Abstract
Backgrounds
A 21-gene expression assay (Oncotype DX™ Recurrence Score (“RS”)) that utilizes RT-PCR is used clinically in early-stage estrogen receptor-positive, HER2-negative breast carcinoma (ER+/HER2− BC) to determine both prognosis with tamoxifen therapy and the utility of adding adjuvant chemotherapy. Use of the assay is associated with reductions in overall chemotherapy usage. This study examined the treatments and outcomes in patients with low recurrence scores.
Methods
We reviewed the institutional database to identify patients with node-negative, ER+/HER2− BC and the 21-gene recurrence score results treated at our center between September 2008 and August 2013.
Results
We identified 1406 consecutive patients with node-negative ER+/HER2− BC and low RS [RS 0–10: n=510; RS 11–17: n=896]. The median age at BC diagnosis was 56 years; 63 (4%) patients were younger than 40 years. Overall, 1361 (97%) of patients received endocrine therapy and 170 (12%) received chemotherapy. The median follow-up time was 46 months. Six patients (0.4%) developed distant metastases (one patient with RS = 5, and five with RS of 11–17). In the RS 11–17 cohort, the absolute rate of distant metastasis among patients <40 years old was 7.1% (3/42), versus 0.2% (2/854) among patients ≥40 years.
Conclusions
Our data document a 0.4% rate of distant metastasis within 5 years of BC diagnosis among patients with node-negative ER+/HER2− BC of RS<18. Patients younger than 40 years at BC diagnosis were observed to have a higher rate of distant metastases. Analysis of data from other studies is necessary to further validate this observation.
Keywords: 21-gene expression assay, recurrence score, low risk, distant metastasis, age
Introduction
Breast cancer is a heterogenous disease that varies greatly in morphology and clinical course. Multiple clinical and pathologic parameters, such as patient age, tumor size and histologic grade, lymph node metastases and estrogen-receptor (ER)/progesterone-receptor (PR)/HER2 status are established prognostic factors. In the past decade, several multigene assays have been developed and showed prognostic value in patients with early-stage breast cancer.1–4 The 21- gene expression assay (Oncotype Dx™, Genomic Health, Redwood City, CA) is the breast cancer multigene assay most widely used in the United States.5
The 21-gene expression assay uses RT-PCR to evaluate the expression of 16 cancer-related genes and 5 reference genes in breast cancer.3 The resulting score (Recurrence Score (“RS”)), derived from the reference-normalized expression of the 16 cancer-related genes, quantifies the risk of distant recurrence at ten years and the benefit of chemotherapy in patients treated with tamoxifen.3 The recurrence score is a continuous variable which has been divided into 3 categories: low risk (RS<18), intermediate risk (RS 18–30), and high risk (RS≥31).3 The predictive value of the assay for the utility of adding chemotherapy to tamoxifen has been partially validated by retrospective analysis of samples from randomized clinical trials.4, 6 The Oncotype DX RS predicted the magnitude of chemotherapy benefit in 651 patients with ER-positive, node-negative breast cancer treated with tamoxifen +/− chemotherapy enrolled in the NASBP B20 trial.4 Analysis of a subset of patients in the SWOG 8814 trial found that the predictive and prognostic value of Oncotype DX RS also applies to patients with ER-positive, node-positive breast cancer.6
The American Society of Clinical Oncology and National Comprehensive Cancer Network (NCCN) currently include the Oncotype DX RS in their recommendations for patients with early stage ER-positive, HER2-negative breast cancer (ER+/HER2− BC).7, 8
Two prospective randomized trials, TailoRx (Trial Assigning IndividuaLized Options for treatment (Rx)) and RxPONDER (Rx for Positive Node, Endocrine Responsive breast cancer) aim to further validate the predictive value of Oncotype DX RS.9–11 TailoRx was specifically designed to evaluate the benefit of chemotherapy in patients with node-negative, ER+/HER2− BC with Oncotype DX RS 11–25 (TailoRx definition of “intermediate” RS).9, 10 Patients with RS 11–25 are randomized to receive hormonal therapy +/− chemotherapy.9, 10 The lower category thresholds were selected to minimize the risk of omitting chemotherapy.10 Patients with RS 0–10 (TailoRx definition of “low” RS) receive hormonal therapy alone. Patients with RS≥26 (TailoRx definition of “high” RS) receive hormonal therapy plus chemotherapy. Thus far the results of TailoRx confirm an extremely low risk of recurrence in patients with RS 0–10, with 99.3% of patients free from distant recurrence of breast cancer at 5 years.10 To date, no prospective data is available for patients in the RS 11–25 group and the risk for patients in the range between the conventional threshold (RS 18) and the tested one (RS 11) is unclear.12
The aims of our study were: 1) to evaluate outcomes in a large cohort of unselected, consecutive patients with early stage node-negative ER+/HER2− BC and Oncotype DX RS <18 who were treated at our center and for whom the Oncotype DX RS was prospectively integrated in the treatment management decision; 2) to characterize the clinicopathological features of the patients who developed distant metastasis in this cohort.
Methods
Study patients
Through a search of our institutional database, we identified patients with ER and/or PR-positive, HER2-negative, node-negative invasive breast carcinomas who had surgical and medical treatment at our center between September 2008 and August 2013. At our institution, all node-negative, ER and/or PR-positive, HER2-negative invasive breast carcinomas measuring ≥0.5 cm in patients medically suitable for chemotherapy and who would potentially agree to such treatment have been routinely submitted for 21-gene expression assay using the standard commercial test since September 2008. Some ER and/or PR-positive, HER2-negative breast carcinomas <0.5 cm in size are also submitted for testing on specific request by the treating clinician. Patients with lymph nodes containing isolated tumor cells (ITC) [pN0(i+)] according to the American Joint Committee on Cancer (AJCC) staging system13 were included in the study. All patients with tumors that failed testing for technical reasons were excluded from the study.
We recorded patient age at breast cancer diagnosis, tumor size, 21-gene expression assay result, patient treatment, and outcome. If a patient had multiple ipsilateral foci of invasive carcinoma or bilateral carcinomas, we recorded the size of the largest tumor and the highest RS. We identified patients with distant metastases through a query of our institutional database. The Institution Review Board approved the study.
Statistical Analysis
Clinicopathologic characteristics of the cohort are summarized using descriptive statistics for the cohort overall. The clinical outcome of interest is distant metastasis free survival which is defined as the time period from diagnosis of breast cancer to the confirmation of a distant metastasis or death from any cause.14 This outcome was described using Kaplan-Meier methods. Due to the small number of events, all analyses are descriptive and exploratory with no formal statistical analysis.
Results
We identified 1406 consecutive patients treated at our center in the study period with stage I and II node-negative [including pN0(i+) (n=74)], ER+/HER2− BC and available 21-gene expression assay results. Of these, 510 (36%) patients had Oncotype DX RS of 0 to 10 and 896 (64%) had RS of 11–17. The clinicopathologic characteristics of the 1406 patients are summarized in Table 1. The median age at breast cancer diagnosis was 56 years (range 22–90). Only 63 (4%) patients were <40 years old at breast cancer diagnosis, 332 (24%) were between 40 and 49 years old, and 1011 (72%) were 50 years or older. The median tumor size was 1.2 cm (range 0.3–5.8).
Table 1.
Clinicopathologic characteristics of the 1406 patients with ER-positive, HER2-negative, node-negative breast carcinoma of recurrence score <18
| Total Patients (n=1406) |
Patients with RS 0–10 (n=510) |
Patients with RS 11–17 (n=896) |
|
|---|---|---|---|
| Age, years | |||
| Median (range) | 56 (22–90) | 60 (25–84) | 55 (22–90) |
| Mean | 57 | 59 | 56 |
| Age distribution, n (%) | |||
| <40 years | 63 (4%) | 21 (4%) | 42 (5%) |
| 40–49 years | 332 (24%) | 111 (22%) | 221 (25%) |
| ≥50 years | 1011 (72%) | 378 (74%) | 633 (71%) |
| Menopausal status, n (%) | |||
| Pre-menopausal | 471 (33%) | 157 (31%) | 314 (35%) |
| Post-menopausal | 902 (64%) | 346 (68%) | 556 (62%) |
| Peri-menopausal | 32 (2%) | 6 (1%) | 26 (3%) |
| Other a | 1 (0.1%) | 1 (0.2%) | 0 |
| Tumor size, cm | |||
| Median (range) | 1.2 (0.3–5.8) | 1.2 (0.4–5.8) | 1.2 (0.3–4.7) |
| Mean | 1.3 | 1.3 | 1.3 |
| Surgery, n (%) | |||
| BCS | 990 (70%) | 337 (66%) | 653 (73%) |
| Mastectomy | 416 (30%) | 173 (34%) | 243 (27%) |
| Radiation after BCS, n (% of BCS) | |||
| Yes | 937 (95%) | 323 (96%) | 614 (94%) |
| No | 53 (5%) | 14 (4%) | 39 (6%) |
| Endocrine therapy, n (%) | 1361 (97%) | 488 (96%) | 873 (97%) |
| Tamoxifen | 536 (38%) | 179 (35%) | 357 (40%) |
| AI | 728 (52%) | 279 (55%) | 449 (50%) |
| Tamoxifen >> AI | 48 (3.4%) | 10 (2%) | 38 (4%) |
| AI >> tamoxifen | 3 (0.2%) | 3 (0.6%) | 0 |
| Tamoxifen + OFS | 23 (1.6%) | 10 (2%) | 13 (1.5%) |
| AI + OFS | 12 (0.9%) | 2 (0.4%) | 10 (1.1%) |
| Tamoxifen >> AI + OFS | 6 (0.4%) | 3 (0.6%) | 3 (0.3%) |
| Other combinations b | 5 (0.4%) | 2 (1%) | 3 (0.3%) |
| Chemotherapy, n (%) | 170 (12%) | 22 (4%) | 148 (17%) |
| Median follow up (range), months | 46 (1–85) | 45 (1–82) | 46 (1–85) |
| Distant metastasis, n (%) | 6 (0.4%) | 1 (0.2%) | 5 (0.6%) |
Abbreviation: BCS, breast conserving surgery; AI, aromatase inhibitor; OFS, ovarian function suppression.
One patient is male.
Other combinations include: tamoxifen + OFS >> AI + OFS (n=2); tamoxifen + OFS >> AI (n=1); AI + OFS >> tamoxifen (n=1); tamoxifen >> OFS >> AI (n=1)
Overall, 990 (70%) patients underwent breast conserving surgery and 416 (30%) had mastectomy. Among patients who underwent breast conserving surgery, 937 (95%) had radiation therapy. A total of 1361 (97%) patients received endocrine therapy, and 170 (12%) received chemotherapy. The median follow up time was 46 months (range 1–85). Six (0.4%) patients developed distant metastases. One (0.1%) patient died of disease 64 months after breast cancer diagnosis, 6 patients died of other causes, and 4 died of unknown causes.
Patients with Low Recurrence Score (RS<18) and Distant Metastases
Six (0.4%) of 1406 patients developed distant metastases within 5 years of initial breast cancer diagnosis. The clinicopathologic characteristics of these 6 patients are listed in details in Table 2. Only one patient was in the RS 0–10 group (RS=5), and 5 patients were in the RS 11–17 group. Pathologic confirmation of metastatic breast carcinoma was obtained for all 6 patients. None of the 6 patients developed locoregional recurrent disease, and none was enrolled in the TAILORx or RxPONDER trials.
Table 2.
Clinicopathologic characteristics of the 6 patients with ER-positive, HER2-negative, node-negative breast carcinoma of recurrence score <18 who developed distant metastasis
| Patients | #1 | #2 | #3 | #4 | #5 | #6 |
|---|---|---|---|---|---|---|
| Age at diagnosis (years) | 50 | 54 | 37 | 71 | 38 | 39 |
| Menopausal status | Pre | Pre | Pre | Post | Pre | Pre |
| Family history of breast/ovarian cancer | No | Yes | No | No | No | Yes |
| Personal history of breast carcinoma | No | Ipsilateral DCIS, 8 years prior | No | Ipsilateral DCIS, 13 years prior | No | No |
| Tumor type | ILC, classical and pleo-morphic | IDC | IDC | IDC a | IDC with focal micro-papillary features | IDC |
| Tumor size (cm) | 2.1 | 1.3 | 2.7 | 2.3 | 1.6 | 2.1 |
| Tumor Grade | 2 | 2 | 2 | 2 | 2 | 3 |
| Multi-focality | Yes | Yes | Yes | Yes | No | No |
| LVI | No | No | No | No | Yes | No |
| ER (%) | 90 | 95 | 95 | 95 | 95 | 95 |
| PR (%) | 30 | 5 | 85 | 75 | 75 | 95 |
| Oncotype DX RS | 5 | 12 | 12 | 13 | 14 | 17 |
| ESR1 expression | 10.2 | 9.3 | 10.8 | 10.7 | 9.2 | 9.5 |
| Surgery | BTM | TM | BTM | BCS | BCS | BTM |
| Radiation Tx | No | No | No | Yes | Yes | No |
| Endocrine Tx | Tamoxifen × 5 years, then BSO, followed by Letrozole | Tamoxifen | Tamoxifen | Arimidex | Nob | Tamoxifen |
| ChemoTx | No | No | CMF | No | No | No |
| Time interval to metastasis (months) | 58 | 41 | 25 | 20 | 48 | 12 |
| Site of metastasis | Bone | Lung, liver, brain, bone | Lung | Bone, liver, brain, lung | Bone, liver | Bone |
| Follow-up (months) | 72 | 53 | 59 | 64 | 71 | 42 |
| Survival | AWD | AWD | AWD | DOD | AWD | AWD |
Abbreviations: RS, recurrence score; ILC, invasive lobular carcinoma; IDC, invasive ductal carcinoma; LVI, lymphovascular invasion; BTM, bilateral total mastectomy; TM, total mastectomy; BCS, breast conserving surgery; Tx, therapy; BSO, bilateral salpingo-oophorectomy; CMF, cyclofosphamide, metotrexate and 5-fluorouracil. AWD, alive with disease; DOD, died of disease.
This patient also had two foci of ILC, measuring 0.6 cm and 0.2 cm respectively.
This patient declined hormonal treatment.
Patients with RS 11–17 and Distant Metastases
Of the 896 patients in the RS 11–17 group, 5 (0.6%) developed distant metastasis. Three of the 5 patients with metastases were younger than 40 years old at breast cancer diagnosis, and 4 were premenopausal. In this consecutively accrued and unselected cohort of patients with node-negative, ER+/HER2− BC and RS 11–17, the absolute incidence of distant metastases among patients with breast cancer diagnosed at age younger than 40 years old is 7.1% (3/42), whereas the absolute incidence of distant metastases among patients ≥40 years is 0.2% (2/854). The median follow up time in patients with breast cancer diagnosis at age younger than 40 years old vs ≥ 40 years was comparable (45.5 vs 45.8 months, p=0.5565). The median tumor size among patients with or without distant metastases was 2.1 cm and 1.2 cm, respectively. Treatment modalities, including the type of surgery (breast conserving surgery vs mastectomy), radiation therapy, endocrine therapy and chemotherapy were similar in patients with or without distant metastasis in this cohort.
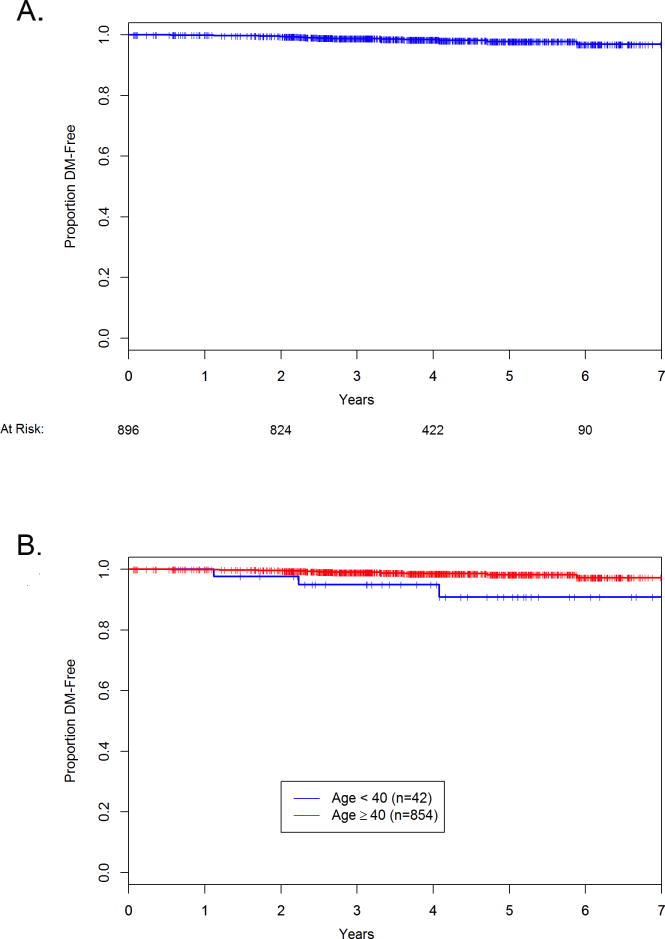
Taking into account the follow-up time, the Kaplan-Meier estimates of distant metastasis free survival overall and by age are shown in Figure 1. These graphs include deaths due to any cause as an event.
Figure 1.
Kaplan-Meier estimates in the analysis of the distant metastasis free survival. A. All 896 patients with recurrence scores of 11–17. B. Analyses by age. The red line is age 40 years or older, blue line is younger than 40 years old.
Discussion
The 21-gene expression assay is increasingly included in the evaluation of early-stage ER+/HER2− BC.15, 16 Multiple studies have assessed its impact on treatment decision-making in patients with breast carcinoma,17–26 reporting a change of chemotherapy recommendations in over 30% of the patients.17, 24 In particular, patients with BC of low RS receive adjuvant endocrine therapy, but no chemotherapy. In this study, we evaluated the characteristics of patients with node-negative, ER+/HER2− BC of low RS who developed distant metastases to identify factors that are associated with distant recurrence in this cohort where chemotherapy may not be routinely recommended.
The overall distant recurrence-free survival rate for patients with node negative ER+/HER2− BC and a low RS (<18) treated at our center was 99.6%. Distant metastases developed in 0.4% of 1406 unselected consecutive patients in this group. Two other studies reported outcome data in large patient populations treated based on the 21-gene recurrence score and found similar incidence of distant recurrence and breast cancer specific mortality in patients with low RS.27, 28 Only one of the 6 patients with distant metastasis in our study had a carcinoma with RS lower than 11. The rate of distant metastasis in the RS 0–10 group was very low (1/510; 0.2%), consistent with the TailoRx results in patients with BC of RS 0–10 reported by Sparano et al.10
Because TailoRx utilized a lower threshold (below 11) rather than 18 to select patients for endocrine therapy alone, it is now an open question as to whether or not higher risk patients within the historical low risk category have similar or significantly higher risk.12 Until the randomized cohort in TailoRx (scores 11–25) is reported, this clinically relevant question will not be definitively answered. In our RS 11–17 cohort, 873/896 (97%) of patients received endocrine therapy, and 148/896 (17%) patients received chemotherapy despite the low RS. Distant recurrence developed in 5/896 (0.6%) patients with median follow-up of 46 months. On exploratory analysis, our results suggest that age under 40 may be a prognostic factor in patients with node-negative ER+/HER2− BC of RS 11–17.
Paik et al. analyzed the impact of age and tumor size on the risk of distant recurrence.3 They reported that patients younger than 50 years old had higher distant recurrence rates at 10 years (21.1% vs. 12.3% respectively, p=0.004) and that patients with tumors ≥2 cm had higher rates of distant recurrence than patients with smaller tumors (17.5% vs. 13.3% respectively; p=0.06). However, age and tumor size lost statistical significance if the 21-gene expression assay result was added to the model.3 In the study by Paik et al,3 50 years of age was used as cutoff for the analysis, and no data were provided on the comparative analysis using other age thresholds. We also found no demonstrable difference in distant metastasis free survival using age cutoff of 50 years. However, our data suggests that patient younger than 40 years of age at breast cancer diagnosis have higher rates of distant recurrence. We note that there were only 16 patients younger than 40 years old in the low RS group in Paik’s study,3 whereas our cohort includes 63 patients under 40 years of age.
Recent results from a larger sample from SEER database do not confirm decreased survival in women <40 with low RS, but the completeness of ascertainment of chemotherapy use in this group is uncertain28. Prospective studies such as TailoRx will provide more definitive information on whether young age remains an independent prognostic factor in women with low RS.
In conclusion, our study identified 6 patients with node-negative ER+/HER2− BC and low 21-gene expression assay results (RS = 0–17) who developed distant recurrence within 5 years of breast carcinoma diagnosis. Our study population is unique, as it consists of a very large, unselected and consecutively accrued cohort of patients treated at a single institution, and for whom the 21 gene expression assay result was prospectively included in the treatment planning. Our results suggest that age younger than 40 years may be a negative prognostic factor even in patients with a low 21-gene expression assay score. Studies with a greater number of events are needed to determine if young age remains significant after adjusting for other variables.
This study has several limitations. First, our center is a tertiary academic institution with a predominant population of higher socioeconomic status and screen detected breast cancer. Therefore the results might not apply to different patient populations. Nonetheless, our study provides a large unselected cohort of patients with clinical follow-up. Second, not all patients in our study had 5 years of follow-up, due to the relatively recent adoption of the 21 gene expression assay. We will continue to follow this cohort of patients and obtain outcome information with long term follow up. Nonetheless, the rate of distant metastasis in patients younger than 40 years old obtained so far will not decrease. Thirdly, this study exploratory and descriptive, as statistical analysis is limited by the low number of events. Our findings, however, are hypothesis-generating, and mandate analysis of distant recurrence in relation to age under 40 when the large prospective randomized trials data become available.
Acknowledgments
We would like to thank Ms. Alicja Wiszowaty, Ms. Angelica Martin, and Ms. Katherine Lopez for their invaluable assistance with data collection.
Funding source:
Research reported in this publication was supported in part by a Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute (Grant No. P30CA008748).
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
Author Contributions:
Hannah Y Wen, MD, PhD: Conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing (original draft), visualization, supervision, project administration, responsible for the overall content
Melissa Krystel-Whittemore, MD: Investigation, writing (original draft)
Sujata Patil, PhD: Methodology, formal analysis
Fresia Pareja, MD, PhD: Investigation
Zenica L Bowser, MS: Investigation, data curation
Maura Dickler, MD: Writing (review and editing)
Larry Norton, MD: Writing (review and editing)
Monica Morrow, MD: Writing (review and editing)
Clifford Hudis, MD: Writing (review and editing)
Edi Brogi, MD, PhD: Conceptualization, methodology, resources, writing (review and editing), supervision
References
- 1.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 2.van’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 3.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 4.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 5.Gyorffy B, Hatzis C, Sanft T, Hofstatter E, Aktas B, Pusztai L. Multigene prognostic tests in breast cancer: past, present, future. Breast Cancer Res. 2015;17:11. doi: 10.1186/s13058-015-0514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 8.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 9.Sparano JA. TAILORx: trial assigning individualized options for treatment (Rx) Clin Breast Cancer. 2006;7:347–350. doi: 10.3816/CBC.2006.n.051. [DOI] [PubMed] [Google Scholar]
- 10.Sparano JA, Gray RJ, Makower DF, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015 doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramsey SD, Barlow WE, Gonzalez-Angulo AM, et al. Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating oncotypeDX-guided management for women with breast cancer involving lymph nodes. Contemp Clin Trials. 2013;34:1–9. doi: 10.1016/j.cct.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudis CA. Biology before Anatomy in Early Breast Cancer–Precisely the Point. N Engl J Med. 2015;373:2079–2080. doi: 10.1056/NEJMe1512092. [DOI] [PubMed] [Google Scholar]
- 13.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th. Springer; 2010. [Google Scholar]
- 14.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 15.Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30:2218–2226. doi: 10.1200/JCO.2011.38.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinan MA, Mi X, Reed SD, Hirsch BR, Lyman GH, Curtis LH. Initial Trends in the Use of the 21-Gene Recurrence Score Assay for Patients With Breast Cancer in the Medicare Population, 2005–2009. JAMA Oncol. 2015;1:158–166. doi: 10.1001/jamaoncol.2015.43. [DOI] [PubMed] [Google Scholar]
- 17.Lo SS, Mumby PB, Norton J, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol. 2010;28:1671–1676. doi: 10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- 18.Ademuyiwa FO, Miller A, O’Connor T, et al. The effects of oncotype DX recurrence scores on chemotherapy utilization in a multi-institutional breast cancer cohort. Breast Cancer Res Treat. 2011;126:797–802. doi: 10.1007/s10549-010-1329-6. [DOI] [PubMed] [Google Scholar]
- 19.Geffen DB, Abu-Ghanem S, Sion-Vardy N, et al. The impact of the 21-gene recurrence score assay on decision making about adjuvant chemotherapy in early-stage estrogen-receptor-positive breast cancer in an oncology practice with a unified treatment policy. Ann Oncol. 2011;22:2381–2386. doi: 10.1093/annonc/mdq769. [DOI] [PubMed] [Google Scholar]
- 20.Partin JF, Mamounas EP. Impact of the 21-gene recurrence score assay compared with standard clinicopathologic guidelines in adjuvant therapy selection for node-negative, estrogen receptor-positive breast cancer. Ann Surg Oncol. 2011;18:3399–3406. doi: 10.1245/s10434-011-1698-z. [DOI] [PubMed] [Google Scholar]
- 21.Albanell J, Gonzalez A, Ruiz-Borrego M, et al. Prospective transGEICAM study of the impact of the 21-gene Recurrence Score assay and traditional clinicopathological factors on adjuvant clinical decision making in women with estrogen receptor-positive (ER+) node-negative breast cancer. Ann Oncol. 2012;23:625–631. doi: 10.1093/annonc/mdr278. [DOI] [PubMed] [Google Scholar]
- 22.Joh JE, Esposito NN, Kiluk JV, et al. The effect of Oncotype DX recurrence score on treatment recommendations for patients with estrogen receptor-positive early stage breast cancer and correlation with estimation of recurrence risk by breast cancer specialists. Oncologist. 2011;16:1520–1526. doi: 10.1634/theoncologist.2011-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eiermann W, Rezai M, Kummel S, et al. The 21-gene recurrence score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use. Ann Oncol. 2013;24:618–624. doi: 10.1093/annonc/mds512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson JJ, Roth JA. The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;141:13–22. doi: 10.1007/s10549-013-2666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinan MA, Mi X, Reed SD, Lyman GH, Curtis LH. Association Between Use of the 21-Gene Recurrence Score Assay and Receipt of Chemotherapy Among Medicare Beneficiaries With Early-Stage Breast Cancer, 2005–2009. JAMA Oncol. 2015;1:1098–1109. doi: 10.1001/jamaoncol.2015.2722. [DOI] [PubMed] [Google Scholar]
- 26.Levine MN, Julian JA, Bedard PL, et al. Prospective Evaluation of the 21-Gene Recurrence Score Assay for Breast Cancer Decision-Making in Ontario. J Clin Oncol. 2015 doi: 10.1200/JCO.2015.62.8503. [DOI] [PubMed] [Google Scholar]
- 27.Stemmer S, Steiner M, Rizel S, et al. First prospective outcome data in 930 patients with more than 5 year median follow up in whom treatment decisions in clinical practice have been made incorporating the 21-Gene Recurrence Score. European Journal of Cancer. 2015;51:S321. [Google Scholar]
- 28.Petkov VIMD, Howlader N, Gliner N, Howe W, Schussler N, Cronin K, Baehner FL, Cress R, Deapen D, Glaser SL, Hernandez BY, Lynch CF, Mueller L, Schwartz AG, Schwartz SM, Stroup A, Sweeney C, Tucker TC, Ward KC, Wiggins C, Wu X, Penberthy L, Shak S. Breast-cancer-specific mortality in patients treated based on the 21-gene assay: a SEER population-based study. NPG Breast Cancer. 2016:2. doi: 10.1038/npjbcancer.2016.17. Article number: 16017. [DOI] [PMC free article] [PubMed] [Google Scholar]