Abstract
OBJECTIVE
There is currently no accepted method of mapping bilateral cochlear-implant (BiCI) users to maximize binaural performance, but the current approach of mapping one ear at a time could produce spatial perceptions that are not consistent with a sound’s physical location in space. The goal of this study was to investigate the perceived intracranial lateralization of bilaterally synchronized electrical stimulation with a range of interaural level differences (ILDs) and to determine a method to produce relatively more centered auditory images when provided multi-electrode stimulation.
DESIGN
Using direct stimulation, lateralization curves were measured in nine BiCI listeners using 1000-pps, 500-ms, constant-amplitude pulse trains with ILDs that ranged from −20 to +20 clinical current units (CUs). The stimuli were presented bilaterally at 70–80% of the dynamic range (%DR) on single- or multiple-electrode pairs. For the multi-electrode pairs, the ILD was applied consistently across all the pairs. The lateralization response range and the bias magnitude at 0-CU ILD (i.e., the number of CUs needed to produce a centered auditory image) were computed. Then the levels that elicit a centered auditory image with single-electrode stimulation were used with multi-electrode stimulation to determine if this produced fewer significant biases at 0-CU ILD. Lastly, a multi-channel ILD processing model was used to predict lateralization for the multi-electrode stimulation from the single-electrode stimulation.
RESULTS
BiCI listeners often perceived both single- and multi-electrode stimulation at 0-CU ILD as not intracranially centered. For single-electrode stimulation, 44% of the lateralization curves had relatively large (≥5 CU) bias magnitudes. For the multi-electrode stimulation, 25% of the lateralization curves had large bias magnitudes. After centering the single-electrode pairs, the percentage of multi-electrode combinations that produced large biases significantly decreased to only 4% (p<0.001, McNemar’s test). The lateralization with multi-electrode stimulation was well predicted by a model that used an unweighted or weighted average single-electrode lateralization percepts across electrode pairs (87 or 90%, respectively).
CONCLUSION
Current BiCI mapping procedures can produce an inconsistent association between a physical ILD and the perceived location across electrodes for both single- and multi-electrode stimulation. Explicit centering of individual electrode pairs using the perceived centered intracranial location almost entirely corrects this problem and such an approach is supported by our understanding and model of across-frequency ILD processing. Such adjustments might be achieved by clinicians using single-electrode binaural comparisons. Binaural abilities, like sound localization and understanding speech in noise, may be improved if these across-electrode perceptual inconsistencies are removed.
Keywords: cochlear implant, binaural hearing, sound localization, binaural models, interaural level differences
INTRODUCTION
Sound localization is typically better in bilateral cochlear-implant (BiCI) users compared to unilateral cochlear-implant (CI) users (Litovsky et al. 2009; Litovsky et al. 2004; Neuman et al. 2007; Nopp et al. 2004; van Hoesel et al. 2003; Verschuur et al. 2005). However, BiCI users have limited sound localization abilities compared to normal-hearing (NH) individuals (Grantham et al. 2008; Grantham et al. 2007; Litovsky et al. 2012; Majdak et al. 2011). As the number of individuals receiving BiCIs continues to grow (Peters et al. 2010), maximizing the advantages conveyed by BiCIs should become an important objective for the programming, fitting, or “mapping” of BiCIs for an individual user.
According to the “duplex theory” (Rayleigh 1907), NH listeners primarily utilize ITDs for localization of low-frequency sounds (<1500 Hz) and ILDs for high-frequency sounds (>1500 Hz). Given access to both ITD and ILD cues, NH listeners demonstrate a low-frequency ITD dominance in the sound localization of complex broadband stimuli (Macpherson et al. 2002; Wightman et al. 1992). In contrast, BiCI listeners demonstrate an ILD dominance in the sound localization of complex broadband stimuli (Grantham et al. 2008; Seeber et al. 2008). Along these lines, Aronoff et al. (2010) independently manipulated the ITDs and ILDs using non-individualized head-related transfer functions applied to broadband sounds. They presented the virtually spatialized sounds to the unsynchronized clinical processors of the BiCI listeners via the direct audio inputs. They showed that there was no difference in localization performance for BiCI listeners between the “ILD-only condition” (when ILDs were allowed to vary and ITDs were held constant at zero) and the “ILD+ITD condition” (when both ILDs and ITDs were allowed to naturally vary), with the mean root-mean-square (RMS) error of about 25º for both conditions. In contrast, a significantly larger mean RMS error of 58º was found for the “ITD-only condition,” suggesting that the localization performance was greatly diminished when ILDs were held constant and the stimuli contained only varying ITDs. In fact, it is possible that BiCI users may show an ILD dominance even in cases where there is bilateral low-frequency hearing preservation (Dorman et al. 2013).
One explanation for the suboptimal localization performance of BiCI users compared to NH listeners is the degradation and interaural decorrelation (i.e., across ear dissimilarity of the signals) of the localization cues after the electrical stimuli are independently processed and presented to each ear. The lack of synchronization between BiCI sound processors may distort ITDs and ILDs. The fine structure of the stimulus (i.e., the rapid temporal changes in the acoustic signal, the carrier information) is removed in most sound processing strategies, and the low-frequency fine structure is the major contributor to the low-frequency ITD dominance for sound localization in NH listeners (Bernstein et al. 1985; Macpherson and Middlebrooks 2002; Wightman and Kistler 1992). Therefore, ILDs appear to be the only salient azimuthal localization cue available to BiCI users using clinical sound processors (Aronoff et al. 2010) and this highlights the importance of ensuring the availability and accuracy of ILDs to this population.
The conventional CI mapping procedure is to treat the two sound processors independently, which potentially undermines a consistent relationship between the ILDs and location perception. At a clinical appointment for a CI user, mapping involves determining proper comfortable (C), and threshold (T), current levels on each electrode. This is done independently for each ear, with little emphasis on single-electrode bilateral loudness balancing with sequential bilateral stimulation or verification of location percepts with simultaneous bilateral stimulation. Significant ear- and electrode-specific differences in effective electrical current that reaches the auditory nerve are quite common, which is affected by the proximity of the electrode to modiolus, survival of the local spiral ganglion cells, and/or growth of the connective tissue around the electrode (Bierer et al. 2014; Kawano et al. 1998). Further adjustments of C and T levels are often dictated by speech perception and sound quality, often performed globally (i.e., all electrodes in one ear have the levels increased or decreased). However, such global adjustments may also adversely affect the ILDs and perceived spatial locations in BiCI users.
There is increasing evidence that even under highly-controlled experimental conditions the intracranial lateralization perception (i.e., how left or right auditory sound image is heard in the head) of ILDs can show variable response ranges and overall response biases across BiCI listeners. Many studies have tested lateralization abilities in BiCI listeners using bilaterally synchronized research processors that replace the clinical sound processors (i.e., direct stimulation) and stimulate single-electrode pairs to ensure high levels of control over ILDs and ITDs. For example, van Hoesel and Tyler (2003) studied lateralization with single-electrode direct stimulation of 50-pps constant-amplitude pulse trains in five BiCI listeners. They showed that ILD lateralization ranges varied across listeners from 55 to 100% of the available response range when the ITD was held constant at zero. Goupell, Kan, et al. (2013) and Kan et al. (2013) showed a significant variability in BiCI listeners’ ILD lateralization range and variable substantial biases towards an individual ear with single-electrode direct stimulation of 100- and 1000-pps constant-amplitude pulse trains. Kan et al. (2013) also showed that interaural place-of-stimulation mismatch resulted in a lateralization bias toward the ear with more basal stimulation. Lateralization biases can vary with the presentation level on an individual electrode (Goupell, Kan, et al. 2013). Taken together, such inconsistencies of perceived location across and within electrodes could be problematic for encoding the spatial location of a modulated signal like speech as different spectral-temporal portions of the signal might be perceived at different locations.
It is important to note that all of the previously mentioned ILD-based lateralization experiments in BiCI listeners presented the stimuli with single-electrode pair direct stimulation. While such an approach affords the highest level of control (arguably an experimental approach that is the closest to presenting tones to NH listeners over headphones), sounds that a BiCI user would need to localize in everyday situations would be broadband stimuli that activate multiple electrode sites simultaneously. There has been only one direct stimulation study where the perception of ILDs presented on multiple electrodes was investigated in BiCI listeners (Best et al. 2011). They presented 100-pps pulse trains on two electrodes, which were located at opposite ends of the arrays, thus limiting effects of channel interactions. On one electrode, a non-zero ILD was applied (the target); on the other electrode, zero ILD was applied (the interferer). Five of six BiCI listeners demonstrated shallower lateralization slopes in the presence of the spectrally remote interferer, suggesting a central computation or combination of the ILDs across frequency (i.e., binaural interference; McFadden et al. 1976). Therefore, it is possible that inconsistent ILDs across channels may lead to a decrease in performance on multi-electrode ILD processing in BiCI listeners, which could in turn be a limiting factor for other binaural tasks, such as sound localization and binaural unmasking of speech using clinical sound processors. However, it is difficult to extrapolate the results of Best et al. (2011) to conditions with more active electrodes, with electrodes that are more closely spaced, and with non-zero ILDs applied to all of the electrode pairs. In other words, it is still unclear how BiCI users combine ILD information across several electrode sites. Such information is important to understand because the aforementioned problems of relating the physical interaural cues to perception likely become compounded when using realistic broadband sounds that stimulate multiple electrodes because the electrical fields from neighboring electrodes could interact to change the effective ILDs.
The purpose of this work was to advance our knowledge of binaural processing in BiCI users beyond single-electrode stimulation, with the goal to eventually improve the ability of BiCI users to better localize complex sound sources with ILDs. First, we investigated if multi-electrode stimulation produced a change in binaural performance as compared to single-electrode stimulation. Then, we investigated if the multi-electrode stimulation could be better controlled. We hypothesized that centering individual electrode pairs would reduce the number of lateralization biases in multi-electrode stimulation. More broadly, the results of this study describe the extent of the incongruence between the intended delivery and perception of ILDs provided to BiCI users. Finally, we also present control data on lateralization of multi-frequency stimuli in NH listeners and discuss the differences in lateralization ranges and lateralization biases in NH and BiCI listeners.
MATERIALS AND METHODS
Listeners
NH listeners
Eight NH listeners, who ranged in age from 19 to 38 years (2 males, 6 females), were tested in this study as a control group. All NH listeners had hearing thresholds within 20 dB hearing level (HL) at octave frequencies measured between 250 and 8000 Hz, with no more than a 10-dB difference in thresholds between the ears at any tested frequency. The invitation to participate in the study was distributed among students at the University of Maryland, College Park. The first eight listeners who responded to the invitation and met the selection requirements for NH group were included in the study and most of the listeners were paid an hourly wage.
BiCI listeners
Nine BiCI listeners with CI24 and CI512 family of implants (Cochlear Ltd., Sydney, Australia) were tested in this study. The BiCI listeners ranged in age from 45 to 74 years (2 males, 7 females). Eight BiCI listeners were postlingually deafened and received the second CI within 1–10 years from the implantation of the first CI. One listener (CAW) had a congenital hearing loss and was implanted at the age of 47 years. Table 1 shows the hearing history and etiology of hearing loss in the BiCI listeners. Most of the BiCI listeners were paid a stipend for their participation. Note that the age ranges of the NH and BiCI listeners were non-overlapping.
Table 1.
Demographic data, hearing loss history, and the models of the intracochlear implant for nine BiCI listeners.
| Listener | Sex | Age (Yrs) | Age at onset of deafness (Yrs) | Implant Type | CI experience | Etiology of hearing loss | ||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | |||||
| CAD | M | 74 | 62 | CI24R | CI24RE | 9 | 3 | Unknown |
| CAE | F | 61 | 51 | CI24RE | CI24RE | 5 | 7 | Hereditary |
| CAG | F | 64 | 20 | CI24RE | CI24R | 9 | 8 | Unknown |
| CAL | F | 53 | 34 | CI24RE | CI24R | 3 | 10 | Unknown |
| CAP | F | 45 | 38 | CI24RE | CI24RE | 6 | 5 | Unknown |
| CAQ | F | 54 | 44 | CI24RE | CI24RE | 4 | 5 | Unknown |
| CAS | F | 49 | 41 | CI24RE | CI512 | 7 | 3 | Unknown |
| CAW | M | 52 | 0 | CI24RE | CI24RE | 5 | 1 | Congenital |
| CBA | F | 54 | 20 | CI24RE | CI512 | 1 | 2 | Stickler’s syndrome |
Equipment and Stimuli
NH listeners
For the NH listeners, testing was conducted in a double-walled sound-proof booth (IAC, New York). A Tucker-Davis Technologies System 3 (RP2.1, PA5, and HB7, Alachua, FL, USA) was used to deliver the stimuli to insert earphones (ER2, Etymotic, Illinois). Stimuli were generated on a personal computer using Matlab software (version 7.12.0, Mathworks, Natick, MA).
The acoustic stimuli were narrow-band noises with a bandwidth of 10 Hz and center frequencies of 500, 750, or 1000 Hz. A complex stimulus combining all three narrow-band noises was used for a multi-band stimulus. The acoustic stimuli were 500 ms in duration and were temporally shaped by Tukey window with a 10-ms rise/fall time. Each narrow-band noise had a 65 dB-A level, as measured on a sound level meter (Brüel & Kjær Sound & Vibration Measurement A/S, Naerum, Denmark). For the multi-band stimulus, each individual noise band was presented at 65 dB-A. The stimuli had the following ILDs applied: 0, ±3, ±6, ±9, ±12, or ±18 dB. Positive ILDs are defined as having a larger level in the right ear. The ILDs were applied by increasing the level in one ear by ILD/2 and reducing the level in the other ear by ILD/2. For the multi-band stimulus, the ILDs were applied consistently across all three noise bands.
BiCI listeners
Stimuli for these experiments were generated on a personal computer using Matlab software (version 7.12.0, Mathworks, Natick, MA). The Nucleus Implant Communicator software (NIC2, Cochlear Ltd., Macquarie University, Sydney, Australia) and L34 research sound processors were used to deliver bilaterally synchronized electrical pulse trains to the BiCIs. The stimuli were constant-amplitude, 500-ms pulse trains, where the pulses were presented at a rate of 1000 pulses per second (pps). The pulses were biphasic with a 25-μs phase duration and an 8-μs phase gap, and were delivered via monopolar stimulation mode. The stimuli were presented at 80% of the dynamic range (DR), which was defined as the difference between the T (threshold) and C (the loud, but comfortable level) for the listeners1. The stimulation levels in CU for each electrode can be calculated by finding 20% of the DR in CU and subtracting this value from the C level. This method allowed us to lower the levels for multi-electrode stimulation for the same proportion of the DR for all listeners to avoid uncomfortably loud stimuli. The stimuli had the following ILDs applied: 0, ±2, ±5, ±10, or ±20 CUs. ILDs were applied by reducing the level on an electrode (e.g., a +20-CU ILD reduced the level from 80%DR in the left ear while the level in the right ear remained unchanged). In cases where the DR of the ear was less than 20 CUs, additional current was applied to the other ear (Goupell, Kan, et al. 2013). Positive ILDs were defined as a relatively higher current in %DR in the right ear (i.e., the current in the left ear was reduced) and negative ILDs were defined as a relatively higher current in %DR in the left ear. ILDs were applied in CUs because it is the most clinically relevant unit (clinicians adjust levels in CUs), it afforded the most controlled changes in levels (round off error from using %DR could be problematic for small DR electrodes and small ILDs), and CUs can be converted to %DR during the data analysis. The largest ILDs that were used (±20 CUs) corresponded to 53%DR on average, with a range from 24 to 182%DR depending on the electrode and listener, which can be seen in Table 2.
Table 2.
C levels and DRs for each electrode pair and each BiCI listener.
| Listener | Electrode | C level (CUs) | DR (CUs) | 20 CU converted to %DR | |||
|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | ||
| CAD | 4 | 195 | 183 | 60 | 42 | 33 | 48 |
| 8 | 211 | 197 | 63 | 83 | 32 | 24 | |
| 12 | 216 | 204 | 60 | 63 | 33 | 32 | |
| 16 | 220 | 206 | 64 | 68 | 31 | 29 | |
| 20 | 219 | 202 | 53 | 58 | 38 | 34 | |
| CAE | 4 | 154 | 140 | 55 | 51 | 36 | 39 |
| 8 | 172 | 162 | 63 | 54 | 32 | 37 | |
| 12 | 171 | 168 | 53 | 51 | 38 | 39 | |
| 16 | 172 | 164 | 55 | 49 | 36 | 41 | |
| 20 | 180 | 178 | 52 | 48 | 38 | 42 | |
| CAG | 4 | 178 | 229 | 35 | 46 | 57 | 43 |
| 8 | 210 | 240 | 42 | 46 | 48 | 43 | |
| 12 | 212 | 240 | 47 | 40 | 43 | 50 | |
| 16 | 222 | 242 | 55 | 40 | 36 | 50 | |
| 20 | 201 | 234 | 54 | 37 | 37 | 54 | |
| CAL | 4 | 163 | 216 | 46 | 48 | 43 | 42 |
| 8 | 183 | 211 | 46 | 37 | 43 | 54 | |
| 12 | 189 | 229 | 49 | 37 | 41 | 54 | |
| 16 | 189 | 228 | 39 | 16 | 51 | 125 | |
| 20 | 192 | 220 | 39 | 16 | 51 | 125 | |
| CAP | 4 | 137 | 166 | 50 | 40 | 40 | 50 |
| 8 | 150 | 169 | 42 | 47 | 48 | 43 | |
| 12 | 143 | 157 | 35 | 22 | 57 | 91 | |
| 16 | 142 | 152 | 40 | 14 | 50 | 143 | |
| 20 | 144 | 165 | 27 | 16 | 74 | 125 | |
| CAQ | 4 | 138 | 127 | 20 | 29 | 100 | 69 |
| 8 | 138 | 153 | 28 | 42 | 71 | 48 | |
| 12 | 138 | 150 | 29 | 52 | 69 | 38 | |
| 16 | 143 | 137 | 35 | 28 | 57 | 71 | |
| 20 | 142 | 140 | 32 | 31 | 63 | 65 | |
| CAS | 4 | 183 | 150 | 65 | 51 | 31 | 39 |
| 8 | 194 | 166 | 68 | 57 | 29 | 35 | |
| 12 | 188 | 186 | 56 | 67 | 36 | 30 | |
| 16 | 191 | 178 | 53 | 63 | 38 | 32 | |
| 20 | 192 | 170 | 46 | 11 | 43 | 182 | |
| CAW | 4 | 206 | 255 | 52 | 57 | 38 | 35 |
| 8 | 197 | 247 | 43 | 47 | 47 | 43 | |
| 12 | 183 | 219 | 59 | 50 | 34 | 40 | |
| 16 | 172 | 206 | 48 | 60 | 42 | 33 | |
| 20 | 162 | 184 | 51 | 55 | 39 | 36 | |
| CBA | 4 | 166 | 163 | 23 | 28 | 87 | 71 |
| 8 | 175 | 177 | 30 | 38 | 67 | 53 | |
| 12 | 181 | 170 | 39 | 31 | 51 | 65 | |
| 16 | 158 | 166 | 28 | 36 | 71 | 56 | |
| 20 | 151 | 166 | 17 | 24 | 118 | 83 | |
Five electrode pairs were chosen for single- and multi-electrode stimulation: 4, 8, 12, 16, and 20, where electrode 4 was located in the relatively basal part of the cochlea and electrode 20 was located in the relatively apical part, therefore stimulating higher and lower places, respectively. The electrode pairs were number-matched (as is typically done in the clinic), not pitch-matched (as is typically done in BiCI research). Since mismatches >3 mm have shown to significantly change lateralization and fusion percepts in BiCI listeners (Goupell 2015; Goupell, Stoelb, et al. 2013; Kan et al. 2015; Kan et al. 2013), we verified that none of the BiCI listeners in this study had pitch mismatch magnitudes >3 mm in separate pitch-matching experiments. By using number-matched electrode pairs we also kept the spacing between electrodes constant and thus better controlled the amount of channel interactions between electrode pairs.
Procedure
The methods and procedures were approved by the University of Maryland Institutional Review Board. Each listener received a uniform set of instructions briefly describing the task. Before the beginning of the experiment, the listeners were familiarized with the experimental screen, which included an image of a face with a transparent horizontal bar. The bar was positioned across the middle of the face and extended from one ear to another. The listeners initiated each trial by pressing a button and were allowed to repeat the stimulus as many times as necessary. The listeners reported the lateralization of the auditory image by adjusting a cursor on the horizontal bar on the screen until it matched the intracranial position of the image. The bar had a linear scale with values that ranged from −10 (completely in the left ear) to 0 (centered in the head) to +10 (completely in the right ear), although the numerical value of scale was not disclosed to the listeners. If multiple auditory images were perceived, the listeners also had the option to indicate the location of the additional image(s) (up to three total). There was no “correct” answer in the subjective lateralization task and therefore no response feedback was provided to the listeners.
NH listeners
Lateralization was measured in the NH listeners using four different stimuli (four conditions). Three of them consisted of narrow-band noises with a center frequency of 500, 750, or 1000 Hz, and one was a multi-band stimulus, which combined all three frequency bands. First, a familiarization block was administered, which consisted of five trials per condition for the four conditions and 11 ILDs. After completion of the block, the listeners reported that they fully understood the procedure and format of the test to an experimenter. Then, listeners were presented blocks of four conditions. Each block consisted of one to four conditions with 5–10 trials per condition, with no more than 600 trials per block. The number of trials in a block was chosen by the listener depending on whether they preferred shorter or longer experimental blocks. The order of the blocks and conditions within each block were randomized. Each listener completed 20 trials per condition and ILD.
BiCI listeners
To set the current levels for the experiment, mapping procedures for the BiCI listeners followed those outlined in Litovsky et al. (2012) and generally followed current clinical practice for mapping of BiCIs. Briefly, T and C levels were obtained for each of the five electrodes in each ear using a conventional mapping procedure. The T level was defined as the threshold of audibility of electrical stimulation. The C level was the stimulation level that was most comfortable and tolerable when listening to for long periods of time. Then the C levels were loudness balanced within each ear by sequentially playing 500-ms pulse trains on each of the five electrodes tested with an inter-stimulus interval of 100 ms. Adjustments were made until the C levels at all five electrodes in this ear were perceived as equally loud. Such unilateral loudness balancing adjustments never exceeded 5 CUs. Neither sequential nor simultaneous bilateral loudness balancing was performed because it does not occur during a clinical mapping appointment.
Lateralization was measured in the BiCI listeners using 21 different stimuli. There were five one-electrode conditions (electrode pairs 4,8,12,16, and 20) and eight multi-electrode conditions [five two-electrode (pairs 8–12, 12–16, 4–12, 12–20, and 4–20), two three-electrode (pairs 4–12–20 and 8–12–16), and one five-electrode (pairs 4–8–12–16–20)]. In addition, for the multi-electrode conditions, the current levels on each individual electrode pair were either unadjusted (called “uncentered”) or adjusted using the single-electrode lateralization data so that the 0-CU ILD point was perceived as centered in the head (called “centered”).
Then a familiarization block was administered, which consisted of five trials per condition for the five single-electrode conditions and 11 ILDs per condition. After completion of the familiarization block, the listeners reported that they fully understood the procedure and format of the test to an experimenter. Second, all five single-electrode conditions were presented in a randomized order. There were four blocks with five trials per condition and ILD, resulting in 20 trials per condition and ILD. Third, eight multi-electrode conditions (two-, three-, and five-electrodes) were presented in a randomized order. Blocks of the multi-electrode conditions consisted of two trials per condition and ILD. The multi-electrode condition blocks consisted of either “uncentered” or “centered” current levels. The order of blocks with uncentered and centered conditions was counterbalanced across listeners. In all conditions, 20 trials were collected for each ILD in each condition. The randomization of conditions and counterbalancing of the pre- and post-centering multi-electrode conditions were done to avoid possible order effects (e.g., changes in responses due to fatigue).
The rationale for presenting single-electrode conditions before the multi-electrode conditions was that the centering procedure for the multi-electrode conditions required a complete set of single-electrode lateralization data. Therefore, randomizing single-electrode conditions and centered multi-electrode conditions was not possible. It would have been possible to randomize the single- and uncentered multi-electrode conditions. However, the most clinically relevant question is the effectiveness of the centering procedure, and therefore it was more desirable to counterbalance the uncentered and centered multi-electrode conditions.
Data Analyses
Lateralization responses in NH and BiCI listeners
The lateralization response patterns were compared within subjects, within subject groups, and across subject groups. Lateralization responses for the first response for each condition in NH and BiCI listeners were fit using a least-square regression assuming a four-parameter cumulative Gaussian function. In the cases where the subject responded with multiple auditory images, the other responses were ignored. Specifically, the function was of the form
| (1) |
where x is the ILD, erf is the error function, and A, σ, μX, and μY are the four free parameters used to optimized to fit the data (Goupell, Kan, et al. 2013). In the NH listeners, the lateralization responses to single- and multi-electrode stimulation were compared using a two-way repeated-measures analysis of variance (ANOVA) with factors frequency band (500, 750, 1000 Hz, and multi) and ILD. In the BiCI listeners, the data showed substantial inter-subject variability and therefore inferential statistics were not performed because they were not particularly informative or elucidating.
Lateralization ranges in NH and BiCI listeners
The extents of laterality were compared across our groups of listeners. The lateralization range for single and multi-band/electrode conditions was defined as the difference between the left-most average response and the right-most average response. Since the response bar had a linear scale from −10 (left-most edge) to +10 (right-most edge), the maximum possible lateralization range was 20. We presented a relatively large range of ILDs to both the NH (±18 dB) and BiCI (±20 CUs) listeners, which produce lateralization ranges of about 15–20 (Litovsky et al. 2010; Yost 1981).
To facilitate the comparison of the lateralization ranges of the NH and BiCI listeners, the ILDs were expressed as %DR. Note that there is no direct way to compare electrical and acoustical changes in level, and therefore such an approach should be viewed with an appropriate amount of skepticism. Nonetheless, such a comparison greatly facilitates interpretation of the data, even if it is only a rough approximation.
The DR for NH listeners was conservatively assessed to be 70 dB calculated from a threshold of 0-dB hearing level (HL; average threshold of hearing) to 70-dB HL, which is a comfortably loud level for the narrow-band noises presented at frequencies 500, 750, or 1000 Hz, and thus comparable to a C level of BiCI listeners. An 18-dB ILD presented to NH listeners in this study would utilize 26% of the DR of 70 dB in one ear and therefore 52% of the DR for a full span of ±18 dB for both ears (18 + 18 dB = 36 dB ILD range; 36 dB/70 dB=52%DR). Because the lateralization range was measured from the average left-most to the average right-most response (from −10 to 10), we used a full span of ILDs (from −18 to 18 dB ILD in NH listeners and from −20 dB to +20 dB in BiCI listeners) to calculate the %DR that was required to elicit the observed lateralization range in each listener.
The DR of BiCI listeners was measured as a range between T and C levels. The %DR was measured separately in each ear and then summed because the DR was different across the ears in many BiCI listeners. For multi-electrode conditions the %DR in each ear was calculated based on the electrode pair with the largest DR. The ILD spans expressed as %DR were used to better compare the lateralization ranges across the BiCI listeners. This was done to test the possibility that a chosen span of ILDs in BiCI and NH listeners was not optimal in producing the largest lateralization range in each listener, or the skewness in lateralization range data in BiCI listeners could affect the interpretation of the results. On average, ±20 CUs was 53%DR (24%DR minimum; 182%DR maximum; see Table 2).
The lateralization range data from the BiCI listeners were not normally distributed and did not meet the assumption of homogeneity. Therefore, the data for all comparison groups (single- and multi-band/electrode conditions, NH and BiCI listeners) were analyzed using a non-parametric Kruskal-Wallis test, followed by Mann-Whitney tests with Bonferroni correction for between-group comparisons (based on p=0.05, six pairwise comparisons for four groups yielded a corrected level of significance of 0.0083).
Auditory image bias values at 0-dB/CU ILD in NH and BiCI listeners
This analysis sought to quantify the biases of stimuli that were intended to be centered in the head, and compare this across groups. Auditory image 0-dB/CU ILD bias values were estimated to determine if a 0-dB/CU ILD was perceived as located in the center of the head. In NH listeners, the location of the intracranial auditory image is considered to be centered in the head when the stimuli are presented at 0-dB ILD (Yost 1981), although small deviations may result from headphone placement or perceptual biases (Mills 1960; Yost 1981). Headphone placement was likely not an issue in this study because the NH listeners used insert earphones. In BiCI listeners, the location of the intracranial auditory image is not centered in the head when the stimuli are presented at 0-CU ILD at a pair of electrodes (Goupell, Kan, et al. 2013). Non-centered 0-CU ILD images seem to result from place of stimulation mismatch (Kan et al. 2013), although differential neural survival across the ears may explain such a phenomenon. Non-centered 0-CU ILD auditory images can occur even when the levels are carefully sequentially loudness balanced (Fitzgerald et al. 2015).
Bias magnitudes were calculated from the point at which the fit crossed the perceptual intracranial lateralization midline, rounded to the nearest integer. Most of the time, this corresponded to the free parameter μX in Eq. 1. The distributions of 0-dB/CU ILD bias magnitudes were presented as histograms with a bin size of 2 dB/CU for single- and multi-band/electrode conditions. The bias magnitudes from a 0-dB/CU ILD were measured in dB/CU, as well as in %DR. For the BiCI listeners, since they had unequal DRs across the ears, the bias calculation used the DR in the ear which corresponded to the direction of the bias. For example, for a BiCI listener with a 40-CU DR in the left ear and a 25-CU DR in the right ear, a 5-CU ILD bias toward the left ear would correspond to a –5-CU/40-CU = –12.5%DR bias (biases toward the left ear are defined as negative and toward the right ear are defined as positive, following the convention of the direction of the ILD).
Centering of auditory images in multi-electrode stimulation in BiCI listeners
In an attempt to consistently produce a centered perception of the multi-electrode stimulation in the BiCI listeners, current levels on individual electrode pairs were adjusting based on the bias magnitudes estimated in single-electrode stimulation. For example, if the bias magnitude on the single-electrode pair was –5-CU, suggesting a perceptual bias toward the right ear, the current level in the right ear was reduced by 5 CUs. The adjustment was always done by reducing the current in the ear with the same direction as the bias. The amount of the required adjustment never exceeded the DR in the biased ear.
Two hypotheses were tested to assess the differences in 0-CU ILD bias magnitudes under different conditions in the BiCI listeners. The first null hypothesis was that there was no difference in 0-CU ILD bias magnitude distributions following single- vs multi-electrode stimulation. The second null hypothesis was that there was no difference in 0-CU ILD bias magnitude distributions following multi-electrode stimulation before vs after the centering procedure. The differences in bias magnitude distributions were tested using a Kolmogorov-Smirnov test. Finally, a McNemar’s test (a non-parametric paired version of the χ2 test) with Yates' continuity correction was used to assess the efficacy of the centering procedure by reducing the number of significant bias magnitudes in multi-electrode stimulation.
Modeling of multi-electrode lateralization in BiCI listeners using response average and variance
There is presently no formal model that predicts the across-frequency processing of ILDs. Our data, with the potentially inconsistent lateralization perception across different frequency regions in the BiCI listeners, could be used to formulate such a model. Our model had two components. First, we wanted to account for the possibility that some frequencies would contribute more to the lateralization percept than others. This was realized by attempting to weight electrodes more heavily with more consistent (i.e., lower variance) responses obtained from the perceptual experiment. Second, there needed to be an across-frequency combination operation. We used the across-frequency combination operations thought to be used in ITD processing, namely summation or multiplication across frequency channels (Pena et al. 2000; Saberi et al. 1998; Shackleton et al. 1992; Stern et al. 1988; Trahiotis et al. 1989).
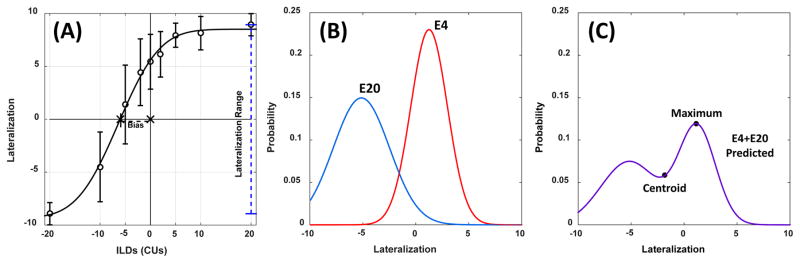
Figure 1B shows an example of our modeling approach. First, we created Gaussian distributions with unity area, where the mean and variance of the distribution was based on the observed mean and variance of the perceptual responses at each ILD in each listener. An example set of two modeled distributions for the responses of two electrode pairs that would comprise a two-pair combination is shown in Fig. 1B, where the distribution on the left represents the modeled responses from electrode pair 20 at 0-CU ILD and the distribution on the right represents the modeled responses from electrode pair 4 at 0-CU ILD for listener CAL.
Figure 1.
Panel A shows an example lateralization curve as a function of ILD. The example data show the average and standard deviations for each measurement. The data are fit with a cumulative Gaussian function. Two summary metrics, the bias in the perceived center position and the lateralization range, are shown. Panels B and C shows an example of the modeling approach, where the average and variance of the single-electrode lateralization responses (panel B) are summed and then the centroid or maximum of the resulting distribution (panel C) are used to predict a multi-electrode lateralization response.
Then the distributions from the two or more pairs were then summed or multiplied, the previous two-electrode example shown in Fig. 1C. The centroid of the combined distribution and the maximum value of the resulting curve were used for predicting the extent of laterality at each ILD in the multi-electrode lateralization. This resulted in four models: (1) summation centroid, (2) summation maximum, (3) multiplication centroid, and (4) multiplication maximum. For the example in Figs. 1B and 1C, the summation centroid model essentially averages percepts across frequency. The predicted lateralization percept from the combination of electrode 20 (lateralization = −5.18) and electrode 4 (lateralization = +1.23) is a lateralization = −1.98. In contrast, the summation maximum model would produce higher weighting in multi-electrode stimulation for electrode pairs with a smaller response variability and thus predict a lateralization = 0.12.
The amount of variance accounted for each model was estimated using the equation presented by Bernstein et al. (1994):
| (2) |
where Oi and Pi represent observed and predicted lateralization at each ILD, respectively, and Om represents the mean of the observed values across ILDs.
Modeling of multi-electrode lateralization in BiCI listeners using weighed response averages
A second approach to modeling the lateralization data was to disregard the variance of the perceptual results and use only the average responses. Five free parameters or weights were included to combine single-electrode responses to predict the multi-electrode responses. This resulted in a matrix of eight equations for the uncentered multi-electrode conditions. The weights were allowed to freely vary from 0 to 1 for each individual listener with the constraint that the sum of the weights equaled 1. The set of weights that maximized the percent variance in Eq. 2 was reported.
RESULTS
Number of perceived auditory images in NH and BiCI listeners
Although presented with an option to indicate a number of sound sources for the complex stimulus (ranging from one to three), NH listeners always indicated that they heard a single sound source and BiCI listeners indicated a single sound source in 98.7% of the trials.2
Single- and multi-band lateralization in NH listeners
Figure 2 shows the average single- and multi-band lateralization responses for the NH listeners, as well as fits to the data. The lateralization responses are similar across all three single-band conditions and no significant change in performance when the multi-band noise was used. A two-way repeated-measures ANOVA with factors band (500, 750, 1000 Hz, and multi) and ILD showed no significant main effect of band [F(3,21)=1.32, p=0.29]. There was a significant main effect of ILD [F(10,70)=263.0, p<0.001] and a significant band × ILD interaction [F(30,210)=3.13, p<0.001], which occurred because the slope of the lateralization function was shallower for the 750- and 1000-Hz single-band conditions compared to the 500-Hz single-band and multi-band conditions.
Figure 2.
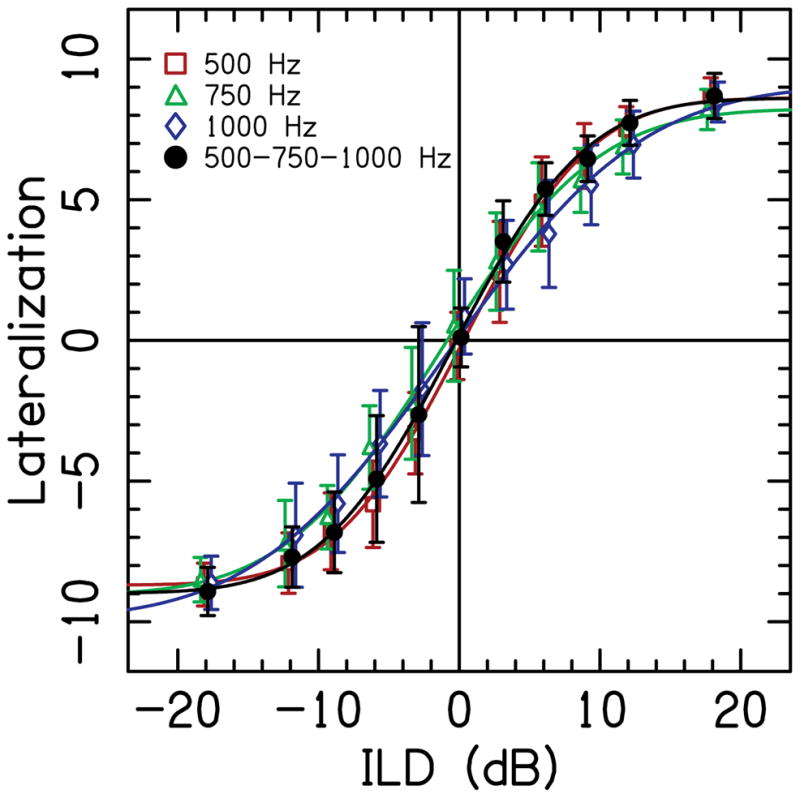
Average lateralization data for NH listeners as a function of ILD. Symbols represent the average response for the single narrow-band noises with a center frequency of 500 Hz (circles), 750 Hz (squares), and 1000 Hz (triangles), and for the multi-band stimulus (diamonds). Error bars show ±1 standard deviation over the 20 trials. Solid curved lines are cumulative Gaussian fits to the average data.
The lateralization range for the single-band stimuli was very similar across NH listeners and on average was 17.1 (±1.4, SD), ranging from 14.9 to 19.4 (74 to 97% of the maximum possible range). The mean lateralization range for the multi-band stimuli was 17.6 (±1.6, SD), ranging from 14.7 to 19.5 (73 to 98% of the maximum possible range), which was essentially the same as the lateralization range for single-band stimuli.
Single- and multi-electrode lateralization in BiCI listeners
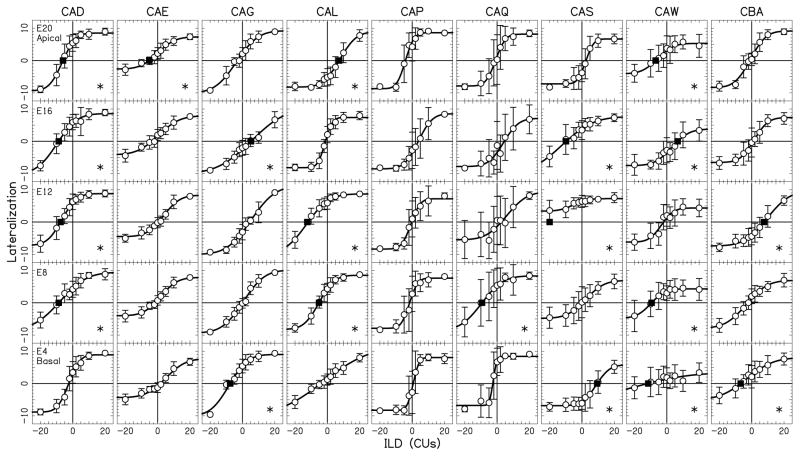
Figures 3, 4, and 5 show individual lateralization data obtained in 9 BiCI listeners for the one-electrode pair conditions, two-electrode pair conditions, and three-/five-electrode pair conditions, respectively. Each column in the figures shows the data from one listener and each row describes the data for one single- or multi-electrode condition across all the listeners. The average lateralization range across all the one-electrode pair conditions was 14.1 (±3.4, SD), ranging from 4.0 to 19.0 (20 to 95% of the maximum possible range). The average lateralization range across all multi-electrode combinations was 14.7 (±2.8, SD), ranging from 8.2 to 19.4 (41 to 97% of the maximum possible range). There was no statistically significant difference for lateralization ranges in two-, three-, and five-electrode combinations (Kruskal-Wallis test, p>0.05) and the data from these combinations were grouped together as multi-electrode combinations for further analysis. The average lateralization ranges, as well as average left- and rightmost responses in each BiCI listener from single- and multi-electrode stimulation are presented in Table 3.
Figure 3.
Lateralization data as a function of ILD on single electrode pairs 20, 16, 12, 8, and 4. Individual listeners are shown in different columns, while the rows show the data for each of the electrode pairs. Scale on y-axis is the lateralization response, from the left-most response (-10) to the right-most response (10). Open circle symbols represent the average response at each ILD and error bars show ±1 standard deviation over the 20 trials. Solid curved lines are cumulative Gaussian fits to the average data. Asterisks in the right low corner of the panels indicate the pairs with a ≥5 CUs bias magnitude at 0-CU ILD. Filled squares symbols show the bias values ≥5 CUs, which were derived as the x-axis (middle line) intersection point of the lateralization curves.
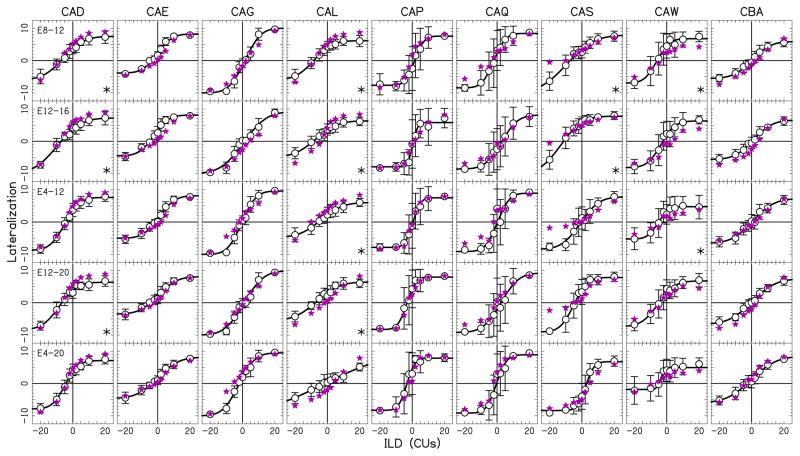
Figure 4.
Lateralization data as a function of ILD for two-electrode pairs. All conventions are similar to that in Fig. 3. Filled stars show responses predicted by summation centroid model.
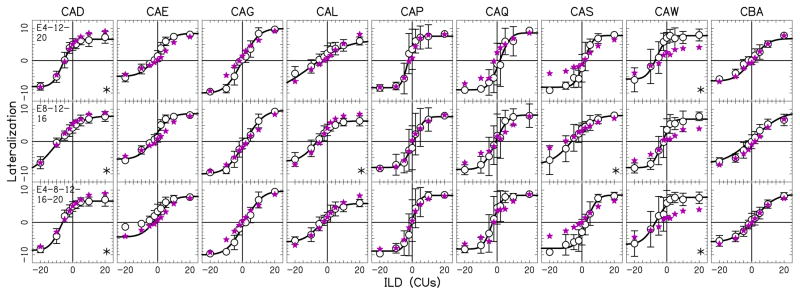
Figure 5.
Lateralization data as a function of ILD for three- and five-electrode pairs. All conventions are similar to that in Fig. 4.
Table 3.
Average left-most and right-most lateralization responses and average lateralization ranges in each BICI listener. The lateralization range was calculated for the full tested range of ±20-CU ILD and for 56%DR. Also reported is ±1 standard deviation.
| Listener | Single-electrode combinations | Multi-electrode combinations | ||||||
|---|---|---|---|---|---|---|---|---|
| Left-Most Response | Right-Most Response | Full Lat. Range | Lat. Range (56%DR) | Left-Most Response | Right-Most Response | Full Lat. Range | Lat. Range(56%DR) | |
| CAD | −7.5±1.5 | 9.0±0.2 | 16.5±1.7 | 13.9±1.0 | −7.3±1.1 | 7.3±0.4 | 14.6±1.2 | 12.2±0.9 |
| CAE | −4.3±0.9 | 7.5±0.5 | 11.8±1.0 | 8.8±1.0 | −4.8±0.7 | 7.9±0.2 | 12.7±0.9 | 9.3±0.7 |
| CAG | −9.2±0.2 | 8.7±1.1 | 17.9±1.3 | 10.9±0.5 | −9.6±0.1 | 9.5±0.3 | 19.1±0.3 | 11.7±0.2 |
| CAL | −7.0±1.3 | 8.2±0.5 | 15.2±1.2 | 7.4±2.2 | −4.7±0.7 | 5.9±0.4 | 10.6±0.8 | 5.7±0.7 |
| CAP | −8.2±0.1 | 8.2±0.3 | 16.4±0.2 | 7.2±2.7 | −8.3±0.7 | 8.0±0.4 | 16.2±1.0 | 7.3±1.0 |
| CAQ | −7.1±1.3 | 8.2±0.8 | 15.3±1.4 | 6.7±0.6 | −8.7±0.3 | 8.7±0.6 | 17.4±0.8 | 8.2±0.6 |
| CAS | −4.2±4.6 | 6.7±0.9 | 10.9±4.1 | 7.3±3.4 | −7.7±1.6 | 7.8±0.4 | 15.5±1.5 | 11.7±1.1 |
| CAW | −4.6±2.3 | 4.0±0.6 | 8.5±2.4 | 6.2±1.9 | −6.2±1.9 | 6.9±1.1 | 13.1±2.6 | 9.7±1.8 |
| CBA | −6.6±1.7 | 7.4±0.9 | 14.1±2.0 | 5.7±1.3 | −5.7±0.3 | 7.0±0.8 | 12.7±0.9 | 5.3±0.6 |
Comparison of lateralization ranges between NH and BiCI listeners
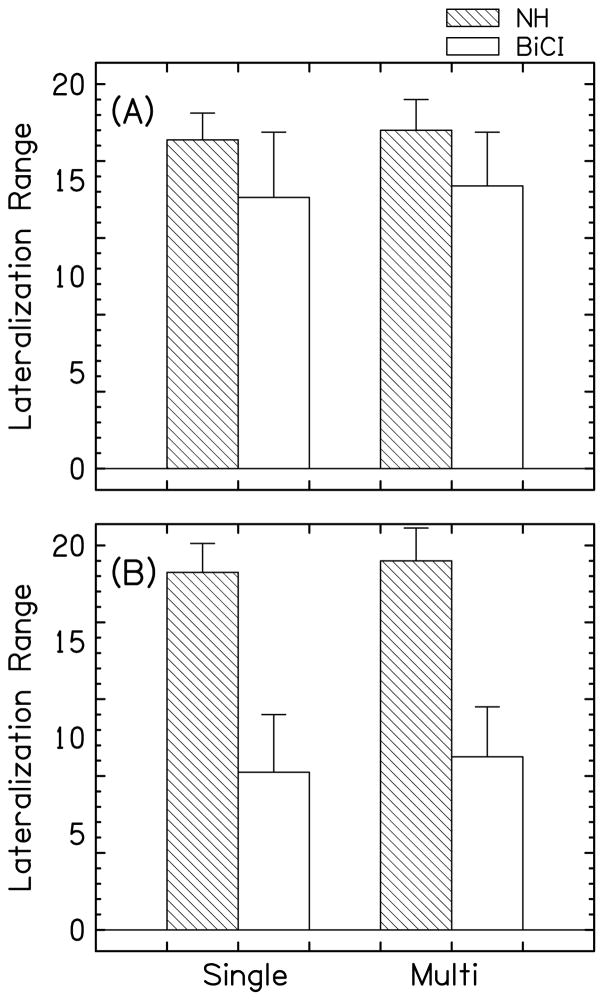
A Kruskal-Wallis analysis on the lateralization ranges in NH and BiCI listeners with single- and multi-band/electrode stimuli showed that the difference in the median values among the four groups studied was statistically significant (H=24.8, df=3, p<0.001). Subsequent Mann-Whitney tests with Bonferroni correction for between-group comparisons were performed. The median values of the lateralization ranges in the groups of BiCI listeners (both single- and multi-stimulation) were significantly smaller as compared to the groups of NH listeners (p<0.0083). Note that neither group utilized the full lateralization response range, and yet there was a saturation in responses at the largest ILDs in both groups. There was no significant difference in lateralization range between single- and multi-band/electrode stimuli within each group (p>0.05 for both).
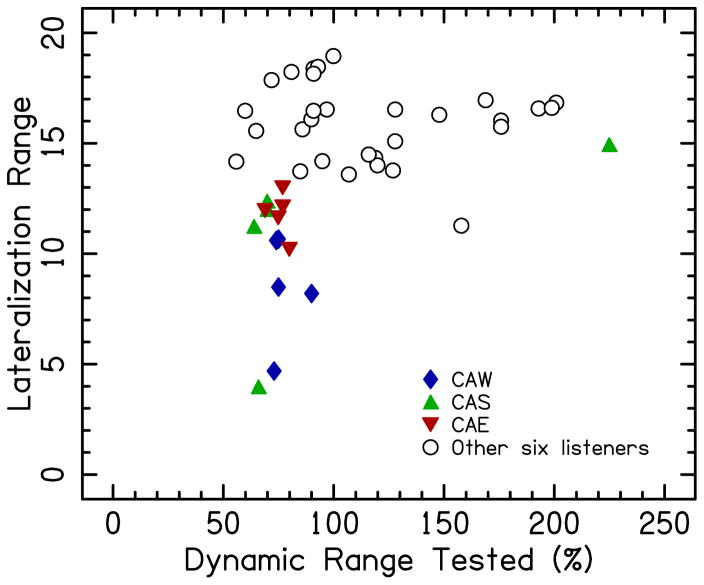
Lateralization range data from single-electrode conditions in BiCI listeners had an asymmetrical distribution with a substantial negative skew (−1.03). Further analysis showed that this asymmetry in the distribution could be attributed to the substantially smaller lateralization ranges measured in three of the BiCI listeners. This difference was independent of the intracochlear location of the stimulated electrode pair. Two of these three listeners had a smaller than the group average lateralization range on all five single-electrode pairs (CAE: average=11.8±1.0, SD; CAW: average=8.5±2.4, SD) and one listener had a smaller range on four out of five electrode pairs (CAS: average=10.9±4.1, SD). These three BiCI listeners generally had smaller %DRs tested with a ±20-CU ILD span, which is shown in Fig. 6. In other words, these three listeners had large dynamic ranges and 20-CU ILDs were relatively small ILDs in terms of %DR.
Figure 6.
Lateralization range on single-electrode pairs as a function of the dynamic range (DR) computed from the span of ILDs from −20 CUs (left ear louder) to +20 CUs (right ear louder). The data show that most of the responses with smaller lateralization ranges belong to only three BiCI listeners (filled symbols), while most of the response with larger lateralization ranges belong to other six listeners (open circles). Three listeners who showed consistently smaller lateralization ranges were also tested with a smaller fraction of their dynamic range as compared to the other six listeners.
On average, 20-CU ILD corresponded to 53% of the DR across the ears for the BiCI listeners. To estimate the DR for a full span of ILD from −20 to +20 CUs (from the left to the right ear), we added %DR for 20 CUs in the left and %DR for 20 CUs in the right ear, which, on average, was 106% DR. In other words, on average, to move the auditory image from the left-most position to the middle of the head in BiCI listeners we stimulated 53%DR, and moving the auditory image from the left-most position to the right-most position would require 106%DR. In contrast, in NH listeners the full lateralization range (moving the auditory image from one ear to another) required 36-dB ILD (or 51% DR) in our experiment.
The smallest %DR tested for ±20-CU ILD occurred for listener CAD, electrode pair 8, which was 56%DR. This value is almost twice as small as compared to the average value across all the BiCI listeners (106%). To test the possibility that a smaller lateralization range in BiCI listeners in our study was influenced by a smaller percentage of the DR tested in three listeners with smaller lateralization ranges described earlier, we calculated the lateralization range at 56%DR. The lateralization ranges in NH and BiCI listeners are presented in Fig. 7, with the full measured ranges in panel A and with the range at 56%DR in panel B.
Figure 7.
Panel A shows the lateralization ranges in NH and BiCI listeners in both single- and multi-band/electrode stimulation. Panel B shows the lateralization ranges utilizing 56%DR.
A Kruskal-Wallis analysis followed by Mann-Whitney tests with a Bonferroni correction for between-group comparisons was performed on the 56%DR lateralization ranges in NH and BiCI listeners with single- and multi-band/electrode stimuli. When the responses from all the NH and BiCI listeners were evaluated within 56%DR, the lateralization ranges were significantly larger for NH listeners as compared to the BiCI listeners (H=81.4, df=3, p<0.001). These data demonstrate that when stimulated at the ILD range of 56%DR, NH listeners maintained their full lateralization range, while BiCI listeners responded within less than half of the available lateralization range, suggesting that BiCI listeners require a significantly larger proportion of their DR stimulated to show a full range of lateralization responses as compared to NH listeners. Electrode pairs with substantially different lateralization ranges were observed across all five single-electrode pairs, with no systematic difference in lateralization ranges along the intracochlear array.
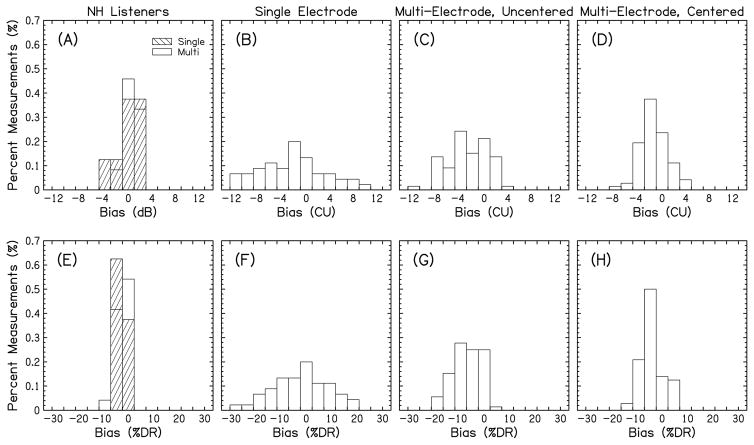
Auditory image bias values at 0-dB/CU ILD
The distribution of the lateralization responses at 0-dB ILD in the group of NH listeners in our study is shown in Fig. 8A, with biases measured in dB, and Fig. 8E, with biases expressed as %DR, again, assuming the DR for an average NH listener between 0 dB HL (minimum audibility per average threshold definition) and 70 dB HL (a loud, but comfortable level in a NH listener). The narrow distribution of the responses around the 0-dB ILD value suggests the consistent perception of the centered image following single- and multi-band stimulation in NH listeners.
Figure 8.
Histograms of the ILD bias values necessary to center an auditory image in NH listeners (panels A and E), and in BiCI listeners (panels B, C, D, F, G, and H). The bias values are presented either in dB or CUs (upper row) or as %DR (bottom row)
The distribution of the 0-CU ILD bias values following single-electrode stimulation in BiCI listeners is presented in Fig. 8 (panel B, in CUs and panel F, in %DR) and suggests a substantial across- and within-listener variability in the intracranial perception of the auditory image at different ILDs. Fitzgerald et al. (2015) showed that measurement variability in the auditory image lateralization responses does not exceed 5-CU ILDs. Using this metric, the bias magnitudes at 0-CU ILD in our study were equal or greater than 5 CUs in 44% of the single-electrode pair measurements. In multi-electrode stimulation (Fig. 8C in CUs and 8G in %DR), the percentages of bias magnitudes equal or greater than 5 CUs were smaller compared to the single-electrode stimulation (25 vs. 44%), although there was no significant difference between the two distributions (Kolmogorov-Smirnov test; p>0.05). For multi-electrode stimulation, there was also no difference between bias distributions on two-, three-, and five-electrode pairs, and data from these conditions were grouped together as multi-electrode combinations for further analysis. In general, the BiCI listeners who had at least three single-electrode pairs with significant biases (four of nine in our study, indicated with the asterisks in Figs. 3, 4, and 5), also showed a number of significant biases in multi-electrode lateralization.
The magnitude of the auditory image bias at 0-CU ILD obtained earlier was also used to estimate the importance of absolute C levels and/or their difference between the ears in the lateralization ability in BiCI listeners. C levels and DR for each electrode pair used in the study are presented in Table 2. C levels were consistently higher in one ear compared to the other on all five electrode pairs in seven of nine listeners. In listeners CAQ and CBA, C levels in one ear were higher on three electrode pairs out of five tested and were lower on the other two pairs. A large proportion of listeners with greater C levels in one ear raised a question if single-electrode stimulation would result in a lateralization bias toward that ear. Our data showed no correlation (r7=0.16, p>0.05) of the ≥5-CU bias magnitudes at 0-CU ILD in single-electrode stimulation with the C levels in the ear with the larger current. Moreover, in approximately half of the single-electrode combinations with ≥5-CU bias magnitude, the bias was toward the ear with a smaller C level (55%). There was also no correlation (p>0.05) between the bias magnitudes at 0-CU ILD and the C level differences between the ears, suggesting that absolute C levels or the frequently observed difference in C levels across the ears cannot reliably predict the direction of the biases in BiCI listeners.
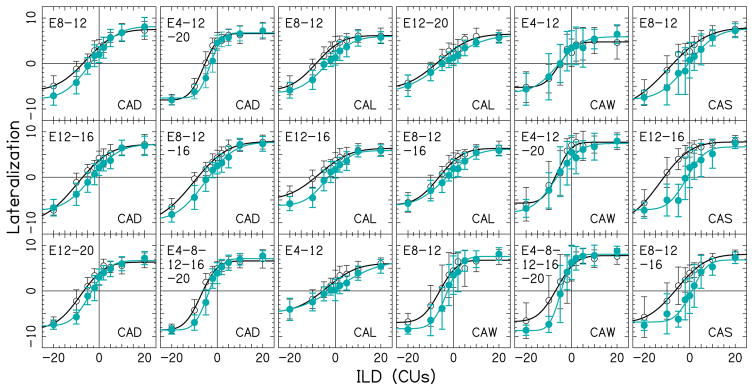
Centering of auditory image in multi-electrode stimulation
We attempted to produce a centered perception of the multi-electrode stimulation by adjusting the current levels on individual electrode pairs, which should reduce the width of the distribution in Figs. 8B and 8F. As predicted, centering individual electrode pairs by reducing the levels in the biased ear resulted in more centered lateralization curves in multi-electrode stimulation, as shown in Fig. 9, and reduced biases at 0-CU ILD as shown in Figs. 8D and 8H. A Kolmogorov-Smirnov test showed that the 0-CU ILD bias distribution in multi-electrode stimulation after the centering procedure was significantly different from the bias distribution prior to centering (p=0.0087). Following the centering procedure, the number of multi-electrode combinations with ≥5-CU bias magnitudes decreased from 25 to 4%. A non-parametric McNemar’s test also showed a significant decrease in the proportion of the large bias values pre- and post-centering procedure [χ²(1)=11.53, p<0.001], suggesting that the centering procedure was successful in reducing the number of the bias values equal or greater than 5 CUs. Specifically, a change from relatively large to small bias values was observed in 15 multi-electrode combinations out of 18 multi-electrode combinations that had large biases prior to centering. In listener CAW, the bias values in two multi-electrode combinations did not change (thus remaining ≥5-CU ILD) and in one multi-electrode combination the bias value significantly increased following the centering procedure.
Figure 9.
Lateralization data as a function of ILD for multi-electrode pairs with significant bias values at 0-CUs ILD. All conventions are similar to that in Fig. 3. Open circles show the data from Figs. 4 and 5 that had significant bias magnitudes. Filled circles show the lateralization data after applying the centering procedure on the single electrode pairs.
Modeling of BiCI multi-electrode ILD-based lateralization
Two modeling approaches were attempted to explain the multi-electrode lateralization responses from the single-electrode lateralization responses. The first approach utilized the response averages and variance, and used four different models to try to explain the multi-electrode responses. The amount of variance accounted for by the model was computed for two-electrode stimulation alone, three-electrode stimulation alone, five-electrode stimulation alone, and the across all conditions. This was done for each individual listener and then including all the listeners. The amount of variance that was accounted for by the summation centroid and the summation maximum models are reported in Table 4. Both models accounted for >70% of variance in the data when combined across the listeners, although a significant amount of within and across listener variability was observed in individual multi-electrode conditions. The multiplication centroid and multiplication maximum models were inferior to the respective summation models.
Table 4.
The percentage of the variance explained for the multi-electrode lateralization conditions by the summation centroid and summation maximum models. Values are reported for each individual listener and the whole group. Values are also reported for just the two-electrode, three-electrode, and five-electrode conditions, as well as the whole group of multi-electrode conditions.
| Listener | Summation Centroid (Percent variance explained) | Summation Maximum (Percent variance explained) | ||||||
|---|---|---|---|---|---|---|---|---|
| Two electrodes | Three electrodes | Fiv electrodes e | All conditions | Two electrodes | Three electrodes | Five electrodes | All conditions | |
| CAD | 89.4 | 95.5 | 94.9 | 91.8 | 85.0 | 93.1 | 91.9 | 88.1 |
| CAE | 87.4 | 84.7 | 92.6 | 87.3 | 82.5 | 80.3 | 86.9 | 82.4 |
| CAG | 91.5 | 92.8 | 89.9 | 91.6 | 88.0 | 89.5 | 98.4 | 89.7 |
| CAL | 57.4 | 85.2 | 76.6 | 68.3 | 0 | 1.6 | 0 | 0 |
| CAP | 94.2 | 96.8 | 97.5 | 95.3 | 92.1 | 96.6 | 97.8 | 94.0 |
| CAQ | 88.4 | 82.4 | 90.7 | 87.4 | 84.0 | 75.2 | 84.0 | 82.8 |
| CAS | 80.9 | 79.9 | 65.0 | 78.9 | 60.7 | 80.4 | 40.5 | 63.7 |
| CAW | 79.3 | 62.0 | 38.8 | 68.3 | 79.3 | 68.1 | 38.6 | 70.4 |
| CBA | 86.1 | 94.7 | 98.3 | 90.3 | 68.2 | 82.1 | 92.9 | 75.6 |
| All listeners | 87.2 | 86.6 | 85.3 | 86.8 | 77.2 | 80.4 | 78.4 | 78.2 |
When comparing the models, the multiplication models and the summation maximum model performed poorly when the responses from the individual electrodes were well separated (as occurred in Fig. 1B). Inspection of Table 4 shows that the summation maximum model almost systematically produced smaller percent variance values and that listener CAL was noteworthy in that the summation maximum model could not explain any of the variance. The summation centroid model accounted for the largest percentage of the variance in the data when including all the conditions (86.8%, Table 4). For these reasons, we concluded that the summation centroid model was superior to the others. To see the specific model predictions, the predicted values based on summation centroid model are shown by the star symbols in Figs. 4 and 5.
The summation centroid model could not explain the last 13% of variance in the data. A source of the deviations might be a factor related to electrode-neural interface, meaning that some areas of the cochlea will have better neural survival and thus may have a representation that is weighted more strongly in an across-frequency central combination of lateralization. Therefore, to further explore the role of individual electrode pairs in multi-electrode lateralization, a second modeling attempt was performed. Because the summation centroid model disregards the response variance, we simple performed an across-frequency averaging of the response averages after individual weighting. The weights of each electrode pair in multi-electrode lateralization response were allowed to freely vary in order to maximize the percentage of variance accounted for in Eq. 2. Because free parameters were added to the model, it is guaranteed that the percent variance explained will be the same or higher than in the unweighted model. The maximum percent variance accounted for with this new modeling approach differed from the summation centroid model (i.e., unweighted version of the model where the individual weights were all 0.2). Specifically, it increased the percentage of variance explained by 4.9% on average when considered all the conditions, ranging from 0.5 to 13.4%, which is shown in Table 5. The largest changes were 13.4 and 11.5% variance explained in listeners CAL and CAW, respectively, the two listeners who had the smallest percent variances explained by the unweighted model. Table 5 also reports the weights that maximize the percent variance explained. Some listeners like CAD and CAQ had relatively equal weights. Some listeners like CAL and CAW had a single electrode with a particularly large weight, 0.77 and 0.91, respectively. The electrodes that had the highest weights were idiosyncratic, but on average the lowest weight was electrode 12, located in the middle of the array; only listener CAP had a weight >0.2 for electrode 12. The highest weight was electrode 20, the most apical electrode; a clear deviation from this pattern would be listener CAL who had a weight of 0.04 for this electrode.
Table 5.
Comparison of modeling results for the unweighted and weighted versions of the averaging model. The weight for electrode 4 is w4, electrode 8 is w8, electrode 12 is w12, electrode 16 is w16, and electrode 20 is w20. The final rows including all listeners in the percent variance explained calculation utilizes their individual weights that maximized percent variance, the same that are reported in the previous rows in this table.
| Listener | Percent variance explained | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| w4 | w8 | w12 | w16 | w20 | Two Electrodes | Three Electrodes | Five Electrodes | All Conditions | ||
| CAD | Unweighted | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 89.4 | 95.5 | 94.9 | 91.8 |
| Weighted | 0.15 | 0.32 | 0.08 | 0.24 | 0.20 | 90.0 | 96.0 | 94.7 | 92.2 | |
| CAE | Unweighted | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 87.4 | 84.7 | 92.6 | 87.3 |
| Weighted | 0.00 | 0.40 | 0.02 | 0.48 | 0.10 | 92.4 | 89.7 | 95.1 | 91.9 | |
| CAG | Unweighted | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 91.4 | 92.8 | 89.9 | 91.6 |
| Weighted | 0.06 | 0.39 | 0.21 | 0.15 | 0.18 | 92.7 | 95.6 | 93.8 | 93.5 | |
| CAL | Unweighted | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 57.4 | 85.2 | 76.6 | 68.3 |
| Weighted | 0.77 | 0.08 | 0.08 | 0.04 | 0.04 | 77.5 | 87.6 | 84.7 | 81.6 | |
| CAP | Unweighted | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 94.2 | 96.8 | 97.5 | 95.3 |
| Weighted | 0.14 | 0.00 | 0.26 | 0.21 | 0.38 | 96.1 | 97.9 | 95.4 | 96.5 | |
| CAQ | Unweighted | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 88.4 | 82.4 | 90.7 | 87.4 |
| Weighted | 0.08 | 0.20 | 0.18 | 0.16 | 0.39 | 88.6 | 85.4 | 90.4 | 88.2 | |
| CAS | Unweighted | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 80.9 | 79.9 | 65.0 | 78.9 |
| Weighted | 0.10 | 0.10 | 0.07 | 0.13 | 0.60 | 79.3 | 93.2 | 90.1 | 84.8 | |
| CAW | Unweighted | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 79.2 | 62.0 | 38.9 | 68.3 |
| Weighted | 0.00 | 0.05 | 0.05 | 0.00 | 0.91 | 85.0 | 73.0 | 75.3 | 79.8 | |
| CBA | Unweighted | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 86.1 | 94.7 | 98.3 | 90.3 |
| Weighted | 0.12 | 0.30 | 0.07 | 0.42 | 0.09 | 90.9 | 98.7 | 98.4 | 94.1 | |
| All listeners | Unweighted | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 87.2 | 86.6 | 85.3 | 86.8 |
| Weighted | -- | -- | -- | -- | -- | 89.6 | 91.2 | 92.1 | 90.4 | |
DISCUSSION
Previous ILD-based lateralization studies in BiCI listeners have focused mostly on single-electrode stimulation (e.g., Litovsky et al. 2010). At the same time, realistic sound sources are more likely to activate multiple electrodes in each ear, producing a broadband stimulus. In NH listeners and in non-reverberant environments, ITDs play a larger role in localization of broadband stimuli as compared to ILDs (Macpherson and Middlebrooks, 2002). In contrast in BiCI listeners, ILDs play a larger role in localization of broadband stimuli as compared to ITDs (Aronoff et al. 2010). Therefore, we investigated how ILDs are processed in multi-electrode stimulation in BiCI listeners.
Lateralization in NH and BiCI listeners
Figure 2 shows that NH listeners demonstrate very similar ILD lateralization curves when presented narrowband noises over headphones, and that NH listeners perceive 0-dB ILD as nearly intracranially centered. There was a small but significant ILD × condition interaction, which was the result of a steeper lateralization function for the 500-Hz and multi-band condition compared to the 750- and 1000-Hz conditions. Yost (1981) showed little to no effect of frequency for ILD lateralization curves using 200-, 500-, 1000-, 2000-, and 5000-Hz tones in four NH listeners. The results in this study are very similar to those in Yost (1981); our significant interaction perhaps a result of more listeners and increased statistical power. In contrast, significant main effects of frequency have been shown before in ILD lateralization. For example, Bernstein et al. (2011) showed ILD lateralization for 4000-Hz modulated tones was larger than for 500-Hz modulated tones in three NH listeners. It is clear that future studies should investigate these frequency effects, in both single- and multi-band conditions.
Compared to the NH control listeners, the BiCI listeners showed remarkably more variability in their ILD lateralization curves. Some variability was expected given that the BiCI listeners had different dynamic ranges, but the amount of variability was noteworthy, as the listeners were presented stimuli with time-synchronized direct stimulation. All BiCI listeners demonstrated an ability to lateralize stimuli based on ILDs in both single- (Fig. 3) and multi-electrode stimulation (Figs. 4 and 5). Most of our BiCI listeners showed large lateralization ranges, similar to the NH control listeners (Fig. 2). However, three BiCI listeners (CAE, CAS, and CAW) showed consistently smaller lateralization ranges across almost all of the electrode pairs tested. The lateralization range, measured from the left-most to the right-most reported position of the auditory image, varied from 20 to 97% of the maximum possible lateralization range across all the BiCI listeners (Figs. 3–7; Table 3). Such a result is similar to that in another study by van Hoesel and Tyler (2003), who showed variability in the observed lateralization ranges in five BiCI listeners, spanning from 55 to 100% of the available lateralization range.
The lateralization ranges in NH listeners were significantly larger than in BiCI listeners, in both single- and multi-electrode/band stimulation, and even when there was an attempt to match the ILD testing range (in %DR) for the two groups (Fig. 7). To explain the smaller lateralization range in the BiCI listeners, we found that three BiCI listeners with smaller lateralization ranges also had the smallest fraction of the DR tested as compared to the other BiCI listeners (Fig. 6). Therefore, it is possible that a 20-CU ILD was insufficient to ensure a full lateralization range in those listeners. On the other hand, other listeners that were tested at comparable %DRs showed larger lateralization ranges (Fig. 6). In addition, note that the majority of the responses in those three poorer performing BiCI listeners, as well as in the remaining six BiCI listeners, were practically saturated at the extreme ILD values indicating that a further increase in the ILDs would likely not result in the increased lateralization range. When the lateralization ranges were compared at 56%DR across all the listeners (the smallest fraction of the DR tested in this study, listener CAD on electrode pair 8), significantly greater values were still observed in the NH group. This result may suggest that BiCI users demonstrate a reduced lateralization ability compared to NH listeners; however, more careful experiments would need to be performed to truly validate this statement.
Admittedly, the comparison across NH and BiCI listeners is complicated as there is no way to fairly equate ILDs in acoustic dB to any electrical unit of measurement. Therefore, we recommend that all the group comparisons be viewed cautiously. The main purpose of the NH listeners was to provide a control data set that showed that 0-dB ILDs are heard as mostly intracranially centered, which was achieved. We also note that the NH and BiCI listeners had non-overlapping age ranges. Therefore, there are potential effects of aging that were not controlled for in this study. However, previous work has shown that aging may not produce large effects in the ability to process ILDs (Babkoff et al. 2002).
Auditory image bias at 0-CU ILD reduced after centering procedure in BiCI listeners
Another important finding in the BiCI listeners was that 0-CU ILD was not consistently perceived as centered in the head, which is unlike the NH listeners for 0-dB ILD. In fact, 44% of the single-electrode pair measurements had a bias magnitude of at least 5 CU in the BiCI listeners (Fig. 8). These results are in line with the findings of Goupell, Kan, et al. (2013) and Fitzgerald et al. (2015), where both reports demonstrated a number of relatively large 0-CU ILD bias magnitudes in BiCI listeners. For the multi-electrode lateralization, the percentage of bias magnitudes that were at least 5 CUs was reduced from 44% in single-electrode lateralization to 25%, but this change was not statistically significant. It may be that the multi-electrode stimulation resulted in blurry auditory images, but not completely unfused (Blauert et al. 1986; Fitzgerald et al. 2015; Kan et al. 2013), and that this produced little overall change in the average location of the auditory image. Explicit centering of the single-electrode pairs with bias magnitudes at least 5 CUs did significantly reduce the percentage of large bias magnitudes for the multi-electrode conditions to only 4% (Figs. 8 and 9). This result suggests that improving location cues at a single electrode pair may provide more consistent binaural information across the array.
Modeling of multi-electrode ILD-based lateralization
The above results can be successfully understood by the summation centroid model (Fig. 1C), which explained up to 86.8% percent of the variance in the multi-electrode lateralization data (Table 4). Because this model is simply an unweighted averaging operation across single-electrode pairs, we attempted a second version of this model that including weights on the individual electrode pairs. This improved the percent variance explained to 90.4% (Table 5). The modeling suggests that across-frequency ILD processing might be a relatively simple averaging operation, with the possibility that some electrodes carry more weight than others. The highest weighted electrode tended to be the most apical one (electrode 20) and the lowest weighted electrode tended to be the middle one (electrode 12); however, Table 5 clearly shows deviations from these trends. Such a result might be explained by local neural survival and the electrode-neural interface (Bierer and Nye 2014; Long et al. 2014) could shape an individual BiCI listener’s across-frequency ILD weighting.
The proposed model of across-frequency ILD processing makes at least two predictions. First, a more centered image in multi-electrode lateralization would be observed in combinations with opposite direction biases of the lateralization curves on single-electrode pairs, and that averaging would result in a more centered values. The shifts in multi-electrode combinations with the same direction of the bias in single-electrode pairs practically remained the same. Evidence for such phenomena can be observed in Figs. 4 and 5. Second, if there is inconsistency in a location perception across electrodes, this will bias the perception to an incorrect location. Remember, BiCI listeners demonstrate an ILD dominance in sound localization (Aronoff et al. 2010), in contrast to NH listeners who demonstrate a low-frequency ITD dominance (Wightman and Kistler 1992). For BiCI listeners, ILDs are likely very different across frequency because the head naturally produces frequency-dependent ILDs (Feddersen et al. 1957; Macaulay et al. 2010) and the ILDs possible altered by microphone response patterns and unsyncronized automatic gain controls (Musa-Shufani et al. 2006; van Hoesel 2012). In the absence of potent low-frequency ITDs to dominate the localization perception, our data and model suggest that BiCI listeners may have localization responses that are biased to the midline because low-frequency ILDs are small and most BiCI listeners weight electrodes equally or most on the apical electrode. In such a case, a strategy to convert ITDs to ILDs at low frequencies (Brown 2014) might be particularly advantageous for localization and binaural processing for BiCI users.
Implications for BiCI mapping
Audiologists map BiCIs as two independent devices. Our data suggest that such an approach could lead to an inconsistent perception of ILDs. Furthermore, the problem of inconsistent ILDs might be rectified by utilizing centering of individual electrode pairs. Previous reports show that centering does not affect single-electrode ITD, ILD, and interaural correlation change just-noticeable differences in BiCI listeners (Goupell 2015; Goupell, Stoelb, et al. 2013; Kan et al. 2015). The importance of centering for multi-electrode stimulation may be more than that for single-electrode stimulation because of potential inconsistencies in perceived location across frequency. If so, removing such inconsistencies has the potential to produce objective improvements in sound localization and speech understanding in noise for BiCI listeners using clinical processors as well as subjective impressions of the sound such as a more fused and punctate auditory image perception.
In addition, a main tool of an audiologist is adjusting levels of single electrodes. Bilateral level comparisons and centering of single electrodes are presently not performed in clinical appointments, but would be possible with some minor changes to technology. Therefore, the results of this work have potential to translate to the clinic and may improve binaural capabilities of BiCI users. Although, this approach would be a deviation from the unilateral loudness balancing, so far neither within-, nor across-ear loudness balancing showed a consistent perception of the centered image in BiCI listeners (Fitzgerald et al. 2015; Goupell, Kan, et al. 2013). A within-ear balancing is usually done in the clinic by simply sweeping the C levels across the electrodes in each ear, and we performed a within-ear balancing in our study as well, but still observed a significant number of non-centered lateralization curves in our listeners. Across-ear loudness balancing also did not ensure the perception of the centered image as well (Fitzgerald et al. 2015) suggesting that loudness balancing does not solve the problems with localization in BiCI listeners. However, if the changes in levels are too great following centering procedure, this could potentially decrease the audibility of the stimulus in one ear. In such an instance, there would be a competition between maximizing binaural performance and maximizing speech understanding. The changes in C levels that were required for centering in this study rarely exceeded 10 CUs for one ear and were always adjusted by decreasing the C level in the leading ear. However, if a large number of electrodes needed significant adjustment, the C levels could be decreased in one ear and increased in the other with the hope that speech understanding would not be negatively impacted. Future research should aim to examine the tradeoff in speech understanding and binaural processing from ILD centering.
Acknowledgments
We would like to thank Tanvi Thakkar, Sarah Shin, and Mikayla Abrams who helped collect data for this study. We would like to thank Tanvi Thakkar and Alan Kan for suggestions about previous versions on this manuscript. This study was supported by NIH Grant R01 DC014948 (Goupell), P30 DC004664 (Center of Comparative Evolutionary Biology of Hearing core grant), and AAA Student Summer Fellowship Award (Stakhovskaya).
Footnotes
Listener CAQ could not tolerate the multi-electrode stimulation at 80%DR and therefore the stimuli were presented at 70%DR.
Listener CAG heard multiple sound sources in 11% of the trials, with 10% of them reported as two sources and 1% as three sources. The majority of multi-source stimuli were heard in a three-electrode combination with widely spaced electrode pairs and in the combination with five electrode pairs.
Listener CAS had a bias magnitude on electrode pair 12 that was greater than the horizontal axis show (~24 CU) and the marker for significant bias offset on this pair was placed at the lowest value of the x-axis.
Portions of this study were presented at the 36th and 38th MidWinter Meetings of the Association for Research in Otolaryngology in Baltimore, MD and 2013 Conference on Implantable Auditory Prostheses at Lake Tahoe, CA.
Financial Disclosures/Conflicts of Interest: None
References
- Aronoff JM, Yoon YS, Freed DJ, et al. The use of interaural time and level difference cues by bilateral cochlear implant users. J Acoust Soc Am. 2010;127:EL87–EL92. doi: 10.1121/1.3298451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babkoff H, Muchnik C, Ben-David N, et al. Mapping lateralization of click trains in younger and older populations. Hear Res. 2002;165:117–127. doi: 10.1016/s0378-5955(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Trahiotis C. Lateralization of low-frequency, complex waveforms: The use of envelope-based temporal disparities. J Acoust Soc Am. 1985;77:1868–1880. doi: 10.1121/1.391938. [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Trahiotis C. The effect of nonsimultaneous on-frequency and off-frequency cues on the detection of a tonal signal masked by narrow-band noise. J Acoust Soc Am. 1994;95:920–930. doi: 10.1121/1.408401. [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Trahiotis C. Lateralization produced by interaural intensitive disparities appears to be larger for high- vs low-frequency stimuli. J Acoust Soc Am. 2011;1:EL15–EL20. doi: 10.1121/1.3528756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best V, Laback B, Majdak P. Binaural interference in bilateral cochlear-implant listeners. J Acoust Soc Am. 2011;130:2939–2950. doi: 10.1121/1.3641400. [DOI] [PubMed] [Google Scholar]
- Bierer JA, Nye AD. Comparisons between detection threshold and loudness perception for individual cochlear implant channels. Ear Hear. 2014;35:641–651. doi: 10.1097/AUD.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauert J, Lindemann W. Spatial mapping of intracranial auditory events for various degrees of interaural coherence. J Acoust Soc Am. 1986;79:806–813. doi: 10.1121/1.393471. [DOI] [PubMed] [Google Scholar]
- Brown CA. Binaural enhancement for bilateral cochlear implant users. Ear Hear. 2014;35:580–584. doi: 10.1097/AUD.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman MF, Spahr AJ, Loiselle L, et al. Localization and speech understanding by a patient with bilateral cochlear implants and bilateral hearing preservation. Ear Hear. 2013;34:245–248. doi: 10.1097/AUD.0b013e318269ce70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feddersen WE, Sandel TT, Teas DC, et al. Localization of high-frequency tones. J Acoust Soc Am. 1957;29:988–991. [Google Scholar]
- Fitzgerald MB, Kan A, Goupell MJ. Bilateral loudness balancing and distorted spatial perception in recipients of bilateral cochlear implants. Ear Hear. 2015 doi: 10.1097/AUD.0000000000000174. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupell MJ. Interaural correlation-change discrimination in bilateral cochlear-implant users: Effects of interaural frequency mismatch, centering, and age of onset of deafness. J Acoust Soc Am. 2015;137:1282–1297. doi: 10.1121/1.4908221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupell MJ, Kan A, Litovsky RY. Typical mapping procedures can produce non-centered auditory images in bilateral cochlear-implant users. J Acoust Soc Am. 2013;133:EL101–EL107. doi: 10.1121/1.4776772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupell MJ, Stoelb C, Kan A, et al. Effect of mismatched place-of-stimulation on the salience of binaural cues in conditions that simulate bilateral cochlear-implant listening. J Acoust Soc Am. 2013;133:2272–2287. doi: 10.1121/1.4792936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham DW, Ashmead DH, Ricketts TA, et al. Interaural time and level difference thresholds for acoustically presented signals in post-lingually deafened adults fitted with bilateral cochlear implants using CIS+ processing. Ear Hear. 2008;29:33–44. doi: 10.1097/AUD.0b013e31815d636f. [DOI] [PubMed] [Google Scholar]
- Grantham DW, Ashmead DH, Ricketts TA, et al. Horizontal-plane localization of noise and speech signals by postlingually deafened adults fitted with bilateral cochlear implants. Ear Hear. 2007;28:524–541. doi: 10.1097/AUD.0b013e31806dc21a. [DOI] [PubMed] [Google Scholar]
- Kan A, Litovsky RY, Goupell MJ. Effects of interaural pitch-matching and auditory image centering on binaural sensitivity in cochlear-implant users. Ear Hear. 2015;36:e62–e68. doi: 10.1097/AUD.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan A, Stoelb C, Litovsky RY, et al. Effect of mismatched place-of-stimulation on binaural fusion and lateralization in bilateral cochlear-implant users. J Acoust Soc Am. 2013;134:2923–2936. doi: 10.1121/1.4820889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano A, Seldon HL, Clark GM, et al. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Oto-Laryngol. 1998;118:313–326. doi: 10.1080/00016489850183386. [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Goupell MJ, Godar S, et al. Studies on bilateral cochlear implants at the University of Wisconsin's Binaural Hearing and Speech Laboratory. J Am Acad Audiol. 2012;23:476–494. doi: 10.3766/jaaa.23.6.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Jones GL, Agrawal S, et al. Effect of age at onset of deafness on binaural sensitivity in electric hearing in humans. J Acoust Soc Am. 2010;127:400–414. doi: 10.1121/1.3257546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Parkinson A, Arcaroli J. Spatial hearing and speech intelligibility in bilateral cochlear implant users. Ear Hear. 2009;30:419–431. doi: 10.1097/AUD.0b013e3181a165be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Parkinson A, Arcaroli J, et al. Bilateral cochlear implants in adults and children. Arch Otolaryngol Head Neck Surg. 2004;130:648–655. doi: 10.1001/archotol.130.5.648. [DOI] [PubMed] [Google Scholar]
- Long CJ, Holden TA, McClelland GH, et al. Examining the electro-neural interface of cochlear implant users using psychophysics, CT scans, and speech understanding. J Assoc Res Otolaryngol. 2014;15:293–304. doi: 10.1007/s10162-013-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay EJ, Hartmann WM, Rakerd B. The acoustical bright spot and mislocalization of tones by human listeners. J Acoust Soc Am. 2010;127:1440–1449. doi: 10.1121/1.3294654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson EA, Middlebrooks JC. Listener weighting of cues for lateral angle: The duplex theory of sound localization revisited. J Acoust Soc Am. 2002;111:2219–2236. doi: 10.1121/1.1471898. [DOI] [PubMed] [Google Scholar]
- Majdak P, Goupell MJ, Laback B. Two-dimensional localization of virtual sound sources in cochlear-implant listeners. Ear Hear. 2011;32:198–208. doi: 10.1097/AUD.0b013e3181f4dfe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG. Lateralization of high frequencies based on interaural time differences. J Acoust Soc Am. 1976;59:634–639. doi: 10.1121/1.380913. [DOI] [PubMed] [Google Scholar]
- Mills AW. Lateralization of high-frequency tones. J Acoust Soc Am. 1960;32:132–134. [Google Scholar]
- Musa-Shufani S, Walger M, von Wedel H, et al. Influence of dynamic compression on directional hearing in the horizontal plane. Ear Hear. 2006;27:279–285. doi: 10.1097/01.aud.0000215972.68797.5e. [DOI] [PubMed] [Google Scholar]
- Neuman AC, Haravon A, Sislian N, et al. Sound-direction identification with bilateral cochlear implants. Ear Hear. 2007;28:73–82. doi: 10.1097/01.aud.0000249910.80803.b9. [DOI] [PubMed] [Google Scholar]
- Nopp P, Schleich P, D'Haese P. Sound localization in bilateral users of MED-EL COMBI 40/40+ cochlear implants. Ear Hear. 2004;25:205–214. doi: 10.1097/01.aud.0000130793.20444.50. [DOI] [PubMed] [Google Scholar]
- Pena JL, Konishi M. Cellular mechanisms for resolving phase ambiguity in the owl's inferior colliculus. Proc Natl Acad Sci U S A. 2000;97:11787–11792. doi: 10.1073/pnas.97.22.11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BR, Wyss J, Manrique M. Worldwide trends in bilateral cochlear implantation. Laryngoscope. 2010;120:S17–44. doi: 10.1002/lary.20859. [DOI] [PubMed] [Google Scholar]
- Rayleigh L. On our perception of sound direction. Phil Mag. 1907;13:214–232. [Google Scholar]
- Saberi K, Takahashi Y, Konishi M, et al. Effects of interaural decorrelation on neural and behavioral detection of spatial cues. Neuron. 1998;21:789–798. doi: 10.1016/s0896-6273(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Seeber BU, Fastl H. Localization cues with bilateral cochlear implants. J Acoust Soc Am. 2008;123:1030–1042. doi: 10.1121/1.2821965. [DOI] [PubMed] [Google Scholar]
- Shackleton TM, Meddis R, Hewitt MJ. Across frequency integration in a model of lateralization. J Acoust Soc Am. 1992;91:2276–2283. [Google Scholar]
- Stern RM, Zeiberg AS, Trahiotis C. Lateralization of complex binaural stimuli: A weighted-image model. J Acoust Soc Am. 1988;84:156–165. doi: 10.1121/1.396982. [DOI] [PubMed] [Google Scholar]
- Trahiotis C, Stern RM. Lateralization of bands of noise: Effects of bandwidth and differences of interaural time and phase. J Acoust Soc Am. 1989;86:1285–1293. doi: 10.1121/1.398743. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJM. Contrasting benefits from contralateral implants and hearing aids in cochlear implant users. Hear Res. 2012;288:100–113. doi: 10.1016/j.heares.2011.11.014. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJM, Tyler RS. Speech perception, localization, and lateralization with bilateral cochlear implants. J Acoust Soc Am. 2003;113:1617–1630. doi: 10.1121/1.1539520. [DOI] [PubMed] [Google Scholar]
- Verschuur CA, Lutman ME, Ramsden R, et al. Auditory localization abilities in bilateral cochlear implant recipients. Otol Neurotol. 2005;26:965–971. doi: 10.1097/01.mao.0000185073.81070.07. [DOI] [PubMed] [Google Scholar]
- Wightman FL, Kistler DJ. The dominant role of low-frequency interaural time differences in sound localization. J Acoust Soc Am. 1992;91:1648–1661. doi: 10.1121/1.402445. [DOI] [PubMed] [Google Scholar]
- Yost WA. Lateral position of sinusoids presented with interaural intensive and temporal differences. J Acoust Soc Am. 1981;70:397–409. [Google Scholar]