] [PubMed] [Google Scholar]
- 18.Calder P. C. (2015) Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 1851, 469–484 [DOI] [PubMed] [Google Scholar]
- 19.Morris M. C., Brockman J., Schneider J. A., Wang Y., Bennett D. A., Tangney C. C., van de Rest O. (2016) Association of seafood consumption, brain mercury level, and APOE ε4 status with brain neuropathology in older adults. JAMA 315, 489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jicha G. A., Markesbery W. R. (2010) Omega-3 fatty acids: potential role in the management of early Alzheimer’s disease. Clin. Interv. Aging 5, 45–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cederholm T., Salem N. Jr., Palmblad J. (2013) ω-3 fatty acids in the prevention of cognitive decline in humans. Adv. Nutr. 4, 672–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annweiler C., Llewellyn D. J., Beauchet O. (2013) Low serum vitamin D concentrations in Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimers Dis. 33, 659–674 [DOI] [PubMed] [Google Scholar]
- 23.Huang T. L., Zandi P. P., Tucker K. L., Fitzpatrick A. L., Kuller L. H., Fried L. P., Burke G. L., Carlson M. C. (2005) Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology 65, 1409–1414 [DOI] [PubMed] [Google Scholar]
- 24.Freund-Levi Y., Eriksdotter-Jönhagen M., Cederholm T., Basun H., Faxén-Irving G., Garlind A., Vedin I., Vessby B., Wahlund L. O., Palmblad J. (2006) Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch. Neurol. 63, 1402–1408 [DOI] [PubMed] [Google Scholar]
- 25.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiala M., Terrando N., Dalli J. (2015) Specialized pro-resolving mediators from omega-3 fatty acids improve amyloid-β phagocytosis and regulate inflammation in patients with minor cognitive impairment. J. Alzheimers Dis. 48, 293–301 [DOI] [PubMed] [Google Scholar]
- 27.Bredesen D. E. (2014) Reversal of cognitive decline: a novel therapeutic program. Aging (Albany, N.Y.) 6, 707–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bredesen D. E., Amos E. C., Canick J., Ackerley M., Raji C., Fiala M., Ahdidan J. (2016) Reversal of cognitive decline in Alzheimer’s disease. Aging (Albany, N.Y.) 8, 1250–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serhan C. N., Chiang N., Dalli J. (2015) The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 27, 200–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serhan C. N., Petasis N. A. (2011) Resolvins and protectins in inflammation resolution. Chem. Rev. 111, 5922–5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serhan C. N., Krishnamoorthy S., Recchiuti A., Chiang N. (2011) Novel anti-inflammatory--pro-resolving mediators and their receptors. Curr. Top. Med. Chem. 11, 629–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L., Wu Y., Wang Y., Wu J., Song L., Xian W., Yuan S., Pei L., Shang Y. (2014) Resolvin D1 promotes the interleukin-4-induced alternative activation in BV-2 microglial cells. J. Neuroinflammation 11, 72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sica A., Mantovani A. (2012) Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schif-Zuck S., Gross N., Assi S., Rostoker R., Serhan C. N., Ariel A. (2011) Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur. J. Immunol. 41, 366–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Girodet P. O., Nguyen D., Mancini J. D., Hundal M., Zhou X., Israel E., Cernadas M. (2016) Alternative macrophage activation is increased in asthma [E-pub ahead of print]. Am. J. Respir. Cell Mol. Biol. doi: 0.1165/rcmb.2015-0295OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Croasdell A., Thatcher T. H., Kottmann R. M., Colas R. A., Dalli J., Serhan C. N., Sime P. J., Phipps R. P. (2015) Resolvins attenuate inflammation and promote resolution in cigarette smoke-exposed human macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L888–L901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue J., Schmidt S. V., Sander J., Draffehn A., Krebs W., Quester I., De Nardo D., Gohel T. D., Emde M., Schmidleithner L., Ganesan H., Nino-Castro A., Mallmann M. R., Labzin L., Theis H., Kraut M., Beyer M., Latz E., Freeman T. C., Ulas T., Schultze J. L. (2014) Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raine A., Portnoy J., Liu J., Mahoomed T., Hibbeln J. R. (2015) Reduction in behavior problems with omega-3 supplementation in children aged 8-16 years: a randomized, double-blind, placebo-controlled, stratified, parallel-group trial. J. Child Psychol. Psychiatry 56, 509–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizwicki M. T., Menegaz D., Zhang J., Barrientos-Durán A., Tse S., Cashman J. R., Griffin P. R., Fiala M. (2012) Genomic and nongenomic signaling induced by 1α,25(OH)2-vitamin D3 promotes the recovery of amyloid-β phagocytosis by Alzheimer’s disease macrophages. J. Alzheimers Dis. 29, 51–62 [DOI] [PubMed] [Google Scholar]
- 40.Petersen R. C., Doody R., Kurz A., Mohs R. C., Morris J. C., Rabins P. V., Ritchie K., Rossor M., Thal L., Winblad B. (2001) Current concepts in mild cognitive impairment. Arch. Neurol. 58, 1985–1992 [DOI] [PubMed] [Google Scholar]
- 41.Duara R., Loewenstein D. A., Greig M. T., Potter E., Barker W., Raj A., Schinka J., Borenstein A., Schoenberg M., Wu Y., Banko J., Potter H. (2011) Pre-MCI and MCI: neuropsychological, clinical, and imaging features and progression rates. Am. J. Geriatr. Psychiatry 19, 951–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jofre-Monseny L., Loboda A., Wagner A. E., Huebbe P., Boesch-Saadatmandi C., Jozkowicz A., Minihane A. M., Dulak J., Rimbach G. (2007) Effects of apoE genotype on macrophage inflammation and heme oxygenase-1 expression. Biochem. Biophys. Res. Commun. 357, 319–324 [DOI] [PMC free article] [PubMed] [Google Scholar]