Abstract
Objective
Explore the association between following a Dietary Approaches to Stop Hypertension (DASH)-accordant diet and kidney end-points among urban adults.
Design
Prospective cohort study.
Setting & Participants
1,534 participants of the Healthy Aging in Neighborhoods of Diversity across the Life Span study with a baseline estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73m2.
Exposure
DASH diet accordance determined via a score based on 9 target nutrients.
Main Outcome Measure
Rapid kidney function decline (eGFR decline > 3 mL/min/1.73 m2 per year), incident CKD (follow-up eGFR <60 mL/min/1.73 m2), and eGFR decline ≥ 25%
Measurements
Multinomial logistic regression.
Results
Participants’ mean age was 48 years and 59% were African American. Median DASH score was 1.5 (range 0–8). Over a median of 5 years, 13.4% experienced rapid eGFR decline, including 15.2% among participants not following a DASH-accordant diet (score ≤1) and 12.0% with higher accordance (score >1) (p=0.08). Outcomes varied by hypertension status. Following adjustment for sociodemographic and clinical factors, including total energy intake, low DASH diet accordance was associated with rapid eGFR decline among participants with hypertension [Risk Ratio (RR) 1.68, 95% Confidence Interval (CI) 1.17–2.42] but not among those without hypertension (RR 0.83, 95% CI 0.56–1.24); p interaction 0.001. There was no statistically significant association between DASH diet accordance and incident CKD or eGFR decline ≥ 25%. Results were similar when DASH diet accordance was analyzed in tertiles.
Conclusions
Among urban adults, low accordance to a DASH-type diet was not associated with incident CKD, but was associated with higher risk of rapid eGFR decline among those with hypertension, yet not among those without hypertension. Further study of dietary patterns as a potential target for improving kidney outcomes among high-risk populations is warranted.
Keywords: HANDLS, Chronic Kidney Disease, Hypertension, Epidemiology, Lifestyle
Introduction
In the past several decades, dietary patterns, one of the most modifiable lifestyle/behavioral factors, have been linked to risk for several chronic diseases.1 Diets rich in vegetables, fruits and soy while low in fats and meats have been concluded as healthy dietary patterns2 and have shown protective associations with morbidity and mortality.3,4
The Dietary Approaches to Stop Hypertension (DASH) dietary pattern features high intake of vegetables, fruits, and low-fat dairy products with reduced total and saturated fat, cholesterol and sugar-sweetened products.5 It has been shown to lower blood pressure in pre-hypertensive and hypertensive adults.6 A DASH-like diet has also been associated with lower risks of cardiovascular and cerebrovascular disease in observational studies.7 Among kidney outcomes examined, accordance to a DASH-like diet was associated with slower estimated glomerular filtration rate (eGFR) decline among older white women with preserved kidney function.8 Higher diet quality (based on Dietary Guidelines for Americans score) was associated with both lower odds of incident chronic kidney disease (CKD) and eGFR decline.9 In addition, an intervention study found that a dietary pattern rich in fruits and vegetables, similar to the DASH diet, attenuated markers of kidney injury among persons with hypertensive nephropathy.10 However, the association of the DASH dietary pattern with kidney end-points has not been examined among urban populations, where DASH diet accordance may be quite low.11 Understanding the relationship between dietary patterns and kidney function decline could inform behavioral interventions targeting populations at high risk for CKD.
In this study, we investigated the association between patient-reported accordance to the DASH diet and rapid kidney function decline, incident CKD, and eGFR decline ≥ 25% among a racially and socio-economically diverse, middle-aged, urban population.
Subjects and Methods
Study Design and Population
We examined longitudinal data from the National Institute on Aging (NIA), Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study-- a population-based cohort study. Participants were community-dwelling African Americans and whites age 30–64 years at enrollment, drawn from 13 neighborhoods, each of which composed of contiguous U.S. census tracts in Baltimore City, Maryland. Household enrollment was from 2004 to 2008. Participants were followed until death or the end of their follow-up visit when they were censored. The median follow-up for censored participants was 5 years (range 1–8 years). Follow-up measures were performed from July 2009 to July 2013. Each participant provided informed consent. The National Institute of Environmental Health Sciences, National Institutes of Health, approved the study protocol.12
The total HANDLS study population was 3,720. We restricted our sample to participants who underwent baseline serum creatinine (n=2,744), baseline dietary intake measurements (n=2,058) and had an eGFR ≥60 mL/min per 1.73 m2 at baseline (n = 1940). We further excluded those who survived but did not undergo a follow up serum creatinine measurement (remaining n=1534). Compared to the excluded individuals, our study sample was comparable in age, race and poverty status; but was less likely to be male (42.0 vs. 47.7%, P = 0.001).
Measurements
Exposure
Participants’ non-prescribed accordance to a DASH-like diet was determined using 24-hour self-reported food intake information gathered using the validated US Department of Agriculture’s Automated Multiple Pass Method (AMPM), Versions 2.3 – 2.6, a computerized methodology, on 2 days separated by 7–10 days.13 This method was supplemented by measurement aids (e.g. measuring cups, spoons, etc.) to assist in estimating accurate quantities of foods and beverages. Both dietary recalls were administered in-person by trained interviewers. The dietary recalls were coded using Survey Net, matching foods consumed with codes in the Food and Nutrient Database for Dietary Studies, Version 3. Energy and selected nutrient intakes were calculated for each recall day.14 There were no significant differences in energy or nutrient intakes between the first and second recall days. The recalls represented both weekend and weekday consumption patterns and no differences existed between energy and nutrient intakes by day of the week.
For this study, the mean nutrient values were used to assess DASH diet accordance as per the patient-reported DASH diet score developed by Mellen et al among participants of the National Health and Nutrition Examination Survey 1988–1994 and 1999–2004 periods who self-reported hypertension.15 Mellen et al identified DASH goals for 8 target nutrients (total fat, saturated fat, protein, fiber, cholesterol, calcium, magnesium, and potassium). Although dietary sodium was held constant in the original DASH study16, sodium was included among the target nutrients. Nutrient goals were then indexed to total energy intake (with the exception of macronutrients), and the DASH score was generated by the sum of all nutrient targets met (maximum score, 9). Individuals with intake meeting a goal intermediate between the DASH goal and the nutrient content of the DASH control diet were given a score of 0.5 for that nutrient. Individuals meeting approximately half of the DASH targets (DASH score ≥4.5) were considered DASH accordant.15
Outcomes
Our primary outcomes of interest were rapid kidney function decline, incident CKD, and eGFR decline ≥ 25%. Kidney endpoints were determined by eGFR calculated from serum creatinine using the CKD Epidemiology Collaboration creatinine-based equation.17 For 9% of participants, creatinine was measured once at baseline and once at follow-up at the NIA Clinical Research Branch Core Laboratory using a modified kinetic Jaffe method (CREA method, Dade Dimension X-Pand Clinical Chemistry System, Siemens Healthcare Diagnostics Inc., Newark, DE); and was measured for the remainder of participants at Quest Diagnostics, Inc. by isotope dilution mass spectrometry (Olympus America Inc., Melville, NY) and standardized to the reference laboratory at the Cleveland Clinic. Rapid kidney function decline was defined as a decrease in eGFR > 3 mL/min/1.73 m2 per year during follow-up.18 Incident CKD was defined as follow-up eGFR below 60 mL/min per 1.73 m2.19 Estimated GFR decline ≥ 25% from baseline was also examined.19
Covariates
Race was self-reported (African American or white) during the initial household survey, where additional socio-demographic data including age, sex, level of educational attainment, report of a regular source of health care, health insurance coverage and poverty status were also assessed. Poverty status was defined as a self-reported annual household income below 125% of the 2004 Department of Health and Human Services poverty guideline (family of 4 earning <$23,562).20 Tobacco use was defined as participants’ self-report of smoking status. Height and weight were measured and used to calculate body mass index (BMI). A mobile research vehicle (MRV) was the site of health care provider ascertained medical history and physical examination. Fasting venous blood specimen were also collected on the MRV and analyzed at the NIA Clinical Research Branch Core Laboratory (Baltimore, MD) and Quest Diagnostics, Inc. (Baltimore, MD and Chantilly, VA).
Each participant underwent sitting and standing blood pressure measurements on each arm using the brachial artery auscultation method with an inflatable cuff of appropriate size. Seated measurements were made after five minutes of rest in a seated position, with feet flat on the floor and legs uncrossed. Standing measurements were made after the participant had been standing for at least two minutes. Hypertension was defined as an average of seated and standing systolic blood pressure ≥ 140 mmHg, an average of seated and standing diastolic blood pressure ≥ 90 mmHg 21, a history of blood pressure medication use, or a self-report of hypertension. Diabetes mellitus was defined as a fasting plasma glucose concentration of ≥126 mg/dl (7.0 mmol/l), or self-report of diabetes.
Statistical Analysis
Due to the low overall DASH diet accordance in the HANDLS study11 and non-normal distribution of DASH scores, we dichotomized participants into two groups around a score of 1.0, which was the 25th percentile for accordance. Thus, DASH scores ≤1 were considered low DASH accordance and those >1, higher DASH accordance. Participant characteristics stratified by DASH diet accordance were compared using χ2 tests or Fisher’s exact tests for categorical variables, and analysis of variance (ANOVA) for continuous variables. Descriptive statistics and χ2 square tests were used to compare the unadjusted occurrence of rapid kidney function decline, the unadjusted occurrence of incident CKD, and the unadjusted occurrence of eGFR decline ≥ 25%, separately, by DASH diet accordance. Multinomial logistic regression was performed to evaluate the odds of rapid eGFR decline, incident CKD, and eGFR decline ≥ 25%, separately, across DASH accordance groups that were conditional on the competing outcome of death. Multinomial logistic regression was used to account for the potential survival bias associated with repeat assessment of biomarkers over time.22 Given previous reports documenting that dietary patterns vary by race23 and socioeconomic status11, and given that hypertension and diabetes are both diet-sensitive chronic conditions, we analyzed each of these factors as potential effect modifiers. We tested the interaction of each potential effect modifier, separately, with DASH diet accordance. Potential confounders considered were those associated with dietary patterns and/or kidney function decline in previous studies, including socio-demographic characteristics (age, race, sex, poverty status, and education level), comorbid conditions (hypertension, diabetes, and systolic blood pressure) and health behaviors (tobacco use and total energy intake). For outcomes with low event rates, we examined a parsimonious set of variables (age, sex, race, and poverty status). To further explore the relationship between DASH diet accordance and kidney function decline we (a) descriptively compared nutrient intakes between participants with and without rapid eGFR decline, and (b) used multivariable logistic regression to test the association between individual DASH nutrient scores (range 0–1) and kidney outcomes (all individual DASH nutrients were included in the model, adjusted for each other).
We performed three sensitivity analyses. First, we repeated our primary models with DASH diet accordance stratified by tertiles. Second, we restricted our sample to participants with available urinary albumin and creatinine data (n = 983), replicated our analysis for the rapid eGFR decline models and further adjusted for urine albumin-to-creatinine ratio (ACR). Third, for kidney outcomes, we restricted to participants with creatinine measured at the same laboratory at baseline and follow-up (n = 1408).
In all analyses, the possibility of confounding by U.S. census tract was controlled with fixed effects modeling, clustered on neighborhood. Statistical analyses were performed using Stata software, version 13 (StataCorp, College Station, TX). A two-sided P <0.05 was used as the level of significance for all tests.
Results
Participant Characteristics and DASH Diet Accordance
Among 1,534 participants, DASH diet accordance was minimal, with a median score of 1.5 and interquartile range (IQR) of 1 to 2.5. Only 5.7% of participants reported dietary patterns consistent with the diet (DASH score ≥ 4.5), as in our previous report.11 Examination of participant characteristics comparing low and higher DASH accordance groups revealed that male sex, African American race, poverty, fewer years of education, tobacco use and higher level of total energy intake were each more prevalent among the low DASH accordance group than the higher accordance group (P<0.05 for all). Median baseline eGFR was comparable between the groups. (Table 1)
TABLE 1.
Participant Characteristics by Dietary Approaches to Stop Hypertension (DASH) Diet £ Accordance (N = 1534)
| Participant Characteristics | N | DASH Diet Accordance
|
P value | |
|---|---|---|---|---|
| Higher (Accordance scores 1.5–8.0) n = 886 (58%) | Low (Accordance scores 0–1.0) n = 648 (42%) | |||
| African American race | 1534 | 481 (54.3) | 419 (64.7) | <0.001 |
| Age, years, mean (SD) | 1534 | 47.8 (9.4) | 47.6 (8.6) | 0.678 |
| Male sex | 1534 | 347 (39.2) | 297 (45.8) | 0.009 |
| Education, years, mean (SD) | 1532 | 12.9 (3.3) | 12.3 (2.9) | <0.001 |
| Regular source of health care | 1534 | 596 (67.3) | 406 (62.7) | 0.061 |
| Insurance | 1534 | 622 (70.2) | 434 (67.0) | 0.178 |
| Poverty | 1534 | 338 (38.2) | 291 (44.9) | 0.008 |
| Tobacco use | 1462 | <0.001 | ||
| Current | 675 | 353 (41.7) | 322 (52.3) | |
| Former | 304 | 181 (21.4) | 123 (20.0) | |
| None | 483 | 312 (36.9) | 171 (27.8) | |
| BMI, kg/m2, mean (SD) | 1532 | 29.8 (7.8) | 29.7 (7.6) | 0.753 |
| eGFR ml/min/1.73m2** | 1534 | 95 (82 – 108) | 96 (83 – 108) | 0.579 |
| ObesityΨ | 1534 | 376 (42.4) | 266 (41.1) | 0.601 |
| Systolic BP (mm/Hg) | 1523 | 119 (19) | 120 (19) | 0.151 |
| Hypertension | 1529 | 380 (43.0) | 272 (42.1) | 0.753 |
| Diabetes | 1529 | 138 (15.6) | 100 (15.5) | 0.955 |
| Total energy intake, kilocalories/day, mean(SD) | 1533 | 1863 (898) | 2233 (972) | <0.001 |
Abbreviations: SD, standard deviation; BMI, body mass index; BP, blood pressure.
Individuals meeting the DASH target for a nutrient received a score of 1 while those who achieved the intermediate target for a nutrient received a score of 0.5 for that nutrient, for a total possible score of 9.
Median (Interquartile Range) presented.
Obesity was defined as BMI ≥30 kg/m2.
DASH Diet Accordance and Rapid eGFR Decline
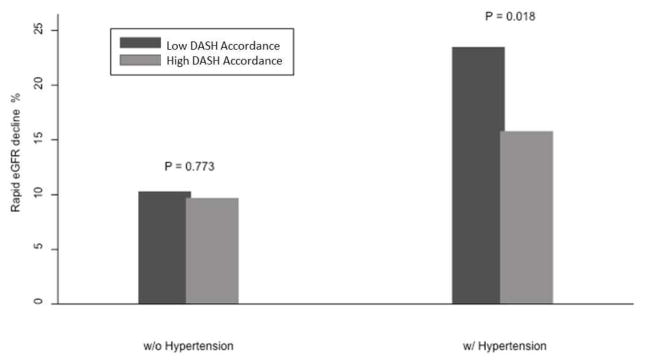
A total of 1443 participants survived and completed follow-up creatinine measures (91 died during follow-up). Among the survivors, 193 (13.4%) experienced rapid eGFR decline over 6,673 person years, including 12.0% of the higher DASH and 15.2% of the low DASH accordance groups. Compared to higher DASH accordance, low DASH accordance was not associated with a greater risk of rapid eGFR decline [unadjusted risk ratio (RR) 1.31, 95% Confidence Interval (CI) 0.99–1.78; adjusted RR 1.22, 95% CI 0.92–1.63 (adjusted for age, sex, race, poverty status, tobacco use, education level, hypertension, diabetes, systolic blood pressure and total energy intake)]. Effect modification by hypertension status was noted (P interaction 0.008), and therefore we stratified our subsequent models by hypertension status (Table 2). The unadjusted association between DASH diet accordance and rapid eGFR decline was statistically significant among hypertensive participants yet not among non-hypertensives (Figure 1). These associations persisted following adjustment for age, sex, race, poverty, smoking status, education, diabetes, systolic blood pressure and total energy intake (Table 3).
TABLE 2.
Participant Characteristics by Hypertension Status and Dietary Approaches to Stop Hypertension (DASH) £ Diet Accordance (N = 1529)
| Participant Characteristics | Non-HTN N = 877 (57%) |
HTN N = 652 (43%) |
||||||
|---|---|---|---|---|---|---|---|---|
| All | Higher DASH | Low DASH | P value | All | Higher DASH | Low DASH | P value | |
| African American race | 480 (54.7) | 245 (48.7) | 235 (62.8) | <0.001 | 416 (63.8) | 234 (61.6) | 182 (66.9) | 0.162 |
| Age, years, mean(SD) | 45.0 (8.6) | 45.1 (8.9) | 44.9 (8.1) | 0.721 | 51.3 (8.4) | 51.3 (8.9) | 51.3 (7.8) | 0.965 |
| Male sex | 388 (44.2) | 210 (41.8) | 178 (47.6) | 0.805 | 255 (39.1) | 137 (36.1) | 11888 (43.4) | 0.059 |
| Education, years, mean(SD) | 12.9 (3.2) | 13.3 (3.4) | 12.3 (3.0) | <0.001 | 12.2 (3.0) | 12.3 (3.1) | 12.2 (2.8) | 0.650 |
| Regular source of health care | 508 (57.9) | 294 (58.5) | 214 (57.2) | 0.715 | 492 (75.5) | 300 (79.0) | 192 (70.6) | 0.014 |
| Insurance | 569 (64.9) | 333 (66.2) | 236 (63.1) | 0.341 | 482 (3.9) | 286 (75.3) | 196 (72.1) | 0.358 |
| Poverty | 335 (38.2) | 179 (35.6) | 156 (41.7) | 0.065 | 291 (44.6) | 157 (41.3) | 134 (49.3) | 0.044 |
| Tobacco use | 0.020 | 0.003 | ||||||
| Current | 417 (50.0) | 218 (45.8) | 199 (55.6) | 258 (41.1) | 135 (36.5) | 123 (47.7) | ||
| Former | 139 (16.7) | 85 (17.9) | 54 (15.1) | 165 (26.3) | 96 (26.0) | 69 (26.7) | ||
| None | 278 (33.3) | 173 (36.3) | 105 (29.3) | 205 (32.6) | 139 (37.6) | 66 (25.6) | ||
| BMI, kg/m2, mean(SD) | 27.9 (6.7) | 27.8 (6.7) | 27.9 (6.7) | 0.830 | 32.3 (8.2) | 32.5 (8.3) | 32.1 (8.2) | 0.598 |
| eGFR ml/min/1.73m2** | 97 (84 – 110) | 97 (83 – 110) | 97 (86 – 109) | 0.583 | 93 (80 – 104) | 93 (80 – 103) | 92 (81 – 105) | 0.880 |
| ObesityΨ | 278 (31.7) | 158 (31.4) | 120 (32.1) | 0.832 | 361 (55.4) | 216 (56.8) | 145 (53.3) | 0.371 |
| Systolic BP (mm/Hg) | 112 (14) | 112 (14) | 112 (14) | 0.641 | 130 (20) | 128 (21) | 131 (18) | 0.053 |
| Diabetes | 57 (6.5) | 34 (6.8) | 23 (6.2) | 0.735 | 181 (27.8) | 104 (27.4) | 77 (28.3) | 0.807 |
| Total energy intake, kilocalories/day, mean (SD) | 2103 (957) | 1964 (908) | 2289 (991) | <0.001 | 1909 (924) | 1727 (866) | 2162 (945) | <0.001 |
Abbreviations: HTN, hypertension; SD, standard deviation; BMI, body mass index; BP, blood pressure.
Individuals meeting the DASH target for a nutrient received a score of 1 while those who achieved the intermediate target for a nutrient received a score of 0.5 for that nutrient, for a total possible score of 9.
Median (Inter Quartile Range) was presented instead of mean (SD).
Obesity was defined as BMI ≥30 kg/m2.
FIGURE 1.
Rapid eGFR Decline by Hypertension Status and Dietary Approaches to Stop Hypertension (DASH) Diet Accordance. Rapid eGFR decline defined as eGFR decrease > 3 mL/min per 1.73 m2 per year during follow-up.
TABLE 3.
Multinomial Logistic Regression Analyses for Associations between Low Dietary Approaches to Stop Hypertension (DASH) £ Accordance (Compared to Higher Accordance) and Rapid eGFR Decline* (N = 1529)
| Model | Variables Included | non-HTN N = 877 N events = 93 |
HTN N = 652 N events = 100 |
P interaction |
|---|---|---|---|---|
|
| ||||
| Risk Ratio (95% Confidence Interval) | Risk Ratio (95% Confidence Interval) | Between HTN and DASH accordance groups | ||
| 1 | Low versus Higher DASH Accordance | 0.97 (0.66, 1.44) | 1.80 (1.33, 2.44) | 0.001 |
| 2 | + Age, Sex, Race | 0.94 (0.61, 1.44) | 1.80 (1.33, 2.45) | 0.001 |
| 3 | + Poverty Status, Tobacco Use, Education level | 0.87 (0.60, 1.25) | 1.74 (1.22, 2.47) | 0.001 |
| 4 | + Diabetes | 0.87 (0.60, 1.26) | 1.71 (1.19, 2.44) | 0.001 |
| 5 | + Systolic BP | 0.89 (0.61, 1.29) | 1.68 (1.17, 2.42) | 0.001 |
| 6 | + Total Energy Intake | 0.83 (0.56, 1.24) | 1.68 (1.17, 2.42) | 0.001 |
Abbreviations: HTN, hypertension; BP, blood pressure.
Individuals meeting the DASH target for a nutrient received a score of 1 while those who achieved the intermediate target for a nutrient received a score of 0.5 for that nutrient, for a total possible accordance score of 9. Persons with accordance scores falling between 0–1.0 were defined as low DASH accordance, while those with higher DASH accordance had scores between 1.5 and 8.0.
Rapid eGFR decline defined as eGFR decrease > 3 mL/min per 1.73 m2 per year during follow-up.
Nutrient intakes differed between participants with and without rapid eGFR decline. (Table 4) The rapid eGFR decline group had lower magnesium and calcium intake, and protein comprised a lower proportion of their diets than those without rapid eGFR decline (P<0.05 for all). Potassium intake was also lower among the rapid eGFR decline group (P 0.10). Multivariable logistic regression revealed, among all participants, that meeting the DASH diet protein target of ≥18% of total energy intake was associated with lesser odds of rapid eGFR decline (OR 0.64 95% CI 0.41 – 0.99). Among hypertensive participants, meeting the protein target and meeting the sodium target were both independently associated with lesser odds of rapid kidney function decline (protein OR 0.53 95% CI 0.29 – 0.99; sodium OR 0.37 95% CI 0.15 – 0.89). No significant association between individual DASH nutrient targets and rapid eGFR decline was identified among non-hypertensive participants.
TABLE 4.
Dietary Approaches to Stop Hypertension (DASH) £ Diet Accordant Nutrient Intakes by eGFR Decline Status at Follow Up* (N = 1443)
| DASH nutrients | DASH Target | eGFR decline ≤ 3 mL/min per 1.73 m2 per year N = 1250 |
eGFR decline > 3 mL/min per 1.73 m2 per year N = 193 |
P value |
|---|---|---|---|---|
| Saturated fat, % energy | ≤ 6 | 12 | 11 | 0.43 |
| Total Fat, % energy | ≤ 27 | 35 | 35 | 0.55 |
| Protein, % energy | ≥ 18 | 16 | 15 | 0.01 |
| Cholesterol, mg/1000kcal | ≤ 71.4 | 1632 | 152 | 0.11 |
| Fiber, g/1000 kcal | ≥ 14.8 | 6.4 | 5.9 | 0.07 |
| Magnesium, mg/1000 kcal | ≥ 238 | 126 | 119 | 0.03 |
| Calcium, mg/1000 kcal | ≥ 590 | 380 | 350 | 0.04 |
| Potassium, mg/1000 kcal | ≥ 2238 | 1,167 | 1,119 | 0.10 |
| Sodium, mg/1000 kcal | ≤ 1143 | 1589 | 1579 | 0.77 |
| Total DASH score | 9.0 | 1.8 | 1.6 | 0.06 |
| DASH, % adherent (total score ≥4.5) | -- | 5.9 | 4.6 | 0.43 |
Individuals meeting the DASH target for a nutrient received a score of 1 while those who achieved the intermediate target for a nutrient received a score of 0.5 for that nutrient, for a total possible accordance score of 9. Individuals with a total DASH score ≥ 4.5 are considered as DASH adherent.
Rapid eGFR decline defined as eGFR decrease > 3 mL/min per 1.73 m2 per year during follow-up.
DASH Diet Accordance and Incident CKD or eGFR Decline ≥ 25%
Fully, 38 (2.6%) developed incident CKD (eGFR <60) and 65 (4.5%) experienced eGFR decline ≥ 25% during follow-up. After adjustment for age, sex, race and poverty status, low DASH accordance was not associated with risk of either outcome (Table 5). No effect modification was identified.
TABLE 5.
Multinomial Logistic Regression Analyses for Associations between Dietary Approaches to Stop Hypertension (DASH) Diet £ Accordance and Incident Chronic Kidney Disease (CKD) or eGFR Decline ≥ 25%* (N = 1534)
| Model | Variable Included | Incident CKD (eGFR < 60 at follow-up)* | eGFR decline ≥ 25% |
|---|---|---|---|
|
| |||
| N events = 38 | N event = 65 | ||
|
| |||
| Risk Ratio (95% Confidence Interval) | |||
| 1 | Low versus Higher DASH Accordance | 1.52 (0.92, 2.52) | 1.23 (0.75, 2.02) |
| 2 | + age, sex, race | 1.49 (0.84, 2.63) | 1.36 (0.78, 2.37) |
| 3 | + poverty status* | 1.48 (0.84, 2.61) | 1.30 (0.76, 2.23) |
Individuals meeting the DASH target for a nutrient received a score of 1 while those who achieved the intermediate target for a nutrient received a score of 0.5 for that nutrient, for a total possible accordance score of 9. Persons with accordance scores falling between 0–1.0 were defined as low DASH accordance, while those with higher DASH accordance had scores between 1.5 and 8.0.
Incident CKD was defined as follow-up eGFR below 60 mL/min per 1.73 m2.
Sensitivity Analyses
Analyses categorizing DASH diet accordance into tertiles [tertile 1: DASH score 0–1, n=648; tertile 2: DASH score 1.5–2, n=445 and tertile 3: DASH score 2.5–7.5, n=441) yielded similar results to our primary models. There was no statistically significant association between DASH diet accordance and rapid eGFR decline or incident CKD. Effect modification by hypertension status was noted for rapid eGFR decline (P interaction 0.001). Among hypertensive participants, the adjusted RR comparing DASH tertile 1 to 3 was 1.49 (95% CI 0.98–2.25); while among non-hypertensive participants, the adjusted RR was 0.89 (95% CI 0.49–1.64).
A total of 983 (64%) participants had available urinary ACR data. Compared to the primary analysis cohort, this subset was comparable in age and gender, yet had fewer African Americans (56% vs. 64%) and less poverty (31% vs. 59%). Here, there was no association between low DASH accordance and rapid eGFR decline (adjusted RR 0.86, 95% CI 0.73–1.00, following adjustment for age, sex, race, poverty status, tobacco use, education level, hypertension, and diabetes). After further adjustment for baseline ACR, results were unchanged (adjusted RR 0.84, 95% CI 0.71–1.00). Stratified analyses by hypertension status, and adjusted for age, sex, race, poverty status, tobacco use, education and diabetes status, yielded an adjusted RR (comparing low DASH accordance to higher accordance) of 1.06, 95% CI 0.71–1.57 among hypertensive participants; and an adjusted RR 0.65, 95% CI 0.50–0.84 among non-hypertensive participants. Further adjustment for baseline ACR again did not change the magnitude or direction of the associations. Among participants who had follow-up creatinine measured at the same laboratory as their baseline creatinine (n=1408), results were similar to our primary analyses, both before and after stratification by hypertension (data not shown).
Discussion
Among this racially-balanced urban sample of adults, we observed that low accordance to a DASH-like diet was not associated with incident CKD or eGFR decline ≥ 25% during 5 years of follow up, but was associated with greater odds of rapid kidney function decline among individuals with hypertension. Among normotensive individuals, we found no association between DASH diet accordance and kidney function decline. Our findings persisted after adjustment for socio-demographic factors and did not vary by race, poverty status or diabetes status.
Previous studies have documented an association between healthful dietary patterns and kidney outcomes, including the DASH diet.8 Cross-sectional reports suggest accordance to the Mediterranean diet, which shares several characteristics with the DASH diet, is associated with lower prevalence of reduced eGFR among older 24 and middle-aged 11 populations. Greater diet quality, in general, has been associated with a lower prevalence of reduced kidney function among older adults.25 Our work extends that of previous studies by examining the relationship between DASH diet accordance and longitudinal kidney outcomes among a middle-aged, urban population with preserved eGFR at baseline.
If confirmed in other samples, a potential explanation for an association between DASH diet accordance and favorable kidney outcomes could relate to the low dietary acid load (DAL) of the DASH diet. DAL is determined by the difference between endogenously produced nonvolatile acid and absorbed alkali precursors, and must be excreted by the kidney to maintain acid-base balance.26 The estimated potential renal acid load (PRAL) of the DASH diet is −25.5 mEq/day 27, which is much lower than the 50 to 75 mEq/day observed in several general populations.28,29 Mechanisms underlying the association of DAL and kidney injury may include tubular toxicity of elevated ammonium concentration and increased angiotensin II activity with succeeding acidification of the distal nephron. 30 Several studies have documented potential kidney benefits of a diet low in DAL. 23,31,32 Additionally, the association we found solely among participants with hypertension may be explained by the beneficial effect of the DASH diet on vascular injury and endothelial dysfunction.33
In our study, most nutrients included in the DASH diet score were comparably consumed between participants who did and did not experience rapid eGFR decline, except for protein, magnesium and calcium. Protein comprised a slightly lower percentage of daily nutrient intakes among participants with rapid eGFR decline as compared to those without; and overall and among hypertensive participants, meeting the DASH diet protein target was associated with lesser odds of rapid kidney function decline. Given the lack of clarity on the potential benefits and harms of protein intake for the preservation of kidney function 34, future longitudinal studies are warranted that, in particular, examine the source of protein (animal versus plant) in the context of overall healthful dietary patterns.
Significantly lower magnesium intakes were observed among individuals who experienced rapid eGFR decline, which is consistent with findings of associations between low serum magnesium 35 and low magnesium intake36 with greater risk of incident CKD. A previous observational study also reported that higher serum magnesium was associated with less endothelial dysfunction, and separately, with higher eGFR, suggesting that magnesium may protect against endothelial damage.37 This finding supports the hypothesis that the vasodilatory effects of micronutrients, like magnesium, might contribute to the beneficial effects of the DASH diet on endothelial function. Significantly lower calcium intake was reported among participants who developed rapid eGFR decline. Similar to magnesium, calcium has also been noted to have favorable effects on endothelial function.38
While sodium consumption was not significantly different between participants who did or didn’t experience rapid kidney function decline, meeting the DASH sodium target was independently associated with lesser odds of rapid eGFR decline among hypertensive participants. This finding suggests that reduced sodium intake, which is known to favorably influence blood pressure control, might have protective effects on eGFR decline. A recently published study among persons with CKD enrolled in the Chronic Renal Insufficiency Cohort (CRIC), found that higher urinary sodium excretion (a correlate of sodium intake) was associated with greater risk of CKD progression39. These results and ours argue that sodium intake may be an important contributor to outcomes among persons at high risk for eGFR decline.
Our study had limitations. First, we were missing complete data for some participants, and in particular, had fewer males in our study sample than in the full HANDLS cohort. We believe that our estimates may be conservative as the association of low DASH diet accordance with rapid eGFR decline was slightly stronger among hypertensive males in our sample as compared to females (Supplementary Table A). Second our results may have been influenced by the use of the DASH score by Mellen et al. Others have operationalized DASH diet accordance differently, and some clinical outcomes have been found to be differentially associated with these various indices.40,41 Third, data on some important factors related to dietary intake and potentially to CKD, such as food additives 42, nutritional supplements 43, availability of healthful foods in neighborhood stores and physical activity 44 were unavailable. Fourth, some level of error has been associated with the measurement of food consumption. The energy intake measurement we used has been reported to underreport energy intake for both normal weight (by less than 3%) and overweight (by 16%) subjects 13, compared to the doubly labeled water technique, as we previously described.11 Fifth, because of variability in serum creatinine measures, changes based on only two data points could be due to random variation.45 Sixth, we lacked 24-hour urine collections to allow for direct analyses of DAL (estimated by net acid excretion) as a potential mediator of the association of dietary patterns with kidney outcomes. Future studies in this area are warranted.
Our findings could have important implications. The U.S. Preventive Services Task Force has recommended behavioral counseling interventions in primary care settings to promote a healthful diet and physical activity for cardiovascular disease prevention in adults.46 Towards diminishing the kidney disease burden in the U.S., our study provides evidence of potential benefits of a DASH-like dietary pattern for CKD prevention among hypertensive patients.
In conclusion, DASH diet accordance was low among this middle-aged urban population. Low DASH diet accordance was not associated with incident CKD or eGFR decline ≥ 25%, but was associated with greater risk for rapid kidney function decline among hypertensive individuals, yet not among non-hypertensive individuals. The role of diet quality in determining risk for kidney disease is worthy of further investigation.
Practical Application
Accordance to a Dietary Approaches to Stop Hypertension (DASH) diet may be associated with lower risk of rapid kidney function decline among individuals with hypertension. This finding suggests that diet quality may play an important role in determining kidney outcomes among individuals with risk factors for CKD.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health (NIH). Dr. Crews was supported by grant K23DK097184 from the National Institute of Diabetes and Digestive and Kidney Diseases, NIH.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest relevant to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boeing H, Bechthold A, Bub A, et al. Critical review: vegetables and fruit in the prevention of chronic diseases. European journal of nutrition. 2012;51(6):637–663. doi: 10.1007/s00394-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kontogianni MD, Panagiotakos DB. Dietary patterns and stroke: a systematic review and remeta-analysis. Maturitas. 2014;79(1):41–47. doi: 10.1016/j.maturitas.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Maghsoudi Z, Azadbakht L. How dietary patterns could have a role in prevention, progression, or management of diabetes mellitus? Review on the current evidence. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences. 2012;17(7):694–709. [PMC free article] [PubMed] [Google Scholar]
- 4.Reedy J, Krebs-Smith SM, Miller PE, et al. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. The Journal of nutrition. 2014;144(6):881–889. doi: 10.3945/jn.113.189407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyson CC, Nwankwo C, Lin PH, Svetkey LP. The Dietary Approaches to Stop Hypertension (DASH) eating pattern in special populations. Current hypertension reports. 2012;14(5):388–396. doi: 10.1007/s11906-012-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svetkey LP, Simons-Morton D, Vollmer WM, et al. Effects of dietary patterns on blood pressure: subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Archives of internal medicine. 1999;159(3):285–293. doi: 10.1001/archinte.159.3.285. [DOI] [PubMed] [Google Scholar]
- 7.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Archives of internal medicine. 2008;168(7):713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 8.Lin J, Fung TT, Hu FB, Curhan GC. Association of dietary patterns with albuminuria and kidney function decline in older white women: a subgroup analysis from the Nurses’ Health Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57(2):245–254. doi: 10.1053/j.ajkd.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster MC, Hwang SJ, Massaro JM, Jacques PF, Fox CS, Chu AY. Lifestyle Factors and Indices of Kidney Function in the Framingham Heart Study. American journal of nephrology. 2015;41(4–5):267–274. doi: 10.1159/000430868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goraya N, Simoni J, Jo CH, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(3):371–381. doi: 10.2215/CJN.02430312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crews DC, Kuczmarski MF, Miller ER, 3rd, Zonderman AB, Evans MK, Powe NR. Dietary Habits, Poverty, and Chronic Kidney Disease in an Urban Population. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2014 doi: 10.1053/j.jrn.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis. 2010;20(3):267–275. [PMC free article] [PubMed] [Google Scholar]
- 13.Moshfegh AJ, Rhodes DG, Baer DJ, et al. The US Department of Agriculture Automated Multiple- Pass Method reduces bias in the collection of energy intakes. The American journal of clinical nutrition. 2008;88(2):324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- 14.Agricultural Research Service. [Accessed May 12, 2014];FSRGUFaNDfDS, 3.0. 3.0. http://www.ars.usda.gov/Services/docs.htm?docid=12089.
- 15.Mellen PB, Gao SK, Vitolins MZ, Goff DC., Jr Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Archives of internal medicine. 2008;168(3):308–314. doi: 10.1001/archinternmed.2007.119. [DOI] [PubMed] [Google Scholar]
- 16.Sacks FM, Obarzanek E, Windhauser MM, et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol. 1995;5(2):108–118. doi: 10.1016/1047-2797(94)00055-x. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiramoto JS, Katz R, Peralta CA, et al. Inflammation and coagulation markers and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA) American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;60(2):225–232. doi: 10.1053/j.ajkd.2012.02.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bash LD, Coresh J, Kottgen A, et al. Defining incident chronic kidney disease in the research setting: The ARIC Study. Am J Epidemiol. 2009;170(4):414–424. doi: 10.1093/aje/kwp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. [Accessed June 3rd, 2014];The 2004 Health and Human Services Poverty Guidelines. 2004 http://aspe.hhs.gov/poverty/04poverty.shtml.
- 21.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 22.Choi S, Huang X. Maximum likelihood estimation of semiparametric mixture component models for competing risks data. Biometrics. 2014;70(3):588–598. doi: 10.1111/biom.12167. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee T, Crews DC, Wesson DE, et al. Dietary acid load and chronic kidney disease among adults in the United States. BMC Nephrol. 2014;15:137. doi: 10.1186/1471-2369-15-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Jimenez-Moleon JJ, Lindholm B, et al. Mediterranean diet, kidney function, and mortality in men with CKD. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(9):1548–1555. doi: 10.2215/CJN.01780213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gopinath B, Harris DC, Flood VM, Burlutsky G, Mitchell P. A better diet quality is associated with a reduced likelihood of CKD in older adults. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2013;23(10):937–943. doi: 10.1016/j.numecd.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Lennon EJ, Lemann J., Jr Influence of diet composition on endogenous fixed acid production. The American journal of clinical nutrition. 1968;21(5):451–456. doi: 10.1093/ajcn/21.5.451. [DOI] [PubMed] [Google Scholar]
- 27.Scialla JJ, Anderson CA. Dietary acid load: a novel nutritional target in chronic kidney disease? Advances in chronic kidney disease. 2013;20(2):141–149. doi: 10.1053/j.ackd.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engberink MF, Bakker SJ, Brink EJ, et al. Dietary acid load and risk of hypertension: the Rotterdam Study. The American journal of clinical nutrition. 2012;95(6):1438–1444. doi: 10.3945/ajcn.111.022343. [DOI] [PubMed] [Google Scholar]
- 29.Gannon RH, Millward DJ, Brown JE, et al. Estimates of daily net endogenous acid production in the elderly UK population: analysis of the National Diet and Nutrition Survey (NDNS) of British adults aged 65 years and over. The British journal of nutrition. 2008;100(3):615–623. doi: 10.1017/S0007114508901240. [DOI] [PubMed] [Google Scholar]
- 30.Wesson DE, Simoni J, Broglio K, Sheather S. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. American journal of physiology Renal physiology. 2011;300(4):F830–837. doi: 10.1152/ajprenal.00587.2010. [DOI] [PubMed] [Google Scholar]
- 31.Goraya N, Simoni J, Jo CH, Wesson DE. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014;86(5):1031–1038. doi: 10.1038/ki.2014.83. [DOI] [PubMed] [Google Scholar]
- 32.Goraya N, Simoni J, Jo C, Wesson DE. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012;81(1):86–93. doi: 10.1038/ki.2011.313. [DOI] [PubMed] [Google Scholar]
- 33.Rifai L, Pisano C, Hayden J, Sulo S, Silver MA. Impact of the DASH diet on endothelial function, exercise capacity, and quality of life in patients with heart failure. Proceedings (Baylor University Medical Center) 2015;28(2):151–156. doi: 10.1080/08998280.2015.11929216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikizler TA. Dietary protein restriction in CKD: the debate continues. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;53(2):189–191. doi: 10.1053/j.ajkd.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Tin A, Grams ME, Maruthur NM, et al. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney international. 2014 doi: 10.1038/ki.2014.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farhadnejad H, Asghari G, Mirmiran P, Yuzbashian E, Azizi F. Micronutrient Intakes and Incidence of Chronic Kidney Disease in Adults: Tehran Lipid and Glucose Study. Nutrients. 2016;8(4) doi: 10.3390/nu8040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanbay M, Yilmaz MI, Apetrii M, et al. Relationship between serum magnesium levels and cardiovascular events in chronic kidney disease patients. American journal of nephrology. 2012;36(3):228–237. doi: 10.1159/000341868. [DOI] [PubMed] [Google Scholar]
- 38.Chen Q, Zhang Y, Tong M, et al. Pre-treatment with calcium prevents endothelial cell activation induced by multiple activators, necrotic trophoblastic debris or IL-6 or preeclamptic sera: possible relevance to the pathogenesis of preeclampsia. Placenta. 2013;34(12):1196–1201. doi: 10.1016/j.placenta.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 39.He J, Mills KT, Appel LJ, et al. Urinary Sodium and Potassium Excretion and CKD Progression. Journal of the American Society of Nephrology : JASN. 2016;27(4):1202–1212. doi: 10.1681/ASN.2015010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez-Cornago A, Sanchez-Villegas A, Bes-Rastrollo M, et al. Relationship between adherence to Dietary Approaches to Stop Hypertension (DASH) diet indices and incidence of depression during up to 8 years of follow-up. Public Health Nutr. 2016:1–10. doi: 10.1017/S1368980016001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller PE, Cross AJ, Subar AF, et al. Comparison of 4 established DASH diet indexes: examining associations of index scores and colorectal cancer. The American journal of clinical nutrition. 2013;98(3):794–803. doi: 10.3945/ajcn.113.063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutierrez OM. Sodium- and phosphorus-based food additives: persistent but surmountable hurdles in the management of nutrition in chronic kidney disease. Advances in chronic kidney disease. 2013;20(2):150–156. doi: 10.1053/j.ackd.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grubbs V, Plantinga LC, Tuot DS, et al. Americans’ use of dietary supplements that are potentially harmful in CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61(5):739–747. doi: 10.1053/j.ajkd.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Painter P, Roshanravan B. The association of physical activity and physical function with clinical outcomes in adults with chronic kidney disease. Current opinion in nephrology and hypertension. 2013;22(6):615–623. doi: 10.1097/MNH.0b013e328365b43a. [DOI] [PubMed] [Google Scholar]
- 45.Selvin E, Juraschek SP, Eckfeldt J, Levey AS, Inker LA, Coresh J. Within-person variability in kidney measures. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61(5):716–722. doi: 10.1053/j.ajkd.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. [Accessed May 30th, 2014];Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults: Behavioral Counseling. 2012 http://www.uspreventiveservicestaskforce.org/uspstf/uspsphys.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.