Abstract
Podocytes play a key role in diabetic nephropathy pathogenesis, but alteration of their metabolism remains unknown in human kidney. By using a conditionally differentiating human podocyte cell line, we addressed the functional and molecular changes in podocyte energetics during in vitro development or under high glucose conditions. In 5 mM glucose medium, we observed a stepwise activation of oxidative metabolism during cell differentiation that was characterized by peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α)–dependent stimulation of mitochondrial biogenesis and function, with concomitant reduction of the glycolytic enzyme content. Conversely, when podocytes were cultured in high glucose (20 mM), stepwise oxidative phosphorylation biogenesis was aborted, and a glycolytic switch occurred, with consecutive lactic acidosis. Expression of the master regulators of oxidative metabolism transcription factor A mitochondrial, PGC-1α, AMPK, and serine–threonine liver kinase B1 was altered by high glucose, as well as their downstream signaling networks. Focused transcriptomics revealed that myocyte-specific enhancer factor 2C (MEF2C) and myogenic factor 5 (MYF5) expression was inhibited by high glucose levels, and endoribonuclease-prepared small interfering RNA–mediated combined inhibition of those transcription factors phenocopied the glycolytic shift that was observed in high glucose conditions. Accordingly, a reduced expression of MEF2C, MYF5, and PGC-1α was found in kidney tissue sections that were obtained from patients with diabetic nephropathy. These findings obtained in human samples demonstrate that MEF2C-MYF5–dependent bioenergetic dedifferentiation occurs in podocytes that are confronted with a high-glucose milieu.—Imasawa, T., Obre, E., Bellance, N., Lavie, J., Imasawa, T., Rigothier, C., Delmas, Y., Combe, C., Lacombe, D., Benard, G., Claverol, S., Bonneu, M., Rossignol, R. High glucose repatterns human podocyte energy metabolism during differentiation and diabetic nephropathy.
Keywords: mitochondria, MEF2C, human kidney
One in 2 patients with diabetes suffers from diabetic nephropathy, the leading cause of chronic kidney disease worldwide (1). Previous studies have revealed that glomerular visceral epithelial cells, namely podocytes, could play a key role in the pathogenesis of diabetic nephropathy (2–5). Podocytes have complex structures, which are known as foot processes, slit diaphragm, and the focal adhesion complex, all of which are altered in diabetic nephropathy (2–5).
Because a considerable number of mitochondria have been observed in the cell body and in the narrow peripheral foot processes, it is thought that podocytes could be sensitive to an alteration of oxidative metabolism (6, 7). Furthermore, in cases of mitochondrial disorders, podocytes lose the interdigitating foot processes and accumulate dysmorphic mitochondria (8, 9). Such foot process effacement resembles an early morphologic change of diabetic nephropathy (2); therefore, understanding the peculiarities of podocyte energy metabolism, notably in diabetic conditions, could provide novel insights into the pathogenesis of diabetic nephropathy and the role of mitochondria (10, 11).
We hypothesized that high glucose levels could alter mitochondrial function in human podocytes, as found in pancreatic β-cells (12), muscle cells (13), adipocytes (14), or neurons (15), which should lead to diabetic complications (16). Because podocytes dedifferentiate in the process of injuries as diabetic nephropathy (17, 18), we investigated podocyte energetics and proteome remodeling during normal differentiation as well as under hyperglycemic stress.
MATERIALS AND METHODS
Cell culture
Human podocytes, previously described (19), were used in our study. These cells began to differentiate after switching from 33° to 37°C and were fully differentiated 15 d after this switch (19, 20). Podocytes were cultured in RPMI 1640 medium that was supplemented with 10% fetal calf serum, insulin (10 μg/ml; Sigma-Aldrich, Lyon, France), transferrin (5.5 μg/ml; Sigma-Aldrich), selenium (5 ng/ml; Sigma-Aldrich), and penicillin-streptomycin 1X (100×; Thermo Fisher Scientific, Waltham, MA, USA), with 5 mM glucose from d 0 (day of temperature switch) to d 7 (7 d after temperature switch). We confirmed that podocytes at d 15 expressed nephrin, podocin, and podocalyxin by Western blot. To observe mitochondrial network morphology, MitoTracker Red CMXRos 100 nM (Thermo Fisher Scientific) was added to culture medium and cells were incubated at 37°C for 25 min (21).
Cellular oxygen consumption rate
Endogenous cellular oxygen consumption rate was monitored in intact podocytes at 37°C in a 1-ml thermostatically controlled chamber (1.0 × 107 cells/ml/run) that was equipped with a Clark oxygen electrode (Hansatech Instruments Ltd., Norfolk, United Kingdom). Endogenous respiratory rate was expressed in nanomolar O2 per min per 105 cells. Data were collected from 3 independent flasks for each condition.
Cellular and mitochondrial ATP synthesis
Podocytes were collected and resuspended in culture medium at a concentration of 5 × 105 cells/ml. Cell suspension (0.1 ml/tube) was incubated at 37°C for 45 min with or without 5 μl of 100 mM antimycin (Sigma-Aldrich) to block mitochondrial respiration and ATP synthesis. After incubation, cells were treated with a cell lysis buffer (ATP Bioluminescence Assay Kit CLS II; Roche, Basel, Switzerland) and ATP content that was released into the medium was measured by bioluminescence (21). ATP produced by mitochondria was calculated as the total ATP content minus the ATP content measured in the presence of antimycin.
Lactate concentration
Cell culture medium was deproteinized with a 10-kDa MW cut-off spin filter to remove lactate dehydrogenase. Lactate concentration was determined by using an enzymatic assay (Lactate Assay Kit; Sigma-Aldrich).
Mitochondrial biogenesis evaluation
We measured the mitochondrial DNA (mtDNA) content and mRNA levels of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α). After collecting cultured podocytes, total DNA was extracted by using a DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA, USA), and total RNA was extracted by using an RNeasy Plus Mini Kit (Qiagen). First-strand cDNA was synthesized in a 20-μl volume using 1 μg total RNA, with 50 units of M-MuLV Reverse Transcriptase (RNase H−) and Oligo d(T)16 (Applied Biosystems, Foster City, CA, USA). Real-time quantitative PCR for nuclear DNA, mtDNA, and PGC-1α mRNA was performed by using IQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) on a thermocycler CFX96 Touch Real-Time PCR system (Bio-Rad), as previously described (22). Normalized units to the endogenous references (RPLP0 and GUSB) were calculated by using the CFX Manager Software program (Bio-Rad).
Measurement of enzymatic activities of respiratory chain complexes and citrate synthase
All assays were performed in 200 µl of Tp II:Mannitol buffer (225 mM mannitol, 75 mM saccharose, 10 mM TrisHCl, 0.1 mM EDTA, pH 7.2). All results were normalized by protein concentration (BCA protein assay; Pierce, Rockford, IL, USA). We determined several respiratory chain complexes (complex III and IV) and citrate synthase enzymatic activities, as previously described (23).
Western blotting
Western blot was performed as previously described (22) by using the following antibodies: PGC-1α (P-19; sc-5815, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA), nuclear respiratory factor-1 (NRF-1; ab55744, 1:500; Abcam, Cambridge, United Kingdom), transcription factor A, mitochondrial (TFAM; ab119684, 1:500; Abcam), AMPK (2532, 1:1000; Cell Signaling Technology, Danvers, MA, USA), phospho-AMPK-α for the detection of endogenous AMPKα only when phosphorylated at threonine 172 (2531, 1:1000: Cell Signaling Technology), serine–threonine liver kinase B1 (LKB1; ab15095, 1:100; Abcam), and mammalian target of rapamycin (mTOR; ab87540, 1:200; Abcam).
Reactive oxygen species measurement
Changes in reactive oxygen species levels were monitored by using the CM-H2DCFDA probe (C6827; Thermo Fisher Scientific). Cells were plated in 96 wells (20,000 cells/well). The probe was added to cell medium and incubated for 30 min at 37°C. Of H2O2, 10 µl was used a positive control. Fluorescence was measured on a Xenius Spectrofluorometer (Safas, Monaco, France).
Label-free quantitative proteomics
This analysis was performed by the proteomic facility of Bordeaux University (http://www.cgfb.u-bordeaux2.fr/en/synthese-proteome). The steps of sample preparation and protein digestion, nano-scale liquid chromatography–tandem mass spectrometry analysis, and database search and results processing were performed as previously described (24). Label-free quantitative data analysis was performed by using Progenesis Liquid Chromatography–Tandem Mass Spectrometry 4.0 software (Nonlinear Dynamics, Newcastle, United Kingdom). Data processing included the following steps: 1) feature detection, 2) feature alignment, 3) volume integration for 2–6 charge-state ions, 4) normalization of the total protein abundance, 5) import of sequence information, 6) ANOVA test at the peptide level and filtering for features P < 0.05, 7) calculation of protein abundance (sum of the volume of corresponding peptides), and 8) ANOVA test at the protein level and filtering for features P < 0.05. Of note, only nonconflicting features and unique peptides were considered for calculation at the protein level. We selected proteins that showed >20% change in their expression levels with statistical significance (P < 0.05) between 2 groups (n = 3 in each group). Furthermore, these proteins were categorized according to their functions by using the Kyoto Encyclopedia of Genes and Genomes analysis in the search tool for the retrieval of interacting genes/proteins (STRING) database (http://string-db.org). A more global analysis of the data was performed via use of Ingenuity Pathway Analysis (Qiagen).
Analysis by quantitative PCR microarray
In this study, we used 2 different quantitative PCR microarrays: Human Glucose Metabolism RT2 Profiler PCR Array (Qiagen) and Human Transcription Factors RT2 Profiler PCR Array (Qiagen).
Analyses by endoribonuclease-prepared small interfering RNA
At d 12, we transfected cells with endoribonuclease-prepared small interfering RNA (esiRNA) under 4 conditions: esiRNA enhanced green fluorescent protein, esiRNA myogenic factor 5 (MYF5), esiRNA myocyte-specific enhancer factor 2C (MEF2C), and esiRNA MYF5 plus MEF2C. Human esiRNA from Sigma-Aldrich and Dharmafect transfection reagent provided by Dharmacon RNA Technologies (Lafayette, CO, USA) were used. We prepared 5 µM esiRNA solutions in serum-free medium, mixed them with Dharmafect transfection reagent, and incubated them at room temperature for 20 min. Then, antibiotic-free complete medium was added for a final esiRNA concentration of 25 nM. We removed the medium in flasks and added the appropriate esiRNA solution for each condition. Podocytes were cultured in 5 mM glucose. At d 15, protein from podocytes was extracted.
Analyses of human kidney samples
Kidney biopsied tissues from 5 patients with diabetic nephropathy and 5 normal participants were used in this study. Primary antibodies were PGC-1α (P-19; sc-5815; Santa Cruz Biotechnology) and pyruvate kinase muscle type 2 (PKM2; D78A4; #4053; Cell Signaling Technology), as well as MYF5 (C-20, sc-302; Santa Cruz Biotechnology) and MEF2C (ab191428; Abcam). Numbers of PKM2-positive podocytes per glomerulus were counted. In addition, we calculated mean percentages of PGC-1α-, MYF5-, and MEF2C-positive nuclei of total nuclei per glomeruli; more than 10 glomeruli per patient were counted in these analyses. This study was approved by the ethical committee of Chiba-East Hospital.
RESULTS
Activation of oxidative energy metabolism during human podocyte in vitro differentiation
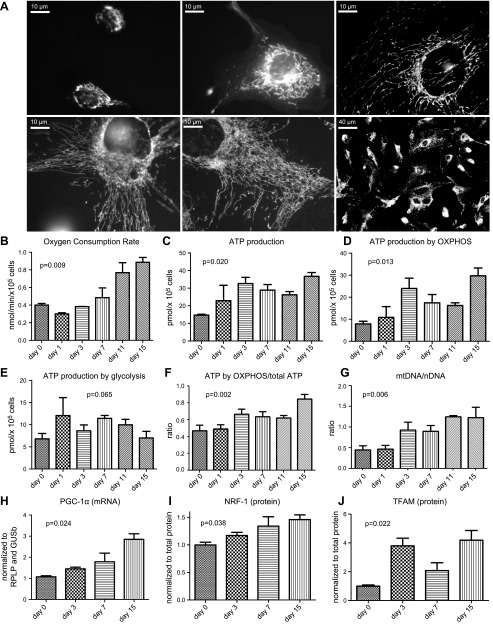
Upon induction of podocyte differentiation (19, 20), cell morphology changed from a small, circular cell to a reticulated one, which became mature at d 15. The size of the mitochondrial network also increased progressively (Fig. 1A). Oxygen consumption rate (Fig. 1B) increased significantly during cell differentiation (P < 0.01, ANOVA analysis), with an increment factor of 2.2 after 15 d of growth in 5 mM glucose medium. Cellular ATP content (Fig. 1C) also increased between d 0 and 15 (P < 0.05, ANOVA analysis), and this increase was attributed to oxidative phosphorylation (oxphos), but not glycolysis (Fig. 1D–F). Accordingly, the amount of mtDNA increased during differentiation (Fig. 1G). Likewise, PGC-1α mRNA levels increased significantly (Fig. 1H), with a factor of 2.7 between d 0 and 15. In addition, NRF-1 and TFAM mRNA expression also increased by factors of 1.5 and 4.2, respectively (Fig. 1I, J).
Figure 1.
Podocyte physiology and energetics during differentiation. A) Representative images of cultured, differentiating podocytes at the day of switching to 37°C (d 0), at 3 d after the switch (d 3), and at d 7, 11, and 15 (mature podocytes). The mitochondrial network (white tubules) was stained with MitotrackerGreen. B) Oxygen consumption rate (nmol/min/×105 cells) at each differentiation stage. C) Changes of cellular ATP content (pmol/×105 cells) during differentiation. D) Changes of ATP content produced by oxphos (pmol/×105 cells) during differentiation. E) Changes of ATP content produced by glycolysis (pmol/×105 cells) during differentiation. F) Contribution of oxphos to total ATP production. G) mtDNA/nDNA (nuclear DNA) ratio determined by using quantitative PCR. H) mRNA content of PGC-1α measured by quantitative PCR. I) NRF-1 expression determined by Western blotting. K) TFAM expression determined by Western blotting. Histograms indicate means ± sd. P values by 1-way ANOVA are indicated in graphs.
Molecular changes in bioenergetic machineries during human podocyte differentiation
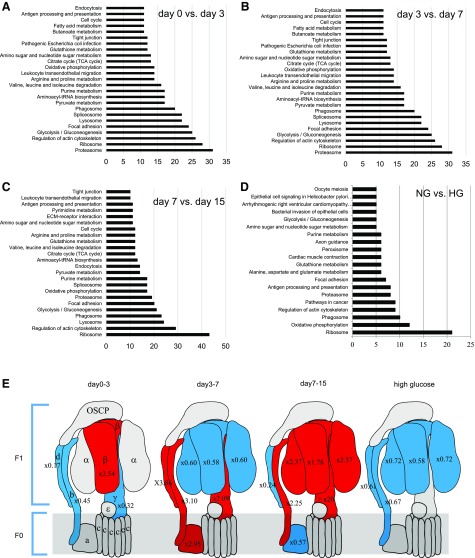
To investigate the molecular changes in podocytes undergoing differentiation, we compared the proteome between d 0–3, 3–7, and 7–15 (Table 1). Approximately 8% of the proteins whose expression levels changed within those 3 periods belonged to mitochondria. Among proteins that increased between d 0–3 and 3–7 (Fig. 2A, B), the proteasome comprised the largest population (>30 proteins). Then, >10 proteins associated with glycolysis/gluconeogenesis, oxphos, and the tricarboxylic acid (TCA) cycle also increased during both periods. From d 7–15 (Fig. 2C), a considerable number of proteins (>40) that were associated with ribosome biogenesis and function increased, which suggested an intense phase of protein translation and cellular reprogramming. In particular, enzymes that were involved in glycolysis/gluconeogenesis (>20 proteins), oxphos (>15 proteins), branched-chain amino acid degradation (>10 proteins), and the TCA cycle (>10 proteins) increased their content by a factor above +20% (P < 0.05).
TABLE 1.
Summary of results by quantitative proteomics
| Stage (d) |
||||
|---|---|---|---|---|
| Results | 0–3 d | 3–7 d | 7–15 d | NG vs. HG |
| Mitochondrial protein/total protein | 104/1451 (7.2) | 124/1579 (7.9) | 102/1416 (7.2) | 40/520 (7.7) |
| Increased mitochondrial protein/increased total protein | 55/549 (10.0) | 84/967 (8.7) | 61/672 (9.1) | 9/230 (3.9) |
| Decreased mitochondrial protein/decreased total protein | 49/902 (5.4) | 40/612 (6.5) | 41/744 (5.5) | 31/290 (10.7) |
Number of proteins whose expression levels changed at 3 stages of podocyte differentiation and between podocytes cultured in the normal glucose and HG media [n/N (%)]. These results were obtained by using mass spectrometry–based quantitative proteomics. Only proteins that showed >20% change in their expression levels with statistical significance between 2 groups were selected. NG, normal glucose. P < 0.05.
Figure 2.
A–C) Differential proteomic analysis of podocytes from d 0 to 3 (A), from d 3 to 7 (B), and from d 7 to 15 (C). Proteins that showed >20% change in their expression levels with statistical significance (P < 0.05) were selected and categorized by Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. Graphs show the number of proteins that belong to the top 25 categories. D) Only decreased proteins (>20% change with statistical significance) in HG compared with those in HG were categorized into functional groups by KEGG analysis. Bars show the number of decreased proteins that belong to the top 20 categories. E) Schematic representation of complex V and changes in subunit expression during podocyte differentiation and on HG condition. Increased proteins are shown in red, decreased in blue (fold change is indicated in each subunit). ECM, extracellular matrix.
Sequential construction of the oxphos system during human podocyte differentiation
Components of oxphos did not increase simultaneously during the 3 phases of differentiation introduced above (Table 2). Subunits of respiratory chain complex IV and V were increased at later stages compared with complex I. In particular, synthesis of the α- and β-subunits of the F1 catalytic-domain of the F1F0ATP synthase complex was increased at the final stages of podocyte differentiation (Table 2 and Fig. 2E). Proteomic analysis also revealed that most glycolytic enzymes were decreased during podocyte differentiation (Table 2). In accordance with an oxidative shift, proteins that were involved in the fueling of oxphos with fatty acid, amino acids, or ketones were also up-regulated (Table 2).
TABLE 2.
Changes of proteins involved in energy metabolism at 3 stages during podocyte differentiation or between podocytes cultured in the normal glucose and HG mediums
| Stage (d) |
||||
|---|---|---|---|---|
| Changes of energy metabolism in podocytes | 0–3 | 3–7 | 7–15 | HG/NG |
| Krebs cycle | ||||
| Pyruvate dehydrogenase E1 component subunit β | − | |||
| Isocitrate dehydrogenase | −− | |||
| Aconitate hydratase | −− | |||
| Complex I | ||||
| NADH-ubiquinone oxidoreductase 75-kDa subunit | +++ | + | − | uc |
| NADH dehydrogenase iron-sulfur protein 2 | −−− | +++ | −−− | uc |
| NADH dehydrogenase iron-sulfur protein 3 | ++++ | uc | ++ | uc |
| NADH dehydrogenase iron-sulfur protein 8 | +++++ | −− | +++ | uc |
| NADH dehydrogenase 1 α subcomplex subunit 4 | uc | uc | +++ | uc |
| NADH dehydrogenase 1 α subcomplex subunit 5 | −−− | ++ | uc | uc |
| NADH dehydrogenase 1 α subcomplex subunit 9 | uc | ++ | uc | uc |
| NADH dehydrogenase 1 α subcomplex subunit 10 | +++++ | − | uc | uc |
| NADH-ubiquinone oxidoreductase chain 1 | −−−− | +++ | −−− | uc |
| NADH-ubiquinone oxidoreductase chain 4 | −−−− | +++++ | −−−−− | uc |
| NADH-ubiquinone oxidoreductase chain 5 | uc | ++++ | −−− | uc |
| NADH dehydrogenase flavoprotein 2 | uc | ++++ | −−− | uc |
| Complex II | ||||
| Succinate dehydrogenase flavoprotein subunit | +++++ | + | uc | uc |
| Complex III | ||||
| Cytochrome b-c1 complex subunit 1 | −−− | + | −−− | −− |
| Cytochrome b-c1 complex subunit 2 | +++++ | uc | uc | uc |
| Cytochrome b-c1 complex subunit 7 | uc | +++ | +++ | uc |
| Complex IV | ||||
| Cytochrome c oxidase subunit 2 | + | −− | ++ | uc |
| Cytochrome c oxidase subunit 4 isoform 1 | −−−− | +++ | ++ | −− |
| Cytochrome c oxidase subunit 5A | −−− | +++ | +++ | uc |
| Cytochrome c oxidase subunit 5B | uc | uc | +++++ | −− |
| Cytochrome c oxidase subunit 6C | −−− | +++ | +++ | −−− |
| Cytochrome c oxidase subunit 7A2 | uc | uc | ++++ | uc |
| HIG1 domain family member 1A | +++++ | uc | +++++ | uc |
| Complex V | ||||
| α | uc | −− | +++ | − |
| β | +++ | −− | ++ | −− |
| γ | −−− | +++ | ++++ | uc |
| a | uc | +++ | − | uc |
| b | −−− | +++ | +++ | − |
| d | −−−− | +++ | −−− | −− |
| f | −−−− | +++ | uc | uc |
| g | −−−−− | ++++ | ++++ | uc |
| O | uc | ++ | +++ | uc |
| F1 complex assembly factor 1 | uc | −−− | +++ | uc |
| Glycolysis | ||||
| Hexokinase | −−− | + | −− | uc |
| Glucokinase | +++++ | +++ | −−−− | +++ |
| Phosphoglucose isomerase | −−− | +++ | −−− | uc |
| Phosphofructokinase | −−− | − | − | + |
| Aldolase | −−− | ++ | − | uc |
| Triose phosphate isomerase | −−−− | +++ | −−− | uc |
| Glyceraldehyde phosphate dehydrogenase | −−− | +++ | −−− | ++ |
| Phosphoglycerate kinase | −− | +++ | ++ | ++ |
| Phosphoglyceromutase | + | −− | +++ | uc |
| Enolase α | −−− | +++ | −−− | uc |
| Enolase γ | +++ | +++ | −−− | uc |
| Pyruvate kinase | +++ | uc | − | +++ |
| Lactate dehydrogenase | −−−− | +++ | −−− | uc |
| β-Oxidation | ||||
| Acyl-CoA synthetase | uc | ++++ | uc | uc |
| Acyl-CoA dehydrogenase (very long chain) | −−− | +++ | −−− | + |
| Acyl-CoA dehydrogenase (medium chain) | +++ | +++ | −−−− | uc |
| Enoyl-CoA hydratase | +++ | +++ | +++ | uc |
| 3-Hydroxyacyl-CoA dehydrogenase | +++ | ++ | +++ | uc |
| β-Ketoacyl-CoA thiolase | + | +++ | +++ | + |
| Ketone body degradation | ||||
| Succinyl-CoA transferase | +++++ | + | +++ | uc |
| Amino acid degradation | ||||
| Serine hydroxymethyltransferase | −−− | −−− | +++ | + |
| Glutamate dehydrogenase 1 | +++ | −−− | +++ | −− |
| Glutaminolysis | ||||
| Glutamate dehydrogenase 1 | +++ | −−− | +++ | −−− |
| Glutaminase | −−− | −−− | uc | −−−− |
| ATP/ADP translocator | ||||
| Adenine nucleotide translocase 2 | uc | −−− | uc | −− |
These data were obtained by using quantitative proteomics. Only proteins that showed >20% change in their expression levels with statistical significance (P < 0.05) between 2 groups were selected. NG, normal glucose; uc, no change during the indicated period; +++++, >10× change; ++++, 5–10×; +++, 2–5×; ++, 1.5–2.0×; +, 1.2–1.5×; −−−−−, <0.1×; −−−−, 0.1–0.2×; −−−, 0.2–0.5×; −−, 0.5–0.67×; −, 0.67–0.8×.
A glycolytic switch occurs in podocytes under high glucose conditions
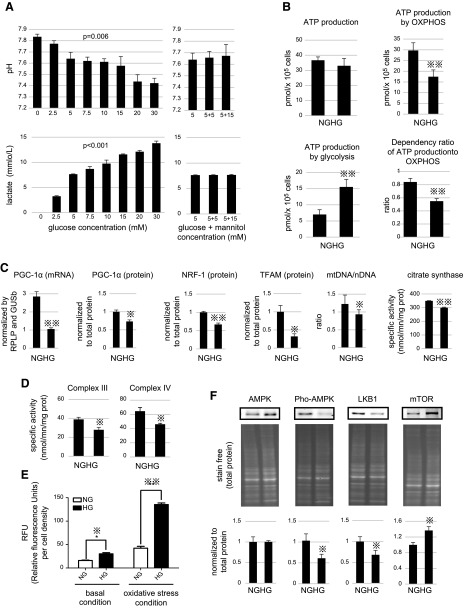
Premature podocytes that were cultured from d 7 to 15 in media with 8 different glucose concentrations showed an increase in lactate levels (Fig. 3A) that was suggestive of a glycolytic switch. Although hyperglycemia is clinically observed when glucose concentration ranges above 7 mM, diabetes symptoms do not typically become noticeable until 15–20 mM. To mimic this situation, we conducted in vitro studies with 20 mM glucose. Podocyte dependency on oxphos for cellular ATP production was decreased in high glucose (HG; 20 mM), whereas that of glycolysis increased compared with podocytes cultured in normal glucose (5 mM; Fig. 3B). This metabolic switch was explained, in part, by a reduction of mitochondrial biogenesis, as PGC-1α, NRF-1, and TFAM expression as well as mtDNA content were reduced in HG conditions (Fig. 3C). Accordingly, activity of citrate synthase, an indicator of mitochondrial active content, was also decreased in those conditions (Fig. 3C).
Figure 3.
A) Analyses of culture medium from d 11–13 (left: glucose concentrations 0, 2.5, 5, 7.5, 10, 15, 20, and 30 mM from left to right, respectively; right: culture medium with 5 mM glucose, with 5 mM glucose and 5 mM mannitol, and with 5 mM glucose and 15 mM mannitol from left to right, respectively). Representative images of the culture medium (top). pH of the culture media (middle). Lactate levels in culture media (bottom). P values by 1-way ANOVA are indicated in graphs. B) Measurement of podocyte ATP content when cultured in normal glucose (NG; 5 mM) or HG (20 mM) condition. Total ATP, mitochondrial ATP, and glycolytic ATP content in podocytes, and contribution of oxphos to the total ATP content were analyzed. C) PGC-1α mRNA expression was determined by quantitative PCR. PGC-1α, NRF-1, and TFAM expression were determined by Western blotting. mtDNA/nDNA (nuclear DNA) ratio in podocytes cultured in HG or NG medium was also analyzed by quantitative PCR. Citrate synthase enzymatic activities, as a quantitative marker for the content of intact mitochondria, were also measured in podocytes cultured in NG or HG. D) Enzymatic activities of complexes III and IV in podocytes cultured in NG or HG were analyzed. E) Changes in reactive oxygen species (ROS) levels of podocytes cultured in NG or HG were monitored with or without oxidative stress condition. F) Expression level of total AMPK, Thr172-phosphorylated AMPK, LKB1, and mTOR in podocytes cultured in HG or NG medium were analyzed by Western blotting. GUSb, beta glucuronidase; RPLP, ribosomal phosphoprotein. Histograms indicate means ± sd. *P < 0.05, **P < 0.01, unpaired Student's t test.
HG triggers metabolic reprogramming in human podocytes in vitro
We performed a differential proteomic analysis between podocytes that were grown in 20 mM (HG) or those grown in 5 mM glucose (normal glucose) between d 7 15. Thirty-one mitochondrial proteins were decreased in podocytes that were cultured under HG (Table 1). Oxphos was severely impacted, with >10 proteins reduced. Key components of intermediate metabolism, such as glutaminolysis or TCA cycle, were altered (Table 2). On the one hand, at the level of the respiratory chain, key components of complexes III, IV, and V were decreased (Table 2), as well as key components of ADP phosphorylation, such as ATPsynthase (Fig. 2E), ANT2, and the mitochondrial phosphate carrier. Measurement of respiratory chain enzyme activities confirmed a reduction for complexes III and IV (Fig. 3D). On the other hand, the content of 5 glycolytic enzymes was increased in HG conditions (Table 2). Lactate dehydrogenase A was also increased in HG (+48%), which was consistent with the elevated lactate production described in Fig. 3A. Lastly, proteomic analysis showed that antioxidant enzymes, such as catalase (+86%), glutathione S transferase (+65%), glutathione S transferase ω 1 (+21%), thioredoxin reductase (+59%), and peroxredoxin 2 (+51%) were increased in hyperglycemia. This could be consecutive to the higher steady-state of H2O2 levels that were measured in the HG conditions (Fig. 3E).
Regulation of HG-mediated podocyte glycolytic switch
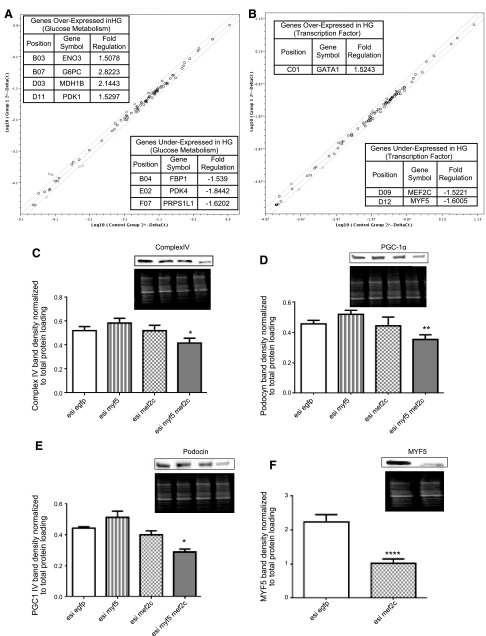
First, we observed an alteration of the LKB1-AMPK pathway—a master regulator of oxphos—in podocytes that were grown under HG conditions (Fig. 3F). Analysis of the mRNA content of 84 genes that are involved in glucose metabolism (Fig. 4A) also showed that hyperglycemia stimulated glycolysis but also activated expression of the oxphos inhibitor, pyruvate dehydrogenase kinase 1 (PDK1) (25). Search for potential transcription factors involved in metabolic reprogramming induced by hyperglycemia (Fig. 4B) showed a decrease in MEF2C and MYF5. A previous study revealed that MEF2C controls the expression of numerous components of oxidative metabolism, notably PGC-1α (26). Accordingly, the concomitant down-regulation of MEF2C and MYF5 by using esiRNA in human podocytes triggered a significant down-regulation of respiratory chain complex IV (Fig. 4C) and PGC1-α (Fig. 4D). In these conditions, dual inhibition of MEF2C and MYF5 also altered podocin expression (Fig. 4E). Of note, use of a single esiRNA against MEF2C or MYF5 alone did not change the levels of complex IV, PGC-1α, or podocin (Fig. 4C–E). Such a cooperative effect between MEF2C and MYF5 was previously described for the synergistic transactivation of a myogenin (27). We further observed that MEF2C controlled MYF5 expression (Fig. 4F), whereas the opposite did not occur (data not shown). In addition to energy metabolism and the role of MEF2C-MYF5 cooperation in reducing PGC-1α and oxphos, changes in the cellular signaling pathways of podocytes challenged with HG are listed in Table 3. As discussed above, NRF-2–mediated oxidative stress response was identified by Ingenuity Pathway Analysis software as activated under HG conditions, as well as glycolysis and mitochondrial dysfunction.
Figure 4.
A, B) Changes of mRNA level of 84 genes involved in glucose metabolism (A) or DNA transcription (B) between podocytes cultured in normal glucose (NG) or HG as analyzed by quantitative PCR microarray. Tables in panels show genes whose expression levels were statistically different between both groups. A scheme of the main regulatory network identified in podocyte remodeling by hyperglycemia. C–F) Complex IV (C), PGC-1α (D), and podocin (E) expression of podocytes cultured with esiRNA of EGFP, MYF5 (F), MEF2C, and MYF5 + MEF2C were determined by Western blotting.
TABLE 3.
Summary of the top biochemical pathways and transcriptional regulators involved in energy metabolism impacted by hyperglycemic condition (Ingenuity Pathway Analysis)
| Name | P | Overlap or no. of molecules |
|---|---|---|
| Glycolysis I | 2.98E-05 | 16.7% |
| NRF-2–mediated oxidative stress response | 1.60E-04 | 3.9% |
| Mitochondrial dysfunction | 1.78E-03 | 3.6% |
| Alyl hydrocarbon receptor signaling | 5.11E-03 | 3.4% |
| Renal necrosis/cell death | 1.12E-02 | 1.9% |
| Hereditary disorder | 1.51E-02 | 54 |
| Cellular growth and proliferation | 1.43E-02 | 59 |
| Kidney failure | 4.52E-01 | 4 |
| Renal inflammation | 1.73E-01 | 4 |
| Renal nephritis | 1.73E-01 | 4 |
| Nephritis | 8.72E-02 | 2 |
| Renal necrosis/cell death | 4.91E-01 | 5 |
| Potential regulators (transcriptional) | ||
| Estrogen-related receptor α | 2.08E-06 | |
| cAMP-responsive element binding protein 3-like 1 | 4.67E-05 | |
| Functional networks | Score | |
| Cell death and survival, cellular development, cellular growth and proliferation | 50 | |
| Skeletal and muscular disorders, hereditary disorder, development disorder | 40 | |
| Top proteins up-regulated (fold-change) | ||
| TOP2A (4.011); DDX42 (2.389); IPO5 (1.893); CSNK2A1 (1.746); TGM2 (1.715); IGF2R (1.654); CALU (1.654); PSNB3 (1.636); MYL12A (1.608); RNF213 (1.580) | ||
| Top proteins down-regulated | ||
| HSPE1 (−2.439); UCHL1 (−2.185); MAN2A1 (−2.141); HADHA (−2.138); CYB5R3 (−2.100); ITGAV (−2.077); ATP5B (−2.073); ABCE1 (−2.060); FLNC (−1.976); LDHB (−1.947) | ||
Investigation of the glycolytic switch in diabetic nephropathy kidney samples
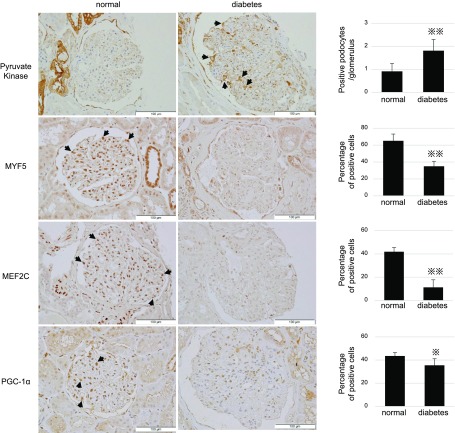
Because proteomics showed up-regulation of the glycolytic enzyme, pyruvate kinase M2 podocyte, under HG (Table 2), and given the role of this enzyme in anabolic glycolysis (28), we measured PKM2 expression in human kidney sections (Fig. 5). Intense labeling was found in podocytes and Bowman’s capsules in glomeruli, and PKM2-positive podocytes were significantly more numerous in diabetic kidney patients (Fig. 5). Furthermore, expression of MYF5 and MEF2C, which were detected as decreased transcriptomic factors in our in vitro study, was significantly decreased in patients with diabetic nephropathy (Fig. 5). Likewise, intensities of PGC-1α expression were also down-regulated in samples from patients with diabetic nephropathy (Fig. 5).
Figure 5.
Representative images of immunostaining of kidney-biopsied specimens from normal participants and patients with diabetic nephropathy. Arrows indicate positive podocytes. Percentages of positive cells for PGC-1a, MYF5, and MEF2 per total cells in glomeruli were calculated. For pyruvate kinase, numbers of positive podocytes per glomerulus were counted. *P < 0.05, **P < 0.01, unpaired Student's t test.
DISCUSSION
Mitochondria are increasingly recognized as key players in acquired renal diseases (29), and Reidy et al. (30) recently reviewed their role in diabetic kidney disease initiation. In the present study, we described the metabolic reprogramming of human podocytes that are confronted with HG levels and we demonstrated the role of MEF2C and MYF5 in the glycolytic switch. Our work has implications for the fundamental understanding of human podocyte differentiation, but also for the study of diabetic nephropathy, as we could validate some of the in vitro findings on human kidney samples that were obtained from patients with diabetes. Regarding the analysis of the determinants of podocyte differentiation, our study has relevance as podocyte dedifferentiation may occur in the context of podocyte injuries (17, 18). Moreover, previous analyses of the alteration of mitochondrial bioenergetics were performed in diabetic podocytes that were obtained from rats and mice, which could differ from human podocytes (31, 32) or between different mouse strains (33). Moreover, the situation is unclear in mice podocytes, as Wang et al. (34) reported an alteration of oxphos by hyperglycemia, whereas other authors showed the opposite (10, 35). In contrast with mice or rat podocyte studies, our data were obtained from human podocytes and from kidney sections from patients with diabetic nephropathy.
A novel finding of our study is that MEF2C and MYF5 were involved in the bioenergetic dedifferentiation of human podocytes toward oxphos, and that hyperglycemia altered these transcription factors. In addition, we described a sequential change in the energetic machinery without precedent. We found that glycolytic enzymes are immediately repressed when podocyte differentiation occurs and that respiratory chain proteins increase in a specific order. In particular, the components of the F1F0-ATP synthase complex are only built up when the rest of the machinery has been produced, which could potentially be to avoid hydrolysis of glycolytic ATP by this complex. A recent study showed that the respiratory chain complex III assembly depends on the energy state of mitochondria (36); thus, feedback mechanisms could also control synthesis of ATP synthases and the overall maturation of oxphos. Another feature of podocyte differentiation resides in the stimulation of oxidative fueling processes. Fatty acids and branched-chain amino acids are excellent fuels for oxphos in skeletal muscle and the heart, and our findings suggest that those energy substrates could also play an important role in podocyte physiology (37). Our findings indicate that onset of oxphos that was observed during normal podocyte differentiation (5 mM glucose) required expression of both MEF2C and MYF5, but also that of TFAM, NRF-1, and PGC-1α (38).
The second part of our work focused on the toxic effect of HG levels (in vitro) and of hyperglycemia (in vivo) on podocyte bioenergetic apparatus. Podocytes that were exposed to 20 mM glucose not only aborted the bioenergetic differentiation process discussed above, but they also shifted to a different program that favored glycolysis (bioenergetic dedifferentiation). Ateration in mitochondrial F1F0-ATP synthase subunits might impact mitochondrial structure (39), dynamics, and function (40) in podocytes, thereby leading to foot process effacement as an early pathologic change in diabetic nephropathy (2). Of interest, knockdown of MEF2C and MYF5 also triggered the reduction of podocin expression in our in vitro study, which suggested a more global dedifferentiation process caused by hyperglycemia. Glycolytic switch observed in podocytes that were grown in 20 mM glucose resembles the Warburg effect described in cancer cells wherein a reduction of PGC-1α content and mitochondrial biogenesis was previously reported (41). Increase in oxidative stress triggered by HG growth conditions in our in vitro study also correlates with the consequences of hyperglycemia in humans (42). In different models, mechanisms of HG-mediated reactive oxygen species increase involved the alteration of mitochondrial respiration (43), which suggested the importance of restoring mitochondrial bioenergetics in this disease. In our study, human podocytes that were confronted with HG showed an increase in oxidative stress despite up-regulation of several antioxidant defenses proteins. In patients with diabetes, advanced glycation end products that induced increased oxidative stress were considered therapeutic targets for using Kremezin and benfotiamine, 2 U.S. Food and Drug Administration–approved advanced glycation end product inhibitors (44).
Regarding the signaling mechanisms that are involved in hyperglycemia-induced metabolic shift in podocytes, we observed that HG and hyperglycemia reduced the expression of MEF2C, which participates in control of the expression of numerous components of oxidative metabolism, notably PGC-1α (41). Previous findings demonstrated the association between MEF2C and the AMPK axis during exercise training in skeletal muscle (45), and our findings revealed that HG altered both the activation of AMPK and the protein content of AMPK activator LKB1 (24, 46, 47). Of interest, we observed that only a combined inhibition of MEF2C and MYF5 expression with esiRNA was required to block the oxphos shift. This could suggest that both transcription factors cooperate at the site of gene transcription to induce expression of target genes. In agreement with our findings, a synergistic transactivation of a myogenin by Myf5 and MEF2 was previously demonstrated (27). The recently identified RNA-binding activity of MYF5 (48) might also suggest the need to conduct further genetic investigations on the collaboration between MEF2C and MYF5. In addition, we observed up-regulation of PDK1, a critical component of the Warburg effect that is capable of shutting down glucose-dependent oxphos in situations of pseudohypoxia (49) or in conditions of high fatty acid and HG intake in pancreatic β-cells (50). The link between MEF2C and PDK1 remains to be established. Lastly, our signaling study showed that mTOR expression was inhibited in podocytes that were cultured in HG medium, as expected in conditions of LKB1-AMPK axis activation. This is consistent with previous studies that have shown that mTOR is activated in podocytes in diabetic nephropathy (51, 52). Recently, mTOR inactivation (52, 53), restoration of the LKB1-AMPK activity (54), and PGC-1α activation (55) were proposed as novel potential therapeutic options for diabetic nephropathy. Our findings further suggest that stimulation of MEF2C and MYF5 expression could permit the restoration of podocyte bioenergetics and mitochondrial biogenesis in these conditions.
ACKNOWLEDGMENTS
The authors thank Welsh GI, Academic and Children's Renal Unit (Bristol University, Southmead Hospital, Bristol, United Kingdom) for providing the human podocytes, and Dr. Akira Hiwatashi (Tsukuba University, Tsukuba, Japan) and Mori Tachibana (National Hospital Organization Chiba-East Hospital) for valuable technical advice and assistance. This work was supported by the Japan Society for the Promotion of Science (KAKENHI) Grant 80348276 (to T.I), a grant from the National Hospital Organization of Japan (to T.I.), and the French Association Against Myopathies. The authors declare no conflicts of interest.
Glossary
- esiRNA
endoribonuclease-prepared small interfering RNA
- LKB1
serine–threonine liver kinase B1
- MEF2C
myocyte-specific enhancer factor 2C
- mtDNA
mitochondrial DNA
- mTOR
mammalian target of rapamycin
- MYF5
myogenic factor 5
- NRF-1
nuclear respiratory factor-1
- oxphos
oxidative phosphorylation
- PDK1
pyruvate dehydrogenase kinase 1
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator 1α
- PKM2
pyruvate kinase muscle type 2
- TCA
tricarboxylic acid
- TFAM
transcription factor A, mitochondrial
AUTHOR CONTRIBUTIONS
Tos. Imasawa performed most of the experiments, designed the study, and wrote the manuscript; E. Obre, N. Bellance, J. Lavie, Tom. Imasawa, and G. Benard performed cell culture, cell staining, quantitative PCR, Western blotting, and quantitative PCR microarray, and analyzed the data; C. Rigothier, Y. Delmas, C. Combe, and D. Lacombe performed and organized the generation of cell culture system and designed a part of this study; S. Claverol and M. Bonneu performed all proteomics studies; and R. Rossignol performed most of the bioinformatics studies and largely participated in constructing study design and methods as well as writing the manuscript.
REFERENCES
- 1.Dronavalli S., Duka I., Bakris G. L. (2008) The pathogenesis of diabetic nephropathy. Nat. Clin. Pract. Endocrinol. Metab. 4, 444–452 [DOI] [PubMed] [Google Scholar]
- 2.Gassler N., Elger M., Kränzlin B., Kriz W., Gretz N., Hähnel B., Hosser H., Hartmann I. (2001) Podocyte injury underlies the progression of focal segmental glomerulosclerosis in the fa/fa Zucker rat. Kidney Int. 60, 106–116 [DOI] [PubMed] [Google Scholar]
- 3.Hara M., Yamagata K., Tomino Y., Saito A., Hirayama Y., Ogasawara S., Kurosawa H., Sekine S., Yan K. (2012) Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: establishment of a highly sensitive ELISA to detect urinary podocalyxin. Diabetologia 55, 2913–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J. J., Kwak S. J., Jung D. S., Kim J. J., Yoo T. H., Ryu D. R., Han S. H., Choi H. Y., Lee J. E., Moon S. J., Kim D. K., Han D. S., Kang S. W. (2007) Podocyte biology in diabetic nephropathy. Kidney Int. Suppl. 72(106), S36–S42 [DOI] [PubMed] [Google Scholar]
- 5.Ihalmo P., Wessman M., Kaunisto M. A., Kilpikari R., Parkkonen M., Forsblom C., Holthöfer H., Groop P. H.; FinnDiane Study Group (2008) Association analysis of podocyte slit diaphragm genes as candidates for diabetic nephropathy. Diabetologia 51, 86–90 [DOI] [PubMed] [Google Scholar]
- 6.Imasawa T., Rossignol R. (2013) Podocyte energy metabolism and glomerular diseases. Int. J. Biochem. Cell Biol. 45, 2109–2118 [DOI] [PubMed] [Google Scholar]
- 7.Abe Y., Sakairi T., Kajiyama H., Shrivastav S., Beeson C., Kopp J. B. (2010) Bioenergetic characterization of mouse podocytes. Am. J. Physiol. Cell Physiol. 299, C464–C476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Güçer S., Talim B., Aşan E., Korkusuz P., Ozen S., Unal S., Kalkanoğlu S. H., Kale G., Cağlar M. (2005) Focal segmental glomerulosclerosis associated with mitochondrial cytopathy: report of two cases with special emphasis on podocytes. Pediatr. Dev. Pathol. 8, 710–717 [DOI] [PubMed] [Google Scholar]
- 9.Diomedi-Camassei F., Di Giandomenico S., Santorelli F. M., Caridi G., Piemonte F., Montini G., Ghiggeri G. M., Murer L., Barisoni L., Pastore A., Muda A. O., Valente M. L., Bertini E., Emma F. (2007) COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J. Am. Soc. Nephrol. 18, 2773–2780 [DOI] [PubMed] [Google Scholar]
- 10.Stieger N., Worthmann K., Teng B., Engeli S., Das A. M., Haller H., Schiffer M. (2012) Impact of high glucose and transforming growth factor-β on bioenergetic profiles in podocytes. Metabolism 61, 1073–1086 [DOI] [PubMed] [Google Scholar]
- 11.Sharma K., Karl B., Mathew A. V., Gangoiti J. A., Wassel C. L., Saito R., Pu M., Sharma S., You Y. H., Wang L., Diamond-Stanic M., Lindenmeyer M. T., Forsblom C., Wu W., Ix J. H., Ideker T., Kopp J. B., Nigam S. K., Cohen C. D., Groop P. H., Barshop B. A., Natarajan L., Nyhan W. L., Naviaux R. K. (2013) Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J. Am. Soc. Nephrol. 24, 1901–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed M., Muhammed S. J., Kessler B., Salehi A. (2010) Mitochondrial proteome analysis reveals altered expression of voltage dependent anion channels in pancreatic β-cells exposed to high glucose. Islets 2, 283–292 [DOI] [PubMed] [Google Scholar]
- 13.Mootha V. K., Lindgren C. M., Eriksson K. F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstråle M., Laurila E., Houstis N., Daly M. J., Patterson N., Mesirov J. P., Golub T. R., Tamayo P., Spiegelman B., Lander E. S., Hirschhorn J. N., Altshuler D., Groop L. C. (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 [DOI] [PubMed] [Google Scholar]
- 14.Gao C. L., Zhu C., Zhao Y. P., Chen X. H., Ji C. B., Zhang C. M., Zhu J. G., Xia Z. K., Tong M. L., Guo X. R. (2010) Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol. Cell. Endocrinol. 320, 25–33 [DOI] [PubMed] [Google Scholar]
- 15.Leinninger G. M., Edwards J. L., Lipshaw M. J., Feldman E. L. (2006) Mechanisms of disease: mitochondria as new therapeutic targets in diabetic neuropathy. Nat. Clin. Pract. Neurol. 2, 620–628 [DOI] [PubMed] [Google Scholar]
- 16.Russell N. D., Cooper M. E. (2015) 50 years forward: mechanisms of hyperglycaemia-driven diabetic complications. Diabetologia 58, 1708–1714 [DOI] [PubMed] [Google Scholar]
- 17.May C. J., Saleem M., Welsh G. I. (2014) Podocyte dedifferentiation: a specialized process for a specialized cell. Front. Endocrinol. (Lausanne) 5, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S. Y., Huang P. H., Yang A. H., Tarng D. C., Yang W. C., Lin C. C., Chen J. W., Schmid-Schönbein G., Lin S. J. (2014) Matrix metalloproteinase-9 deficiency attenuates diabetic nephropathy by modulation of podocyte functions and dedifferentiation. Kidney Int. 86, 358–369 [DOI] [PubMed] [Google Scholar]
- 19.Saleem M. A., O’Hare M. J., Reiser J., Coward R. J., Inward C. D., Farren T., Xing C. Y., Ni L., Mathieson P. W., Mundel P. (2002) A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 13, 630–638 [DOI] [PubMed] [Google Scholar]
- 20.Rigothier C., Auguste P., Welsh G. I., Lepreux S., Deminière C., Mathieson P. W., Saleem M. A., Ripoche J., Combe C. (2012) IQGAP1 interacts with components of the slit diaphragm complex in podocytes and is involved in podocyte migration and permeability in vitro. PLoS One 7, e37695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benard G., Bellance N., James D., Parrone P., Fernandez H., Letellier T., Rossignol R. (2007) Mitochondrial bioenergetics and structural network organization. J. Cell Sci. 120, 838–848 [DOI] [PubMed] [Google Scholar]
- 22.Melser S., Chatelain E. H., Lavie J., Mahfouf W., Jose C., Obre E., Goorden S., Priault M., Elgersma Y., Rezvani H. R., Rossignol R., Bénard G. (2013) Rheb regulates mitophagy induced by mitochondrial energetic status. Cell Metab. 17, 719–730 [DOI] [PubMed] [Google Scholar]
- 23.Medja F., Allouche S., Frachon P., Jardel C., Malgat M., Mousson de Camaret B., Slama A., Lunardi J., Mazat J. P., Lombès A. (2009) Development and implementation of standardized respiratory chain spectrophotometric assays for clinical diagnosis. Mitochondrion 9, 331–339 [DOI] [PubMed] [Google Scholar]
- 24.Fessart D., Martin-Negrier M. L., Claverol S., Thiolat M. L., Crevel H., Toussaint C., Bonneu M., Muller B., Savineau J. P., Delom F. (2014) Proteomic remodeling of proteasome in right heart failure. J. Mol. Cell. Cardiol. 66, 41–52 [DOI] [PubMed] [Google Scholar]
- 25.Velpula K. K., Bhasin A., Asuthkar S., Tsung A. J. (2013) Combined targeting of PDK1 and EGFR triggers regression of glioblastoma by reversing the Warburg effect. Cancer Res. 73, 7277–7289 [DOI] [PubMed] [Google Scholar]
- 26.Czubryt M. P., McAnally J., Fishman G. I., Olson E. N. (2003) Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha) and mitochondrial function by MEF2 and HDAC5. Proc. Natl. Acad. Sci. USA 100, 1711–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johanson M., Meents H., Ragge K., Buchberger A., Arnold H. H., Sandmöller A. (1999) Transcriptional activation of the myogenin gene by MEF2-mediated recruitment of myf5 is inhibited by adenovirus E1A protein. Biochem. Biophys. Res. Commun. 265, 222–232 [DOI] [PubMed] [Google Scholar]
- 28.Yang L., Xie M., Yang M., Yu Y., Zhu S., Hou W., Kang R., Lotze M. T., Billiar T. R., Wang H., Cao L., Tang D. (2014) PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat. Commun. 5, 4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emma F., Montini G., Parikh S. M., Salviati L. (2016) Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat. Rev. Nephrol. 12, 267–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reidy K., Kang H. M., Hostetter T., Susztak K. (2014) Molecular mechanisms of diabetic kidney disease. J. Clin. Invest. 124, 2333–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheval L., Pierrat F., Rajerison R., Piquemal D., Doucet A. (2012) Of mice and men: divergence of gene expression patterns in kidney. PLoS One 7, e46876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien L. L., Guo Q., Lee Y., Tran T., Benazet J. D., Whitney P. H., Valouev A., McMahon A. P. (2016) Differential regulation of mouse and human nephron progenitors by the Six family of transcriptional regulators. Development 143, 595–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walkin L., Herrick S. E., Summers A., Brenchley P. E., Hoff C. M., Korstanje R., Margetts P. J. (2013) The role of mouse strain differences in the susceptibility to fibrosis: a systematic review. Fibrogenesis Tissue Repair 6, 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W., Wang Y., Long J., Wang J., Haudek S. B., Overbeek P., Chang B. H., Schumacker P. T., Danesh F. R. (2012) Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 15, 186–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coimbra T. M., Janssen U., Gröne H. J., Ostendorf T., Kunter U., Schmidt H., Brabant G., Floege J. (2000) Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int. 57, 167–182 [DOI] [PubMed] [Google Scholar]
- 36.Ostojić J., Panozzo C., Lasserre J. P., Nouet C., Courtin F., Blancard C., di Rago J. P., Dujardin G. (2013) The energetic state of mitochondria modulates complex III biogenesis through the ATP-dependent activity of Bcs1. Cell Metab. 18, 567–577 [DOI] [PubMed] [Google Scholar]
- 37.Sieber J., Jehle A. W. (2014) Free fatty acids and their metabolism affect function and survival of podocytes. Front. Endocrinol. (Lausanne) 5, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hock M. B., Kralli A. (2009) Transcriptional control of mitochondrial biogenesis and function. Annu. Rev. Physiol. 71, 177–203 [DOI] [PubMed] [Google Scholar]
- 39.Paumard P., Vaillier J., Coulary B., Schaeffer J., Soubannier V., Mueller D. M., Brèthes D., di Rago J. P., Velours J. (2002) The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 21, 221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carraro M., Giorgio V., Šileikytė J., Sartori G., Forte M., Lippe G., Zoratti M., Szabò I., Bernardi P. (2014) Channel formation by yeast F-ATP synthase and the role of dimerization in the mitochondrial permeability transition. J. Biol. Chem. 289, 15980–15985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jose C., Bellance N., Rossignol R. (2011) Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim. Biophys. Acta 1807, 552–561 [DOI] [PubMed] [Google Scholar]
- 42.Marfella R., Quagliaro L., Nappo F., Ceriello A., Giugliano D. (2001) Acute hyperglycemia induces an oxidative stress in healthy subjects. J. Clin. Invest. 108, 635–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishikawa T., Araki E. (2007) Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 9, 343–353 [DOI] [PubMed] [Google Scholar]
- 44.Lambers Heerspink H. J., de Zeeuw D. (2013) Novel drugs and intervention strategies for the treatment of chronic kidney disease. Br. J. Clin. Pharmacol. 76, 536–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGee S. L., Hargreaves M. (2006) Exercise and skeletal muscle glucose transporter 4 expression: molecular mechanisms. Clin. Exp. Pharmacol. Physiol. 33, 395–399 [DOI] [PubMed] [Google Scholar]
- 46.Lizcano J. M., Göransson O., Toth R., Deak M., Morrice N. A., Boudeau J., Hawley S. A., Udd L., Mäkelä T. P., Hardie D. G., Alessi D. R. (2004) LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zong H., Ren J. M., Young L. H., Pypaert M., Mu J., Birnbaum M. J., Shulman G. I. (2002) AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA 99, 15983–15987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panda A. C., Abdelmohsen K., Martindale J. L., Di Germanio C., Yang X., Grammatikakis I., Noh J. H., Zhang Y., Lehrmann E., Dudekula D. B., De S., Becker K. G., White E. J., Wilson G. M., de Cabo R., Gorospe M. (2016) Novel RNA-binding activity of MYF5 enhances Ccnd1/Cyclin D1 mRNA translation during myogenesis. Nucleic Acids Res. 44, 2393–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim J. W., Tchernyshyov I., Semenza G. L., Dang C. V. (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 [DOI] [PubMed] [Google Scholar]
- 50.Xu J., Han J., Epstein P. N., Liu Y. Q. (2006) Regulation of PDK mRNA by high fatty acid and glucose in pancreatic islets. Biochem. Biophys. Res. Commun. 344, 827–833 [DOI] [PubMed] [Google Scholar]
- 51.Gödel M., Hartleben B., Herbach N., Liu S., Zschiedrich S., Lu S., Debreczeni-Mór A., Lindenmeyer M. T., Rastaldi M. P., Hartleben G., Wiech T., Fornoni A., Nelson R. G., Kretzler M., Wanke R., Pavenstädt H., Kerjaschki D., Cohen C. D., Hall M. N., Rüegg M. A., Inoki K., Walz G., Huber T. B. (2011) Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Invest. 121, 2197–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inoki K., Mori H., Wang J., Suzuki T., Hong S., Yoshida S., Blattner S. M., Ikenoue T., Rüegg M. A., Hall M. N., Kwiatkowski D. J., Rastaldi M. P., Huber T. B., Kretzler M., Holzman L. B., Wiggins R. C., Guan K. L. (2011) mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Invest. 121, 2181–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan K., Ito N., Nakajo A., Kurayama R., Fukuhara D., Nishibori Y., Kudo A., Akimoto Y., Takenaka H. (2012) The struggle for energy in podocytes leads to nephrotic syndrome. Cell Cycle 11, 1504–1511 [DOI] [PubMed] [Google Scholar]
- 54.Lee M. J., Feliers D., Sataranatarajan K., Mariappan M. M., Li M., Barnes J. L., Choudhury G. G., Kasinath B. S. (2010) Resveratrol ameliorates high glucose-induced protein synthesis in glomerular epithelial cells. Cell. Signal. 22, 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan Y., Huang S., Wang W., Wang Y., Zhang P., Zhu C., Ding G., Liu B., Yang T., Zhang A. (2012) Activation of peroxisome proliferator-activated receptor-γ coactivator 1α ameliorates mitochondrial dysfunction and protects podocytes from aldosterone-induced injury. Kidney Int. 82, 771–789 [DOI] [PubMed] [Google Scholar]