Abstract
In an effort to inhibit the response to vascular injury that leads to intimal hyperplasia, this study investigated the in vivo efficacy of intraluminal delivery of thrombospondin-2 (TSP-2) small interfering RNA (siRNA). Common carotid artery (CCA) balloon angioplasty injury was performed in rats. Immediately after denudation, CCA was transfected intraluminally (15 min) with one of the following: polyethylenimine (PEI)+TSP-2 siRNA, saline, PEI only, or PEI+control siRNA. CCA was analyzed at 24 h or 21 d by using quantitative real-time PCR and immunohistochemistry. TSP-2 gene and protein expression were significantly up-regulated after endothelial denudation at 24 h and 21 d compared with contralateral untreated, nondenuded CCA. Treatment with PEI+TSP-2 siRNA significantly suppressed TSP-2 gene expression (3.1-fold) at 24 h and TSP-2 protein expression, cell proliferation, and collagen deposition up to 21 d. These changes could be attributed to changes in TGF-β and matrix metalloproteinase-9, the downstream effectors of TSP-2. TSP-2 knockdown induced anti-inflammatory M2 macrophage polarization at 21 d; however, it did not significantly affect intima/media ratios. In summary, these data demonstrate effective siRNA transfection of the injured arterial wall and provide a clinically effective and translationally applicable therapeutic strategy that involves nonviral siRNA delivery to ameliorate the response to vascular injury.—Bodewes, T. C. F., Johnson, J. M., Auster, M., Huynh, C., Muralidharan, S., Contreras, M., LoGerfo, F. W., Pradhan-Nabzdyk, L. Intraluminal delivery of thrombospondin-2 small interfering RNA inhibits the vascular response to injury in a rat carotid balloon angioplasty model.
Keywords: vascular remodeling, arterial injury, siRNA, extracellular matrix protein, intimal hyperplasia
Peripheral artery disease (PAD) affects 8.5 million Americans age >40 yr and is most commonly a result of atherosclerosis, with patients often presenting with claudication, ischemic rest pain, or nonhealing wounds (1). PAD is associated with significant morbidity and mortality, and with an aging population, the incidence of patients with PAD is expected to rise. Endovascular and surgical revascularization procedures are often necessary to alleviate ischemic symptoms after failed medical treatment. Whereas endovascular interventions are increasingly used, autologous vein grafts are preferred in select patients and associated with significantly lower long-term amputation rates and improved overall survival (2, 3). Durability of vascular interventions, however, is limited by the development of intimal hyperplasia (IH) that leads to restenosis. IH is a result of a complex pathologic response to damaging stimuli, such as injury, inflammation, or shear stress that triggers macrophage infiltration, as well as proliferation and migration of numerous cells to the intima (4–6). As a result, delayed failure rates occur in approximately 30–35% of all vein grafts within the first postoperative year (7). IH has been a target of experimental therapies to improve long-term patency rates; however, thus far, no effective treatment has been developed to selectively target IH after vascular procedures.
To better understand the pathophysiology of graft failure, we have analyzed cell-specific and time-dependent genomic alterations to arterial injury. Whereas inflammatory cytokines dominated the early response, genes that involve proliferation and extracellular remodeling were subsequently identified (8). In prior studies, we found thrombospondin-2 (TSP-2) to be substantially up-regulated in neointimal tissue of prosthetic grafts and identified it as a high-profile target (9). Although TSP-2 is associated with wound healing and angiogenesis, its distinct role in IH remains unclear.
As an extracellular matrix (ECM) protein, TSP-2 functions primarily as an inhibitor of angiogenesis and a modulator of cell–matrix interactions, yet it does not contribute to structural stability of the ECM. Of interest, wounds of TSP-2–null mice exhibit highly vascularized granulation tissue and abnormal organization of collagen fibrils, which results in accelerated wound healing and minimal scarring (10–12). Because IH is a result of an excessive pathologic wound healing response to injury, gene knockout of TSP-2 may inhibit the initial alterations, which leads to failure of vascular interventions.
In prior studies using small interfering RNA (siRNA), we demonstrated effective transfection of human aortic smooth muscle cells, thereby silencing TSP-2 with consequent protein knockdown in vitro for up to 30 d (9). Accordingly, the purpose of this study was to evaluate the efficacy of in vivo localized intraluminal delivery of siRNA to the arterial wall in a rat carotid artery balloon angioplasty injury model. Specific objectives were to confirm, in an in vivo model, effective delivery of siRNA with knockdown of TSP-2 gene transcription and protein translation and to analyze TSP-2–dependent pathways that may contribute to inflammation and IH.
MATERIALS AND METHODS
siRNA design and transfection agent
The rat-specific TSP-2 and the nontargeting control siRNA were obtained from Thermo Fisher Scientific (Waltham, MA, USA). To mediate efficient siRNA delivery, polyethylenimine (PEI; in vivo jetPEI; Polyplus, Strasbourg, France) was used as a transfection reagent according to manufacturer instructions. Information about siRNA is provided in Supplemental Table 1.
Animal model and surgical procedure
Protocols for this study were approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center, and all animals were treated in compliance with the Guide for the Care and use of Laboratory Animals [(National Institutes of Health (NIH), Bethesda, MD, USA)]. Both male and female adult Wistar rats (Charles River, Indianapolis, IN, USA) were used at an ideal weight of 400–450 and 275–300 g, respectively (n = 7 per group). After a 48-h acclimation period, rats were anesthetized with isoflurane (2.5–3%) via an induction chamber and sustained by conical mask. Respiratory rate and anesthesia were monitored throughout the entire procedure. With the animal in dorsal decumbency, one of the common carotid arteries (CCAs) was dissected and exposed from the sternal notch to the bifurcation via a midline neck incision. Once arterial control was obtained, a 2F Fogarty catheter was introduced through an arteriotomy in the external carotid artery. To ensure complete circumferential endothelial denudation, the balloon was advanced, inflated, and withdrawn toward the entry point with constant rotation. This procedure was repeated for a total of 3 passages. Subsequently, the balloon-injured region of the CCA was infused intraluminally with either saline, PEI only, nontargeting control siRNA with PEI (PEI+control siRNA; 50 μM in 50–75μl), or TSP-2 siRNA with PEI (PEI+TSP-2 siRNA; 25 μM in 50–75 μl). For adequate transfection, arterial segments were isolated with vascular clamps and distended for 15 min. Transfection time of 15 min was selected, taking into consideration the time and technical constraints within the operating room. Then, CCA was flushed with saline. Once hemostasis was confirmed, the wound was approximated with a subcuticular running suture (5-0 Vicryl Plus). Meloxicam was administered as postoperative analgesia, with an additional dose 24-h later (1 mg/kg i.p.).
Tissue harvest
At 24 h and 21 d after the initial operative procedure, rats were euthanized. Denuded CCA along with contralateral nonoperated control CCA, which will be referred to as nondenuded CCA, were harvested. While under deep anesthesia, a midline thoracotomy was made for cardiac saline perfusion to clear arterial vessels of blood. Denuded and nondenuded CCA were then fixed in formalin 10% at 4°C for 24 h. Before embedding in paraffin, each CCA was cut at the midpoint and, as a result, analysis started at the midsegment of balloon-injured arteries.
Quantitative real-time PCR
RNA was isolated and extracted with the Recoverall Total Nucleic Acid Isolation Kit (Thermo Fisher Scientific). Upon purification, samples were quantified by using a NanoDrop spectrophotometer. Equal amounts of total RNA from the different samples were subjected to standard quantitative real-time PCR method. Rat-specific primers for TSP-2 and the housekeeping gene, β2-microglobulin, were obtained from Integrated DNA Technologies (Coralville, IA, USA). TSP-2 mRNA levels were normalized to β2-microglobulin levels and all gene amplifications reactions were carried out in duplicate. The 2−ΔΔCt method was used to calculate relative TSP-2 mRNA expression levels (relative to nondenuded contralateral CCA). Information about the reagents and TSP-2 primer sequences is provided in Supplemental Table 1.
Histology
Paraffin-embedded cross-sections of 6 μm were obtained for histology and immunohistochemical analysis. IH was determined with the Verhoef-van Giesson stain by measuring the intima/media area ratio. To provide a representative sample, 6 equally separated cross-sections were analyzed per CCA to average along the length of the injured artery. Collagen deposition was assessed with a Masson’s Trichrome stain and presented as a percentage of the total area. Information about the reagents is provided in Supplemental Table 1.
Immunohistochemical analysis
Protein expression
Standard fluorescence immunohistochemistry protocol was followed to evaluate protein expression of TSP-2, TGF-β, and matrix metalloproteinase-9 (MMP-9). Information about specific antibodies and reagents is provided in Supplemental Table 1. To quantify protein expression, we used the following arbitrary scoring system: density of expression scoring: 1, minimal; 2, mild; 3, moderate; 4, strong; and 5, extensive; dissemination of expression scoring: 1, minimal expression in intima, media, or adventitia; 2, limited expression in 1 cell layer; 3, limited expression in 2 cell layers; 4, expression in all cell layers; and 5, extensive expression throughout the specimen. TSP-2 protein expression was quantified by using both density and dissemination scoring system. TGF-β protein expression was quantified by using only the density scoring system. Positive staining of MMP-9 was predominantly in the intima, and, thus, only the density scoring system was applied. Data for TSP-2 and TGF-β are presented as arbitrary scores, whereas data for MMP-9 are presented as a percentage of total intima area.
Cellular proliferation
Proliferative cells were detected with a commercial Ki-67 assay. Standard immunohistochemical staining protocol was performed with 3,3′-diaminobenzidine substrate buffer and counterstained with hematoxylin, after which sections were mounted. Data are presented as number of positive cells per square millimeter. Information about reagents is provided in Supplemental Table 1.
Macrophage infiltration
Infiltration was assessed with anti-CD68, a panmacrophage marker. Polarization of macrophages was determined by costaining of DAPI, anti-CD68, and anti-iNOS for detection of M1-macrophages and DAPI, anti-CD68, and anti-CD206 for M2-macrophages on 2 separate cross-sections. Standard fluorescence immunohistochemistry protocol was followed, and information about antibodies is provided in Supplemental Table 1. Quantification was performed by counting the number of positive cells per square millimeter of the specimen. For all immunohistochemical stains, 2 equally separated cross-sections were analyzed per specimen. Analysis was conducted by 2 observers in a blinded fashion. ImageJ (v1.49; NIH) was used as analysis software.
Statistical analysis
Differences between nondenuded and denuded CCA were analyzed by using the paired sample Student’s t test, as these were tested within the same animal. To assess the effect of treatment in denuded arteries, 1-way ANOVA was performed for multiple comparisons, followed by Dunnett’s post hoc test, with PEI+TSP-2 siRNA as the category group. Normally distributed continuous variables are presented as means ± sem. All tests were 2-sided and data was considered statistically significant at P < 0.05. Statistical analysis was performed by using IBM SPSS Statistics 23 (SPSS, Chicago, IL, USA).
RESULTS
Effect of arterial denudation and subsequent TSP-2 siRNA transfection on TSP-2 gene expression
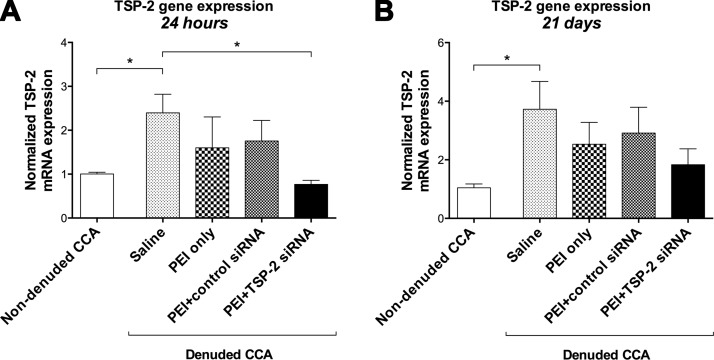
TSP-2 gene expression was compared between denuded saline-treated CCA and contralateral nondenuded CCA by quantitative real-time PCR at 24 h and 21 d. To further investigate whether intraluminal delivery of siRNA alters gene expression of TSP-2 in vivo, CCA were analyzed at 24 h and 21 d after denudation and siRNA transfection. PCR data are normalized to nondenuded CCA and subsequently expressed as a fold change.
Twenty-four hours after denudation, TSP-2 gene expression was significantly up-regulated in denuded CCA (2.40 ± 0.42) compared with nondenuded CCA (1.00 ± 0.04; P = 0.006; Fig. 1A). Twenty-one days after denudation, up-regulation of TSP-2 mRNA was maintained in denuded CCA (3.73 ± 0.95) compared with nondenuded controls (1.04 ± 0.13; P = 0.028; Fig. 1B).
Figure 1.
Relative gene expression of TSP-2. Fold change in TSP-2 mRNA expression in all groups relative to nondenuded contralateral CCA at 24 h (A) and 21 d (B). Data are presented as means ± sem; n = 5. *P < 0.05.
Twenty-four hours after denudation and transfection, PEI+TSP-2 siRNA treatment significantly reduced gene expression of TSP-2 (0.77 ± 0.09) compared with saline (2.40 ± 0.42; P = 0.045). There were no significant differences between PEI+TSP-2 siRNA and PEI only (1.60 ± 0.71; P = 0.461) or PEI+control siRNA (1.76 ± 0.47; P = 0.294) treatment (Fig. 1A). Twenty-one days after denudation and siRNA transfection, PEI+TSP-2 siRNA treatment only showed a trend toward reduced gene expression (1.83 ± 0.54), with no significant differences compared with saline (3.73 ± 0.95; P = 0.206), PEI only (2.53 ± 0.75; P = 0.880), or PEI+control siRNA (2.92 ± 0.88; P = 0.682) treatment (Fig. 1B).
These data suggest that TSP-2 gene expression was significantly up-regulated after endothelial denudation in the rat carotid balloon injury model, thus validating the model. Whereas gene silencing was not sustained over a 21-d period, these data suggest that a single 15-min localized intraluminal infusion of target-specific siRNA is effective at achieving short-term gene silencing.
Effect of arterial denudation and subsequent TSP-2 siRNA transfection on TSP-2 protein expression
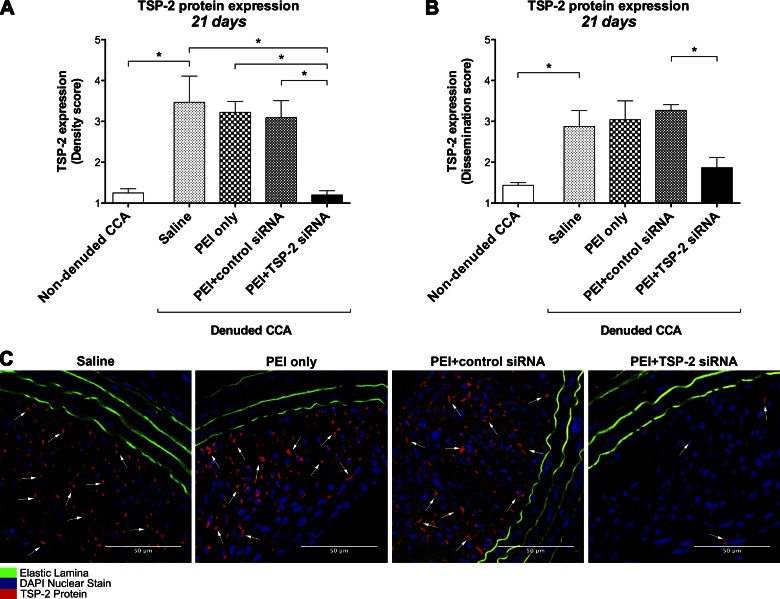
TSP-2 protein expression was compared between denuded saline-treated CCA and contralateral nondenuded CCA by immunohistochemistry at 21 d. To further investigate whether intraluminal delivery of siRNA alters TSP-2 protein expression, CCA were analyzed at 21 d after denudation and siRNA transfection. Quantification of TSP-2 protein expression was based on density and dissemination of TSP-2 expression in CCA. Data are expressed as arbitrary scores.
Twenty-one days after denudation, TSP-2 protein density was increased in denuded CCA (3.47 ± 0.64) compared with nondenuded CCA (1.25 ± 0.10; P = 0.040; Fig. 2A). In addition, dissemination of TSP-2 protein was higher in denuded CCA (2.88 ± 0.39) compared with nondenuded CCA (1.44 ± 0.06; P = 0.043; Fig. 2B).
Figure 2.
TSP-2 protein expression. A, B) Quantification of TSP-2 protein expression in all groups at 21 d on the basis of density (A) and dissemination scores (B). Data are expressed as arbitrary scores (1–5) and presented as means ± sem; n = 4. C) Representative images at 21 d of all denuded CCA stained for TSP-2 protein (red), nuclei (blue), and elastic lamina (green autofluorescence). Original magnification, ×400. Scale bars, 50 μm. *P < 0.05.
Twenty-one days after denudation and siRNA transfection, PEI+TSP-2 siRNA treatment significantly reduced the density of TSP-2 protein expression (1.20 ± 0.11) compared with saline (3.47 ± 0.64; P = 0.007), PEI only (3.22 ± 0.27; P = 0.022), or PEI+control siRNA (3.09 ± 0.41; P = 0.020) treatment (Fig. 2A). Similarly, transfection with PEI+TSP-2 siRNA significantly reduced the dissemination of TSP-2 protein expression (1.86 ± 0.25) compared with PEI+control siRNA (3.27 ± 0.14; P = 0.019), whereas near significant differences were observed with saline (2.88 ± 0.39; P = 0.094) or PEI only (3.04 ± 0.46; P = 0.069) treatment (Fig. 2B). Representative images of TSP-2 protein expression are depicted in Fig. 2C.
These data suggest that TSP-2 protein expression was significantly up-regulated up to 21 d after endothelial denudation in the rat carotid balloon injury model, thus validating the model. Although significant gene silencing of TSP-2 was not sustained by PEI+TSP-2 siRNA treatment over a 21-d period, TSP-2 protein knockdown was achieved after a single intraluminal transfection with TSP-2 siRNA.
Effect of arterial denudation and subsequent TSP-2 siRNA transfection on cell proliferation
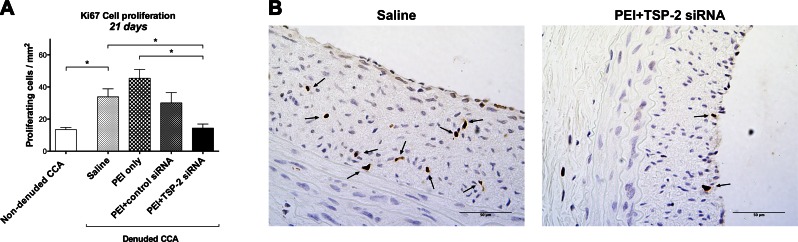
Cellular proliferation is one of the key events in the development of IH (13). Immunostaining for Ki-67, a marker of cellular proliferation, was performed in all CCA at 21 d. Data are expressed as number of proliferating cells per square millimeter.
Twenty-one days after denudation, cell proliferation was significantly increased in denuded saline CCA (33.8 ± 5.0) compared with nondenuded CCA (13.4 ± 1.4; P = 0.017; Fig. 3A).
Figure 3.
Ki-67 immunostaining for cell proliferation. A) Quantitative analysis of cell proliferation in all groups at 21 d expressed as the number of cells per square millimeter. Data are presented as means ± sem; n = 4. B) Representative images at 21 d of denuded CCA in the saline and PEI+TSP-2 siRNA group. Proliferating cells are indicated with black arrows. Original magnification, ×400. Scale bars, 50 μm. *P < 0.05.
Twenty-one days after denudation and transfection, PEI+TSP-2 siRNA treatment significantly decreased proliferation (14.4 ± 2.5) compared with saline (33.8 ± 5.0; P = 0.048) or PEI only (45.4 ± 5.5; P = 0.003) treatment. There was no significant difference observed between PEI+TSP-2 siRNA and PEI+control siRNA (30.1 ± 6.4; P = 0.119) treatment (Fig. 3A). Representative images of cellular proliferation are depicted in Fig. 3B.
These data suggest that arterial denudation increased cell proliferation, and this increase in proliferation could be curtailed by single intraluminal transfection with TSP-2 siRNA.
Effect of arterial denudation and subsequent TSP-2 siRNA transfection on collagen deposition
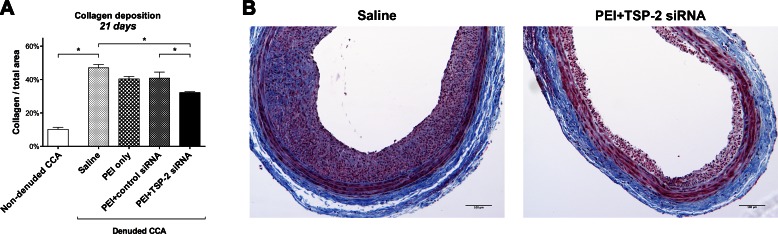
The final lesion of IH is made up of collagen (14). Hence, collagen deposition was measured by using Masson’s Trichrome stain at 21 d. Data are presented as a percentage stained per square millimeter.
Twenty-one days after denudation, collagen deposition was significantly increased in denuded saline CCA (47.0 ± 2.0%) compared with nondenuded CCA (10.2 ± 1.2%; P < 0.001; Fig. 4A).
Figure 4.
Collagen deposition. A) Quantitative analysis of collagen deposition in all groups at 21 d expressed as a percentage of total area of the cross-section. Data are presented as means ± sem; n = 4. B) Representative images at 21 d of Masson’s Trichrome–stained denuded CCA in the saline and PEI+TSP-2 siRNA groups. Collagen (blue); cytoplasm, keratin, and muscle (red); and nuclei (black). Original magnification, ×100. Scale bars, 100 μm. *P < 0.05.
Twenty-one days after denudation and transfection, PEI+TSP-2 siRNA treatment significantly reduced collagen deposition (32.2 ± 0.5%) compared with saline (47.0 ± 2.0%; P = 0.001) or PEI+control siRNA (40.9 ± 3.6%; P = 0.041) treatment, whereas a near significant difference was observed with PEI only (40.4 ± 1.5%; P = 0.056) treatment (Fig. 4A). Representative images of collagen deposition are depicted in Fig. 4B.
These data suggest that arterial denudation increased collagen deposition, and this increase in collagen could be mitigated by single intraluminal transfection with TSP-2 siRNA.
Effect of arterial denudation and subsequent TSP-2 siRNA transfection on macrophage infiltration
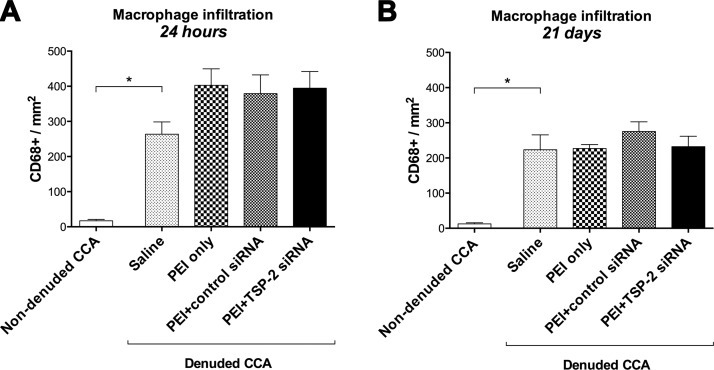
To identify early and long-term inflammatory events, we examined CCA for infiltration of macrophages at 24 h and 21 d after transfection by immunostaining for CD68 antigen. Data are expressed as CD68+ cells per square millimeter.
Denudation triggered macrophage activation in denuded saline CCA both at 24 h (263.5 ± 50.3) and 21 d (223.4 ± 62.6) compared with contralateral nondenuded CCA (16.8 ± 3.7; P = 0.004; and 12.5 ± 3.1; P = 0.022, respectively; Fig. 5A, B).
Figure 5.
Total macrophage infiltration. Quantification of total macrophage infiltration in all groups expressed as CD68+ cells at 24 h (A) and 21 d (B). Data are presented as means ± sem; n = 4–6. *P < 0.05.
Twenty-four hours after denudation and transfection, extensive macrophage infiltration was observed in all treatment groups. PEI+TSP-2 siRNA (394.7 ± 63.6) treatment showed no significant differences compared with saline (263.5 ± 50.3; P = 0.316), PEI only (402.9 ± 63.1; P = 0.999), or PEI+control siRNA (378.9 ± 77.6; P = 0.996) treatment (Fig. 5A).
Twenty-one days after denudation and transfection, the increase in macrophage infiltration that was observed at 24 h with PEI only, PEI+control siRNA, or PEI+TSP-2 siRNA treatment was significantly diminished. Similar to 24 h, after 21 d, macrophage infiltration with PEI+TSP-2 siRNA (232.0 ± 39.8) treatment was not significantly different compared with saline (223.4 ± 62.6; P = 0.998), PEI only (227.0 ± 15.8; P > 0.99), or PEI+control siRNA (275.3 ± 39.9; P = 0.887) treatment (Fig. 5B).
These data suggest that arterial denudation increased macrophage infiltration, and this increase in macrophage infiltration could not be suppressed by single intraluminal transfection with TSP-2 siRNA.
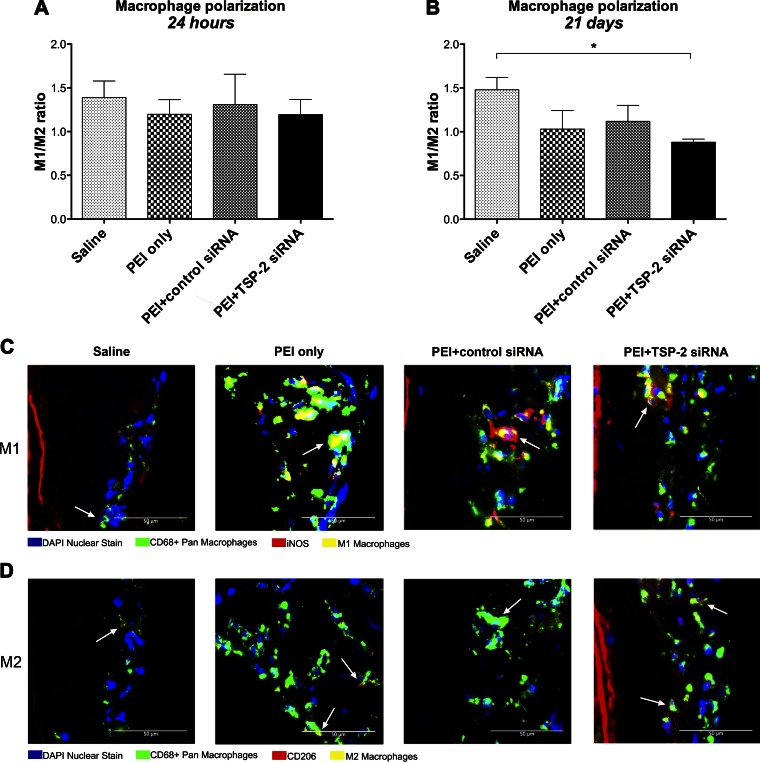
Effect of TSP-2 siRNA transfection on macrophage polarization in denuded CCA
To further evaluate the phenotype of infiltrating macrophages, we measured M1 and M2 macrophage polarization in denuded CCA at 24 h and 21 d after denudation and transfection. Quantification of polarization was performed by calculating the M1/M2 ratio, which represents macrophage heterogeneity. Any value >1 correlates with a proinflammatory phenotype, and a value <1 correlates with an anti-inflammatory phenotype of scavenging and remodeling. Data are expressed as a ratio.
Twenty-four hours after denudation and transfection, all treatment groups demonstrated a proinflammatory phenotype with M1/M2 ratios >1. Moreover, PEI+TSP-2 siRNA (1.19 ± 0.17) treatment was not significantly different from saline (1.39 ± 0.19; P = 0.880), PEI only (1.20 ± 0.17; P > 0.99), or PEI+control siRNA (1.31 ± 0.35; P = 0.970) treatment (Fig. 6A).
Figure 6.
Macrophage polarization. A, B) Quantification of macrophage polarization in all denuded CCA expressed as M1/M2 ratio at 24 h (A) and 21 d (B). Data are presented as means ± sem; n = 4. C, D) Representative images at 21 d of denuded CCA in all groups depicting M1 and M2 macrophage polarization. Proinflammatory M1 macrophages were costained with DAPI (blue), CD68 (green), and iNOS (red) (C), whereas anti-inflammatory protissue repair M2 macrophages were costained with DAPI (blue), CD68 (green), and CD206 (red) (D). White arrows indicate the respective macrophages. Original magnification, ×400. Scale bars, 50 μm. *P < 0.05.
Twenty-one days after denudation and transfection, PEI+TSP-2 siRNA treatment had a significantly lower M1/M2 ratio (0.88 ± 0.04) compared with saline (1.48 ± 0.14; P = 0.049), whereas no significant differences were observed with PEI only (1.03 ± 0.21; P = 0.842) or PEI+control siRNA (1.12 ± 0.18; P = 0.590) treatment (Fig. 6B). Representative images of M1 and M2 macrophage infiltration are depicted in Fig. 6C, D.
These data suggest that, at 24 h, a single intraluminal transfection with TSP-2 siRNA could not improve the M1/M2 ratio; however, by 21 d, it could significantly reduce the M1/M2 ratio. Of interest, although total macrophage counts were similar between saline and PEI+TSP-2 siRNA treatment, the latter treatment group demonstrated increased anti-inflammatory M2 macrophages.
Effect of arterial denudation and subsequent TSP-2 siRNA transfection on regulation of TGF-β and MMP-9
Possible downstream targets of TSP-2, TGF-β and MMP-9, which are known to play a role in vascular injury, were investigated.
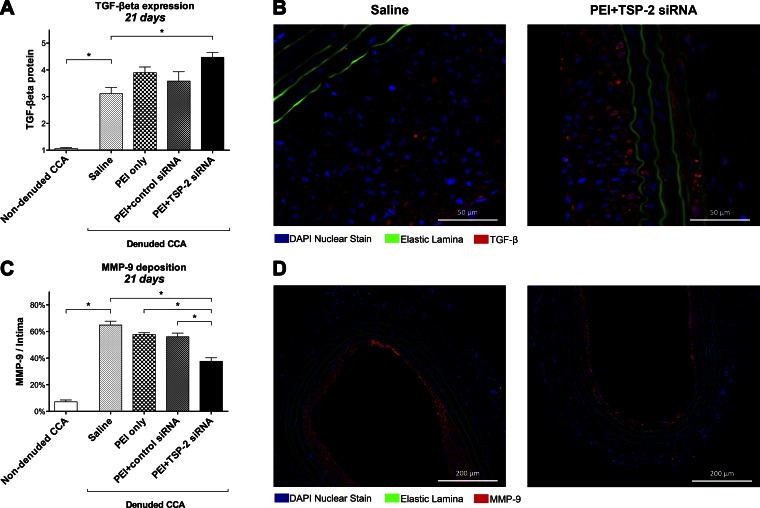
We analyzed TGF-β protein expression at 21 d by using immunohistochemistry. Quantification of TGF-β protein expression was based on density of protein stained in CCA. Data are expressed as arbitrary scores.
Twenty-one days after denudation, TGF-β protein expression was significantly increased in denuded saline CCA (3.11 ± 0.22) compared with nondenuded CCA (1.06 ± 0.04; P = 0.003; Fig. 7A).
Figure 7.
Downstream targets. A) Quantification of TGF-β protein expression in all groups at 21 d on the basis of arbitrary density scores (1–5). Data are presented as means ± sem; n = 4. B) Representative images at 21 d of denuded CCA in the saline and PEI+TSP-2 siRNA groups stained for TGF-β (red), nuclei (blue), and elastic lamina (green autofluorescence). Original magnification, ×400. Scale bars, 50 μm. C) Quantification of MMP-9 expression in all groups 21 d expressed as a percentage of the intima. Data are presented as means ± sem; n = 4. D) Representative images at 21 d of denuded CCA in the saline and PEI+TSP-2 siRNA groups stained for MMP-9 (red), nuclei (blue), and elastic lamina (green autofluorescence). Original magnification, ×100. Scale bars, 200 μm. *P < 0.05.
Twenty-one days after denudation and siRNA transfection, PEI+TSP-2 siRNA treatment significantly increased TGF-β expression (4.47 ± 0.18) compared with saline (3.11 ± 0.22; P = 0.012) treatment. No significant differences were found compared with PEI only (3.90 ± 0.21; P = 0.340) and PEI+control siRNA (3.58 ± 0.35; P = 0.097) treatment (Fig. 7A). Representative images of TGF-β protein expression are depicted in Fig. 7B.
We analyzed MMP-9 protein expression at 21 d by using immunohistochemistry. Data are presented as a percentage stained per square millimeter of the intima, as positive staining was observed solely in the intima of CCA.
Twenty-one days after denudation, MMP-9 expression was significantly increased in denuded saline CCA (64.9 ± 2.9%) compared with nondenuded CCA (7.1 ± 1.4%; P < 0.001; Fig. 7C).
Twenty-one days after denudation and transfection, PEI+TSP-2 siRNA treatment significantly reduced MMP-9 expression (37.6 ± 2.6%) compared with saline (64.9 ± 2.9%; P < 0.001), PEI only (57.6 ± 1.4%; P < 0.001), and PEI+control siRNA (56.0 ± 2.8%; P = 0.001) treatment (Fig. 7C). Representative images of MMP-9 protein expression are depicted in Fig. 7D.
These data suggest that arterial denudation increased TGF-β and MMP-9 protein expression up to 21 d. A single intraluminal transfection with TSP-2 siRNA further increased TGF-β expression while it reduced MMP-9 expression, which indicated that TSP-2 could play a role in the modulation of these 2 proteins in an arterial injury model.
Effect of arterial denudation and subsequent TSP-2 siRNA transfection on IH
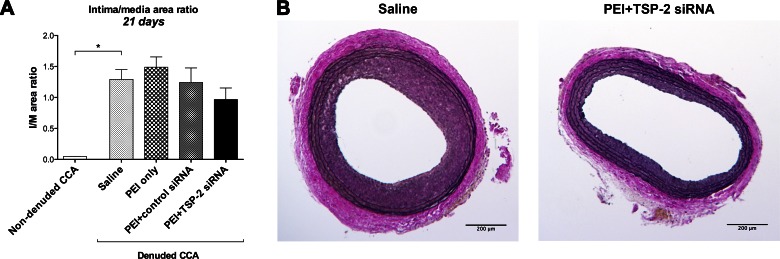
Finally, we evaluated the effect of TSP-2 silencing on IH by measuring intima/media area ratios using Verhoef-van Giesson stain at 21 d.
Twenty-one days after denudation, intima/media area ratio was significantly increased in denuded saline CCA (1.29 ± 0.16) compared with nondenuded CCA (0.049 ± 0.00; P = 0.001; Fig. 8A).
Figure 8.
Morphometric analysis. A) Intima/media area ratios in all groups at 21 d. Data are presented as means ± sem; n = 7. B) Representative cross-sections at 21 d of denuded CCA in the saline and PEI+TSP-2 siRNA groups. Sections were stained with Verhoef-van Giesson. Intima was defined by the area between lumen and inner elastic lamina, whereas the area between the inner and outer elastic lamina represents the media. Original magnification, ×40. Scale bars, 200 μm. *P < 0.05.
Twenty-one days after denudation and transfection, PEI+TSP-2 siRNA treatment reduced intima/media area ratio (0.97 ± 0.19) compared with saline (1.29 ± 0.16; P = 0.480), PEI only (1.49 ± 0.16; P = 0.147), or PEI+control siRNA (1.24 ± 0.23; P = 0.605) treatment. However, none of these differences was statistically significant (Fig. 8A). Representative images of intima/media ratios are depicted in Fig. 8B.
These data suggest that arterial denudation led to IH development by 21 d; however, a single intraluminal transfection with TSP-2 siRNA was not sufficient to significantly suppress IH development.
DISCUSSION
ECM proteins are recognized as important modulators that contribute to arterial restenosis and graft failure, impairing remodeling and matrix stability (15). TSP-2 is an ECM protein that is primarily produced by smooth muscle cells and fibroblasts and is recognized as an ECM modulator after injury. Recent studies have associated TSP-2 with tissue repair, collagen synthesis, bone growth, and angiogenesis. In 1999, Kyriakides et al. (12) reported deposition of discontinuous collagen fibrils, highly vascularized granulation tissue, and enhanced cellularity in wounds that lacked TSP-2. As a result, these wounds demonstrated an accelerated rate of healing compared with wild-type mice. In addition, TSP-2–null mice were associated with induced endothelial cell migration, which led to enhanced neovascularization, as well as impaired platelet aggregation, which would be important in prevention of thromboembolic events (16).
The present study expands on our previous in vitro and ex vivo findings that demonstrated efficient transfection of endothelial and smooth muscle cells with siRNA that resulted in consistent and reproducible silencing of target genes (17, 18). Our current study provides further evidence of effective gene silencing with siRNA in an animal model. We demonstrated that localized intraluminal gene knockdown of TSP-2 by siRNA, possibly via TGF-β- and MMP-9–dependent pathways, resulted in significant inhibition of protein expression, cell proliferation, and collagen deposition, persisting up to 21 d after transfection. Furthermore, TSP-2 knockdown induces anti-inflammatory repair and remodeling in a balloon-injured arterial wall.
Numerous studies have demonstrated efficacious silencing of genes in IH animal models via perivascular application or systemic administration of siRNA, whereas only a limited number of studies were successful with localized intraluminal transfection (19–22). Of these studies, prolonged transfection times were applied, whereas other reports used viral vectors for more adequate transfection. In contrast, these results demonstrate, for the first time to our knowledge, that both gene and protein expression of TSP-2 are significantly inhibited after localized intraluminal transfection of only 15 min with a nonviral delivery system. Although there were no differences in gene expression at 21 d, a prolonged inhibition of TSP-2 protein expression was demonstrated in the PEI+TSP-2 siRNA-treated group. These results clearly indicate effective delivery of siRNA to the arterial wall under clinically applicable conditions. Moreover, localized intraluminal transfection poses a viable alternative to avoid systemic effects, which are known concerns of sustained-release perivascular and systemic delivery. Administration of high doses to achieve sufficient transfection and exposure of nontarget tissues could affect siRNA specificity and induce unintended systemic effects (23). Findings of this study are relevant to gene therapy safety and the feasibility of clinical application within the time and technical constraints of the operating room.
Collagen deposition at 21 d was significantly reduced in the arterial wall after TSP-2 silencing. These results are consistent with previous reports of accelerated wound healing and support a disordered organization of collagenous matrix in the absence of TSP-2. Specifically, it has been shown that TSP-2 interacts with several ECM proteins, including collagen, fibrinogen, and fibronectin. Structural matrix abnormalities and the complexity in which TSP-2 modulates the ECM are partially elucidated in dermal fibroblasts. In TSP-2–null mice, tissue-transglutaminase and MMP-2 were implicated in the degradation of many matrix proteins, including collagen. Its subsequent inhibitors reversed proteolysis and increased cross-linking of the ECM (24). Our data supports these findings and suggests modulating capabilities of TSP-2 on downstream regulating ECM proteins, in particular TSP-2–mediated reduction of MMP-9, which may maintain vessel circumference. In addition, convincing evidence exists that MMP-9 contributes to arterial lesion growth and impaired wound healing by regulating smooth muscle cell migration and replication (25–27). Therefore, lower MMP-9 levels might alter the severity of IH. Regulation of MMP-9 by TSP-2 has not been extensively studied; however, Krady et al. (28) demonstrated that TSP-2 seems to regulate extracellular levels of MMP-9 in a spatiotemporal fashion in response to ischemic injury in a mouse hindlimb model. Although we found reduced MMP-9 protein levels after TSP-2 knockdown, others have reported increased levels of MMP-2 and MMP-9 in TSP-2–null mice (28, 29). The reason for this discrepancy is unclear but emphasizes the fragile equipoise of catabolic degradation and up-regulation of MMPs through their subsequent tissue inhibitors.
In contrast to the present results that show significant inhibition of cell proliferation after TSP-2 knockdown in an animal model, our previous in vitro study could not demonstrate a similar correlation for smooth muscle cells (9). Through Rac-regulated modulation, TSP-2 synthesis has been shown to inhibit cell proliferation of endothelial cells, yet it is hypothesized that thrombospondins stimulate cell growth in smooth muscle cells (30, 31). Recently, by using human aortic vascular smooth muscle cells, TSP-2 was associated with 3.1-fold rise in chemotaxis compared with serum-free media that resulted in a 50% increase of proliferation in vitro (32). These results further support the idea of proliferative cell inhibition by TSP-2 siRNA that may potentially attenuate IH formation.
Furthermore, we present evidence that siRNA-mediated knockdown of TSP-2 affects macrophage infiltration and polarization in the latter stages of IH. In the PEI+TSP-2 siRNA-treated group, we observed an M2 polarization at 21 d that is associated with an anti-inflammatory phenotype. The spectrum of functional phenotypes of macrophages is induced by various cytokines, of which IL-4, but also TGF-β, plays a key role (33). Whereas TSP-1, a different isoform of TSP, has been shown to activate latent TGF-β that functions as an anti-inflammatory cytokine, it is suggested that TSP-2 competitively inhibits activation of TGF-β (34). The current study provides evidence that TSP-2 silencing leads to higher levels of TGF-β. It is likely that this increase in TGF-β may promote the anti-inflammatory M2 macrophage phenotype that is known to induce repair and remodeling. Seemingly contradictory findings are reported regarding the role of TGF-β in restenosis. Whereas some studies found that overexpression of TGF-β after arterial injury decreased neointima formation, the majority suggest that TGF-β increases smooth muscle cell proliferation and migration (35). It is important to note that this paradox is also reported in malignancies (tumor suppressor vs. promoter) and our study adds to the growing body of literature on the temporal and context-dependent function of TGF-β. Nevertheless, TSP-2 modulates TGF-β downstream most likely via TSP-1–mediated activation, which could be critical for macrophage–vessel interactions. Further studies will be needed to understand the role of TSP-2–dependent TGF-β induction in arterial injury.
siRNAs are extremely hydrophilic and are quickly degraded in serum, which contributes to their short half-lives in vivo. Various chemical modifications, transfection agents, and delivery methods have been evaluated to increase stability and transfection. In the present study, we used PEI as a transfection agent to enhance entry of siRNA into the cell and achieve increased silencing of the target gene. Of interest, our results indicate enhanced cell proliferation and increased IH for the PEI only group. Although PEI combined with target siRNA exerts protective vascular effects, PEI only may induce specific alterations in the transcriptome and increase expression of off-target genes (36). Our research group recently identified 213 differentially expressed genes that are modulated as a result of transfection with PEI only. Major genes that are linked to vascular dysregulation primarily include inflammation-related and cell proliferative alterations (e.g., IL1A, STAT-4, CCL8, SFN, and CSF2) (37). These data are supported by our current findings of increased CD68+ macrophage infiltration at 24 h of all PEI combined treatment groups compared with saline. These off-target effects of PEI are significant and warrant the development of efficacious, yet nontoxic, siRNA delivery agents.
Contrary to expectations, this study did not find a significant reduction of IH after TSP-2 knockdown. This outcome could be attributed to the complex pathogenesis of IH involving crosstalk among various cellular and molecular pathways or to the smaller animal sample size. Whereas single gene knockout highlights the potential of siRNA, simultaneous silencing of multiple genes represents a plausible method to effectively attack IH. Our research group has already demonstrated multiple gene silencing in vitro and will undertake further studies to investigate this hypothesis in vivo (38).
This study has limitations that should be addressed. First, we used a rat carotid balloon angioplasty injury model to mimic diseased vessels after surgical manipulation. To better comprehend the processes that lead to atherosclerosis and IH, an animal model of graft failure would be preferred. However, the rat carotid balloon injury model serves as a valuable proof-of-concept study to verify in vitro results. Furthermore, in addition to the current study that demonstrates promising results extending to 21 d, further research is needed to investigate the long-term effects of TSP-2 silencing on vascular restenosis.
In summary, we demonstrated that siRNA-mediated knockdown of TSP-2 gene expression after vascular injury in an in vivo animal model effectively modulates TGF-β and MMP-9 that are known to contribute to IH. Effective target-specific gene silencing with shortened localized intraluminal transfection times is feasible under clinically applicable conditions. This work justifies further research of local siRNA treatment for IH and vascular restenosis.
ACKNOWLEDGMENTS
This work was funded, in part, by U.S. National Institutes of Health, National Heart, Lung, and Blood Institute Grants 5 R01-HL086741, 5 R01-HL021796, 5 T32-HL007734, and 1 T35-HL110843-01A1 (to F.W.L.); and 1 R21-HL133821 (to L.P.-N.). This work was also supported by the Dutch Foundation “De Drie Lichten.”
Glossary
- CCA
common carotid artery
- ECM
extracellular matrix
- IH
intimal hyperplasia
- MMP-9
matrix metalloproteinase-9
- PAD
peripheral artery disease
- PEI
polyethylenimine
- siRNA
small interfering RNA
- TSP-2
thrombospondin-2
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
T. C. F. Bodewes, F. W. LoGerfo, and L. Pradhan-Nabzdyk designed research; T. C. F. Bodewes and L. Pradhan-Nabzdyk analyzed data; T. C. F. Bodewes, J. M. Johnson, M. Auster, S. Muralidharan, and M. Contreras performed research; T. C. F. Bodewes, F. W. LoGerfo, and L. Pradhan-Nabzdyk wrote the paper; and T. C. F. Bodewes, J. M. Johnson, M. Auster, C. Huynh, M. Contreras, F. W. LoGerfo, and L. Pradhan-Nabzdyk revised the paper.
REFERENCES
- 1.Mozaffarian D., Benjamin E. J., Go A. S., Arnett D. K., Blaha M. J., Cushman M., de Ferranti S., Després J. P., Fullerton H. J., Howard V. J., Huffman M. D., Judd S. E., Kissela B. M., Lackland D. T., Lichtman J. H., Lisabeth L. D., Liu S., Mackey R. H., Matchar D. B., McGuire D. K., Mohler E. R. III, Moy C. S., Muntner P., Mussolino M. E., Nasir K., Neumar R. W., Nichol G., Palaniappan L., Pandey D. K., Reeves M. J., Rodriguez C. J., Sorlie P. D., Stein J., Towfighi A., Turan T. N., Virani S. S., Willey J. Z., Woo D., Yeh R. W., Turner M. B.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2015) Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 131, e29–e322 [DOI] [PubMed] [Google Scholar]
- 2.Bradbury A. W., Adam D. J., Bell J., Forbes J. F., Fowkes F. G., Gillespie I., Ruckley C. V., Raab G. M.; BASIL trial Participants (2010) Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J. Vasc. Surg. 51, 5S–17S [DOI] [PubMed] [Google Scholar]
- 3.Suckow B. D., Kraiss L. W., Stone D. H., Schanzer A., Bertges D. J., Baril D. T., Cronenwett J. L., Goodney P. P.; Vascular Study Group of New England (2013) Comparison of graft patency, limb salvage, and antithrombotic therapy between prosthetic and autogenous below-knee bypass for critical limb ischemia. Ann. Vasc. Surg. 27, 1134–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies M. G., Hagen P. O. (2011) Reprinted article “Pathophysiology of vein graft failure: a review”. Eur. J. Vasc. Endovasc. Surg. 42, S19–S29 [DOI] [PubMed] [Google Scholar]
- 5.Tseng C. N., Karlöf E., Chang Y. T., Lengquist M., Rotzius P., Berggren P. O., Hedin U., Eriksson E. E. (2014) Contribution of endothelial injury and inflammation in early phase to vein graft failure: the causal factors impact on the development of intimal hyperplasia in murine models. PLoS One 9, e98904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan S., George S. J., Berry C., Baker A. H. (2012) Vein graft failure: current clinical practice and potential for gene therapeutics. Gene Ther. 19, 630–636 [DOI] [PubMed] [Google Scholar]
- 7.Conte M. S., Bandyk D. F., Clowes A. W., Moneta G. L., Seely L., Lorenz T. J., Namini H., Hamdan A. D., Roddy S. P., Belkin M., Berceli S. A., DeMasi R. J., Samson R. H., Berman S. S.; PREVENT III Investigators (2006) Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J. Vasc. Surg. 43, 742–751, discussion 751 [DOI] [PubMed] [Google Scholar]
- 8.Bhasin M., Huang Z., Pradhan-Nabzdyk L., Malek J. Y., LoGerfo P. J., Contreras M., Guthrie P., Csizmadia E., Andersen N., Kocher O., Ferran C., LoGerfo F. W. (2012) Temporal network based analysis of cell specific vein graft transcriptome defines key pathways and hub genes in implantation injury. PLoS One 7, e39123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida S., Nabzdyk C. S., Pradhan L., LoGerfo F. W. (2011) Thrombospondin-2 gene silencing in human aortic smooth muscle cells improves cell attachment. J. Am. Coll. Surg. 213, 668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bornstein P. (2001) Thrombospondins as matricellular modulators of cell function. J. Clin. Invest. 107, 929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyriakides T. R., Zhu Y. H., Smith L. T., Bain S. D., Yang Z., Lin M. T., Danielson K. G., Iozzo R. V., LaMarca M., McKinney C. E., Ginns E. I., Bornstein P. (1998) Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J. Cell Biol. 140, 419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyriakides T. R., Tam J. W., Bornstein P. (1999) Accelerated wound healing in mice with a disruption of the thrombospondin 2 gene. J. Invest. Dermatol. 113, 782–787 [DOI] [PubMed] [Google Scholar]
- 13.Hao H., Gabbiani G., Bochaton-Piallat M. L. (2003) Arterial smooth muscle cell heterogeneity: implications for atherosclerosis and restenosis development. Arterioscler. Thromb. Vasc. Biol. 23, 1510–1520 [DOI] [PubMed] [Google Scholar]
- 14.Strauss B. H., Robinson R., Batchelor W. B., Chisholm R. J., Ravi G., Natarajan M. K., Logan R. A., Mehta S. R., Levy D. E., Ezrin A. M., Keeley F. W. (1996) In vivo collagen turnover following experimental balloon angioplasty injury and the role of matrix metalloproteinases. Circ. Res. 79, 541–550 [DOI] [PubMed] [Google Scholar]
- 15.Farb A., Kolodgie F. D., Hwang J. Y., Burke A. P., Tefera K., Weber D. K., Wight T. N., Virmani R. (2004) Extracellular matrix changes in stented human coronary arteries. Circulation 110, 940–947 [DOI] [PubMed] [Google Scholar]
- 16.Calabro N. E., Kristofik N. J., Kyriakides T. R. (2014) Thrombospondin-2 and extracellular matrix assembly. Biochim. Biophys. Acta 1840, 2396–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monahan T. S., Andersen N. D., Martin M. C., Malek J. Y., Shrikhande G. V., Pradhan L., Ferran C., LoGerfo F. W. (2009) MARCKS silencing differentially affects human vascular smooth muscle and endothelial cell phenotypes to inhibit neointimal hyperplasia in saphenous vein. FASEB J. 23, 557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen N. D., Chopra A., Monahan T. S., Malek J. Y., Jain M., Pradhan L., Ferran C., LoGerfo F. W. (2010) Endothelial cells are susceptible to rapid siRNA transfection and gene silencing ex vivo. J. Vasc. Surg. 52, 1608–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malabanan K. P., Kanellakis P., Bobik A., Khachigian L. M. (2008) Activation transcription factor-4 induced by fibroblast growth factor-2 regulates vascular endothelial growth factor-A transcription in vascular smooth muscle cells and mediates intimal thickening in rat arteries following balloon injury. Circ. Res. 103, 378–387 [DOI] [PubMed] [Google Scholar]
- 20.Zuojun H., Lingyu H., Wei H., Henghui Y., Chonggang Z., Jingsong W., Mian W., Yong L., Shenming W. (2012) Interference of IP-10 expression inhibits vascular smooth muscle cell proliferation and intimal hyperplasia in carotid artery: a new insight in the prevention of restenosis. Cell Biochem. Biophys. 62, 125–135 [DOI] [PubMed] [Google Scholar]
- 21.Suwanabol P. A., Seedial S. M., Shi X., Zhang F., Yamanouchi D., Roenneburg D., Liu B., Kent K. C. (2012) Transforming growth factor-β increases vascular smooth muscle cell proliferation through the Smad3 and extracellular signal-regulated kinase mitogen-activated protein kinases pathways. J. Vasc. Surg. 56, 446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L., Yu F., Wang L., Zheng J., Du Y., Huang Y., Liu B., Wang X., Kong W. (2015) ADAMTS-7 promotes vascular smooth muscle cells proliferation in vitro and in vivo. Sci. China Life Sci. 58, 674–681 [DOI] [PubMed] [Google Scholar]
- 23.Bumcrot D., Manoharan M., Koteliansky V., Sah D. W. (2006) RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat. Chem. Biol. 2, 711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agah A., Kyriakides T. R., Bornstein P. (2005) Proteolysis of cell-surface tissue transglutaminase by matrix metalloproteinase-2 contributes to the adhesive defect and matrix abnormalities in thrombospondin-2-null fibroblasts and mice. Am. J. Pathol. 167, 81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng L., Mantile G., Pauly R., Nater C., Felici A., Monticone R., Bilato C., Gluzband Y. A., Crow M. T., Stetler-Stevenson W., Capogrossi M. C. (1998) Adenovirus-mediated gene transfer of the human tissue inhibitor of metalloproteinase-2 blocks vascular smooth muscle cell invasiveness in vitro and modulates neointimal development in vivo. Circulation 98, 2195–2201 [DOI] [PubMed] [Google Scholar]
- 26.Cho A., Reidy M. A. (2002) Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ. Res. 91, 845–851 [DOI] [PubMed] [Google Scholar]
- 27.Tellechea A., Leal E. C., Kafanas A., Auster M. E., Kuchibhotla S., Ostrovsky Y., Tecilazich F., Baltzis D., Zheng Y., Carvalho E., Zabolotny J. M., Weng Z., Petra A., Patel A., Panagiotidou S., Pradhan-Nabzdyk L., Theoharides T. C., Veves A. (2016) Mast cells regulate wound healing in diabetes. Diabetes 65, 2006–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krady M. M., Zeng J., Yu J., MacLauchlan S., Skokos E. A., Tian W., Bornstein P., Sessa W. C., Kyriakides T. R. (2008) Thrombospondin-2 modulates extracellular matrix remodeling during physiological angiogenesis. Am. J. Pathol. 173, 879–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maclauchlan S., Skokos E. A., Agah A., Zeng J., Tian W., Davidson J. M., Bornstein P., Kyriakides T. R. (2009) Enhanced angiogenesis and reduced contraction in thrombospondin-2-null wounds is associated with increased levels of matrix metalloproteinases-2 and -9, and soluble VEGF. J. Histochem. Cytochem. 57, 301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopes N., Gregg D., Vasudevan S., Hassanain H., Goldschmidt-Clermont P., Kovacic H. (2003) Thrombospondin 2 regulates cell proliferation induced by Rac1 redox-dependent signaling. Mol. Cell. Biol. 23, 5401–5408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majack R. A., Cook S. C., Bornstein P. (1986) Control of smooth muscle cell growth by components of the extracellular matrix: autocrine role for thrombospondin. Proc. Natl. Acad. Sci. USA 83, 9050–9054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helkin A., Maier K. G., Gahtan V. (2015) Thrombospondin-1, -2 and -5 have differential effects on vascular smooth muscle cell physiology. Biochem. Biophys. Res. Commun. 464, 1022–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantovani A., Biswas S. K., Galdiero M. R., Sica A., Locati M. (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 229, 176–185 [DOI] [PubMed] [Google Scholar]
- 34.Murphy-Ullrich J. E., Poczatek M. (2000) Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 11, 59–69 [DOI] [PubMed] [Google Scholar]
- 35.Suwanabol P. A., Kent K. C., Liu B. (2011) TGF-β and restenosis revisited: a Smad link. J. Surg. Res. 167, 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merkel O. M., Beyerle A., Beckmann B. M., Zheng M., Hartmann R. K., Stöger T., Kissel T. H. (2011) Polymer-related off-target effects in non-viral siRNA delivery. Biomaterials 32, 2388–2398 [DOI] [PubMed] [Google Scholar]
- 37.Raof N. A., Rajamani D., Chu H. C., Gurav A., Johnson J. M., LoGerfo F. W., Pradhan-Nabzdyk L., Bhasin M. (2016) The effects of transfection reagent polyethyleneimine (PEI) and non-targeting control siRNAs on global gene expression in human aortic smooth muscle cells. BMC Genomics 17, 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen N. D., Monahan T. S., Malek J. Y., Jain M., Daniel S., Caron L. D., Pradhan L., Ferran C., Logerfo F. W. (2007) Comparison of gene silencing in human vascular cells using small interfering RNAs. J. Am. Coll. Surg. 204, 399–408 [DOI] [PubMed] [Google Scholar]