Abstract
In this study, we report for the first time that the primary cilium acts as a crucial sensor for electrical field stimulation (EFS)–enhanced osteogenic response in osteoprogenitor cells. In addition, primary cilia seem to functionally modulate effects of EFS-induced cellular calcium oscillations. Primary cilia are organelles that have recently been implicated to play a crucial sensor role for many mechanical and chemical stimuli on stem cells. Here, we investigate the role of primary cilia in EFS-enhanced osteogenic response of human adipose-derived stem cells (hASCs) by knocking down 2 primary cilia structural proteins, polycystin-1 and intraflagellar protein-88. Our results indicate that structurally integrated primary cilia are required for detection of electrical field signals in hASCs. Furthermore, by measuring changes of cytoplasmic calcium concentration in hASCs during EFS, our findings also suggest that primary cilia may potentially function as a crucial calcium-signaling nexus in hASCs during EFS.—Cai, S., Bodle, J. C., Mathieu, P. S., Amos, A., Hamouda, M., Bernacki, S., McCarty, G., Loboa, E. G. Primary cilia are sensors of electrical field stimulation to induce osteogenesis of human adipose-derived stem cells.
Keywords: polycystin-1, intraflagellar protein-88, siRNA knockdown, signal transduction, calcium oscillations
Tissue engineering is a promising therapeutic approach to treat large bone defects (1), and it provides distinct advantages over traditional autograft and allograft methods, overcoming challenges of limited tissue sources and potential graft-vs.-host rejection (2). To facilitate full regeneration of artificial bone tissues, various biophysical and biochemical stimuli have been developed to enhance the bone phenotype (3–5). Among them, electrical field stimulation (EFS) has been found to be a promising manipulation and activation method to create osteoprogenitor cells (6–9). EFS has several potential advantages over chemical, mechanical, or other physical stimuli, including the absence of possible immunogenic bioagents and use of less complicated equipment (10, 11).
Although EFS-enhanced cell proliferation and osteogenic differentiation have been widely reported, the underlying cellular transduction mechanisms that control cell response are unclear (12–15). The difficulty of identifying exact mechanisms comes from the fact that biologic effects of electrical stimulation are highly dependent on the stimulation parameters, such as applied frequency, electrical field strength, and cell types (11, 16), and several distinct transduction mechanisms exist. For example, previous investigators have shown that electrical fields can open ion channels and elevate intracellular Ca2+ concentrations (16–18). Other studies have proposed that electrical fields can activate or redistribute cell surface receptors (13). Furthermore, it has been proposed that electrical stimulation can affect cells by acting as an electromechanical force (19, 20).
Despite numerous reports on these different theories, there are few studies that have reported on how cells sense EFS. It is unclear which cell structures in osteoprogenitor cells function as the major sensors of electrical field signals. Limited understanding of the cellular detection mechanism has hindered the widespread application of electrical stimulation in tissue engineering.
Recently, primary cilia have been implicated as a crucial sensor for many cellular mechano- or chemoresponses (21–25). Primary cilium is an organelle that can be found in most cell types in the human body and it colocalizes with a variety of receptor proteins. It is believed that primary cilia can be easily accessed and affected by multiple extracellular modulators (26).
Primary cilia have been shown to be critical in bone formation processes (27–29), with mutations in primary ciliary structural proteins resulting in severe developmental defects (30, 31). Recent studies have reported that cilia can detect exogenous stimuli, transducing these signals to multiple cellular biochemical activities, such as proliferation, differentiation, and migration (32–34).
Existing literature (21–23, 26, 35, 36) indicates that the primary cilium is a likely potential sensor for EFS in osteoprogenitor cells; therefore, the goal of this study was to determine the role of primary cilia in electrical field–enhanced osteogenic differentiation of human adipose-derived stem cells (hASCs), a relevant progenitor cell model. hASCs are actively investigated as a promising cell source for autologous replacement therapies in tissue engineering. They are relatively abundant and exhibit multipotent capability to differentiate into a variety of mesenchymal lineages (37). We hypothesized that the primary cilium plays a critical role in the sensation and transduction of electrical field signals in hASCs.
MATERIALS AND METHODS
Interdigitated electrode fabrication
Custom interdigitated electrodes (IDEs) were used as electrical stimulation devices for all experiments. IDEs described here contain 2 contact pads, each connected to 25 digits. Each digit is 100-µm wide, and the spacing between digits is 100 µm. IDEs were fabricated by using microfabrication. In brief, IDEs were produced by using UV-lithography and wet etching as follows. After cleaning, glass slides of 1 mm thickness (VWR International, Radnor, PA, USA) were coated with 15 nm Cr and 60 nm Au, using an e-beam evaporator. Slides were then spin coated with hexamethyldisilazane (Microchem, Newton, MA, USA) and AZ5214-IR photoresist (Microchem) using a spin coater (Laurell Technologies, North Wales, PA, USA), followed by a bake at 90°C for 60 s on a hot plate. Slides were exposed to UV light for 15 s (Optical Associates, San Jose, CA, USA) by using a chrome mask with the desired IDE pattern and were hard baked at 115°C for 45 s. Slides were then flood exposed to UV light for 40 s without the mask and were developed in MIF CD-26 (Microchem). Excess metals were removed by using standard chrome and gold etchants (Sigma-Aldrich, St. Louis, MO, USA), and the remaining photoresist was removed in acetone. Electrodes were connected in parallel by using conductive silver epoxy (Ted Pella, Redding, CA, USA) and 250-µm-diameter silver wire (Goodfellow, Coraopolis, PA, USA). Silver wires were attached to contact pads on the IDE with silver epoxy and were soldered to gold pin connectors (Newark Electronics, Richfield, OH, USA). Matching gold pin connectors were soldered to Bayonet Neill–Concelman connectors inside the tissue culture flask to link electrodes to the external electrical stimulation. Assembled electrodes—inside tissue culture flasks—were sterilized with ethylene oxide (Andersen Sterilizers, Haw River, NC, USA).
IDEs were connected to a signal generator (33220A; Agilent Technologies, Santa Clara, CA, USA). Stimulus intensity of 10 mV (peak-to-peak) was used to generate the calculated electrical fields of 1 V/cm on cells (12, 38). Waveform and intensity were confirmed with an oscilloscope. IDEs provided a stable and consistent electric field, both parallel to and above the surface of the electrode, up to a height of 100 µm (12). Spacing between digits and digit width were both 100 µm. The diminutive spacing (100 µm) between electrode fingers allowed physiologically relevant fields to be obtained with the application of low voltages, which eliminated potential adverse electrochemical effects.
Cell isolation and culture
hASCs were derived from waste adipose tissue that was acquired from voluntary abdominoplasty procedures. All adipose tissue was obtained at the University of North Carolina–Chapel Hill in accordance with an approved institutional review board protocol (IRB 04-1622). Detailed processes for isolation of hASCs have been previously reported from our laboratory (39). An hASC superlot that was derived from 5 female individual donors (age 24–81 yr) were used for all experiments in this work (40). After isolation, hASCs were propagated in complete growth medium until 80% confluency (up to 1 wk). Complete growth medium contained Eagle’s Minimum Essential Medium, alphamodified, and supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. Obtained hASCs were cryopreserved as passage 0. For experiments in this work, hASCs at passage 3 were expanded in flasks to 80% confluence (up to 1 wk) and were then trypsinized, resuspended, and seeded directly onto electrodes at a density of 20,000 cells/cm2. hASC-seeded electrodes were then cultured in 1 ml complete growth medium for 24 h before being exposed to subsequent treatments and/or electrical stimulation.
EFS
Cell-seeded electrodes were placed in 1 ml osteogenic differentiation medium (ODM) and stimulated with 1 V/cm electrical field at a frequency of 1 Hz for 4 h/d unless otherwise indicated. ODM contained complete growth medium with the addition of 50 µM ascorbic acid, 0.1 µM dexamethasone, and 10 mM β-glycerolphosphate. These parameters were selected on the basis of our previous study (12), in which we found that this stimulation promoted osteogenic differentiation and calcium accretion of hASCs. For calcium channel blocker studies, different ion channel inhibitors—with concentrations of 10 µM for gadolinium and 20 µM for verapamil (9, 14)—were added into ODM before applying electrical fields.
Cell viability and calcium accretion
hASC viability was determined with a Live/Dead Assay Cytotoxicity Kit (Molecular Probes, Eugene, OR, USA) for mammalian cells. Total calcium concentration was quantified by using an absorbance assay. In brief, cells were rinsed with PBS and digested in 0.5 N HCl overnight. Supernatants were then assayed by using the Calcium Liquicolor kit (Stanbio Laboratory, Boerne, TX, USA). Calcium concentration was determined on the basis of absorbance values that were read at 550 nm and were compared with a standard curve that was generated by using CaCl2 per manufacturer’s instructions. Calcium data were normalized to total protein quantified by using the bicinchoninic acid absorbance assay. To visualize calcium accretion, calcium deposits were stained by using Alizarin Red S that was applied directly to electrodes after a 30-min fixation in 10% formalin. Images were captured with a Leica (Wetzlar, Germany) EZ 4D Digital Scope.
RNA extraction and gene expression analyses
Quantitative RT-PCR analysis was used to determine relative mRNA expression levels of lineage-specific gene markers for osteogenic gene markers [Runx2 (runt-related transcription factor) and BMP-2 (bone morphogenetic protein-2)] and genes for cilia structural proteins [PKD1 (polycystin-1; PC1) and IFT88 (intraflagellar protein-88)]. RNA cell lysate samples were collected and run through a Qiashredder column (Qiagen, Valencia, CA, USA) to be homogenized. Total RNA was extracted by using the RNeasy Mini Kit (Qiagen). RNA concentrations were measured by using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). To synthesize first strand cDNA, 50 ng RNA was used with the Superscript III RT primers kit (Thermo Fisher Scientific) in a reverse transcriptase reaction. Taqman Gene expression assays with predetermined primer-probe sets were used to amplify cDNA (Assays-on-Demand; Applied Biosystems, Foster City, CA, USA). All gene expression profiles were normalized to glyceraldehyde-3-phosphate dehydrogenase (Assay HS99999905_M1) in an ABI Prism 7000 system.
Immunostaining
hASC-seeded IDEs were fixed in 10% formalin and then blocked in PBS solution that contained 0.2% Triton X-100 and 5.0% bovine serum albumin. IDEs were then incubated in primary antibody solution in humidified chambers overnight at 4°C. Primary antibody solution contained 0.2% Triton X-100, 0.5% bovine serum albumin, acetylated α-tubulin antibody (diluted 1:100; Sigma-Aldrich), and/or Runx2 antibody (1:100; Abcam, Cambridge, MA, USA) in PBS. Electrodes were then incubated in a solution that contained Alexa Fluor 488 secondary antibody (1:250; Molecular Probes), phalloidin 594 (1:500) or Alexa Fluor 594 (1:250) secondary antibody, and DAPI (1:500) stain solutions. Samples were incubated for 3 h at room temperature, then rinsed in PBS and mounted on glass slides by using Prolong Gold Mounting Media (Molecular Probes). Fluorescent microscopy (DM5500B; Leica) was used to visualize cytoskeletal organization, cell differentiation markers, and primary cilia conformation. Three images were taken with different filters to delineate DAPI (blue), Alexa Fluor 488 (green), and Alexa Fluor 594 (red), respectively. Optimized exposure, gain, and intensity experiment settings were determined by using the quick LUT function with compatible LAS-AF (Leica) software. Images were taken at ×40 magnification, 500–1000 ms exposure time (depending on filter), and a lamp intensity of 4. Semiquantitative image analysis with ImageJ (National Institutes of Health, Bethesda, MD, USA) was used to measure the frequency of cilia expression. For each condition, n > 300 cells, as identified by DAPI staining, on multiple (n > 3) electrodes were analyzed.
Small interfering RNA knockdown
hASC-seeded electrodes were transfected by Lipofectamine 2000 (Thermo Fisher Scientific) with PKD1 or IFT88 Stealth RNAi small interfering RNA (siRNA; Thermo Fisher Scientific) for gene knockdown. Stealth RNAi siRNA Negative Control was used as a control. A ratio of 20 pmol siRNA with 1.0 µl Lipofectamine 2000 per electrode in 1 ml serum-free Opti-MEM-I transfection medium was prepared according to the manufacturer’s protocol. hASCs were transfected for 24 h in siRNA-Lipofectamine solution, then cultured in fresh complete growth medium for another 24 h before electrical stimulations.
Calcium imaging
hASC-seeded electrodes were imaged to examine transient electrical field–elicited changes in cellular cytoplasmic Ca2+ concentration by using calcium sensitive dye. Specifically, cell-seeded electrodes were incubated for 1 h at 37°C in 1 ml PBS that contained 0.25 µl of the vital dye Cell Tracker Red CMTPX (Thermo Fisher Scientific) and 4 µl of the Ca2+-sensitive dye Fluo-4 AM (Thermo Fisher Scientific). Electrodes were then rinsed with PBS and incubated for 30 min with 1 ml dye-free PBS to allow Fluo-4 de-esterification for retention in cells. Electrodes were then transferred into a cell culture dish, immersed with fresh PBS, and mounted on the stage of a fluorescent microscope (Leica DM5500B). A ×20 objective was used to image hASC-seeded electrodes. A series of images with a time interval of 10 s were captured automatically by compatible LAS-AF software for an initial control (unstimulated) period of 8 min and a subsequent 8-min electrical field stimulation period. To calculate the average calcium fluorescence intensity for each group, multiple (n > 3) experiments on independent electrodes were performed.
Data analyses
Experiments were performed with at least 3 independent technical replicates (i.e., electrodes) for each experimental group. For quantitative RT-PCR analyses, 3 technical replicates for each sample were performed (n = 3). For calcium imaging analyses, MatLab software (MathWorks, Natick, MA, USA) was used to analyze changes in calcium dye fluorescence intensity of all cells in the field of view to generate line graphs of changes in cytoplasmic Ca2+. Statistical differences between treated samples, such as stimulated, and control (nonstimulated) at each experimental condition were analyzed by using Student’s t test. Quantitative data are presented as means ± sem. A value of P < 0.05 was considered significantly different.
RESULTS
EFS promotes osteogenesis of hASCs without compromising hASC viability
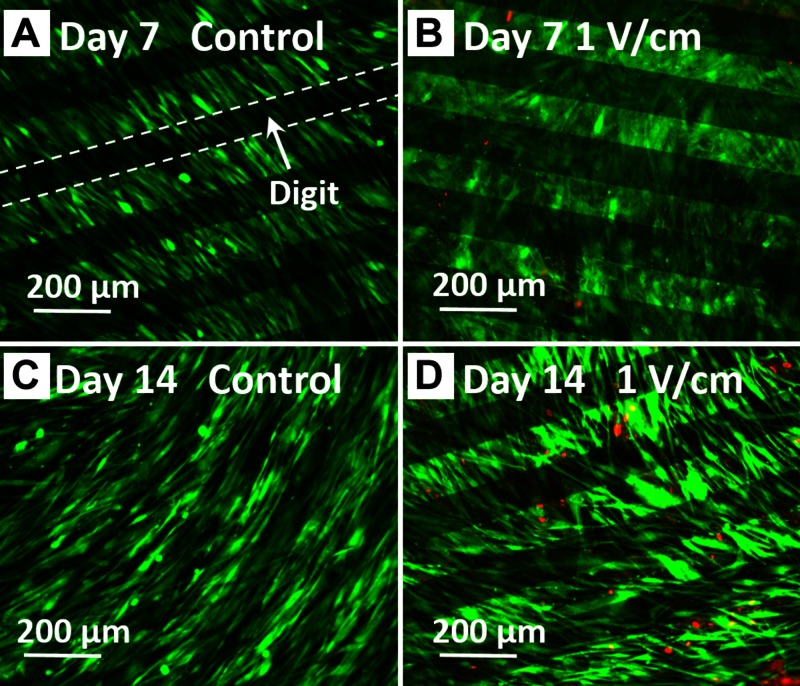
We first confirmed that hASCs that were cultured on IDEs (Fig. 1) under 1 V/cm electrical field at 1 Hz for 4 h/d remained viable. After 14 d of stimulation, hASCs exhibited high viability for all groups. Very few dead cells were present on d 7 and 14 (Fig. 2). These images also show that hASCs were able to adhere and spread on the surface of IDEs, maintaining a characteristic fibroblastic shape.
Figure 1.

IDE. The bright (white) regions are glass, whereas the dark regions are metal pads and electrode array.
Figure 2.
hASC viability after up to 14 d of EFS. hASCs exhibited high viability after up to 14 d exposure to 1 V/cm electric fields at 1 Hz for 4 h/d. Observed by Live/Dead staining (green, live cells; red, dead cells) after electrical stimulation at respective timepoints.
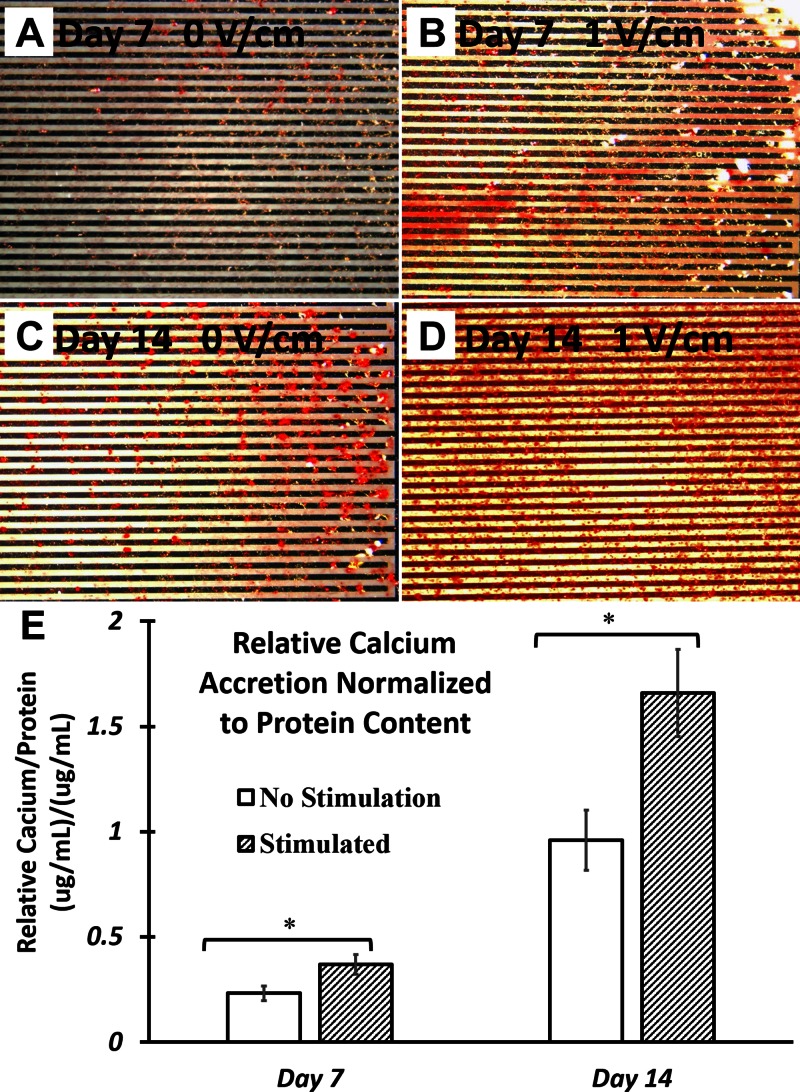
To confirm the stimulatory effect of 1 V/cm electrical field in promoting osteogenic differentiation of hASCs, cell-mediated calcium accretion was quantified. hASCs that were exposed to 1 V/cm electrical field at 1 Hz for 4 h/d significantly increased calcium accretion compared with nonstimulated controls (Fig. 3A–D). By d 7 and 14, mineralized calcium of hASCs that were exposed to 1V/cm electrical field was increased by 49.8 and 73.1%, respectively, compared with nonstimulated controls at the same timepoints (Fig. 3E).
Figure 3.
hASC calcium accretion after up to 14 d of EFS. A–D) Degree of hASC mineralization was determined by Alizarin Red staining, for cell-seeded IDE after 7 d culture without EFS (A), 7 d culture with EFS (B), 14 d culture without EFS (C), and 1 d culture with EFS (D). E) Quantified calcium accretion was measured and normalized to bicinchoninic acid protein content. hASCs were cultured in ODM and exposed to 1 V/cm electric field at 1 Hz for 4 h/d. Calcium accretion in hASCs that were exposed to EFS was significantly increased compared with nonstimulated controls at both timepoints evaluated. Error bars = sem, Student’s t test compared stimulated samples with nonstimulated control samples at each timepoint. *P < 0.05.
EFS simultaneously up-regulated mRNA expression of hASC osteogenic markers and primary cilia structural protein
To investigate whether primary cilia might play a role in sensing and transducing electrical field signals in hASC osteogenesis, we sought to determine the effects of EFS on primary cilium formation during hASC osteogenic differentiation. To accomplish this, by using quantitative RT-PCR, we tracked mRNA expression of hASC osteogenic markers, Runx2 and BMP-2, as well as genes that encode for primary cilia–associated proteins, PC1 (or PKD1) and IFT88, over a time course of 7 d.
We found that EFS simultaneously up-regulated mRNA expression of osteogenic differentiation markers and primary cilia structural protein, PC1, at d 1 and 7. Specifically, we found that 1 V/cm electrical field at 1 Hz significantly improved expression of these 2 osteogenic markers. As shown, after 4 h of electrical stimulation, both Runx2 (Fig. 4A) and BMP-2 (Fig. 4B) mRNA expression in hASCs was significantly increased. Seven days of EFS significantly up-regulated Runx2 mRNA expression at all timepoints tested and significantly up-regulated BMP-2 mRNA expression at all timepoints except d 5. mRNA expression of PKD1 (Fig. 4C) in hASCs was also significantly up-regulated by EFS by 6- and 2-fold at d 1 and 5, respectively, compared with nonstimulated controls.
Figure 4.
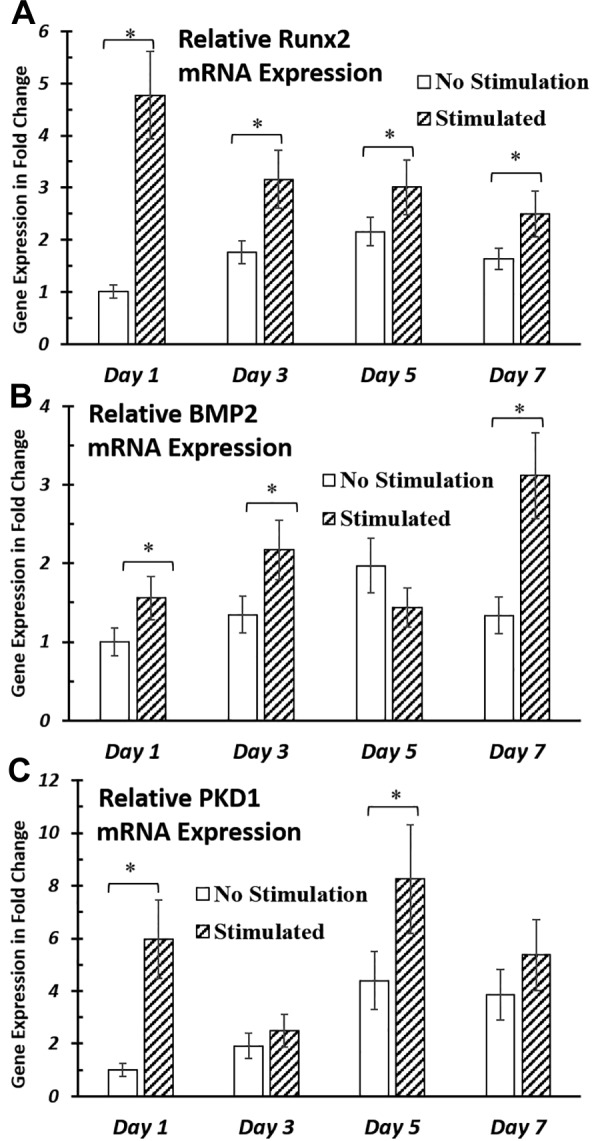
Gene expression of osteogenic markers and primary cilia structural proteins after up to 7 d of EFS. A, B) EFS significantly improved gene expression of osteogenic markers Runx2 (A) and BMP-2 (B) in hASCs during up to 7 d of stimulation. C) mRNA expression of primary cilia structural protein, PC1, was up-regulated by EFS at timepoints d 1 and 7. hASC-seeded IDEs were cultured in ODM and stimulated with 1 V/cm electric field at 1 Hz for 4 h/d. RNA was extracted and analyzed at the end of electrical stimulations for respective timepoints. mRNA expressions were normalized to nonstimulated samples at d 1. Error bars = sem, Student’s t test compared stimulated samples with nonstimulated control samples at each timepoint. *P < 0.05.
As PC1 is a protein that is associated with ciliary function and structure (41), it is expected that its up-regulation would have an effect on final cilia structure; however, the frequency of cilia expression that was observed in the hASC population when evaluated with immunostaining was not significantly changed (data not shown). mRNA expression of IFT88, another primary cilia structural protein (42), was not significantly up-regulated by EFS (data not shown).
Primary cilia are required for EFS-induced osteogenic responses in hASCs
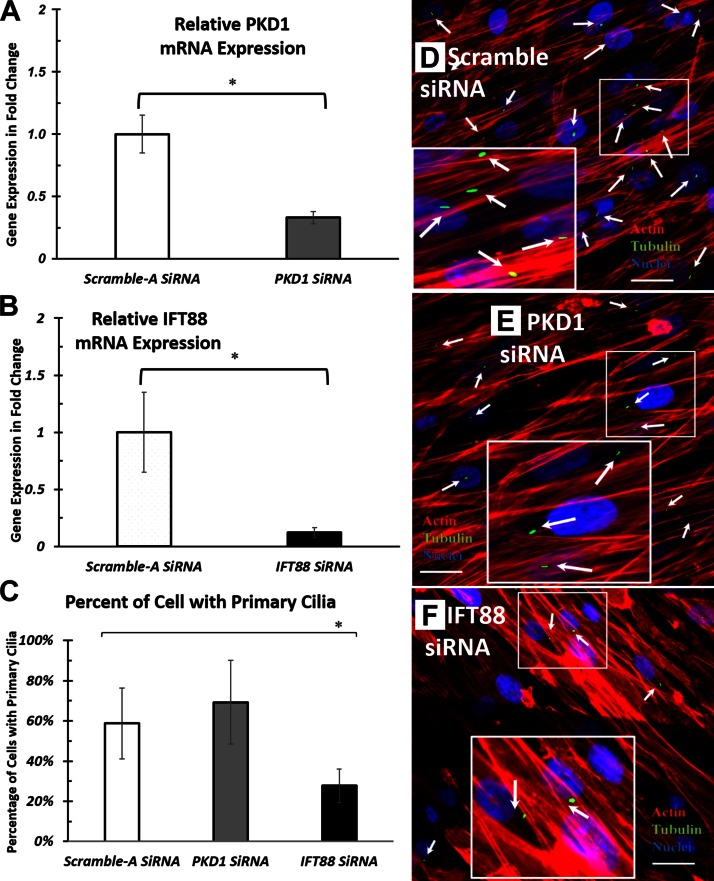
To investigate the function of primary cilia in hASCs during electrical stimulation, we abrogated the mRNA expression of 2 cilia-associated proteins of interest, PC1 and IFT88, by using siRNA transfection. The diminished mRNA expression of PKD1, the gene that encodes PC1 protein, and IFT88 was confirmed by real-time RT-PCR. siRNA transfection of PKD1 and IFT88 yielded a minimum of 65% knockdown efficiency (Fig. 5A, B). Because PC1 and IFT88 are primary cilia–associated proteins, we expected that their siRNA knockdown would have an effect on final cilia structure. To determine whether this was true, after PKD1 and IFT88 knockdown, we assessed the frequency of primary cilia that was observed in the hASC population via immunostaining (Fig. 5E, F). We found that IFT88 knockdown significantly reduced the frequency of primary cilia observed compared with negative controls. PKD1 knockdown did not significantly alter the frequency of cilia observed.
Figure 5.
Effects of PKD1 and IFT88 siRNA knockdown on hASC primary cilia. A, B) Gene expression of cilia-associated structural proteins, PKD1 (A) and IFT88 (B). C) Percentage of hASCs observed with cilia structure. D–F) Representative fluorescence images of hASC primary cilia after negative control siRNA (D), PKD1 siRNA (E), and IFT siRNA knockdown (F). White arrows indicate the immunostained primary cilia observed under fluorescent microscope. RNA extraction and immunostaining were performed after 72 h culture in ODM after siRNA knockdown. mRNA expressions were normalized to negative control siRNA transfected samples. Error bars = sem, Student’s t test compared siRNA knockdown samples with negative control siRNA-treated samples. *P < 0.05.
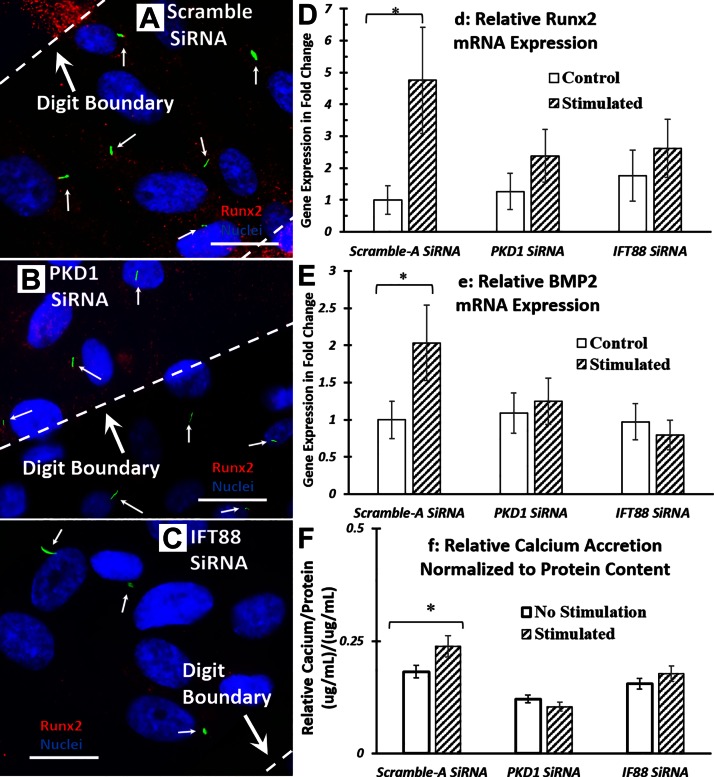
To determine the role of primary cilia in electrical field–induced hASC osteogenesis, after PKD1 and IFT88 knockdown, osteogenic gene expression in hASCs was analyzed by real-time RT-PCR. We probed for gene expression of Runx2 (Fig. 6D) and gene expression of BMP-2 (Fig. 6E). We found that after siRNA knockdown by either PKD1 or IFT88, electrical stimulation no longer significantly up-regulated mRNA expression of Runx2 and BMP2.
Figure 6.
Electrical field–induced hASC osteogenic response is affected by siRNA knockdown of primary cilia proteins. A–C) Fluorescence images of EFS-stimulated hASCs after negative control siRNA (A), PKD1 siRNA (B), and IFT siRNA knockdown (C). White arrows indicate the immunostained primary cilia observed under fluorescent microscope. D, E) Expression of osteogenic gene markers, Runx2 (D) and BMP-2 (E), in hASCs after knockdown and 4 h of electrical stimulation. hASC-seeded IDEs were stimulated with 1 V/cm electric field at 1 Hz for 4 h/d in ODM culture. RNA was extracted and analyzed at the end of electrical stimulation. mRNA expressions were normalized to negative control siRNA transfected nonstimulated samples. After siRNA knockdown, electrical field–enhanced osteogenic response was significantly diminished. F) Calcium accretion after siRNA knockdown and 7 d of electrical stimulation. Error bars = sem, Student’s t test compared stimulated samples with nonstimulated control samples at each treatments. *P < 0.05.
To confirm the involvement of primary cilia during EFS, the effects of PKD1 and IFT88 knockdown on hASC-mediated mineralization after 7 d of electrical stimulation were studied. We found that EFS-enhanced calcium accretion in hASCs was diminished after PKD1 and IFT88 knockdown compared with controls (Fig. 6F).
siRNA knockdown of primary cilia structural proteins does not diminish EFS-induced Ca2+ influx
To extend our study, we investigated the role of primary cilia in the electrical field signal transduction process in hASCs. Previously, we and others have reported that electrical stimulation can alter the cytoplasmic Ca2+ oscillation patterns (12, 13, 43, 44). It is generally thought that modulated intracellular Ca2+ levels can act as a second messenger to cause multiple transcriptional responses (9, 45, 46). Thus, we tested the role of cilia-associated proteins for EFS-altered intracellular Ca2+ oscillation patterns. We hypothesized that primary cilia transduce electrical field signals by controlling cytoplasmic Ca2+ oscillation patterns and that only cells that possess whole primary cilia structure could display Ca2+ influx during electrical stimulation.
To test our hypotheses, after knockdown of primary cilia structural proteins, we measured the changes of cytoplasmic calcium concentration in hASCs during electrical stimulation. Unexpectedly, calcium signal oscillation patterns during electrical stimulation were the same for hASCs with or without siRNA knockdown. As shown in Fig. 7, calcium fluorescence levels varied over time; however, there was no identifiable change in either the magnitude or trend of calcium oscillation patterns after siRNA knockdown of cilia structural proteins.
Figure 7.
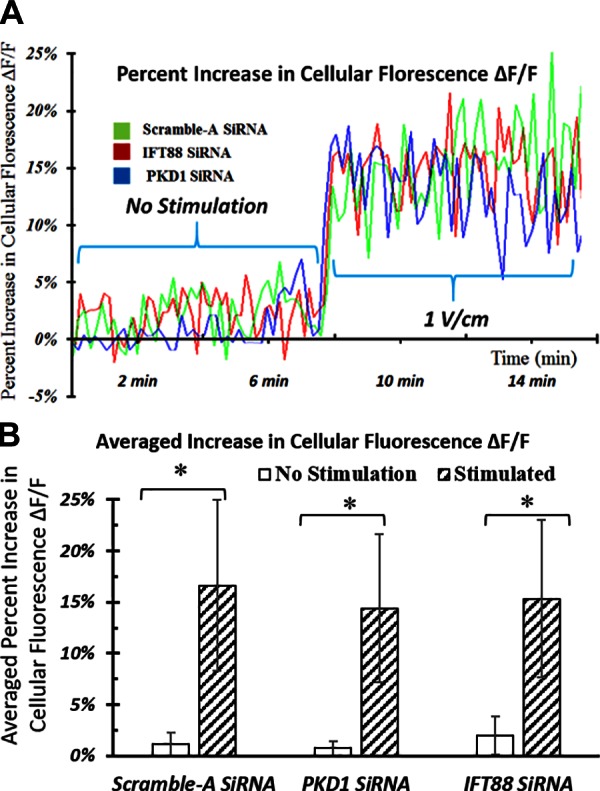
Effects of siRNA knockdown on transient EFS-induced cytoplasmic calcium oscillations. A) Graphical representation of the changes in calcium fluorescence intensity from all cells in the field of view on an IDE in a representative experiment. B) Changes of average calcium fluorescence intensity from multiple parallel experiments. Increase in hASC cytoplasmic Ca2+ concentration induced by transient electrical stimulation is not affected by siRNA knockdown. Error bars = sem, Student’s t test compared stimulated samples with nonstimulated control samples at each treatment condition. *P < 0.05.
Primary cilia in hASCs transduce EFS signals independent of stretch-activated Ca2+ channels
We have shown that cilia-associated structural protein PC1 plays a key role in sensing electrical field signals. PC1, together with PC2, are key components of the polycystin complex, which is thought to function as a stretch-activated calcium channel (41). In response to physical stimulation, the PC1/PC2-coupled channel can open and import extracellular Ca2+ into the cell, which elevates cytoplasmic Ca2+ concentration and triggers multiple biochemical responses (47). Recent studies have shown that the voltage-activated calcium channel is also involved in cilia sensing of physical stimulation (48, 49), and that PC1 may participate in this activation process (50, 51).
Therefore, to further study the primary cilia signal transduction mechanism, we investigated the involvement of stretch-activated ion channels and voltage-gated ion channels during the cilia sensation process of EFS. To do this, we blocked the stretch-activated channels or voltage-gated channels by using gadolinium and verapamil, respectively. We then looked at their interactive effects with knockdown of cilia structural proteins on the osteogenic response of hASCs. We hypothesized that cilia-associated proteins sense electrical field signals in a fashion that is similar to how they detect mechanical stimulations, that is, as a result of colocalization of calcium channel PC1/PC2 complex in primary cilia.
We found after blocking the voltage-gated channels, further knocking down primary cilia proteins did not affect electrical stimulation–induced osteogenic response in hASCs (Fig. 8A). In contrast, after blocking stretch-activated calcium channels in hASCs, there was still a significant decrease in stimulation effect when further knocking down primary cilia structural proteins (Fig. 8B). These results indicate that primary cilia may transduce the effects of EFS via a voltage-gated calcium channel or a voltage-modulated mechanism, potentially independent of stretch-activated Ca2+ channels.
Figure 8.
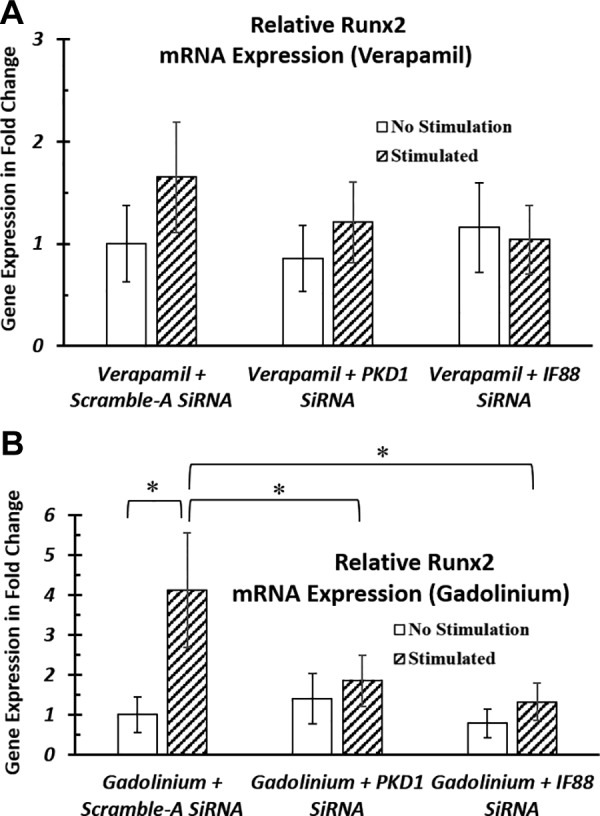
Involvement of calcium channels during primary cilia–mediated EFS. A, B) After siRNA knockdown, hASCs were transferred into ODM that was supplemented with verapamil (A), a voltage-gated ion channel blocker, or gadolinium (B), a stretch-activated ion channel blocker. hASCs were stimulated with 1 V/cm electric field at 1 Hz for 4 h. After blocking the voltage-gated channels, further knocking down primary cilia proteins did not affect electrical stimulation–induced osteogenic response in hASCs (A). In contrast, after blocking stretch-activated calcium channels in hASCs, there was still a significant decrease in stimulation effect when further knocking down primary cilia structural proteins (B). RNA was extracted and analyzed at the end of electrical stimulation. mRNA expressions were normalized to negative control siRNA-transfected nonstimulated samples. *P < 0.05.
DISCUSSION
This study demonstrated that primary cilia proteins seem to play a critical role in mediating electrical stimulation–induced osteogenic differentiation of hASCs. In addition, we have shown that primary cilia transduce electrical field signals that are involved in voltage-gated calcium channels and that this process is independent of stretch-activated calcium channels. Furthermore, we have shown that knockdown of primary cilia structural proteins does not diminish electrical stimulation–induced Ca2+ influx in hASCs.
We found that EFS simultaneously up-regulated mRNA expression of primary cilia structural protein, PC1, and osteogenic differentiation markers, Runx2 and BMP2. Recently, a feedback mechanism was reported that indicated that physical and chemical stimuli can confer changes to primary cilia structures (52, 53). Investigators proposed that changes in primary cilia structure may be an adaptive mechanism to modulate cilia sensory functions to adjust cellular exposure to external sensory signals (53). Up-regulation of mRNA expression of cilia protein and osteogenic differentiation markers by EFS suggests that primary cilia proteins may potentially play an adaptive role in sensing and translating electrical field signals to osteogenic response in hASCs. However, it should be noted that up-regulation of primary cilia–associated protein genes do not necessarily correlate with distinctive changes in cilia structure. Further study of cilia orientation, length, or morphology is required to illustrate the detailed influence of electrical stimulation on cilia structure.
We further showed that structurally integrated primary cilia proteins are required for EFS-enhanced osteogenic responses in hASCs. We found that knockdown of either IFT88 or PKD1, the gene that encodes PC1 protein, resulted in hASCs no longer expressing any up-regulation in Runx2 and BMP-2 osteogenic gene expression upon stimulation. IFT88 is protein that is critical for the maintenance of proper cilia structure and function (42). It is responsible for transporting proteins into the cilium axoneme, building the cilium structure, and transporting functional cilia proteins (42). PKD1 is required for skeletogenesis in the embryo, and mutations of this gene may cause defects in skeletal development (29). Thus, disruption of electrical field–enhanced Runx2 and BMP-2 gene expression after knockdown of either IFT88 or PKD1 indicates that these cilium-associated proteins are highly involved in osteogenic responses to electrical stimulation. Our results also hint that IFT88 may have more dynamic functions in hASC fate determination than regulating protein transport in the primary cilium.
Of note, after IFT88 or PKD1 knockdown, although hASCs lost their response to electrical field stimulation, the frequency of cilia observed on the hASCs was not decreased proportionally. There was a 50% decrease in cilia observation frequency after IFT88 siRNA knockdown; however, no significant change was observed in cilia expression with PKD1 siRNA knockdown. This is likely because IFT88 and PKD1 knockdown disrupts the function of cilia, though some fraction of cilia structures may remain (22, 54).
Of interest, after PKD1 or IFT88 siRNA knockdown of hASCs, although the electrical field–enhanced osteogenic response is completely lost, the transient increase of cytoplasmic Ca2+ concentration in response to stimulation remains intact. Our results indicated that electrical stimulation–induced transient intracellular Ca2+ increases likely do not ensure the osteogenic response in hASCs. Modulation in intracellular Ca2+ levels is generally thought to act as a second messenger, which can further cause the multiple downstream transcriptional responses (9, 45, 46). The discrepancy can be explained by 2 possible mechanisms.
One possible mechanism suggests that the primary cilium may serve as a crucial stimuli signal integrator in hASCs. Electrical field–induced cytoplasmic Ca2+ is one of the preconditions in the cilia sensation process for electrical stimulation. This argument can be supported by several similar observations in primary cilia sensation studies (35, 54). It has been found that knockdown of cilia proteins PC1 or PC2 in osteocytes did not affect cytosolic Ca2+ peaks, but completely blocked the stimulation-induced osteogenic response (54). Another report found that primary cilia function as a calcium signaling nexus, and that intracellular Ca2+ release is one of the prerequisites in primary cilia sensory activity (35). Thus, our findings imply that cilia protein knockdown disrupts the signal integrator activity of the cilia, which leads to the diminished electrical field–induced osteogenic response in hASCs, despite intracellular calcium activity.
Another possible explanation, which is supported by recent studies, is that during physical or chemical stimulations, the primary cilium can form a separated Ca2+ microdomain with a calcium concentration that is distinct from that in cytosol (36, 48). Delling et al. (36) reported that the PC1/PC2 complex can act as a ciliary calcium channel to mediate calcium concentration in primary cilia without substantially altering the global cytoplasmic calcium concentration. Elevated ciliary calcium can directly affect signaling pathways (36); thus, it is highly possible that siRNA knockdown of cilia proteins modulates cilia calcium under electrical stimulation concomitant with an altered osteogenic response, whereas no changes in cytoplasmic calcium can be detected as a result of limitation in the sensitivity of the Fluo-4 assay. To illustrate the detailed signal transduction mechanism in cilia during electrical stimulation, further detailed studies on the localized calcium change in cilia are needed but are beyond the scope of this study.
We have demonstrated that primary cilia transduce electrical field signals likely via a voltage-activated calcium channel–dependent mechanism and independent of stretch-activated channels in hASCs. This is consistent with previous evidence that after blocking voltage-activated calcium channels, electrical stimulation–induced osteogenic response is fully diminished (16–18, 45). These results contrast with studies of primary cilia activity on cells that were cultured under mechanical stimulation. It is believed that primary cilia transduce physical stimulation, at least in part, via stretch-activated channels (35, 47). Thus, our results suggest a novel mechanism through which primary cilia transduce electrical stimuli in a method that is different from how they detect mechanical signals. To better understand how primary cilia differentially transduce EFS and mechanical signals, future work should investigate primary cilia and cytoplasmic calcium oscillation in the presence of both inhibitors and cilia knockdown during EFS.
CONCLUSIONS
In this work, we have shown that structurally integrated primary cilia proteins are required for electrical field–enhanced osteogenic responses in hASCs. Furthermore, we showed that primary cilia transduce electrical field signals independent of stretch-activated calcium channels, which were believed to be involved in the transduction activity of primary cilia exposed to mechanical stimulations. These results suggest that primary cilia differentially transduce mechanical and electrical stimuli. Finally, we showed that knockdown of primary cilia–associated proteins does not diminish electrical stimulation–induced Ca2+ influx in hASCs. Taken together, our data implicate the primary cilium as a crucial calcium signaling nexus in hASCs during EFS and that this structure may be required for downstream calcium-induced cellular biochemical responses. In summary, our findings have shown that the primary cilium is an indispensable sensing and signal transduction organelle for EFS in osteoprogenitor cells.
ACKNOWLEDGMENTS
This work was supported by U.S. National Science Foundation/Chemical, Bioengineering, Environmental, and Transport Systems Grant 1133427 and National Institutes of Health, National Institute of Biomedical Imaging and Bioengineering Grant 1R03-EB008790.
Glossary
- BMP-2
bone morphogenetic protein-2
- EFS
electrical field stimulation
- hASC
human adipose-derived stem cell
- IDE
interdigitated electrode
- IFT88
intraflagellar protein-88
- ODM
osteogenic differentiation medium
- PC1
polycystin-1
- Runx2
runt-related transcription factor
- siRNA
small interfering RNA
AUTHOR CONTRIBUTIONS
S. Cai, J. C. Bodle, P. S. Mathieu, and E. G. Loboa designed the study; S. Cai, P. S. Mathieu, A. Amos, and M. Hamouda performed research; S. Cai, J. C. Bodle, S. Bernacki, G. McCarty, and E. G. Loboa analyzed data; and S. Cai, J. C. Bodle, S. Bernacki, G. McCarty, and E. G. Loboa wrote the paper.
REFERENCES
- 1.Dimitriou R., Jones E., McGonagle D., Giannoudis P. V. (2011) Bone regeneration: current concepts and future directions. BMC Med. 9, 66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saadoun S., Macdonald C., Bell B. A., Papadopoulos M. C. (2011) Dangers of bone graft substitutes: lessons from using GeneX. J. Neurol. Neurosurg. Psychiatry 82, e3 [DOI] [PubMed] [Google Scholar]
- 3.Dvir T., Timko B. P., Kohane D. S., Langer R. (2011) Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 6, 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riehl B. D., Park J. H., Kwon I. K., Lim J. Y. (2012) Mechanical stretching for tissue engineering: two-dimensional and three-dimensional constructs. Tissue Eng. Part B Rev. 18, 288–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore N. M., Lin N. J., Gallant N. D., Becker M. L. (2011) Synergistic enhancement of human bone marrow stromal cell proliferation and osteogenic differentiation on BMP-2-derived and RGD peptide concentration gradients. Acta Biomater. 7, 2091–2100 [DOI] [PubMed] [Google Scholar]
- 6.Hwang S. J., Song Y. M., Cho T. H., Kim R. Y., Lee T. H., Kim S. J., Seo Y. K., Kim I. S. (2012) The implications of the response of human mesenchymal stromal cells in three-dimensional culture to electrical stimulation for tissue regeneration. Tissue Eng. Part A 18, 432–445 [DOI] [PubMed] [Google Scholar]
- 7.Zhang J., Neoh K. G., Hu X., Kang E. T., Wang W. (2013) Combined effects of direct current stimulation and immobilized BMP-2 for enhancement of osteogenesis. Biotechnol. Bioeng. 110, 1466–1475 [DOI] [PubMed] [Google Scholar]
- 8.Hu W. W., Hsu Y. T., Cheng Y. C., Li C., Ruaan R. C., Chien C. C., Chung C. A., Tsao C. W. (2014) Electrical stimulation to promote osteogenesis using conductive polypyrrole films. Mater. Sci. Eng. C 37, 28–36 [DOI] [PubMed] [Google Scholar]
- 9.Kim I. S., Song J. K., Song Y. M., Cho T. H., Lee T. H., Lim S. S., Kim S. J., Hwang S. J. (2009) Novel effect of biphasic electric current on in vitro osteogenesis and cytokine production in human mesenchymal stromal cells. Tissue Eng. Part A 15, 2411–2422 [DOI] [PubMed] [Google Scholar]
- 10.Balint R., Cassidy N. J., Cartmell S. H. (2013) Electrical stimulation: a novel tool for tissue engineering. Tissue Eng. Part B Rev. 19, 48–57 [DOI] [PubMed] [Google Scholar]
- 11.Hronik-Tupaj M., Kaplan D. L. (2012) A review of the responses of two- and three-dimensional engineered tissues to electric fields. Tissue Eng. Part B Rev. 18, 167–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCullen S. D., McQuilling J. P., Grossfeld R. M., Lubischer J. L., Clarke L. I., Loboa E. G. (2010) Application of low-frequency alternating current electric fields via interdigitated electrodes: effects on cellular viability, cytoplasmic calcium, and osteogenic differentiation of human adipose-derived stem cells. Tissue Eng. Part C Methods 16, 1377–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun S., Liu Y., Lipsky S., Cho M. (2007) Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J. 21, 1472–1480 [DOI] [PubMed] [Google Scholar]
- 14.Brighton C. T., Wang W., Seldes R., Zhang G., Pollack S. R. (2001) Signal transduction in electrically stimulated bone cells. J. Bone Joint Surg. Am. 83-A, 1514–1523 [DOI] [PubMed] [Google Scholar]
- 15.Banks T. A., Luckman P. S., Frith J. E., Cooper-White J. J. (2015) Effects of electric fields on human mesenchymal stem cell behaviour and morphology using a novel multichannel device. Integr. Biol. 7, 693–712 [DOI] [PubMed] [Google Scholar]
- 16.Zhang J., Li M., Kang E. T., Neoh K. G. (2016) Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater. 32, 46–56 [DOI] [PubMed] [Google Scholar]
- 17.Zayzafoon M. (2006) Calcium/calmodulin signaling controls osteoblast growth and differentiation. J. Cell. Biochem. 97, 56–70 [DOI] [PubMed] [Google Scholar]
- 18.Bergh J. J., Xu Y., Farach-Carson M. C. (2004) Osteoprotegerin expression and secretion are regulated by calcium influx through the L-type voltage-sensitive calcium channel. Endocrinology 145, 426–436 [DOI] [PubMed] [Google Scholar]
- 19.Brownell W. E., Qian F., Anvari B. (2010) Cell membrane tethers generate mechanical force in response to electrical stimulation. Biophys. J. 99, 845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart F. X. (2010) Cytoskeletal forces produced by extremely low-frequency electric fields acting on extracellular glycoproteins. Bioelectromagnetics 31, 77–84 [DOI] [PubMed] [Google Scholar]
- 21.Basten S. G., Giles R. H. (2013) Functional aspects of primary cilia in signaling, cell cycle and tumorigenesis. Cilia 2, 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodle J. C., Rubenstein C. D., Phillips M. E., Bernacki S. H., Qi J., Banes A. J., Loboa E. G. (2013) Primary cilia: the chemical antenna regulating human adipose-derived stem cell osteogenesis. PLoS One 8, e62554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hierck B. P., Van der Heiden K., Alkemade F. E., Van de Pas S., Van Thienen J. V., Groenendijk B. C., Bax W. H., Van der Laarse A., Deruiter M. C., Horrevoets A. J., Poelmann R. E. (2008) Primary cilia sensitize endothelial cells for fluid shear stress. Dev. Dyn. 237, 725–735 [DOI] [PubMed] [Google Scholar]
- 24.Hoey D. A., Kelly D. J., Jacobs C. R. (2011) A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem. Biophys. Res. Commun. 412, 182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bimonte S., De Angelis A., Quagliata L., Giusti F., Tammaro R., Dallai R., Ascenzi M. G., Diez-Roux G., Franco B. (2011) Ofd1 is required in limb bud patterning and endochondral bone development. Dev. Biol. 349, 179–191 [DOI] [PubMed] [Google Scholar]
- 26.Hoerner C., Stearns T. (2013) Remembrance of cilia past. Cell 155, 271–273 [DOI] [PubMed] [Google Scholar]
- 27.Temiyasathit S., Jacobs C. R. (2010) Osteocyte primary cilium and its role in bone mechanotransduction. Ann. N. Y. Acad. Sci. 1192, 422–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tummala P., Arnsdorf E. J., Jacobs C. R. (2010) The role of primary cilia in mesenchymal stem cell differentiation: a pivotal switch in guiding lineage commitment. Cell. Mol. Bioeng. 3, 207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao Z. S., Quarles L. D. (2010) Role of the polycytin-primary cilia complex in bone development and mechanosensing. Ann. N. Y. Acad. Sci. 1192, 410–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh E. C., Katsanis N. (2012) Cilia in vertebrate development and disease. Development 139, 443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goetz S. C., Anderson K. V. (2010) The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 11, 331–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bijlsma M. F., Damhofer H., Roelink H. (2012) Hedgehog-stimulated chemotaxis is mediated by smoothened located outside the primary cilium. Sci. Signal. 5, ra60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen A. M., Jacobs C. R. (2013) Emerging role of primary cilia as mechanosensors in osteocytes. Bone 54, 196–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan J. L., Zhou J., Ma H. P., Ma X. N., Gao Y. H., Shi W. G., Fang Q. Q., Ren Q., Xian C. J., Chen K. M. (2015) Pulsed electromagnetic fields promote osteoblast mineralization and maturation needing the existence of primary cilia. Mol. Cell. Endocrinol. 404, 132–140 [DOI] [PubMed] [Google Scholar]
- 35.Lee K. L., Guevarra M. D., Nguyen A. M., Chua M. C., Wang Y., Jacobs C. R. (2015) The primary cilium functions as a mechanical and calcium signaling nexus. Cilia 4, 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delling M., DeCaen P. G., Doerner J. F., Febvay S., Clapham D. E. (2013) Primary cilia are specialized calcium signalling organelles. Nature 504, 311–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bodle J. C., Hanson A. D., Loboa E. G. (2011) Adipose-derived stem cells in functional bone tissue engineering: lessons from bone mechanobiology. Tissue Eng. Part B Rev. 17, 195–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim J. H., McCullen S. D., Piedrahita J. A., Loboa E. G., Olby N. J. (2013) Alternating current electric fields of varying frequencies: effects on proliferation and differentiation of porcine neural progenitor cells. Cell. Reprogram. 15, 405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernacki S. H., Wall M. E., Loboa E. G. (2008) Isolation of human mesenchymal stem cells from bone and adipose tissue. Methods Cell Biol. 86, 257–278 [DOI] [PubMed] [Google Scholar]
- 40.Bodle J. C., Teeter S. D., Hluck B. H., Hardin J. W., Bernacki S. H., Loboa E. G. (2014) Age-related effects on the potency of human adipose-derived stem cells: creation and evaluation of superlots and implications for musculoskeletal tissue engineering applications. Tissue Eng. Part C Methods 20, 972–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J. (2009) Polycystins and primary cilia: primers for cell cycle progression. Annu. Rev. Physiol. 71, 83–113 [DOI] [PubMed] [Google Scholar]
- 42.Pazour G. J., Dickert B. L., Vucica Y., Seeley E. S., Rosenbaum J. L., Witman G. B., Cole D. G. (2000) Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozkucur N., Monsees T. K., Perike S., Do H. Q., Funk R. H. (2009) Local calcium elevation and cell elongation initiate guided motility in electrically stimulated osteoblast-like cells. PLoS One 4, e6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khatib L., Golan D. E., Cho M. (2004) Physiologic electrical stimulation provokes intracellular calcium increase mediated by phospholipase C activation in human osteoblasts. FASEB J. 18, 1903–1905 [DOI] [PubMed] [Google Scholar]
- 45.Xu J., Wang W., Clark C. C., Brighton C. T. (2009) Signal transduction in electrically stimulated articular chondrocytes involves translocation of extracellular calcium through voltage-gated channels. Osteoarthritis Cartilage 17, 397–405 [DOI] [PubMed] [Google Scholar]
- 46.Li J. K., Lin J. C., Liu H. C., Sun J. S., Ruaan R. C., Shih C., Chang W. H. (2006) Comparison of ultrasound and electromagnetic field effects on osteoblast growth. Ultrasound Med. Biol. 32, 769–775 [DOI] [PubMed] [Google Scholar]
- 47.Sharif-Naeini R., Folgering J. H., Bichet D., Duprat F., Lauritzen I., Arhatte M., Jodar M., Dedman A., Chatelain F. C., Schulte U., Retailleau K., Loufrani L., Patel A., Sachs F., Delmas P., Peters D. J., Honoré E. (2009) Polycystin-1 and -2 dosage regulates pressure sensing. Cell 139, 587–596 [DOI] [PubMed] [Google Scholar]
- 48.Jin X., Mohieldin A. M., Muntean B. S., Green J. A., Shah J. V., Mykytyn K., Nauli S. M. (2014) Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell. Mol. Life Sci. 71, 2165–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleene S. J., Van Houten J. L. (2014) Electrical signaling in motile and primary cilia. Bioscience 64, 1092–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nauli S. M., Alenghat F. J., Luo Y., Williams E., Vassilev P., Li X., Elia A. E., Lu W., Brown E. M., Quinn S. J., Ingber D. E., Zhou J. (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129–137 [DOI] [PubMed] [Google Scholar]
- 51.Delmas P., Nomura H., Li X., Lakkis M., Luo Y., Segal Y., Fernández-Fernández J. M., Harris P., Frischauf A. M., Brown D. A., Zhou J. (2002) Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. J. Biol. Chem. 277, 11276–11283 [DOI] [PubMed] [Google Scholar]
- 52.Wann A. K., Knight M. M. (2012) Primary cilia elongation in response to interleukin-1 mediates the inflammatory response. Cell. Mol. Life Sci. 69, 2967–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGlashan S. R., Knight M. M., Chowdhury T. T., Joshi P., Jensen C. G., Kennedy S., Poole C. A. (2010) Mechanical loading modulates chondrocyte primary cilia incidence and length. Cell Biol. Int. 34, 441–446 [DOI] [PubMed] [Google Scholar]
- 54.Malone A. M., Anderson C. T., Tummala P., Kwon R. Y., Johnston T. R., Stearns T., Jacobs C. R. (2007) Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc. Natl. Acad. Sci. USA 104, 13325–13330 [DOI] [PMC free article] [PubMed] [Google Scholar]