Abstract
BACKGROUND
Animal models of peripheral neuropathy produced by a number of manipulations are assessed for the presence of pathological pain states such as allodynia. While stimulus-induced behavioral assays are frequently used and important to examine allodynia (i.e. sensitivity to light mechanical touch; von Frey fiber test) other measures of behavior that reflect overall function are not only complementary to stimulus-induced responsive measures, but are also critical to gain a complete understanding of the effects of the pain model on quality of life, a clinically relevant aspect of pain on general function. Voluntary wheel running activity in rodent models of inflammatory and muscle pain is emerging as a reliable index of general function that extends beyond stimulus-induced behavioral assays. Clinically, reports of increased pain intensity occur at night, a period typically characterized with reduced activity during the diurnal cycle. We therefore examined in rats whether alterations in wheel running activity were more robust during the inactive phase compared to the active phase of their diurnal cycle in a widely used rodent model of chronic peripheral neuropathic pain, the sciatic nerve chronic constriction injury (CCI) model.
METHODS
In adult male Sprague Dawley rats, baseline (BL) hindpaw threshold responses to light mechanical touch were assessed using the von Frey test prior to measuring BL activity levels using freely accessible running wheels (1 hr/day for 7 sequential days) to quantify distance traveled. Running wheel activity BL values are expressed as total distance traveled (m). The overall experimental design was: following BL measures, rats underwent either sham or CCI surgery followed by repeated behavioral re-assessment of hindpaw thresholds and wheel running activity levels for up to 18 days after surgery. Specifically, separate groups of rats were assessed for wheel running activity levels (1 hr total/trial) during the onset (within first 2 hrs) of either the (1) inactive (n=8/gp) or (2) active (n = 8/gp) phase of the diurnal cycle. An additional group of CCI-treated rats (n = 8/gp) were exposed to a locked running wheel to control for the potential effects of wheel running exercise on allodynia. The 1-hr running wheel trial period was further examined at discrete 20-min intervals to identify possible pattern differences in activity during the first, middle and last portion of the 1-hr trial. The effect of neuropathy on activity levels were assessed by measuring the change from their respective BLs to distance traveled in the running wheels.
RESULTS
While wheel running distances between groups were not different at BL from rats examined during either the inactive phase of the diurnal cycle or active phase of the diurnal cycle, sciatic nerve CCI reduced running wheel activity levels compared to sham-operated controls during the inactive phase. Additionally, compared to sham controls, bilateral low threshold mechanical allodynia was observed at all time-points after surgical induction of neuropathy in rats with free-wheel and locked-wheel access. Allodynia in CCI compared to shams was replicated in rats whose running wheel activity was examined during the active phase of the diurnal cycle. Conversely, no significant reduction in wheel running activity was observed in CCI-treated rats compared to sham controls at any timepoint when activity levels were examined during the active diurnal phase. Lastly, running wheel activity patterns within the 1 hr trial period during the inactive phase of the diurnal cycle were relatively consistent throughout each 20 min phase.
CONCLUSIONS
Compared to non-neuropathic sham controls, a profound and stable reduction of running wheel activity was observed in CCI rats during the inactive phase of the diurnal cycle. A concurrent robust allodynia persisted in all rats regardless of when wheel running activity was examined or whether they ran on wheels, suggesting that acute wheel running activity does not alter chronic low intensity mechanical allodynia as measured using the von Frey fiber test. Overall, these data support that acute wheel running exercise with limited repeated exposures does not itself alter allodynia and offers a behavioral assay complementary to stimulus-induced measures of neuropathic pain.
Introduction
Chronic peripheral neuropathic pain is typically caused by a primary lesion or disease of the somatosensory system induced by diverse underlying etiologies that can persist from months to years.[1, 2] Patients with neuropathic pain frequently present with abnormal sensation (i.e. hypoesthesia) or hypersensitivity of skin areas innervated by damaged peripheral nerves. The quality of pain often accompanying these abnormalities is commonly described as paroxysmal shooting pain and/or crawling or tingling sensations that can be spontaneous, ongoing or movement-evoked.[1, 3] Stimulus-induced peripheral neuropathic pain includes allodynia (pain in response to normally non-painful stimuli) or hyperalgesia to mildly noxious stimuli.[4] Clinical stimulus-evoked allodynia (light moving stimuli), referred to as mechanical dynamic allodynia, is commonly seen under peripheral neuropathic conditions,[5] and associated preclinical animal models of peripheral neuropathy are frequently assessed for the presence of mechanical dynamic allodynia.[6]
While up to 20% of patients with neuropathic pain have indicated stimulus-evoked mechanical dynamic allodynia,[7] patients with neuropathic pain also experience substantial quality of life deficits such as sleep disturbances, reduced mobility and disrupted daily activities.[8–11] Additionally, several reports reveal that neuropathic pain worsens at specific times throughout the day.[12–14] A more recent study in patients with painful diabetic neuropathy or post-herpetic neuralgia found individuals report greater pain intensity at night[15], suggesting that under some neuropathic conditions, pain intensity and related quality of life deficits may fluctuate within discrete phases of the diurnal cycle.
In an attempt to capture quality-of-life alterations across the diurnal cycle in rodent pain models, several studies have examined a wide array of behaviors ranging from eating and drinking to activity levels as reflected by voluntary wheel running activity and/or other tests of locomotor function.[16–19] While long-lasting hindpaw stimulus-evoked allodynia is produced following hindpaw inflammation in rodent peripheral inflammatory models, activity levels assessed by distance traveled from wheel running during the active cycle of the diurnal period appear to only be transiently decreased.[16, 17, 19]
Other studies have examined alterations in quality-of life measures by assessing activity levels following damage to peripheral nerves leading to neuropathy (i.e. allodynia). A variety of manipulations in rodent models induce allodynia and hyperalgesia in response to acute hindpaw stimuli. For example, peripheral neuropathic pain models in rodents include ligating peripheral nerves, referred to as chronic constriction injury (CCI) of the sciatic nerve, or include partial ligation of two of three branches (sural and common peroneal) of the sciatic nerve referred to as the spared nerve injury (SNI) model. Frequently, CCI or SNI applied in animal models induce long-lasting stimulus-evoked mechanical allodynia.[18] A comprehensive battery of behavioral quality-of-life indices including home cage activity monitored throughout the diurnal cycle revealed that CCI induced decreases in activity throughout the active phase of the diurnal cycle that persisted into the first few hours (early phase) of the inactive phase of the diurnal cycle[19] Notably, this study did not include voluntary wheel running activity. Overall, these results suggest that activity levels corresponding to quality-of-life alterations may be observable during a discrete time window at the onset of the inactive phase of the diurnal cycle. This possibility is particularly intriguing given that pain patients report greater pain intensity at night when activity levels are typically lowest.[15]
While quality-of-life parameters such as activity levels in rodent models of chronic peripheral neuropathy are sorely needed to complement stimulus-induced measures of allodynia and hyperalgesia, activity can itself impact allodynia and hyperalgesia. Indeed, in experimental models of rodent neuropathy, regular exercise by voluntary wheel running significantly reduced allodynia and hyperalgesia.[20] This report reveals that by engaging in regular exercise on in-cage voluntary wheels, over a period of 8 weeks rodents demonstrate a significant reduction in chronic muscle pain.[20] Thus wheel-running activity can itself directly alter pain and activity/quality-of life measures under ongoing neuropathic pain conditions. However, reduced exposure to exercise by limiting access to 5 days[20], exerts little impact on allodynia, suggesting that a discrete window of voluntary wheel running exposure can be explored and potentially utilized without altering pain-related measures of activity and allodynia.
Given that voluntary wheel running activity in rats with CCI has not previously been reported, and activity levels are a critical complement to stimulus-induced assessment of allodynia in models of neuropathy, wheel running activity was examined in the current report while attempting to avoid the potential effects of exercise on activity levels and chronic allodynia. To achieve this, the following was implemented in rats: (1) minimal exposure time to running wheels while achieving baseline assessment of wheel-running activity, and (2) examination of running wheel activity during the early inactive phase of the diurnal cycle to maximize potential differences in activity levels between healthy controls and neuropathic rats, as pain-related behaviors may increase during the inactive phase of the diurnal cycle. Therefore, the goal of the present study was to determine the effect of peripheral neuropathy on an index of quality-of-life, wheel running activity levels at the early-onset of the inactive phase of the diurnal cycle in rats. For comparison, effects of peripheral neuropathy on wheel running activity during the early onset of the active phase of the diurnal cycle were also examined applying the same approach in minimizing exposure to the running wheels. Behavioral assessment of hindpaw sensitivity to light touch (allodynia) was performed concurrently to determine whether acute access to exercise wheels during the active or inactive phase of the light cycle altered hindpaw responses to neuropathic pain in rats.
Methods
Animals
All experiments were performed on Sprague Dawley rats (300–425g, upon arrival; Harlan Labs). Rats were housed in temperature (23 +/− 3°C) and light (12 h light:12 h dark) controlled rooms with standard rodent chow and water available ad libitum. All procedures and protocols were approved by the University of New Mexico Institutional Animal Care and Use Committee, and conducted in accordance to the guidelines recommended by the International Association for the Study of Pain for handling and ethical use of laboratory animals.20
Chronic constriction injury (CCI) surgery
Following baseline (BL) behavioral assessment, the surgical procedure for chronic constriction of the sciatic nerve was performed identically as previously described.[21, 22] Briefly, under isoflurane anesthesia (5% volume in 2.5% in oxygen), the low back and dorsal left thigh was shaved and cleaned with Bacti-Stat AE solution (EcoLab Healthcare Division, Mississuaga, Ontario, Canada). Using aseptic procedures, the sciatic nerve was carefully isolated using sterile glass rods after gentle blunt dissection of overlying muscle, and loosely ligated with 4 chromic gut sutures (Ethicon, Somerville, NJ). The overlying muscle was sutured closed with two sterile silk sutures (Ethicon, Somerville, NJ), and the overlying skin was closed with wound clips. Sham-operated rats underwent the identical manipulation with the exception that the sciatic nerve was not ligated. Animal body weight was recorded and recovery from anesthesia was observed within 10 minutes. Experimenters were blinded to the randomized assignment of animals with respect to group allocation.
Wheel running activity assessment
Within the first hour of the active (night, n=6/gp) or inactive (light, n=8/gp) cycle, rats were removed from their home cages and placed into polycarbonate cages (20.5 cm wide × 36.5 cm long × 14 cm high) with free access to stainless steel rat activity wheels (diameter 23 cm; width 5 cm) with a ball-bearing axle (Lafayette Instruments, Indiana, USA), located within the same colony room. The wheel could be turned in either direction, and the absolute value of distances travelled was recorded. The activity cages were contained within the testing room and wheels were connected to a computer that automatically recorded distance travelled by each animal during the 1 hour evaluation session, a time period previously used in acute voluntary wheel activity studies.[16] However, data sampling allowed for precise recording and analysis at up to one sample per second. In a pilot study, analysis was undertaken using 5 minute recording bins (data not shown). Upon qualitative observations from the 1-hr wheel running activity session, three 20-min phases of activity with revealed potentially different activity patterns. Therefore, three 20-min phases were additionally examined to identify possible phase-related differences within the 1 hour period. A motionless, silent experimenter was present in the room during all evaluations to make note of instances when hindpaws were inadvertently misplaced potentially producing unintended injury, in particular, of ipsilateral hindpaws following CCI. No such instances were observed.
All data were automatically recorded on a computer using Activity Wheel Monitor 5.0 software (Lafayette Instrument Company, IN) and were analyzed using GraphPad Prism 6.0 software (GraphPad Software Inc., CA). Rats were habituated daily for 7 consecutive 1 hour sessions after the initiation of the day (inactive) or night (active) cycle. Exclusion criteria included rats chewing their feet (an indication of nerve damage, which is a rare complication of CCI). All animals remained healthy and completed the experiment. The habituation sessions were used to ensure familiarity with the wheels and to determine whether stable distances travel by wheel activity prior to surgical manipulation can be achieved within the narrow time-window of the inactive phase of the diurnal cycle. The final habituation recording session was taken as the baseline (BL) level, and intra-animal changes (δ meters travelled) from this level were analyzed on each testing day following surgery. After BL values were obtained, rats were assigned to one of three groups: a) the injured free-wheel running group, b) the injured locked-wheel group, or c) the sham operant free-wheel group.
Based on prior reports demonstrating largely different distances traveled from wheel running activity during the active and inactive diurnal cycles, ranging from 200 m – 600 m and 50 m – 100 m, respectively[17, 19, 23] (supporting initial observations during habituation training of the current report), an analysis of wheel running distances between active and inactive cycles was not further considered. A priori, the most informative comparisons at the time of baseline training are within each experimental group to determine that stable wheel running distances were achieved within the narrow time-window of assessment and prior to subsequent surgical manipulation.
Mechanical allodynia assessment
The von Frey test sensory test was performed within the sciatic innervation area of the hind paws as previously described, 30 min prior to the inactive (light) voluntary wheel running sessions.23 Rats were habituated to the wire mesh for 4 consecutive days at the beginning of the light cycle. Baseline recordings were subsequently taken on 3 consecutive days prior to surgery. Low threshold mechanical allodynia was assessed as previously described[22, 24–26] with assessment occurring on days 3, 10, 16 and 18 after surgery. Low threshold mechanical hindpaw responses were assessed for the active (night) free-wheel groups in the morning. Three BLs were averaged to generate an overall single BL value for each rat prior to CCI or sham surgery. Hindpaw thresholds were reassessed on days 3, 10, 14, 16 and 18 after experimental manipulations for each experiment. Paw withdrawal thresholds were carried out as previously described[22, 26]. Briefly, a logarithmic series of 10 calibrated Semmes-Weinstein monofilaments (von Frey hairs; Stoelting, Wood Dale, IL, USA) was applied randomly to the left and right hindpaw. The log stiffness of the hairs ranged from 3.61 (0.407 g) to 5.18 (15.136 g). Response thresholds for each rat are complete when a fiber elicits a 100% paw withdrawal response (3/3 stimulus presentations). As previously described, absolute paw withdrawal thresholds were calculated,[22, 26, 27] by fitting a Gaussian integral psychometric function using a maximum-likelihood fitting method.[28, 29] Hindpaw thresholds following surgical manipulation are expressed as a change from BL (Delta Absolute Log Stimulus (mg × 10).
Statistical Analysis
All statistical comparisons were performed with GraphPad Prism 6.0 (GraphPad Software Inc, La Jolla CA). The threshold for statistical significance was set a priori at α = 0.05 for all comparisons. For inactive cycle and active cycle habituation to wheels, as assessed by distance traveled with repeated exposure to the wheel-running environment, a repeated measures ANOVA followed by Tukey’s multiple comparisons’ post hoc test (detailed further below) was used for pre-surgery differences. Between-subject factors of surgical manipulation (Sham vs. CCI) × timepoint (consecutive days 1–7; as within-subjects factor) interactions in distance traveled were analyzed. A priori, distance traveled during the active and inactive cycles during habituation were expected to be different based on known differences in activity levels at different phases of the diurnal cycle[17, 19, 23]. Therefore, comparisons of distance traveled between active- and inactive-phase diurnal groups were not considered informative to the key points of this study hypothesis and not further considered. However, a priori comparisons of distances traveled within each group at repeated timepoints during habituation are considered informative because reliable increases in distance traveled prior to subsequent experimental manipulation supports the feasibility of further examining this behavior within a discrete diurnal window of time.
Following surgical manipulation, wheel running activity and von Frey hindpaw threshold responses were analyzed with repeated-measures ANOVA. For distance traveled from wheel running activity, group (Sham surgery vs. CCI) × timpoint (3, 10, 14 and 18) interactions were performed for rats examined during the inactive phase, and separately, during the active phase of the diurnal cycle. Surgical treatment (Sham vs. CCI) were analyzed as the between subjects factor and time (3, 10, 14 and 18) as a within-subject factor. Groups were compared, a priori, at each timepoint within the inactive or active phase of the diurnal cycle because stable consistent activity levels are a critical element in this report to determine that this behavior is a reliable indicator of diminished quality-of-life due to chronic pain. Thus, ongoing reduced activity levels that coincide with, and are as sensitive to, well-characterized chronic alterations in mechanical light touch broaden the capacity to evaluate “pain” in experimental animals.
To control the Type I error rate during multiple comparisons, reported with adjusted P-values, Tukey’s test was applied for post hoc examination of possible group differences in wheel activity distance at specific timepoints during the repeated habituation period. As such, 2 groups (Sham vs. CCI) × timepoints (1–7 habituation days) result in the outcome variables for comparisons during habituation training for the inactive, and separately, active diurnal cycle. Similarly, Šidák’s comparison tests were applied to analyze post hoc the effect of group differences (Sham vs. CCI) at specific timepoints (days 3,10, 14 and 18 post surgery) for wheel activity distance during the active, and separately, inactive diurnal phase. For von Frey hindpaw sensitivity data, group differences (sham + unlocked wheel vs. CCI + locked wheel vs. CCI + unlocked wheel) × specific timepoints (days 2, 9, 13 and 15 and 17 post surgery) for each group was examined during the active, and separately, the inactive diurnal cycle. The threshold for statistical significance was set a priori at α = 0.05 for all sets of multiple comparisons.
Study statistical power was confirmed via a sensitivity analysis performed with G*Power 3.1.9.2 software[30]. Based on previous data, a correlation among repeated measures of at least 0.8 and nonsphericity correction of at least 0.9 were assumed. With these conservative assumptions, the sample size used in the current report (8 rats/group; 2 groups; 5 timepoint iterations) is sufficient with typical 80% power at α = 0.05 to detect a group (CCI vs. Sham; for rats examined during the inactive cycle, and separately, rats examined during the active cycle) × timepoint (BL, 3, 10, 14, 16 and 18) interaction on voluntary wheel activity that represents as little as 3.4% of the overall observed variation. As noted, however, the assumptions for repeated-measures correlation and nonsphericity were conservative, so the actual detectable effect size was likely to be smaller, suggesting that any clinically-important distinction between groups was likely to be detected in this study.
Results
Acute voluntary wheel habituation activity during the inactive diurnal phase
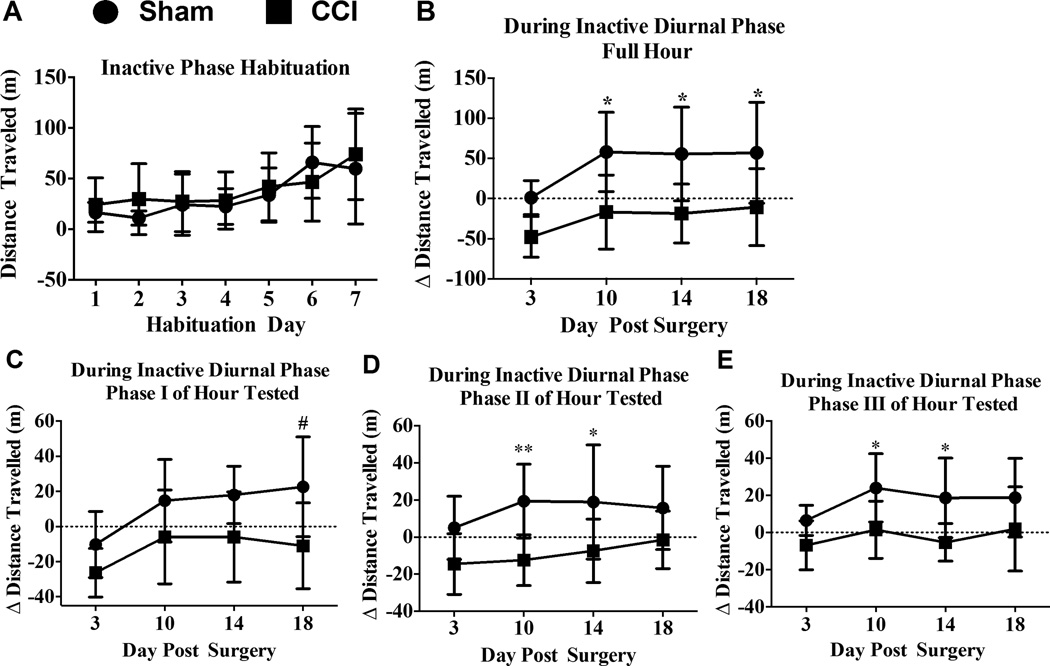
Rats in sham- and CCI-treated groups showed habituation-related changes over time in distance traveled from wheel running activity during the inactive phase of the diurnal cycle (Fig. 1A) (F(6,84) = 11.82; P < 0.0001). Rats were exposed either daily or nightly for a 1 hr habituation sessions for 7 consecutive days/nights, respectively. Running wheel activity examined during the inactive phase of the diurnal cycle revealed within group time-related changes. Specifically, activity levels of sham rats increased from Day 1 to Day 6 and 7 (1 vs. 6: 95% CI [−82.18, −16.7], p < 0.002, and 1 vs. 7: 95% CI [−76.1, −10.62], p < 0.002), increased from Day 2 to Day 6 and 7 (2 vs. 6: 95% CI −[87.72, −22.24], p < 0.0005, and 2 vs. 7: 95% CI [−81.64, −16.16], p < 0.0005), increased from Day 3 to 6 and 7 (3 vs. 6: 95% CI [−74.49, −9.017], p < 0.05, and 3 vs. 7: 95% CI [−68.41, −2.937], p < 0.05), and increased from Day 4 to 6 and 7 (4 vs. 6: 95% CI [−76.19, −10.72], p< 0.02; and 4 vs. 7: 95% CI [−70.11, −4.637], p< 0.02). In addition, activity levels of rats prior to CCI treatment increased from Day 1, 2, 3 and 4 to Day 7 (1 vs. 7: 95% CI [−82.73, −17.25], p < 0.002; 2 vs. 7: 95% CI [−77.15, −11.67], p < 0.002; 3 vs. 7 (95% CI [−79.53, −14.06], p < 0.002; and 4 vs. 7: 95% CI [−78.41, −12.93], p < 0.002). These results support that reliable increases in distance can be achieved at BL by wheel running activity even when assessment occurs within a narrow time-window at early-onset of the inactive phase of the diurnal cycle.
Figure 1. The effects of sciatic neuropathy (CCI) on voluntary wheel running activity during the inactive phase of the diurnal cycle.
(A) Total distance traveled (m) steadily increased over a 7-day sequential habituation period of a fixed interval 1-hr/day free-access period to running wheels, while no differences were observed between groups (N = 8/gp) at any timepoint. Tukey’s test for timepoint comparisons and Šidák’ test for group comparisons at each time point. Statistical significance was set with alpha at p <0.05. (B) One hour wheel running activity after either sham surgery or unilateral sciatic nerve chronic constriction injury (CCI) revealed reduced activity in CCI rats relative to their baseline (BL) activity levels (BL indicated by a dashed horizontal line at ‘0’). Significant difference between sham- and CCI-treated rats using Tukey’s test for post-hoc contrasts. ** P < 0.01, # P < 0.02. (C, D and E) Within the one-hour activity assessment, three 20-min phases were analyzed with each phase revealing decreases in distance traveled after CCI at 1 or 2 of the 4 timepoints examined. Rats with CCI traveled the least on Day 18 during the first 20-min (phase I) of the 1-hr testing period. However, during the following 40 min (phase II and III), CCI rats consistently traveled less than sham controls on Day 10 and 14 after surgical manipulation. Significant differences between sham- and CCI-treated rats using Tukey’s test for post-hoc contrasts. ** P < 0.01, # P < 0.02, * P < 0.05.
Acute voluntary wheel habituation activity during the active diurnal phase
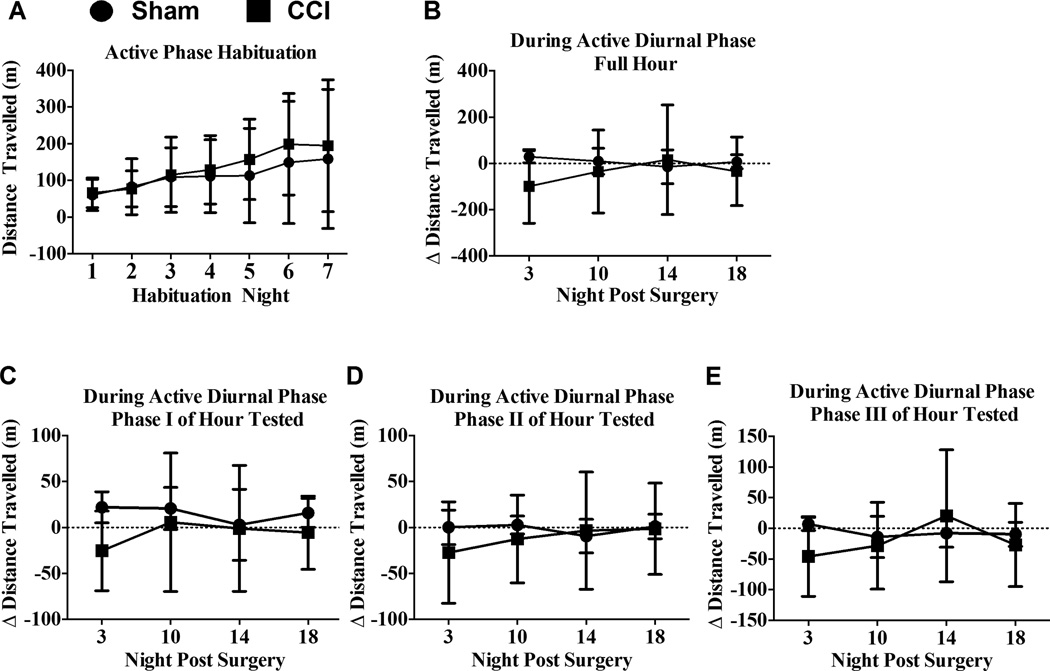
Rats in sham- and CCI-treated groups showed habituation-related changes over time in distance traveled from wheel running activity during the active phase of the diurnal cycle (Fig. 2A) (F(6,60) = 4.783; P = 0.0005). With groups examined for activity levels during the active phase prior to surgical manipulation, within group time-related increases were revealed only in the CCI group from Night 1 to Night 6 and 7 (1 vs. 6, 95% CI [−252.8, −11.94], p < 0.05; 1 vs. 7, 95% CI [−248.9, −8.075], p < 0.05), and increased from Night 2 to Night 6 (95% CI [−242.2, −1.322], p < 0.05). Prior to surgical manipulation, no differences were observed between CCI and sham groups while undergoing repeated habituation for the inactive phase (F(1,14) = 1.257; p = 0.19,) or the active (F(1,10) = 0.1507; p = 0.70) phase. As with BL data from activity during the inactive phase of the diurnal cycle, these results support that reliable distance increases can be achieved at BL by wheel running activity when assessment occurs within a narrow active phase time-window.
Figure 2. The effects of sciatic neuropathy (CCI) on voluntary wheel running activity during the active phase of the diurnal cycle.
(A) Total distance traveled (m) increased over a 7-day sequential habituation period of a fixed interval 1-hr/day free-access period to running wheels, while no differences were observed between groups (N = 6/gp) at any timepoint. Tukey’s test for timepoint comparisons and Šidák’ test for group comparisons at each time point. Statistical significance was set with alpha at p <0.05. (B) One hour wheel running activity during the active diurnal cycle relative to BL levels was similar between sham or unilateral CCI. Šidák’ test for group comparisons at each timepoint revealed no group differences. Statistical significance at p <0.05. (C, D and E) Comparison of distance traveled within three 20-min phases of the 1 hr wheel-running testing period during the active diurnal cycle revealed that CCI rats traveled similar distances to that observed with sham rats. Thus, CCI did not significantly alter wheel running on the indicated nights tested post-surgery as revealed by Šidák’ test for group comparisons at each timepoint with statistical significance set at p <0.05.
Effects of CCI on acute exposure to voluntary wheel activity during inactive and active diurnal phases
Examining voluntary wheel running activity in rats tested during the early portion of their inactive diurnal cycle revealed a main effect of surgical treatment relative to BL (F(1,14) = 12.88; p = 0.003) and a main effect of time (F(3,42) = 8.25; p < 0.0005) (Fig 1B). However, no interaction between surgical treatment and time on distance from wheel activity was observed (p = 0.6060). Wheel running activity during the rats’ inactive cycle was greater in non-neuropathic sham-treated rats compared to CCI rats on Day 10 (95% CI [21.06, 128.8], p < 0.01), Day 14 (95% CI [20.39, 128.1], p < 0.01), and Day 18 (95% CI [13.83, 121.6], p < 0.02). Surprisingly, wheel running activity in a separate group of rats examined during the active phase of their diurnal cycle revealed no differences between sham and CCI treatment (F(1,10) = 0.3983, p = 0.54) or over time (F(1,10) = 0.49, p = 0.5421) (Fig 2B).
Within wheel running trial: phase-specific wheel running activity
To characterize potential phasic patterns of activity within the 1-hour access period, wheel activity was analyzed in three equal phases (20 min duration/phase) in rats examined during the inactive phase, and in a separate group of animals, during the active phase of the diurnal cycle. During Phase I of rats examined at the onset of their inactive cycle, a reduction in wheel running activity due to CCI was observed (F(1,14) = 7.150; p = 0.0182) that slightly increased in both treatment groups during the 18-day timecourse (F(3,42) = 8.242; p < 0.001). However, differences between sham and CCI were observed only on day 18 (95% CI [6.823, 60.46], p < 0.05). Additionally, rats examined during phases II and III of the testing hour during their inactive cycle revealed an overall reduction in wheel running activity in CCI-induced neuropathic rats compared to sham non-neuropathic rats (F(1,14) = 8.816; p < 0.01; (F(1,14) = 8.771; p = 0.01, respectively) (Fig. 1C–E). In phase II, decreased wheel running in CCI rats compared to shams was observed on Day 10 (95% CI [8.447, 55.15], p = 0.01) and also on Day14 (95% CI [2.978, 49.68], p < 0.05), but not at other timepoints (Fig. 1D). Similarly, specific timepoints examined during phase III revealed diminished activity in CCI rats compared to shams on Day 10 (95% CI [2.323, 42.7], p = 0.05) and Day 14 (95% CI [3.779, 44.15], p < 0.05) (Fig 1E). A separate examination of each of the three phases during the active cycle of rats revealed no overall differences in wheel running activity between sham and CCI rats (phase I: (F(1,10) = 0.8795; p = 0.37); phase II: (F(1,10) = 0.2231; p = 0.22): phase III (F(1,10) = 0.2159; p = 0.65) (Fig 2C–E). Combined, these data suggest that a lack of group differences between controls and chronic neuropathic rats observed on Day 3 is delayed, which may be due to required recovery time from sham surgical manipulations that CCI rats are unable to overcome, as persistent group differences were observed by Day 10 and longer. Surprisingly, examining rats during the active portion of their diurnal cycle creates a disadvantage for observing potential activity levels in experimental rats. It is important to note that an entirely separate group of rats were examined during their active diurnal cycle reducing the possibility of stress-induced over-testing thereby potentially masking possible effects of sciatic neuropathy on activity.
Light touch mechanical allodynia: von Frey test
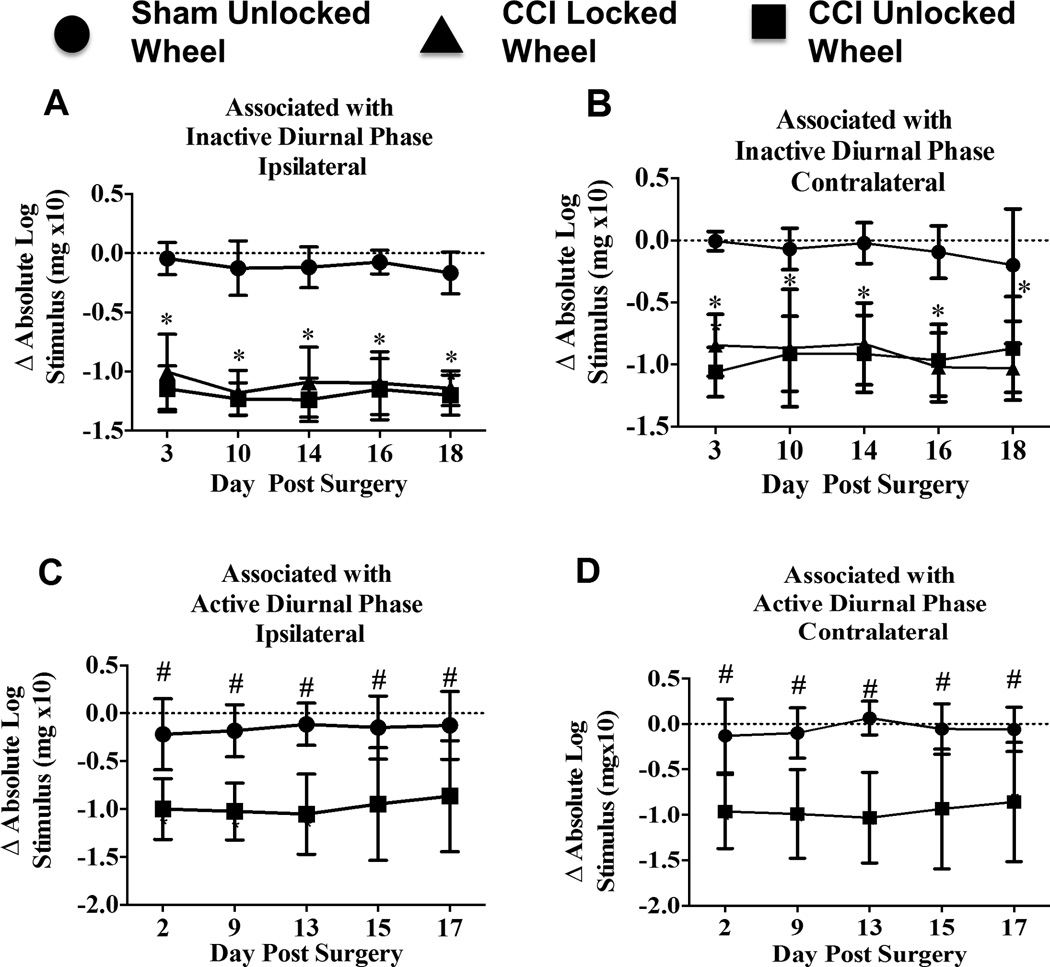
Sciatic nerve ligation produced long-lasting bilateral allodynia in rats examined for wheel running activity during their inactive and active diurnal cycle. In the “inactive diurnal” groups, robust hindpaw sensitization differences between sham and CCI rats having had 1-hr access to either a locked wheel or an unlocked wheel persisted throughout the entire timecourse following surgical manipulation. A main effect of surgical treatment on hindpaw response thresholds was observed ipsilateral (F(2,21) = 169.9; P < 0.0001) and contralateral (F(2,21) = 88.89; P < 0.0001) to the nerve manipulation (Fig 3A, B). Ipsilateral hindpaw responses from sham-treated rats remained stable and similar to their pretreatment baseline thresholds compared to Day 3 CCI + locked wheel (95% CI [0.7104, 1.203], p <0.0001) and CCI + unlocked free-wheel (95% CI [0.8535 to 1.347, p < 0.0001), and respectively on Day 10 (95% CI [0.8075, 1.300], p < 0.0001; 95% CI [0.8589, 1.352], p < 0.0001), Day 14 (95% CI [0.7242, 1.217], p < 0.0001; 95% CI [0.8727, 1.366] p < 0.0001), Day 16 (95% CI [0.7758, 1.269], p < 0.0001; 95% CI [0.8283, 1.321]), and Day 18 (95% CI [0.7273, 1.22], p < 0.0001; 95% CI [0.7859, 1.279] p < 0.0001) (Fig 3A). Similar differences were observed in sensitivity of the contralateral hindpaw between sham-treated rats compared to Day 3 CCI + locked wheel (95% CI [0.4879, 1.189], p < 0.0001) and CCI + unlocked free-wheel (95% CI [0.7045, 1.405], p < 0.0001), and respectively on Day 10 (95% CI [0.4478, 1.148], p < 0.0001; 95% CI [0.4937, 1.194], p < 0.0001), Day 14 (95% CI [0.4605, 1.161], p < 0.0001; 95% CI 0.5407 to 1.241, p < 0.0001), Day 16 (95% CI [0.5768, 1.277], p < 0.0001; 95% CI [0.5206, 1.221, p < 0.0001), and Day 18 (95% CI [0.4787, 1.179], p < 0.0001; 95% CI [0.3196, 1.02], p < 0.0001) (Fig 3B). No significant differences were observed between the CCI + locked wheel and CCI + free-wheel groups on sensitivity from the ipsilateral (F(1,14) = 1.751; p = 0.21) or contralateral hindpaws (F(1,14) = 0.1006; P = 0.7558) (Fig 3A, B).
Figure 3. Hindpaw threshold sensitivity throughout the 18-day timecourse remains stable.
(A, B) Compared to hindpaw threshold responses relative to BL levels in sham-treated rats, CCI leads to robust bilateral and persistent allodynia that is unaltered by wheel running activity occurring during the inactive portion of the diurnal cycle. No hindpaw threshold differences between CCI – locked wheel and CCI – unlocked wheel throughout the timecourse were observed. Tukey’s test for post-hoc contrasts between each group at each time were performed, and Šidák’ post-hoc comparisons were performed between each CCI group at each time. ** P < 0.001. (C, D) This separate group of rats replicated our prior results of bilateral hindpaw sensitivity following CCI compared to sham controls, which verifies (1) stable ongoing allodynia was present in rats that were also examined for wheel running activity during the active phase of the diurnal cycle, and (2) this paradigm of acute exposure to active-cycle voluntary wheel running activity does not alter the pattern of ongoing allodynia. Šidák’ post-hoc comparisons were performed between CCI and sham groups at each time. # P < 0.01.
Similarly, analysis of bilateral hindpaw sensitivity examined in rats associated with the “active diurnal” phase revealed robust ipsilateral allodynia in CCI compared to sham rats throughout the timecourse (F(1,10) = 15.32; p= 0.0029), which was also observed on the contralateral hindpaws (F(1,10) = 14.23; P = 0.0036) (Fig. 3C, D). Bilateral hindpaw responses from sham-treated rats were similar to their pretreatment baseline thresholds compared to CCI, as CCI + 1 hr access to an unlocked free-wheel revealed greater hindpaw sensitivity on Day 2 post-surgical manipulation (Ipsilateral: 95% CI [0.1749, 1.385], p < 0.01; Contralateral: 95% CI [0.1555, 1.509], p < 0.01), as well as on Day 9 (Ipsilateral: 95% CI [0.237,1.447], p < 0.01; Contralateral: 95% [CI 0.2137, 1.568], p < 0.01), Day 13 (Ipsilateral: 95% CI [0.3335, 1.543], p = 0.001; Contralateral: 95% CI [0.4208, 1.775], p < 0.001), Day 15 (Ipsilateral: 95% CI [0.1945, 1.404], p < 0.01; Contralateral 95% CI [0.2034, 1.557], p < 0.01), and Day 17 (Ipsilateral: 95% CI [0.1341, 1.344], p < 0.01; Contralateral: 95% CI [0.1216, 1.476], p < 0.01) (Fig. 3C, D).
The results indicate that acute access to wheels during either the inactive or active phase of the diurnal cycle does not modify the degree or duration of sensory allodynia associated with peripheral neuropathy.
Discussion
The studies in the current report were aimed at identifying an optimal method (acute wheel running exposure) and time of diurnal phase whereby activity levels could be examined in rats with unilateral sciatic nerve damage induced by chronic constriction injury (CCI). CCI is a widely used rodent model of chronic peripheral neuropathy that is generated by the loose ligation of chromic gut sutures around one intact sciatic nerve leading to local inflammation plus slight constriction.[21] The current study not only revealed robust allodynia throughout the time-course, as expected, but also modulated activity levels relative to sham from respective baselines as assessed by distance traveled in running wheels only during the inactive phase of the diurnal cycle. This report demonstrates that CCI-induced neuropathy in rats produces significant modulation of voluntary wheel activity. The onset of reduced wheel running activity during the inactive portion of the diurnal cycle occurred with verification of bilateral mechanical allodynia, which persisted in all CCI-treated rats whose activity levels were examined during the active and inactive phase of the diurnal cycle. Importantly, while activity levels in rats examined during the inactive phase of the diurnal cycle were stable (~100 m/hr) prior to surgical induction of sciatic neuropathy, these pre-manipulation levels were sufficient to detect a consistent reduction in activity that coincided with allodynia for 18 days after induction of sciatic neuropathy. Stable consistent activity levels are a critical element in this study to determine whether this endpoint can be indicative of diminished quality-of-life due to chronic pain. Ongoing changes in activity levels coinciding with allodynia can broaden the capacity of future studies to evaluate “pain” in experimental animals.
Rats are more active in the dark phase of their cycle[31], and this heightened activity may mask the effects of neuropathic pain on voluntary wheel running levels. Repeated exposure to activity wheels has been shown to lead to progressively increasing distances traveled over time. It is possible that heightened overall wheel activity results from increased exposure to activity.[32] It is also possible that exercise-induced restorative mechanisms suppress movement-evoked pain28 potentially involving regions of entorhinal cortex (EC) previously implicated in wheel running activity[32]. Interestingly, lesioned EC is sufficient to exacerbate chronic pain[33] and produces both hyperphagy and hypoactivity.[34]
Interestingly, the amount of pain experienced under peripheral neuropathies is elevated during human inactive cycles[15], and heightened pain-like responses are present, as measured by rodent stimulus-induced response assays (allodynia and hyperalgeisa).[35] Therefore, testing during the rodent’s inactive cycle may be the optimal time in which to examine activity levels. Differences in wheel running activity observed in each of the three 20-minute phases suggest that, even if there are phase-related changes, the entire hour provides the most robust reflection of overall activity changes.
The acute exposure paradigm described in the current report did not result in exercise-induced modulation of mechanical allodynia. This is an important consideration given that repeated voluntary exercise training has been shown to reduce formalin and nerve injury-induced pain in rats compared to their sedentary counterparts.[20] These data suggest that acute voluntary wheel running activity may confer an additional benefit of screening for pain-like behaviors, particularly when testing new animal models of neuropathy. For example, preclinical rodent models of conditions involving peripheral nerve sensitization include chronic osteoarthritis of the knee[36], lumbar radiculopathy[37, 38] or diabetic neuropathy.[39] The automated data acquisition associated with the acute running wheel exposure model currently reported confers an additional advantage of ensuring a relatively simple means to ensure complete experimenter objectivity at multiple time points.
Voluntary wheel running activity selectively assays movement-evoked distal nociceptive input based on modulation of nerve signals arising from the affected anatomical area, which is supported by findings that a) that injection of intraplantar but not L1 dorsum CFA suppressed wheel running activity in mouse[16], b) intra-plantar but not intra-tail carrageenan suppressed wheel activity in rats[40] and c) intra-articular injections of the blood-brain barrier impermeant neurotoxin, botulinum, completely restored wheel running activity in rats with arthritis induced by intra-articular CFA in mice.[41] Additionally, activity levels examined by voluntary wheel running may be particularly important in the development of novel rodent models of discogenic neuropathic pain[42] as alternative hind-paw allodynia and hyperalgesia assays may not fully capture the spectrum of pain behaviors from neuropathy in this model.
While voluntary wheel running is proposed to be a clinically relevant endpoint to assess supra-spinal processes that lead to behaviors such a decision making and physical activity that reflect impacts on quality-of-life of the pain experience[16, 18, 19, 23], a recent report argues the possibility that voluntary wheel activity is a different measure of hindpaw evoked mechanical sensitivity induced by hindpaw inflammation rather than more complicated cortical processes[17]. Experiments revealed that while the widely used model of hindpaw subcutaneous complete Freund’s adjuvant (CFA) induced a 7-day allodynia and decreased wheel running activity for 2 days, CFA injections into hindpaw-independent sights failed to alter wheel running activity. Specifically, CFA injections into the lumbar dorsal surface at the L1 dorsum dermatome, which leaves the L5 hindpaw dermatomal distribution undisturbed, did not alter wheel running activity at any time after injection. These data suggest that hindpaw inflammatory pain from CFA leads to diminished wheel running activity due heightened sensitivity at the hindpaw eliciting avoidance behaviors of stimulus-evoked pain. However, a more recent report demonstrated in rats with experimental autoimmune encephalomyelitis (EAE), which is a rodent model of multiple sclerosis, robust allodynia and a profound reduction of wheel running activity that occurred prior to the onset of EAE-induced motor weakness. Allodynia and decreased wheel activity levels coincided with decreased social exploration[43], a test of anxiety in rodents[44, 45]. Notably, hindpaws are not directly affected in EAE and in this report, rats were allowed voluntary unrestricted access to in-cage running wheels. Thus, while hindpaw hypersensitivity to running wheels cannot be ruled out, affective-like qualities in the rat (anxiety) may reflect overall physical and affective dimensions such as lethargy, anxiety, and depression frequently observed in people with multiple sclerosis[46]. Interestingly, following hindpaw intraplantar formalin, pain behavioral responses such as paw licking/flinching were observed within minutes that were absent in intraplantar CFA, while wheel running activity in intraplantar formalin was unaltered[17]. Based on these reports, wheel running activity may serve as an endpoint for avoidance behavior from painful stimuli, but depending on the animal model, may also reveal affective dimensions of pain.
Various mechanical stimulus-evoked responses such as the von Frey fiber test do not always serve as predictors of wheel running activity in local inflammatory animal models of pathological pain, and consequently investigators suggest that the von Frey fiber test may not be a useful endpoint/strategy to assess clinical allodynia and drugs that alleviate discomfort[16]. Using intraplantar CFA, the effects of a range of non-steroidal anti-inflammatory drugs or morphine given peripherally primarily altered wheel running activity without modifying allodynia assessed by the von Frey fiber test[16]. The authors speculate that the von Fiber test may not be sufficiently sensitive to detect subtle changes in stimulus evoked inflammatory pain compared to the functional changes observed in running wheel activity. While this may be possible for hindpaw inflammatory pain models, other reports demonstrate nerve-injury induced pain can be assessed by mechanical hypersensitivity using the von Frey fiber tests with concurrent assessment of activity levels using voluntary running wheels[19, 23], whereby von Frey allodynia outlasted running wheel activity. A separate recent study revealed that in rats with onset EAE, intrathecal transgene application of the anti-inflammatory cytokine IL-10 gene profoundly reversed decreases in wheel running activity and simultaneously blunted the development of von Frey low threshold mechanical allodynia, suggesting that von Frey fiber testing can act as an important complementary endpoint to wheel running activity. In further support of complementary von Frey fiber testing to wheel running activity, a separate study of spared nerve injury demonstrated persistent 4-wk allodynia identified by responses to von Frey fibers that corresponded to elevated nociceptor membrane excitability as examined by whole-cell patch clamp electrophysiology[47]. These studies are striking and support the application of the von Fiber test for low threshold mechanical allodynia as a complementary endpoint to activity levels in animal models of peripheral neuropathy.
In summary, the long-lasting reduction of voluntary wheel running activity produced by CCI reported here was observed during the onset the rat’s inactive diurnal cycle. The data are in contrast to previously reported transient suppression of wheel activity, albeit from a distinctly different animal model; peripheral inflammatory pain. This suggests behavioral assays used to assess various aspects of neuropathic pain can include an examination of activity levels in addition to classically applied touch-evoked sensory responses (i.e. thermal Hargreaves test or the von Frey test for light touch allodynia). Acute, time-restricted voluntary wheel running activity may serve as a novel complementary assay of peripheral neuropathic pain. Our basic findings show that alterations in levels of voluntary running wheel activity, as a quality-of-life index in a rodent model, are sufficiently sensitive to reflect chronic neuropathy during the rat’s inactive diurnal cycle.
Acknowledgments
This work was supported by: (1) NIH DA018156, (2) UNM SOM Research Allocation Grants, (3) UNM Department of Anesthesiology Research Fund
Footnotes
None of the authors have any conflicts of interest.
References
- 1.Dworkin RH, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 2.Jensen TS, et al. A new definition of neuropathic pain. Pain. 2011;152(10):2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Freeman R, et al. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain. 2014;155(2):367–376. doi: 10.1016/j.pain.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Baron R, Forster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol. 2012;11(11):999–1005. doi: 10.1016/S1474-4422(12)70189-8. [DOI] [PubMed] [Google Scholar]
- 5.Mainero C, et al. Mapping the spinal and supraspinal pathways of dynamic mechanical allodynia in the human trigeminal system using cardiac-gated fMRI. Neuroimage. 2007;35(3):1201–1210. doi: 10.1016/j.neuroimage.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee IO, et al. Evaluation of a novel mouse model of intracisternal strychnine-induced trigeminal allodynia. Can J Anaesth. 2013;60(8):780–786. doi: 10.1007/s12630-013-9975-x. [DOI] [PubMed] [Google Scholar]
- 7.Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150(3):439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Doth AH, et al. The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. Pain. 2010;149(2):338–344. doi: 10.1016/j.pain.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Liedberg GM, Vrethem M. Polyneuropathy, with and without neurogenic pain, and its impact on daily life activities--a descriptive study. Disabil Rehabil. 2009;31(17):1402–1408. doi: 10.1080/09638280802621382. [DOI] [PubMed] [Google Scholar]
- 10.McCarberg BH, Billington R. Consequences of neuropathic pain: quality-of-life issues and associated costs. Am J Manag Care. 2006;12(9 Suppl):S263–S268. [PubMed] [Google Scholar]
- 11.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18(6):350–354. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Bellamy N, Sothern RB, Campbell J. Rhythmic variations in pain perception in osteoarthritis of the knee. J Rheumatol. 1990;17(3):364–372. [PubMed] [Google Scholar]
- 13.Kowanko IC, et al. Domiciliary self-measurement in the rheumatoid arthritis and the demonstration of circadian rhythmicity. Ann Rheum Dis. 1982;41(5):453–455. doi: 10.1136/ard.41.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigas B, et al. The circadian rhythm of biliary colic. J Clin Gastroenterol. 1990;12(4):409–414. doi: 10.1097/00004836-199008000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Odrcich M, et al. Chronobiological characteristics of painful diabetic neuropathy and postherpetic neuralgia: diurnal pain variation and effects of analgesic therapy. Pain. 2006;120(1–2):207–212. doi: 10.1016/j.pain.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Cobos EJ, et al. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153(4):876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grace PM, et al. Suppression of voluntary wheel running in rats is dependent on the site of inflammation: evidence for voluntary running as a measure of hind paw-evoked pain. J Pain. 2014;15(2):121–128. doi: 10.1016/j.jpain.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory NS, et al. An overview of animal models of pain: disease models and outcome measures. J Pain. 2013;14(11):1255–1269. doi: 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urban R, et al. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain. 2011;152(5):990–1000. doi: 10.1016/j.pain.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sluka KA, et al. Regular physical activity prevents development of chronic pain and activation of central neurons. J Appl Physiol. 1985;114(6):725–733. doi: 10.1152/japplphysiol.01317.2012. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett GJ, Xie KY. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 22.Milligan ED, et al. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the Human Immunodeficiency Virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- 23.Kandasamy R, Calsbeek JJ, Morgan MM. Home cage wheel running is an objective and clinically relevant method to assess inflammatory pain in male and female rats. J Neurosci Methods. 2016;263:115–122. doi: 10.1016/j.jneumeth.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dengler EC, et al. Improvement of spinal non-viral IL-10 gene delivery by D-mannose as a transgene adjuvant to control chronic neuropathic pain. J Neuroinflammation. 2014;11:92. doi: 10.1186/1742-2094-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dengler EC, et al. Mesoporous silica-supported lipid bilayers (protocells) for DNA cargo delivery to the spinal cord. Journal of controlled release : official journal of the Controlled Release Society. 2013;168(2):209–224. doi: 10.1016/j.jconrel.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milligan ED, Maier SF, Watkins LR. Sciatic inflammatory neuropathy in the rat: surgical procedures, induction of inflammation, and behavioral testing. Methods in molecular medicine. 2004;99:67–89. doi: 10.1385/1-59259-770-X:067. [DOI] [PubMed] [Google Scholar]
- 27.Chaplan SR, et al. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 28.Harvey LOJ. Efficient estimation of sensory thresholds. Behav. Res. Meth. Instrum. Comput. 1986;18:623–632. [Google Scholar]
- 29.Treutwein B, Strasburger H. Fitting the psychometric function. Perception & psychophysics. 1999;61(1):87–106. doi: 10.3758/bf03211951. [DOI] [PubMed] [Google Scholar]
- 30.Faul F, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 31.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69(6):1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhodes JS, Gammie SC, Garland T., Jr Neurobiology of Mice Selected for High Voluntary Wheel-running Activity. Integr Comp Biol. 2005;45(3):438–455. doi: 10.1093/icb/45.3.438. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, et al. Exacerbation of tonic but not phasic pain by entorhinal cortex lesions. Neurosci Lett. 2014;581:137–142. doi: 10.1016/j.neulet.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Ross JF, Walsh LL, Grossman SP. Some behavioral effects of entorhinal cortex lesions in the albino rat. J Comp Physiol Psychol. 1973;85(1):70–81. doi: 10.1037/h0034864. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Gomez M, et al. Assessing pain threshold in the rat: changes with estrus and time of day. Physiol Behav. 1994;55(4):651–657. doi: 10.1016/0031-9384(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 36.Berger D. Leg discomfort: beyond the joints. Med Clin North Am. 2014;98(3):429–444. doi: 10.1016/j.mcna.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Kawakami M, et al. Experimental lumbar radiculopathy. Behavioral and histologic changes in a model of radicular pain after spinal nerve root irritation with chromic gut ligatures in the rat. Spine (Phila Pa 1976) 1994;19(16):1795–1802. doi: 10.1097/00007632-199408150-00002. [DOI] [PubMed] [Google Scholar]
- 38.Kawakami M, et al. Experimental lumbar radiculopathy. Immunohistochemical and quantitative demonstrations of pain induced by lumbar nerve root irritation of the rat. Spine (Phila Pa 1976) 1994;19(16):1780–1794. doi: 10.1097/00007632-199408150-00001. [DOI] [PubMed] [Google Scholar]
- 39.Edmonds ME. The diabetic foot: pathophysiology and treatment. Clin Endocrinol Metab. 1986;15(4):889–916. doi: 10.1016/s0300-595x(86)80079-2. [DOI] [PubMed] [Google Scholar]
- 40.Loram LC, et al. Behavioural, histological and cytokine responses during hyperalgesia induced by carrageenan injection in the rat tail. Physiol Behav. 2007;92(5):873–880. doi: 10.1016/j.physbeh.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Krug HE, et al. Pain behavior measures to quantitate joint pain and response to neurotoxin treatment in murine models of arthritis. Pain Med. 2009;10(7):1218–1228. doi: 10.1111/j.1526-4637.2009.00711.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim JS, et al. Development of an Experimental Animal Model for Lower Back Pain by Percutaneous Injury-Induced Lumbar Facet Joint Osteoarthritis. J Cell Physiol. 2015;230(11):2837–2847. doi: 10.1002/jcp.25015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Grace PM, et al. Behavioral assessment of neuropathic pain, fatigue, and anxiety in experimental autoimmune encephalomyelitis (EAE) and attenuation by interleukin-10 gene therapy. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9(1):37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 45.Korte SM, De Boer SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463(1–3):163–175. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]
- 46.Fiest KM, et al. Systematic review and meta-analysis of interventions for depression and anxiety in persons with multiple sclerosis. Mult Scler Relat Disord. 2016;5:12–26. doi: 10.1016/j.msard.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Sheahan TD, et al. Voluntary Exercise Training: Analysis of Mice in Uninjured, Inflammatory, and Nerve-Injured Pain States. PLoS One. 2015;10(7):e0133191. doi: 10.1371/journal.pone.0133191. [DOI] [PMC free article] [PubMed] [Google Scholar]