Abstract
Airway goblet cell differentiation and related mucus overproduction are critical processes in the development of respiratory diseases, including asthma. The underlying mechanisms, however, are not fully understood. We identified Runt-related transcription factor 2 (Runx2) as a novel regulator for goblet cell differentiation. Runx2 was up-regulated by 6.4-fold during IL-13-induced goblet cell differentiation of human bronchial epithelial cells. Knockdown of Runx2 attenuated the IL-13-induced differentiation/mucus production by 67%. Mechanistically, Runx2 bound to the promoter of SAM-pointed domain-containing Ets-like factor (SPDEF), a known factor for goblet cell differentiation, resulting in an activation of SPDEF transcription. In vivo, Runx2 was induced by 6.2-fold in pulmonary epithelium of house dust mite–challenged mice. Blockade of Runx2 inhibited the house dust mite–induced goblet cell differentiation with a 75% reduction in mucus overproduction while improving airway responsiveness to methacholine by 41%. More importantly, a 12.3-fold increase in Runx2 expression was observed in human asthma lung epithelium, underlying the potential clinical importance of these findings.—Shi, N., Zhang, J., Chen, S.-Y. Runx2, a novel regulator for goblet cell differentiation and asthma development.
Keywords: mucus overproduction, airway epithelial remodeling, transcriptional activation, SPDEF
Airway epithelial remodeling is a hallmark of respiratory diseases, including asthma, chronic obstructive pulmonary disease, and cystic fibrosis (1). During epithelial remodeling after pulmonary injury, epithelial cells lining the respiratory tract undergo differentiation to goblet cells, also called mucous cells, which are the primary mucus-secreting cell type. The differentiated goblet cells produce and secrete large amounts of mucus; normal ciliary movement is unable to clear the mucus, leading to the airway obstruction, which contributes to the morbidity and mortality of these respiratory disorders (2, 3). It is well established that there is a consistent relationship between asthma and airway goblet cell differentiation (4–6).
Goblet cells are generally not abundant in the conducting airways of the lung. However, acute and chronic inflammatory stimuli such as IL-13 and house dust mites (HDM) can effectively induce goblet cell differentiation, altering the number and activity of goblet cells (7–11). Although numerous recent studies have advanced our understanding of the signaling networks involved in IL-13- and HDM-induced goblet cell differentiation and mucus overproduction in airway epithelium (11–13), the precise molecular mechanisms regulating this common pathologic process remain largely unknown.
Runt-related transcription factors (Runxs) belong to a family of metazoan genes that play important roles in many biologic processes, including hematopoiesis, neurogenesis, bone development, and tumor suppression (14, 15). In mammals, there are 3 Runx family members: Runx1–3. Both Runx1 and Runx3 appear to be associated with the risk of asthma in children with intrauterine smoke exposure (16), whereas Runt-related transcription factor 2 (Runx2) is found to be involved in tumor malignancies attributable to the epithelial–mesenchymal transition (17).
Because bronchial epithelial–mesenchymal transition is an important process during airway epithelial remodeling (18), we were prompted to test if Runx2 was regulated in airway epithelial cells. In the present study, we found that Runx2 was markedly induced in branchial epithelial cells by IL-13 and HDM in vitro and in vivo, respectively. Up-regulation of Runx2 expression was also observed in primary cultured human asthmatic branchial epithelial cells and pulmonary epithelium of patients with asthma. Importantly, knockdown of Runx2 by adenoviral vector–expressing Runx2 short hairpin RNA (Ad-shRunx2) inhibited IL-13-induced goblet cell differentiation and mucus overproduction in human epithelial cells in vitro. In addition, intranasal administration of Ad-shRunx2 significantly decreased HDM-induced airway mucus overproduction in mouse pulmonary epithelium, leading to an improved pulmonary function. Runx2 appeared to activate the gene transcription of SAM-pointed domain-containing Ets-like factor (SPDEF), a factor critical for goblet cell differentiation.
MATERIALS AND METHODS
Animals and HDM challenge
Male C57BL/6 mice (10 wk old) were sensitized subcutaneously with 100 µg of Freund adjuvant–emulsified HDM extract (Greer, Cambridge, MA, USA) (1 mg/ml) in abdomen and groins on d 0 and 14. Starting on d 21, the sensitized mice were challenged intranasally with 50 µg of HDM extract dissolved in PBS (1 mg/ml) daily for 14 d, while PBS was administered as control. For adenoviral vector transduction in mouse lung, we intranasally administered adenoviral vector–expressing scramble (Ad-GFP; green fluorescent protein [GFP]-expressing adenovirus) or adenoviral vector–expressing Runx2 short hairpin RNA (Ad-shRunx2) 1 d before the HDM challenge via a pipette tip. Then the thorax was circumferentially compressed to facilitate adenoviral diffusion to the distal air spaces. Intranasal administration of Ad-shRunx2 or Ad-GFP was repeated 7 d later. The mice were then humanely killed or underwent methacholine challenge 3 d after the last HDM challenge. All animals were housed under conventional conditions in the animal care facilities and received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Georgia.
Cell culture
Human epithelial cell line BEAS-2B was purchased from American Type Culture Collection (Manassas, VA, USA). BEAS-2B cells were cultured in Advanced DMEM/F12 containing 10% fetal bovine serum. Primary normal and diseased (asthmatic) human bronchial epithelial cells (NHBECs and DHBECs, respectively) were purchased from Lonza (Basel, Switzerland) and were cultured in growth factor–supplemented bronchial epithelial growth medium (Lonza). Cells were grown at 37°C in a humidified atmosphere of 5% CO2. NHBECs and DHBECs of 3 or fewer passages with 70 to 80% confluence were used in the experiments.
Construction of adenoviral vector
Runx2 short hairpin (sh) RNA (shRunx2) coding sequences were as follows: 5′–CGCGTCGCTTGATGACTCTAAACCTAGTTTGTTCTTTCAAGAGAAGAACAAACTAGGTTTAGAGTCATCAAGCTTTTTTCCAAA-3′ (top strand) and 5′-AGCTTTTGGAAAAAAGCGCTTGATGACTCTAAACCTAGTTTGTTCTTTCAAGAGAAGAACAAACTAGGTTTAGAGTCATCAAGCG–3′ (bottom strand). Both strands were annealed and ligated into pRNAT-H1.1/Adeno (GenScript Biotech, Piscataway Township, NJ, USA) and digested with MluI and HindIII. Recombinant adenoviral vector was produced by homologous recombination in AD-1-competent cells following the manufacturer’s instructions (Agilent Technologies, Santa Clara, CA, USA). The resultant recombinant digested with PacI was transfected into AD-293 cells with Lipofectamine LTX (Thermo Fisher Scientific, Waltham, MA, USA) to package virus particles expressing shRunx2 (Ad-shRunx2). The adenovirus was purified by gradient density ultracentrifugation of cesium chloride and dialyzed in dialysis buffer (135 mM NaCl, 1 mM MgCl2, 10 mM Tris-HCl, pH 7.5, 10% glycerol). Ad-GFP was used as control.
Histology and immunohistochemistry (IHC)
Mouse lung tissues were collected and immersed in 4% paraformaldehyde in PBS to preserve pulmonary architecture. The left lung of each mouse was embedded in paraffin using standard procedures. Sections (5 μm) were mounted on slides for histologic or IHC analysis. Hematoxylin and eosin staining was used to evaluate lung morphology. Periodic acid–Schiff (PAS) staining was used to visualize mucus secretion and goblet cells. IHC analysis was performed using an indirect method with antibody against the mucin 5AC protein (Abcam, Cambridge, MA, USA), followed by 3,3′-diaminobenzidine and hematoxylin staining.
Western blot analysis
BEAS-2B cells, NHBECs, and DHBECs were lysed, and mouse lungs were homogenized in a lysis buffer (50 mM Tris-HCl, pH 7.5/150 mM NaCl/1% SDS containing protease inhibitor cocktail; Sigma-Aldrich, St. Louis, MO, USA), followed by removal of cell or tissue debris by centrifugation. A total of 10 to 30 µg of proteins were separated by 10% SDS-PAGE and were transferred to PVDF membrane (Bio-Rad, Hercules, CA, USA). Antibodies against Runx2 (Cell Signaling Technology, Danvers, MA, USA), mucin 5AC (Abcam), glyceraldehyde phosphate dehydrogenase (GAPDH; Proteintech, Rosemont, IL, USA), or α-tubulin (Sigma-Aldrich) were used for immunoblotting.
Chromatin immunoprecipitation assay
ChIP assays were performed using a chromatin immunoprecipitation (ChIP) kit (EMD Millipore, Billerica, MA, USA). Growth-arrested NHBECs were treated with IL-13 (50 ng/ml, Cell Signaling Technology) for 24 h. Chromatin complexes were immunoprecipitated with 3 μg of Runx2 antibody or IgG (negative control) according to the manufacturer’s instructions. Semiquantitative and quantitative real-time PCR were performed to amplify human SPDEF promoter region containing the TGTGGT sequence using the primers 5′–CGAGTGAATGAGCGAGTGAA-3′ (forward) and 5′–AACTCTTCATCTCGCGGCT–3′ (reverse).
Reverse transcription and real-time PCR
Total RNA from lung tissues was extracted using Trizol (Thermo Fisher Scientific) according to the manufacturer’s instructions. RNA (1 µg) was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad). Real-time quantitative PCR (qPCR) was performed on a Stratagene Mx3005 qPCR thermocycler (Agilent Technologies) using the SYBR Green Master Kit (Genecopoeia, Rockville, MD, USA). The PCR primers are as follows: Runx2, 5′–TCTGGCCTTCCACTCTCAGT–3′ (forward) and 5′–GACTGGCGGGGTGTAAGTAA–3′ (reverse); and SPDEF, 5′–AATGTGCAGAAGTGGCTCCT-3′ (forward) and 5′–CAGGTGAAGTCCGCTCTTTC–3′ (reverse).
Immunofluorescence staining
Mouse lung tissues were treated similarly to the IHC procedure. Paraffin-embedded sections were incubated overnight with mucin 5AC (Abcam) and Runx2 antibodies (Cell Signaling Technology), followed by incubation with FITC- and tetramethylrhodamine isothiocyanate–conjugated secondary antibodies (Thermo Fisher Scientific) for 1 h, respectively. DAPI was used to stain the nuclei blue. Stained lung tissues were imaged with a Nikon (Tokyo, Japan) microscope.
Human specimens
Clinical specimens from patients with asthma were collected at Taihe Hospital, Hubei University of Medicine. Approval was obtained from the hospital Medical Ethics Committee on Human Investigation. Informed consent was obtained from every subject in this study. Tissues were subjected to histologic and IHC analyses.
Induction and measurement of airway hyperresponsiveness (AHR)
Mice were anesthetized with inhaled isoflurane (3% in O2). A 19-gauge blunt-end needle was inserted into the trachea; the animals were then ventilated mechanically and challenged with increasing doses of nebulized methacholine using a FlexiVent system (Scireq Scientific Respiratory Equipment, Montreal, QC, Canada).
Statistical analysis
All data were evaluated by 2-tailed unpaired Student’s t test or compared by 1-way ANOVA followed by the Fisher t test; data are expressed as means ± sd. A value of P < 0.05 was considered statistically significant.
RESULTS
Runx2 was induced in goblet cell differentiation of human airway epithelial cells in vitro
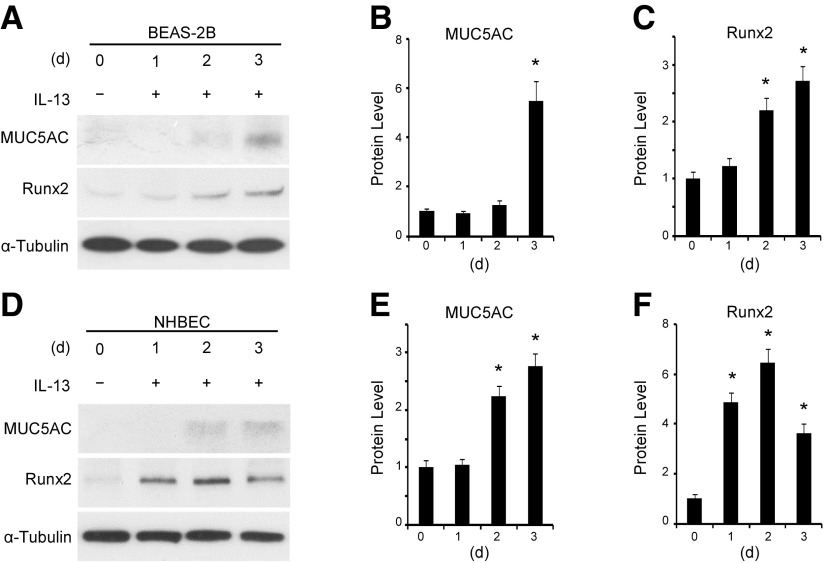
IL-13 is a well-documented factor inducing goblet cell differentiation of airway epithelial cells (8, 19, 20). Mucin 5AC is a major component of airway mucus and a well-established marker for goblet cells (21, 22). When treated with IL-13, BEAS-2B cells expressed mucin 5AC 3 d after treatment, indicating a differentiation of goblet cells (Fig. 1A, B). Interestingly, IL-13 induced Runx2 expression 2 d after the treatment, a time preceding the induction of mucin 5AC (Fig. 1A–C). Moreover, IL-13 also induced primary normal human bronchial epithelial cells (NHBECs) to differentiate to goblet cells, and Runx2 was induced earlier than mucin 5AC in these cells (d 1 vs. 2, Fig. 1D–F), similar to BEAS-2B. These data suggest that Runx2 may be involved in IL-13-induced mucus production and goblet cell differentiation.
Figure 1.
IL-13 induced Runx2 expression along with goblet cell differentiation of human airway epithelial cells. BEAS-2B cells (A–C) and NHBECs (D–F) were treated with vehicle (−) or IL-13 (+, 50 ng/ml) for days as indicated. A, D) Western blot analysis was performed to examine protein expression of mucin 5AC and Runx2. B, C) Quantification of mucin 5AC (B) and Runx2 (C) expression shown in A by normalizing to α-tubulin level, respectively. E, F) Quantification of mucin 5AC (E) and Runx2 (F) expression shown in D by normalizing to α-tubulin level, respectively. *P < 0.01 compared to vehicle-treated group (d 0) for each corresponding protein (n = 3).
Runx2 was required for IL-13–induced goblet-cell differentiation of human airway epithelial cells
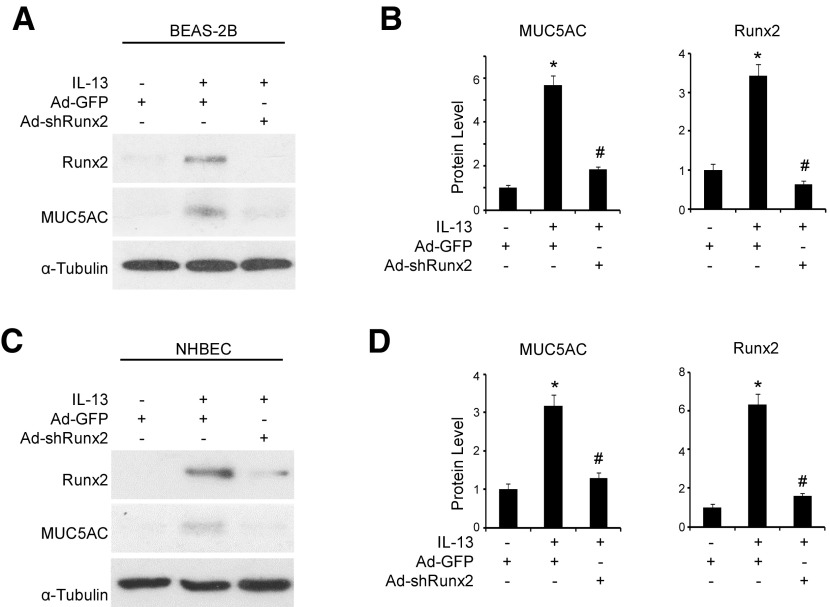
To determine whether Runx2 plays a role in mucus production and goblet cell differentiation, we knocked down Runx2 expression using its specific shRNA (Ad-shRunx2), then examined mucin 5AC expression in IL-13-treated epithelial cells. As shown in Fig. 2A, B, knockdown of Runx2 significantly inhibited IL-13-induced mucin 5AC expression in BEAS-2B cells. Importantly, when we knocked down Runx2 in human primary NHBECs, mucin 5AC expression was also blocked, suggesting an inhibition of goblet cell differentiation (Fig. 2C, D). These data demonstrate that Runx2 is essential for IL-13-induced goblet cell differentiation of human airway epithelial cells in vitro.
Figure 2.
Runx2 was essential for IL-13-induced goblet cell differentiation of human airway epithelial cells. BEAS-2B cells (A, B) and NHBECs (C, D) were transduced with Ad-GFP or Ad-shRunx2 followed by vehicle (−) or IL-13 (+, 50 ng/ml) treatment as indicated for 3 and 2 d, respectively. A, C) Western blot analysis was performed to examine mucin 5AC and Runx2 protein expression. B) Quantification of mucin 5AC and Runx2 expression shown in A by normalizing to α-tubulin level, respectively. D) Quantification of mucin 5AC and Runx2 expression shown in C by normalizing to α-tubulin level, respectively. *P < 0.01 compared to Ad-GFP group with vehicle treatment; #P < 0.01 compared to Ad-GFP group with IL-13 treatment for each corresponding protein (n = 3).
Runx2 mediated IL-13–induced expression of SPDEF during goblet cell differentiation
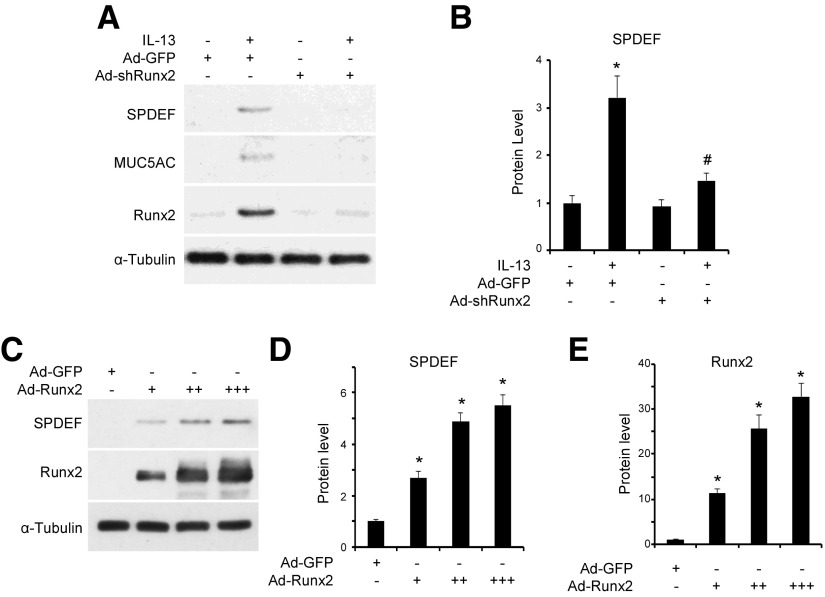
Previous studies have shown that SPDEF plays a critical role in goblet cell differentiation (11, 12, 23). Therefore, we sought to test whether Runx2-mediated goblet cell differentiation is associated with SPDEF. SPDEF expression was induced by IL-13 along with mucin 5AC and Runx2 in NHBECs (Fig. 3A). However, when we knocked down Runx2 to block mucin 5AC expression, SPDEF expression was almost completely diminished (Fig. 3A, B), suggesting that Runx2 is an upstream regulator of SPDEF and is essential for SPDEF expression during goblet cell differentiation. To test whether Runx2 is sufficient or whether it can directly regulate SPDEF expression, we forcefully expressed Runx2 in NHBECs using adenovirus vector expressing Runx2 (Ad-Runx2). As shown in Fig. 3C–E, Runx2 alone promoted SPDEF protein expression in a dose-dependent manner, further demonstrating that Runx2 is a key factor regulating SPDEF expression during goblet cell differentiation.
Figure 3.
Runx2 mediated goblet cell differentiation by regulating SPDEF expression. A, B) Knockdown of Runx2 inhibited IL-3-induced SPDEF and mucin 5AC expression. Serum-starved NHBECs were transduced with Ad-GFP or Ad-shRunx2, followed by treatment with vehicle or IL-13 (50 ng/ml) for 2 d. Western blot analysis was performed to examine expression of proteins as indicated. B) Quantification of SPDEF expression shown by normalizing to α-tubulin level. *P < 0.01 compared to Ad-GFP group without IL-13; #P < 0.01 compared to Ad-GFP group with IL-13 (n = 3). C–E) Runx2 promoted SPDEF protein expression in dose-dependent manner. NHBECs were transduced with Ad-GFP (7 × 109 PFU/ml, 2 µl/ml) or Ad-Runx2 (3 × 1010 PFU/ml: 0.5 µl/ml (+), 1 µl/ml (++), 2 µl/ml (+++)) for 3 d. Western blot analyses (C) were performed to examine Runx2 and SPDEF expression. D, E) Quantification of Runx2 (D) and SPDEF (E) protein expression shown in C by normalizing to α-tubulin level. *P < 0.01 compared to Ad-GFP group for corresponding proteins (n = 3).
Runx2 regulated SPDEF gene transcription by binding to its promoter
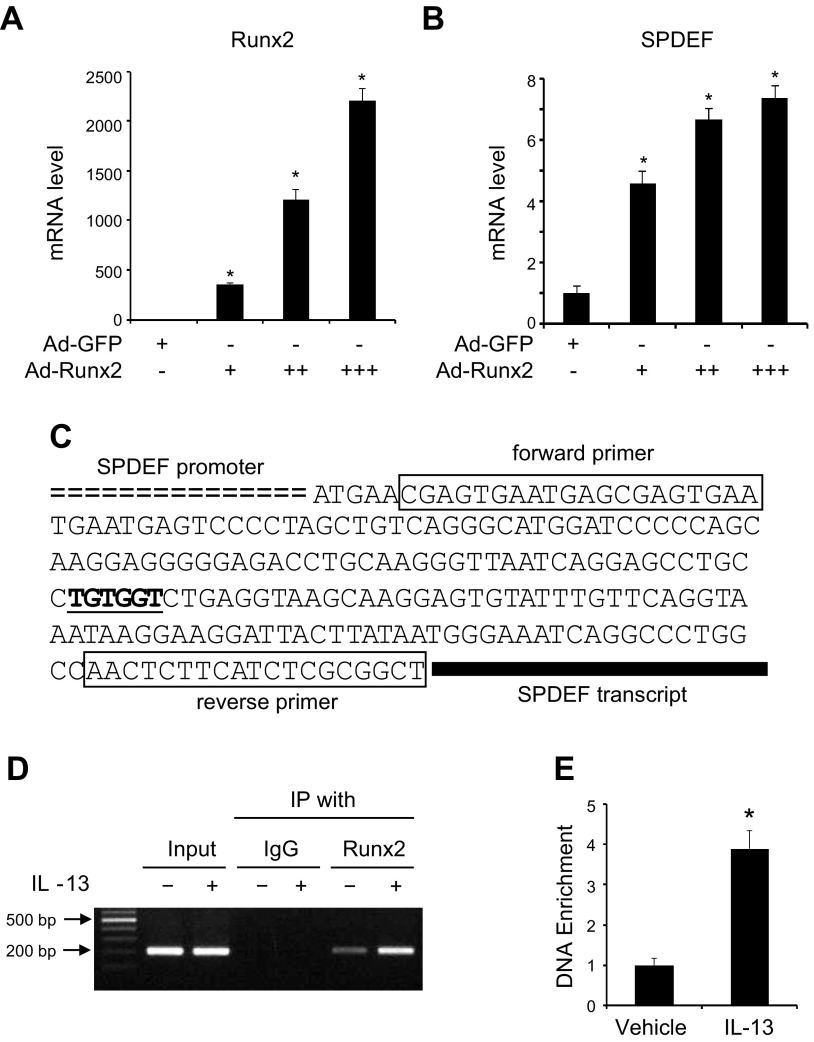
Because Runx2 is a transcription factor, we sought to investigate whether Runx2 regulates SPDEF gene transcription. Thus, we first tested whether Runx2 induces SPDEF mRNA expression. As shown in Fig. 4A, B, forced expression of Runx2 dose-dependently induced SPDEF mRNA expression in NHBECs. To determine how Runx2 regulates SPDEF mRNA expression, we analyzed SPDEF promoter DNA sequence and identified a 6-bp Runx2 consensus binding sequence TGTGGT located between −95 and −100 bp upstream of the SPDEF transcription start site (Fig. 4C) (24). To determine whether Runx2 indeed binds to the consensus sequence in the SPDEF promoter, we performed a ChIP analysis in NHBECs. As shown in Fig. 4D, Runx2 physically interacted with the SPDEF promoter weakly in a chromatin setting in NHBECs before differentiation. IL-13 treatment significantly enhanced the interaction (Fig. 4D, E), suggesting that Runx2 may mediate IL-13-induced goblet cell differentiation through binding to SPDEF promoter, thus enhancing SPDEF transcription.
Figure 4.
Runx2 regulated SPDEF gene transcription by binding to Runx2 binding element (RBE) in SPDEF promoter. A, B) Runx2 promoted SPDEF mRNA expression in dose-dependent manner. NHBECs were transduced with Ad-GFP (7 × 109 PFU/ml, 2 µl/ml) or Ad-Runx2 (3 × 1010 PFU/ml: 0.5 µl/ml (+), 1 µl/ml (++), 2 µl/ml (+++)) for 3 d. Real-time qPCR was performed to examine Runx2 (A) and SPDEF (B) expression. *P < 0.01 compared to Ad-GFP group for corresponding gene, respectively (n = 3). C) Sequence scheme of SPDEF promoter region with RBE underlined indicates RBE sequence. Boxed sequences are primers used in ChIP assay. D, E) ChIP assay detecting Runx2 binding to SPDEF promoter. NHBECs were treated with vehicle (−) or IL-13 (+, 50 ng/ml) for 24 h followed by ChIP with IgG (control) or Runx2 antibody as indicated. Semiquantitative (D) or quantitative (E) PCR was performed to amplify promoter region containing RBE in chromatin setting using primer pair shown in C. Binding affinity of Runx2 to SPDEF promoter was quantified by normalizing to input DNA level (E). *P < 0.01 compared to vehicle-treated group (n = 3).
Runx2 was increased in HDM-induced goblet-cell differentiation in vivo
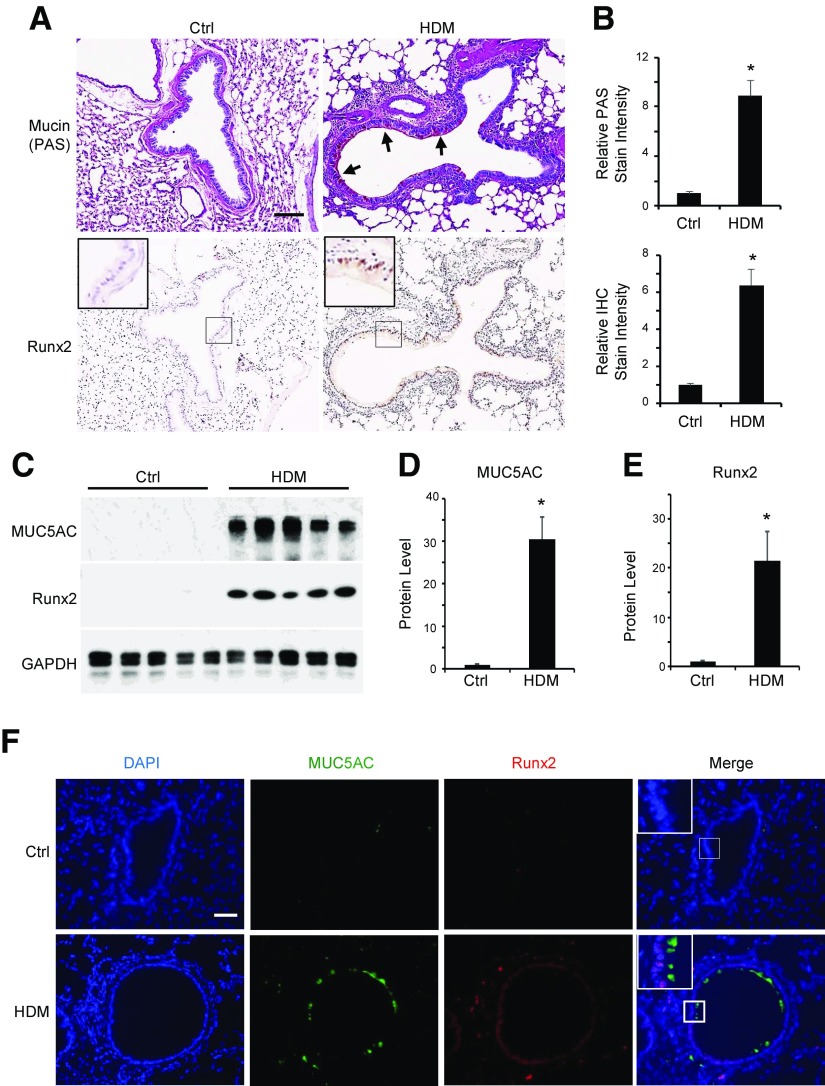
Goblet cell differentiation, as indicated by mucus production, is one of the initial cellular processes during the development of asthma (12, 25). To assess the role of Runx2 in goblet cell differentiation in vivo, we first examined the expression of Runx2 and mucin 5AC in lung tissues of HDM-challenged mice. Consistent with previous observations, mucin production was significantly induced in HDM-challenged mouse pulmonary epithelium (Fig. 5A, B). Importantly, although Runx2 was not expressed in the normal pulmonary epithelium, it was significantly induced in the HDM-challenged epithelium where the mucin was produced, suggesting that Runx2 may be involved in the goblet cell differentiation in vivo. The correlation between Runx2 expression and mucin production was also shown by analyzing the Runx2 and mucin 5AC expression in HDM-challenged lung tissues (Fig. 5C–E). To further assess this correlation in pulmonary epithelium, we tested whether Runx2 and mucin 5AC are expressed in the same epithelial cells after HDM challenge. As shown in Fig. 5F, neither Runx2 nor mucin 5AC was detectable in control mouse pulmonary epithelium. After HDM challenge, however, Runx2 was induced and localized in the nuclei of epithelial cells, whereas mucin 5AC was expressed in the cytoplasm or extracellular matrix of a large portion of Runx2-positive epithelial cells. These results further indicate that Runx2 is involved in HDM-induced goblet-cell differentiation in vivo.
Figure 5.
Runx2 was induced in HDM-induced goblet cell differentiation in mouse lungs. Mice were sensitized and challenged with HDM as described in Materials and Methods. A) Mouse lungs were harvested and sectioned, followed by PAS staining (top) for mucus production and IHC staining for Runx2 expression (bottom). Arrows point to PAS-stained mucin. B) Quantification of mucin production (top) and Runx2 expression (bottom) shown in (A) by normalizing to staining density in control groups (ctrl). *P < 0.01 compared to control (n = 5). C–E) Runx2 correlated with mucin 5AC expression in HDM-challenged mouse lungs. Mouse lungs were collected 3 d after last HDM challenge (PBS as control). Western blot analysis (C) was performed to examine Runx2 and mucin 5AC expression. Mucin 5AC (D) and Runx2 (E) protein levels shown in C were quantified by normalizing to GAPDH level, respectively. *P < 0.01 compared to control (n = 5). F) HDM-induced Runx2 and mucin were co-localized in same pulmonary epithelium. Immunofluorescence staining was performed to display mucin 5AC and Runx2 expression. DAPI stains nuclei. Scale bars, 100 µm.
Runx2 was required for HDM-induced mucus production and goblet cell differentiation in vivo
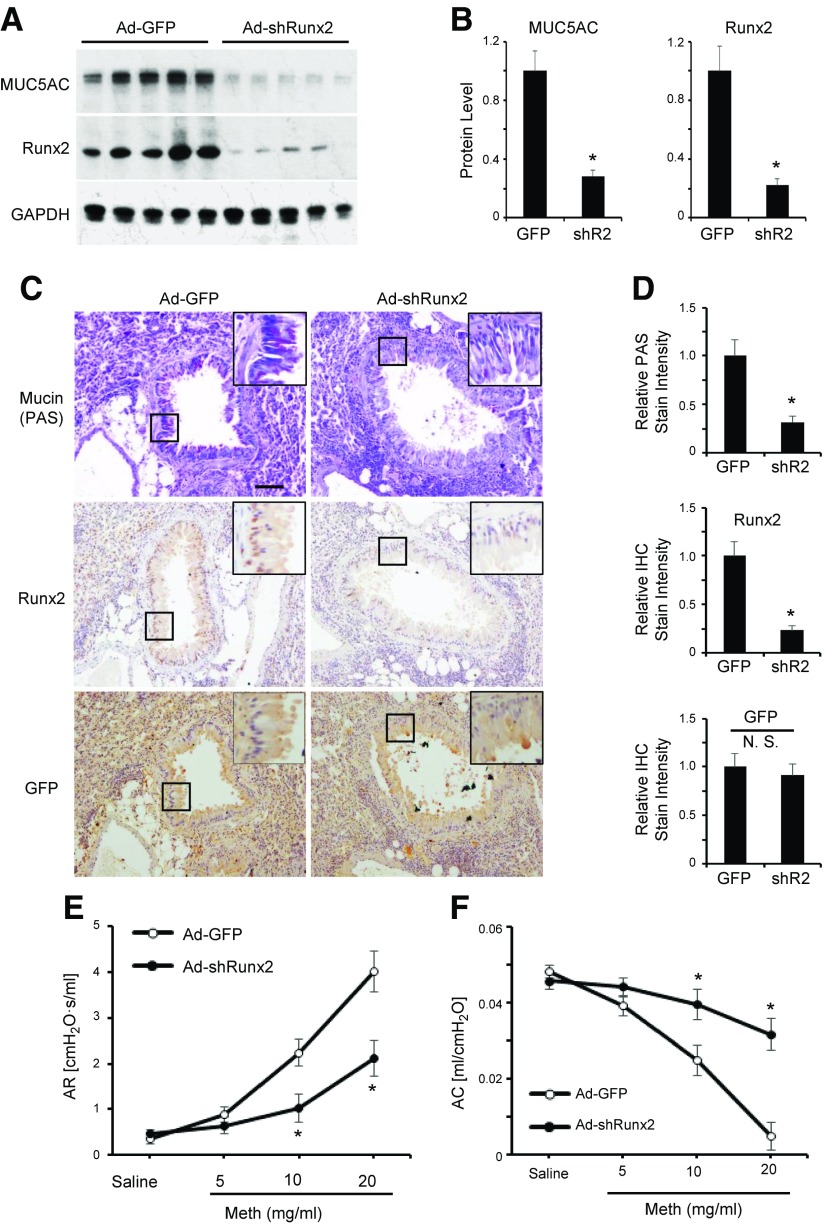
To determine whether Runx2 indeed plays a role in goblet cell differentiation in vivo, we knocked down Runx2 expression in the lungs of HDM-sensitized mice by using adenovirus delivery (26). As shown in Fig. 6A, B, Runx2 expression was effectively blocked by the intranasal introduction of Ad-shRunx2, resulting in a reduction of mucin 5AC expression, demonstrating that Runx2 was essential for the mucin 5AC induction in HDM-challenged mouse lungs. Consistently, knockdown of Runx2 significantly decreased the mucus production in pulmonary epithelium (Fig. 6C, D). IHC staining for GFP showed that the GFP was diffusely expressed in pulmonary epithelium and the interstitial areas, suggesting a high efficacy of adenoviral transduction, leading to the effective blockade of Runx2 expression in epithelium (Fig. 6C, D). Functionally, Runx2 knockdown significantly attenuated the AHR in response to methacholine in HDM-challenged mice (Fig. 6E), whereas airway compliance was greatly enhanced compared to the control adenovirus-treated mice (Fig. 6F). Taken together, our results demonstrated that Runx2 is essential for HDM-induced goblet cell differentiation and mucus production in vivo. Runx2-mediated goblet cell differentiation impairs the lung function, while targeting Runx2 by adenovirus delivery is able to impede the initial epithelium damage during the asthma development.
Figure 6.
Runx2 was essential for HDM-induced goblet cell differentiation and AHR in mouse lungs. A) Knockdown of Runx2 blocked HDM-induced mucin 5AC expression in mouse lungs. Adenovirus expressing GFP (Ad-GFP) or Runx2 shRNA (Ad-shRunx2, 3 × 1010 PFU/ml, 50 µl) were intranasally administered to HDM-sensitized mice as described in Materials and Methods. Lung tissues were collected 3 d after last HDM challenge, and Western blot analysis was performed to examine protein expression as indicated. B) Quantification of protein expression shown in A by normalizing to GAPDH level. *P < 0.01 compared to Ad-GFP (GFP) group (n = 5). C) Knockdown of Runx2 blocked HDM-induced mucin production in pulmonary epithelium. Control (Ad-GFP)- or Runx2 shRNA (Ad-shRNA)-transduced lung sections were processed for PAS staining for mucin, IHC staining for Runx2, and GFP expression for adenovirus delivery as indicated. Scale bar, 100 µm. D) Mucin production and Runx2 and GFP expression were quantified by normalizing to staining intensity in control sections for each individual protein. Relative levels are shown. *P < 0.01 compared to Ad-GFP group for each corresponding protein (n = 5). E, F) Knockdown of Runx2 diminished HDM-induced asthmatic responses to methacholine. Mice were anesthetized, and methacholine (Meth) challenge test was performed by using FlexiVent to measure airway resistance (AR; E) and airway compliance (AC; F) as indicated. *P < 0.01 compared to corresponding Ad-GFP group (n = 5).
Runx2 was induced in human asthmatic bronchial epithelial cells and airway epithelium
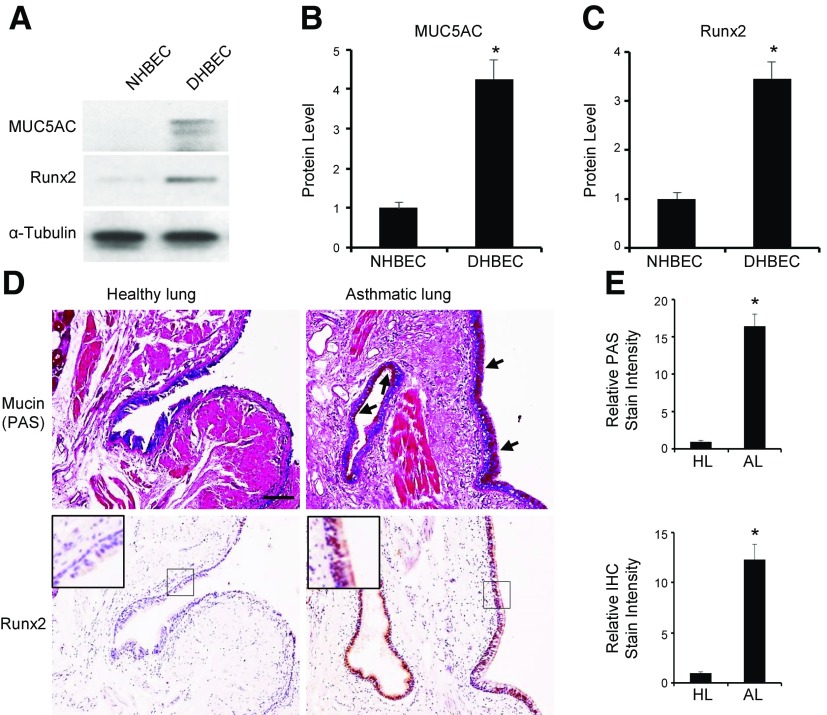
To determine whether the Runx2 function in goblet cell differentiation and asthma development is relevant to human diseases, we first compared the Runx2 expression in the primary bronchial epithelial cells isolated from healthy patients (NHBECs) and patients with asthma (DHBECs). As shown in Fig. 7A–C, Runx2 was almost undetectable in NHBECs but was highly expressed, along with mucin 5AC protein, in DHBECs, indicating that Runx2 expression is correlated with goblet cell differentiation in human lung. To confirm this correlation in human patients, we examined mucus production and Runx2 expression in the same epithelium of lung specimens from healthy control subjects and patients with asthma. It was evident that mucus was produced in asthma lung epithelium, where a high level of Runx2 was expressed (Fig. 7D, E), whereas neither mucus nor Runx2 was produced in healthy lung. These data provided a strong correlation of Runx2 with the development and progression of human asthma. Thus, Runx2 is likely to play a critical role in goblet cell differentiation and epithelium damage in the onset of asthma in humans.
Figure 7.
Runx2 was expressed in DHBECs and pulmonary epithelium of patients with asthma. A–C) Runx2 was expressed along with mucin 5AC in DHBECs, but not NHBECs. NHBECs and DHBECs were cultured for 3 to 4 d to 70 to 80% confluence. Mucin 5AC and Runx2 protein expression was detected by Western blot analysis (A) and quantified by normalizing to α-tubulin level (B, C, respectively). *P < 0.01 compared to NHBECs (n = 3). D, E) Runx2 was highly expressed along with mucus production in pulmonary epithelium of patients with asthma. D) Mucus production was detected by PAS staining for mucin (top), and Runx2 expression was detected by IHC staining (bottom). Arrows point to PAS-stained mucin. Scale bar, 100 µm. E) Quantification of mucin production and Runx2 level in asthmatic lungs (AL) by normalizing to staining intensity in healthy lungs (HL), respectively. Shown are relative levels. *P < 0.01 compared to healthy lungs (n = 5).
DISCUSSION
Airway epithelium is the first line of defense protecting lung against injury. Mucus overproduction due to goblet cell differentiation in the epithelium is a key feature of a number of respiratory diseases, including asthma and chronic obstructive pulmonary disease. We demonstrated that Runx2 is a critical regulator for the goblet cell differentiation and mucus overproduction both in vitro and in vivo. Runx2 is nearly undetectable in normal airway epithelial cells and lung tissue. However, it is significantly up-regulated during goblet cell differentiation induced by IL-13 in vitro and HDM in vivo. Knockdown of Runx2 blocks IL-13-induced goblet cell differentiation of both BEAS-2B cells and NHBECs, demonstrating the role of Runx2 in goblet cell differentiation in vitro. Runx2 is also essential for the goblet cell differentiation and mucus overproduction in vivo because intranasal delivery of Ad-shRunx2 blocks mucin 5AC protein expression in lung epithelium. Most importantly, Runx2 function in lung epithelium is relevant to human disease, because Runx2 expression correlates with mucus production in human epithelium in patients with asthma.
Runx2 appears to regulate goblet cell differentiation by directly binding to the promoter of the SPDEF gene, serving as a transcription factor of SPDEF. IL-13 enhances Runx2 binding affinity to the SPDEF promoter, likely due to the increased Runx2 expression induced by IL-13. Consistent with this finding, ectopic expression of Runx2 increases SPDEF mRNA and protein expression in NHBECs in a dose-dependent manner. Interestingly, although it induces SPDEF expression, Runx2 alone is unable to induce the goblet cell differentiation. Runx2 induces a modest increase in mucin 5AC mRNA expression, but it fails to induce a detectable level of mucin 5AC protein, which is probably because Runx2-induced endogenous SPDEF does not reach a threshold level sufficient to trigger the mucin 5AC protein biosynthesis. Previous studies have shown that a high level of SPDEF is essential for the induction of mucin 5AC protein in epithelial cells (12, 23). Moreover, some Runx2-positive epithelial cells do not show mucin production (Fig. 5), which is indicative of a less mature differentiation of goblet cells. Nevertheless, our results demonstrate that Runx2 is essential, although not sufficient, for IL-13-induced goblet cell differentiation of airway epithelial cells. Other regulators are evidently required for regulating this process.
In addition to inducing goblet cell differentiation, HDM allergy is a common factor inducing asthma in humans or a mouse model. Because mucus overproduction/goblet cell differentiation is an initial and key factor for asthma development (3, 11, 27), Runx2 appears to play an important role in the onset of asthma. Indeed, knockdown of Runx2 in lung reduces HDM-induced asthmatic responses to methacholine challenge (Fig. 6), demonstrating that Runx2 is one of the risk factors mediating the HDM-induced asthma. Interestingly, Runx2 is not exclusively expressed in pulmonary epithelium but is also present in interstitial cells or infiltrated macrophages (Figs. 5 and 6). Whether Runx2 in these cells contributes to asthmatic response or inflammation requires further investigation.
Our study identified Runx2 as a novel regulator for goblet cell differentiation/mucus overproduction in airway epithelial cells both in vitro and in vivo, which is relevant to human asthma development. Targeting Runx2 may be a potential preventive and therapeutic strategy for inflammatory cytokine- or allergen-triggered mucus overproduction/goblet cell differentiation and asthma.
ACKNOWLEDGMENTS
This work was supported, in part, by grants from the U.S. National Institutes of Health, National Heart, Lung, and Blood Institute (HL123302 and HL119053).
Glossary
- Ad-GFP
green fluorescent protein–expressing adenovirus
- Ad-shRunx2
adenoviral vector–expressing Runx2 short hairpin RNA
- AHR
airway hyperresponsiveness
- ChIP
chromatin immunoprecipitation
- DHBEC
diseased human bronchial epithelial cell
- GAPDH
glyceraldehyde phosphate dehydrogenase
- GFP
green fluorescent protein
- HDM
house dust mite
- IHC
immunohistochemistry
- NHBEC
normal human bronchial epithelial cell
- PAS
periodic acid–Schiff
- qPCR
quantitative PCR
- Runx2
Runt-related transcription factor 2
- sh
short hairpin
- SPDEF
SAM-pointed domain-containing Ets-like factor
AUTHOR CONTRIBUTIONS
N. Shi and S.-Y. Chen conceived of and coordinated the study; N. Shi designed, performed, and analyzed the experiments; J. Zhang collected and provided human lung specimens; N. Shi and S.-Y. Chen analyzed the results; N. Shi and S.-Y. Chen wrote the article; and all authors analyzed the results and approved the article’s final version.
REFERENCES
- 1.Fahy J. V., Dickey B. F. (2010) Airway mucus function and dysfunction. N. Engl. J. Med. 363, 2233–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers D. F. (2001) Motor control of airway goblet cells and glands. Respir. Physiol. 125, 129–144 [DOI] [PubMed] [Google Scholar]
- 3.Curran D. R., Cohn L. (2010) Advances in mucous cell metaplasia: a plug for mucus as a therapeutic focus in chronic airway disease. Am. J. Respir. Cell Mol. Biol. 42, 268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ordoñez C. L., Khashayar R., Wong H. H., Ferrando R., Wu R., Hyde D. M., Hotchkiss J. A., Zhang Y., Novikov A., Dolganov G., Fahy J. V. (2001) Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am. J. Respir. Crit. Care Med. 163, 517–523 [DOI] [PubMed] [Google Scholar]
- 5.Thavagnanam S., Parker J. C., McBrien M. E., Skibinski G., Heaney L. G., Shields M. D. (2011) Effects of IL-13 on mucociliary differentiation of pediatric asthmatic bronchial epithelial cells. Pediatr. Res. 69, 95–100 [DOI] [PubMed] [Google Scholar]
- 6.Rogers D. F. (2002) Airway goblet cell hyperplasia in asthma: hypersecretory and anti-inflammatory? Clin. Exp. Allergy 32, 1124–1127 [DOI] [PubMed] [Google Scholar]
- 7.Atherton H. C., Jones G., Danahay H. (2003) IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L730–L739 [DOI] [PubMed] [Google Scholar]
- 8.Kuperman D. A., Huang X., Koth L. L., Chang G. H., Dolganov G. M., Zhu Z., Elias J. A., Sheppard D., Erle D. J. (2002) Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 8, 885–889 [DOI] [PubMed] [Google Scholar]
- 9.Laoukili J., Perret E., Willems T., Minty A., Parthoens E., Houcine O., Coste A., Jorissen M., Marano F., Caput D., Tournier F. (2001) IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J. Clin. Invest. 108, 1817–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren X., Shah T. A., Ustiyan V., Zhang Y., Shinn J., Chen G., Whitsett J. A., Kalin T. V., Kalinichenko V. V. (2013) FOXM1 promotes allergen-induced goblet cell metaplasia and pulmonary inflammation. Mol. Cell. Biol. 33, 371–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajavelu P., Chen G., Xu Y., Kitzmiller J. A., Korfhagen T. R., Whitsett J. A. (2015) Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J. Clin. Invest. 125, 2021–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G., Korfhagen T. R., Xu Y., Kitzmiller J., Wert S. E., Maeda Y., Gregorieff A., Clevers H., Whitsett J. A. (2009) SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Invest. 119, 2914–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambrecht B. N., Hammad H. (2012) The airway epithelium in asthma. Nat. Med. 18, 684–692 [DOI] [PubMed] [Google Scholar]
- 14.Ito Y., Bae S. C., Chuang L. S. (2015) The RUNX family: developmental regulators in cancer. Nat. Rev. Cancer 15, 81–95 [DOI] [PubMed] [Google Scholar]
- 15.Coffman J. A. (2003) Runx transcription factors and the developmental balance between cell proliferation and differentiation. Cell Biol. Int. 27, 315–324 [DOI] [PubMed] [Google Scholar]
- 16.Haley K. J., Lasky-Su J., Manoli S. E., Smith L. A., Shahsafaei A., Weiss S. T., Tantisira K. (2011) RUNX transcription factors: association with pediatric asthma and modulated by maternal smoking. Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L693–L701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tandon M., Gokul K., Ali S. A., Chen Z., Lian J., Stein G. S., Pratap J. (2012) Runx2 mediates epigenetic silencing of the bone morphogenetic protein-3B (BMP-3B/GDF10) in lung cancer cells. Mol. Cancer 11, 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pain M., Bermudez O., Lacoste P., Royer P. J., Botturi K., Tissot A., Brouard S., Eickelberg O., Magnan A. (2014) Tissue remodelling in chronic bronchial diseases: from the epithelial to mesenchymal phenotype. Eur. Respir. Rev. 23, 118– 130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose M. C., Piazza F. M., Chen Y. A., Alimam M. Z., Bautista M. V., Letwin N., Rajput B. (2000) Model systems for investigating mucin gene expression in airway diseases. J. Aerosol Med. 13, 245–261 [DOI] [PubMed] [Google Scholar]
- 20.Kanoh S., Tanabe T., Rubin B. K. (2011) IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin. Exp. Allergy 41, 1747–1756 [DOI] [PubMed] [Google Scholar]
- 21.Tesfaigzi Y. (2008) Regulation of mucous cell metaplasia in bronchial asthma. Curr. Mol. Med. 8, 408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fahy J. V. (2002) Goblet cell and mucin gene abnormalities in asthma. Chest 122(6, Suppl)320S–326S [DOI] [PubMed] [Google Scholar]
- 23.Park K. S., Korfhagen T. R., Bruno M. D., Kitzmiller J. A., Wan H., Wert S. E., Khurana Hershey G. K., Chen G., Whitsett J. A. (2007) SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Invest. 117, 978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim M., Zhong C., Yang S., Bell A. M., Cohen M. B., Roy-Burman P. (2010) Runx2 regulates survivin expression in prostate cancer cells. Lab. Invest. 90, 222–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long A. J., Sypek J. P., Askew R., Fish S. C., Mason L. E., Williams C. M., Goldman S. J. (2006) Gob-5 contributes to goblet cell hyperplasia and modulates pulmonary tissue inflammation. Am. J. Respir. Cell Mol. Biol. 35, 357–365 [DOI] [PubMed] [Google Scholar]
- 26.Wang Y., Manevich Y., Feinstein S. I., Fisher A. B. (2004) Adenovirus-mediated transfer of the 1-cys peroxiredoxin gene to mouse lung protects against hyperoxic injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L1188–L1193 [DOI] [PubMed] [Google Scholar]
- 27.Tsao P. N., Wei S. C., Wu M. F., Huang M. T., Lin H. Y., Lee M. C., Lin K. M., Wang I. J., Kaartinen V., Yang L. T., Cardoso W. V. (2011) Notch signaling prevents mucous metaplasia in mouse conducting airways during postnatal development. Development 138, 3533–3543 [DOI] [PMC free article] [PubMed] [Google Scholar]