Abstract
Corneal avascularity is critical for achieving transparency necessary for proper transmission of light to the lens and visual acuity. Although much is known about angiogenesis and angiostasis, the precise regulation of these processes in the cornea is unclear. MicroRNA (miR)-184, the most abundant corneal epithelial miRNA, has been suggested to function in corneal angiostasis by altering VEGF signaling; however, the mechanism(s) underlying this regulation have not been addressed. Using a combination of in vitro and in vivo assays to evaluate angiogenesis, we demonstrated that human limbal epithelial keratinocytes (HLEKs) engineered to overexpress miR-184 secreted lower amounts of angiogenic mitogens. Human dermal microvascular cells exposed to conditioned medium from miR-184-overexpressing HLEKs were less proliferative and failed to seal linear scratch wounds. The in vivo Matrigel plug assay showed that conditioned medium from miR-184-expressing HLEKs elicited a lesser degree of neovascularization compared with controls. We found that miR-184 directly targets and represses the proangiogenic factors, friend of Gata 2 (FOG2), platelet-derived growth factor (PDGF)-β, and phosphatidic acid phosphatase 2b (PPAP2B). FOG2 regulates VEGF expression, whereas PDGF-β and PPAP2B regulate Akt activity. By attenuating both VEGF and Akt signaling, miR-184 acts as a broad-spectrum negative regulator of corneal angiogenesis.—Park, J. K., Peng, H., Yang, W., Katsnelson, J., Volpert, O., Lavker, R. M. miR-184 exhibits angiostatic properties via regulation of Akt and VEGF signaling pathways.
Keywords: corneal epithelium, limbal epithelium, FOG2, PDGF-β, PPAP2B
Physical protection of the delicate understructures of the eye (e.g., lens, trabecular meshwork, iris, and retina) while maintaining transparency is the major role of the cornea. Protection is achieved, in part, via a continuously renewing stratified squamous epithelium comprised of keratin-filled cells, which support a complex tear film solution (1, 2). The corneal epithelium is sustained by a relatively acellular, dense stroma of tightly packed collagen fibers in a milieu that is devoid of blood vessels (3). This combination of an avascular and acellular stroma allows light to be properly refracted through the lens and onto the retina, thereby ensuring proper vision. The adjacent limbus is highly invested in blood vessels, in part to support the overlying stem cell-enriched limbal epithelium (4–6). Regarding vessel distribution, under normal resting conditions, there is a sharp demarcation at the limbal/corneal junction (7). However, upon perturbation, frequent egress of vessels into the cornea occurs, which ultimately compromises the visual experience and causes a myriad of problems (e.g., inflammation, altered immunity, and transplant rejection) [see Ecoiffier et al. (8)]. Since the earliest recognition of the need for avascularity in the cornea, there has been an ongoing mission to understand the biology of both corneal angiogenesis and angiostasis (9). Although much has been learned, our understanding on what regulates and maintains corneal avascularity is still incomplete.
MicroRNAs (miRs or miRNAs) are an important class of noncoding RNA that regulate diverse biologic processes. Their dysregulation has been linked to human diseases, including cancer (10, 11). Within the cornea, several miRNAs are expressed preferentially in the corneal epithelium and have been shown to regulate lineage specification, cell migration, cell survival, differentiation, and glycogen storage (12–18). Recently, the miR-103/107 family was demonstrated to be limbal epithelium-enriched, regulating slow-cycling, proliferative capacity, and cell-cell communication, all characteristics of epithelial stem cells (19). As in other tissues, changes in corneal miRNA expression have been linked to pathology, such as wound healing [reviewed in Funari et al. (20)], diabetes (21), and familial keratoconus (22, 23).
One of the most interesting miRNAs is miR-184, which is most abundant in the corneal epithelium (24). During wound healing, miR-184 expression was dramatically reduced in the re-epithelialized tissue but was still maintained in the peripheral (unwounded) corneal epithelium (24). In this study, no relationship was observed between miR-184 expression and corneal epithelial proliferation (24). Such a decrease in miR-184 expression was confirmed in an expression-profiling study of corneal epithelial wound healing, where out of 600 candidate miRNAs only miR-184 and miR-204 were dramatically down-regulated (25). One of the first functions described for miR-184 was to antagonize miR-205, thereby maintaining SH2 domain-containing inositol phosphatase 2 (SHIP2) levels in human corneal epithelial cells (18). This was the first instance of one miRNA abrogating the inhibitory function of another. Other functions for miR-184 in the corneal epithelium have been lineage specification (16), controlling familial severe keratoconus and cataract formation (22, 26), and recently angiostasis (27).
The idea that miR-184 might be involved in the suppression of angiogenesis was first suggested by its role in maintaining SHIP2 levels (18). SHIP2, via its ability to negatively regulate Akt signaling (18), was postulated to suppress corneal angiogenesis through inhibition of VEGF (28). This miR-184/VEGF relationship was recently confirmed when miR-184 was observed to down-regulate VEGF in human corneal epithelial cells (27). In this study, a corneal suture technique was used to induce neovascularization in rats, and expression levels of miR-184 were reduced by 75% compared with controls. Conversely, topical administration of miR-184 to corneal-sutured rat corneas significantly reduced corneal neovascularization. miR-184 also suppressed cell proliferation and migration of HUVECs, thus demonstrating miR-184’s antiangiogenic properties (27). However, the mechanisms underlying this angiostatic property were not addressed. Herein, we present direct evidence that miR-184 regulates corneal angiogenesis. We show that human limbal epithelial keratinocytes (HLEKs), engineered to overexpress miR-184, secrete lower amounts of angiogenic mitogens. Furthermore, we demonstrate that miR-184 directly targets and represses the proangiogenic factors friend of Gata 2 (FOG2), platelet-derived growth factor (PDGF)-β, and phosphatidic acid phosphatase 2b (PPAP2B), thereby preventing angiogenesis and maintaining corneal avascularity. Specifically, miR-184 negatively affects VEGF signaling by targeting FOG2. Likewise, PDGF-β and PPAP2B regulate Akt and thus miR-184 blocks Akt signaling via multiple pathways.
MATERIALS AND METHODS
Cell culture, treatment, transfection, and transduction
Primary human corneal epithelial keratinocytes (HCEKs) and HLEKs were isolated from cadaver donor corneas provided by Midwest Eye Bank (Ann Arbor, MI, USA) and cultured in CnT-20 medium with supplements (CellnTech, Bern, Switzerland) on collagen IV-coated plates (Corning, Corning, NY, USA) (19). HeLa cells were obtained from American Type Culture Collection (Manassas, VA, USA) and grown in DMEM with 10% fetal bovine serum. HCEKs were stimulated with 10 ng/ml recombinant human PDGF-BB (R&D Systems, Minneapolis, MN, USA) for 6 h. To overexpress miR-184, microRNA precursor (pre-miRNA-184) mimetic or control oligonucleotide (negative control #1) (Ambion, Austin, TX, USA) was transfected into cells using siRNA transfection reagent (RNAiMax; Thermo Fisher Scientific, Waltham, MA, USA). For lentiviral infections of human pre-miRNA expression construct lenti-miR-184 (System Biosciences, Palo Alto, CA, USA), keratinocytes were transduced with lentiviral supernatants (produced by the NU-SDRC RNA/DNA Delivery Core Facility) for 6 h and switched to fresh culture medium overnight. To silence gene expression, siRNA Smart pools (20 nM) targeting PPAP2B; zinc finger protein, multitype 2 protein (ZFPM2); or a scrambled negative control (Dharmacon, Lafayette, CO, USA) were transiently transfected into cells using RNAiMax. To express constitutively active Akt (CA-Akt), cells were transfected with either empty pcDNA3.1 or pcDNA3.1 vector encoding constitutively CA-Akt kinase using lipofectamine 2000 (Thermo Fisher Scientific).
Mice
Wild-type mice (C57Bl/6) were obtained from Charles River Laboratories (Wilmington, MA, USA). Matrigel samples were excised, immediately embedded in optimal cutting temperature compound, and stored at −80°C. All the procedures involving animals were approved by the Northwestern University Animal Care and Use Committee.
Matrigel plug assay
Growth factor-reduced Matrigel (BD Biosciences, Bedford, MA, USA) was thawed at 4°C and supplemented with conditioned medium (CM) from HLEKs expressing negative control miR or miR-184, and CM from HCEKs after treatment with irrelevant antagomir or antagomir against miR-184 (antago-184) was injected subcutaneously into the laterodorsal abdominal region of 8-wk-old male C57Bl/6 mice. The Matrigel samples were recovered 5 and 10 d after implantation for immunofluorescence staining with an antibody recognizing CD31 (BD Biosciences).
Laser capture microdissection and miRNA quantitative PCR
Eyes from a cadaver donor were embedded in optimal cutting temperature compound and stored at −80°C until sectioning. Basal cells from limbal and corneal epithelia from 5-μm frozen sections were isolated and captured using a Palm laser capture system (Carl Zeiss, Oberkochen, Germany) as previously described (19, 29). Total cellular RNA from laser capture microdissection-captured cells was isolated and purified with an miRNeasy kit (Qiagen, Valencia, CA, USA). miRNA levels were measured using Taqman microRNA Assays (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions.
Antagomirs
Antagomirs were synthesized by Dharmacon. Sequences were:5′-mA*mC*mC*mCmUmUmAmUmCmAmGmUmUmCmUmCmCmGmU*mC*mC*mA-Chol-3′ (antagomir-184), 5′- mG(*)mG(*)mC(*)mAmUmUmCmAmCmCmGmCmGmUmGmCmC(*)mU(*)mU(*)mA-Chol-3′ (irrelevant-antagomir). “mN” represents 2′-O-methyl-modified oligonucleotide, the asterisk represents a phosphorothioate linkage, and “Chol” represents linked cholesterol.
Scratch wound assay
Supernatant (CM) was collected from control and miR-184 lentiviral vector-transduced HLEKs. Green fluorescent protein-expressing human dermal microvascular endothelial cells (HDMECs) were plated in 24-well plates at a density of 2.5 × 105 cells and serum starved overnight using 0.5% fetal bovine serum. CM was applied in HDMECs, and percentage of wound closure was calculated after 8 h.
Western blotting
Western blots were performed as described previously (18). The following antibodies were used: p-Akt (Thr308), Akt (pan) (Cell Signaling Technologies, Danvers, MA, USA), ZFPM2, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), PDGF-β (Abcam), and PPAP2B (Sigma-Aldrich, St. Louis, MO, USA).
Immunocytochemistry and immunohistochemistry
Normal human corneas were obtained from the Illinois Eye Bank. Frozen sections (5 μm) were processed for fluorescence immunostaining as described previously (30). Briefly, sections were fixed with 4% PFA for 10 min. Slides were incubated overnight with an antibody recognizing p-Akt (1:50; Cell Signaling Technologies), PDGF-β (1:50; Abcam), PPAP2B (1:50; Sigma-Aldrich), or ZFPM2 (1:50; Santa Cruz Biotechnology). As isotype controls, rabbit monoclonal or polyclonal IgG (Abcam) was used. DAPI was used to counterstain nuclei. For bromodeoxyuridine (BrdU) staining to detect proliferation, cells were treated with 10 μM BrdU for 1 h, and then the medium was replaced and incubated for an additional hour before fixing in methanol at −20°C for 10 min. Antigen retrieval was performed at 70°C in formamide retrieval solution (1× saline-sodium citrate in formamide) for 30 min. After blocking in PBS containing 0.01% BSA and 0.01% Tween-20, slides were incubated for 30 min with BrdU monoclonal antibody (1:10; Developmental Studies Hybridoma Bank, Iowa City, IA, USA). After washing, slides were incubated with Alexa 555-linked secondary anti-mouse IgG (Vector Laboratories, Burlingame, CA, USA). DAPI was used to counterstain nuclei. Images were acquired on an epifluorescence microscope system (AxioVision Z1; Carl Zeiss) fitted with a digital camera (AxioCam MRm; Carl Zeiss).
Real-time quantitative PCR
Total RNAs were isolated by miRNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. cDNA was prepared using a SuperScript II reverse transcription kit (Thermo Fisher Scientific). Real-time quantitative PCR (qPCR) was performed on a Roche LightCycler 96 System using the Roche FastStart Essential DNA Green Master (Roche Life Science, Indianapolis, IN, USA) according to the manufacturer’s instructions. Primer sequences used in this study were designed by Integrated DNA Technologies (IDT, Skokie, IL, USA) (Supplemental Table S1).
Luciferase reporter constructs and assay
The 3′UTR of miR-184-predicted targets was ligated into the psiCheck-2 vector (Promega, Madison, WI, USA). The Renilla and firefly luciferase activities were measured using the Dual-Luciferase Reporter Assay system (Promega) as previously described (19).
Bioinformatics analysis
To analyze the functions of the predicted target genes of miR-184, predicted target genes were exported from TargetScan 7.0 (Whitehead Institute for Biomedical Research, Cambridge, MA, USA; http://www.targetscan.org/). Functional Annotation Clustering of genes was performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) Functional Annotation Bioinformatics Resources, v6.7 (National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA; https://david.ncifcrf.gov), and data were ordered by Enrichment scores.
Statistical analysis
Values are expressed as means ± sd. The significance of the differences between 2 groups was evaluated by an unpaired Student’s t test. Wound closure was repeated 3 times, and each sample was tested in quadruplicate. All other experiments were performed at least in triplicate.
RESULTS
miR-184 is highly enriched in the corneal vs. limbal epithelial basal cells
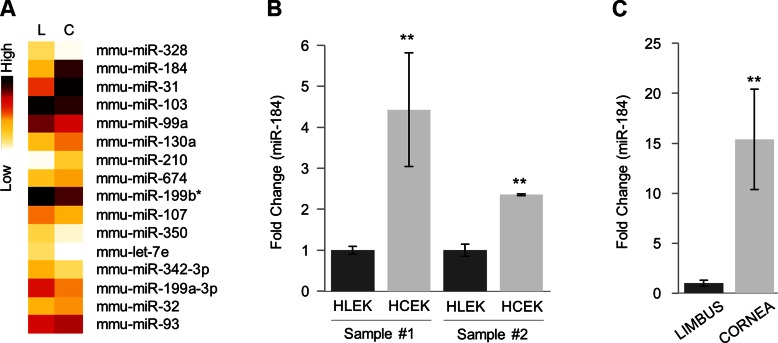
In the initial miRNA profiling studies, the entire mouse corneal epithelium was compared with the footpad epithelium. miR-184 expression was 90-fold greater in the corneal epithelium compared with the footpad epithelium (24). This finding was confirmed by in situ hybridization where an extensive signal for miR-184 was only detected in the corneal epithelium (24). To further focus these findings, we compared limbal vs. corneal epithelium. Relatively pure populations of mouse limbal and corneal basal epithelial cells were isolated using laser capture microdissection, and miRNA expression profiles were obtained and performed using miRcury LNA Universal RT miRNA PCR Rodent Panels I and II (Exiqon, Vedbaek, Denmark) (19). miR-184 expression levels were significantly greater in the corneal basal cells compared with the limbal basal cells (Fig. 1A). When RNA from primary cultures of HLEKs and HCEKs and RNA from human limbal and corneal epithelial basal cells were compared, miR-184 expression was 4-fold greater in HCEKs and 15-fold greater in human corneal basal cells compared with their limbal counterparts (Fig. 1B, C).
Figure 1.
miR-184 is a corneal epithelium-preferred miRNA in mouse and human. A) Heat map showing differentially expressed miRNAs in mouse limbal (L) and corneal (C) epithelial basal cells. Total cellular RNA from laser capture microdissection-isolated cells was extracted, and profiling was performed. B) Mature miR-184 levels were determined by Taqman miRNA assay using total RNA isolated from HLEKs and HCEKs. C) miRNA qPCR analysis of mature miR-184 using total RNA from laser capture microdissection-captured human corneal and limbal epithelium. **P < 0.001.
To interrogate the biologic processes potentially regulated by miR-184, we undertook an in silico analysis and found 730 predicted target transcripts in human. A functional gene annotation of the 730 genes indicated their involvement in blood vessel development as well as multiple signaling pathways (Supplemental Fig. 1A).
miR-184 negatively affects angiogenesis
To validate the prediction that miR-184 plays a direct role in blood vessel development, we tested whether miR-184 would affect the HCEK microenvironment to dampen human angiogenic behaviors of HDMECs, such as migration (31, 32) and proliferation. HLEKs, which contain low levels of miR-184, were transduced with lentiviral miR-184 or a control construct and grown to confluence, and the supernatant (CM) was collected and concentrated. A scratch wound assay was performed on serum-starved HDMECs exposed to the limbus-derived CM (Supplemental Fig. 1B). CM from HLEKs expressing miR-184 caused a 35% reduction in wound closure compared with control CM (Supplemental Fig. 1C).
Another important function required for angiogenesis is proliferation. HDMECs exposed to the limbus-derived CM showed a significant reduction in BrdU incorporation (Supplemental Fig. 1D). Endothelial cells are known to migrate and proliferate in response to angiogenic stimuli (33). Thus, collectively our findings of impaired migration and BrdU incorporation due to CM from cells expressing miR-184 support the idea that this miRNA has angiostatic properties.
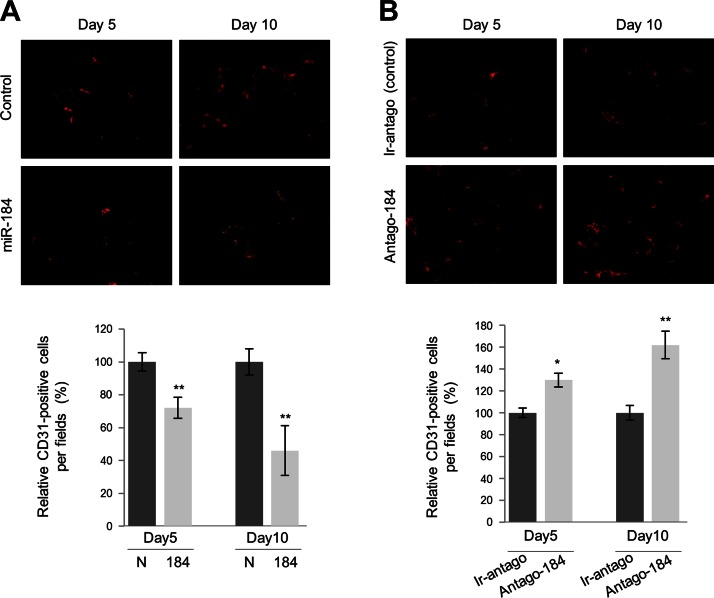
We used Matrigel assay to test the antiangiogenic properties of miR-184 in vivo. Although corneal micropocket assay is a gold standard of the field, because miR-184 is highly expressed in the normal corneal epithelium (24), this would confound the results. Thus, a subcutaneous implantation approach was used where Matrigel plugs CM from HLEKs engineered to overexpress miR-184 were compared with control CM. Angiogenesis (the number of CD31+ cells) was also evaluated in Matrigel plugs containing CM from HCEKs after miR-184 knockdown with antago-184. Five days after subcutaneous implantation of the Matrigel plugs, there was a 30% reduction in CD31+ cells in Matrigel plugs containing CM conditioned by HLEKs overexpressing miR-184 compared with control CM (Fig. 2A). Ten days after subcutaneous implantation, there was a 65% reduction in CD31+ cells (Fig. 2A). Conversely, a 25 and 44% increase in CD31+ cells was detected in plugs supplemented with CM from HCEKs treated with antago-184 compared with control CM at 5 and 10 d after implantation, respectively (Fig. 2B).
Figure 2.
miR-184 inhibits angiogenesis in the Matrigel plug assay in vivo. Effects of miR-184 on angiogenesis were assessed by immunofluorescence staining of Matrigel plugs using a CD31 antibody. A, B) Representative images of CD31 staining in Matrigel plugs containing CM from HLEKs expressing negative control miR (control) or miR-184 (A) and conditioned medium from HCEKs after treatment with irrelevant antagomir (ir-antago, control) or antago-184 (B). Bar graphs show quantification analysis of CD31-positive cells in sections, and the data are expressed as percentage values under input control (d 5), which was assigned a value of 100. *P < 0.05; **P < 0.01.
miR-184 targets FOG2, PDGF-β, and PPAP2B
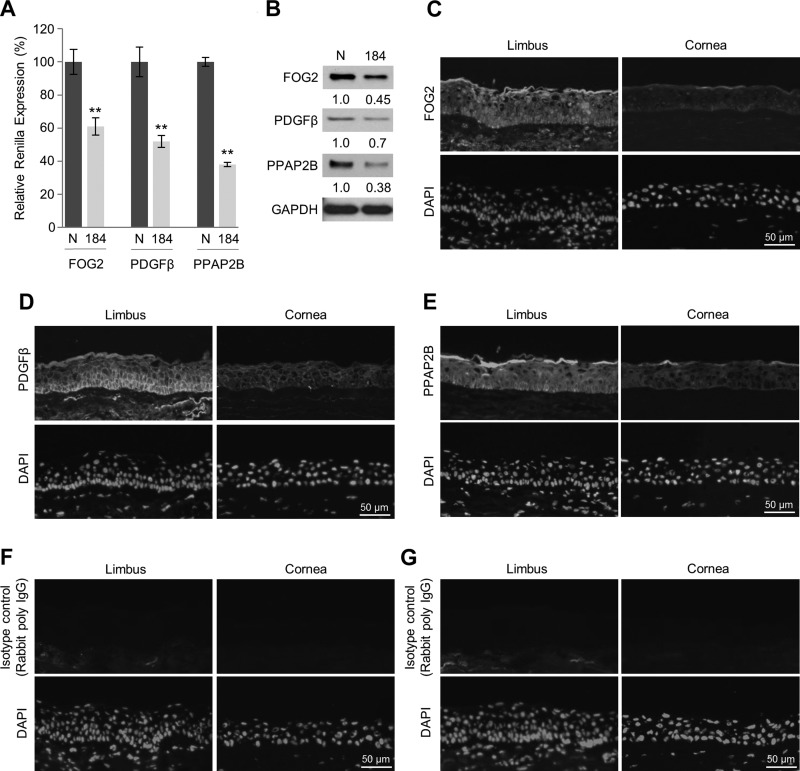
Retardation of wound closure and proliferation of HDMECs by CM from miR-184-expressing HLECs indicates that these limbal keratinocytes secrete lower amounts of trophic factors, including angiogenic mitogens, than control cells. In an attempt to understand further the mechanism, we used in silico and cell-based luciferase assays to find miR-184 targets that were proangiogenic. We performed luciferase assays in HeLa and human telomerase-immortalized corneal epithelial cells to screen 9 potential proangiogenic factors, which are among the predicted targets of miR-184 (Supplemental Fig. 2). Three genes were identified as direct targets of miR-184: friend of GATA2 (FOG2, also known as ZFPM2), which plays a role in the regulation of coronary angiogenesis (34), and platelet-derived growth factor (PDGF)-β, which is essential for the normal recruitment of pericytes by PDGF-βR–expressing endothelial cells, which is required for vascular maturation (32, 35). Another gene, lipid phosphate phosphatase 3 (PPAP2B), which is essential for vascular development and maintenance of vascular integrity (36), is found in keratinocytes. We further confirmed the regulation of FOG2, PDGF-β, and PPAP2B by miR-184 in HLEKs transfected with reporter constructs harboring the 3′UTR of each gene fused to luciferase coding sequence. Each construct was cotransfected into HLEKs with either pre-miRNA control or pre-miR-184. Cell-based luciferase assays revealed that FOG2, PDGF-β, and PPAP2B are indeed targeted miR-184 (Fig. 3A and Supplemental Fig. 3). To confirm the reporter analysis, we ectopically expressed miR-184 in HLEKs and observed a decrease in FOG2, PDGF-β, and PPAP2B proteins (Fig. 3B), which indicates that these proteins were bona fide miR-184 targets. In agreement, immunohistochemical analysis of human limbal (miR-184 low) and corneal (miR-184 high) epithelia revealed that all 3 proteins were readily detected in the limbal epithelium and borderline-detectable in the corneal epithelium (Fig. 3C–G).
Figure 3.
miR-184 targets FOG2, PDGF-β, and PPAP2B. A) Screening the interactions of FOG2, PDGF-β, and PPAP2B using the psiCHECK-2 vector harboring 3′UTR of each gene. Constructs were cotransfected with either miR-control or miR-184 into HLEKs. Twenty-four hours after transfection, firefly and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay System. N, precursor miRNA control; 184, precursor miR-184. **P < 0.01. B) Immunoblotting of FOG2, PDGF-β, and PPAP2B in HLEKs after transfection of precursor miRNA negative control (N) or precursor miR-184 (184). C–G) Tissue immunofluorescence staining with FOG2 (C), PDGF-β (D), and PPAP2B (E) antibodies in the frozen section of human limbal and corneal epithelium. No specific staining was observed in the limbus and cornea stained with isotype control antibodies, rabbit monoclonal antibody IgG (F), and rabbit polyclonal antibody IgG (G).
miR-184 negatively regulates VEGF
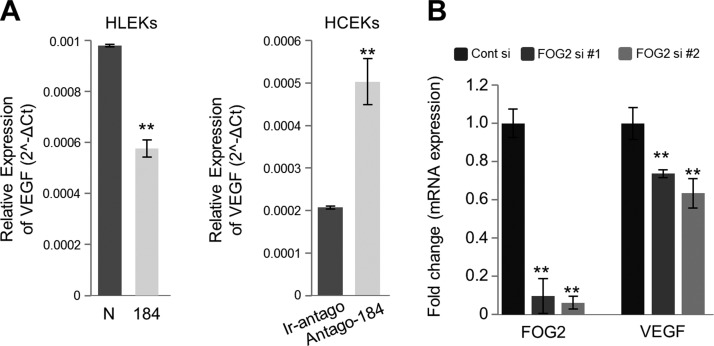
Having established that miR-184 targets and reduces the production of angiostatic factors FOG2, PDGF-β, and PPAP2B, we sought the link between these proteins and vascularization. FOG2 has the potential to affect a number of pathways involved in angiogenesis (37). Furthermore, FOG2 promotes the expression of VEGF (34) and the VEGF receptor (FLK-1) (38), which indicates a major role for this transcriptional regulator in vascular expansion and maintenance. To explore further the relationship between miR-184, VEGF, and FOG2 in corneal and limbal keratinocytes, we first determined that overexpression of miR-184 in HLEKs decreased VEGF levels, whereas knockdown of mir-184 in HCEKs increased VEGF expression (Fig. 4A). Not surprisingly, knockdown of FOG2 in HLEKs using 2 siFOG2 constructs resulted in a decrease in VEGF mRNA, confirming the stimulatory effect of FOG2 on VEGF expression in limbal keratinocytes (Fig. 4B).
Figure 4.
Regulation of VEGF expression by miR-184. A) VEGF levels were determined by qPCR in HLEKs and HCEKs after overexpression or knockdown of miR-184, respectively. B) HLEKs were transfected with siRNAs against FOG2 or scrambled control (Cont si). Forty-eight hours after transfection, total RNA was extracted, and cDNA was synthesized from DNase-treated RNA. Real-time qPCR data of FOG2 and VEGF were normalized to GAPDH. **P < 0.001.
miR-184 attenuates Akt phosphorylation
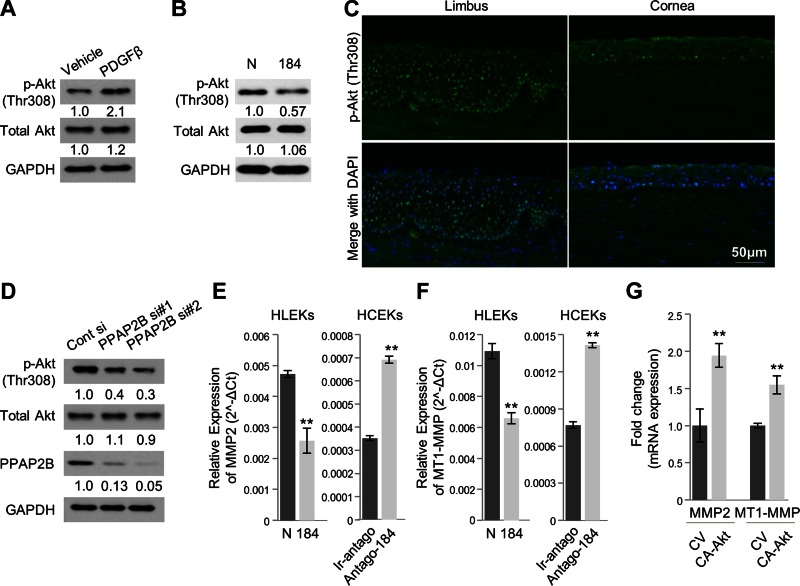
It is widely accepted that activation of Akt initiates a number of signaling pathways, which culminate in angiogenesis (reviewed in refs. 37, 39). On the other hand, it is known that PDGF-β activates Akt signaling (40). Not surprisingly, Akt and p-Akt levels were increased after PDGF-β treatment of HCEKs (Fig. 5A). Having shown that miR-184 decreases PDGF-β expression (Fig. 3B), we also sought to determine the effect of miR-184 on Akt signaling. HLEKs transduced with a pre-miR-184 had markedly decreased p-Akt levels compared with cells transduced with a control pre-miR (Fig. 5B). Immunohistochemical evaluation of p-Akt (Thr308) localization in human limbal and corneal epithelium demonstrated strong nuclear expression throughout the limbal epithelium and markedly lower expression in the corneal epithelium (Fig. 5C).
Figure 5.
miR-184 modulates Akt activity and its downstream gene expression. A) Immunoblotting of phospho-Akt (T308) and total Akt expression in HCEKs after treatment of PDGF-β. B) Immunoblotting of phospho-Akt (T308) and total Akt expression in HLEKs after transfection of precursor miRNA negative control or precursor miR-184. Phospho-Akt was markedly decreased in HLEKs expressing miR-184 compared with controls. C) Tissue immunofluorescence staining with p-Akt antibody in the frozen section of human limbal and corneal epithelium. Phospho-akt was strongly localized in the nucleus of limbal epithelium compared with corneal epithelium. D) Both phospho-Akt (T308) and total Akt protein levels were determined in HLEKs after depletion of PPAP2B using siRNAs. E) Real-time qPCR analysis of MMP2 levels in HLEKs and HCEKs after overexpression or knockdown of miR-184, respectively. F) MT1-MMP levels were determined by qPCR in HLEKs and HCEKs after overexpression or knockdown of miR-184, respectively. G) Real-time qPCR analysis of MMP2 and MT1-MMP in HCEKs followed by transient transfection with either control vector (CV) or a constitutively active Akt (CA-Akt). Expression levels were calculated relative to GAPDH mRNA.
To investigate further the role of miR-184 and Akt signaling, we looked at the effect of PPAP2B because this protein is upstream of Akt (41). Using 2 different siPPAP2B oligo pools, we effectively silenced this gene in HLEKs and significantly decreased p-Akt levels in these cells (Fig. 5D). Collectively, these data suggest that the antiangiogenic activity displayed by miR-184 is due, at least in part, to attenuated Akt activity.
miR-184 alters metalloproteinases
To further define the role of miR-184, Akt signaling, and angiogenesis, we looked at the expression of matrix metalloproteinases (MMPs) downstream of Akt, which have been implicated in angiogenesis (42–44). Overexpression of miR-184 in HLEKs significantly decreased the expression of MMP2, whereas knockdown of miR-184 in HCEKs dramatically increased MMP2 levels (Fig. 5E). Similarly, membrane type 1 MMP (MT1-MMP) levels were decreased in HLEKs that overexpressed miR-184 and increased in HCEKs upon miR-184 knockdown (Fig. 5F). To confirm that MMP levels were directly regulated by Akt in HCEKs, we performed transient transfection with CA-Akt and observed marked increases of MMP2 and MT1-MMP levels compared with control vector (Fig. 5G). Collectively, these data clearly implicate miR-184 as an inhibitor of Akt activation, which can affect matrix remodeling, a critical component of angiogenesis.
DISCUSSION
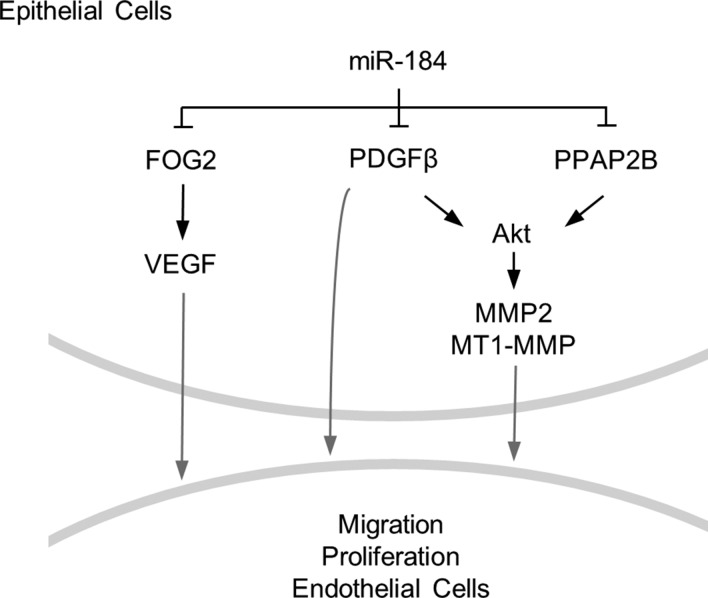
Given that miR-184 is the most abundant corneal epithelial miRNA (24), along with the overarching need to maintain corneal avascularity to insure proper vision, our finding that miR-184 has broad angiostatic properties makes excellent biologic sense. Previously, corneal suturing was reported to down-regulate miR-184, and it was suggested that this miRNA might negatively regulate VEGF signaling (27); however, the mechanisms underlying such regulation were unclear. We now provide direct evidence that miR-184 negatively regulates VEGF signaling by targeting FOG2, the upstream transcriptional regulator of VEGF (38) (Figs. 4 and 6). In addition, we demonstrate that, by targeting PDGF-β and PPAP2B, miR-184 negatively regulates Akt activity (Figs. 5 and 6). Such attenuation of Akt signaling affects angiogenesis by down-regulating metalloproteinases, which are associated with maintenance of vascular structure and remodeling (45). Thus, by attenuating both Akt signaling and VEGF, miR-184 can be considered as a broad-spectrum negative regulator of angiogenesis.
Figure 6.
MiR-184 coordinately regulates aspects of angiogenesis. Schematic diagram showing that miR-184 targets PDGF-β and PPAP2B, which affects the activity of Akt and expression of Akt downstream genes MMP2 and MT1-MMP. Targeting FOG2 by miR-184 contributes to VEGF expression levels. Akt, PDGF-β, MMP2, MT1-MMP, and VEGF affect both proliferation and migration of endothelial cells, which ultimately regulates angiogenesis.
We previously reported that miR-184 had a novel function, which was to negatively modulate the activity of another miRNA (miR-205) to maintain SHIP2 levels in the corneal epithelium (18). This was the first demonstration that an miRNA could interfere with another to ensure the expression of a target protein. We also reported that miR-205 targeted SHIP2, and thus SHIP2 regulation in the corneal epithelium via a miR-184/205 interaction was unique (18). SHIP2 is known to down-regulate the Akt signaling pathway (46–48). Thus, by neutralizing the inhibitory activity of miR-205 on SHIP2, miR-184 negatively regulates Akt signaling in corneal keratinocytes (18). We postulated that miR-184 might be functioning to maintain corneal avascularity via a SHIP2/Akt/VEGF axis (18). We now show that miR-184 can also negatively regulate Akt signaling by directly targeting upstream genes PDGF-β (40) and PPAP2B. A similar suppression of proliferation by miR-184 via inhibition of Akt signaling has been observed in breast cancer cell lines and orthotopic tumor models in vivo (49). Overexpression of miR-184 in several neuroblastoma cell lines has also been reported to decrease proliferative status (50, 51). It should be noted that in some instances miR-184 expression appears to be independent of the proliferative status of the corneal epithelium. For example, 24 h after wounding, numerous BrdU-labeled basal cells as well as a strong signal for miR-184 were seen in nonwounded corneal epithelium adjacent to the wound, suggestive that in this system miR-184 expression is independent of proliferation (24).
Similar to what we reported for miR-184 with respect to being the most abundant miRNA in the corneal epithelium (24), a recent study profiling miRNAs in aqueous humor in human eyes revealed that miR-184 was the second most prevalent miRNA (52). Interestingly, miR-184 levels were too low to be detected in plasma samples when compared with the aqueous humor samples, consistent with the idea that this miRNA may be somewhat “eye-specific” (24, 52). miR-184 has also been shown to down-regulate VEGF in vascular endothelial cells (27). Because miRNAs can be transported to neighboring cells via exosomes (53, 54), it is also possible that miR-184 in aqueous humor can be delivered to the limbal and corneal endothelium to restrain VEGF secretion to the ocular microenvironment under normal conditions. In certain limbal and corneal diseases, loss of miR-184 may lead to more VEGF secretion from the endothelium to the stroma, thereby exacerbating neovascularization. Thus, relatively high levels of miR-184 in the aqueous humor are likely to be another means of maintaining corneal avascularity.
Our finding that miR-184 negatively regulates the expression of MMP2 and MT1-MMP by affecting upstream targets of the Akt signaling pathway may also have implications in the etiology of keratoconus, which is a bilateral thinning of the cornea (55). An epithelial defect resulting in the release of proteolytic enzymes has been considered as a possible mechanism for the thinning (55, 56). Indeed MT1-MMP is elevated in keratoconic corneas (57) and could be involved in matrix degradation by MMP-2 activation (56). Interestingly, a mutation in the seed sequence of miR-184 has been reported to be responsible for familial severe keratoconus (22). Thus, it is possible that one consequence of such a mutated miR-184 is a failure to negatively regulate MT1-MMP and MMP2, leading to up-regulation of these two proteases and resulting in a thinner cornea. Another way that mutated miR-184 might be involved in keratoconus is via promoting vascularization in patients with keratoconus who wear contact lenses (58, 59). An association of keratoconus with contact lens-induced deep stromal neovasculatization has been reported, where of the 9 patients with contact lens-induced deep stromal neovasculatization, 7 had keratoconus as the primary diagnosis (58). Because DNV induced by contact lens wear is relatively rare (58), it is possible that patients with keratoconus are more predisposed to this condition due to miR-184 mutations, which allow for a more angiogenic stromal environment.
CONCLUSIONS
Our findings demonstrate that the ability of miR-184 to negatively regulate proangiogenetic secreted factors (VEGF, PDGF, and MMPs) and consequently to inhibit endothelial proliferation and migration, form a rationale for the development of an in vivo delivery system to introduce miR-184 or antago-184, such as nucleic acid nanoparticle conjugates. Because miR-184 has an inhibitory effect on Akt signaling, such a delivery system will have significant clinical application for treatment of corneal and retinal diseases, such as retinopathy of prematurity, diabetic retinopathy, and age-related macular dystrophy. Because miR-184 has been implicated in certain neoplasias, delivery of miR-184 to solid tumors may not only impair angiogenesis via inhibiting production of VEGF, PDGF, and MMPs but may also induce apoptosis in tumor cells via down-regulation of Akt signaling (18). In addition, delivery of antago-184 will promote angiogenesis and epithelial migration, which will be beneficial in the healing of chronic wounds.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Eye Institute Grants EY017536 and EY019463 (to R.M.L.), EY019463 (to O.V.), and NIH National Cancer Institute Grant CA172669 (to O.V.); and by a Dermatology Foundation Career Development Award (to H.P.). The NU-SDRC is supported by NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR057216. The authors thank Drs. Spiro Getsios and Nihal Kaplan (Northwestern University) for helpful discussions.
Glossary
- antagomir-184
antagomir against miR-184
- BrdU
bromodeoxyuridine
- CA-Akt
constitutively active Akt
- CM
conditioned medium
- FOG2
friend of Gata 2
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HCEK
human corneal epithelial keratinocyte
- HDMEC
human dermal microvascular cell
- HLEK
human limbal epithelial keratinocyte
- miR/miRNA
microRNA
- MMP
matrix metallopeptidase
- MT1-MMP
membrane-type-1 matrix metalloproteinase
- PDGF
platelet-derived growth factor
- PDGF-βR
platelet derived growth factor-β receptor
- PPAP2B
phosphatidic acid phosphatase 2b
- pre-miRNA-control
microRNA precursor negative control
- pre-miRNA-184
microRNA-184 precursor
- qPCR
quantitative PCR
- SHIP2
SH2 domain-containing inositol phosphatase 2
- ZFPM2
zinc finger protein, multitype 2 protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. K. Park, H. Peng, O. Volpert, and R. M. Lavker designed the research; J. K. Park, H. Peng, W. Yang, and J. Katsnelson performed the research; J. K. Park, H. Peng, O. Volpert, and R. M. Lavker analyzed the data; and J. K. Park, H. Peng, and R. M. Lavker wrote the paper.
REFERENCES
- 1.Lavker R. M., Tseng S. C. G., Sun T.-T. (2004) Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp. Eye Res. 78, 433–446 [DOI] [PubMed] [Google Scholar]
- 2.Dilly P. N. (1985) Contribution of the epithelium to the stability of the tear film. Trans. Ophthalmol. Soc. U. K. 104, 381–389 [PubMed] [Google Scholar]
- 3.Cintron C., Covington H., Kublin C. L. (1983) Morphogenesis of rabbit corneal stroma. Invest. Ophthalmol. Vis. Sci. 24, 543–556 [PubMed] [Google Scholar]
- 4.Cotsarelis G., Cheng S. Z., Dong G., Sun T.-T., Lavker R. M. (1989) Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 57, 201–209 [DOI] [PubMed] [Google Scholar]
- 5.Schermer A., Galvin S., Sun T.-T. (1986) Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J. Cell Biol. 103, 49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amitai-Lange A., Altshuler A., Bubley J., Dbayat N., Tiosano B., Shalom-Feuerstein R. (2015) Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells 33, 230–239 [DOI] [PubMed] [Google Scholar]
- 7.Wei Z. G., Wu R. L., Lavker R. M., Sun T. T. (1993) In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia: implications on conjunctival epithelial transdifferentiation and stem cells. Invest. Ophthalmol. Vis. Sci. 34, 1814–1828 [PubMed] [Google Scholar]
- 8.Ecoiffier T., Yuen D., Chen L. (2010) Differential distribution of blood and lymphatic vessels in the murine cornea. Invest. Ophthalmol. Vis. Sci. 51, 2436–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Eye Institute. (2012) Vision research: needs, gaps, and opportunities. U.S. Department of Health and Human Sciences, Washington, D.C. [Google Scholar]
- 10.Ambros V. (2004) The functions of animal microRNAs. Nature 431, 350–355 [DOI] [PubMed] [Google Scholar]
- 11.Hammond S. M. (2006) MicroRNAs as oncogenes. Curr. Opin. Genet. Dev. 16, 4–9 [DOI] [PubMed] [Google Scholar]
- 12.Morgan C. P., Bale T. L. (2012) Sex differences in microRNA regulation of gene expression: no smoke, just miRs. Biol. Sex Differ. 3, 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng H., Hamanaka R. B., Katsnelson J., Hao L. L., Yang W., Chandel N. S., Lavker R. M. (2012) MicroRNA-31 targets FIH-1 to positively regulate corneal epithelial glycogen metabolism. FASEB J. 26, 3140–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng H., Kaplan N., Hamanaka R. B., Katsnelson J., Blatt H., Yang W., Hao L., Bryar P. J., Johnson R. S., Getsios S., Chandel N. S., Lavker R. M. (2012) microRNA-31/factor-inhibiting hypoxia-inducible factor 1 nexus regulates keratinocyte differentiation. Proc. Natl. Acad. Sci. USA 109, 14030–14034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng H., Katsnelson J., Yang W., Brown M. A., Lavker R. M. (2013) FIH-1/c-kit signaling: a novel contributor to corneal epithelial glycogen metabolism. Invest. Ophthalmol. Vis. Sci. 54, 2781–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shalom-Feuerstein R., Serror L., De La Forest Divonne S., Petit I., Aberdam E., Camargo L., Damour O., Vigouroux C., Solomon A., Gaggioli C., Itskovitz-Eldor J., Ahmad S., Aberdam D. (2012) Pluripotent stem cell model reveals essential roles for miR-450b-5p and miR-184 in embryonic corneal lineage specification. Stem Cells 30, 898–909 [DOI] [PubMed] [Google Scholar]
- 17.Yu J., Peng H., Ruan Q., Fatima A., Getsios S., Lavker R. M. (2010) MicroRNA-205 promotes keratinocyte migration via the lipid phosphatase SHIP2. FASEB J. 24, 3950–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J., Ryan D., Getsios S., Oliveira-Fernandes M., Fatima A., Lavker R. (2008) MicroRNA-184 antagonizes microRNA-205 to maintain the lipid phosphatase SHIP2 levels in epithelia. Proc. Natl. Acad. Sci. USA 105, 19300–19305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng H., Park J. K., Katsnelson J., Kaplan N., Yang W., Getsios S., Lavker R. M. (2015) microRNA-103/107 family regulates multiple epithelial stem cell characteristics. Stem Cells 33, 1642–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ljubimov A. V., Saghizadeh M. (2015) Progress in corneal wound healing. Prog. Retin. Eye Res. 49, 17–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funari V. A., Winkler M., Brown J., Dimitrijevich S. D., Ljubimov A. V., Saghizadeh M. (2013) Differentially expressed wound healing-related microRNAs in the human diabetic cornea. PLoS One 8, e84425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes A. E., Bradley D. T., Campbell M., Lechner J., Dash D. P., Simpson D. A., Willoughby C. E. (2011) Mutation altering the miR-184 seed region causes familial keratoconus with cataract. Am. J. Hum. Genet. 89, 628–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lechner J., Bae H. A., Guduric-Fuchs J., Rice A., Govindarajan G., Siddiqui S., Abi Farraj L., Yip S. P., Yap M., Das M., Souzeau E., Coster D., Mills R. A., Lindsay R., Phillips T., Mitchell P., Ali M., Inglehearn C. F., Sundaresan P., Craig J. E., Simpson D. A., Burdon K. P., Willoughby C. E. (2013) Mutational analysis of MIR184 in sporadic keratoconus and myopia. Invest. Ophthalmol. Vis. Sci. 54, 5266–5272 [DOI] [PubMed] [Google Scholar]
- 24.Ryan D. G., Oliveira-Fernandes M., Lavker R. M. (2006) MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol. Vis. 12, 1175–1184 [PubMed] [Google Scholar]
- 25.An J., Chen X., Chen W., Liang R., Reinach P. S., Yan D., Tu L. (2015) MicroRNA expression profile and the role of miR-204 in corneal wound healing. Invest. Ophthalmol. Vis. Sci. 56, 3673–3683 [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann A., Huang Y., Suetsugu-Maki R., Ringelberg C. S., Tomlinson C. R., Del Rio-Tsonis K., Tsonis P. A. (2012) Implication of the miR-184 and miR-204 competitive RNA network in control of mouse secondary cataract. Mol. Med. 18, 528–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zong R., Zhou T., Lin Z., Bao X., Xiu Y., Chen Y., Chen L., Ma J. X., Liu Z., Zhou Y. (2016) Down-regulation of microRNA-184 is associated with corneal neovascularization. Invest. Ophthalmol. Vis. Sci. 57, 1398–1407 [DOI] [PubMed] [Google Scholar]
- 28.Jiang B. H., Zheng J. Z., Aoki M., Vogt P. K. (2000) Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc. Natl. Acad. Sci. USA 97, 1749–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou M., Li X. M., Lavker R. M. (2006) Transcriptional profiling of enriched populations of stem cells versus transient amplifying cells. A comparison of limbal and corneal epithelial basal cells. J. Biol. Chem. 281, 19600–19609 [DOI] [PubMed] [Google Scholar]
- 30.Getsios S., Simpson C. L., Kojima S., Harmon R., Sheu L. J., Dusek R. L., Cornwell M., Green K. J. (2009) Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J. Cell Biol. 185, 1243–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambati B. K., Nozaki M., Singh N., Takeda A., Jani P. D., Suthar T., Albuquerque R. J., Richter E., Sakurai E., Newcomb M. T., Kleinman M. E., Caldwell R. B., Lin Q., Ogura Y., Orecchia A., Samuelson D. A., Agnew D. W., St Leger J., Green W. R., Mahasreshti P. J., Curiel D. T., Kwan D., Marsh H., Ikeda S., Leiper L. J., Collinson J. M., Bogdanovich S., Khurana T. S., Shibuya M., Baldwin M. E., Ferrara N., Gerber H. P., De Falco S., Witta J., Baffi J. Z., Raisler B. J., Ambati J. (2006) Corneal avascularity is due to soluble VEGF receptor-1. Nature 443, 993–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raza A., Franklin M. J., Dudek A. Z. (2010) Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am. J. Hematol. 85, 593–598 [DOI] [PubMed] [Google Scholar]
- 33.Lamalice L., Le Boeuf F., Huot J. (2007) Endothelial cell migration during angiogenesis. Circ. Res. 100, 782–794 [DOI] [PubMed] [Google Scholar]
- 34.Zhou B., Ma Q., Kong S. W., Hu Y., Campbell P. H., McGowan F. X., Ackerman K. G., Wu B., Zhou B., Tevosian S. G., Pu W. T. (2009) Fog2 is critical for cardiac function and maintenance of coronary vasculature in the adult mouse heart. J. Clin. Invest. 119, 1462–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abramsson A., Lindblom P., Betsholtz C. (2003) Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J. Clin. Invest. 112, 1142–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panchatcharam M., Salous A. K., Brandon J., Miriyala S., Wheeler J., Patil P., Sunkara M., Morris A. J., Escalante-Alcalde D., Smyth S. S. (2014) Mice with targeted inactivation of ppap2b in endothelial and hematopoietic cells display enhanced vascular inflammation and permeability. Arterioscler. Thromb. Vasc. Biol. 34, 837–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimmeler S., Zeiher A. M. (2000) Akt takes center stage in angiogenesis signaling. Circ. Res. 86, 4–5 [DOI] [PubMed] [Google Scholar]
- 38.Tevosian S. G., Deconinck A. E., Tanaka M., Schinke M., Litovsky S. H., Izumo S., Fujiwara Y., Orkin S. H. (2000) FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell 101, 729–739 [DOI] [PubMed] [Google Scholar]
- 39.Karar J., Maity A. (2011) PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 4, 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H., Bajraszewski N., Wu E., Wang H., Moseman A. P., Dabora S. L., Griffin J. D., Kwiatkowski D. J. (2007) PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J. Clin. Invest. 117, 730–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humtsoe J. O., Feng S., Thakker G. D., Yang J., Hong J., Wary K. K. (2003) Regulation of cell-cell interactions by phosphatidic acid phosphatase 2b/VCIP. EMBO J. 22, 1539–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang D., Bar-Eli M., Meloche S., Brodt P. (2004) Dual regulation of MMP-2 expression by the type 1 insulin-like growth factor receptor: the phosphatidylinositol 3-kinase/Akt and Raf/ERK pathways transmit opposing signals. J. Biol. Chem. 279, 19683–19690 [DOI] [PubMed] [Google Scholar]
- 43.Zhang D., Brodt P. (2003) Type 1 insulin-like growth factor regulates MT1-MMP synthesis and tumor invasion via PI 3-kinase/Akt signaling. Oncogene 22, 974–982 [DOI] [PubMed] [Google Scholar]
- 44.Risinger G. M. Jr., Hunt T. S., Updike D. L., Bullen E. C., Howard E. W. (2006) Matrix metalloproteinase-2 expression by vascular smooth muscle cells is mediated by both stimulatory and inhibitory signals in response to growth factors. J. Biol. Chem. 281, 25915–25925 [DOI] [PubMed] [Google Scholar]
- 45.Vu T. H., Werb Z. (2000) Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 14, 2123–2133 [DOI] [PubMed] [Google Scholar]
- 46.Sasaoka T., Kikuchi K., Wada T., Sato A., Hori H., Murakami S., Fukui K., Ishihara H., Aota R., Kimura I., Kobayashi M. (2003) Dual role of SRC homology domain 2-containing inositol phosphatase 2 in the regulation of platelet-derived growth factor and insulin-like growth factor I signaling in rat vascular smooth muscle cells. Endocrinology 144, 4204–4214 [DOI] [PubMed] [Google Scholar]
- 47.Sharrard R. M., Maitland N. J. (2007) Regulation of protein kinase B activity by PTEN and SHIP2 in human prostate-derived cell lines. Cell. Signal. 19, 129–138 [DOI] [PubMed] [Google Scholar]
- 48.Sleeman M. W., Wortley K. E., Lai K. M., Gowen L. C., Kintner J., Kline W. O., Garcia K., Stitt T. N., Yancopoulos G. D., Wiegand S. J., Glass D. J. (2005) Absence of the lipid phosphatase SHIP2 confers resistance to dietary obesity. Nat. Med. 11, 199–205 [DOI] [PubMed] [Google Scholar]
- 49.Phua Y. W., Nguyen A., Roden D. L., Elsworth B., Deng N., Nikolic I., Yang J., Mcfarland A., Russell R., Kaplan W., Cowley M. J., Nair R., Zotenko E., O’Toole S., Tan S. X., James D. E., Clark S. J., Kouros-Mehr H., Swarbrick A. (2015) MicroRNA profiling of the pubertal mouse mammary gland identifies miR-184 as a candidate breast tumour suppressor gene. Breast Cancer Res. 17, 83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malzkorn B., Wolter M., Liesenberg F., Grzendowski M., Stühler K., Meyer H. E., Reifenberger G. (2010) Identification and functional characterization of microRNAs involved in the malignant progression of gliomas. Brain Pathol. 20, 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foley N. H., Bray I. M., Tivnan A., Bryan K., Murphy D. M., Buckley P. G., Ryan J., O’Meara A., O’Sullivan M., Stallings R. L. (2010) MicroRNA-184 inhibits neuroblastoma cell survival through targeting the serine/threonine kinase AKT2. Mol. Cancer 9, 83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wecker T., Hoffmeier K., Plötner A., Grüning B. A., Horres R., Backofen R., Reinhard T., Schlunck G. (2016) MicroRNA profiling in aqueous humor of individual human eyes by next-generation sequencing. Invest. Ophthalmol. Vis. Sci. 57, 1706–1713 [DOI] [PubMed] [Google Scholar]
- 53.Salido-Guadarrama I., Romero-Cordoba S., Peralta-Zaragoza O., Hidalgo-Miranda A., Rodríguez-Dorantes M. (2014) MicroRNAs transported by exosomes in body fluids as mediators of intercellular communication in cancer. Onco Targets Ther. 7, 1327–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhuang G., Wu X., Jiang Z., Kasman I., Yao J., Guan Y., Oeh J., Modrusan Z., Bais C., Sampath D., Ferrara N. (2012) Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 31, 3513–3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davidson A. E., Hayes S., Hardcastle A. J., Tuft S. J. (2014) The pathogenesis of keratoconus. Eye (Lond.) 28, 189–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balasubramanian S. A., Pye D. C., Willcox M. D. (2010) Are proteinases the reason for keratoconus? Curr. Eye Res. 35, 185–191 [DOI] [PubMed] [Google Scholar]
- 57.Collier S. A., Madigan M. C., Penfold P. L. (2000) Expression of membrane-type 1 matrix metalloproteinase (MT1-MMP) and MMP-2 in normal and keratoconus corneas. Curr. Eye Res. 21, 662–668 [PubMed] [Google Scholar]
- 58.Shah S., Yeung K. K., Weissman B. A. (1989) Contact lens-related deep stromal neovascularization. Int. Contact Lens Clin. 25, 128–136 [Google Scholar]
- 59.Kymionis G. D., Kontadakis G. A. (2012) Severe corneal vascularization after intacs implantation and rigid contact lens use for the treatment of keratoconus. Semin. Ophthalmol. 27, 19–21 [DOI] [PubMed] [Google Scholar]