Introduction
Liver biopsy remains the gold standard for diagnosing and staging the non-alcoholic steatohepatitis (NASH) subtype of non-alcoholic fatty liver disease (NAFLD) in children and adults.1, 2 The perilipin (PLIN) lipid droplet proteins PLIN1 and PLIN2 have been shown previously to be upregulated in hepatic steatosis and adult NASH3-7 but their differential expression in pediatric NAFLD is unknown. We examined the hepatic expression of PLIN1 and PLIN2 in pediatric NAFLD and compared them to adults with NAFLD and children with steatosis due to Hepatitis C (HepC).
Methods
This study consisting of archived samples was reviewed and granted exempt status by the Institutional Review Board (IRB) at Indiana University. Twenty-five pediatric and twenty-nine adult paraffin-embedded liver sections were immunostained with PLIN1 and PLIN2 antibodies. Liver histology was independently analyzed for steatosis and inflammation and categorized as normal, non-alcoholic fatty liver (NAFL), NASH or HepC. PLIN1 and PLIN2 immunostaining was scored on a qualitative scale of 0 to 5 by a reviewer blinded to patient diagnosis: 0 no staining; 1=<5% staining; 2= 5-10% staining; 3= 11-49% staining; 4= 50-75% staining; 5=>75% staining.
Statistical analysis
Data were analyzed with ANOVA and Neuman-Keuls test. P<0.05 is considered significant.
Results
Clinical characteristics
Among children, five had normal histology, six had NAFL, nine had NASH, and five had HepC. The adults included fifteen with normal livers, three with NAFL and eleven with NASH. Pediatric controls were younger than those with steatotic diseases (7 versus 13, p<0.0005); however there was no difference in age among pediatric NAFL, NASH, and HepC patients. Body mass index (BMI) in pediatric NAFL and NASH patients was twice that of controls (36 vs 17 mg/kg p<0.0001). Patients with HepC had an average BMI of 20.7 mg/kg, intermediate between control and the NAFLD cohorts. The mean age of the adult control and NAFL patients was 49; and 43 in NASH patients (p=0.56). Average BMI among adult NAFL patients was 25 kg/m2 compared with 35 kg/m2 for NASH patients (p=0.009). There was no difference in serum transaminase levels among the pediatric or adult groups.
Hepatic perilipin immunostaining in children and adults
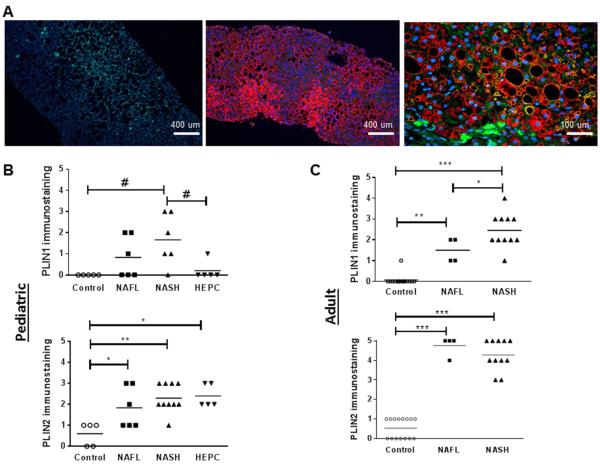
In pediatric controls, PLIN1 immunostaining was absent. PLIN1 was present in 1 child with HepC, mildly expressed in NAFL (0.83±0.4) and increased in NASH (1.67±0.5, P<0.05 vs. control.) Unlike PLIN1, low levels of PLIN2 were detected in the livers of control children (0.6±0.24). Compared with control, PLIN2 staining was more pronounced in NAFL (1.8±0.4, P≤0.01), NASH (2.3±0.2, P≤0.001), and HepC (2.4±0.24, P≤0.01) (Figure).
Figure. Immunoflorescence (IF) of hepatic PLIN1 and PLIN2 in children with NAFL, NASH and HCV and adults with NAFL and NASH.
A. PLIN1, PLIN2 and combined PLIN1/PLIN2 IF liver sections. Left=PLIN1, middle=PLIN2, right=combined B. Quantification of PLIN1 and PLIN2 staining in children C. Quantification of PLIN1 and PLIN2 staining in adults. # p<0.05 *p≤0.01 **p≤0.001 ***p≤0.0001
In adults, PLIN1 immunostaining was detected in only one control adult and was significantly higher in adult NASH compared with NAFL (2.4±0.04 vs.1.5±0.28, P≤0.01). PLIN2 was detected at low levels in controls (0.5±0.13) and significantly increased in both NAFL (4.75±0.25) and NASH (4.27±0.23) (P<0.0001 vs. control) (Figure).
These data suggest that similar to adult patients, PLIN1 is de novo expressed in pediatric NAFLD and significantly increased in NASH. In addition, there is differential expression of PLIN1 in pediatric NASH compared with HepC.
Discussion
NAFLD is one of the few hepatic diseases that impacts both children and adults at alarmingly high rates. Epidemiologic studies show that there is a divergence in risk of disease progression and mortality in patients with NAFL and NASH.1, 8 However, pathologic distinction between these clinical subtypes is challenging.
The use of Perilipin proteins in the histopathologic distinction of pediatric NAFL and NASH has not been described previously. Here, we demonstrate that similar to adults, PLIN2 is an abundant hepatic LD protein that is present in both normal and disease states in children and is therefore a non-specific histologic marker of hepatic steatosis. In contrast, PLIN1 is not detectable in the majority of normal livers, is modestly increased in NAFL and has the highest expression in hepatic steatosis due to NASH compared with control, NAFL and HCV. These data suggest that the combination of PLIN1 and PLIN2 immunostaining may be a useful histologic tool in NAFLD children. Further studies are needed to understand if in a larger cohort of pediatric and adult NAFLD patients this relationship is maintained.
Acknowledgement
This research was supported by N.I.H grants P01-DK-049210 (R.S.A), R01-DK-074627, K24-DK-069290 (N.C), T32-DK-007066, NIH/NIAAA K08-AA021424, RWJ 7158, P30 DK019525 (R.M.C.). This work was supported in part by NIH P30-DK050306 and its core facilities pilot grant program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Study concept and design (RMC, NPC, RSA); Acquisition of data (RMC, RD, KM, MC); Drafting of the manuscript (RMC, NPC, RSA); Critical revision of the manuscript for important intellectual content (all authors); Statistical analysis (RMC, RD); Study supervision (NPC, RSA)
Conflict of Interest: None to declare
References
- 1.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, Lavine JE. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–9. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 3.Chang BH, Li L, Paul A, Taniguchi S, Nannegari V, Heird WC, Chan L. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Molecular and cellular biology. 2006;26:1063–76. doi: 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McManaman JL, Bales ES, Orlicky DJ, Jackman M, MacLean PS, Cain S, Crunk AE, Mansur A, Graham CE, Bowman TA, Greenberg AS. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. Journal of lipid research. 2013;54:1346–59. doi: 10.1194/jlr.M035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straub BK, Stoeffel P, Heid H, Zimbelmann R, Schirmacher P. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology. 2008;47:1936–46. doi: 10.1002/hep.22268. [DOI] [PubMed] [Google Scholar]
- 6.Carr RM, Peralta G, Yin X, Ahima RS. Absence of perilipin 2 prevents hepatic steatosis, glucose intolerance and ceramide accumulation in alcohol-fed mice. PloS one. 2014;9:e97118. doi: 10.1371/journal.pone.0097118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varela GM, Antwi DA, Dhir R, Yin X, Singhal NS, Graham MJ, Crooke RM, Ahima RS. Inhibition of ADRP prevents diet-induced insulin resistance. American journal of physiology. Gastrointestinal and liver physiology. 2008;295:G621–8. doi: 10.1152/ajpgi.90204.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009;50:1282–93. doi: 10.1002/hep.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]