Abstract
Background
While poor adherence to hormonal therapies such as aromatase inhibitors (AIs) is widely documented, less is known about whether health beliefs predict treatment non-adherence. This study aimed to evaluate the relationship between health beliefs (perceived susceptibility to breast cancer, perceived benefits of AI treatment, perceived barriers to AI treatment) and adherence to AIs.
Methods
Postmenopausal women with early stage estrogen receptor positive breast cancer who were currently on an AI completed the three-factor Health Beliefs and Medication Adherence in Breast Cancer (HBMABC) scale and questionnaires about their demographics and symptoms. Adherence data (treatment gaps and premature discontinuation) were abstracted from participants’ medical charts. Logistic regression analyses were conducted to evaluate the relationship between health beliefs and adherence.
Results
Among 437 participants, 93 (21.3%) were non-adherent. Those who perceived greater barriers to their AI treatment were more likely to display AI non-adherence behaviors by the end of their treatment period than those who reported fewer barriers to AI therapy (adjusted odds ratio 1.71, 95% confidence interval: 1.03 – 2.86, p = 0.04). In contrast, perceived susceptibility to cancer recurrence and perceived benefits of AIs did not predict AI adherence. Minorities had lower perceived susceptibility to breast cancer recurrence and higher perceived barriers to AI treatment (p < 0.05 for both).
Conclusions
Greater perceived barriers predicted non-adherence to AIs. Interventions addressing women’s negative beliefs about the challenges of AI treatment are needed to help optimize adherence in breast cancer survivors.
Keywords: aromatase inhibitors, arthralgia, medication non-adherence, patient compliance, psychosocial factors
Condensed abstract
Aromatase inhibitors (AIs) are a life-saving treatment for post-menopausal breast cancer survivors, yet non-adherence is a prevalent problem. Results of this study demonstrate that patients’ health beliefs, a potentially modifiable psychological factor, predict AI adherence over the course of five years of treatment.
Introduction
For many women with breast cancer, treatment does not end with chemotherapy, radiation, or surgery. After completing their primary treatments, survivors of hormone dependent breast cancer are often prescribed oral adjuvant hormonal therapy, including selective estrogen receptor modulators (e.g., tamoxifen, raloxifene) or aromatase inhibitors (AIs). For post-menopausal women, AIs are considered the front-line medication for preventing breast cancer recurrence [1, 2].
Despite the efficacy of AIs, many women do not fully adhere to their medication regimen and even discontinue prematurely. A systematic review of adherence rates to endocrine therapy found that 9–50% of AI patients were non-adherent [3]. The consequences of non-adherence can be devastating: Stopping AI treatment prematurely, as well as taking breaks from the treatment, have each been found to be associated with higher risk of cancer recurrence and mortality [4].
Risk factors for AI non-adherence that have been investigated include demographic, cancer, and symptom variables, including race, age, education level, income, cancer stage, joint pain, etc. [5–9, 3]. Findings from this research enhance our understanding of which patients may be most likely to non-adhere to AIs, but since many of these factors are not modifiable (such as age), or at least cannot be changed by the health care system (such as income), it is important to identify factors on which clinicians can intervene.
One potentially modifiable factor that may play a role in predicting adherence to AIs is health beliefs. Three major components of the health belief model include perceived susceptibility, perceived benefits, and perceived barriers [10]. Each of these reflects key elements of the mental calculations an individual makes before taking action to avoid a negative health outcome. Some or all of these constructs have been shown to be associated with adherence behaviors among breast cancer survivors [11, 12]. For example, breast cancer survivors who perceive tamoxifen to have greater benefits than risks are more likely to adhere to their treatment than those who perceive the opposite [12]. The relationship between health beliefs and AI adherence, however, is currently unknown, and given that tamoxifen and AIs have different side effect profiles, it is reasonable to speculate that survivors may hold different beliefs about AIs [13].
In this study, we hypothesized that breast cancer survivors with greater perceived benefits of AIs, greater perceived susceptibility to breast cancer recurrence, and lower perceived barriers to taking AIs would be more likely to adhere to AIs. As a secondary aim, we also evaluated whether health beliefs differed by key demographic and medical variables.
Participants and Methods
Participants were drawn from the Wellness after Breast Cancer (WABC), an ongoing cohort study of postmenopausal breast cancer survivors who had been prescribed AIs and were consented between March 2008 and July 2009 [14]. We chose this time frame to ensure all participants in this study had an opportunity to complete the five years of prescribed AI therapy. Inclusion criteria for WABC were as follows: (1) female sex, (2) age 18 or older, (3) postmenopausal status, (4) stage I-III hormone receptor positive breast cancer, (5) use of a third-generation AI, (6) completion of all chemotherapy and/or radiotherapy at least one month prior to survey date, and (7) ability to provide informed consent in English. Recruitment took place at a breast cancer clinic at an academic teaching hospital. The study was approved by the Institutional Review Board of the University of Pennsylvania.
Analysis focused on participants who were on an AI at the time of the survey (n = 437). Adherence outcome data and most clinical variables were gathered from participants’ medical charts. Other variables came from the WABC baseline survey that participants completed.
Measures
Adherence
Adherence information was abstracted from participants’ medical charts. Oncology progress notes and telephone calls that dated from the day the participant completed the WABC survey to the end-date of their prescribed AI treatment (typically five years) were searched for medication-related events. Treatment interruptions (defined as any time off an AI during the prescribed treatment) and premature discontinuation (defined as stopping the AI entirely before the prescribed treatment end-date) were both considered forms of non-adherence and grouped together into one binary outcome variable (“adherent” versus “non-adherent”). Discontinuations after breast cancer recurrence or metastasis were not considered non-adherence events.
Premature discontinuation and treatment interruptions were abstracted from medical records. Raters were trained to do the abstractions on a different data set until they achieved strong inter-rater reliability with an experienced rater (kappa statistic ≥ 0.70 for each category). Adherent behavior was coded as 0 and non-adherence was coded as 1. Subsequently, 12.5% of cases were independently re-abstracted by one of two additional raters; interrater reliability was good to excellent (0.78 – 1.00) for both categories of non-adherence.
Health Beliefs
At study entry, all participants completed the Health Beliefs and Medication Adherence in Breast Cancer (HBMABC) scale, a measure adapted from the Champion Health Belief Model Scales (CHBMS) for Mammography Screening (with permission from the author). Each item consists of a Likert-type scale ranging from 1 (strongly disagree) to 5 (strongly agree). To determine the factor structure of the scale, we randomly split the sample into two and conducted an exploratory factor analysis (promax rotation) using the first subsample. We followed this with a confirmatory factor analysis to determine whether the solution from the exploratory analysis fit the data for the second subsample.
For the exploratory factor analysis, the pattern of factor loadings as well as the scree plot of eigen values indicated a three-factor solution. Three items were deleted due to lack of salience (factor loading below 0.4) or because when they were removed, the internal consistency of the subscales improved. Factor loadings and communalities are displayed in Table 1. The three factors identified mapped onto the theoretical constructs that informed the development of the HBMABC: perceived susceptibility, perceived benefits, and perceived barriers. In the context of AIs, susceptibility reflects a survivor’s sense of vulnerability to breast cancer recurrence, perceived benefits represent survivors’ beliefs about the efficacy of AIs, and perceived barriers captures the difficulties survivors feel they face in adhering to their AI treatment.
Table 1.
Health Beliefs Scale Items and Scale Properties
| Item | Factor Loadinga | Communality | Internal Consistencyb |
|---|---|---|---|
| Perceived Susceptibility | 0.87 | ||
| 1. It is likely that my breast cancer will come back | 0.93 | 0.89 | |
| 2. The chances of my breast cancer coming back in the next few years are great |
0.89 | 0.82 | |
| 3. I feel that my breast cancer will come back sometime during my life |
0.79 | 0.61 | |
| Perceived Benefits | 0.77 | ||
| 1. Taking my AI exactly as prescribed will decrease the chance of my breast cancer coming back. |
0.56 | 0.37 | |
| 2. Taking my AI is the best way to avoid my breast cancer coming |
0.87 | 0.75 | |
| 3. Taking my AI will decrease my chances of dying from breast cancer. |
0.95 | 0.89 | |
| Perceived Barriers | 0.83 | ||
| 1. I am afraid to take my AI because I don’t understand how it works. |
0.71 | 0.62 | |
| 2. I don’t know how to go about getting my AI prescription filled on a regular basis. |
0.80 | 0.66 | |
| 3. Taking my AI causes side effects that are embarrassing |
0.75 | 0.53 | |
| 4. I have to take my AI for too many years | 0.74 | 0.56 | |
| 5. Taking my AI is difficult because it causes pain. | 0.81 | 0.62 | |
| 6. Taking my AI is difficult because it costs too much money. |
0.65 | 0.45 | |
| 7. I have difficulty remembering to take my AI. | 0.80 | 0.64 | |
| 8. I am too old to need to take an AI. | 0.74 | 0.58 |
These loadings are based on the exploratory factor anaylsis.
The internal consistency was calculated using the entire sample.
For the confirmatory factor analysis, we examined multiple indices to determine the goodness of fit of the three-factor solution for the second subsample: the X2/df ratio, the Comparative Fit Index (CFI), the Tucker-Lewis Index (TLI), and the root mean square error of approximation (RMSEA). The results indicated that the factor structure had a reasonable fit to the data (X2/df = 2.13; CFI = 0.98; TLI = .98; RMSEA = .07 [90% confidence interval 0.06 – 0.9]) [15]. Cronbach’s alphas for the three subscales (using the entire sample) were 0.87 (Perceived Susceptibility), 0.77 (Perceived Benefits), and 0.83 (Perceived Barriers). The full range of scores was represented on both the Perceived Susceptibility and Perceived Benefits subscales (both 3 to 15) and on the Perceived Barriers subscale (8 to 40). Two of the three subscales were highly skewed, therefore all subscales were dichotomized into low and high categories using a median split. A score of eight or below (out of 15) on the Perceived Susceptibility subscale indicates low perceived susceptibility to cancer recurrence; a score of 11 or below (out of 15) on the Perceived Benefits subscale indicates low perceived benefits to AIs; and a score of 12 or below (out of 40) on the Perceived Barriers subscale indicates low perceived barriers to AI treatment.
Covariates
Race, age, education, marital status, joint pain, clinical comorbidities, and date of last menstrual period were collected via self-report. Joint pain was measured with the worst pain item of the pain intensity subscale of the Brief Pain Inventory. This single item has been found to predict premature discontinuation among breast cancer survivors on AIs [7]. Clinical variables, including cancer stage and prior use of tamoxifen, were abstracted from medical charts.
Statistical Analyses
Univariate analyses using logistic regression were conducted to assess the association between each variable and non-adherence. Univariately significant variables (p < 0.10) were assessed in multivariable logistic regression analyses. Chi-square tests were conducted to determine the relationship between health beliefs, and demographic, medical, and psychosocial variables. All tests were two sided with p < 0.05 indicating statistical significance. Twenty-four participants were lost to follow-up and we were therefore unable to obtain information about their adherence behaviors. Data for these individuals was imputed using multiple imputation. SPSS v22 (IBM, Armonk, NY) was used for all data analysis.
Power analysis
A power analysis using G*Power [16] demonstrated that with a sample of 437 participants, we had 80% power to detect an odds ratio of 1.91 or greater, assuming 30% of the sample was non-adherent and that each health beliefs subscale was moderately associated with covariates in the model. The non-adherence rate of 30% was selected based on rates seen in the literature [3].
Results
Participant Characteristics
Of the 437 participants, 82.6% were White, 14.9% were African American, 1.6% were Asian and 0.9% indicated they were “Other”. The sample was highly educated: Among participants, 35.1% had a graduate or professional degree, 43.6% attended at least some college, and 21.3% had a high school diploma or less. Most of the participants were married or living with a partner (62.6%) and more than half of the sample had been in a postmenopausal state for over 10 years. See Table 2.
Table 2.
Logistic Regression Analysis of Baseline Characteristics and Non-Adherence
| Characteristic | N (%) | % Non-Adherent* | P |
|---|---|---|---|
| Total | 437 (100%) | 21.3% | |
| Age | 0.34 | ||
| >65 | 138 (31.6%) | 23.6% | |
| 55–65 | 201 (46.0%) | 18.1% | |
| <55 | 98 (22.4%) | 24.4% | |
| Race | 0.88 | ||
| White | 360 (82.6%) | 21.3% | |
| Non-White | 76 (17.4%) | 20.5% | |
| Living Status | 0.61 | ||
| Living with partner | 266 (62.6%) | 21.6% | |
| Living alone | 159 (37.4%) | 19.5% | |
| Education | 0.10 | ||
| Graduate school | 153 (35.1%) | 18.3% | |
| Four year college | 105 (24.1%) | 29.1% | |
| Some college | 85 (19.5%) | 16.0% | |
| HS or less | 93 (21.3%) | 21.7% | |
| Prior use of tamoxifen | 0.22 | ||
| No | 290 (66.4%) | 23.1% | |
| Yes | 147 (33.6%) | 17.7% | |
| Years since last menstruation | 0.89 | ||
| > 10 | 242 (56.1%) | 20.7% | |
| 5–10 | 108 (26.1%) | 20.7% | |
| < 5 | 81 (18.8%) | 23.0% | |
| Number of comorbidities | 0.50 | ||
| None | 68 (15.6%) | 20.0% | |
| One | 133 (30.4%) | 25.9% | |
| Two or more | 236 (54.0%) | 19.1% | |
| Years since start of AI | <0.001 | ||
| <1 | 141 (32.3%) | 37.8% | |
| 1–3 | 152 (34.8%) | 19.4% | |
| >3 | 144 (33.0%) | 6.9% | |
| Brief Pain Inventory – Worst Joint Pain | 0.04 | ||
| 0–3 | 277 (64.0%) | 18.1% | |
| ≥ 4 | 156 (36.0%) | 26.8% |
Based on imputed values for missing adherence data.
Predictors of Non-Adherence
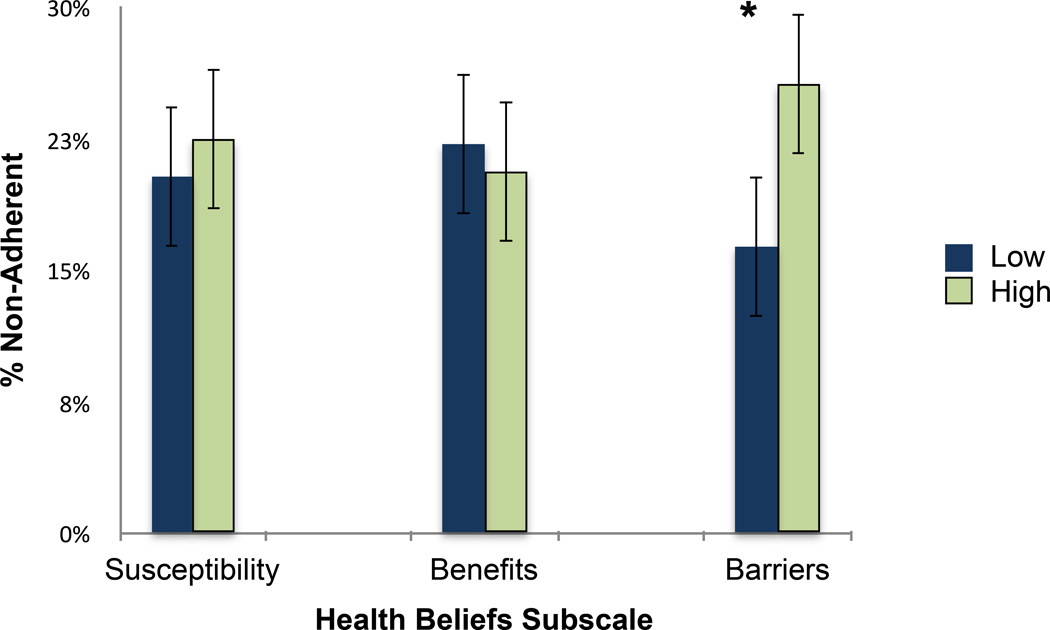
Using multiple imputation, we estimated that a total of 93 (21.3%) participants exhibited some form of non-adherence after completing the survey. Of the three health-belief subscales, perceived barriers was the only subscale that was significantly associated with non-adherence. Those who reported having more barriers to AI treatment were more likely to later non-adhere to AIs (25.7% vs. 16.3%, p = .02). See Figure 1. In univariate analyses, the following covariates were associated with premature discontinuation: joint pain, and years on an AI at the time of the survey (see Table 2). After we included joint pain and time on an AI in the model, perceived barriers remained a statistically significant predictor of non-adherence (adjusted odds ratio 1.71, 95% confidence interval 1.03 – 2.86, p = 0.04; see Table 3), while joint pain was no longer significant (adjusted odds ratio 1.48, 95% confidence interval .89 – 2.46, p = .14).
Figure 1.
Non-adherence by health beliefs subscale.
* p < 0.05
Bars represent 95% confidence intervals.
Table 3.
Analyses of Health Beliefs and Non-Adherence
| Univariate Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Characteristic | Odds Ratio | 95% CI | P | Odds Ratio | 95% CI | P |
| Perceived Barriers | ||||||
| Low | 1.00 | - | - | 1.00 | - | - |
| High | 1.78 | (1.10 – 2.88) | 0.02 | 1.71 | 1.03 – 2.86 | 0.04 |
| Years since start of AI | ||||||
| <1 | 1.00 | - | - | 1.00 | - | - |
| 1–3 | 0.40 | (0.23 – 0.68) | <0.01 | 0.39 | 0.22 – 0.68 | 0.001 |
| > 3 | 0.12 | (0.06 – 0.26) | <0.001 | 0.13 | 0.06 – 0.26 | <0.001 |
| BPI – Worst Joint Pain | ||||||
| 0–3 | 1.00 | - | - | 1.00 | - | - |
| ≥ 4 | 1.65 | (1.03 – 2.67) | 0.04 | 1.48 | 0.89 – 2.46 | 0.14 |
BPI = Brief Pain Inventory; CI = Confidence Interval
The barriers subscale included items about financial barriers, side-effects, difficulty getting refills, and other topics. Exploratory analyses were conducted to examine if any particular items on the perceived barriers subscale drove the relationship between perceived barriers and adherence. When each item was examined independently, with joint pain severity and length of time on AI included in the model, Item 4, “I have to take my AI for too many years” (OR = 1.25, 95% CI: 1.03 – 1.52, p = 0.03) significantly predicted non-adherence, as did Item 5,“Taking my AI is difficult because it causes pain” (OR = 1.30, 95% CI: 1.07 – 1.57, p = 0.009). Survivors who perceived that their pain made taking AIs difficult, or that AI treatment lasted too long, were more likely to show some form of non-adherence. To determine the degree to which Item 5 and the joint pain item were measuring overlapping constructs, we assessed their correlation and found it to be modest (r = 0.32, p < 0.001).
Correlates of Health Beliefs
Women who had lower perceived susceptibility to cancer recurrence were more likely to be non-White (p = .01). Survivors who perceived less benefits to AI therapy were more likely to be above 55 years old (p = .003) and have had more years in menopause (p = .009). Participants with higher perceived barriers to AI treatment were more likely to be non-White (p = .004) and have higher joint pain levels (p = .004). See Table 4.
Table 4.
Relationship Between Health Belief Subscales and Demographic and Medical Variables
| Health Beliefs Subscales | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Susceptibility Subscale | Benefits Subscale | Barriers Subscale | |||||||
| % L | % H | p | % L | % H | p | % L | % H | p | |
| Age | .51 | .003 | .63 | ||||||
| >65 | 55.0 | 45.0 | 47.7 | 52.3 | 48.1 | 51.9 | |||
| 55–65 | 52.1 | 47.9 | 43.9 | 56.1 | 45.2 | 54.8 | |||
| <55 | 59.4 | 40.6 | 26.5 | 73.5 | 51.0 | 49.0 | |||
| Race | .01 | .24 | .004 | ||||||
| White | 52.2 | 47.8 | 39.8 | 60.2 | 50.7 | 49.3 | |||
| Non-White | 68.1 | 31.9 | 47.9 | 52.1 | 31.9 | 68.1 | |||
| Education | .45 | .17 | .77 | ||||||
| HS or less | 49.4 | 50.6 | 37.1 | 62.9 | 44.3 | 55.7 | |||
| Some college | 58.8 | 41.3 | 51.8 | 48.2 | 45.2 | 54.8 | |||
| Four-year college | 59.4 | 40.6 | 40.2 | 59.8 | 47.6 | 52.4 | |||
| Graduate school | 52.8 | 47.2 | 38.4 | 61.6 | 50.7 | 49.3 | |||
| No of comorbidities | .05 | .89 | .94 | ||||||
| None | 41.5 | 58.5 | 39.7 | 60.3 | 47.8 | 52.2 | |||
| One | 54.4 | 45.6 | 42.7 | 57.3 | 46.2 | 53.8 | |||
| Two or more | 58.7 | 41.3 | 40.5 | 59.5 | 48.1 | 51.9 | |||
| Prior use of tamoxifen | .24 | .26 | .06 | ||||||
| No | 56.8 | 43.2 | 43.0 | 57.0 | 44.2 | 55.8 | |||
| Yes | 50.7 | 49.3 | 37.4 | 62.6 | 53.7 | 46.3 | |||
| Years since LMP | .71 | .009 | .89 | ||||||
| > 10 | 52.9 | 47.1 | 47.2 | 52.8 | 46.2 | 53.8 | |||
| 5–10 | 54.9 | 45.1 | 32.4 | 67.6 | 49.1 | 50.9 | |||
| < 5 | 58.2 | 41.8 | 32.5 | 67.5 | 47.5 | 52.5 | |||
| Brief Pain Inventory – Worst Joint Pain |
.31 | .42 | .004 | ||||||
| 0–3 | 56.6 | 43.4 | 39.3 | 60.7 | 53.1 | 46.9 | |||
| ≥ 4 | 51.4 | 48.6 | 43.3 | 56.7 | 38.4 | 61.6 | |||
%L = percent of participants scoring above the median on a particular health beliefs subscale.
%H = percent of participants scoring below the median on a particular health beliefs subscale.
Conclusion
To achieve optimal clinical outcomes for breast cancer survivors, adherence to AI therapy is essential [17]. In this prospective cohort study, we found that participants who perceived greater barriers to AI treatment, as measured by the HBMABC, were more likely to later take breaks from their AI or stop taking it all together. Conversely, perceived susceptibility to breast cancer and perceived benefits of AIs were not predictive of adherence. Importantly, health beliefs were found to differ by socio-demographic characteristics with minority women perceiving less risk of breast cancer recurrence as well as greater perceived barriers to AI treatment.
Our findings are particularly important for several reasons. This study is one of the first to identify a potentially modifiable, psychological predictor of AI non-adherence; addressing women’s perceptions of perceived barriers may help improve their adherence to AIs. These findings also corroborate earlier work that shows that perceived benefits may not be worth targeting to improve AI adherence. An intervention aimed at increasing patients’ knowledge around the importance and benefits of AIs had no significant impact on adherence rates [18]. While it could be that the particular intervention that was implemented was not effective, it appears that survivors who are no longer willing to take AIs are not basing their decision on whether they think AIs are effective; our study showed that perceived barriers rather than perceived benefits predict non-adherence. This is in contrast to tamoxifen adherence, for which beliefs about benefits have been shown to be associated with adherence in an observational study [12]. These findings suggest that the psychological determinants of adherence to tamoxifen and aromatase inhibitors may be different and underscore the importance of studying them as separate outcomes.
The role of pain perception emerged as an important element in our findings. Prior literature has shown that joint pain is a predictor of adherence to AIs [7]. However, in our analysis, joint pain was no longer predictive of adherence when pain beliefs were included in the model. Additionally, pain beliefs and pain intensity were only moderately correlated, suggesting beliefs participants held about pain as a barrier to AI treatment were not completely tied to the degree of pain the participant experienced. These findings imply that cognitions about the experience of pain, as opposed to pain itself, drives adherence behavior. Further research is needed to understand women’s psychological reactions to AI-associated joint pain. Prior work among other patient populations has shown that fear of pain, for example, is a powerful predictor of behavior [19–21]; women who experience greater distress due to pain anticipation may consider AIs more difficult to take. There may be other cognitions triggered by joint pain that are unique to the AI experience that lead to non-adherence. Understanding these reactions is important for the development of intervention content.
Patients’ perceptions that AI treatment is too long were also a barrier to adherence. This perception may reflect ambivalence about whether the benefits of AIs are worth the many years of side effects. Motivational interviewing (MI), which can be delivered as a brief, short-term intervention, has been found to be effective at overcoming patients’ ambivalence about making changes to their daily behaviors [22] and may be effective at promoting AI adherence. In addition, as interventions have shown to help with AI-related joint pain [23, 24], health care providers should work with patients to detect the side effects of AIs early in treatment and engage patients in management of their symptoms.
To our knowledge, the HBMABC is the first instrument to evaluate health beliefs related to AI therapy. Our psychometric analyses support the original factor structure of the health belief model. In addition, we found that health beliefs are associated with a number of demographic and medical variables. Minorities and those with higher levels of joint pain tended to perceive greater barriers to their AI treatment. Although non-Whites are no more likely to discontinue AI treatment than Whites, previous work has shown that non-Whites are less likely to initiate AI treatment [25, 26]. A number of factors have been examined to explain these differences, including lower rates of insurance coverage and a higher presence of comorbidities among minorities, but none have been found to fully explain racial disparities in AI initiation [26]. Health beliefs may help shed light on what is driving poorer rates of AI initiation among minorities as compared to Whites and should be explored in future research.
There are several limitations in this study. Our measurement of adherence is based on abstracting information from notes in participants’ medical records. This process relies on the reports of both participants and their physicians, which may not always be accurate due to recall and response biases. Additionally, due to power considerations, we combined premature discontinuation and treatment interruptions into one outcome, though it is possible that each type of non-adherence is caused by a different psychological process. This is a topic area that requires further research.
There are also a number of limitations due to the timing of the health beliefs assessments. All women included in this study had been on an AI for at least one month when they consented to the study. Therefore, we were unable to study how health beliefs predict AI initiation and non-adherence within the first month of AI usage. Also, women were consented to the study at different points in their AI treatment. Thus, women who discontinued the AI shortly after initiating treatment were underrepresented in our sample given that they had to be on an AI at the survey date to be included in our analyses. It is possible that for these women, the psychological determinants of non-adherence were different than those who discontinued later in treatment. It is also unclear whether participants’ prior adherence behaviors may have impacted their health beliefs, thereby complicating our ability to interpret the direction of the relationship between health beliefs and adherence. A longitudinal study examining health beliefs at the beginning of treatment and at multiple time points over the course of treatment is needed to assess this. Another limitation is that we controlled for joint pain severity, but not other side effects. Lastly, our study was conducted in an academic cancer center limiting its generalizability.
Conclusion
We found that breast cancer survivors’ beliefs about barriers to treatment predicted their AI adherence. Beliefs about AI-related pain and treatment length appeared to be the most salient barriers. Incorporating health beliefs into research and clinical practice may help to elucidate the challenges patients face in both initiation and continued use of AIs, and help optimize clinical outcomes.
Acknowledgments
The authors would like to thank all the breast cancer survivors, physicians, nurse practitioners, and staff for their support. They would like to thank Carrie Stricker for the development of the Health Beliefs and Medication Adherence in Breast Cancer (HBMABC) scale, Karan Ahluwalia and Coby Basal for data abstraction, and all the research assistants who were involved in the data collection and management process.
This research was supported, in part, by grants from the National Institutes of Health/National Cancer Institute; R01 CA158243 and P30 CA008748, The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Ms. Brier was supported by a fellowship grant from the University of Pennsylvania.
Footnotes
Author contributions: Conceptualization - M. Brier, D. Chambless, J. Mao; Methodology - M. Brier, D. Chambless, R. Gross, J. Chen, J. Mao; Software – N/A; Validation - N/A; Formal analysis - M. Brier, J. Chen; Investigation - M. Brier, J. Mao; Resources - D. Chambless, J. Mao; Data curation - M. Brier; Writing – M. Brier, D. Chambless, R. Gross, J. Chen, J. Mao; Visualization - M. Brier, J. Mao; Supervision - D. Chambless, J. Mao; Project administration - M. Brier, D. Chambless, J. Mao; Funding acquisition - J. Mao
Part of this manuscript was presented as a poster-discussion at the 2015 San Antonio Breast Cancer Symposium. Moriah Brier had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest
The authors declare that they have no conflict of interest.
Moriah Brier, Dianne Chambless, Robert Gross, Jinbo Chen, and Jun Mao do not have conflicts of interest to report.
Contributor Information
Moriah J. Brier, Psychology Department, University of Pennsylvania.
Dianne L. Chambless, Psychology Department, University of Pennsylvania.
Robert Gross, University of Pennsylvania School of Medicine and Philadelphia Veterans Administration Medical Center, Philadelphia, PA, USA.
Jinbo Chen, Center for Clinical Epidemiology and Biostatistics, The University of Pennsylvania School of Medicine.
Jun J. Mao, Integrative Medicine Service, Memorial Sloan Kettering Cancer Center.
References
- 1.Boccardo F, Rubagotti A, Puntoni M, Guglielmini P, Amoroso D, Fini A, Paladini G, Mesiti M, Romeo D, Rinaldini M, Scali S, Porpiglia M, Benedetto C, Restuccia N, Buzzi F, Franchi R, Massidda B, Distante V, Amadori D, Sismondi P. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Cncology. 2005;23:5138–5147. doi: 10.1200/JCO.2005.04.120. [DOI] [PubMed] [Google Scholar]
- 2.Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Wardly A, Price KN, Goldhirsch A. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 3.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134:459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai W-Y, Fehrenbacher L, Gomez SL, Miles S, Neugut AI. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanton AL, Petrie KJ, Partridge AH. Contributors to nonadherence and nonpersistence with endocrine therapy in breast cancer survivors recruited from an online research registry. Breast Cancer Res Treat. 2014;145:525–534. doi: 10.1007/s10549-014-2961-3. [DOI] [PubMed] [Google Scholar]
- 6.Bender CM, Gentry AL, Brufsky AM, Casillo FE, Cohen SM, Dailey MM, Donovan HS, Dunbar-Jacob J, Jankowitz RC, Rosenzweig MQ, Sherwood PR, Sereika SM. Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncol Nurs Forum. 2014;41:274–285. doi: 10.1188/14.ONF.274-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chim K, Xie SX, Stricker CT, Li QS, Gross R, Farrar JT, DeMichele A, Mao JJ. Joint pain severity predicts premature discontinuation of aromatase inhibitors in breast cancer survivors. BMC Cancer. 2013;13:401–407. doi: 10.1186/1471-2407-13-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Liew JR, Christensen AJ, de Moor JS. Psychosocial factors in adjuvant hormone therapy for breast cancer: an emerging context for adherence research. J Cancer Surviv. 2014 doi: 10.1007/s11764-014-0374-2. [DOI] [PubMed] [Google Scholar]
- 9.Sawesi S, Carpenter JS, Jones J. Reasons for non-adherence to tamoxifen and aromatase inhibitors for the treatment of breast cancer: a literature review. Clin J Oncol Nurs. 2014;18:1092–1095. doi: 10.1188/14.CJON.E50-E57. [DOI] [PubMed] [Google Scholar]
- 10.Champion V, Skinner C. Health Belief Model. In: Glanz K, Rimer B, Viswanath K, editors. Heal. Behav. Heal. Educ. Theory, Res. Pract. 4th. San Francisco, CA: John Wiley & Sons, Ltd; 2008. pp. 45–65. [Google Scholar]
- 11.Sohl SJ, Moyer A. Tailored interventions to promote mammography screening: a meta-analytic review. Prev Med (Baltim) 2007;45:252–261. doi: 10.1016/j.ypmed.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2004;22:3309–3315. doi: 10.1200/JCO.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 13.Side Effects of Aromatase Inhibitors. Komen.org . [Accessed 14 Aug 2015]; http://ww5.komen.org/BreastCancer/SideEffectsofAromataseInhibitors.html. [Google Scholar]
- 14.Mao JJ, Su HI, Feng R, Donelson ML, Aplenc R, Rebbeck TR, Stanczyk F, DeMichele A. Association of functional polymorphisms in CYP19A1 with aromatase inhibitor associated arthralgia in breast cancer survivors. Breast Cancer Res. 2011;13:R8. doi: 10.1186/bcr2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browne MW, Cudeck R. Alternative ways of assessing model fit. Sociol Methods Res. 1992;21:230–258. [Google Scholar]
- 16.Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behav Res Methods, Instruments Comput. 1996;28:1–11. [Google Scholar]
- 17.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Neugut AI. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. Early. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markopoulos C, Neven P, Tanner M, Marty M, Kreienberg R, Atkins L, Franquet A, Gnant M, Neciosup S. Does patient education work in breast cancer? Final results from the global CARIATIDE study. Futur Oncol. 2015;11:205–217. doi: 10.2217/fon.14.179. [DOI] [PubMed] [Google Scholar]
- 19.Lethem J, Slade PD, Troup JDG, Bentley G. Outline of a fear-avoidance model of exaggerated pain perception. Behav Res Ther. 1983;21:401–408. doi: 10.1016/0005-7967(83)90009-8. [DOI] [PubMed] [Google Scholar]
- 20.Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 21.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52:157–168. doi: 10.1016/0304-3959(93)90127-B. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, Hemmelgarn BR. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12:no–no. doi: 10.1111/j.1467-789X.2011.00892.x. [DOI] [PubMed] [Google Scholar]
- 23.Mao JJ, Xie SX, Farrar JT, Stricker CT, Bowman MA, Bruner D, DeMichele A. A randomised trial of electro-acupuncture for arthralgia related to aromatase inhibitor use. Eur J Cancer. 2014;50:267–276. doi: 10.1016/j.ejca.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irwin ML, Cartmel B, Gross CP, Ercolano E, Li F, Yao X, Fiellin M, Capozza S, Rothbard M, Zhou Y, Harrigan M, Sanft T, Schmitz K, Neogi T, Hershman D, Ligibel J. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol. 2015;33:1104–1111. doi: 10.1200/JCO.2014.57.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheppard VB, Faul LA, Luta G, Clapp JD, Yung RL, Wang JH-Y, Kimmick G, Isaacs C, Tallarico M, Barry WT, Pitcher BN, Hudis C, Winer EP, Cohen HJ, Muss HB, Hurria A, Mandelblatt JS. Frailty and adherence to adjuvant hormonal therapy in older women with breast cancer: CALGB protocol 369901. J Clin Oncol. 2014;32:2318–2327. doi: 10.1200/JCO.2013.51.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K, Mendez J, Guth AA. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]